Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis
Simple Summary
Abstract
1. Hyaluronic Acid
| Cancer Types | HA Category | Clinical Relevance | References |
|---|---|---|---|
| Breast cancer | Serum LMW HA | Lymph node metastasis and angiogenesis | [9] |
| Plasma HA | Tumour progression, poor prognosis, worse response to treatment | [21] | |
| Stromal HA and malignant cell-associated HA | HER2 positivity; elevated tumour size, tumour grading lymph nodes involvement, body mass index and relapse rate; reduced hormone receptor expression, tumour differentiation, overall survival | [22] | |
| [23] | |||
| Colorectal cancer | HA fragments | Early development, cancer progression, lymph nodes metastasis. | [11,24] |
| Pericellular HA | Enhanced invasive capacity. | [25] | |
| HA level | Predictors for OS and DFS | [26] | |
| Ovarian cancer | Pericellular HA | Malignancy; an independent adverse predictor for OS. | [10] |
| HA accumulation | Poor differentiation, metastasis, and aggressive phenotype | [27] | |
| Serum HA before chemotherapy | Chemotherapy resistant; shortened OS and DFS | [28,29] | |
| Endometrial cancer | HA level | Enhanced invasion, tumour grading and lymphatic involvement | [30] |
| Tumour stromal HA | Tumour development | [31] | |
| Peritumour stroma HA | Tumour grade and invasion | [32] | |
| Gastric cancer | HA level | Lymph node metastasis, cancer subtype specific, worse survival outcome. | [33,34] |
| Tumoral HA | Cancer-subtype specific | [35] | |
| Serum HA | Elevated in gastric cancer | [36] | |
| Lung cancer | Baseline plasma HA | Bone metastasis; chemotherapy efficacy | [37] |
| HA content | Histological subtype specific, tumour differentiation, stage, recurrence and DFS | [38] | |
| Tumour HA | Level of malignancy, angiogenesis, patient survival, reflected in sputum | [8] | |
| Mesothelioma | HA level | Increased in pleural fluid | [39] |
| High effusion HA level | Better survival |
2. HA Interacting Molecules
3. Deregulated HAIMs in Solid Tumours
| HA Interacting Molecule | Abnormal Expression | Mutation/Isoform | |
|---|---|---|---|
| Elevation | Decrease | ||
| TSG-6 | High-grade prostate tumour [87], colon cancer [88], ovarian cancer [89] | ||
| ITIH2 | Longer survival in LIHC and colorectal cancer liver metastasis [90,91] | Hepatocellular carcinoma (HCC) cells [92] | |
| ITIH3 | Advanced PDAC [93] | Colorectal, breast, uterine, ovarian, and lung cancers [41,56] | |
| SHAP | Breast, ovarian, and endometrial cancer [94,95,96] | ||
| RHAMM | Breast, ovarian, and pancreatic cancer; lung cancer, endometrial cancer, bladder cancer, hepatocellular carcinoma, and colon cancer [97,98,99,100,101,102,132,133,155] | Pancreatic cancer [132]. | |
| PHBP | Lung adenocarcinomas [103] and non-small-cell lung cancer [104] | Head and neck squamous cell carcinoma [105] | Thyroid cancer [106] |
| HAPLN1 | Pancreas cancer [107], malignant pleural mesothelioma [108], lung cancer [109] | Colorectal cancer [110], malignant gliomas [111] | |
| HAPLN2 | Malignant gliomas [111] | ||
| HAPLN3 | Breast cancer [112,113], clear cell renal cell cancer [114] | Advanced skin cutaneous melanoma [115], cutaneous melanoma [116] | Gene methylation: prostate cancer [117] and de novo metastatic prostate cancer [118] |
| HAPLN4 | Malignant gliomas [111] | ||
| LYVE-1 | Breast, endometrial carcinoma, gastric cancer, malignant vascular tumours, neuroblastoma, and colorectal cancer [119,120,121,122,123,124] | Tongue squamous Cell carcinomas, lung cancer metastasis [125,126] | |
| NCAN | Astrocytoma, glioblastoma, neuroblastoma [127,128,129] | ||
| BCAN | Glioma [50,159] | B/b(Deltag): only present in high-grade glioma [130] | |
| VCAN | Brain tumours, melanomas, osteosarcomas, lymphomas, acute monocytic leukaemia, testicular tumours, breast, prostate, colon, lung, pancreatic, endometrial, ovarian, and oral cancers [131] | ||
4. HAIM Coordinated Cellular Functions
- Dedifferentiation and epithelial mesenchymal transition (EMT)
- Proliferation
- Adhesion
- Invasion and motility
5. HAIMs and Extracellular Matrix Remodelling
6. HAIM and Angiogenesis/Lymphangiogenesis in Cancer
7. HAIM and Immunity in Cancer
8. HAIMs in Distant Metastasis of Cancer Cells
9. Conclusions and Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Passi, A.; Vigetti, D.; Buraschi, S.; Iozzo, R.V. Dissecting the role of hyaluronan synthases in the tumor microenvironment. FEBS J. 2019, 286, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Stankovska, M.; Arnhold, J.; Rychly, J.; Spaltehoz, H.; Gemeiner, P.; Soltes, L. In vitro screening of the action of non-steroidal anti-inflammatory drugs on hypochlorous acid-induced hyaluronan degradation. Polym. Degrad. Stabil. 2007, 92, 644–652. [Google Scholar] [CrossRef]
- Rapta, P.; Valachova, K.; Gemeiner, P.; Soltes, L. High-Molar-Mass Hyaluronan Behavior During Testing Its Radical Scavenging Capacity in Organic and Aqueous Media: Effects of the Presence of Manganese(II) Ions. Chem. Biodivers. 2009, 6, 162–169. [Google Scholar] [CrossRef]
- Bot, P.T.; Hoefer, I.E.; Piek, J.J.; Pasterkamp, G. Hyaluronic acid: Targeting immune modulatory components of the extracellular matrix in atherosclerosis. Curr. Med. Chem. 2008, 15, 786–791. [Google Scholar] [CrossRef]
- Chen, B.; Miller, R.J.; Dhal, P.K. Hyaluronic acid-based drug conjugates: State-of-the-art and perspectives. J. Biomed. Nanotechnol. 2014, 10, 4–16. [Google Scholar] [CrossRef]
- Nykopp, T.K.; Rilla, K.; Tammi, M.I.; Tammi, R.H.; Sironen, R.; Hamalainen, K.; Kosma, V.M.; Heinonen, S.; Anttila, M. Hyaluronan synthases (HAS1-3) and hyaluronidases (HYAL1-2) in the accumulation of hyaluronan in endometrioid endometrial carcinoma. BMC Cancer 2010, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.P.; de Sa, V.K.; Martins, V.; Martins, J.R.M.; Parra, E.R.; Mendes, A.; Andrade, P.C.; Reis, R.M.; Longatto, A.; Oliveira, C.Z.; et al. Tissue hyaluronan expression, as reflected in the sputum of lung cancer patients, is an indicator of malignancy. Braz. J. Med. Biol. Res. 2015, 48, 557–567. [Google Scholar] [CrossRef]
- Wu, M.; Cao, M.; He, Y.; Liu, Y.; Yang, C.; Du, Y.; Wang, W.; Gao, F. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J. 2015, 29, 1290–1298. [Google Scholar] [CrossRef]
- Oliveira-Ferrer, L.; Schmalfeldt, B.; Dietl, J.; Bartmann, C.; Schumacher, U.; Stürken, C. Ovarian Cancer-Cell Pericellular Hyaluronan Deposition Negatively Impacts Prognosis of Ovarian Cancer Patients. Biomedicines 2022, 10, 2944. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, R.; Wu, M.; Liu, Y.; He, Y.; Xu, J.; Yang, C.; Du, Y.; Gao, F. Colorectal cancer-associated ∼6 kDa hyaluronan serves as a novel biomarker for cancer progression and metastasis. FEBS J. 2019, 286, 3148–3163. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cao, M.; Liu, H.; He, Y.; Xu, J.; Du, Y.; Liu, Y.; Wang, W.; Cui, L.; Hu, J.; et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 2012, 287, 43094–43107. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, R.N.; Beebe, D.C. Hyaluronate in vasculogenesis. Science 1983, 220, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Wang, L.; Lu, L.; Li, Y.; Huang, G.; Song, J. ‘Two-faces’ of hyaluronan, a dynamic barometer of disease progression in tumor microenvironment. Discov. Oncol. 2023, 14, 11. [Google Scholar] [CrossRef]
- Horton, M.R.; Olman, M.A.; Bao, C.; White, K.E.; Choi, A.M.; Chin, B.Y.; Noble, P.W.; Lowenstein, C.J. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L707–L715. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Fieber, C.; Baumann, P.; Vallon, R.; Termeer, C.; Simon, J.C.; Hofmann, M.; Angel, P.; Herrlich, P.; Sleeman, J.P. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J. Cell Sci. 2004, 117, 359–367. [Google Scholar] [CrossRef]
- Xu, H.; Ito, T.; Tawada, A.; Maeda, H.; Yamanokuchi, H.; Isahara, K.; Yoshida, K.; Uchiyama, Y.; Asari, A. Effect of hyaluronan oligosaccharides on the expression of heat shock protein 72. J. Biol. Chem. 2002, 277, 17308–17314. [Google Scholar] [CrossRef]
- Peng, C.K.; Wallwiener, M.; Rudolph, A.; Cuk, K.; Eilber, U.; Celik, M.; Modugno, C.; Trumpp, A.; Heil, J.; Marmé, F.; et al. Plasma hyaluronic acid level as a prognostic and monitoring marker of metastatic breast cancer. Int. J. Cancer 2016, 138, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Kosma, V.M.; Sironen, R.; Soini, Y.; Mannermaa, A.; Tumelius, R.; Uljas, E.; Tammi, M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int. J. Cancer 2013, 132, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Ågren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.M. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Klusmeier, S.; Rothley, M.; Dimmler, A.; Sipos, B.; Faller, G.; Thiele, W.; Allgayer, H.; Hohenberger, P.; Post, S.; et al. Accumulation of small hyaluronan oligosaccharides in tumour interstitial fluid correlates with lymphatic invasion and lymph node metastasis. Br. J. Cancer 2014, 111, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Wheeler, M.A.; Wilson, C.M.; Iida, J.; Eng, D.; Simpson, M.A.; McCarthy, J.B.; Bullard, K.M. Hyaluronan facilitates invasion of colon carcinoma cells in vitro via interaction with CD44. Cancer Res. 2004, 64, 4569–4576. [Google Scholar] [CrossRef] [PubMed]
- Ropponen, K.; Tammi, M.; Parkkinen, J.; Eskelinen, M.; Tammi, R.; Lipponen, P.; Ågren, U.; Alhava, E.; Kosma, V.M. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 1998, 58, 342–347. [Google Scholar] [PubMed]
- Hiltunen, E.L.J.; Anttila, M.; Kultti, A.; Ropponen, K.; Penttinen, J.; Yliskoski, M.; Kuronen, A.T.; Juhola, M.; Tammi, R.; Tammi, M.; et al. Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res. 2002, 62, 6410–6413. [Google Scholar] [PubMed]
- Lokman, N.A.; Price, Z.K.; Hawkins, E.K.; Macpherson, A.M.; Oehler, M.K.; Ricciardelli, C. 4-Methylumbelliferone Inhibits Cancer Stem Cell Activation and Overcomes Chemoresistance in Ovarian Cancer. Cancers 2019, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, C.; Ween, M.P.; Lokman, N.A.; Tan, I.A.; Pyragius, C.E.; Oehler, M.K. Chemotherapy-induced hyaluronan production: A novel chemoresistance mechanism in ovarian cancer. BMC Cancer 2013, 13, 476. [Google Scholar] [CrossRef]
- Yabushita, H.; Kishida, T.; Fusano, K.; Kanyama, K.; Zhuo, L.; Itano, N.; Kimata, K.; Noguchi, M. Role of hyaluronan and hyaluronan synthase in endometrial cancer. Oncol. Rep. 2005, 13, 1101–1105. [Google Scholar] [CrossRef]
- Afify, A.M.; Craig, S.; Paulino, A.F.; Stern, R. Expression of hyaluronic acid and its receptors, CD44s and CD44v6, in normal, hyperplastic, and neoplastic endometrium. Ann. Diagn. Pathol. 2005, 9, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Paiva, P.; Van Damme, M.P.; Tellbach, M.; Jones, R.L.; Jobling, T.; Salamonsen, L.A. Expression patterns of hyaluronan, hyaluronan synthases and hyaluronidases indicate a role for hyaluronan in the progression of endometrial cancer. Gynecol. Oncol. 2005, 98, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.L.; Hong, R.T.; Xie, H.J.; Hu, N.Z.; Xu, J.M.; Zhang, W. Significance of elevated levels of collagen type IV and hyaluronic acid in gastric juice and serum in gastric cancer and precancerous lesion. Dig. Dis. Sci. 2011, 56, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Setälä, L.P.; Tammi, M.I.; Tammi, R.H.; Eskelinen, M.J.; Lipponen, P.K.; Ågren, U.M.; Parkkinen, J.; Alhava, E.M.; Kosma, V.M. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br. J. Cancer 1999, 79, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Vizoso, F.; Suarez, C.; Sanz, L.; Rodriguez, J.C.; Roiz, C.; Garcia-Munz, J.L. Relationship of tumoral hyaluronic acid and cathepsin D contents with histological type of gastric carcinoma. Int. J. Biol. Markers 2000, 15, 215–218. [Google Scholar] [CrossRef]
- Aghcheli, K.; Parsian, H.; Qujeq, D.; Talebi, M.; Mosapour, A.; Khalilipour, E.; Islami, F.; Semnani, S.; Malekzadeh, R. Serum hyaluronic acid and laminin as potential tumor markers for upper gastrointestinal cancers. Eur. J. Intern. Med. 2012, 23, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Z.; Hu, X.; Zhang, L.; Liu, Y.; Wang, Y.; Guo, Y.; Zhang, T.; Li, W.; Li, B. Hyaluronic Acid Correlates With Bone Metastasis and Predicts Poor Prognosis in Small-Cell Lung Cancer Patients. Front. Endocrinol. 2021, 12, 785192. [Google Scholar] [CrossRef] [PubMed]
- Pirinen, R.; Tammi, R.; Tammi, M.; Hirvikoski, P.; Parkkinen, J.J.; Johansson, R.; Bohm, J.; Hollmen, S.; Kosma, V.M. Prognostic value of hyaluronan expression in non-small-cell lung cancer: Increased stromal expression indicates unfavorable outcome in patients with adenocarcinoma. Int. J. Cancer 2001, 95, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Creaney, J.; Dick, I.M.; Segal, A.; Musk, A.W.; Robinson, B.W.S. Pleural effusion hyaluronic acid as a prognostic marker in pleural malignant mesothelioma. Lung Cancer 2013, 82, 491–498. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front. Neurosci. 2015, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Morgelin, M.; Paulsson, M.; Heinegard, D.; Aebi, U.; Engel, J. Evidence of a Defined Spatial Arrangement of Hyaluronate in the Central Filament of Cartilage Proteoglycan Aggregates. Biochem. J. 1995, 307, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Banerji, S.; Ni, J.; Wang, S.X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.A.; McCarthy, J.; Turley, E. Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual oncogenic functions? J. Cell Sci. 2008, 121, 925–932. [Google Scholar] [CrossRef]
- Salier, J.P.; Rouet, P.; Raguenez, G.; Daveau, M. The inter-alpha-inhibitor family: From structure to regulation. Biochem. J. 1996, 315 Pt 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi-Miura, N.H.; Yoda, M.; Saito, K.; Takahashi, K.; Tomita, M. Identification of the substrates for plasma hyaluronan binding protein. Biol. Pharm. Bull. 2001, 24, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Islam, S.; Watanabe, H. Versican: A Dynamic Regulator of the Extracellular Matrix. J. Histochem. Cytochem. 2020, 68, 763–775. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Lecticans: Organizers of the brain extracellular matrix. Cell. Mol. Life Sci. 2000, 57, 276–289. [Google Scholar] [CrossRef]
- Lord, M.S.; Melrose, J.; Day, A.J.; Whitelock, J.M. The Inter-α-Trypsin Inhibitor Family: Versatile Molecules in Biology and Pathology. J. Histochem. Cytochem. 2020, 68, 907–927. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Bode-Lesniewska, B.; Dours-Zimmermann, M.T.; Odermatt, B.F.; Briner, J.; Heitz, P.U.; Zimmermann, D.R. Distribution of the large aggregating proteoglycan versican in adult human tissues. J. Histochem. Cytochem. 1996, 44, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78–79, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bai, X.Y.; Li, B.; Li, Y.; Xia, K.; Wang, M.; Li, S.; Wu, H. Plasma Inter-Alpha-Trypsin Inhibitor Heavy Chains H3 and H4 Serve as Novel Diagnostic Biomarkers in Human Colorectal Cancer. Dis. Mark. 2019, 2019, 5069614. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Schwarzler, C.; Gunthert, U. CD44 isoforms during differentiation and development. Bioessays 1995, 17, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Jackson, D.G. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS 2004, 112, 526–538. [Google Scholar] [CrossRef]
- Jackson, D.G.; Prevo, R.; Clasper, S.; Banerji, S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001, 22, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Prevo, R.; Banerji, S.; Ferguson, D.J.; Clasper, S.; Jackson, D.G. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 2001, 276, 19420–19430. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; McCarthy, J.B.; Yazdani, A.; Turley, E.A. Hyaluronan and RHAMM in Wound Repair and the “Cancerization” of Stromal Tissues. BioMed Res. Int. 2014, 2014, 103923. [Google Scholar] [CrossRef] [PubMed]
- He, Z.C.; Mei, L.; Connell, M.; Maxwell, C.A. Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor. Cells 2020, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Hinneh, J.A.; Gillis, J.L.; Moore, N.L.; Butler, L.M.; Centenera, M.M. The role of RHAMM in cancer: Exposing novel therapeutic vulnerabilities. Front. Oncol. 2022, 12, 982231. [Google Scholar] [CrossRef] [PubMed]
- Hatano, H.; Shigeishi, H.; Kudo, Y.; Higashikawa, K.; Tobiume, K.; Takata, T.; Kamata, N. RHAMM/ERK interaction induces proliferative activities of cementifying fibroma cells through a mechanism based on the CD44-EGFR. Lab. Investig. 2011, 91, 379–391. [Google Scholar] [CrossRef]
- Tolg, C.; Hamilton, S.R.; Nakrieko, K.A.; Kooshesh, F.; Walton, P.; McCarthy, J.B.; Bissell, M.J.; Turley, E.A. Rhamm−/− fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J. Cell Biol. 2006, 175, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, H.; Higashikawa, K.; Takechi, M. Role of receptor for hyaluronan-mediated motility (RHAMM) in human head and neck cancers. J. Cancer Res. Clin. Oncol. 2014, 140, 1629–1640. [Google Scholar] [CrossRef]
- Mirzapoiazova, T.; Mambetsariev, N.; Lennon, F.E.; Mambetsariev, B.; Berlind, J.E.; Salgia, R.; Singleton, P.A. HABP2 is a Novel Regulator of Hyaluronan-Mediated Human Lung Cancer Progression. Front. Oncol. 2015, 5, 164. [Google Scholar] [CrossRef]
- Mambetsariev, N.; Mirzapoiazova, T.; Mambetsariev, B.; Sammani, S.; Lennon, F.E.; Garcia, J.G.; Singleton, P.A. Hyaluronic Acid binding protein 2 is a novel regulator of vascular integrity. Arter. Thromb. Vasc. Biol. 2010, 30, 483–490. [Google Scholar] [CrossRef]
- Bost, F.; Diarra-Mehrpour, M.; Martin, J.P. Inter-alpha-trypsin inhibitor proteoglycan family--a group of proteins binding and stabilizing the extracellular matrix. Eur. J. Biochem. 1998, 252, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Salustri, A.; Kimata, K. A physiological function of serum proteoglycan bikunin: The chondroitin sulfate moiety plays a central role. Glycoconj. J. 2002, 19, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.; Birchenough, H.; Ali, T.; Rugg, M.; Waltho, J.; Ievoli, E.; Jowitt, T.; Enghild, J.; Richter, R.; Salustri, A.; et al. Metal Ion-Dependent Heavy Chain Transfer Activity of TSG-6 Mediates Assembly of the Cumulus-Oocyte Matrix. J. Biol. Chem. 2015, 290, 28708–28723. [Google Scholar] [CrossRef]
- Wisniewski, H.G.; Hua, J.C.; Poppers, D.M.; Naime, D.; Vilcek, J.; Cronstein, B.N. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J. Immunol. 1996, 156, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J. Understanding hyaluronan-protein interactions. Glycoforum 2001, 5, A1. [Google Scholar]
- Kohda, D.; Morton, C.J.; Parkar, A.A.; Hatanaka, H.; Inagaki, F.M.; Campbell, I.D.; Day, A.J. Solution structure of the link module: A hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell 1996, 86, 767–775. [Google Scholar] [CrossRef]
- Jadin, L.; Huang, L.; Wei, G.; Zhao, Q.; Gelb, A.B.; Frost, G.I.; Jiang, P.; Shepard, H.M. Characterization of a novel recombinant hyaluronan binding protein for tissue hyaluronan detection. J. Histochem. Cytochem. 2014, 62, 672–683. [Google Scholar] [CrossRef]
- Li, R.; Ji, C.; Dai, M.; Huang, J.; Xu, W.; Ma, Y.; Zhang, H. An update on the role of tumor necrosis factor alpha stimulating gene-6 in inflammatory diseases. Mol. Immunol. 2022, 152, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.; Beckmann, G. The Cub Domain—A Widespread Module in Developmentally-Regulated Proteins. J. Mol. Biol. 1993, 231, 539–545. [Google Scholar] [CrossRef]
- Jiang, Y.; Glasstetter, L.M.; Lerman, A.; Lerman, L.O. TSG-6 (Tumor Necrosis Factor-alpha-Stimulated Gene/Protein-6): An Emerging Remedy for Renal Inflammation. Hypertension 2023, 80, 35–42. [Google Scholar] [CrossRef]
- Spicer, A.P.; Joo, A.; Bowling, R.A. A hyaluronan binding link protein gene family whose members are physically linked adjacent to chrondroitin sulfate proteoglycan core protein genes—The missing links. J. Biol. Chem. 2003, 278, 21083–21091. [Google Scholar] [CrossRef] [PubMed]
- Talts, U.; Kuhn, U.; Roos, G.; Rauch, U. Modulation of extracellular matrix adhesiveness by neurocan and identification of its molecular basis. Exp. Cell Res. 2000, 259, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.C.; Zhang, Y.; Cao, L.; Yang, B.L.; Young, B.; Kiani, C.; Lee, V.; Allan, K.; Yang, B.B. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J. Neuropathol. Exp. Neurol. 1999, 58, 597–605. [Google Scholar] [CrossRef]
- Perissinotto, D.; Iacopetti, P.; Bellina, I.; Doliana, R.; Colombatti, A.; Pettway, Z.; Bronner-Fraser, M.; Shinomura, T.; Kimata, K.; Mörgelin, M.; et al. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development 2000, 127, 2823–2842. [Google Scholar] [CrossRef]
- Zheng, P.S.; Wen, J.; Ang, L.C.; Sheng, W.; Viloria-Petit, A.; Wang, Y.; Wu, Y.; Kerbel, R.S.; Yang, B.B. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004, 18, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, B.B.; Yang, B.L.; Deng, Z.; Fang, L.; Shan, S.W.; Jeyapalan, Z.; Zhang, Y.; Seth, A.; Yee, A.J. Versican G3 domain modulates breast cancer cell apoptosis: A mechanism for breast cancer cell response to chemotherapy and EGFR therapy. PLoS ONE 2011, 6, e26396. [Google Scholar] [CrossRef]
- Garcia, G.E.; Wisniewski, H.G.; Lucia, M.S.; Arevalo, N.; Slaga, T.J.; Kraft, S.L.; Strange, R.; Kumar, A.P. 2-Methoxyestradiol inhibits prostate tumor development in transgenic adenocarcinoma of mouse prostate: Role of tumor necrosis factor-alpha-stimulated gene 6. Clin. Cancer Res. 2006, 12, 980–988. [Google Scholar] [CrossRef]
- Chan, T.-C.; Li, C.-F.; Ke, H.-L.; Wei, Y.-C.; Shiue, Y.-L.; Li, C.-C.; Yeh, H.-C.; Lee, H.-Y.; Huang, S.-K.; Wu, W.-J.; et al. High TNFAIP6 level is associated with poor prognosis of urothelial carcinomas. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 293.e211–293.e224. [Google Scholar] [CrossRef]
- Rachidi, S.M.; Qin, T.; Sun, S.; Zheng, W.J.; Li, Z. Molecular profiling of multiple human cancers defines an inflammatory cancer-associated molecular pattern and uncovers KPNA2 as a uniform poor prognostic cancer marker. PLoS ONE 2013, 8, e57911. [Google Scholar] [CrossRef]
- Kang, X.; Bai, L.; Qi, X.; Wang, J. Screening and identification of key genes between liver hepatocellular carcinoma (LIHC) and cholangiocarcinoma (CHOL) by bioinformatic analysis. Medicine 2020, 99, e23563. [Google Scholar] [CrossRef]
- Niu, L.; Gao, C.; Li, Y. Identification of potential core genes in colorectal carcinoma and key genes in colorectal cancer liver metastasis using bioinformatics analysis. Sci. Rep. 2021, 11, 23938. [Google Scholar] [CrossRef] [PubMed]
- González-Ramón, N.; Alava, M.A.; Sarsa, J.A.; Piñeiro, M.; Escartin, A.; Garcia-Gil, A.; Lampreave, F.; Piñeiro, A. The major acute phase serum protein in pigs is homologous to human plasma kallikrein sensitive PK-120. FEBS Lett. 1995, 371, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Pan, S.; Yan, Y.; Brand, R.E.; Petersen, G.M.; Chari, S.T.; Lai, L.A.; Eng, J.K.; Brentnall, T.A.; Chen, R. Systemic Proteome Alterations Linked to Early Stage Pancreatic Cancer in Diabetic Patients. Cancers 2020, 12, 1534. [Google Scholar] [CrossRef]
- Yabushita, H.; Noguchi, M.; Kishida, T.; Fusano, K.; Noguchi, Y.; Itano, N.; Kimata, K.; Noguchi, M. Hyaluronan synthase expression in ovarian cancer. Oncol. Rep. 2004, 12, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, H.; Iwasaki, K.; Kanyama, K.; Obayashi, Y.; Zhuo, L.; Itano, N.; Kimata, K.; Wakatsuki, A. Clinicopathological Role of Serum-Derived Hyaluronan-Associated Protein (SHAP)-Hyaluronan Complex in Endometrial Cancer. Obs. Gynecol. Int. 2011, 2011, 739150. [Google Scholar] [CrossRef]
- Peng, S.T.; Su, C.H.; Kuo, C.C.; Shaw, C.F.; Wang, H.S. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int. J. Oncol. 2007, 31, 1119–1126. [Google Scholar]
- Schatz-Siemers, N.; Chen, Y.T.; Chen, Z.M.; Wang, D.R.; Ellenson, L.H.; Du, Y.C.N. Expression of the Receptor for Hyaluronic Acid-Mediated Motility (RHAMM) in Endometrial Cancer is Associated With Adverse Histologic Parameters and Tumor Progression. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Wang, D.R.; Chen, X.; Tang, L.H.; Verma, A.; Chen, Z.M.; Kim, B.J.; Selesner, L.; Robzyk, K.; Zhang, G.; et al. Function and clinical relevance of RHAMM isoforms in pancreatic tumor progression. Mol. Cancer 2019, 18, 92. [Google Scholar] [CrossRef]
- Buttermore, S.T.; Hoffman, M.S.; Kumar, A.; Champeaux, A.; Nicosia, S.V.; Kruk, P.A. Increased RHAMM expression relates to ovarian cancer progression. J. Ovarian Res. 2017, 10, 66. [Google Scholar] [CrossRef]
- Mele, V.; Sokol, L.; Kölzer, V.H.; Pfaff, D.; Muraro, M.G.; Keller, I.; Stefan, Z.; Centeno, I.; Terracciano, L.M.; Dawson, H.; et al. The hyaluronan-mediated motility receptor RHAMM promotes growth, invasiveness and dissemination of colorectal cancer. Oncotarget 2017, 8, 70617–70629. [Google Scholar] [CrossRef]
- Morera, D.S.; Hennig, M.S.; Talukder, A.; Lokeshwar, S.D.; Wang, J.; Garcia-Roig, M.; Ortiz, N.; Yates, T.J.; Lopez, L.E.; Kallifatidis, G.; et al. Hyaluronic acid family in bladder cancer: Potential prognostic biomarkers and therapeutic targets. Br. J. Cancer 2017, 117, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, K.; Gao, Y. The critical role of B4GALT4 in promoting microtubule spindle assembly in HCC through the regulation of PLK1 and RHAMM expression. J. Cell. Physiol. 2022, 237, 617–636. [Google Scholar] [CrossRef]
- Wang, K.K.; Liu, N.; Radulovich, N.; Wigle, D.A.; Johnston, M.R.; Shepherd, F.A.; Minden, M.D.; Tsao, M.S. Novel candidate tumor marker genes for lung adenocarcinoma. Oncogene 2002, 21, 7598–7604. [Google Scholar] [CrossRef] [PubMed]
- Chong, I.W.; Chang, M.Y.; Chang, H.C.; Yu, Y.P.; Sheu, C.C.; Tsai, J.R.; Hung, J.Y.; Chou, S.H.; Tsai, M.S.; Hwang, J.J.; et al. Great potential of a panel of multiple hMTH1, SPD, ITGA11 and COL11A1 markers for diagnosis of patients with non-small cell lung cancer. Oncol. Rep. 2006, 16, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, K.; Katsuno, S.; Hashimoto, S.; Oguchi, T.; Suzuki, N.; Asamura, K.; Usami, S. Differences in the expression of genes between normal tissue and squamous cell carcinomas of head and neck using cancer-related gene cDNA microarray. Acta Otolaryngol. 2006, 126, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Gara, S.K.; Jia, L.; Merino, M.J.; Agarwal, S.K.; Zhang, L.; Cam, M.; Patel, D.; Kebebew, E. Germline HABP2 Mutation Causing Familial Nonmedullary Thyroid Cancer. N. Engl. J. Med. 2015, 373, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, L.; De Angelis Rigotti, F.; Vaquero-Siguero, N.; Donato, E.; Espinet, E.; Moll, I.; Alsina-Sanchis, E.; Bohnenberger, H.; Fernandez-Florido, E.; Mulfarth, R.; et al. HAPLN1 potentiates peritoneal metastasis in pancreatic cancer. Nat. Commun. 2023, 14, 2353. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Goparaju, C.M.; Ivanov, S.V.; Nonaka, D.; Cruz, C.; Beck, A.; Lonardo, F.; Wali, A.; Pass, H.I. Protumorigenic role of HAPLN1 and its IgV domain in malignant pleural mesothelioma. Clin. Cancer Res. 2009, 15, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, X.; He, Y.; Wang, Y.; Shen, J.; Wang, S.; You, Q.; Zhai, J.; Shen, L. Cancer-associated fibroblasts-derived HAPLN1 promotes tumour invasion through extracellular matrix remodeling in gastric cancer. Gastric Cancer 2022, 25, 346–359. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Marshall, J.E.; Gong, M.; Zhao, Y.; Dua, K.; Hansbro, P.M.; Xu, J.; Liu, G. Loss of Hyaluronan and Proteoglycan Link Protein-1 Induces Tumorigenesis in Colorectal Cancer. Front. Oncol. 2021, 11, 754240. [Google Scholar] [CrossRef]
- Sim, H.; Hu, B.; Viapiano, M.S. Reduced expression of the hyaluronan and proteoglycan link proteins in malignant gliomas. J. Biol. Chem. 2009, 284, 26547–26556. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.J.; Chien, S.Y.; Lin, C.; Chan, S.E.; Tsai, H.T.; Chen, D.R. Significant elevation of CLDN16 and HAPLN3 gene expression in human breast cancer. Oncol. Rep. 2010, 24, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Santuario-Facio, S.K.; Cardona-Huerta, S.; Perez-Paramo, Y.X.; Trevino, V.; Hernandez-Cabrera, F.; Rojas-Martinez, A.; Uscanga-Perales, G.; Martinez-Rodriguez, J.L.; Martinez-Jacobo, L.; Padilla-Rivas, G.; et al. A New Gene Expression Signature for Triple Negative Breast Cancer Using Frozen Fresh Tissue before Neoadjuvant Chemotherapy. Mol. Med. 2017, 23, 101–111. [Google Scholar] [CrossRef]
- Ding, Y.; Xiong, S.; Chen, X.; Pan, Q.; Fan, J.; Guo, J. HAPLN3 inhibits apoptosis and promotes EMT of clear cell renal cell carcinoma via ERK and Bcl-2 signal pathways. J. Cancer Res. Clin. Oncol. 2023, 149, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lei, S.; Luo, X.; Jiang, C.; Li, Z. The value of cuproptosis-related differential genes in guiding prognosis and immune status in patients with skin cutaneous melanoma. Front. Pharmacol. 2023, 14, 1129544. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Liu, H.; Lu, J. Development of a Four-mRNA Expression-Based Prognostic Signature for Cutaneous Melanoma. Front. Genet. 2021, 12, 680617. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, C.; Mundbjerg, K.; Vestergaard, E.M.; Lamy, P.; Wild, P.; Schulz, W.A.; Arsov, C.; Visakorpi, T.; Borre, M.; Hoyer, S.; et al. DNA methylation signatures for prediction of biochemical recurrence after radical prostatectomy of clinically localized prostate cancer. J. Clin. Oncol. 2013, 31, 3250–3258. [Google Scholar] [CrossRef]
- Bjerre, M.T.; Norgaard, M.; Larsen, O.H.; Jensen, S.O.; Strand, S.H.; Ostergren, P.; Fode, M.; Fredsoe, J.; Ulhoi, B.P.; Mortensen, M.M.; et al. Epigenetic Analysis of Circulating Tumor DNA in Localized and Metastatic Prostate Cancer: Evaluation of Clinical Biomarker Potential. Cells 2020, 9, 1362. [Google Scholar] [CrossRef]
- Gao, F.; Lu, Y.M.; Cao, M.L.; Liu, Y.W.; He, Y.Q.; Wang, Y. Expression and quantification of LYVE-1 in human colorectal cancer. Clin. Exp. Med. 2006, 6, 65–71. [Google Scholar] [CrossRef]
- Ozmen, F.; Ozmen, M.M.; Ozdemir, E.; Moran, M.; Seçkin, S.; Guc, D.; Karaagaoglu, E.; Kansu, E. Relationship between LYVE-1, VEGFR-3 and CD44 gene expressions and lymphatic metastasis in gastric cancer. World J. Gastroenterol. 2011, 17, 3220–3228. [Google Scholar] [CrossRef]
- Ramani, P.; Dungwa, J.V.; May, M.T. LYVE-1 upregulation and lymphatic invasion correlate with adverse prognostic factors and lymph node metastasis in neuroblastoma. Virchows Arch. 2012, 460, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bono, P.; Wasenius, V.M.; Heikkila, P.; Lundin, J.; Jackson, D.G.; Joensuu, H. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin. Cancer Res. 2004, 10, 7144–7149. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Z.; Gao, F.; Meng, X.Y. High density of peritumoral lymphatic vessels is a potential prognostic marker of endometrial carcinoma: A clinical immunohistochemical method study. BMC Cancer 2010, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Edwards, J.R.; Espinosa, O.; Banerji, S.; Jackson, D.G.; Athanasou, N.A. Expression of a lymphatic endothelial cell marker in benign and malignant vascular tumors. Hum. Pathol. 2004, 35, 857–861. [Google Scholar] [CrossRef]
- Nunomiya, K.; Shibata, Y.; Abe, S.; Inoue, S.; Igarashi, A.; Yamauchi, K.; Kimura, T.; Aida, Y.; Nemoto, T.; Sato, M.; et al. Relationship between Serum Level of Lymphatic Vessel Endothelial Hyaluronan Receptor-1 and Prognosis in Patients with Lung Cancer. J. Cancer 2014, 5, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Mukae, S.; Tsuda, H.; Sawada, A.; Okazaki, Y.; Nagai, K.; Akishima-Fukasawa, Y.; Ohki, H.; Ishikawa, Y.; Ishii, T.; et al. Prognostic value of LYVE-1-positive lymphatic vessel in tongue squamous cell carcinomas. Anticancer Res. 2010, 30, 1897–1903. [Google Scholar] [PubMed]
- Varga, I.; Hutoczki, G.; Petras, M.; Scholtz, B.; Miko, E.; Kenyeres, A.; Toth, J.; Zahuczky, G.; Bognar, L.; Hanzely, Z.; et al. Expression of invasion-related extracellular matrix molecules in human glioblastoma versus intracerebral lung adenocarcinoma metastasis. Cent. Eur. Neurosurg. 2010, 71, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Hutóczki, G.; Szemcsák, C.D.; Zahuczky, G.; Tóth, J.; Adamecz, Z.; Kenyeres, A.; Bognár, L.; Hanzély, Z.; Klekner, A. Brevican, neurocan, tenascin-C and versican are mainly responsible for the invasiveness of low-grade astrocytoma. Pathol. Oncol. Res. 2012, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Kishida, S.; Tsubota, S.; Sakamoto, K.; Cao, D.; Kiyonari, S.; Ohira, M.; Kamijo, T.; Narita, A.; Xu, Y.; et al. Neurocan, an extracellular chondroitin sulfate proteoglycan, stimulates neuroblastoma cells to promote malignant phenotypes. Oncotarget 2017, 8, 106296–106310. [Google Scholar] [CrossRef]
- Viapiano, M.S.; Bi, W.L.; Piepmeier, J.; Hockfield, S.; Matthews, R.T. Novel tumor-specific isoforms of BEHAB/Brevican identified in human malignant gliomas. Cancer Res. 2005, 65, 6726–6733. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Feng, J.; Chen, X.; Wang, D.; Wong, M.; Zhang, G.; Na, J.; Zhang, T.; Chen, Z.; Chen, Y.-T.; et al. High levels of truncated RHAMM cooperate with dysfunctional p53 to accelerate the progression of pancreatic cancer. Cancer Lett. 2021, 514, 79–89. [Google Scholar] [CrossRef]
- Wang, C.; Thor, A.D.; Moore, D.H.; Zhao, Y.; Kerschmann, R.; Stern, R.; Watson, P.H.; Turley, E.A. The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated protein kinase and is a significant parameter in breast cancer progression. Clin. Cancer Res. 1998, 4, 567–576. [Google Scholar]
- Obayashi, Y.; Yabushita, H.; Kanyama, K.; Noguchi, M.; Zhuo, L.; Kimata, K.; Wakatsuki, A. Role of serum-derived hyaluronan-associated protein-hyaluronan complex in ovarian cancer. Oncol. Rep. 2008, 19, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Augustin, F.; Fiegl, M.; Schmid, T.; Pomme, G.; Sterlacci, W.; Tzankov, A. Receptor for hyaluronic acid-mediated motility (RHAMM, CD168) expression is prognostically important in both nodal negative and nodal positive large cell lung cancer. J. Clin. Pathol. 2015, 68, 368–373. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Huang, L.W.; Li, J.; Wang, P. Bioinformatics analysis reveals immune prognostic markers for overall survival of colorectal cancer patients: A novel machine learning survival predictive system. BMC Bioinform. 2022, 23, 124. [Google Scholar] [CrossRef]
- Liu, B.; Liu, T.; Liu, Y.; Feng, X.; Jiang, X.; Long, J.; Ye, S.; Chen, D.; Wang, J.; Yang, Z. TSG-6 promotes Cancer Cell aggressiveness in a CD44-Dependent Manner and Reprograms Normal Fibroblasts to create a Pro-metastatic Microenvironment in Colorectal Cancer. Int. J. Biol. Sci. 2022, 18, 1677–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Tang, B.X.; Hu, J.P.; Fang, X.; Bian, H.Z.; Han, J.L.; Hou, C.X.; Sun, F. Neutrophils correlate with hypoxia microenvironment and promote progression of non-small-cell lung cancer. Bioengineered 2021, 12, 8872–8884. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Liu, X.Y.; Qiu, X.W.; Niu, Z.H.; Dong, W.; Song, Y.P. Prognostic roles of a novel basement membranes-related gene signature in lung adenocarcinoma. Front. Genet. 2023, 14, 1100560. [Google Scholar] [CrossRef]
- Harigaya, T.; Ogawa, H.; Tsunoda, S.; Nagasawa, H. The mRNA expression of neurocan, a brain-specific chondroitin sulfate proteoglycan, in neoplastic mammary glands in mice. Zool. Sci. 1996, 13, 665–668. [Google Scholar] [CrossRef]
- Salmikangas, M.E.K. Neurocan as a Prognostic Marker in Merkel Cell Carcinoma. Master’s Thesis, Faculty of Medicine, University of Helsinki, Helsinki, Finland, 2020. [Google Scholar]
- Suwiwat, S.; Ricciardelli, C.; Tammi, R.; Tammi, M.; Auvinen, P.; Kosma, V.M.; LeBaron, R.G.; Raymond, W.A.; Tilley, W.D.; Horsfall, D.J. Expression of extracellular matrix components versican, chondroitin sulfate, tenascin, and hyaluronan, and their association with disease outcome in node-negative breast cancer. Clin. Cancer Res. 2004, 10, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Ricciardelli, C.; Mayne, K.; Sykes, P.J.; Raymond, W.A.; McCaul, K.; Marshall, V.R.; Horsfall, D.J. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin. Cancer Res. 1998, 4, 963–971. [Google Scholar] [PubMed]
- Pirinen, R.; Leinonen, T.; Böhm, J.; Johansson, R.; Ropponen, K.; Kurnpulainen, E.; Kosma, V.M. Versican in nonsmall cell lung cancer:: Relation to hyaluronan, clinicopathologic factors, and prognosis. Hum. Pathol. 2005, 36, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pukkila, M.; Kosunen, A.; Ropponen, K.; Virtaniemi, J.; Kellokoski, J.; Kumpulainen, E.; Pirinen, R.; Nuutinen, J.; Johansson, R.; Kosma, V.M. High stromal versican expression predicts unfavourable outcome in oral squamous cell carcinoma. J. Clin. Pathol. 2007, 60, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Labropoulou, V.T.; Theocharis, A.D.; Ravazoula, P.; Perimenis, P.; Hjerpe, A.; Karamanos, N.K.; Kalofonos, H.P. Versican but not decorin accumulation is related to metastatic potential and neovascularization in testicular germ cell tumours. Histopathology 2006, 49, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Kodama, J.; Hasengaowa; Kusumoto, T.; Seki, N.; Matsuo, T.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Versican expression in human cervical cancer. Eur. J. Cancer 2007, 43, 1460–1466. [Google Scholar] [CrossRef]
- Kodama, J.; Hasengaowa; Kusumoto, T.; Seki, N.; Matsuo, T.; Ojima, Y.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Prognostic significance of stromal versican expression in human endometrial cancer. Ann. Oncol. 2007, 18, 269–274. [Google Scholar] [CrossRef]
- Song, J.; Wei, R.; Huo, S.; Liu, C.; Liu, X. Versican enrichment predicts poor prognosis and response to adjuvant therapy and immunotherapy in gastric cancer. Front. Immunol. 2022, 13, 960570. [Google Scholar] [CrossRef] [PubMed]
- Abetamann, V.; Kern, H.F.; Elsasser, H.P. Differential expression of the hyaluronan receptors CD44 and RHAMM in human pancreatic cancer cells. Clin. Cancer Res. 1996, 2, 1607–1618. [Google Scholar]
- Li, H.; Guo, L.; Li, J.W.; Liu, N.; Qi, R.; Liu, J. Expression of hyaluronan receptors CD44 and RHAMM in stomach cancers: Relevance with tumor progression. Int. J. Oncol. 2000, 17, 927–932. [Google Scholar] [CrossRef]
- Rein, D.T.; Roehrig, K.; Schondorf, T.; Lazar, A.; Fleisch, M.; Niederacher, D.; Bender, H.G.; Dall, P. Expression of the hyaluronan receptor RHAMM in endometrial carcinomas suggests a role in tumour progression and metastasis. J. Cancer Res. Clin. Oncol. 2003, 129, 161–164. [Google Scholar] [CrossRef]
- Teder, P.; Bergh, J.; Heldin, P. Functional hyaluronan receptors are expressed on a squamous cell lung carcinoma cell line but not on other lung carcinoma cell lines. Cancer Res. 1995, 55, 3908–3914. [Google Scholar]
- Song, J.M.; Im, J.; Nho, R.S.; Han, Y.H.; Upadhyaya, P.; Kassie, F. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol. Carcinog. 2019, 58, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Messam, B.J.; Tolg, C.; McCarthy, J.B.; Nelson, A.C.; Turley, E.A. RHAMM Is a Multifunctional Protein That Regulates Cancer Progression. Int. J. Mol. Sci. 2021, 22, 10313. [Google Scholar] [CrossRef] [PubMed]
- Jauch, A.S.; Wohlfeil, S.A.; Weller, C.; Dietsch, B.; Hafele, V.; Stojanovic, A.; Kittel, M.; Nolte, H.; Cerwenka, A.; Neumaier, M.; et al. Lyve-1 deficiency enhances the hepatic immune microenvironment entailing altered susceptibility to melanoma liver metastasis. Cancer Cell Int. 2022, 22, 398. [Google Scholar] [CrossRef]
- Kemmochi, S.; Yamamichi, S.; Shimamoto, K.; Onda, N.; Hasumi, K.; Suzuki, K.; Mitsumori, K.; Shibutani, M. Lac color inhibits development of rat thyroid carcinomas through targeting activation of plasma hyaluronan-binding protein. Exp. Biol. Med. 2012, 237, 728–738. [Google Scholar] [CrossRef]
- Melo-Hanchuk, T.D.; Colleti, C.; Saito, A.; Mendes, M.C.S.; Carvalheira, J.B.C.; Vassallo, J.; Kobarg, J. Intracellular hyaluronic acid-binding protein 4 (HABP4): A candidate tumor suppressor in colorectal cancer. Oncotarget 2020, 11, 4325–4337. [Google Scholar] [CrossRef]
- Lu, R.; Wu, C.; Guo, L.; Liu, Y.; Mo, W.; Wang, H.; Ding, J.; Wong, E.T.; Yu, M. The role of brevican in glioma: Promoting tumor cell motility in vitro and in vivo. BMC Cancer 2012, 12, 607. [Google Scholar] [CrossRef]
- Tang, J.X.; Chen, Q.; Li, Q.; He, Y.H.; Xiao, D. Exosomal mRNAs and lncRNAs involved in multiple myeloma resistance to bortezomib. Cell Biol. Int. 2021, 45, 965–975. [Google Scholar] [CrossRef]
- Huynh, M.; Chang, H.Y.; Lisiero, D.N.; Ong, I.M.; Kashyap, T.; Callander, N.S.; Miyamoto, S. HAPLN1 confers multiple myeloma cell resistance to several classes of therapeutic drugs. PLoS ONE 2022, 17, e0274704. [Google Scholar] [CrossRef]
- Pan, S.; Cheng, L.H.; White, J.T.; Lu, W.; Utleg, A.G.; Yan, X.W.; Urban, N.D.; Drescher, C.W.; Hood, L.; Lin, B.Y. Quantitative Proteomics Analysis Integrated with Microarray Data Reveals That Extracellular Matrix Proteins, Catenins, and P53 Binding Protein 1 Are Important for Chemotherapy Response in Ovarian Cancers. Omics J. Integr. Biol. 2009, 13, 345–354. [Google Scholar] [CrossRef]
- Pibuel, M.A.; Poodts, D.; Molinari, Y.; Diaz, M.; Amoia, S.; Byrne, A.; Hajos, S.; Lompardia, S.; Franco, P. The importance of RHAMM in the normal brain and gliomas: Physiological and pathological roles. Br. J. Cancer 2023, 128, 12–20. [Google Scholar] [CrossRef]
- Zlobec, I.; Terracciano, L.; Tornillo, L.; Günthert, U.; Vuong, T.; Jass, J.R.; Lugli, A. Role of RHAMM within the hierarchy of well-established prognostic factors in colorectal cancer. Gut 2008, 57, 1413–1419. [Google Scholar] [CrossRef]
- Ireland, H.; Miller, G.J.; Webb, K.E.; Cooper, J.A.; Humphries, S.E. The factor VII activating protease G511E (Marburg) variant and cardiovascular risk. Thromb. Haemost. 2004, 92, 986–992. [Google Scholar] [CrossRef]
- Sedding, D.; Daniel, J.M.; Muhl, L.; Hersemeyer, K.; Brunsch, H.; Kemkes-Matthes, B.; Braun-Dullaeus, R.C.; Tillmanns, H.; Weimer, T.; Preissner, K.T.; et al. The G534E polymorphism of the gene encoding the factor VII-activating protease is associated with cardiovascular risk due to increased neointima formation. J. Exp. Med. 2006, 203, 2801–2807. [Google Scholar] [CrossRef]
- Mohamedi, Y.; Fontanil, T.; Cobo, T.; Cal, S.; Obaya, A.J. New Insights into ADAMTS Metalloproteases in the Central Nervous System. Biomolecules 2020, 10, 403. [Google Scholar] [CrossRef]
- Sakko, A.J.; Ricciardelli, C.; Mayne, K.; Tilley, W.D.; LeBaron, R.G.; Harosfall, D.J. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor β1. Cancer Res. 2001, 61, 926–930. [Google Scholar]
- Nikitovic, D.; Zafiropoulos, A.; Katonis, P.; Tsatsakis, A.; Theocharis, A.D.; Karamanos, N.K.; Tzanakakis, G.N. Transforming growth factor-β as a key molecule triggering the expression of versican isoforms V0 and V1, hyaluronan synthase-2 and synthesis of hyaluronan in malignant osteosarcoma cells. IUBMB Life 2006, 58, 47–53. [Google Scholar] [CrossRef]
- Arslan, F.; Bosserhoff, A.K.; Nickl-Jockschat, T.; Doerfelt, A.; Bogdahn, U.; Hau, P. The role of versican isoforms V0/V1 in glioma migration mediated by transforming growth factor-β2. Br. J. Cancer 2007, 96, 1560–1568. [Google Scholar] [CrossRef]
- Wight, T.N.; Day, A.J.; Kang, I.; Harten, I.A.; Kaber, G.; Briggs, D.C.; Braun, K.R.; Lemire, J.M.; Kinsella, M.G.; Hinek, A.; et al. V3: An enigmatic isoform of the proteoglycan versican. Am. J. Physiol. Cell Physiol. 2023, 325, C519–C537. [Google Scholar] [CrossRef]
- von Spreckelsen, N.; Fadzen, C.M.; Hartrampf, N.; Ghotmi, Y.; Wolfe, J.M.; Dubey, S.; Yang, B.Y.; Kijewski, M.F.; Wang, S.; Farquhar, C.; et al. Targeting glioblastoma using a novel peptide specific to a deglycosylated isoform of brevican. Adv. Ther. 2021, 4, 2000244. [Google Scholar] [CrossRef] [PubMed]
- Nischalke, H.D.; Lutz, P.; Krämer, B.; Söhne, J.; Müller, T.; Rosendahl, J.; Fischer, J.; Berg, T.; Hittatiya, K.; Fischer, H.P.; et al. A common polymorphism in the NCAN gene is associated with hepatocellular carcinoma in alcoholic liver disease. J. Hepatol. 2014, 61, 1073–1079. [Google Scholar] [CrossRef]
- Fontanil, T.; Mohamedi, Y.; Moncada-Pazos, A.; Cobo, T.; Vega, J.A.; Cobo, J.L.; García-Suárez, O.; Cobo, J.; Obaya, Á.J.; Cal, S. Neurocan is a New Substrate for the ADAMTS12 Metalloprotease: Potential Implications in Neuropathies. Cell. Physiol. Biochem. 2019, 52, 1003–1016. [Google Scholar] [CrossRef]
- Strand, S.H.; Orntoft, T.F.; Sorensen, K.D. Prognostic DNA methylation markers for prostate cancer. Int. J. Mol. Sci. 2014, 15, 16544–16576. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.L.; Yang, B.; Yang, X.; Zhang, S.; Turley, M.; Samuel, S.; Lange, L.A.; Wang, C.; Curpen, G.D.; Savani, R.C.; et al. Overexpression of the hyaluronan receptor RHAMM is transforming and is also required for H-ras transformation. Cell 1995, 82, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, G.; Shalaby, M.; Elangovan, S.; Petti, L.; Roda, G.; Restelli, S.; Arena, V.; Ungaro, F.; Fiorino, G.; Day, A.J.; et al. Hyaluronan Accelerates Intestinal Mucosal Healing through Interaction with TSG-6. Cells 2019, 8, 1074. [Google Scholar] [CrossRef]
- Coles, C.H.; Shen, Y.; Tenney, A.P.; Siebold, C.; Sutton, G.C.; Lu, W.; Gallagher, J.T.; Jones, E.Y.; Flanagan, J.G.; Aricescu, A.R. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science 2011, 332, 484–488. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef]
- Kirkham, D.L.; Pacey, L.K.; Axford, M.M.; Siu, R.; Rotin, D.; Doering, L.C. Neural stem cells from protein tyrosine phosphatase sigma knockout mice generate an altered neuronal phenotype in culture. BMC Neurosci. 2006, 7, 50. [Google Scholar] [CrossRef]
- Gaviglio, A.L.; Knelson, E.H.; Blobe, G.C. Heparin-binding epidermal growth factor-like growth factor promotes neuroblastoma differentiation. FASEB J. 2017, 31, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, D.; Ru, Y.B.; Weinhaus, B.; Cash, S.; Theodorescu, D.; Guin, S. CD44 and RHAMM are essential for rapid growth of bladder cancer driven by loss of Glycogen Debranching Enzyme (AGL). BMC Cancer 2016, 16, 713. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.H.; An, J.H.; Ko, B.G.; Kim, K.B.; Youn, H.Y. Hypoxia Increases the Proliferative and Metastatic Ability of Canine Mammary Tumor Cells via Up-regulation of TSG-6. Anticancer Res. 2022, 42, 5803–5812. [Google Scholar] [CrossRef]
- Romano, B.; Elangovan, S.; Erreni, M.; Sala, E.; Petti, L.; Kunderfranco, P.; Massimino, L.; Restelli, S.; Sinha, S.; Lucchetti, D.; et al. TNF-Stimulated Gene-6 Is a Key Regulator in Switching Stemness and Biological Properties of Mesenchymal Stem Cells. Stem Cells 2019, 37, 973–987. [Google Scholar] [CrossRef]
- Lee, J.H.; An, J.H.; Youn, H.Y. Tumour necrosis factor stimulated gene 6 intrinsically regulates PD-L1 expressions in breast cancer cells, leading to modulation of tumour microenvironment. Vet. Comp. Oncol. 2023, 21, 255–269. [Google Scholar] [CrossRef]
- Wu, M.; Du, Y.; Liu, Y.; He, Y.; Yang, C.; Wang, W.; Gao, F. Low Molecular Weight Hyaluronan Induces Lymphangiogenesis through LYVE-1-Mediated Signaling Pathways. PLoS ONE 2014, 9, e92857. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Lee, D.Y.; Yee, A.; Cao, L.; Zhang, Y.; Kiani, C.; Yang, B.B. Versican protects cells from oxidative stress-induced apoptosis. Matrix Biol. 2005, 24, 3–13. [Google Scholar] [CrossRef]
- LaPierre, D.P.; Lee, D.Y.; Li, S.Z.; Xie, Y.Z.; Zhong, L.; Sheng, W.; Deng, Z.; Yang, B.B. The ability of versican to simultaneously cause apoptotic resistance and sensitivity. Cancer Res. 2007, 67, 4742–4750. [Google Scholar] [CrossRef]
- Assmann, V.; Jenkinson, D.; Marshall, J.F.; Hart, I.R. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. J. Cell Sci. 1999, 112 Pt 22, 3943–3954. [Google Scholar] [CrossRef]
- Yang, B.; Yang, B.L.; Savani, R.C.; Turley, E.A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, L.; Yang, B.L.; Yang, B.B. The G3 domain of versican enhances cell proliferation via epidermal growth factor-like motifs. J. Biol. Chem. 1998, 273, 21342–21351. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Angello, J.C.; Wight, T.N. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 1999, 19, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Etscheid, M.; Beer, N.; Dodt, J. The hyaluronan-binding protease upregulates ERK1/2 and PI3K/Akt signalling pathways in fibroblasts and stimulates cell proliferation and migration. Cell. Signal. 2005, 17, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, S.; Coulter, J.A.; Tibbits, P.; O’Grady, A.; McFarlane, C.; Montgomery, N.; Hill, A.; McCarthy, H.O.; Young, L.S.; Kay, E.W.; et al. CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget 2015, 6, 11465–11476. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Hwang, D.; Lee, D.; Kim, J.H.; Kim, S.Y.; Lim, D.S. MRTF potentiates TEAD-YAP transcriptional activity causing metastasis. EMBO J. 2017, 36, 520–535. [Google Scholar] [CrossRef]
- Tsubaki, M.; Genno, S.; Takeda, T.; Matsuda, T.; Kimura, N.; Yamashita, Y.; Morii, Y.; Shimomura, K.; Nishida, S. Rhosin Suppressed Tumor Cell Metastasis through Inhibition of Rho/YAP Pathway and Expression of RHAMM and CXCR4 in Melanoma and Breast Cancer Cells. Biomedicines 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Gares, S.L.; Pilarski, L.M. Balancing thymocyte adhesion and motility: A functional linkage between beta1 integrins and the motility receptor RHAMM. Dev. Immunol. 2000, 7, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Kouvidi, K.; Berdiaki, A.; Nikitovic, D.; Katonis, P.; Afratis, N.; Hascall, V.C.; Karamanos, N.K.; Tzanakakis, G.N. Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J. Biol. Chem. 2011, 286, 38509–38520. [Google Scholar] [CrossRef]
- Hu, B.; Kong, L.L.; Matthews, R.T.; Viapiano, M.S. The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. J. Biol. Chem. 2008, 283, 24848–24859. [Google Scholar] [CrossRef]
- Yang, B.L.; Zhang, Y.; Cao, L.; Yang, B.B. Cell adhesion and proliferation mediated through the G1 domain of versican. J. Cell. Biochem. 1999, 72, 210–220. [Google Scholar] [CrossRef]
- Touab, M.; Villena, J.; Barranco, C.; Arumi-Uria, M.; Bassols, A. Versican is differentially expressed in human melanoma and may play a role in tumor development. Am. J. Pathol. 2002, 160, 549–557. [Google Scholar] [CrossRef]
- Sakko, A.J.; Ricciardelli, C.; Mayne, K.; Suwiwat, S.; LeBaron, R.G.; Marshall, V.R.; Tilley, W.D.; Horsfall, D.J. Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res. 2003, 63, 4786–4791. [Google Scholar]
- Zhao, S.; Chen, C.; Chang, K.; Karnad, A.; Jagirdar, J.; Kumar, A.P.; Freeman, J.W. CD44 Expression Level and Isoform Contributes to Pancreatic Cancer Cell Plasticity, Invasiveness, and Response to Therapy. Clin. Cancer Res. 2016, 22, 5592–5604. [Google Scholar] [CrossRef]
- Hanagiri, T.; Shinohara, S.; Takenaka, M.; Shigematsu, Y.; Yasuda, M.; Shimokawa, H.; Nagata, Y.; Nakagawa, M.; Uramoto, H.; So, T.; et al. Effects of hyaluronic acid and CD44 interaction on the proliferation and invasiveness of malignant pleural mesothelioma. Tumour Biol. 2012, 33, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.; Rogers, M.J.; Chellaiah, M.A. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol. Cancer 2007, 6, 18. [Google Scholar] [CrossRef]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Cao, W.; Sadashivaiah, K.; Chen, W.; Schneider, A.; Chellaiah, M.A. Promising noninvasive cellular phenotype in prostate cancer cells knockdown of matrix metalloproteinase 9. Sci. World J. 2013, 2013, 493689. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014, 14, 342–356. [Google Scholar] [CrossRef]
- Bellerby, R.; Smith, C.; Kyme, S.; Gee, J.; Gunthert, U.; Green, A.; Rakha, E.; Barrett-Lee, P.; Hiscox, S. Overexpression of Specific CD44 Isoforms Is Associated with Aggressive Cell Features in Acquired Endocrine Resistance. Front. Oncol. 2016, 6, 145. [Google Scholar] [CrossRef]
- Akiyama, Y.; Jung, S.; Salhia, B.; Lee, S.P.; Hubbard, S.; Taylor, M.; Mainprize, T.; Akaishi, K.; van Furth, W.; Rutka, J.T. Hyaluronate receptors mediating glioma cell migration and proliferation. J. Neuro-Oncol. 2001, 53, 115–127. [Google Scholar] [CrossRef]
- Twarock, S.; Tammi, M.I.; Savani, R.C.; Fischer, J.W. Hyaluronan Stabilizes Focal Adhesions, Filopodia, and the Proliferative Phenotype in Esophageal Squamous Carcinoma Cells. J. Biol. Chem. 2010, 285, 23274–23282. [Google Scholar] [CrossRef]
- Zhang, H.; Kelly, G.; Zerillo, C.; Jaworski, D.M.; Hockfield, S. Expression of a cleaved brain-specific extracellular matrix protein mediates glioma cell invasion. J. Neurosci. 1998, 18, 2370–2376. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Russell, D.L.; Ween, M.P.; Mayne, K.; Suwiwat, S.; Byers, S.; Marshall, V.R.; Tilley, W.D.; Horsfall, D.J. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J. Biol. Chem. 2007, 282, 10814–10825. [Google Scholar] [CrossRef]
- Cattaruzza, S.; Schiappacassi, M.; Kimata, K.; Colombatti, A.; Perris, R. The globular domains of PG-M/versican modulate the proliferation-apoptosis equilibrium and invasive capabilities of tumor cells. FASEB J. 2004, 18, 779–781. [Google Scholar] [CrossRef]
- Yee, A.J.; Akens, M.; Yang, B.L.; Finkelstein, J.; Zheng, P.S.; Deng, Z.; Yang, B. The effect of versican G3 domain on local breast cancer invasiveness and bony metastasis. Breast Cancer Res. 2007, 9, R47. [Google Scholar] [CrossRef]
- Wu, R.L.; Huang, L.; Zhao, H.C.; Geng, X.P. Hyaluronic acid in digestive cancers. J. Cancer Res. Clin. Oncol. 2017, 143, 1–16. [Google Scholar] [CrossRef]
- Choi-Miura, N.-H.; Tobe, T.; Sumiya, J.-i.; Nakano, Y.; Sano, Y.; Mazda, T.; Tomita, M. Purification and Characterization of a Novel Hyaluronan-Binding Protein (PHBP) from Human Plasma: It Has Three EGF, a Kringle and a Serine Protease Domain, Similar to Hepatocyte Growth Factor Activator. J. Biochem. 1996, 119, 1157–1165. [Google Scholar] [CrossRef]
- Siebert, J.R.; Conta Steencken, A.; Osterhout, D.J. Chondroitin sulfate proteoglycans in the nervous system: Inhibitors to repair. Biomed. Res. Int. 2014, 2014, 845323. [Google Scholar] [CrossRef]
- Oohashi, T.; Edamatsu, M.; Bekku, Y.; Carulli, D. The hyaluronan and proteoglycan link proteins: Organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp. Neurol. 2015, 274, 134–144. [Google Scholar] [CrossRef]
- Eriksen, G.V.; Carlstedt, I.; Morgelin, M.; Uldbjerg, N.; Malmstrom, A. Isolation and characterization of proteoglycans from human follicular fluid. Biochem. J. 1999, 340 Pt 3, 613–620. [Google Scholar] [CrossRef]
- Zhuo, L.; Hascall, V.C.; Kimata, K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J. Biol. Chem. 2004, 279, 38079–38082. [Google Scholar] [CrossRef] [PubMed]
- Selbi, W.; de la Motte, C.A.; Hascall, V.C.; Day, A.J.; Bowen, T.; Phillips, A.O. Characterization of hyaluronan cable structure and function in renal proximal tubular epithelial cells. Kidney Int. 2006, 70, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Perides, G.; Asher, R.A.; Lark, M.W.; Lane, W.S.; Robinson, R.A.; Bignami, A. Glial hyaluronate-binding protein: A product of metalloproteinase digestion of versican? Biochem. J. 1995, 312 Pt 2, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Dehghan, A. The Expression and Functional Significance of Vascular Endothelial-Cadherin, CD44, and Vimentin in Oral Squamous Cell Carcinoma. J. Int. Soc. Prev. Community Dent. 2018, 8, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Z.; Zhang, D.F.; Yao, L.L.; Zhao, X.L.; Zhao, X.M.; Gu, Q.; Dong, X.Y.; Liu, F.; Sun, J.Y.; Zheng, X. Vasculogenic mimicry and cancer stem-like cell markers are associated with poor prognosis of non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2016, 9, 11523–11535. [Google Scholar]
- Jelicic, J.; Balint, M.T.; Jovanovic, M.P.; Boricic, N.; Micev, M.; Stojsic, J.; Antic, D.; Andjelic, B.; Bila, J.; Balint, B.; et al. The Role of Lymphocyte to Monocyte Ratio, Microvessel Density and HiGH CD44 Tumor Cell Expression in Non Hodgkin Lymphomas. Pathol. Oncol. Res. 2016, 22, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.Y.; Savani, R.C.; Fehrenbach, M.; Lyons, C.; Zhang, L.; Coukos, G.; DeLisser, H.M. Involvement of endothelial CD44 during in vivo angiogenesis. Am. J. Pathol. 2006, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Coenen, M.J.H.; Damen, C.A.; Hellwig, S.M.M.; van Weering, D.H.J.; Vooys, W.; Blijham, G.H.; Groenewegen, G. CD44 is involved in tumor angiogenesis; an activation antigen on human endothelial cells. Blood 1997, 90, 1150–1159. [Google Scholar] [CrossRef]
- Savani, R.C.; Cao, G.Y.; Pooler, P.M.; Zaman, A.; Zhou, Z.; DeLisser, H.M. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J. Biol. Chem. 2001, 276, 36770–36778. [Google Scholar] [CrossRef]
- Park, D.; Kim, Y.; Kim, H.; Kim, K.; Lee, Y.S.; Choe, J.; Hahn, J.H.; Lee, H.; Jeon, J.; Choi, C.; et al. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGF beta receptor interaction via CD44-PKC delta. Mol. Cells 2012, 33, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Matou-Nasri, S.; Gaffney, J.; Kumar, S.; Slevin, M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and γ-adducin. Int. J. Oncol. 2009, 35, 761–773. [Google Scholar] [CrossRef][Green Version]
- Song, H.B.; Park, S.Y.; Ko, J.H.; Park, J.W.; Yoon, C.H.; Kim, D.H.; Kim, J.H.; Kim, M.K.; Lee, R.H.; Prockop, D.J.; et al. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol. Ther. 2018, 26, 162–172. [Google Scholar] [CrossRef]
- Baranova, N.S.; Inforzato, A.; Briggs, D.C.; Tilakaratna, V.; Enghild, J.J.; Thakar, D.; Milner, C.M.; Day, A.J.; Richter, R.P. Incorporation of pentraxin 3 into hyaluronan matrices is tightly regulated and promotes matrix cross-linking. J. Biol. Chem. 2014, 289, 30481–30498. [Google Scholar] [CrossRef]
- Spinelli, F.M.; Vitale, D.L.; Icardi, A.; Caon, I.; Brandone, A.; Giannoni, P.; Saturno, V.; Passi, A.; García, M.; Sevic, I. Hyaluronan preconditioning of monocytes/macrophages affects their angiogenic behavior and regulation of TSG-6 expression in a tumor type-specific manner. FEBS J. 2019, 286, 3433–3449. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yee, A.J. Versican V2 isoform enhances angiogenesis by regulating endothelial cell activities and fibronectin expression. FEBS Lett. 2013, 587, 185–192. [Google Scholar] [CrossRef]
- Koyama, H.; Hibi, T.; Isogai, Z.; Yoneda, M.; Fujimori, M.; Amano, J.; Kawakubo, M.; Kannagi, R.; Kimata, K.; Taniguchi, S.; et al. Hyperproduction of hyaluronan in Neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment—Possible involvement of versican/PG-M. Am. J. Pathol. 2007, 170, 1086–1099. [Google Scholar] [CrossRef]
- Nandi, A.; Estess, P.; Siegelman, M.H. Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J. Biol. Chem. 2000, 275, 14939–14948. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Simopoulos, C.; Gatter, K.C.; Harris, A.L.; Jackson, D.G. LYVE-1 immunohistochemical assessment of lymphangiogenesis in endometrial and lung cancer. J. Clin. Pathol. 2005, 58, 202–206. [Google Scholar] [CrossRef]
- Nishida-Fukuda, H.; Araki, R.; Shudou, M.; Okazaki, H.; Tomono, Y.; Nakayama, H.; Fukuda, S.; Sakaue, T.; Shirakata, Y.; Sayama, K.; et al. Ectodomain Shedding of Lymphatic Vessel Endothelial Hyaluronan Receptor 1 (LYVE-1) Is Induced by Vascular Endothelial Growth Factor A (VEGF-A). J. Biol. Chem. 2016, 291, 10490–10500. [Google Scholar] [CrossRef]
- Wong, H.L.; Jin, G.; Cao, R.; Zhang, S.; Cao, Y.; Zhou, Z. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun. 2016, 7, 10824. [Google Scholar] [CrossRef] [PubMed]
- Sethy, C.; Goutam, K.; Das, B.; Dash, S.R.; Kundu, C.N. Nectin-4 promotes lymphangiogenesis and lymphatic metastasis in breast cancer by regulating CXCR4-LYVE-1 axis. Vasc. Pharmacol. 2021, 140, 106865. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Li, Y.W.; Wu, J.L.; Johnson, F.E.; Huang, J.S. Development of the LYVE-1 gene with an acidic-amino-acid-rich (AAAR) domain in evolution is associated with acquisition of lymph nodes and efficient adaptive immunity. J. Cell. Physiol. 2018, 233, 2681–2692. [Google Scholar] [CrossRef]
- Jackson, D.G. Hyaluronan in the lymphatics: The key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol. 2019, 78–79, 219–235. [Google Scholar] [CrossRef]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef]
- Cao, T.V.; La, M.; Getting, S.J.; Day, A.J.; Perretti, M. Inhibitory effects of TSG-6 Link module on leukocyte-endothelial cell interactions in vitro and in vivo. Microcirculation 2004, 11, 615–624. [Google Scholar] [CrossRef]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.; Day, A.J.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Guo, X.; Yan, X.; Tian, Z.; Jiang, W.; He, X. TSG-6 inhibits IL-1beta-induced inflammatory responses and extracellular matrix degradation in nucleus pulposus cells by activating the PI3K/Akt signaling pathway. J. Orthop. Surg. Res. 2022, 17, 572. [Google Scholar] [CrossRef]
- Zhao, M.; Yoneda, M.; Ohashi, Y.; Kurono, S.; Iwata, H.; Ohnuki, Y.; Kimata, K. Evidence for the covalent binding of SHAP, heavy chains of inter-alpha-trypsin inhibitor, to hyaluronan. J. Biol. Chem. 1995, 270, 26657–26663. [Google Scholar] [CrossRef]
- de la Motte, C.A.; Hascall, V.C.; Drazba, J.; Bandyopadhyay, S.K.; Strong, S.A. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: Inter-alpha-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 2003, 163, 121–133. [Google Scholar] [CrossRef]
- Eba, H.; Murasawa, Y.; Iohara, K.; Isogai, Z.; Nakamura, H.; Nakamura, H.; Nakashima, M. The anti-inflammatory effects of matrix metalloproteinase-3 on irreversible pulpitis of mature erupted teeth. PLoS ONE 2012, 7, e52523. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Ringhoffer, M.; Taniguchi, M.; Schmitt, A.; Kirchner, D.; Krahn, G.; Heilmann, V.; Gschwend, J.; Bergmann, L.; Dohner, H.; et al. Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp. Hematol. 2002, 30, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Willemen, Y.; Van den Bergh, J.M.J.; Bonte, S.M.; Anguille, S.; Heirman, C.; Stein, B.M.H.; Goossens, H.; Kerre, T.; Thielemans, K.; Peeters, M.; et al. The tumor-associated antigen RHAMM (HMMR/CD168) is expressed by monocyte-derived dendritic cells and presented to T cells. Oncotarget 2016, 7, 73960–73970. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Zhang, D.M.; Deng, H.X.; Peng, F.; Wei, Y.Q. Antitumor and anti-angiogenesis immunity induced by CR-SEREX-identified Xenopus RHAMM. Cancer Sci. 2010, 101, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, L.M.; Masellissmith, A.; Belch, A.R.; Yang, B.; Savani, R.C.; Turley, E.A. Rhamm, a Receptor for Hyaluronan-Mediated Motility, on Normal Human-Lymphocytes, Thymocytes and Malignant B-Cells—A Mediator in B-Cell Malignancy. Leuk. Lymphoma 1994, 14, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Messam, B.J.A.; McCarthy, J.B.; Nelson, A.C.; Turley, E.A. Hyaluronan Functions in Wound Repair That Are Captured to Fuel Breast Cancer Progression. Biomolecules 2021, 11, 1551. [Google Scholar] [CrossRef]
- Gaudreau, P.O.; Negrao, M.V.; Mitchell, K.G.; Reuben, A.; Corsini, E.M.; Li, J.; Karpinets, T.V.; Wang, Q.; Diao, L.; Wang, J.; et al. Neoadjuvant Chemotherapy Increases Cytotoxic T Cell, Tissue Resident Memory T Cell, and B Cell Infiltration in Resectable NSCLC. J. Thorac. Oncol. 2021, 16, 127–139. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Kaur, A.; Ecker, B.L.; Douglass, S.M.; Kugel, C.H.; Webster, M.R.; Almeida, F.V.; Somasundaram, R.; Hayden, J.; Ban, E.; Ahmadzadeh, H.; et al. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. 2019, 9, 64–81. [Google Scholar] [CrossRef]
- Lin, X.; Hessenow, R.; Yang, S.; Ma, D.; Yang, S. A seven-immune-genes risk model predicts the survival and suitable treatments for patients with skin cutaneous melanoma. Heliyon 2023, 9, e20234. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, D.; Duan, H.; Rao, Q.; Qian, Y.; Chen, Q.; Du, X.; Ni, H.; Wang, S. Identification of Pyroptosis-Relevant Signature in Tumor Immune Microenvironment and Prognosis in Skin Cutaneous Melanoma Using Network Analysis. Stem Cells Int. 2023, 2023, 3827999. [Google Scholar] [CrossRef]
- Wight, T.N.; Kang, I.; Evanko, S.P.; Harten, I.A.; Chang, M.Y.; Pearce, O.M.T.; Allen, C.E.; Frevert, C.W. Versican-A Critical Extracellular Matrix Regulator of Immunity and Inflammation. Front. Immunol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Wang, W.; Xu, G.L.; Jia, W.D.; Ma, J.L.; Li, J.S.; Ge, Y.S.; Ren, W.H.; Yu, J.H.; Liu, W.B. Ligation of TLR2 by Versican: A Link Between Inflammation and Metastasis. Arch. Med. Res. 2009, 40, 321–323. [Google Scholar] [CrossRef]
- Kim, S.; Takahashi, H.; Lin, W.W.; Descargues, P.; Grivennikov, S.; Kim, Y.; Luo, J.L.; Karin, M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009, 457, 102–106. [Google Scholar] [CrossRef]
- Gill, S.; Wight, T.N.; Frevert, C.W. Proteoglycans: Key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2010, 293, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N.; Kinsella, M.G.; Evanko, S.P.; Potter-Perigo, S.; Merrilees, M.J. Versican and the regulation of cell phenotype in disease. Biochim. Biophys. Acta 2014, 1840, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Potter-Perigo, S.; Bollyky, P.L.; Nepom, G.T.; Wight, T.N. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012, 31, 90–100. [Google Scholar] [CrossRef]
- Wight, T.N.; Kang, I.; Merrilees, M.J. Versican and the control of inflammation. Matrix Biol. 2014, 35, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.X.; Wang, Y.Y.; Cao, D.; Wang, X.; Jiang, M.; Li, M.; Yan, X.; Li, Y.; Liu, Y.Y.; et al. Versican silencing improves the antitumor efficacy of endostatin by alleviating. its induced inflammatory and immunosuppressive changes in the tumor microenvironment. Oncol. Rep. 2015, 33, 2981–2991. [Google Scholar] [CrossRef]
- Jaworski, D.M.; Kelly, G.M.; Hockfield, S. Intracranial injury acutely induces the expression of the secreted isoform of the CNS-specific hyaluronan-binding protein BEHAB brevican. Exp. Neurol. 1999, 157, 327–337. [Google Scholar] [CrossRef]
- Silva, R.V.; Biskup, K.; Zabala-Jouvin, J.K.; Batzdorf, C.S.; Stellmach, C.; Morr, A.S.; Sack, I.; Ludwig, A.; Blanchard, V.; Infante-Duarte, C. Brain inflammation induces alterations in glycosaminoglycan metabolism and subsequent changes in CS-4S and hyaluronic acid. Int. J. Biol. Macromol. 2023, 230, 123214. [Google Scholar] [CrossRef]
- Zhuo, L.; Kanamori, A.; Kannagi, R.; Itano, N.; Wu, J.; Hamaguchi, M.; Ishiguro, N.; Kimata, K. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 2006, 281, 20303–20314. [Google Scholar] [CrossRef]
- Tighe, R.M.; Garantziotis, S. Hyaluronan interactions with innate immunity in lung biology. Matrix Biol. 2019, 78–79, 84–99. [Google Scholar] [CrossRef]
- Dunsch, A.K.; Hammond, D.; Lloyd, J.; Schermelleh, L.; Gruneberg, U.; Barr, F.A. Dynein light chain 1 and a spindle-associated adaptor promote dynein asymmetry and spindle orientation. J. Cell Biol. 2012, 198, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Liu, M.; Cousteils, K.; Telmer, P.; Alam, K.; Ma, J.; Mendina, L.; McCarthy, J.B.; Morris, V.L.; Turley, E.A. Cell-specific expression of the transcriptional regulator RHAMM provides a timing mechanism that controls appropriate wound re-epithelialization. J. Biol. Chem. 2020, 295, 5427–5448. [Google Scholar] [CrossRef]
- Kouvidi, K.; Nikitovic, D.; Berdiaki, A.; Tzanakakis, G.N. Hyaluronan/RHAMM interactions in mesenchymal tumor pathogenesis: Role of growth factors. Adv. Cancer Res. 2014, 123, 319–349. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Selzer, M.G. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. J. Biol. Chem. 2000, 275, 27641–27649. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, C.; Hoare, K.; Owens, R.; Hohn, H.P.; Hook, M.; Moore, D.; Cripps, V.; Austen, L.; Nance, D.M.; Turley, E.A. Molecular Cloning of a Novel Hyaluronan Receptor That Mediates Tumor Cell Motility. J. Cell Biol. 1992, 117, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.B.; Tolg, C.; Peart, T.; Symonette, C.; Veiseh, M.; Umoh, J.U.; Holdsworth, D.W.; McCarthy, J.B.; Luyt, L.G.; Bissell, M.J.; et al. Receptor for hyaluronan mediated motility (RHAMM/HMMR) is a novel target for promoting subcutaneous adipogenesis. Integr. Biol. 2017, 9, 223–237. [Google Scholar] [CrossRef]
- Nakano, M.; Taguchi, R.; Kikushige, Y.; Isobe, T.; Miyawaki, K.; Mizuno, S.; Tsuruta, N.; Hanamura, F.; Yamaguchi, K.; Yamauchi, T.; et al. RHAMM marks proliferative subpopulation of human colorectal cancer stem cells. Cancer Sci. 2023, 114, 2895–2906. [Google Scholar] [CrossRef]
- Ecker, B.L.; Kaur, A.; Douglass, S.M.; Webster, M.R.; Almeida, F.V.; Marino, G.E.; Sinnamon, A.J.; Neuwirth, M.G.; Alicea, G.M.; Ndoye, A.; et al. Age-Related Changes in HAPLN1 Increase Lymphatic Permeability and Affect Routes of Melanoma Metastasis. Cancer Discov. 2019, 9, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Pukkila, M.J.; Kosunen, A.S.; Virtaniemi, J.A.; Kumpulainen, E.J.; Johansson, R.T.; Kellokoski, J.K.; Nuutinen, J.; Kosma, V.M. Versican expression in pharyngeal squamous cell carcinoma: An immunohistochemical study. J. Clin. Pathol. 2004, 57, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Joshi, N.; Choi, H.; Ryu, S.; Hahn, M.; Catena, R.; Sadik, H.; Argani, P.; Wagner, P.; Vahdat, L.T.; et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012, 72, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Prevo, R.; Steers, G.; Roberts, H.; Leek, R.D.; Kimura, T.; Kameoka, S.; Nishikawa, T.; Kobayashi, M.; Jackson, D.G.; et al. A quantitative analysis of lymphatic vessels in human breast cancer, based on LYVE-1 immunoreactivity. Br. J. Cancer 2005, 93, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lee, S.K.; Song, M.; Zhang, T.; Han, M.S.; Chen, Y.T.; Chen, Z.; Ma, X.; Tung, C.H.; Du, Y.N. RHAMM(B)-mediated bifunctional nanotherapy targeting Bcl-xL and mitochondria for pancreatic neuroendocrine tumor treatment. Mol. Ther. Oncolytics 2021, 23, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Chen, Y.; Chen, J.; Yang, S.; Gao, F.; Underhill, C.B.; Creswell, K.; Zhang, L. A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis. Cancer Res. 2003, 63, 5685–5690. [Google Scholar]
- Akentieva, N.P.; Shushanov, S.S. [Inhibitory effect of RHAMM-target peptides on invasion of breast cancer cells]. Vopr. Onkol. 2016, 62, 831–838. [Google Scholar]
- Akentieva, N.P.; Shushanov, S.S. [RHAMM (receptor hyaluronan-mediated motility)-target peptides induce apoptosis in prostate cancer cells]. Vopr. Onkol. 2016, 62, 514–518. [Google Scholar] [PubMed]
- Amano, T.; Kajiwara, K.; Yoshikawa, K.; Morioka, J.; Nomura, S.; Fujisawa, H.; Kato, S.; Fujii, M.; Fukui, M.; Hinoda, Y.; et al. Antitumor effects of vaccination with dendritic cells transfected with modified receptor for hyaluronan-mediated motility mRNA in a mouse glioma model. J. Neurosurg. 2007, 106, 638–645. [Google Scholar] [CrossRef]
- Giannopoulos, K.; Dmoszynska, A.; Kowal, M.; Rolinski, J.; Gostick, E.; Price, D.A.; Greiner, J.; Rojewski, M.; Stilgenbauer, S.; Dohner, H.; et al. Peptide vaccination elicits leukemia-associated antigen-specific cytotoxic CD8+ T-cell responses in patients with chronic lymphocytic leukemia. Leukemia 2010, 24, 798–805. [Google Scholar] [CrossRef]
- Casalegno-Garduno, R.; Meier, C.; Schmitt, A.; Spitschak, A.; Hilgendorf, I.; Rohde, S.; Hirt, C.; Freund, M.; Putzer, B.M.; Schmitt, M. Immune responses to RHAMM in patients with acute myeloid leukemia after chemotherapy and allogeneic stem cell transplantation. Clin. Dev. Immunol. 2012, 2012, 146463. [Google Scholar] [CrossRef]
- Wang, L.; Feng, L.; Liu, L.; Han, J.; Zhang, X.; Li, D.; Liu, J.; Wang, Y.; Zuo, J.; Fan, Z. Joint effect of THBS2 and VCAN accelerating the poor prognosis of gastric cancer. Aging 2023, 15, 1343–1357. [Google Scholar] [CrossRef]
- Linares, J.; Varese, M.; Sallent-Aragay, A.; Mendez, A.; Palomo-Ponce, S.; Iglesias, M.; Batlle, E.; Pisonero, J.; Montagut, C.; Giralt, E.; et al. Peptide-Platinum(IV) Conjugation Minimizes the Negative Impact of Current Anticancer Chemotherapy on Nonmalignant Cells. J. Med. Chem. 2023, 66, 3348–3355. [Google Scholar] [CrossRef]
- Hu, F.; Dzaye, O.; Hahn, A.; Yu, Y.; Scavetta, R.J.; Dittmar, G.; Kaczmarek, A.K.; Dunning, K.R.; Ricciardelli, C.; Rinnenthal, J.L.; et al. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages Toll-like receptor 2 signaling. Neuro-oncology 2015, 17, 200–210. [Google Scholar] [CrossRef]
- Hirani, P.; Gauthier, V.; Allen, C.E.; Wight, T.N.; Pearce, O.M.T. Targeting Versican as a Potential Immunotherapeutic Strategy in the Treatment of Cancer. Front. Oncol. 2021, 11, 712807. [Google Scholar] [CrossRef]
- Anstee, J.E.; Feehan, K.T.; Opzoomer, J.W.; Dean, I.; Muller, H.P.; Bahri, M.; Cheung, T.S.; Liakath-Ali, K.; Liu, Z.; Choy, D.; et al. LYVE-1(+) macrophages form a collaborative CCR5-dependent perivascular niche that influences chemotherapy responses in murine breast cancer. Dev. Cell 2023, 58, 1548–1561.e1510. [Google Scholar] [CrossRef]
- Ulndreaj, A.; Li, A.; Chen, Y.; Besla, R.; Pacheco, S.; Althagafi, M.G.; Cybulsky, M.I.; Lindsay, T.; Robbins, C.S.; Byrne, J.S. Adventitial recruitment of Lyve-1− macrophages drives aortic aneurysm in an angiotensin-2-based murine model. Clin. Sci. 2021, 135, 1295–1309. [Google Scholar] [CrossRef]
- Elfstrum, A.K.; Rumahorbo, A.H.; Reese, L.E.; Nelson, E.V.; McCluskey, B.M.; Schwertfeger, K.L. LYVE-1-expressing macrophages modulate the hyaluronan-containing extracellular matrix in the mammary stroma and contribute to mammary tumor growth. Cancer Res. Commun. 2024. [Google Scholar] [CrossRef]
- Leung, K. AlexaFluor 488-conjugated antibody against lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1). In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Leung, K. 124I-Labeled antibody against lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1). In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Zhang, G.L.; Qian, C.; Zhang, S.Z.; Tuo, Y.H.; Zeng, B.Y.; Ji, Y.X.; Wang, Y.Z. Effect of conditioned medium from neural stem cells on glioma progression and its protein expression profile analysis. World J. Stem Cells 2020, 12, 1396–1409. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, Y.; Liu, H.; Zhang, Y.; Wang, L.; Zhang, H. Identification of Potential Prognostic Genes for Neuroblastoma. Front. Genet. 2018, 9, 589. [Google Scholar] [CrossRef]
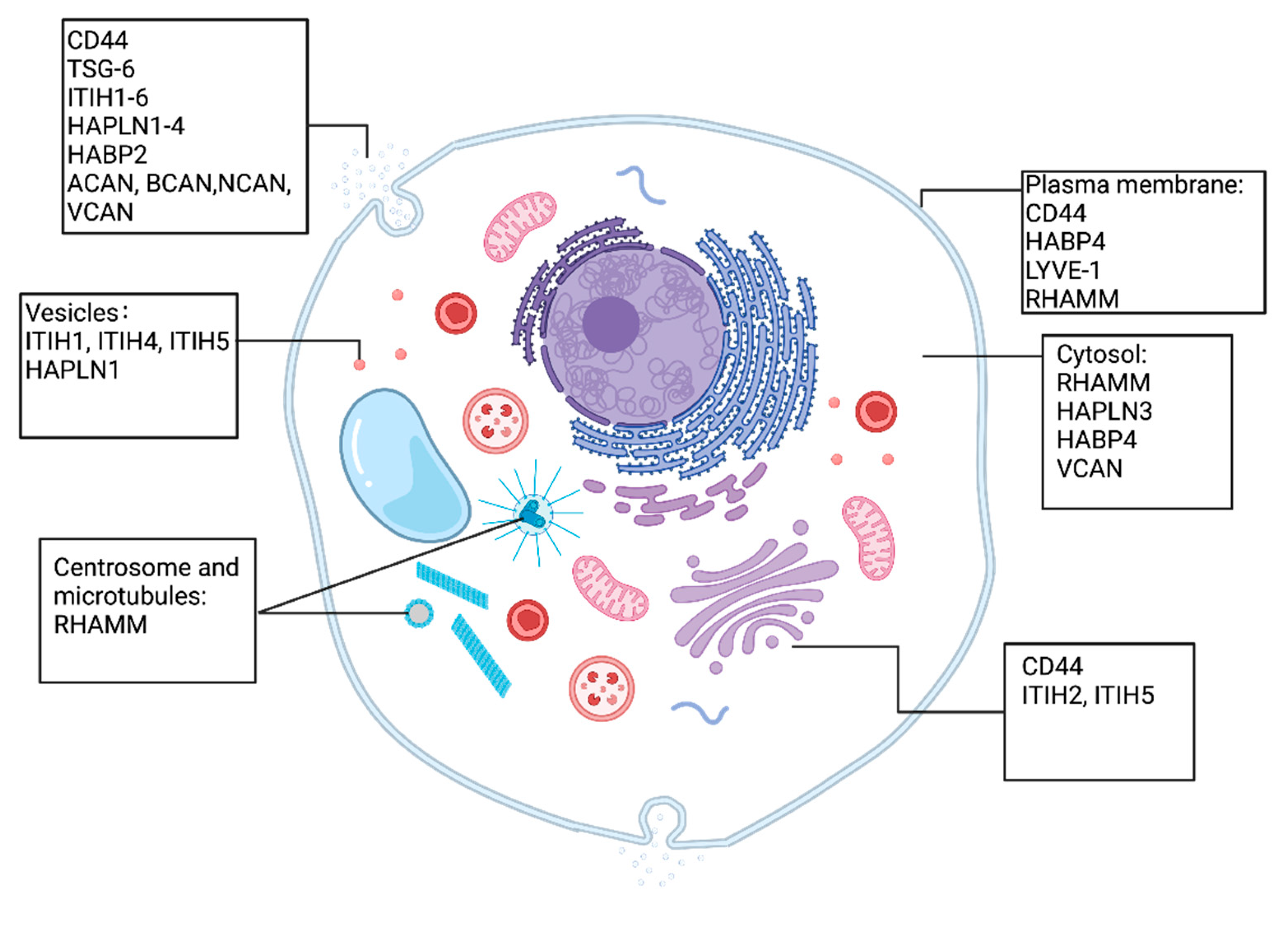
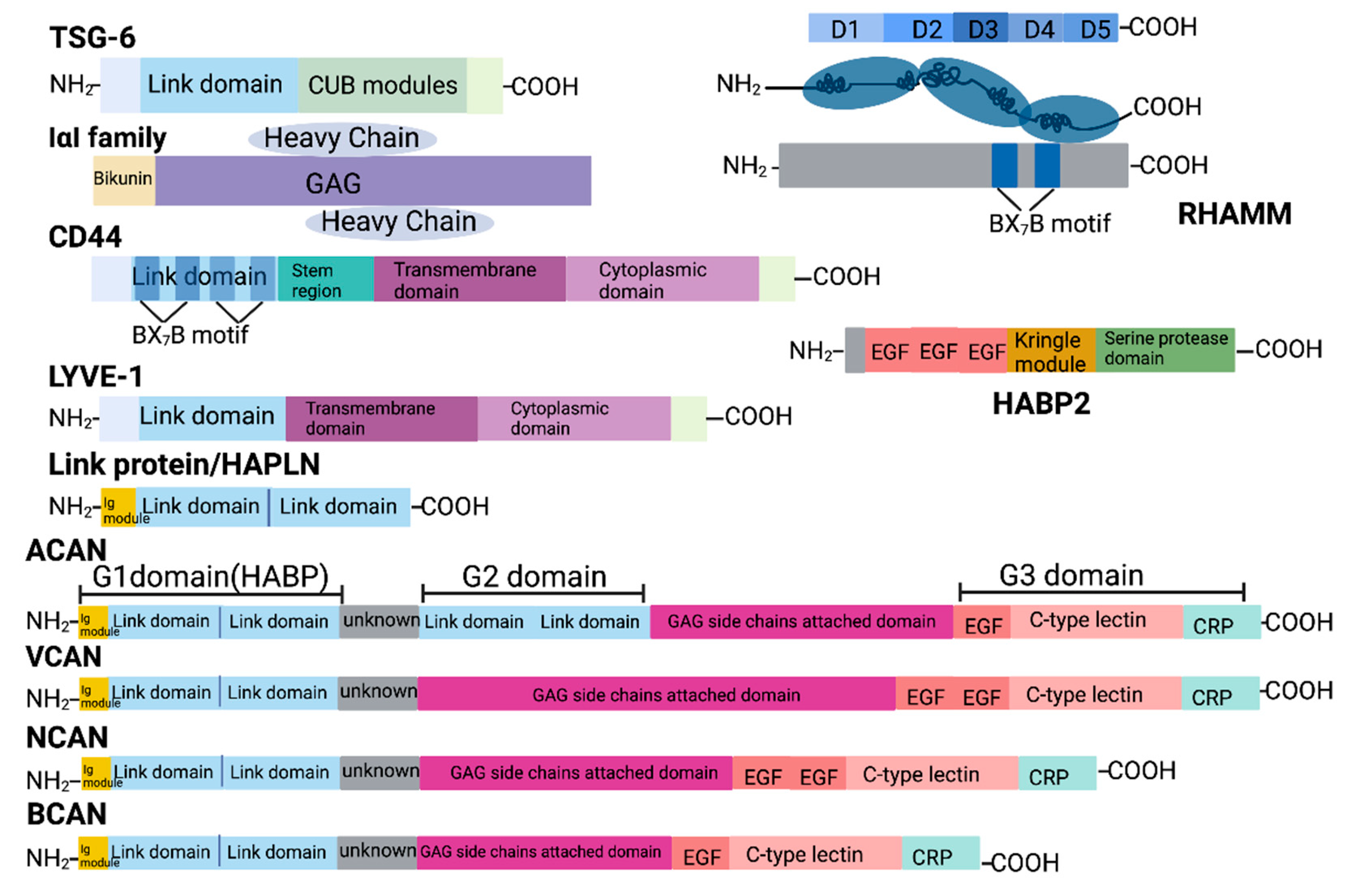
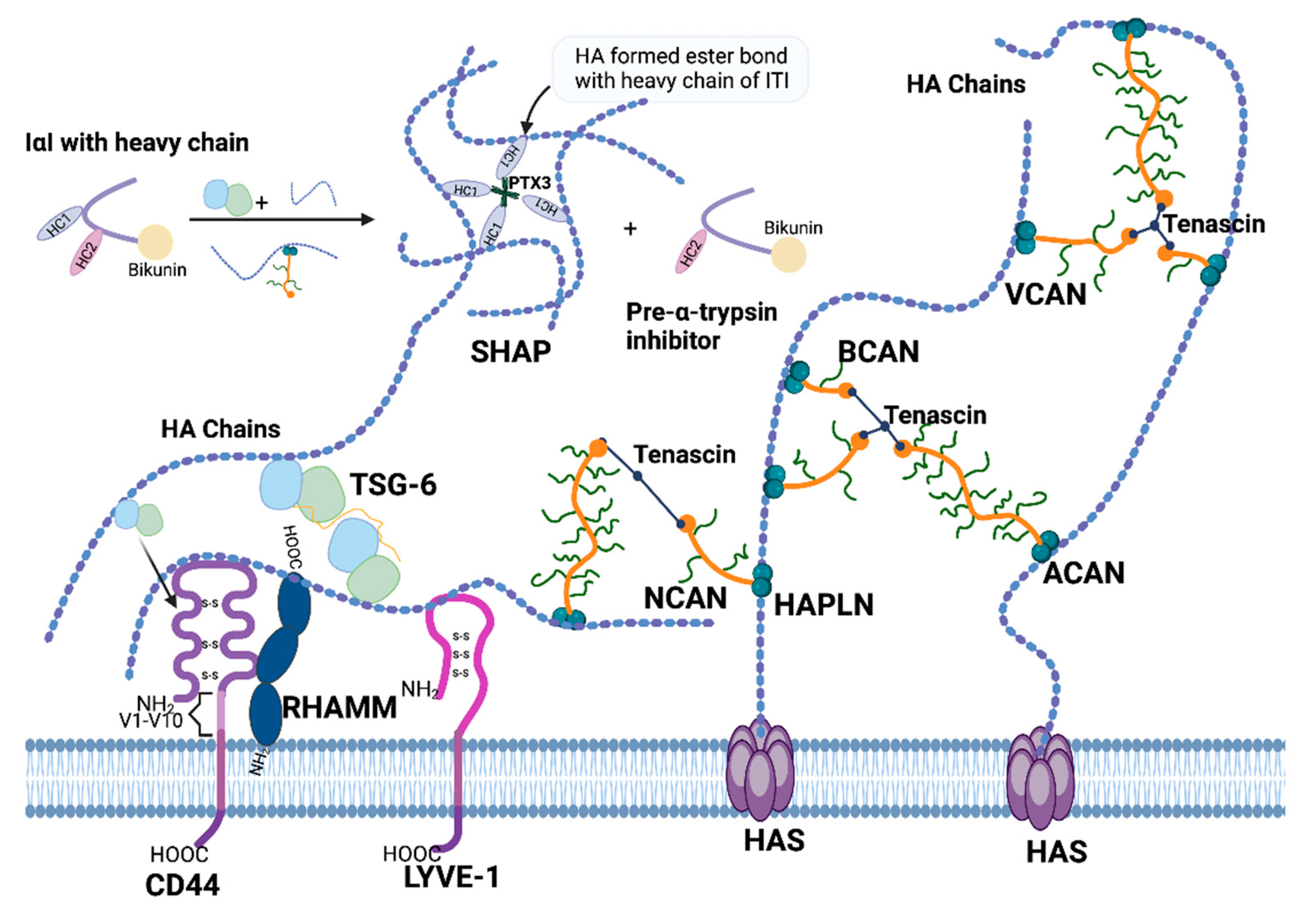
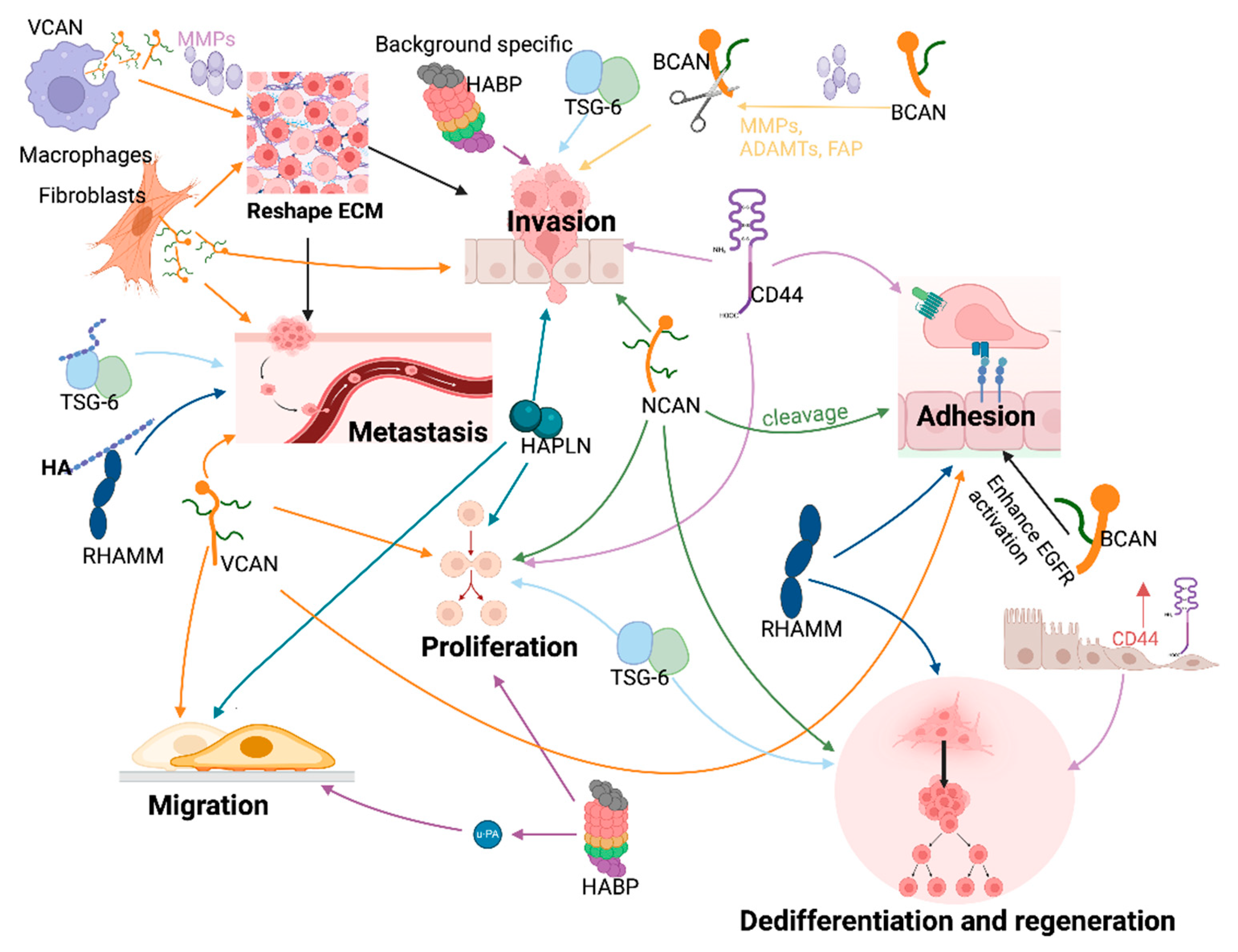
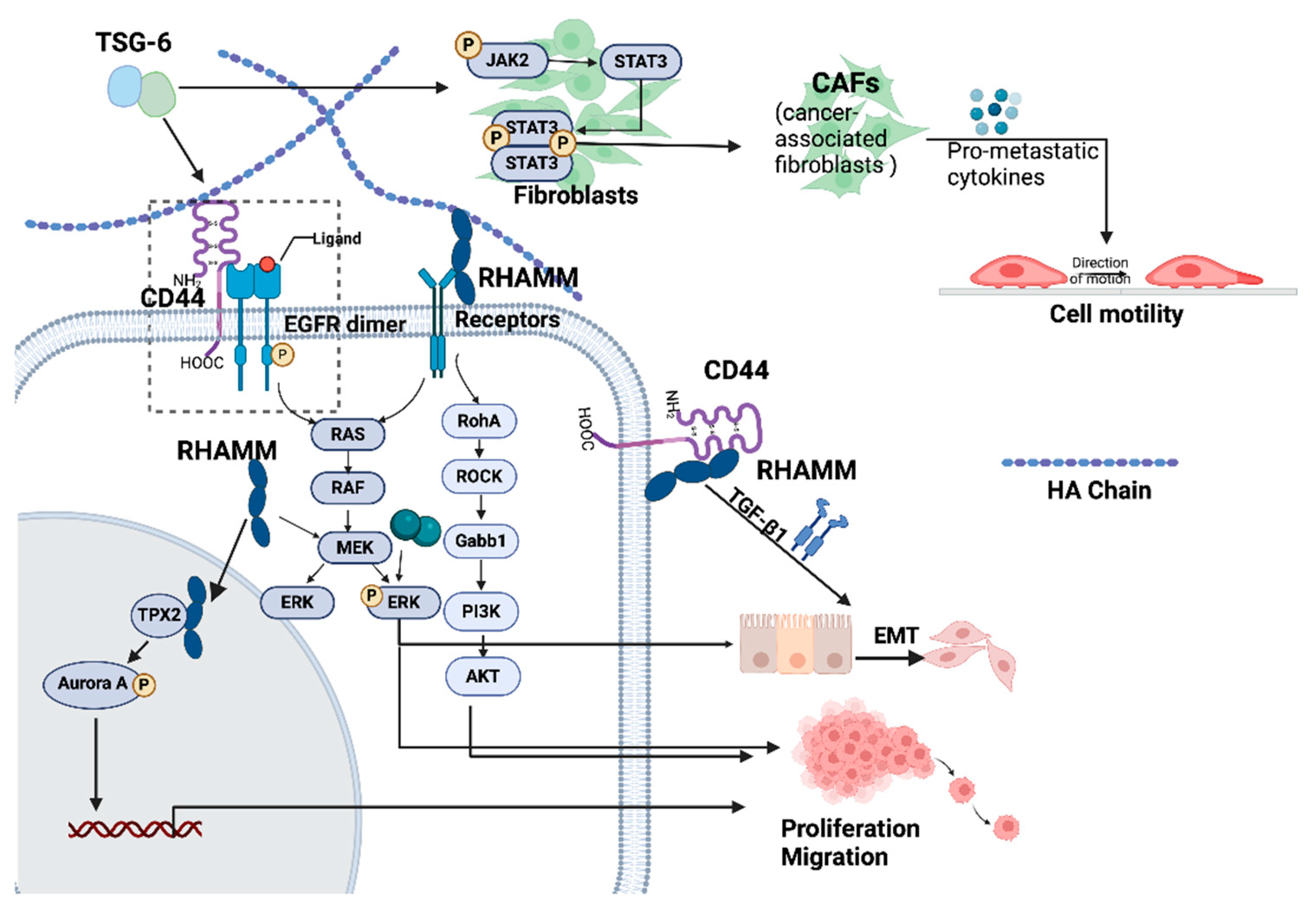
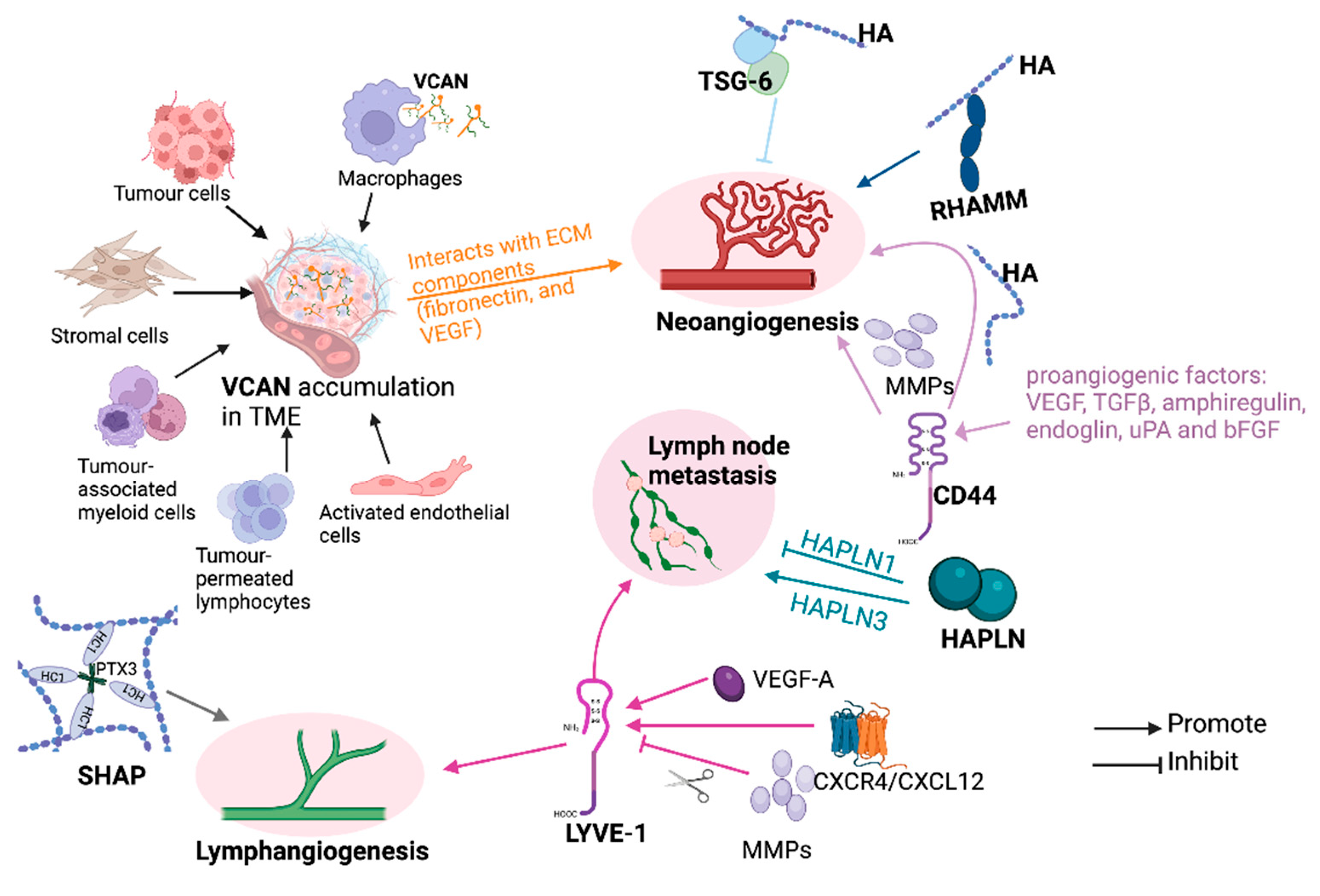
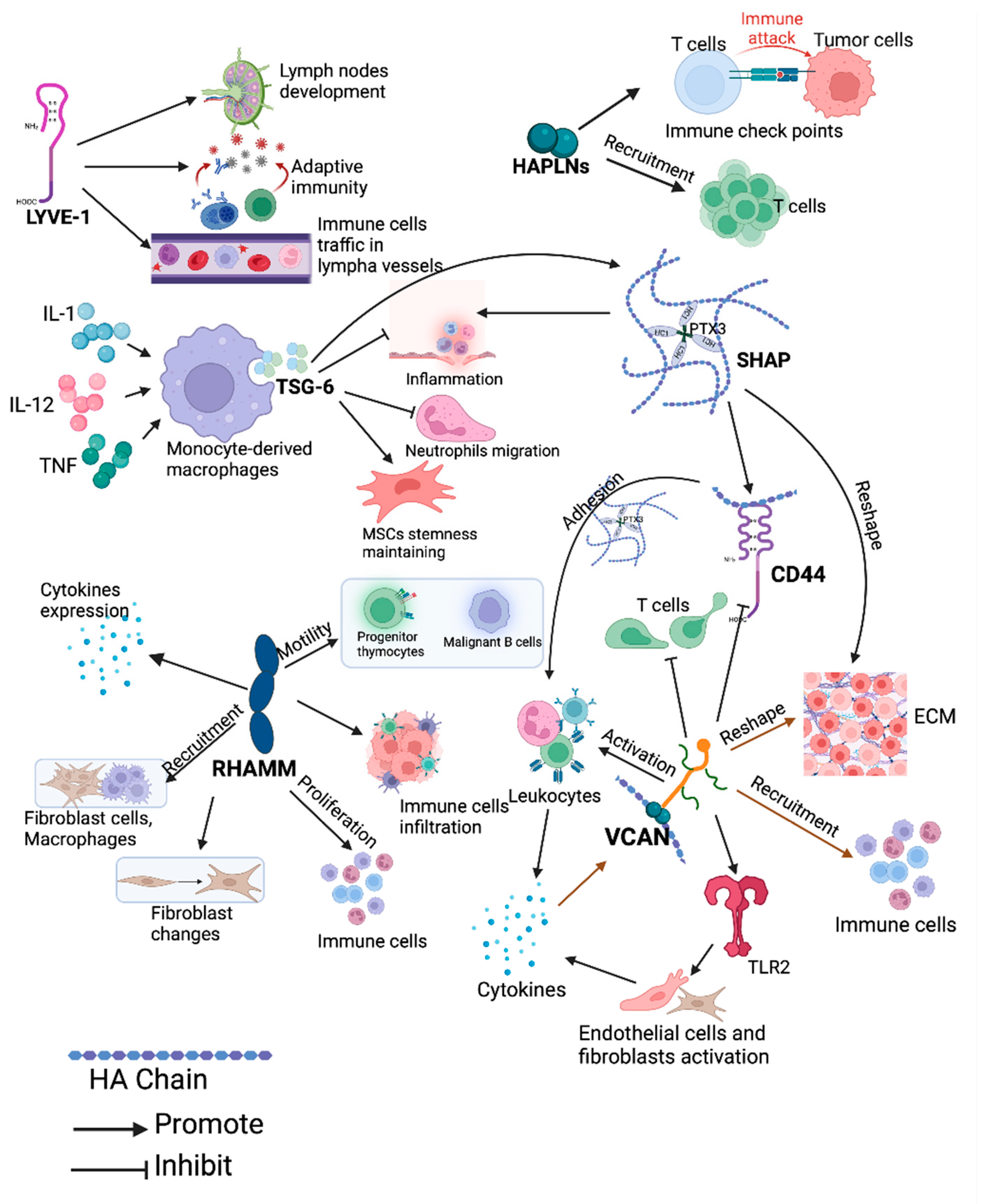
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Benedikt, J.; Ye, L. Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis. Cancers 2024, 16, 1907. https://doi.org/10.3390/cancers16101907
Xu Y, Benedikt J, Ye L. Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis. Cancers. 2024; 16(10):1907. https://doi.org/10.3390/cancers16101907
Chicago/Turabian StyleXu, Yali, Johannes Benedikt, and Lin Ye. 2024. "Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis" Cancers 16, no. 10: 1907. https://doi.org/10.3390/cancers16101907
APA StyleXu, Y., Benedikt, J., & Ye, L. (2024). Hyaluronic Acid Interacting Molecules Mediated Crosstalk between Cancer Cells and Microenvironment from Primary Tumour to Distant Metastasis. Cancers, 16(10), 1907. https://doi.org/10.3390/cancers16101907






