Oncologic Outcome of Robotic-Assisted and Laparoscopic Sentinel Node Biopsy in Endometrial Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
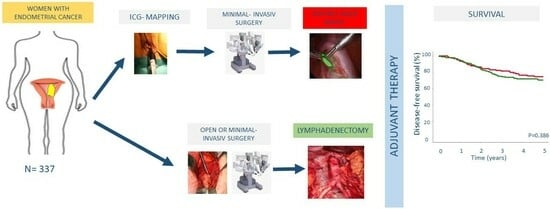
2. Materials and Methods
2.1. Patients
2.2. SLNB and LND
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ignatov, A.; Papathemelis, T.; Ivros, S.; Ignatov, T.; Ortmann, O.; Eggemann, H. Comparison of survival of patients with endometrial cancer undergoing sentinel node biopsy alone or systematic lymphadenectomy. Arch. Gynecol. Obstet. 2020, 302, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- ASTEC Study Group; Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Cheung, M.K.; Huh, W.K.; Osann, K.; Husain, A.; Teng, N.N.; Kapp, D.S. Therapeutic role of lymph node resection in endometrioid corpus cancer: A study of 12,333 patients. Cancer 2006, 107, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Cragun, J.M.; Havrilesky, L.J.; Calingaert, B.; Synan, I.; Secord, A.A.; Soper, J.T.; Clarke-Pearson, D.L.; Berchuck, A. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J. Clin. Oncol. 2005, 23, 3668–3675. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ho, C.M.; Chen, Y.L.; You, S.L.; Chen, C.A.; Cheng, W.F. Impact of lymphadenectomy in uterine endometrioid carcinoma. Eur. J. Surg. Oncol. 2013, 39, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, X.; Cui, M.; Wang, J. Sentinel Lymph Node Mapping in Endometrial Cancer: A Comprehensive Review. Front. Oncol. 2021, 11, 701758. [Google Scholar] [CrossRef]
- Bogani, G.; Giannini, A.; Vizza, E.; Di Donato, V.; Raspagliesi, F. Sentinel node mapping in endometrial cancer. J. Gynecol. Oncol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef]
- Goncalves, B.T.; Dos Reis, R.; Ribeiro, R.; Moretti-Marques, R.; Schamme, F.K.; Oliveira, G.S.; Tsunoda, A.T.; Alvarenga-Bezerra, V.; Lopes, A.; Pastore, C.B.P.; et al. Does sentinel node mapping impact morbidity and quality of life in endometrial cancer? Int. J. Gynecol. Cancer 2023, 33, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Tanaka, T.; Murakami, H.; Tsuchihashi, H.; Toji, A.; Daimon, A.; Miyamoto, S.; Nishie, R.; Ueda, S.; Hashida, S.; et al. Lymphatic Complications Following Sentinel Node Biopsy or Pelvic Lymphadenectomy for Endometrial Cancer. J. Clin. Med. 2023, 12, 4540. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Dowdy, S.C.; Cliby, W.A.; Ghezzi, F.; Rossetti, D.; Mariani, A. Role of pelvic and para-aortic lymphadenectomy in endometrial cancer: Current evidence. J. Obstet. Gynaecol. Res. 2014, 40, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Papadia, A.; Buda, A.; Casarin, J.; Di Donato, V.; Gasparri, M.L.; Plotti, F.; Pinelli, C.; Paderno, M.C.; Lopez, S.; et al. Sentinel node mapping vs. sentinel node mapping plus back-up lymphadenectomy in high-risk endometrial cancer patients: Results from a multi-institutional study. Gynecol. Oncol. 2021, 161, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.; Gomez-Hidalgo, N.R.; Garcia-Pineda, V.; Bebia, V.; Fernandez-Gonzalez, S.; Alonso, P.; Rodriguez-Gomez, T.; Fuste, P.; Gracia-Segovia, M.; Lorenzo, C.; et al. Accuracy and Survival Outcomes after National Implementation of Sentinel Lymph Node Biopsy in Early Stage Endometrial Cancer. Ann. Surg. Oncol. 2023, 30, 7653–7662. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Rosati, A.; Maglietta, G.; Vargiu, V.; Scarpelli, E.; Cosentino, F.; Sozzi, G.; Chiantera, V.; Ghi, T.; Scambia, G.; et al. Long-term survival outcomes in high-risk endometrial cancer patients undergoing sentinel lymph node biopsy alone versus lymphadenectomy. Int. J. Gynecol. Cancer 2023, 33, 1013–1020. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, L.; Chen, X.; Wen, Q.; Shao, Z.; Tang, X.; Shi, X.; Wang, J.; Zhang, Y.; Zhu, T. A multicenter noninferior randomized controlled study of sentinel lymph node biopsy alone versus sentinel lymph node biopsy plus lymphadenectomy for patients with stage I endometrial cancer, INSEC trial concept. BMC Cancer 2023, 23, 1184. [Google Scholar] [CrossRef]
- Khemworapong, K.; Jaishuen, A.; Srichaikul, P.; Inthasorn, P.; Viriyapak, B.; Achariyapota, V.; Jareemit, N.; Warnnissorn, M.; Hanamornroongruang, S.; Sukmee, J. The fluorescence imaging for laparoscopic and laparotomic endometrial sentinel lymph node biopsy (FILLES) trial: Siriraj gynecologic sentinel node of endometrial cancer (SiGN-En) study. J. Surg. Oncol. 2023, 1–7. [Google Scholar] [CrossRef]
- Koh, K.M.L.; Ng, Z.Y.; Chin, F.H.X.; Wong, W.L.; Wang, J.; Lim, Y.K. Comparing Surgical and Oncological Outcomes between Indocyanine Green (ICG) Sentinel Lymph Node Mapping with Routine Lymphadenectomy in the Surgical Staging of Early-Stage Endometrioid Endometrial Cancer. Obstet. Gynecol. Int. 2023, 2023, 9949604. [Google Scholar] [CrossRef]
- Salman, L.; Cusimano, M.C.; Marchocki, Z.; Ferguson, S.E. Sentinel lymph node mapping in endometrial cancer: Current evidence and practice. J. Surg. Oncol. 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.G.Z.; Davidson, B.; Bjerre Trent, P.; Eyjolfsdottir, B.; Dahl, G.F.; Wang, Y.; Staff, A.C. Update on Sentinel Lymph Node Biopsy in Surgical Staging of Endometrial Carcinoma. J. Clin. Med. 2021, 10, 3094. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Schlappe, B.A.; Weaver, A.L.; Ducie, J.A.; Eriksson, A.G.Z.; Dowdy, S.C.; Cliby, W.A.; Glaser, G.E.; Soslow, R.A.; Alektiar, K.M.; Makker, V.; et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: A sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy. Gynecol. Oncol. 2018, 151, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.B.; Zivanovic, O.; Zhou, Q.; Leitao, M.M., Jr.; Levine, D.A.; Soslow, R.A.; Alektiar, K.M.; Makker, V.; Iasonos, A.; Abu-Rustum, N.R. Survival of Patients with Uterine Carcinosarcoma Undergoing Sentinel Lymph Node Mapping. Ann. Surg. Oncol. 2016, 23, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Basaran, D.; Bruce, S.; Aviki, E.M.; Mueller, J.J.; Broach, V.A.; Cadoo, K.; Soslow, R.A.; Alektiar, K.M.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Sentinel lymph node mapping alone compared to more extensive lymphadenectomy in patients with uterine serous carcinoma. Gynecol. Oncol. 2020, 156, 70–76. [Google Scholar] [CrossRef]
- Schlappe, B.A.; Weaver, A.L.; McGree, M.E.; Ducie, J.; Zahl Eriksson, A.G.; Dowdy, S.C.; Cliby, W.A.; Glaser, G.E.; Abu-Rustum, N.R.; Mariani, A.; et al. Multicenter study comparing oncologic outcomes after lymph node assessment via a sentinel lymph node algorithm versus comprehensive pelvic and paraaortic lymphadenectomy in patients with serous and clear cell endometrial carcinoma. Gynecol. Oncol. 2020, 156, 62–69. [Google Scholar] [CrossRef]
- Ignatov, A.; Lebius, C.; Ignatov, T.; Ivros, S.; Knueppel, R.; Papathemelis, T.; Ortmann, O.; Eggemann, H. Lymph node micrometastases and outcome of endometrial cancer. Gynecol. Oncol. 2019, 154, 475–479. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Gomez, J.D.; Alektiar, K.M.; Soslow, R.A.; Hensley, M.L.; Leitao, M.M., Jr.; Gardner, G.J.; Sonoda, Y.; Chi, D.S.; Barakat, R.R. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol. Oncol. 2009, 115, 236–238. [Google Scholar] [CrossRef]
- Rutten, H.; Verhoef, C.; van Weelden, W.J.; Smits, A.; Dhanis, J.; Ottevanger, N.; Pijnenborg, J.M.A. Recurrent Endometrial Cancer: Local and Systemic Treatment Options. Cancers 2021, 13, 6275. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Emons, G.; Kim, J.W.; Weide, K.; de Gregorio, N.; Wimberger, P.; Trillsch, F.; Gabriel, B.; Denschlag, D.; Kommoss, S.; Aydogdu, M.; et al. Endometrial Cancer Lymphadenectomy Trial (ECLAT) (pelvic and para-aortic lymphadenectomy in patients with stage I or II endometrial cancer with high risk of recurrence; AGO-OP.6). Int. J. Gynecol. Cancer 2021, 31, 1075–1079. [Google Scholar] [CrossRef]



| SnB | LAD | p-Value | |
|---|---|---|---|
| Total | 150 (44.5%) | 187 (55.5%) | |
| Age, median | 67 (42–86) | 67 (33–81) | 0.804 |
| Stage | |||
| I | 114 (76.0%) | 127 (67.9%) | 0.217 |
| II | 15 (10.0%) | 29 (15.5%) | |
| III | 21 (14.0%) | 31 (16.6%) | |
| Grading | |||
| Low | 102 (68.9%) | 89 (47.6%) | <0.001 |
| High | 46 (31.1%) | 98 (52.4%) | |
| Myometrium invasion | |||
| <50% | 70 (49%) | 49 (27.5%) | <0.001 |
| >50% | 73 (51.0%) | 129 (72.5%) | |
| LVSI | |||
| Negative | 110 (73.8%) | 39 (26.2%) | 0.393 |
| Positive | 125 (69.1%) | 56 (30.9%) | |
| Extirpated LN, median | 3 (2–27) | 42 (6–88) | <0.001 |
| LN-Status | |||
| Negative | 123 (82.0%) | 156 (83.4%) | 0.772 |
| Positive | 27 (18.0%) | 31 (16.6%) | |
| Adjuvant Therapy | |||
| No | 86 (57.3%) | 96 (51.3%) | 0.748 |
| Radiotherapy | 17 (11.3%) | 25 (13.4%) | |
| Chemotherapy | 10 (6.7%) | 14 (7.5%) | |
| Both | 37 (24.7%) | 52 (27.8%) |
| SnB | LND | p-Value | |
|---|---|---|---|
| Total | 37 (24.7%) | 54 (59.3%) | 0.459 |
| Local | 14 (9.3%) | 12 (6.4%) | 0.412 |
| Regional lymph nodes | 5 (3.3%) | 11 (5.9%) | 0.313 |
| Paraaortic/distant lymph nodes | 4 (2.7%) | 7 (3.7%) | 0.760 |
| Abdominal | 8 (5.3%) | 9 (4.8%) | 1.000 |
| Distant | 12 (8%) | 25 (13.4%) | 0.160 |
| Variable | Patients with Negative Lymph Nodes | Patients with Positive Lymph Nodes | ||||||
|---|---|---|---|---|---|---|---|---|
| DFS | OS | DFS | OS | |||||
| HR, (CI 95%) | p-Value | HR, (CI 95%) | p-Value | HR, (CI 95%) | p-Value | HR, (CI 95%) | p-Value | |
| Approach | 0.172 | 0.058 | 0.257 | 0.496 | ||||
| SLNB | Reference | Reference | Reference | Reference | ||||
| LND | 1.75 (0.79–3.90) | 2.47 (0.97–6.30) | 1.16 (0.71–3.68) | 1.39 (0.54–356) | ||||
| Age | 0.97 (0.94–1.01) | 0.142 | 0.99 (0.96–1.04) | 0.868 | 0.97 (0.93–1.000) | 0.052 | 0.99 (0.96–1.02) | 0.544 |
| Histology | c | 0.008 | 0.031 | 0.327 | 0.902 | |||
| Type I | Reference | Reference | Reference | Reference | ||||
| Type II | 2.70 (1.30–5.61) | 2.11 (1.07–4.15) | 1.35 (0.74–2.49) | 0.95 (0.45–2.00) | ||||
| Stage | 0.004 | 0.041 | 0.002 | 0.016 | ||||
| FIGO I/II | Reference | Reference | Reference | Reference | ||||
| FIGO III | 4.56 (1.64–12.64) | 3.07 (1.04–9.02) | 3.80 (1.61–8.95) | 3.01 (1.23–7.32) | ||||
| Grading | 0.093 | 0.47 | 0.65 | 0.739 | ||||
| Low | Reference | Reference | Reference | Reference | ||||
| High | 0.39 (0.13–1.17) | 0.69 (0.25–1.90) | 0.77 (0.24–2.41) | 0.83 (0.27–2.56) | ||||
| LVSI | 0.528 | 0.382 | 0.774 | 0.807 | ||||
| Negative | Reference | Reference | Reference | Reference | ||||
| Positive | 0.72 (0.26–1.98) | 1.53 (0.59–3.94) | 1.17 (0.40–3.41) | 0.88 (0.30–2.56) | ||||
| Adjuvant therapy | 0.103 | 0.631 | 0.006 | 0.003 | ||||
| None | Reference | References | Reference | Reference | ||||
| RT | 2.06 (0.96–3.41) | 0.86 (0.32–2.27) | 0.44 (0.10–2.26) | 0.42 (0.10–1.69) | ||||
| RT + CT | 2.28 (0.85–6.16) | 1.28 (0.46–3.56) | 0.22 (0.07–0.64) | 0.18 (0.06–0.55) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatov, A.; Mészáros, J.; Ivros, S.; Gennari, P.; Ignatov, T. Oncologic Outcome of Robotic-Assisted and Laparoscopic Sentinel Node Biopsy in Endometrial Cancer. Cancers 2023, 15, 5894. https://doi.org/10.3390/cancers15245894
Ignatov A, Mészáros J, Ivros S, Gennari P, Ignatov T. Oncologic Outcome of Robotic-Assisted and Laparoscopic Sentinel Node Biopsy in Endometrial Cancer. Cancers. 2023; 15(24):5894. https://doi.org/10.3390/cancers15245894
Chicago/Turabian StyleIgnatov, Atanas, József Mészáros, Stylianos Ivros, Paolo Gennari, and Tanja Ignatov. 2023. "Oncologic Outcome of Robotic-Assisted and Laparoscopic Sentinel Node Biopsy in Endometrial Cancer" Cancers 15, no. 24: 5894. https://doi.org/10.3390/cancers15245894
APA StyleIgnatov, A., Mészáros, J., Ivros, S., Gennari, P., & Ignatov, T. (2023). Oncologic Outcome of Robotic-Assisted and Laparoscopic Sentinel Node Biopsy in Endometrial Cancer. Cancers, 15(24), 5894. https://doi.org/10.3390/cancers15245894