Stratification of Homologous Recombination Deficiency-Negative High-Grade Ovarian Cancer by the Type of Peritoneal Spread into Two Groups with Distinct Survival Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Ethical Approval
2.3. Type of Peritoneal Spread
2.4. SNP-Array and Data Analysis
2.5. Scoring (Integrated HRD Score)
2.6. Statistical Analysis
3. Results
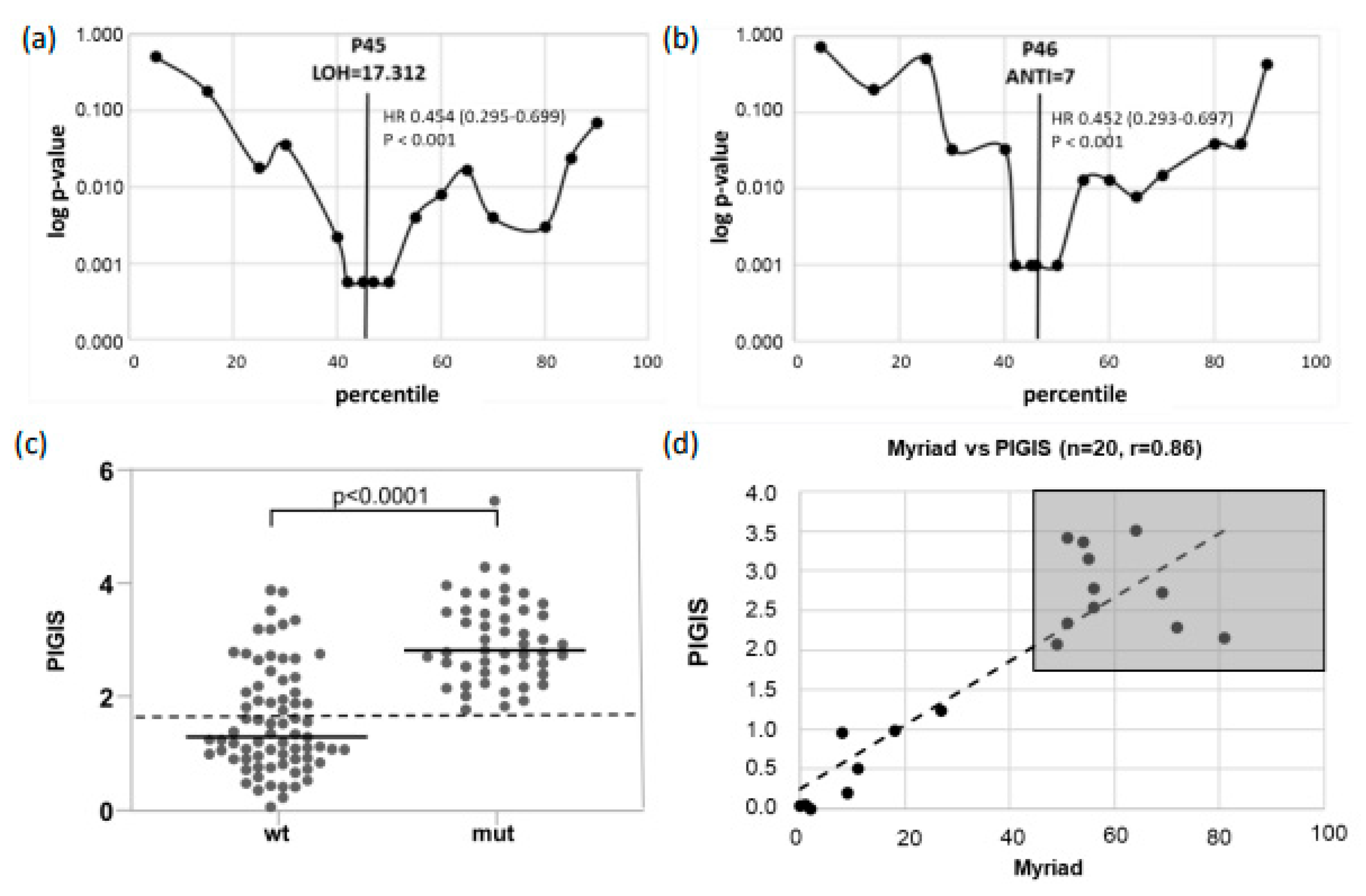
3.1. Setup of the Integrated HRD Score
3.2. Determination of a Diagnostic Threshold of PIGIS
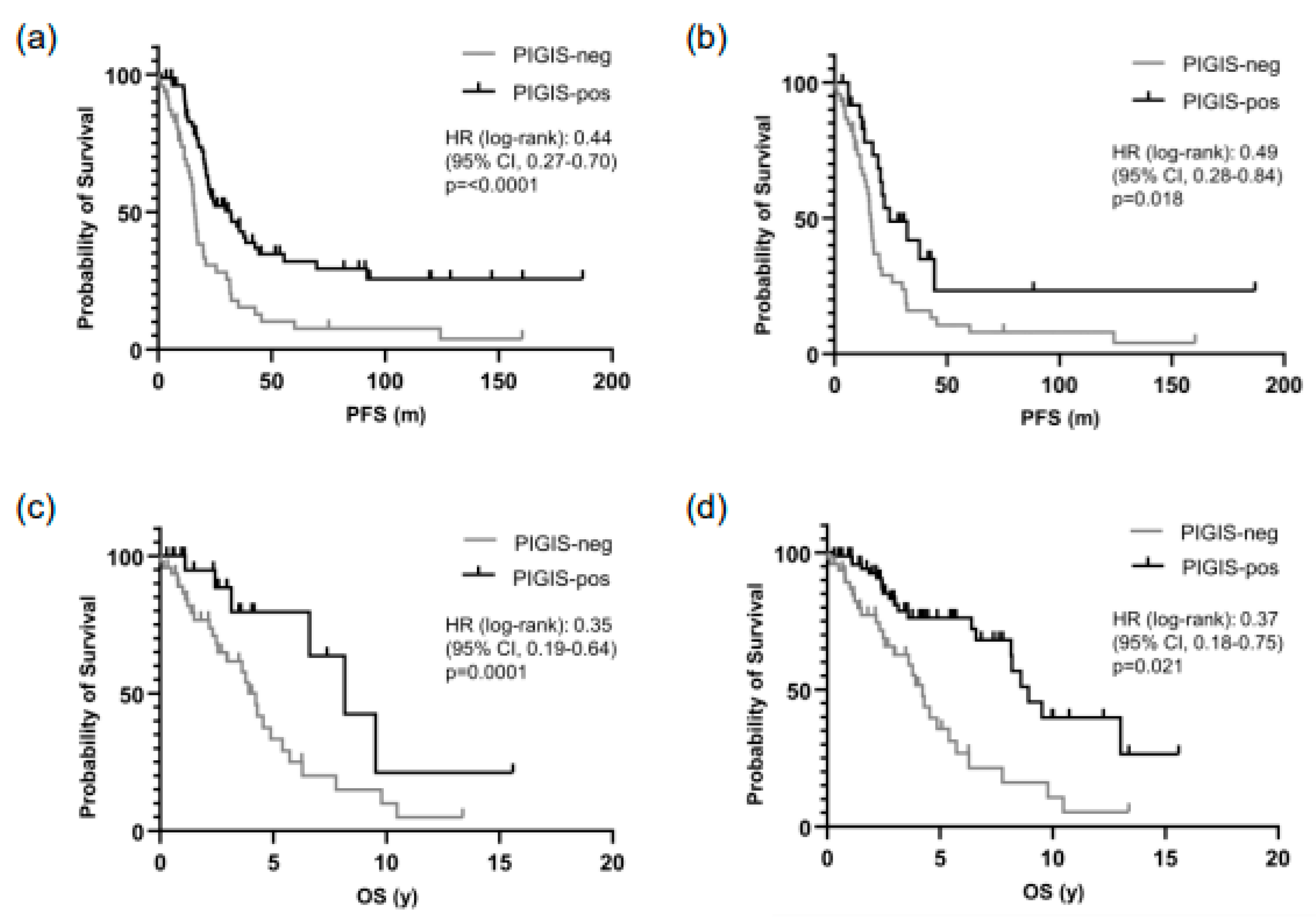
3.3. Patients with PIGIS (HRD)-Positive Tumors Have Better Prognosis
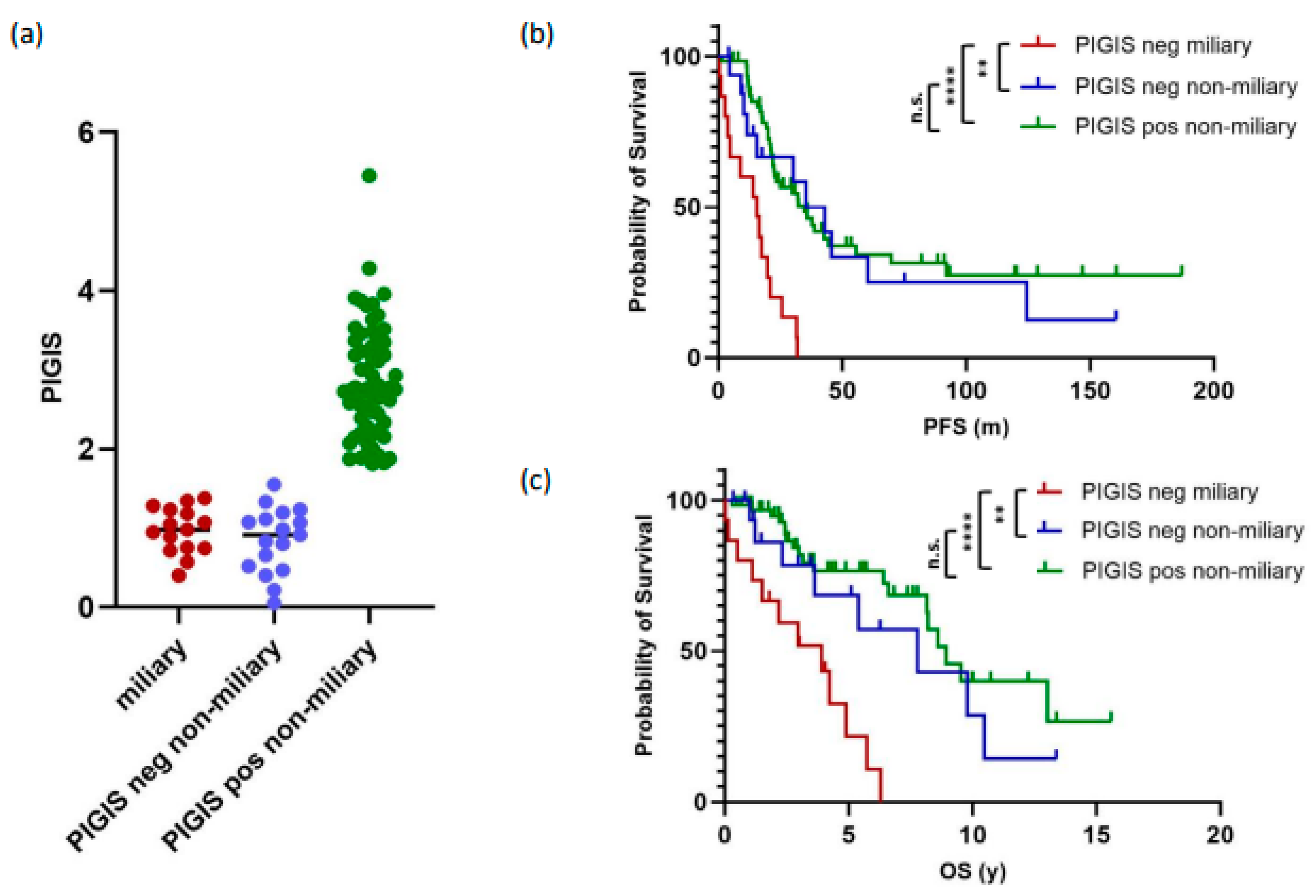
3.4. Tumors with Miliary Spread Are Exclusively PIGIS-Negative and Have a Poorer Prognosis
3.5. Residual Disease and Survival Correlates with Peritoneal Spread, but Not with HRD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Cancer Research Fund International. Ovarian Cancer Statistics. 2020. Available online: https://www.wcrf.org/cancer-trends/ovarian-cancer-statistics/ (accessed on 25 May 2023).
- National Cancer Institute, Surveillance, Epidemology, and End Results Program. Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 30 April 2024).
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef]
- Auer, K.; Bachmayr-Heyda, A.; Aust, S.; Grunt, T.W.; Pils, D. Comparative transcriptome analysis links distinct peritoneal tumor spread types, miliary and non-miliary, with putative origin, tubes and ovaries, in high grade serous ovarian cancer. Cancer Lett. 2017, 388, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Auer, K.; Bachmayr-Heyda, A.; Aust, S.; Sukhbaatar, N.; Reiner, A.T.; Grimm, C.; Horvat, R.; Zeillinger, R.; Pils, D. Peritoneal tumor spread in serous ovarian cancer-epithelial mesenchymal status and outcome. Oncotarget 2015, 6, 17261–17275. [Google Scholar] [CrossRef] [PubMed]
- Eng, K.H.; Morrell, K.; Starbuck, K.; Spring-Robinson, C.; Khan, A.; Cleason, D.; Akman, L.; Zsiros, E.; Odunsi, K.; Szender, J.B. Prognostic value of miliary versus non-miliary sub-staging in advanced ovarian cancer. Gynecol. Oncol. 2017, 146, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bachmayr-Heyda, A.; Auer, K.; Sukhbaatar, N.; Aust, S.; Deycmar, S.; Reiner, A.T.; Polterauer, S.; Dekan, S.; Pils, D. Small RNAs and the competing endogenous RNA network in high grade serous ovarian cancer tumor spread. Oncotarget 2016, 7, 39640–39653. [Google Scholar] [CrossRef]
- Egger, E.K.; Buchen, M.A.; Recker, F.; Stope, M.B.; Strunk, H.; Mustea, A.; Marinova, M. Predicting incomplete cytoreduction in patients with advanced ovarian cancer. Front. Oncol. 2022, 12, 1060006. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, G.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Costantini, B.; Margariti, P.A.; Gueli Alletti, S.; Cosentino, F.; et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 2016, 59, 22–33. [Google Scholar] [CrossRef]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; DiSaia, P.; Gabra, H.; Glenn, P.; et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Pal, T.; Permuth-Wey, J.; Betts, J.A.; Krischer, J.P.; Fiorica, J.; Arango, H.; LaPolla, J.; Hoffman, M.; Martino, M.A.; Wakeley, K.; et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 2005, 104, 2807–2816. [Google Scholar] [CrossRef]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.; Rosen, B.; Bradley, L.; Fan, I.; Tang, J.; Li, S.; Zhang, S.; Shaw, P.A.; et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: A kin-cohort study in Ontario, Canada. J. Natl. Cancer Inst. 2006, 98, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.T.; et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.L.; Tan, D.S.P. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: Do we need it? ESMO Open 2021, 6, 100144. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.A.; Irshad, S.; Grigoriadis, A.; Tutt, A.N.J. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Uzilov, A.V.; Ding, W.; Fink, M.Y.; Antipin, Y.; Brohl, A.S.; Davis, C.; Lau, C.Y.; Pandya, C.; Shah, H.; Kasai, Y.; et al. Development and clinical application of an integrative genomic approach to personalized cancer therapy. Genome Med. 2016, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Stecklein, S.R.; Jensen, R.A. Identifying and exploiting defects in the Fanconi anemia/BRCA pathway in oncology. Transl. Res. 2012, 160, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Fernández-Serra, A.; López-Reig, R.; Márquez, R.; Gallego, A.; de Sande, L.M.; Yubero, A.; Pérez-Segura, C.; Ramchandani-Vaswani, A.; Barretina-Ginesta, M.P.; Mendizábal, E.; et al. The Scarface Score: Deciphering Response to DNA Damage Agents in High-Grade Serous Ovarian Cancer-A GEICO Study. Cancers 2023, 15, 3030. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Drew, Y.; Kristeleit, R.S. Homologous recombination deficiency and ovarian cancer. Eur. J. Cancer 2016, 60, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Abkevich, V.; Timms, K.M.; Hennessy, B.T.; Potter, J.; Carey, M.S.; Meyer, L.A.; Smith-McCune, K.; Broaddus, R.; Lu, K.H.; Chen, J.; et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer 2012, 107, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Birkbak, N.J.; Wang, Z.C.; Kim, J.Y.; Eklund, A.C.; Li, Q.; Tian, R.; Bowman-Colin, C.; Li, Y.; Greene-Colozzi, A.; Iglehart, J.D.; et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012, 2, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Loverix, L.; Vergote, I.; Busschaert, P.; Vanderstichele, A.; Venken, T.; Boeckx, B.; Harter, P.; Brems, H.; Van Nieuwenhuysen, E.; Pignata, S.; et al. PARP inhibitor predictive value of the Leuven HRD test compared with Myriad MyChoice CDx PLUS HRD on 468 ovarian cancer patients from the PAOLA-1/ENGOT-ov25 trial. Eur. J. Cancer 2023, 188, 131–139. [Google Scholar] [CrossRef]
- Murthy, P.; Muggia, F. PARP inhibitors: Clinical development, emerging differences, and the current therapeutic issues. Cancer Drug Resist. 2019, 2, 665–679. [Google Scholar] [CrossRef]

| Inclusion criteria | BRCA1/2 mutational status |
| HGOC histology | |
| Exclusion criteria | FIGO Stage Ia |
| Variant of uncertain significance in BRCA1/2 | |
| Unclear or to low tumor cell content in the sample | |
| Patients lost in follow-up | |
| Death unrelated to ovarian cancer diagnosis (2nd malignancy, accident, etc.) | |
| Complete documentation of treatment not available | |
| Peritoneal spread type not characterized |
| Variable | Patients (n = 122) | |
|---|---|---|
| n | % | |
| ECOG Performance Status | ||
| 0 | 86 | 70.5 |
| 1–2 | 33 | 27.2 |
| ≥3 | 3 | 2.3 |
| Histologic Subtype | ||
| HGS | 109 | 89.3 |
| Endometrioid | 13 | 10.7 |
| Grading | ||
| Grade 2 | 42 | 34.4 |
| Grade 3 | 80 | 65.6 |
| FIGO Classification | ||
| Ic | 5 | 4.1 |
| IIa–IIb | 5 | 4.1 |
| IIIa–IIIc | 88 | 72.1 |
| IVa | 10 | 8.2 |
| IVb | 14 | 11.5 |
| Variable | Patients n = 122 | |
|---|---|---|
| n | % | |
| Surgery | ||
| PDS | 95 | 77.9 |
| IDS | 27 | 22.1 |
| Residual Disease | ||
| No residual disease | 93 | 76.2 |
| Residual < 1 cm | 24 | 19.7 |
| Any residual | 5 | 4.1 |
| Adjuvant CTx | ||
| Carbo + Pacli | 99 | 81.1 |
| Carbo Mono | 6 | 4.9 |
| Carbo + PLD | 3 | 2.5 |
| Cis + Pacli ip | 8 | 6.6 |
| Carbo + Pacli + IO | 6 | 4.9 |
| Maintenance | ||
| No therapy | 56 | 45.9 |
| Bev | 48 | 39.3 |
| PARPi | 4 | 3.3 |
| Bev + PARPi | 8 | 6.6 |
| Bev + IO | 4 | 3.3 |
| Bev + PARPi + IO | 2 | 1.6 |
| Recurrence | ||
| No | 38 | 31.1 |
| Yes | 84 | 68.9 |
| Platinum Option * | ||
| No (PFI < 6 Mo) | 15 | 17.9 |
| Yes (PFI ≥ 6 Mo) | 69 | 82.1 |
| Death | ||
| No | 73 | 59.8 |
| Yes | 49 | 40.2 |
| Mutational Status | ||
| WT | 73 | 59.8 |
| BRCA1 | 34 | 27.9 |
| BRCA2 | 10 | 8.2 |
| RAD51C | 5 | 4.1 |
| Peritoneal Spread | ||
| Non-miliary | 91 | 74.6 |
| Miliary | 31 | 25.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnaiter, S.; Schamschula, E.; Laschtowiczka, J.; Fiegl, H.; Zschocke, J.; Zeimet, A.; Wimmer, K.; Reimer, D. Stratification of Homologous Recombination Deficiency-Negative High-Grade Ovarian Cancer by the Type of Peritoneal Spread into Two Groups with Distinct Survival Outcomes. Cancers 2024, 16, 2129. https://doi.org/10.3390/cancers16112129
Schnaiter S, Schamschula E, Laschtowiczka J, Fiegl H, Zschocke J, Zeimet A, Wimmer K, Reimer D. Stratification of Homologous Recombination Deficiency-Negative High-Grade Ovarian Cancer by the Type of Peritoneal Spread into Two Groups with Distinct Survival Outcomes. Cancers. 2024; 16(11):2129. https://doi.org/10.3390/cancers16112129
Chicago/Turabian StyleSchnaiter, Simon, Esther Schamschula, Juliane Laschtowiczka, Heidelinde Fiegl, Johannes Zschocke, Alain Zeimet, Katharina Wimmer, and Daniel Reimer. 2024. "Stratification of Homologous Recombination Deficiency-Negative High-Grade Ovarian Cancer by the Type of Peritoneal Spread into Two Groups with Distinct Survival Outcomes" Cancers 16, no. 11: 2129. https://doi.org/10.3390/cancers16112129
APA StyleSchnaiter, S., Schamschula, E., Laschtowiczka, J., Fiegl, H., Zschocke, J., Zeimet, A., Wimmer, K., & Reimer, D. (2024). Stratification of Homologous Recombination Deficiency-Negative High-Grade Ovarian Cancer by the Type of Peritoneal Spread into Two Groups with Distinct Survival Outcomes. Cancers, 16(11), 2129. https://doi.org/10.3390/cancers16112129






