Tumour Heterogeneity and the Consequent Practical Challenges in the Management of Gastroenteropancreatic Neuroendocrine Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Tumour Heterogeneity in GEP-NENs
3. Morphologic Heterogeneity in GEP-NENs
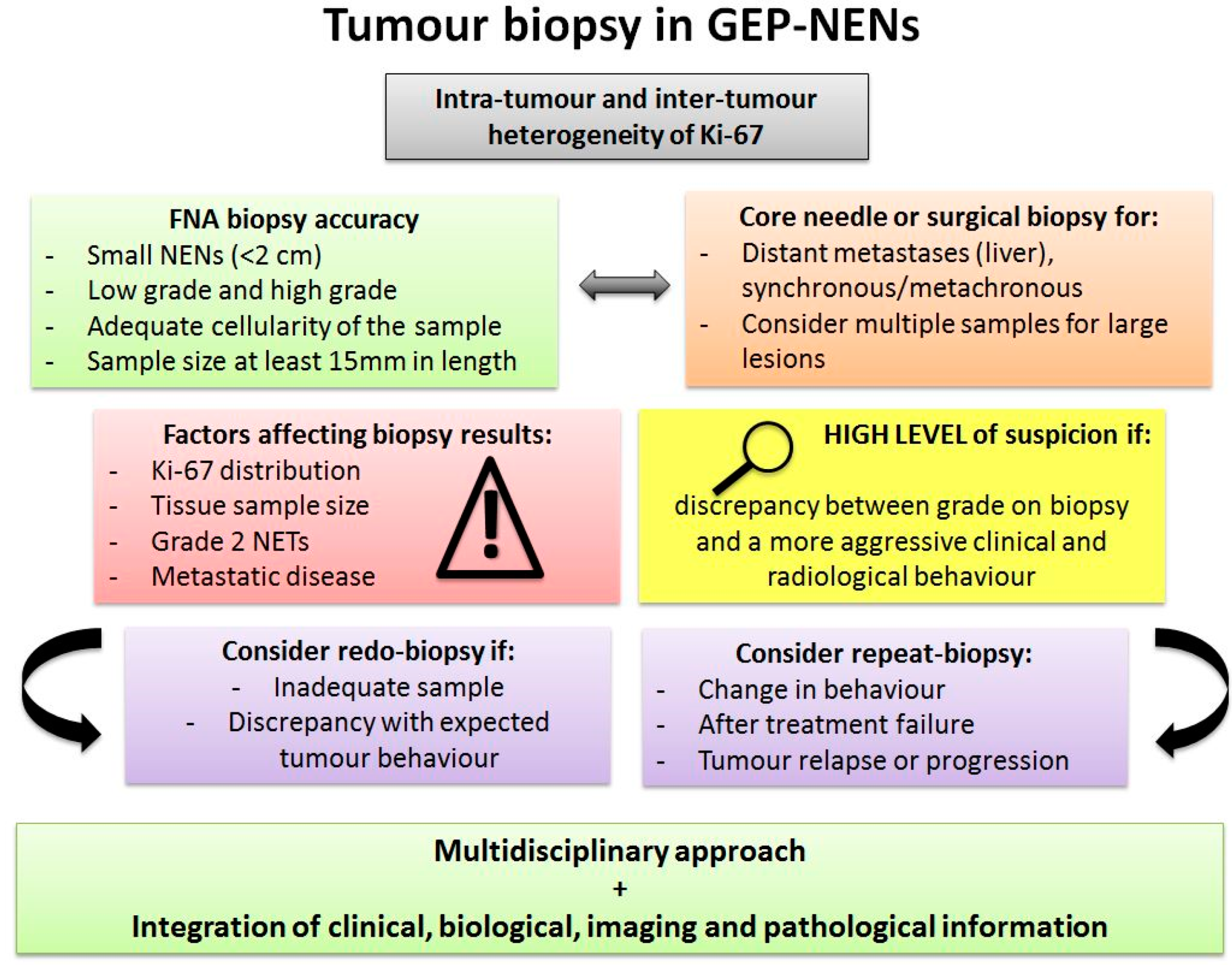
4. Tumour Tissue Heterogeneity and the Impact on Biopsy
5. Radioligand Therapy
6. Somatostatin Analogues Therapy
7. Cell Signalling Pathway Heterogeneity: The mTOR Pathway
8. Present and Future Strategies
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, J.; Dang, H.; Wang, X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp. Mol. Med. 2018, 50, e416. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovly, C.M.; Salama, A.K.S.; Salgia, R. Tumor Heterogeneity and Therapeutic Resistance. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e585–e593. [Google Scholar] [CrossRef] [PubMed]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vito, A.; El-Sayes, N.; Mossman, K.L. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells 2020, 9, 992. [Google Scholar] [CrossRef]
- Davies, A.E.; Albeck, J.G. Microenvironmental Signals and Biochemical Information Processing: Cooperative Determinants of Intratumoral Plasticity and Heterogeneity. Front. Cell Dev. Biol. 2018, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Brodt, P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin. Cancer Res. 2016, 22, 5971–5982. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Jiang, M.; Wang, H.; Sun, H.; Zhu, J.; Zhao, W.; Fang, Q.; Yu, J.; Chen, P.; Wu, S.; et al. A narrative review of tumor heterogeneity and challenges to tumor drug therapy. Ann. Transl. Med. 2021, 9, 1351. [Google Scholar] [CrossRef]
- Diaz-Cano, S.J. Tumor heterogeneity: Mechanisms and bases for a reliable application of molecular marker design. Int. J. Mol. Sci. 2012, 13, 1951–2011. [Google Scholar] [CrossRef]
- Klimstra, D.; Klöppel, G.; La Rosa, S. Classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours of Digestive System, 5th ed.; IARC Publications: Lyon, France, 2019; pp. 16–19. [Google Scholar]
- Rogoza, O.; Megnis, K.; Kudrjavceva, M.; Gerina-Berzina, A.; Rovite, V. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int. J. Mol. Sci. 2022, 23, 1447. [Google Scholar] [CrossRef]
- Briest, F.; Grabowski, P. PI3K-AKT-mTOR-signaling and beyond: The complex network in gastroenteropancreatic neuroendocrine neoplasms. Theranostics 2014, 4, 336–365. [Google Scholar] [CrossRef] [Green Version]
- Patel, Y.C. Somatostatin and Its Receptor Family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Veenstra, M.J.; de Herder, W.W.; Feelders, R.A.; Hofland, L.J. Targeting the somatostatin receptor in pituitary and neuroendocrine tumors. Expert Opin. Ther. Targets 2013, 17, 1329–1343. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sänger, J.; Baum, R.P.; Prasad, V.; Hommann, M. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 2012, 5, 187–194. [Google Scholar]
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.; Bussolati, G. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Sahani, D.V.; Bonaffini, P.A.; Castillo, C.F.D.; Blake, M.A. Gastroenteropancreatic Neuroendocrine Tumors: Role of Imaging in Diagnosis and Management. Radiology 2013, 266, 38–61. [Google Scholar] [CrossRef] [Green Version]
- Graf, J.; Pape, U.-F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef]
- Ortega, C.; Wong, R.K.S.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, A.; Metser, U. Quantitative 68Ga-DOTATATE PET/CT Parameters for the Prediction of Therapy Response in Patients with Progressive Metastatic Neuroendocrine Tumors Treated with 177Lu-DOTATATE. J. Nucl. Med. 2021, 62, 1406–1414. [Google Scholar] [CrossRef]
- Shi, C.; Morse, M.A. Mechanisms of Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2022, 14, 6114. [Google Scholar] [CrossRef] [PubMed]
- Charoenpitakchai, M.; Liu, E.; Zhao, Z.; Koyama, T.; Huh, W.J.; Berlin, J.; Hande, K.; Walker, R.; Shi, C. In liver metastases from small intestinal neuroendocrine tumors, SSTR2A expression is heterogeneous. Virchows Arch. 2017, 470, 545–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cives, M.; Strosberg, J. The Expanding Role of Somatostatin Analogs in Gastroenteropancreatic and Lung Neuroendocrine Tumors. Drugs 2015, 75, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, L.H.; Klimstra, D.S. Effect of Tumor Heterogeneity on the Assessment of Ki67 Labeling Index in Well-differentiated Neuroendocrine Tumors Metastatic to the Liver: Implications for Prognostic Stratification. Am. J. Surg. Pathol. 2011, 35, 853–860. [Google Scholar] [CrossRef]
- Nuñez-Valdovinos, B.; Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Capdevila, J.; Castaño-Pascual, Á.; Benavent, M.; Pi Barrio, J.J.; Teule, A.; Alonso, V.; Custodio, A.; et al. Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE). Oncologist 2018, 23, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Jiang, C.; Zhang, Q.; Qi, C.; Yao, H.; Lin, R. Clinicopathological heterogeneity between primary and metastatic sites of gastroenteropancreatic neuroendocrine neoplasm. Diagn. Pathol. 2020, 15, 108. [Google Scholar] [CrossRef]
- Zhang, W.H.; Gao, H.L.; Liu, W.S.; Qin, Y.; Ye, Z.; Lou, X.; Wang, F.; Zhang, Y.; Chen, X.M.; Chen, J.; et al. A real-life treatment cohort of pancreatic neuroendocrine tumors: High-grade increase in metastases confers poor survival. Front. Endocrinol. 2022, 13, 941210. [Google Scholar] [CrossRef]
- Furukawa, T.; Ozaka, M.; Takamatsu, M.; Takazawa, Y.; Inamura, K.; Inoue, Y.; Mie, T.; Takeda, T.; Kanata, R.; Kasuga, A.; et al. Ki-67 Labeling Index Variability Between Surgically Resected Primary and Metastatic Hepatic Lesions of Gastroenteropancreatic Neuroendocrine Neoplasms. Int. J. Surg. Pathol. 2021, 29, 475–481. [Google Scholar] [CrossRef]
- Shi, C.; Gonzalez, R.S.; Zhao, Z.; Koyama, T.; Cornish, T.C.; Hande, K.R.; Walker, R.; Sandler, M.; Berlin, J.; Liu, E.H. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am. J. Clin. Pathol. 2015, 143, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Borga, C.; Dal Pozzo, C.A.; Trevellin, E.; Bergamo, F.; Murgioni, S.; Milanetto, A.C.; Pasquali, C.; Cillo, U.; Munari, G.; Martini, C.; et al. mTOR pathway and somatostatin receptors expression intratumor-heterogeneity in ileal NETs. Endocr. Relat. Cancer 2021, 28, 449–456. [Google Scholar] [CrossRef]
- El-Sayes, N.; Vito, A.; Mossman, K. Tumor Heterogeneity: A Great Barrier in the Age of Cancer Immunotherapy. Cancers 2021, 13, 806. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsatou, M.; Kyriakopoulos, G.; Mavroeidi, V.; Kostopoulou, A.; Atlan, K.; Theocharis, S.; Rindi, G.; Grossman, A.B.; Grozinsky-Glasberg, S.; et al. Distinctive features of pancreatic neuroendocrine neoplasms exhibiting an increment in proliferative activity during the course of the disease. Endocrine 2021, 72, 279–286. [Google Scholar] [CrossRef]
- Botling, J.; Lamarca, A.; Bajic, D.; Norlén, O.; Lönngren, V.; Kjaer, J.; Eriksson, B.; Welin, S.; Hellman, P.; Rindi, G.; et al. High-Grade Progression Confers Poor Survival in Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 891–898. [Google Scholar] [CrossRef]
- Grillo, F.; Valle, L.; Ferone, D.; Albertelli, M.; Brisigotti, M.P.; Cittadini, G.; Vanoli, A.; Fiocca, R.; Mastracci, L. KI-67 heterogeneity in well differentiated gastro-entero-pancreatic neuroendocrine tumors: When is biopsy reliable for grade assessment? Endocrine 2017, 57, 494–502. [Google Scholar] [CrossRef]
- Trikalinos, N.A.; Chatterjee, D.; Lee, J.; Liu, J.; Williams, G.; Hawkins, W.; Hammill, C. Accuracy of Grading in Pancreatic Neuroendocrine Neoplasms and Effect on Survival Estimates: An Institutional Experience. Ann. Surg. Oncol 2020, 27, 3542–3550. [Google Scholar] [CrossRef]
- Walter, D.; Harter, P.N.; Battke, F.; Winkelmann, R.; Schneider, M.; Holzer, K.; Koch, C.; Bojunga, J.; Zeuzem, S.; Hansmann, M.L.; et al. Genetic heterogeneity of primary lesion and metastasis in small intestine neuroendocrine tumors. Sci. Rep. 2018, 8, 3811. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Turner, G.; King, B.; Jones, L.; Culliford, D.; McCance, D.; Ardill, J.; Johnston, B.T.; Poston, G.; Rees, M.; et al. Midgut neuroendocrine tumours with liver metastases: Results of the UKINETS study. Endocr. Relat. Cancer 2009, 16, 885–894. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [Green Version]
- Karpathakis, A.; Dibra, H.; Pipinikas, C.; Feber, A.; Morris, T.; Francis, J.; Oukrif, D.; Mandair, D.; Pericleous, M.; Mohmaduvesh, M.; et al. Prognostic Impact of Novel Molecular Subtypes of Small Intestinal Neuroendocrine Tumor. Clin. Cancer Res. 2016, 22, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Francis, J.M.; Kiezun, A.; Ramos, A.H.; Serra, S.; Pedamallu, C.S.; Qian, Z.R.; Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Karpathakis, A.; et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 2013, 45, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Karpathakis, A.; Dibra, H.; Pipinikas, C.; Feber, A.; Morris, T.; Francis, J.; Oukrif, D.; Mandair, D.; Pericleous, M.; Mohmaduvesh, M.; et al. Progressive epigenetic dysregulation in neuroendocrine tumour liver metastases. Endocr. Relat. Cancer 2017, 24, L21–L25. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Li, J.; Malamutmann, E.; Schmid, K.-W.; Bockisch, A.; Broelsch, C.E. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. BJS 2009, 96, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.C.; Drymousis, P.; Flora, R.; Goldin, R.; Spalding, D.; Frilling, A. Role of Ki-67 Proliferation Index in the Assessment of Patients with Neuroendocrine Neoplasias Regarding the Stage of Disease. World J. Surg. 2014, 38, 1353–1361. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Spyroglou, A.; Kykalos, S.; Daskalakis, K.; Kyriakopoulos, G.; Sotiropoulos, G.C.; Kaltsas, G.A.; Grossman, A.B. Changing biological behaviour of NETs during the evolution of the disease: Progress on progression. Endocr. Relat. Cancer 2021, 28, R121–R140. [Google Scholar] [CrossRef]
- Schmid, H.A.; Lambertini, C.; van Vugt, H.H.; Barzaghi-Rinaudo, P.; Schäfer, J.; Hillenbrand, R.; Sailer, A.W.; Kaufmann, M.; Nuciforo, P. Monoclonal Antibodies against the Human Somatostatin Receptor Subtypes 1–5: Development and Immunohistochemical Application in Neuroendocrine Tumors. Neuroendocrinology 2012, 95, 232–247. [Google Scholar] [CrossRef]
- Feijtel, D.; Doeswijk, G.N.; Verkaik, N.S.; Haeck, J.C.; Chicco, D.; Angotti, C.; Konijnenberg, M.W.; de Jong, M.; Nonnekens, J. Inter and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics 2021, 11, 491–505. [Google Scholar] [CrossRef]
- Lamberti, G.; Brighi, N.; Maggio, I.; Manuzzi, L.; Peterle, C.; Ambrosini, V.; Ricci, C.; Casadei, R.; Campana, D. The Role of mTOR in Neuroendocrine Tumors: Future Cornerstone of a Winning Strategy? Int. J. Mol. Sci. 2018, 19, 747. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.R.; Li, T.; Ter-Minassian, M.; Yang, J.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.; de Reuver, P.R.; Gill, P.; Andrici, J.; D’Urso, L.; Mittal, A.; Pavlakis, N.; Clarke, S.; Samra, J.S.; Gill, A.J. Somatostatin Receptor SSTR-2a Expression Is a Stronger Predictor for Survival Than Ki-67 in Pancreatic Neuroendocrine Tumors. Medicine 2015, 94, e1281. [Google Scholar] [CrossRef]
- Puranik, A.D.; Dromain, C.; Fleshner, N.; Sathekge, M.; Pavel, M.; Eberhardt, N.; Zengerling, F.; Marienfeld, R.; Grunert, M.; Prasad, V. Target Heterogeneity in Oncology: The Best Predictor for Differential Response to Radioligand Therapy in Neuroendocrine Tumors and Prostate Cancer. Cancers 2021, 13, 3607. [Google Scholar] [CrossRef]
- Iravani, A.; Parihar, A.S.; Akhurst, T.; Hicks, R.J. Molecular imaging phenotyping for selecting and monitoring radioligand therapy of neuroendocrine neoplasms. Cancer Imaging 2022, 22, 25. [Google Scholar] [CrossRef]
- Popa, O.; Taban, S.M.; Pantea, S.; Plopeanu, A.D.; Barna, R.A.; Cornianu, M.; Pascu, A.A.; Dema, A.L.C. The new WHO classification of gastrointestinal neuroendocrine tumors and immunohistochemical expression of somatostatin receptor 2 and 5. Exp. Ther. Med. 2021, 22, 1179. [Google Scholar] [CrossRef]
- Righi, L.; Volante, M.; Tavaglione, V.; Billè, A.; Daniele, L.; Angusti, T.; Inzani, F.; Pelosi, G.; Rindi, G.; Papotti, M. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: A clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann. Oncol. 2010, 21, 548–555. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Watkins, J.; Marelli, L.; Khan, K.; Caplin, M.E. Expression of Somatostatin and Dopamine 2 Receptors in Neuroendocrine Tumours and the Potential Role for New Biotherapies. Neuroendocrinology 2009, 89, 308–314. [Google Scholar] [CrossRef]
- Fonti, R.; Panico, M.; Pellegrino, S.; Pulcrano, A.; Vastarella, L.A.; Hakkak, A.; Giuliano, M.; Palmieri, G.; De Placido, S.; Del Vecchio, S. Heterogeneity of SSTR2 Expression Assessed By 68Ga-DOTATOC PET/CT Using Coefficient of Variation in Patients With Neuroendocrine Tumors. J. Nucl. Med. 2022, 63, 1509–1514. [Google Scholar] [CrossRef]
- Graham, M.M. Chapter 80—Quantification of Radiotracer Uptake Into Tissue. In Molecular Imaging, 2nd ed.; Ross, B.D., Gambhir, S.S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1613–1624. [Google Scholar] [CrossRef]
- Yu, J.; Li, N.; Li, J.; Lu, M.; Leal, J.P.; Tan, H.; Su, H.; Fan, Y.; Zhang, Y.; Zhao, W.; et al. The Correlation Between [68Ga]DOTATATE PET/CT and Cell Proliferation in Patients With GEP-NENs. Mol. Imaging Biol. 2019, 21, 984–990. [Google Scholar] [CrossRef]
- Partelli, S.; Rinzivillo, M.; Maurizi, A.; Panzuto, F.; Salgarello, M.; Polenta, V.; Delle Fave, G.; Falconi, M. The Role of Combined 68Ga-DOTANOC and 18FDG PET/CT in the Management of Patients with Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2014, 100, 293–299. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Polverari, G.; Peterle, C.; Diodato, S.; Ricci, C.; Allegri, V.; Casadei, R.; Tomassetti, P.; Fanti, S. Prognostic Value of 68Ga-DOTANOC PET/CT SUVmax in Patients with Neuroendocrine Tumors of the Pancreas. J. Nucl. Med. 2015, 56, 1843–1848. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, M.; Oberauer, A.; Dobrozemsky, G.; Decristoforo, C.; Putzer, D.; Kendler, D.; Uprimny, C.; Kovacs, P.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-Octreotide PET for Assessing Response to Somatostatin-Receptor–Mediated Radionuclide Therapy. J. Nucl. Med. 2009, 50, 1427–1434. [Google Scholar] [CrossRef] [Green Version]
- Haug, A.R.; Auernhammer, C.J.; Wängler, B.; Schmidt, G.P.; Uebleis, C.; Göke, B.; Cumming, P.; Bartenstein, P.; Tiling, R.; Hacker, M. 68Ga-DOTATATE PET/CT for the Early Prediction of Response to Somatostatin Receptor–Mediated Radionuclide Therapy in Patients with Well-Differentiated Neuroendocrine Tumors. J. Nucl. Med. 2010, 51, 1349–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsótér, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, L.; Du, T.; Yang, J.; Cai, L.; Liu, H.; Chen, Y. Analysis of the Diagnostic Efficacy of DOTATATE Imaging Combined with CGA and BSP Detection Mode for NEN Patients with Bone Metastasis. Biomed. Res. Int. 2022, 2022, 6279826. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-Fluorodeoxyglucose Positron Emission Tomography Predicts Survival of Patients with Neuroendocrine Tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Bahri, H.; Laurence, L.; Edeline, J.; Leghzali, H.; Devillers, A.; Raoul, J.-L.; Cuggia, M.; Mesbah, H.; Clement, B.; Boucher, E.; et al. High Prognostic Value of 18F-FDG PET for Metastatic Gastroenteropancreatic Neuroendocrine Tumors: A Long-Term Evaluation. J. Nucl. Med. 2014, 55, 1786–1790. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, I.; Steffen, I.G.; Wedel, F.; Lougovski, A.; Marnitz, S.; Derlin, T.; Amthauer, H.; Buchert, R.; Hofheinz, F.; Brenner, W. Asphericity of pretherapeutic tumour FDG uptake provides independent prognostic value in head-and-neck cancer. Eur. Radiol. 2014, 24, 2077–2087. [Google Scholar] [CrossRef]
- Hatt, M.; Majdoub, M.; Vallières, M.; Tixier, F.; Le Rest, C.C.; Groheux, D.; Hindié, E.; Martineau, A.; Pradier, O.; Hustinx, R.; et al. 18F-FDG PET Uptake Characterization Through Texture Analysis: Investigating the Complementary Nature of Heterogeneity and Functional Tumor Volume in a Multi–Cancer Site Patient Cohort. J. Nucl. Med. 2015, 56, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Kubota, K.; Okasaki, M.; Minamimoto, R.; Miyata, Y.; Morooka, M.; Nakajima, K.; Sato, T. Lesion-based analysis of (18)F-FDG uptake and (111)In-Pentetreotide uptake by neuroendocrine tumors. Ann. Nucl. Med. 2014, 28, 1004–1010. [Google Scholar] [CrossRef] [Green Version]
- Laffi, A.; Colandrea, M.; Buonsanti, G.; Frassoni, S.; Bagnardi, V.; Spada, F.; Pisa, E.; Barberis, M.; Rubino, M.; Grana, C.M.; et al. A Retrospective Analysis of the Correlation between Functional Imaging and Clinical Outcomes in Grade 3 Neuroendocrine Tumors (NETs G3). Diagnostics 2021, 11, 2401. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Alshammari, A.; Michopoulou, S.; Skoura, E.; Naik, K.; Maragkoudakis, E.; Mohmaduvesh, M.; Al-Harbi, M.; Belda, M.; Caplin, M.E.; et al. Comparison of the Impact of 68Ga-DOTATATE and 18F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Laffi, A.; Spada, F.; Bagnardi, V.; Frassoni, S.; Pisa, E.; Rubino, M.; Barberis, M.; Fazio, N. Gastroenteropancreatic grade 3 neuroendocrine tumors: A single entity or a heterogeneous group? A retrospective analysis. J. Endocrinol. Investig. 2022, 45, 317–325. [Google Scholar] [CrossRef]
- Kayani, I.; Bomanji, J.B.; Groves, A.; Conway, G.; Gacinovic, S.; Win, T.; Dickson, J.; Caplin, M.; Ell, P.J. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008, 112, 2447–2455. [Google Scholar] [CrossRef]
- Nilica, B.; Waitz, D.; Stevanovic, V.; Uprimny, C.; Kendler, D.; Buxbaum, S.; Warwitz, B.; Gerardo, L.; Henninger, B.; Virgolini, I.; et al. Direct comparison of (68)Ga-DOTA-TOC and (18)F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Karfis, I.; Marin, G.; Levillain, H.; Drisis, S.; Muteganya, R.; Critchi, G.; Taraji-Schiltz, L.; Guix, C.A.; Shaza, L.; Elbachiri, M.; et al. Prognostic value of a three-scale grading system based on combining molecular imaging with (68)Ga-DOTATATE and (18)F-FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasias. Oncotarget 2020, 11, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Frilling, A.; Herrmann, K.; Kämmerer, D.; Toumpanakis, C. Neuroendocrine Tumors of the Gastrointestinal Tract. Visc. Med. 2017, 33, 368–371. [Google Scholar] [CrossRef]
- Carideo, L.; Prosperi, D.; Panzuto, F.; Magi, L.; Pratesi, M.S.; Rinzivillo, M.; Annibale, B.; Signore, A. Role of Combined [(68)Ga]Ga-DOTA-SST Analogues and [(18)F]FDG PET/CT in the Management of GEP-NENs: A Systematic Review. J. Clin. Med. 2019, 8, 1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, R.; Xie, Q.; Yu, F.; Du, C.; Yao, X.; Zang, S.; Zhang, C.; Zhang, P.; Shao, G.; Yang, Z.; et al. Detection of Bone Metastases by 68Ga-DOTA-SSAs and 18F-FDG PET/CT: A Two-Center Head-to-Head Study of Gastroenteropancreatic Neuroendocrine Neoplasms. Contrast Media Mol. Imaging 2022, 2022, 1750132. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Johnson, S.J.; French, J.J. Correlation of Ki-67 indices from biopsy and resection specimens of neuroendocrine tumours. Ann. R. Coll. Surg. Engl. 2017, 99, 193–197. [Google Scholar] [CrossRef]
- Rebours, V.; Cordova, J.; Couvelard, A.; Fabre, M.; Palazzo, L.; Pierre Vullierme, M.; Hentic, O.; Sauvanet, A.; Aubert, A.; Bedossa, P.; et al. Can pancreatic neuroendocrine tumour biopsy accurately determine pathological characteristics? Dig. Liver Dis. 2015, 47, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Albertelli, M.; Grillo, F.; Lo Calzo, F.; Puliani, G.; Rainone, C.; Colao, A.A.L.; Faggiano, A. Pathology Reporting in Neuroendocrine Neoplasms of the Digestive System: Everything You Always Wanted to Know but Were Too Afraid to Ask. Front. Endocrinol. 2021, 12, 680305. [Google Scholar] [CrossRef]
- Fazio, N.; Milione, M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: New insights and treatment implications. Cancer Treat. Rev. 2016, 50, 61–67. [Google Scholar] [CrossRef]
- Scott, A.T.; Howe, J.R. Management of Small Bowel Neuroendocrine Tumors. J. Oncol. Pract. 2018, 14, 471–482. [Google Scholar] [CrossRef]
- Sigel, C.S. Advances in the cytologic diagnosis of gastroenteropancreatic neuroendocrine neoplasms. Cancer Cytopathol. 2018, 126, 980–991. [Google Scholar] [CrossRef] [Green Version]
- Hirabayashi, K.; Zamboni, G.; Nishi, T.; Tanaka, A.; Kajiwara, H.; Nakamura, N. Histopathology of gastrointestinal neuroendocrine neoplasms. Front. Oncol. 2013, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Kalantri, S.; Bakshi, P.; Verma, K. Grading of pancreatic neuroendocrine tumors on endoscopic ultrasound-guided fine-needle aspiration using Ki-67 index and 2017 World Health Organization criteria: An analysis of 32 cases. CytoJournal 2020, 17, 21. [Google Scholar] [CrossRef]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Farrell, J.M.; Pang, J.C.; Kim, G.E.; Tabatabai, Z.L. Pancreatic neuroendocrine tumors: Accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014, 122, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Abi-Raad, R.; Lavik, J.-P.; Barbieri, A.L.; Zhang, X.; Adeniran, A.J.; Cai, G. Grading Pancreatic Neuroendocrine Tumors by Ki-67 Index Evaluated on Fine-Needle Aspiration Cell Block Material. Am. J. Clin. Pathol. 2019, 153, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Díaz del Arco, C.; Díaz Pérez, J.Á.; Ortega Medina, L.; Sastre Valera, J.; Fernández Aceñero, M.J. Reliability of Ki-67 Determination in FNA Samples for Grading Pancreatic Neuroendocrine Tumors. Endocr. Pathol. 2016, 27, 276–283. [Google Scholar] [CrossRef]
- Piani, C.; Franchi, G.M.; Cappelletti, C.; Scavini, M.; Albarello, L.; Zerbi, A.; Giorgio Arcidiacono, P.; Bosi, E.; Manzoni, M.F. Cytological Ki-67 in pancreatic endocrine tumours: An opportunity for pre-operative grading. Endocr. Relat. Cancer 2008, 15, 175–181. [Google Scholar] [CrossRef]
- Larghi, A.; Capurso, G.; Carnuccio, A.; Ricci, R.; Alfieri, S.; Galasso, D.; Lugli, F.; Bianchi, A.; Panzuto, F.; De Marinis, L.; et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest. Endosc. 2012, 76, 570–577. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamao, K.; Hijioka, S.; Bhatia, V.; Mizuno, N.; Hara, K.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Evaluation of Ki-67 index in EUS–FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy 2014, 46, 32–38. [Google Scholar] [CrossRef]
- Appelstrand, A.; Bergstedt, F.; Elf, A.K.; Fagman, H.; Hedenström, P. Endoscopic ultrasound-guided side-fenestrated needle biopsy sampling is sensitive for pancreatic neuroendocrine tumors but inadequate for tumor grading: A prospective study. Sci. Rep. 2022, 12, 5971. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Hikichi, T.; Suzuki, R.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; Konno, N.; Waragai, Y.; Asama, H.; et al. Efficacy of endoscopic ultrasonography-guided fine needle aspiration for pancreatic neuroendocrine tumor grading. World J. Gastroenterol. 2015, 21, 8118–8124. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, Y.; An, S.; Kim, S.J.; Kim, J.Y.; Kim, S.-Y.; Hwang, D.W.; Park, D.H.; Lee, S.S.; Kim, S.C.; et al. Grading by the Ki-67 Labeling Index of Endoscopic Ultrasound–Guided Fine Needle Aspiration Biopsy Specimens of Pancreatic Neuroendocrine Tumors Can Be Underestimated. Pancreas 2018, 47, 1296–1303. [Google Scholar] [CrossRef]
- Unno, J.; Kanno, A.; Masamune, A.; Kasajima, A.; Fujishima, F.; Ishida, K.; Hamada, S.; Kume, K.; Kikuta, K.; Hirota, M.; et al. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand. J. Gastroenterol. 2014, 49, 1367–1374. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, H.S.; Lee, K.J.; Jang, S.I.; Lee, W.J.; Hwang, J.-H.; Cho, C.M.; Park, C.-H.; Han, J.; Woo, S.M.; et al. Malignant potential of small pancreatic neuroendocrine neoplasm and its risk factors: A multicenter nationwide study. Pancreatology 2021, 21, 208–214. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Zhu, H. Fine-Needle Aspiration Biopsy of Neuroendocrine Neoplasms in the Liver: A 9-Year Retrospective, Single Institutional Study. Am. J. Clin. Pathol. 2019, 152, S94. [Google Scholar] [CrossRef]
- Saeed, O.A.M.; Cramer, H.; Wang, X.; Wu, H.H. Fine needle aspiration cytology of hepatic metastases of neuroendocrine tumors: A 20-year retrospective, single institutional study. Diagn. Cytopathol. 2018, 46, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frilling, A.; Sotiropoulos, G.C.; Li, J.; Kornasiewicz, O.; Plöckinger, U. Multimodal management of neuroendocrine liver metastases. HPB 2010, 12, 361–379. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, Y.; He, X.; Li, W. Using percutaneous computed tomography-guided core needle biopsy of liver metastases from gastroenteropancreatic neuroendocrine tumors to identify inter-tumor grading classification heterogeneity. Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Ramirez, R.A.; Beyer, D.T.; Wang, Y.-Z.; Ramcharan, T.; Ricks, M.J.; Boudreaux, J.P.; Woltering, E. Liver needle biopsies and prediction of histologic tumor grade for midgut neuroendocrine tumors. J. Clin. Oncol. 2016, 34, e15664. [Google Scholar] [CrossRef]
- Heong, V.; Tay, D.; Goh, S.E.; Wee, B.; Tan, T.Z.; Soo, R.; Pang, B.; Lim, D.; Gopinathan, A.; Ow, S.; et al. Whole Exome Sequencing of Multi-Regional Biopsies from Metastatic Lesions to Evaluate Actionable Truncal Mutations Using a Single-Pass Percutaneous Technique. Cancers 2020, 12, 1599. [Google Scholar] [CrossRef]
- Grillo, F.; Albertelli, M.; Brisigotti, M.P.; Borra, T.; Boschetti, M.; Fiocca, R.; Ferone, D.; Mastracci, L. Grade Increases in Gastroenteropancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology 2016, 103, 452–459. [Google Scholar] [CrossRef]
- Adesoye, T.; Daleo, M.A.; Loeffler, A.G.; Winslow, E.R.; Weber, S.M.; Cho, C.S. Discordance of Histologic Grade Between Primary and Metastatic Neuroendocrine Carcinomas. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S817–S821. [Google Scholar] [CrossRef] [Green Version]
- Keck, K.J.; Choi, A.; Maxwell, J.E.; Li, G.; O’Dorisio, T.M.; Breheny, P.; Bellizzi, A.M.; Howe, J.R. Increased Grade in Neuroendocrine Tumor Metastases Negatively Impacts Survival. Ann. Surg. Oncol. 2017, 24, 2206–2212. [Google Scholar] [CrossRef]
- Numbere, N.; Huber, A.R.; Shi, C.; Cates, J.M.M.; Gonzalez, R.S. Should Ki67 immunohistochemistry be performed on all lesions in multifocal small intestinal neuroendocrine tumours? Histopathology 2019, 74, 424–429. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Öberg, K.; Knigge, U.; Kwekkeboom, D.; Perren, A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii124–vii130. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Gilson, P.; Merlin, J.L.; Harlé, A. Deciphering Tumour Heterogeneity: From Tissue to Liquid Biopsy. Cancers 2022, 14, 1384. [Google Scholar] [CrossRef]
- Merola, E.; Perren, A.; Rinke, A.; Zerbi, A.; McNamara, M.G.; Arsenic, R.; Fazio, N.; de Herder, W.; Valle, J.W.; Gress, T.M.; et al. High rate of Ki-67 increase in entero-pancreatic NET relapses after surgery with curative intent. J. Neuroendocrinol. 2022, 34, e13193. [Google Scholar] [CrossRef]
- Vyas, M.; Tang, L.H.; Rekhtman, N.; Klimstra, D.S. Alterations in Ki67 Labeling Following Treatment of Poorly Differentiated Neuroendocrine Carcinomas. Am. J. Surg. Pathol. 2020, 45, 25–34. [Google Scholar] [CrossRef]
- Blesl, A.; Krones, E.; Pollheimer, M.J.; Haybaeck, J.; Wiesspeiner, U.; Lipp, R.W.; Kump, P. Downgrading of a G3 Neuroendocrine Tumor to a G2 Tumor: Can First-Line Cytotoxic Chemotherapy Change the Tumor Biology? Case Rep. Oncol. 2017, 10, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- de Mestier, L.; Armani, M.; Cros, J.; Hentic, O.; Rebours, V.; Cadiot, G.; Sauvanet, A.; Couvelard, A.; Lebtahi, R.; Ruszniewski, P. Lesion-by-lesion correlation between uptake at FDG PET and the Ki67 proliferation index in resected pancreatic neuroendocrine tumors. Dig. Liver Dis. 2019, 51, 1720–1724. [Google Scholar] [CrossRef]
- Majala, S.; Seppänen, H.; Kemppainen, J.; Sundström, J.; Schalin-Jäntti, C.; Gullichsen, R.; Schildt, J.; Mustonen, H.; Vesterinen, T.; Arola, J.; et al. Prediction of the aggressiveness of non-functional pancreatic neuroendocrine tumors based on the dual-tracer PET/CT. EJNMMI Res. 2019, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Fikri, A.S.F.; Abdul, J.N.; Ramdave, S.; Syafik-Eid, R. The value of the FDG-GaTate and proliferation marker (ki-67) in the assessment of neuroendocrine tumours (NETs). Cancer Imaging 2015, 15, P53. [Google Scholar] [CrossRef] [Green Version]
- Bucau, M.; Laurent-Bellue, A.; Poté, N.; Hentic, O.; Cros, J.; Mikail, N.; Rebours, V.; Ruszniewski, P.; Lebtahi, R.; Couvelard, A. 18F-FDG Uptake in Well-Differentiated Neuroendocrine Tumors Correlates with Both Ki-67 and VHL Pathway Inactivation. Neuroendocrinology 2018, 106, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, S.; Kato, H.; Shiraha, H.; Tsutsumi, K.; Yamamoto, N.; Matsumoto, K.; Tomoda, T.; Uchida, D.; Akimoto, Y.; Mizukawa, S.; et al. Dynamic computed tomography is useful for prediction of pathological grade in pancreatic neuroendocrine neoplasm. J. Gastroenterol. Hepatol. 2017, 32, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Reidy-Lagunes, D.; Anthony, L.B.; Bertino, E.M.; Brendtro, K.; Chan, J.A.; Chen, H.; Jensen, R.T.; Kim, M.K.; Klimstra, D.S.; et al. Consensus Guidelines for the Management and Treatment of Neuroendocrine Tumors. Pancreas 2013, 42, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Peptide Receptor Radionuclide Therapy with Radiolabelled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Hope, T.A.; Bodei, L.; Chan, J.A.; El-Haddad, G.; Fidelman, N.; Kunz, P.L.; Mailman, J.; Menda, Y.; Metz, D.C.; Mittra, E.S.; et al. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of (177)Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2020, 61, 222–227. [Google Scholar] [CrossRef]
- Cremonesi, M.; Ferrari, M.; Bodei, L.; Tosi, G.; Paganelli, G. Dosimetry in Peptide Radionuclide Receptor Therapy: A Review. J. Nucl. Med. 2006, 47, 1467–1475. [Google Scholar]
- Albertelli, M.; Dotto, A.; Di Dato, C.; Malandrino, P.; Modica, R.; Versari, A.; Colao, A.; Ferone, D.; Faggiano, A.; Nike. PRRT: Identikit of the perfect patient. Rev. Endocr. Metab. Disord. 2021, 22, 563–579. [Google Scholar] [CrossRef]
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Willinek, W.; Grünwald, F.; Guhlke, S.; Biersack, H.-J.; Sabet, A. Predictors of Long-Term Outcome in Patients with Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy with 177Lu-Octreotate. J. Nucl. Med. 2014, 55, 183–190. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, E.; Kersemans, V.; Allen, P.D.; Terry, S.Y.A.; Torres, J.B.; Mosley, M.; Smart, S.; Lee, B.Q.; Falzone, N.; Vallis, K.A.; et al. Imaging DNA Damage Repair In Vivo After (177)Lu-DOTATATE Therapy. J. Nucl. Med. 2020, 61, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Wetz, C.; Genseke, P.; Apostolova, I.; Furth, C.; Ghazzawi, S.; Rogasch, J.M.M.; Schatka, I.; Kreissl, M.C.; Hofheinz, F.; Grosser, O.S.; et al. The association of intra-therapeutic heterogeneity of somatostatin receptor expression with morphological treatment response in patients undergoing PRRT with [177Lu]-DOTATATE. PLoS ONE 2019, 14, e0216781. [Google Scholar] [CrossRef]
- Wetz, C.; Apostolova, I.; Steffen, I.G.; Hofheinz, F.; Furth, C.; Kupitz, D.; Ruf, J.; Venerito, M.; Klose, S.; Amthauer, H. Predictive Value of Asphericity in Pretherapeutic [111In]DTPA-Octreotide SPECT/CT for Response to Peptide Receptor Radionuclide Therapy with [177Lu]DOTATATE. Mol. Imaging Biol. 2017, 19, 437–445. [Google Scholar] [CrossRef]
- Bakker, W.H.; Albert, R.; Bruns, C.; Breeman, W.A.P.; Hofland, L.J.; Marbach, P.; Pless, J.; Pralet, D.; Stolz, B.; Koper, J.W.; et al. [111In-DTPA-D-Phe1]-octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: Synthesis, radiolabeling and in vitro validation. Life Sci. 1991, 49, 1583–1591. [Google Scholar] [CrossRef]
- Bol, K.; Haeck, J.C.; Groen, H.C.; Niessen, W.J.; Bernsen, M.R.; de Jong, M.; Veenland, J.F. Can DCE-MRI Explain the Heterogeneity in Radiopeptide Uptake Imaged by SPECT in a Pancreatic Neuroendocrine Tumor Model? PLoS ONE 2013, 8, e77076. [Google Scholar] [CrossRef] [Green Version]
- Asselin, M.-C.; O’Connor, J.P.B.; Boellaard, R.; Thacker, N.A.; Jackson, A. Quantifying heterogeneity in human tumours using MRI and PET. Eur. J. Cancer 2012, 48, 447–455. [Google Scholar] [CrossRef]
- Montelius, M.; Jalnefjord, O.; Spetz, J.; Nilsson, O.; Forssell-Aronsson, E.; Ljungberg, M. Multiparametric MR for non-invasive evaluation of tumour tissue histological characteristics after radionuclide therapy. NMR Biomed. 2019, 32, e4060. [Google Scholar] [CrossRef] [Green Version]
- Haeck, J.C.; Bol, K.; de Ridder, C.M.A.; Brunel, L.; Fehrentz, J.A.; Martinez, J.; van Weerden, W.M.; Bernsen, M.R.; de Jong, M.; Veenland, J.F. Imaging heterogeneity of peptide delivery and binding in solid tumors using SPECT imaging and MRI. EJNMMI Res. 2016, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Refardt, J.; Zandee, W.T.; Brabander, T.; Feelders, R.A.; Franssen, G.J.H.; Hofland, L.J.; Christ, E.; de Herder, W.W.; Hofland, J. Inferior outcome of neuroendocrine tumor patients negative on somatostatin receptor imaging. Endocr. Relat. Cancer 2020, 27, 615–624. [Google Scholar] [CrossRef]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr. Relat. Cancer 2020, 27, R67–R77. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.A.; Fazio, N.; Granberg, D.; Grozinsky-Glasberg, S.; Ahmadzadehfar, H.; Grana, C.M.; Zandee, W.T.; Cwikla, J.; Walter, M.A.; Oturai, P.S.; et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr. Relat. Cancer 2019, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Hicks, R.J. PRRT for higher-grade neuroendocrine neoplasms: What is still acceptable? Curr. Opin. Pharmacol. 2022, 67, 102293. [Google Scholar] [CrossRef] [PubMed]
- Thang, S.P.; Lung, M.S.; Kong, G.; Hofman, M.S.; Callahan, J.; Michael, M.; Hicks, R.J. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—A single-institution retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging. 2018, 45, 262–277. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Zhang, Q.; Lin, Y.; Jiang, C.; Yao, H.; Hou, X.; Chen, M.; Lin, R.; Chen, J. Concordance Between the Ki-67 Index Cutoff Value of 55% and Differentiation in Neuroendocrine Tumor and Neuroendocrine Carcinoma in Grade 3 Pancreatic Neuroendocrine Neoplasms. Pancreas 2020, 49, 1378–1382. [Google Scholar] [CrossRef]
- Ruhwedel, T.; Rogasch, J.M.M.; Huang, K.; Jann, H.; Schatka, I.; Furth, C.; Amthauer, H.; Wetz, C. The Prognostic Value of the De Ritis Ratio for Progression-Free Survival in Patients with NET Undergoing [(177)Lu]Lu-DOTATOC-PRRT: A Retrospective Analysis. Cancers 2021, 13, 635. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Herder, W.W.d.; Kam, B.L.; Eijck, C.H.v.; Essen, M.v.; Kooij, P.P.; Feelders, R.A.; Aken, M.O.v.; Krenning, E.P. Treatment With the Radiolabeled Somatostatin Analog [177Lu-DOTA0,Tyr3]Octreotate: Toxicity, Efficacy, and Survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [Green Version]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [Green Version]
- Katona, B.W.; Roccaro, G.A.; Soulen, M.C.; Yang, Y.X.; Bennett, B.J.; Riff, B.P.; Glynn, R.A.; Wild, D.; Nicolas, G.P.; Pryma, D.A.; et al. Efficacy of Peptide Receptor Radionuclide Therapy in a United States-Based Cohort of Metastatic Neuroendocrine Tumor Patients: Single-Institution Retrospective Analysis. Pancreas 2017, 46, 1121–1126. [Google Scholar] [CrossRef]
- Assi, H.A.; Hornbacker, K.; Shaheen, S.; Wittenberg, T.; Silberman, R.; Kunz, P.L. Rapid Progression After 177Lu-DOTATATE in Patients With Neuroendocrine Tumors. Pancreas 2021, 50, 890–894. [Google Scholar] [CrossRef]
- O’Dorisio, T.M.; Harris, A.G.; O’Dorisio, M.S. Evolution of Neuroendocrine Tumor Therapy. Surg. Oncol. Clin. N. Am. 2020, 29, 145–163. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Strosberg, J.; Kvols, L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2010, 16, 2963–2970. [Google Scholar] [CrossRef]
- Zamora, V.; Cabanne, A.; Salanova, R.; Bestani, C.; Domenichini, E.; Marmissolle, F.; Giacomi, N.; O’Connor, J.; Méndez, G.; Roca, E. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig. Liver Dis. 2010, 42, 220–225. [Google Scholar] [CrossRef]
- Pavel, M.; O’’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Okuwaki, K.; Kida, M.; Mikami, T.; Yamauchi, H.; Imaizumi, H.; Miyazawa, S.; Iwai, T.; Takezawa, M.; Saegusa, M.; Watanabe, M.; et al. Clinicopathologic characteristics of pancreatic neuroendocrine tumors and relation of somatostatin receptor type 2A to outcomes. Cancer 2013, 119, 4094–4102. [Google Scholar] [CrossRef]
- Watanabe, H.; Fujishima, F.; Komoto, I.; Imamura, M.; Hijioka, S.; Hara, K.; Yatabe, Y.; Kudo, A.; Masui, T.; Tsuchikawa, T.; et al. Somatostatin Receptor 2 Expression Profiles and Their Correlation with the Efficacy of Somatostatin Analogues in Gastrointestinal Neuroendocrine Tumors. Cancers 2022, 14, 775. [Google Scholar] [CrossRef]
- Butturini, G.; Bettini, R.; Missiaglia, E.; Mantovani, W.; Dalai, I.; Capelli, P.; Ferdeghini, M.; Pederzoli, P.; Scarpa, A.; Falconi, M. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr.-Relat. Cancer 2006, 13, 1213–1221. [Google Scholar] [CrossRef] [Green Version]
- Pinato, D.J.; Tan, T.M.; Toussi, S.T.; Ramachandran, R.; Martin, N.; Meeran, K.; Ngo, N.; Dina, R.; Sharma, R. An expression signature of the angiogenic response in gastrointestinal neuroendocrine tumours: Correlation with tumour phenotype and survival outcomes. Br. J. Cancer 2014, 110, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Zarebczan, B.; Chen, H. Signaling mechanisms in neuroendocrine tumors as targets for therapy. Endocrinol. Metab. Clin. N. Am. 2010, 39, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramon y Cajal, S.; Castellvi, J.; Hümmer, S.; Peg, V.; Pelletier, J.; Sonenberg, N. Beyond molecular tumor heterogeneity: Protein synthesis takes control. Oncogene 2018, 37, 2490–2501. [Google Scholar] [CrossRef] [Green Version]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Milella, M.; Ciuffreda, L. Role of mTOR Signaling in Tumor Microenvironment: An Overview. Int. J. Mol. Sci. 2018, 19, 2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missiaglia, E.; Dalai, I.; Barbi, S.; Beghelli, S.; Falconi, M.; Della Peruta, M.; Piemonti, L.; Capurso, G.; Di Florio, A.; delle Fave, G.; et al. Pancreatic Endocrine Tumors: Expression Profiling Evidences a Role for AKT-mTOR Pathway. J. Clin. Oncol. 2010, 28, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Catena, L.; Bajetta, E.; Milione, M.; Ducceschi, M.; Valente, M.; Dominoni, F.; Colonna, V. Mammalian target of rapamycin expression in poorly differentiated endocrine carcinoma: Clinical and therapeutic future challenges. Target. Oncol. 2011, 6, 65–68. [Google Scholar] [CrossRef]
- Unni, N.; Arteaga, C.L. Is Dual mTORC1 and mTORC2 Therapeutic Blockade Clinically Feasible in Cancer? JAMA Oncol. 2019, 5, 1564–1565. [Google Scholar] [CrossRef]
- Zanini, S.; Renzi, S.; Giovinazzo, F.; Bermano, G. mTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs). Front. Endocrinol. 2020, 11, 562505. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Sabatini, D.M. Rapamycin inhibits mTORC1, but not completely. Autophagy 2009, 5, 725–726. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.; Kulke, M. Targeting the mTOR Signaling Pathway in Neuroendocrine Tumors. Curr. Treat. Options Oncol. 2014, 15, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Pobłocki, J.; Jasińska, A.; Syrenicz, A.; Andrysiak-Mamos, E.; Szczuko, M. The Neuroendocrine Neoplasms of the Digestive Tract: Diagnosis, Treatment and Nutrition. Nutrients 2020, 12, 1437. [Google Scholar] [CrossRef]
- Bhaoighill, M.N.; Dunlop, E.A. Mechanistic target of rapamycin inhibitors: Successes and challenges as cancer therapeutics. CDR 2019, 2, 1069–1085. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.G.; Scott, A.T.; Li, G.; Sherman, S.K.; Ear, P.H.; Howe, J.R. Metastatic pancreatic neuroendocrine tumors have decreased somatostatin expression and increased Akt signaling. Surgery 2021, 169, 155–161. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Adhikari, L.J.; Lloyd, R.V.; Rubin, J.; Haluska, P.; Carboni, J.M.; Gottardis, M.M.; Ames, M.M. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr. Relat. Cancer 2010, 17, 623–636. [Google Scholar] [CrossRef]
- Han, X.; Ji, Y.; Zhao, J.; Xu, X.; Lou, W. Expression of PTEN and mTOR in pancreatic neuroendocrine tumors. Tumor Biol. 2013, 34, 2871–2879. [Google Scholar] [CrossRef]
- Sato, S.; Tsuchikawa, T.; Nakamura, T.; Sato, N.; Tamoto, E.; Okamura, K.; Shichinohe, T.; Hirano, S. Impact of the tumor microenvironment in predicting postoperative hepatic recurrence of pancreatic neuroendocrine tumors. Oncol. Rep 2014, 32, 2753–2759. [Google Scholar] [CrossRef] [Green Version]
- Gelsomino, F.; Casadei-Gardini, A.; Caputo, F.; Rossi, G.; Bertolini, F.; Petrachi, T.; Spallanzani, A.; Pettorelli, E.; Kaleci, S.; Luppi, G. mTOR Pathway Expression as Potential Predictive Biomarker in Patients with Advanced Neuroendocrine Tumors Treated with Everolimus. Cancers 2020, 12, 1201. [Google Scholar] [CrossRef]
- Kasajima, A.; Pavel, M.; Darb-Esfahani, S.; Noske, A.; Stenzinger, A.; Sasano, H.; Dietel, M.; Denkert, C.; Röcken, C.; Wiedenmann, B.; et al. mTOR expression and activity patterns in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2011, 18, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Carpizo, D.R.; Harris, C.R. Genetic Drivers of Ileal Neuroendocrine Tumors. Cancers 2021, 13, 5070. [Google Scholar] [CrossRef] [PubMed]
- Svejda, B.; Kidd, M.; Kazberouk, A.; Lawrence, B.; Pfragner, R.; Modlin, I.M. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor–mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer 2011, 117, 4141–4154. [Google Scholar] [CrossRef] [PubMed]
- Geis, C.; Fendrich, V.; Rexin, P.; Di Fazio, P.; Bartsch, D.K.; Ocker, M.; Quint, K.; Heverhagen, A.E. Ileal neuroendocrine tumors show elevated activation of mammalian target of rapamycin complex. J. Surg. Res. 2015, 194, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Zakka, K.; Nagy, R.; Drusbosky, L.; Akce, M.; Wu, C.; Alese, O.B.; El-Rayes, B.F.; Kasi, P.M.; Mody, K.; Starr, J.; et al. Blood-based next-generation sequencing analysis of neuroendocrine neoplasms. Oncotarget 2020, 11, 1749–1757. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Falconi, M.; Filosso, P.L.; Frilling, A.; Malczewska, A.; Toumpanakis, C.; Valk, G.; Pacak, K.; Bodei, L.; et al. A multigenomic liquid biopsy biomarker for neuroendocrine tumor disease outperforms CgA and has surgical and clinical utility. Ann. Oncol. 2021, 32, 1425–1433. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Frilling, A.; Falconi, M.; Filosso, P.L.; Malczewska, A.; Kitz, A. Molecular Genomic Assessment Using a Blood-based mRNA Signature (NETest) is Cost-effective and Predicts Neuroendocrine Tumor Recurrence With 94% Accuracy. Ann. Surg. 2021, 274, 481–490. [Google Scholar] [CrossRef]
- Bodei, L.; Raj, N.; Do, R.K.; Mauguen, A.; Krebs, S.; Reidy-Lagunes, D.; Schöder, H. Interim analysis of a prospective validation of two blood-based genomic assessments (PPQ and NETest) to determine clinical efficacy of 177Lu-DOTATATE in neuroendocrine tumors. J. Nucl. Med. 2022, 64, 8A. [Google Scholar] [CrossRef]
- Baslan, T.; Hicks, J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat. Rev. Cancer 2017, 17, 557–569. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Liu, C.; Yang, J.; Lin, Q.; Zheng, S.; Chen, C.; Zhou, Q.; Chen, R. Single-cell RNA sequencing reveals spatiotemporal heterogeneity and malignant progression in pancreatic neuroendocrine tumor. Int. J. Biol. Sci. 2021, 17, 3760–3775. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; Dafydd, D.a.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.-M.; Orton, M.R. Radiomics in Oncology: A Practical Guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xu, N.; Song, J. Decoding intra-tumoral spatial heterogeneity on radiological images using the Hilbert curve. Insights Imaging 2021, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Dionisi, B.; Thuillier, P.; Ciuffreda, L.; Piovesan, A.; Fioroni, F.; et al. 68Ga-DOTATOC PET/CT-Based Radiomic Analysis and PRRT Outcome: A Preliminary Evaluation Based on an Exploratory Radiomic Analysis on Two Patients. Front. Med. 2021, 7, 601853. [Google Scholar] [CrossRef]
- Gu, D.; Hu, Y.; Ding, H.; Wei, J.; Chen, K.; Liu, H.; Zeng, M.; Tian, J. CT radiomics may predict the grade of pancreatic neuroendocrine tumors: A multicenter study. Eur. Radiol. 2019, 29, 6880–6890. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yang, P.; Huang, R.; Xu, L.; Wang, J.; Liu, W.; Zhang, L.; Wan, D.; Huang, Q.; Lu, Y.; et al. A Combined Nomogram Model to Preoperatively Predict Histologic Grade in Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2019, 25, 584–594. [Google Scholar] [CrossRef] [Green Version]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Del Rivero, J.; Ramirez, R.A.; Soares, H.P.; Li, D. Treatment Sequencing Strategies in Advanced Neuroendocrine Tumors: A Review. Cancers 2022, 14, 5248. [Google Scholar] [CrossRef]
- Chan, T.G.; O’Neill, E.; Habjan, C.; Cornelissen, B. Combination Strategies to Improve Targeted Radionuclide Therapy. J. Nucl. Med. 2020, 61, 1544–1552. [Google Scholar] [CrossRef]
- Clarke, C.N.; Evans, D.B. Treatment sequencing for pancreatic neuroendocrine tumors: Daring to challenge the status quo. J. Gastrointest. Oncol. 2020, 11, 545–547. [Google Scholar] [CrossRef]


| Terminology | Differentiation | Grade | Mitotic Rate (Mitoses/2 mm2) * | Ki-67 Index % ** |
|---|---|---|---|---|
| NET, G1 | Well differentiated | Low | <2 | <3% |
| NET, G2 | Intermediate | 2–20 | 3–20% | |
| NET, G3 | High | >20 | >20% | |
| NEC, small-cell type (SCNEC) | Poorly differentiated | High | >20 | >20% |
| NEC, large-cell type (LCNEC) | >20 | >20% | ||
| MiNEN | Well or poorly differentiated | Variable | Variable | Variable |
| Authors | Site | mTOR | Other Findings |
|---|---|---|---|
| Tran et al. [177] | Metastatic (nodal and liver) pancreatic | ↑ | ↓SSTR |
| Borga et al. [32] | Metastatic (nodal and liver) ileal | ↑ (++ liver) | ↑SSTR5 (liver) intra-spot SSTR2A/SSTR5 expression heterogeneity |
| Karphatakis et al. [43] | Metastatic (liver) small intestine | ↑ | - |
| Gilbert et al. [178] | NENs (including small intestine) | ↑primary ↓metastases | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reccia, I.; Pai, M.; Kumar, J.; Spalding, D.; Frilling, A. Tumour Heterogeneity and the Consequent Practical Challenges in the Management of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2023, 15, 1861. https://doi.org/10.3390/cancers15061861
Reccia I, Pai M, Kumar J, Spalding D, Frilling A. Tumour Heterogeneity and the Consequent Practical Challenges in the Management of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers. 2023; 15(6):1861. https://doi.org/10.3390/cancers15061861
Chicago/Turabian StyleReccia, Isabella, Madhava Pai, Jayant Kumar, Duncan Spalding, and Andrea Frilling. 2023. "Tumour Heterogeneity and the Consequent Practical Challenges in the Management of Gastroenteropancreatic Neuroendocrine Neoplasms" Cancers 15, no. 6: 1861. https://doi.org/10.3390/cancers15061861







