Priming of Colorectal Tumor-Associated Fibroblasts with Zoledronic Acid Conjugated to the Anti-Epidermal Growth Factor Receptor Antibody Cetuximab Elicits Anti-Tumor Vδ2 T Lymphocytes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. TAF Isolation from Tumor Specimens
2.2. ZA and Cet-ZA ADC
2.3. CRC-TAF/T Cell Co-Cultures
2.4. Vδ2 T Cell Proliferation
2.5. Immunofluorescence and Flow Cytometry
2.6. Confocal Microscopy
2.7. Cytotoxicity Assay and Interferon γ (IFNγ) Production
2.8. Immunohistochemistry (IHC) and Digital Image Analysis
2.9. Statistical Analysis
3. Results
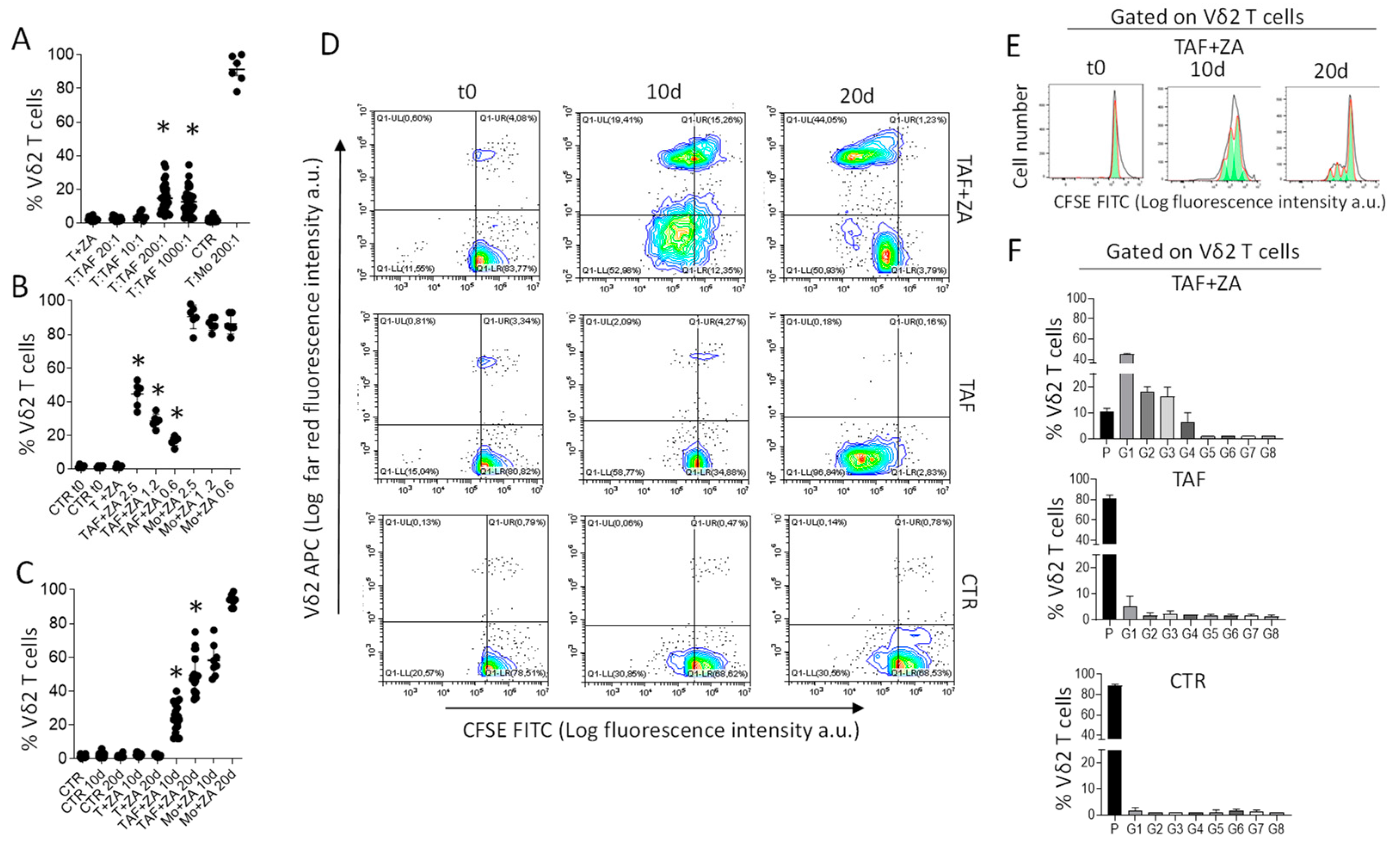
3.1. CRC-TAF Can Stimulate the Expansion of Vδ2 T Cells with Effector Phenotype
3.2. Cet-ZA ADC Reacts with CRC-TAF and Induces Vδ2 Cytotoxic Effector T Cells
3.3. CRC-TAF Express In Situ BTN3A Molecules in Stromal Areas within the Tumor
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazennec, G.; Jorgensen, C. Concise review: Adult multipotent stromal cells and cancer: Risk or benefit? Stem Cells 2008, 26, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Krampera, M. mesenchymal stromal cell “licensing”: A multistep process. Leukemia 2011, 25, 1408–1414. [Google Scholar] [CrossRef] [Green Version]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumour growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmiewtz, J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T cell unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef] [Green Version]
- Poggi, A.; Musso, A.; Dapino, I.; Zocchi, M.R. Mechanisms of tumour escape from immune system: Role of mesenchymal stromal cells. Immunol. Lett. 2014, 159, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Miyazaki, E.; Enzan, H. Differential expression of high molecular weight caldesmon in colorectal pericryptal fibroblasts and tumour stroma. J. Clin. Pathol. 1999, 52, 785–786. [Google Scholar] [CrossRef] [Green Version]
- Cirri, P.; Chiarugi, P. Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012, 31, 195–208. [Google Scholar] [CrossRef]
- Raffaghello, L.; Dazzi, F. Classification and biology of tumour associated stromal cells. Immunol. Lett. 2015, 168, 175–182. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Barnas, J.L.; Simpson-Abelson, M.R.; Brooks, S.P.; Kelleher, R.J.; Bankert, R.B. Reciprocal functional modulation of the activation of T lymphocytes and fibroblasts derived from human solid tumours. J. Immunol. 2010, 185, 2681–2692. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.M.; Dwyer, R.M.; Joyce, M.R.; Kerin, M.J. Mesenchymal stem cells in the colorectal tumour microenvironment: Recent progress and implications. Int. J. Cancer 2012, 131, 1–7. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, G.; Heijltjes, M.; Houston, A.M.; Rani, S.; Ritter, T.; Egan, L.J.; Ryan, A.E. Mesenchymal stromal cells (MSCs) and colorectal cancer—A troublesome twosome for the anti-tumour immune response? Oncotarget 2016, 7, 60752–60774. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, M.; Kruger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumour associated fibroblasts improves cancer chemotherapy by increasing intratumoural drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Zhao, Y.; Yang, H.; Tong, A.; Zhao, C.; Shi, H.; Li, Y.; Wang, Z.; Wei, Y. Immunotherapy of tumour with vaccine based on basic fibroblast growth factor-activated fibroblasts. J. Cancer Res. Clin. Oncol. 2014, 140, 271–280. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Liu, L.; Yu, J.; Ren, X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol. Ther. 2012, 13, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Poggi, A.; Varesano, S.; Zocchi, M.R. How to hit mesenchymal stromal cells and make the tumour microenvironment immunostimulant rather than immunosuppressive. Front. Immunol. 2018, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Zocchi, M.R.; Costa, D.; Venè, R.; Tosetti, F.; Ferrari, N.; Minghelli, S.; Benelli, R.; Scabini, S.; Romairone, E.; Catellani, S.; et al. Zoledronate can induce colorectal cancer microenvironment expressing BTN3A1 to stimulate effector γδ T cells with anti-tumour activity. Oncoimmunology 2017, 6, e1278099. [Google Scholar] [CrossRef]
- Costa, D.; Venè, R.; Benelli, R.; Romairone, E.; Scabini, S.; Catellani, S.; Rebesco, B.; Mastracci, L.; Grillo, F.; Minghelli, S.; et al. Targeting the Epidermal Growth Factor Receptor Can Counteract the Inhibition of Natural Killer Cell Function Exerted by Colorectal Tumour-Associated Fibroblasts. Front. Immunol. 2018, 9, 1150. [Google Scholar] [CrossRef]
- Corvaisier, M.; Moreau-Aubry, A.; Diez, E.; Bennouna, J.; Mosnier, J.F.; Scotet, E.; Bonneville, M.; Jotereau, F. V. gamma 9V delta 2 T cell response to colon carcinoma cells. J. Immunol. 2005, 175, 5481–5488. [Google Scholar] [CrossRef] [Green Version]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Meraviglia, S.; Lo Presti, E.; Tosolini, M.; La Mendola, C.; Orlando, V.; Todaro, M.; Catalano, V.; Stassi, G.; Cicero, G.; Vieni, S.; et al. Distinctive features of tumour-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology 2017, 6, e1347742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumour cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, H.; Wang, L.; Kamath, A.; Bukowski, J.F. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood 2001, 98, 1616–1618. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, S.; Kumar, A.; Wan, G.; Ramanjaneyulu, G.S.; Cavallari, M.; El Daker, S.; Beddoe, T.; Theodossis, A.; Williams, N.K.; Gostick, E.; et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 2013, 14, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Cano, C.E.; Pasero, C.; De Gassart, A.; Kerneur, C.; Gabriac, M.; Fullana, M.; Granarolo, E.; Hoet, R.; Scotet, E.; Rafia, C.; et al. BTN2A1, an immune checkpoint targeting Vγ9Vδ2 T cell cytotoxicity against malignant cells. Cell Rep. 2021, 36, 109359. [Google Scholar] [CrossRef]
- Santini, D.; Vespasiani Gentilucci, U.; Vincenzi, B.; Picardi, A.; Vasaturo, F.; La Cesa, A.; Onori, N.; Scarpa, S.; Tonini, G. The antineoplastic role of bisphosphonates: From basic research to clinical evidence. AnnOncol 2003, 14, 1468–1476. [Google Scholar] [CrossRef]
- Clézardin, P.; Fournier, P.; Boissier, S.; Peyruchaud, O. In vitro and in vivo anti-tumour effects of bisphopshonates. Curr. Med. Chem. 2003, 10, 173–180. [Google Scholar] [CrossRef]
- Santolaria, T.; Robard, M.; Léger, A.; Catros, V.; Bonneville, M.; Scotet, E. Repeated systemic administration of aminobisphosphonates and human Vγ9Vδ2 T cells efficiently control tumour development in vivo. J. Immunol. 2013, 191, 1993–2000. [Google Scholar] [CrossRef]
- Musso, A.; Zocchi, M.R.; Poggi, A. Relevance of the mevalonate biosynthetic pathway in the regulation of bone marrow mesenchymal stromal cell-mediated effects on T-cell proliferation and B-cell survival. Haematologica 2011, 96, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Varesano, S.; Zocchi, M.R.; Poggi, A. Zoledronate triggers Vδ2 T cells to destroy and kill spheroids of colon carcinoma: Quantitative image analysis of three-dimensional cultures. Front. Immunol. 2018, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Di Mascolo, D.; Varesano, S.; Benelli, R.; Mollica, H.; Salis, A.; Zocchi, M.R.; Decuzzi, P.; Poggi, A. Nanoformulated Zoledronic Acid Boosts the Vδ2 T Cell Immunotherapeutic Potential in Colorectal Cancer. Cancers 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benelli, R.; Costa, D.; Salvini, L.; Tardito, S.; Tosetti, F.; Villa, F.; Zocchi, M.R.; Poggi, A. Targeting of colorectal cancer organoids with zoledronic acid conjugated to the anti-EGFR antibody cetuximab. J. Immunother. Cancer 2022, in press. [Google Scholar] [CrossRef]
- Benelli, R.; Venè, R.; Minghelli, S.; Carlone, S.; Gatteschi, B.; Ferrari, N. Celecoxib induces proliferation and Amphiregulin production in colon subepithelial myofibroblasts, activating erk1-2 signaling in synergy with EGFR. Cancer Lett. 2013, 328, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Prevosto, C.; Zancolli, M.; Canevali, P.; Zocchi, M.R.; Poggi, A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 2007, 92, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Locatelli, I.; D’Arrigo, C.; Mora, M.; Vozzi, G.; De Acutis, A.; Pece, R.; Tavella, S.; Costa, D.; Poggi, A.; et al. Lysyl-Oxidase Dependent Extracellular Matrix Stiffness in Hodgkin Lymphomas: Mechanical and Topographical Evidence. Cancers 2022, 14, 259. [Google Scholar] [CrossRef]
- Dieli, F.; Poccia, F.; Lipp, M.; Sireci, G.; Caccamo, N.; Di Sano, C.; Salerno, A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J. Exp. Med. 2003, 198, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Musso, A.; Catellani, S.; Canevali, P.; Tavella, S.; Venè, R.; Boero, S.; Pierri, I.; Gobbi, M.; Kunkl, A.; Ravetti, J.L.; et al. Aminobisphosphonate prevent the inhibitory effect exerted by lymph node stromal cells on antitumour Vδ2 T lymphocytes in non-Hodgkin lymphomas. Haematologica 2014, 99, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. mAbs 2021, 13, 1951427. [Google Scholar] [CrossRef]
- Olatunji, F.P.; Herman, J.W.; Kesic, B.N.; Olabode, D.; Berkman, C.E. A click-ready pH-triggered phosphoramidate-based linker for controlled-release of monomethyl auristatin E. Tetrahedron. Lett. 2020, 61, 152398. [Google Scholar] [CrossRef]
- Angelini, D.F.; Borsellino, G.; Poupot, M.; Diamantini, A.; Poupot, R.; Bernardi, G.; Poccia, F.; Fournié, J.J.; Battistini, L. FcγRIII discriminates between 2 subsets of Vγ9Vγδ2 effector cells with different responses and activation pathways. Blood 2004, 104, 1801–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohrt, H.E.; Houot, R.; Marabelle, A.; Cho, H.J.; Osman, K.; Goldstein, M.; Levy, R.; Brody, J. Combination strategies to enhance antitumour ADCC. Immunotherapy 2012, 4, 511–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, P.J.; Rajpal, A. Designing antibodies as therapeutics. Cell 2022, 185, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvey, C.M.; Lau, R.; Sanchez, A.; Sun, R.X.; Fong, E.J.; Doche, M.E.; Chen, O.; Jusuf, A.; Lenz, H.J.; Larson, B.; et al. Anti-EGFR Therapy Induces EGF Secretion by Cancer-Associated Fibroblasts to Confer Colorectal Cancer Chemoresistance. Cancers 2020, 12, 1393. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez, J.L.C.; Benelli, R.; Costa, D.; Campioli, A.; Tavella, S.; Zocchi, M.R.; Poggi, A. Priming of Colorectal Tumor-Associated Fibroblasts with Zoledronic Acid Conjugated to the Anti-Epidermal Growth Factor Receptor Antibody Cetuximab Elicits Anti-Tumor Vδ2 T Lymphocytes. Cancers 2023, 15, 610. https://doi.org/10.3390/cancers15030610
Fernandez JLC, Benelli R, Costa D, Campioli A, Tavella S, Zocchi MR, Poggi A. Priming of Colorectal Tumor-Associated Fibroblasts with Zoledronic Acid Conjugated to the Anti-Epidermal Growth Factor Receptor Antibody Cetuximab Elicits Anti-Tumor Vδ2 T Lymphocytes. Cancers. 2023; 15(3):610. https://doi.org/10.3390/cancers15030610
Chicago/Turabian StyleFernandez, Jordi Leonardo Castrillo, Roberto Benelli, Delfina Costa, Alessio Campioli, Sara Tavella, Maria Raffaella Zocchi, and Alessandro Poggi. 2023. "Priming of Colorectal Tumor-Associated Fibroblasts with Zoledronic Acid Conjugated to the Anti-Epidermal Growth Factor Receptor Antibody Cetuximab Elicits Anti-Tumor Vδ2 T Lymphocytes" Cancers 15, no. 3: 610. https://doi.org/10.3390/cancers15030610




