Exploring Spatial Heterogeneity of Immune Cells in Nasopharyngeal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Cohort
2.2. Digital Spatial Profiling (DSP)
2.3. Statistical Analysis
2.4. Quantification of CD45 and CD8 Expression by Immunofluorescence Microscopy
2.5. Transcriptomics Analysis
3. Results
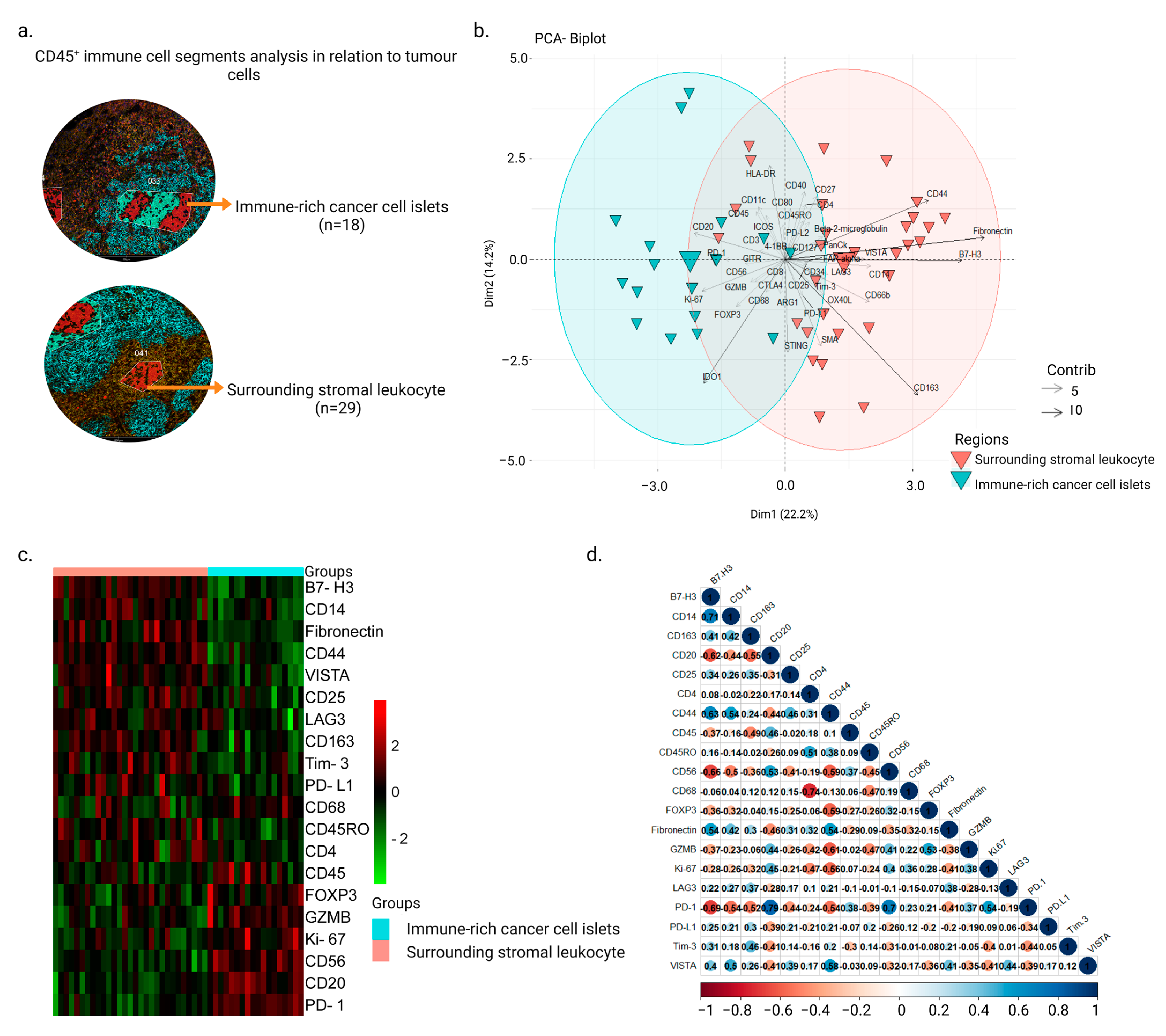
3.1. Intra-Tumor Spatial Profile of NPC for Selected CD45+ Regions Show an Inhibitory Profile in Surrounding Stromal Regions
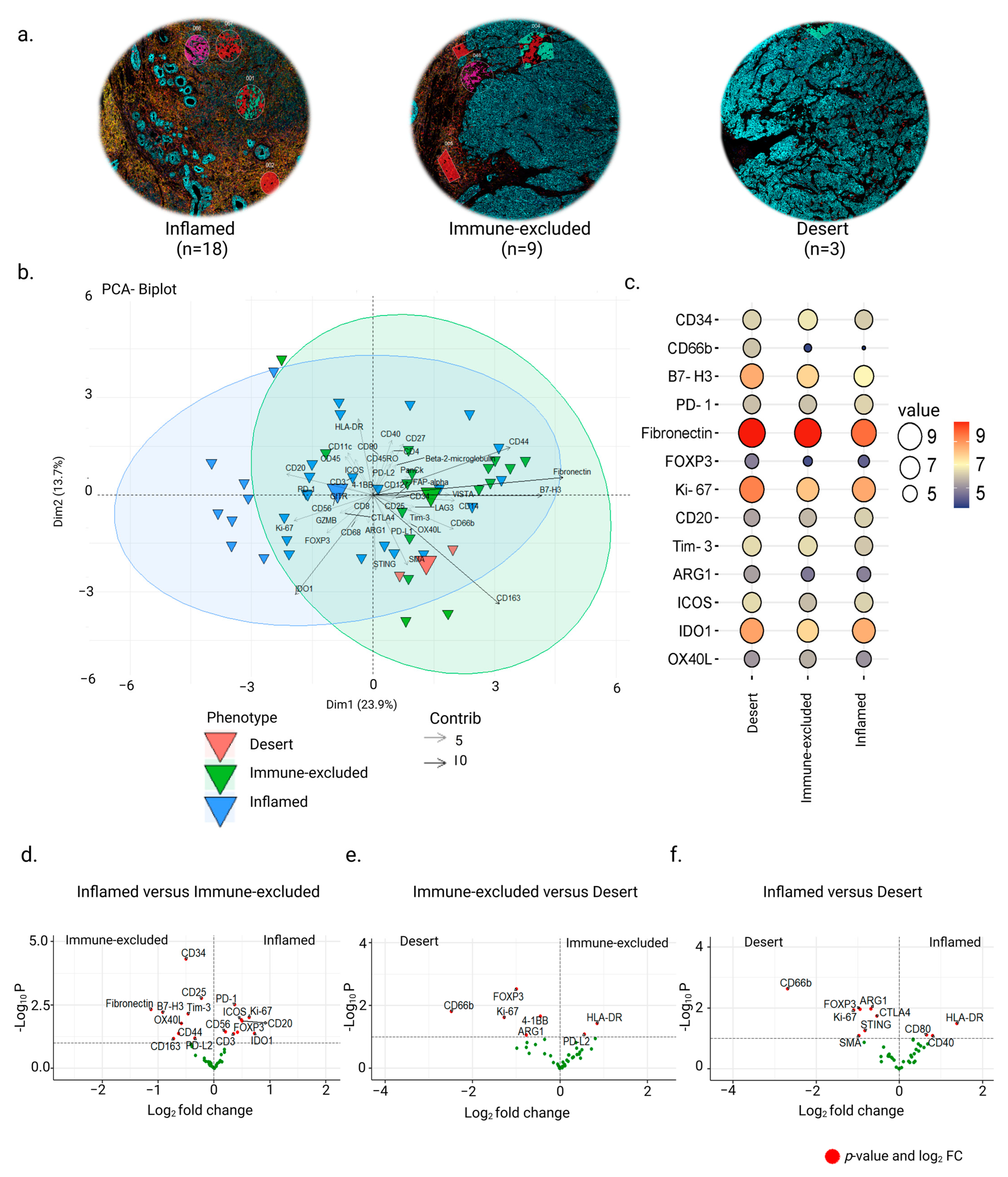
3.2. Inter-Tumor Protein Expression Associated with Three Different Phenotypes
3.3. CD11c and IDO1 Proteins Are Associated with Favorable Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.-P.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cheng, W.; Li, H.; Liu, X. The global, regional, national burden of nasopharyngeal cancer and its attributable risk factors (1990–2019) and predictions to 2035. Cancer Med. 2022, 11, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Lui, V.W.Y.; Bruce, J.P.; Pugh, T.J.; Lo, K.W. Translational genomics of nasopharyngeal cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 84–100. [Google Scholar] [CrossRef]
- Lee, H.M.; Okuda, K.S.; González, F.E.; Patel, V. Current Perspectives on Nasopharyngeal Carcinoma. Hum. Cell Transform. 2019, 1164, 11–34. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Review. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Selitsky, S.R.; Marron, D.; Mose, L.E.; Parker, J.S.; Dittmer, D.P. Epstein-Barr Virus-Positive Cancers Show Altered B-Cell Clonality. Msystems 2018, 3, e00081-18. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.C.M.; Tsao, S.W.; Tsang, C.M. Interplay of Viral Infection, Host Cell Factors and Tumor Microenvironment in the Pathogenesis of Nasopharyngeal Carcinoma. Cancers 2018, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Q.; Chen, Y.-P.; Zhang, Y.; Jiang, W.; Liu, N.; Yun, J.-P.; Sun, Y.; He, Q.-M.; Tang, X.-R.; Wen, X.; et al. Prognostic significance of tumor-infiltrating lymphocytes in nondisseminated nasopharyngeal carcinoma: A large-scale cohort study. Int. J. Cancer 2018, 142, 2558–2566. [Google Scholar] [CrossRef]
- Yu, Y.; Ke, L.; Lv, X.; Ling, Y.H.; Lu, J.; Liang, H.; Qiu, W.; Huang, X.; Liu, G.; Li, W.; et al. The prognostic significance of carcinoma-associated fibroblasts and tumor-associated macrophages in nasopharyngeal carcinoma. Cancer Manag. Res. 2018, 10, 1935–1946. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Azuma, K.; Kawahara, A.; Sasada, T.; Matsuo, N.; Kakuma, T.; Kamimura, H.; Maeda, R.; Hattori, C.; On, K.; et al. Prognostic stratification of patients with nasopharyngeal carcinoma based on tumor immune microenvironment. Head Neck 2018, 40, 2007–2019. [Google Scholar] [CrossRef]
- Liu, S.-L.; Bian, L.-J.; Liu, Z.-X.; Chen, Q.-Y.; Sun, X.-S.; Sun, R.; Luo, D.-H.; Li, X.-Y.; Xiao, B.-B.; Yan, J.-J.; et al. Development and validation of the immune signature to predict distant metastasis in patients with nasopharyngeal carcinoma. J. Immunother. Cancer 2020, 8, e000205. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, J.-H.; Li, W.-F.; Li, H.-J.; Chen, D.-P.; Zhang, C.-J.; Lv, J.-W.; Wang, Y.-Q.; Li, X.-M.; Li, J.-Y.; et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020, 30, 1024–1042. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhang, Y.; Jiang, W.; Chen, Y.-P.; Xu, S.-Y.; Liu, N.; Zhao, Y.; Li, L.; Lei, Y.; Hong, X.-H.; et al. Development and validation of an immune checkpoint-based signature to predict prognosis in nasopharyngeal carcinoma using computational pathology analysis. J. Immunother. Cancer 2019, 7, 298. [Google Scholar] [CrossRef]
- Hernandez, S.; Lazcano, R.; Serrano, A.; Powell, S.; Kostousov, L.; Mehta, J.; Khan, K.; Lu, W.; Solis, L.M. Challenges and Opportunities for Immunoprofiling Using a Spatial High-Plex Technology: The NanoString GeoMx® Digital Spatial Profiler. Front. Oncol. 2022, 12, 2946. [Google Scholar] [CrossRef]
- Nilsson, J.S.; Sobti, A.; Swoboda, S.; Erjefält, J.S.; Forslund, O.; Lindstedt, M.; Greiff, L. Immune Phenotypes of Nasopharyngeal Cancer. Cancers 2020, 12, 3428. [Google Scholar] [CrossRef]
- Lewis, M.; Goldmann, K.; Sciacca, E.; Cubut, C.; Surace, A. glmmSeq: General Linear Mixed Models for Gene-Level Differential Expression; R Package Version: 2021. Available online: https://github.com/myles-lewis/glmmSeq (accessed on 5 July 2022).
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z. Complex heatmap visualization. Imeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling; R Package Version: 2019. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 1 August 2022).
- Alboukadel Kassambara, M.K.; Przemyslaw, B. Survminer: Survival Analysis and Visualization. 2022. Available online: https://rpkgs.datanovia.com/survminer/index.html (accessed on 21 September 2022).
- Terry, M.; Therneau, T.L.; Elizabeth, A.; Cynthia, C. A Package for Survival Analysis in R. Available online: https://CRAN.R-project.org/package=survival (accessed on 21 September 2022).
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M.; The Cancer Genome Atlas Research Network. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-convex Polygons. arXiv 2018, arXiv:1806.03535. [Google Scholar] [CrossRef] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Yang, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 2021, 184, 792–809.e23. [Google Scholar] [CrossRef]
- Ooft, M.L.; van Ipenburg, J.A.; Braunius, W.W.; Zuur, C.I.; Koljenović, S.; Willems, S.M. Prognostic role of tumor infiltrating lymphocytes in EBV positive and EBV negative nasopharyngeal carcinoma. Oral Oncol. 2017, 71, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ooft, M.L.; Van Ipenburg, J.A.; Sanders, M.E.; Kranendonk, M.; Hofland, I.; De Bree, R.; Koljenović, S.; Willems, S.M. Prognostic role of tumour-associated macrophages and regulatory T cells in EBV-positive and EBV-negative nasopharyngeal carcinoma. J. Clin. Pathol. 2018, 71, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, M.; Jin, F. The detection and significance of T cells in nasopharyngeal carcinoma patients. J. Cancer Res. Ther. 2018, 14, S331–S335. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.S.; Abolhalaj, M.; Lundberg, K.; Lindstedt, M.; Greiff, L. Dendritic cell subpopulations in nasopharyngeal cancer. Oncol. Lett. 2019, 17, 2557–2561. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, H.; Lu, J.; Bi, P.; Wang, F.; Liu, X.; Zhang, B.; Luo, Y.; Li, X. Tumor cells induced-M2 macrophage favors accumulation of Treg in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2017, 10, 8389–8401. [Google Scholar]
- Liu, Y.; He, S.; Wang, X.-L.; Peng, W.; Chen, Q.-Y.; Chi, D.-M.; Chen, J.-R.; Han, B.-W.; Lin, G.-W.; Li, Y.-Q.; et al. Tumour heterogeneity and intercellular networks of nasopharyngeal carcinoma at single cell resolution. Nat. Commun. 2021, 12, 741. [Google Scholar] [CrossRef]
- Jin, S.; Li, R.; Chen, M.-Y.; Yu, C.; Tang, L.-Q.; Liu, Y.-M.; Li, J.-P.; Liu, Y.-N.; Luo, Y.-L.; Zhao, Y.; et al. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res. 2020, 30, 950–965. [Google Scholar] [CrossRef]
- Gong, L.; Kwong, D.L.-W.; Dai, W.; Wu, P.; Li, S.; Yan, Q.; Zhang, Y.; Zhang, B.; Fang, X.; Liu, L.; et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat. Commun. 2021, 12, 1540. [Google Scholar] [CrossRef]
- Zhang, L.; MacIsaac, K.D.; Zhou, T.; Huang, P.-Y.; Xin, C.; Dobson, J.R.; Yu, K.; Chiang, D.Y.; Fan, Y.; Pelletier, M.; et al. Genomic Analysis of Nasopharyngeal Carcinoma Reveals TME-Based Subtypes. Mol. Cancer Res. 2017, 15, 1722–1732. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Q.; Chen, L.; Mao, Y.-P.; Li, Y.-Q.; Jiang, W.; Xu, S.-Y.; Zhang, Y.; Chen, Y.-P.; Li, X.-M.; He, Q.-M.; et al. Prognostic value of immune score in nasopharyngeal carcinoma using digital pathology. J. Immunother. Cancer 2020, 8, e000334. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Li, E.; Zhang, G.; Wang, X.; Tang, T.; Bai, X.; Liang, T. VISTA: An immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 83. [Google Scholar] [CrossRef]
- Xu, R.-H.; Mai, H.-Q.; Chen, Q.-Y.; Chen, D.; Hu, C.; Yang, K.; Wen, J.; Li, J.-G.; Shi, Y.; Jin, F.; et al. JUPITER-02: Randomized, double-blind, phase III study of toripalimab or placebo plus gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (NPC). J. Clin. Oncol. 2021, 39, LBA2. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, S.; Li, J.; Hu, C.; Xu, M.; Li, W.; Zhou, T.; Shen, L.; Wu, H.; Lang, J.; et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021, 22, 1162–1174. [Google Scholar] [CrossRef]
- Jung, K.H.; LoRusso, P.; Burris, H.; Gordon, M.; Bang, Y.-J.; Hellmann, M.D.; Cervantes, A.; de Olza, M.O.; Marabelle, A.; Hodi, F.S.; et al. Phase I Study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 3220–3228. [Google Scholar] [CrossRef] [Green Version]
- Yonesaka, K.; Haratani, K.; Takamura, S.; Sakai, H.; Kato, R.; Takegawa, N.; Takahama, T.; Tanaka, K.; Hayashi, H.; Takeda, M.; et al. B7-H3 Negatively Modulates CTL-Mediated Cancer Immunity. Clin. Cancer Res. 2018, 24, 2653–2664. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-Y.; Twu, C.-W.; Liu, Y.-C.; Lin, H.-H.; Chen, C.-J.; Lin, J.-C. Fibronectin promotes nasopharyngeal cancer cell motility and proliferation. Biomed. Pharmacother. 2019, 109, 1772–1784. [Google Scholar] [CrossRef]
- Ma, L.-J.; Lee, S.-W.; Lin, L.-C.; Chen, T.-J.; Chang, I.-W.; Hsu, H.-P.; Chang, K.-Y.; Huang, H.-Y.; Li, C.-F. Fibronectin overexpression is associated with latent membrane protein 1 expression and has independent prognostic value for nasopharyngeal carcinoma. Tumor Biol. 2014, 35, 1703–1712. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, Z.-X.; Li, H.-W.; Luo, H.-Q.; Yang, D.-H.; Zhou, H.-C.; Jiang, D.-X.; Zhan, D.-C.; Yang, L.; Liang, X.-Y.; et al. Circulating Tumor Cells and Fibronectin 1 in the Prognosis of Nasopharyngeal Carcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820909911. [Google Scholar] [CrossRef] [Green Version]
- Pokrývková, B.; Šmahelová, J.; Dalewská, N.; Grega, M.; Vencálek, O.; Šmahel, M.; Nunvář, J.; Klozar, J.; Tachezy, R. ARG1 mRNA Level Is a Promising Prognostic Marker in Head and Neck Squamous Cell Carcinomas. Diagnostics 2021, 11, 628. [Google Scholar] [CrossRef]
- Chang, C.I.; Liao, J.; Kuo, L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001, 61, 1100–1106. [Google Scholar]

| Antibody | Clone | Supplier |
|---|---|---|
| CD8 | C8/144B | Dako/Agilent |
| CD8-AF666/Cy5 | OTI3H6 | Origene |
| CD45-AF615/Texas Red | 2B11 + PD7/26 | Novus Biologicals |
| Pan-Cytokeratin-AF568/Cy3 | AE1 + AE3 | Novus Biologicals |
| SYTO™ 13 | Invitrogen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobti, A.; Sakellariou, C.; Nilsson, J.S.; Askmyr, D.; Greiff, L.; Lindstedt, M. Exploring Spatial Heterogeneity of Immune Cells in Nasopharyngeal Cancer. Cancers 2023, 15, 2165. https://doi.org/10.3390/cancers15072165
Sobti A, Sakellariou C, Nilsson JS, Askmyr D, Greiff L, Lindstedt M. Exploring Spatial Heterogeneity of Immune Cells in Nasopharyngeal Cancer. Cancers. 2023; 15(7):2165. https://doi.org/10.3390/cancers15072165
Chicago/Turabian StyleSobti, Aastha, Christina Sakellariou, Johan S. Nilsson, David Askmyr, Lennart Greiff, and Malin Lindstedt. 2023. "Exploring Spatial Heterogeneity of Immune Cells in Nasopharyngeal Cancer" Cancers 15, no. 7: 2165. https://doi.org/10.3390/cancers15072165
APA StyleSobti, A., Sakellariou, C., Nilsson, J. S., Askmyr, D., Greiff, L., & Lindstedt, M. (2023). Exploring Spatial Heterogeneity of Immune Cells in Nasopharyngeal Cancer. Cancers, 15(7), 2165. https://doi.org/10.3390/cancers15072165






