Safety and Efficacy of Laser Interstitial Thermal Therapy as Upfront Therapy in Primary Glioblastoma and IDH-Mutant Astrocytoma: A Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Search Results
3.2. Patient Demographics and Tumor Characteristics
3.2.1. IDH-Wildtype Glioblastoma
3.2.2. IDH-Mutant Astrocytoma
3.3. Postoperative Outcomes
3.3.1. IDH-Wildtype Glioblastoma
3.3.2. IDH-Mutant Astrocytoma
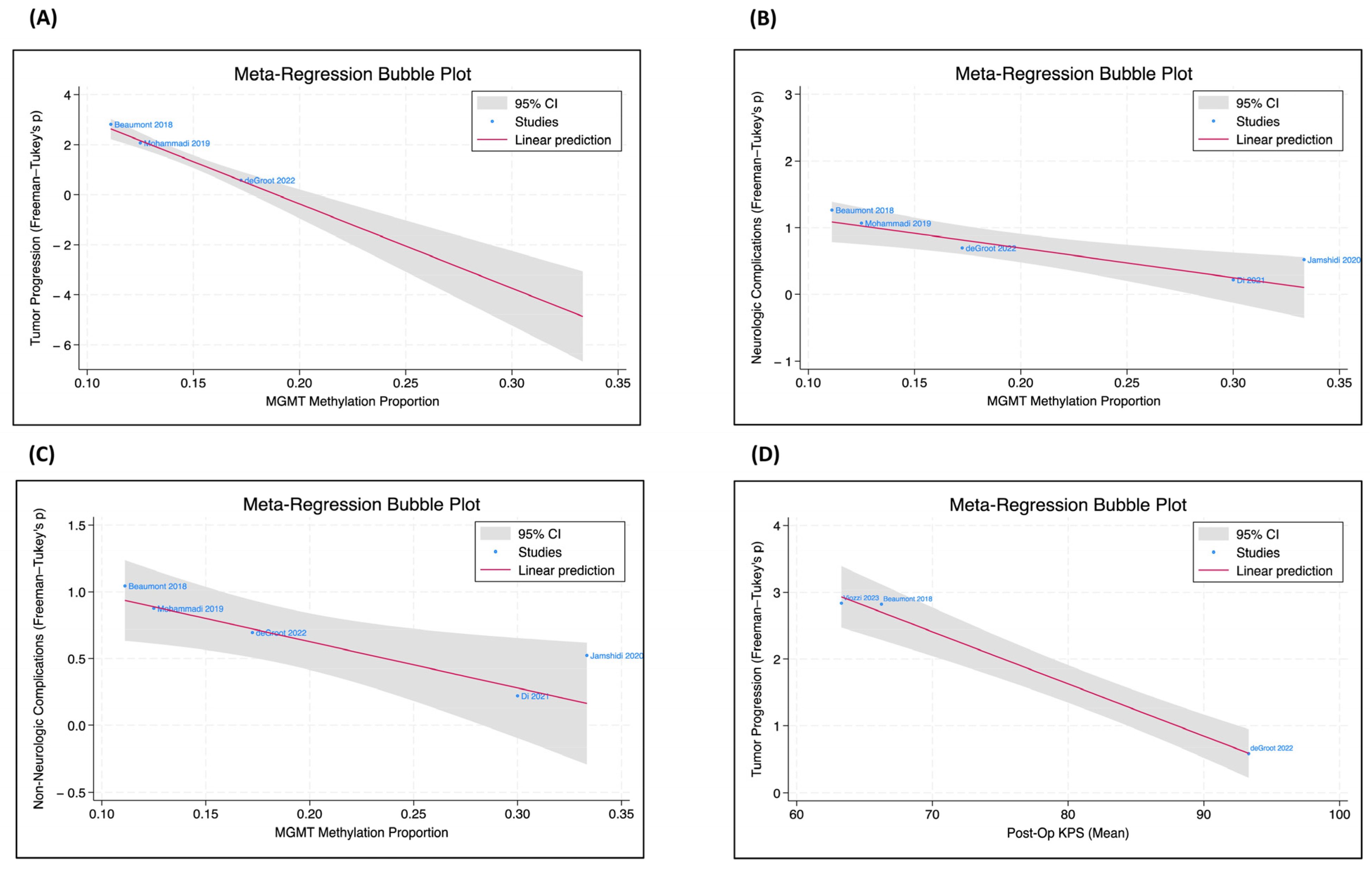
3.4. Meta-Regression Analysis
3.5. Study Heterogeneity
3.6. Adjuvant Therapy in Combination with LITT
3.7. Publication Bias
4. Discussion
4.1. Survival and Function
4.2. Extent of Ablation
4.3. Postoperative Complications
4.4. Adjuvant Therapies
4.5. Limitations and Future Steps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System Tumours: A Practical Update on What Neurosurgeons Need to Know—A Minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical Implications of the 2021 Edition of the WHO Classification of Central Nervous System Tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- McNamara, C.; Mankad, K.; Thust, S.; Dixon, L.; Limback-Stanic, C.; D’Arco, F.; Jacques, T.S.; Löbel, U. 2021 WHO Classification of Tumours of the Central Nervous System: A Review for the Neuroradiologist. Neuroradiology 2022, 64, 1919–1950. [Google Scholar] [CrossRef]
- Wen, P.Y.; Packer, R.J. The 2021 WHO Classification of Tumors of the Central Nervous System: Clinical Implications. Neuro-Oncology 2021, 23, 1215–1217. [Google Scholar] [CrossRef]
- Johnson, D.R.; Giannini, C.; Vaubel, R.A.; Morris, J.M.; Eckel, L.J.; Kaufmann, T.J.; Guerin, J.B. A Radiologist’s Guide to the 2021 WHO Central Nervous System Tumor Classification: Part I—Key Concepts and the Spectrum of Diffuse Gliomas. Radiology 2022, 304, 494–508. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, Therapeutic, and Prognostic Implications of the 2021 World Health Organization Classification of Tumors of the Central Nervous System. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef]
- Lakhani, D.A.; Sabsevitz, D.S.; Chaichana, K.L.; Quiñones-Hinojosa, A.; Middlebrooks, E.H. Current State of Functional MRI in the Presurgical Planning of Brain Tumors. Radiol. Imaging Cancer 2023, 5, e230078. [Google Scholar] [CrossRef]
- Shah, S.; Ivey, N.; Matur, A.; Andaluz, N. Intraoperative Fluorophores: An Update on 5-Aminolevulinic Acid and Sodium Fluorescein in Resection of Tumors of the Central Nervous System and Metastatic Lesions-A Systematic Review and Meta-Analysis. Tomography 2023, 9, 1551–1567. [Google Scholar] [CrossRef]
- Osborn, A.G.; Louis, D.N.; Poussaint, T.Y.; Linscott, L.L.; Salzman, K.L. The 2021 World Health Organization Classification of Tumors of the Central Nervous System: What Neuroradiologists Need to Know. Am. J. Neuroradiol. 2022, 43, 928–937. [Google Scholar] [CrossRef]
- Xue, F.; Chen, T.; Sun, H. Postoperative Outcomes of Magnetic Resonance Imaging (MRI)-Guided Laser Interstitial Thermal Therapy (LITT) in the Treatment of Drug-Resistant Epilepsy: A Meta-Analysis. Med. Sci. Monit. 2018, 24, 9292–9299. [Google Scholar] [CrossRef]
- Salem, U.; Kumar, V.A.; Madewell, J.E.; Schomer, D.F.; De Almeida Bastos, D.C.; Zinn, P.O.; Weinberg, J.S.; Rao, G.; Prabhu, S.S.; Colen, R.R. Neurosurgical Applications of MRI Guided Laser Interstitial Thermal Therapy (LITT). Cancer Imaging 2019, 19, 65. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Ivan, M.E.; Mohammadi, A.M.; De Deugd, N.; Reyes, J.; Rodriguez, G.; Shah, A.; Barnett, G.H.; Komotar, R.J. Laser Ablation of Newly Diagnosed Malignant Gliomas: A Meta-Analysis. Neurosurgery 2016, 79 (Suppl. S1), S17–S23. [Google Scholar] [CrossRef]
- Chen, C.; Lee, I.; Tatsui, C.; Elder, T.; Sloan, A.E. Laser Interstitial Thermotherapy (LITT) for the Treatment of Tumors of the Brain and Spine: A Brief Review. J. Neurooncol. 2021, 151, 429–442. [Google Scholar] [CrossRef]
- de Groot, J.F.; Kim, A.H.; Prabhu, S.; Rao, G.; Laxton, A.W.; Fecci, P.E.; O’Brien, B.J.; Sloan, A.; Chiang, V.; Tatter, S.B.; et al. Efficacy of Laser Interstitial Thermal Therapy (LITT) for Newly Diagnosed and Recurrent IDH Wild-Type Glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac040. [Google Scholar] [CrossRef]
- Patel, T.R.; Chiang, V.L.S. Laser Interstitial Thermal Therapy for Treatment of Post-Radiosurgery Tumor Recurrence and Radiation Necrosis. Photonics Lasers Med. 2014, 3, 95–105. [Google Scholar] [CrossRef]
- Lerner, E.C.; Edwards, R.M.; Wilkinson, D.S.; Fecci, P.E. Laser Ablation: Heating up the Anti-Tumor Response in the Intracranial Compartment. Adv. Drug Deliv. Rev. 2022, 185, 114311. [Google Scholar] [CrossRef]
- Fabiano, A.J.; Alberico, R.A. Laser-Interstitial Thermal Therapy for Refractory Cerebral Edema from Post-Radiosurgery Metastasis. World Neurosurg. 2014, 81, 652.e1–652.e4. [Google Scholar] [CrossRef]
- Bown, S.G. Phototherapy of Tumors. World J. Surg. 1983, 7, 700–709. [Google Scholar] [CrossRef]
- Aizer, A.A.; Lamba, N.; Ahluwalia, M.S.; Aldape, K.; Boire, A.; Brastianos, P.K.; Brown, P.D.; Camidge, D.R.; Chiang, V.L.; Davies, M.A.; et al. Brain Metastases: A Society for Neuro-Oncology (SNO) Consensus Review on Current Management and Future Directions. Neuro-Oncology 2022, 24, 1613–1646. [Google Scholar] [CrossRef] [PubMed]
- Desclides, M.; Ozenne, V.; Bour, P.; Faller, T.; Machinet, G.; Pierre, C.; Chemouny, S.; Quesson, B. Real-Time Automatic Temperature Regulation during In Vivo MRI-Guided Laser-Induced Thermotherapy (MR-LITT). Sci. Rep. 2023, 13, 3279. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R.T.; Wang, A.C.; Miller, J.W.; Novotny, E.J.; Ojemann, J.G. Stereotactic Laser Ablation for Hypothalamic and Deep Intraventricular Lesions. Neurosurg. Focus 2016, 41, E10. [Google Scholar] [CrossRef] [PubMed]
- Medvid, R.; Ruiz, A.; Komotar, R.J.; Jagid, J.R.; Ivan, M.E.; Quencer, R.M.; Desai, M.B. Current Applications of MRI-Guided Laser Interstitial Thermal Therapy in the Treatment of Brain Neoplasms and Epilepsy: A Radiologic and Neurosurgical Overview. Am. J. Neuroradiol. 2015, 36, 1998–2006. [Google Scholar] [CrossRef]
- Kim, A.H.; Tatter, S.; Rao, G.; Prabhu, S.; Chen, C.; Fecci, P.; Chiang, V.; Smith, K.; Williams, B.J.; Mohammadi, A.M.; et al. Laser Ablation of Abnormal Neurological Tissue Using Robotic NeuroBlate System (LAANTERN): 12-Month Outcomes and Quality of Life After Brain Tumor Ablation. Neurosurgery 2020, 87, E338–E346. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Semonche, A.; Eichberg, D.G.; Borowy, V.; Luther, E.; Sarkiss, C.A.; Morell, A.; Mahavadi, A.K.; Ivan, M.E.; Komotar, R.J. The Role of Laser Interstitial Thermal Therapy in Surgical Neuro-Oncology: Series of 100 Consecutive Patients. Neurosurgery 2020, 87, 266–275. [Google Scholar] [CrossRef]
- Boop, S.; Bonda, D.; Randle, S.; Leary, S.; Vitanza, N.; Crotty, E.; Novotny, E.; Friedman, S.; Ellenbogen, R.G.; Durfy, S.; et al. A Comparison of Clinical Outcomes for Subependymal Giant Cell Astrocytomas Treated with Laser Interstitial Thermal Therapy, Open Surgical Resection, and mTOR Inhibitors. Pediatr. Neurosurg. 2023, 58, 150–159. [Google Scholar] [CrossRef]
- Yudkoff, C.; Mahtabfar, A.; Piper, K.; Judy, K. Safety and Efficacy of Salvage Therapy with Laser Interstitial Thermal Therapy for Malignant Meningioma Refractory to Cesium-131 Brachytherapy: Illustrative Case. J. Neurosurg. Case Lessons 2022, 4, CASE22379. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.C.; Basil, G.W.; Diaz, R.J.; Komotar, R.J. The Safety of Bevacizumab Administered Shortly after Laser Interstitial Thermal Therapy in Glioblastoma: A Case Series. World Neurosurg. 2018, 117, e588–e594. [Google Scholar] [CrossRef]
- Butt, O.H.; Zhou, A.Y.; Huang, J.; Leidig, W.A.; Silberstein, A.E.; Chheda, M.G.; Johanns, T.M.; Ansstas, G.; Liu, J.; Talcott, G.; et al. Corrigendum to: A Phase II Study of Laser Interstitial Thermal Therapy Combined with Doxorubicin in Patients with Recurrent Glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac042. [Google Scholar] [CrossRef]
- Fomchenko, E.I.; Leelatian, N.; Darbinyan, A.; Huttner, A.J.; Chiang, V.L. Histological Changes Associated with Laser Interstitial Thermal Therapy for Radiation Necrosis: Illustrative Cases. J. Neurosurg. Case Lessons 2022, 4, CASE21373. [Google Scholar] [CrossRef]
- Salehi, A.; Paturu, M.R.; Patel, B.; Cain, M.D.; Mahlokozera, T.; Yang, A.B.; Lin, T.-H.; Leuthardt, E.C.; Yano, H.; Song, S.-K.; et al. Therapeutic Enhancement of Blood-Brain and Blood-Tumor Barriers Permeability by Laser Interstitial Thermal Therapy. Neuro-Oncol. Adv. 2020, 2, vdaa071. [Google Scholar] [CrossRef] [PubMed]
- Balança, B.; Meiller, A.; Bezin, L.; Dreier, J.P.; Marinesco, S.; Lieutaud, T. Altered Hypermetabolic Response to Cortical Spreading Depolarizations after Traumatic Brain Injury in Rats. J. Cereb. Blood Flow Metab. 2017, 37, 1670–1686. [Google Scholar] [CrossRef]
- Hu, L.S.; Brat, D.J.; Bloch, O.; Ramkissoon, S.; Lesser, G.J. The Practical Application of Emerging Technologies Influencing the Diagnosis and Care of Patients with Primary Brain Tumors. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e35–e46. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.; Rommel, F.; Kondakci, M.; Gorol, M.; Willers, R.; Bilzer, T. Locoregional Opening of the Rodent Blood-Brain Barrier for Paclitaxel Using Nd:YAG Laser-Induced Thermo Therapy: A New Concept of Adjuvant Glioma Therapy? Lasers Surg. Med. 2003, 33, 75–80. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Leuthardt, E.C.; Duan, C.; Kim, M.J.; Campian, J.L.; Kim, A.H.; Miller-Thomas, M.M.; Shimony, J.S.; Tran, D.D. Hyperthermic Laser Ablation of Recurrent Glioblastoma Leads to Temporary Disruption of the Peritumoral Blood Brain Barrier. PLoS ONE 2016, 11, e0148613. [Google Scholar] [CrossRef]
- Demeule, M.; Régina, A.; Jodoin, J.; Laplante, A.; Dagenais, C.; Berthelet, F.; Moghrabi, A.; Béliveau, R. Drug Transport to the Brain: Key Roles for the Efflux Pump P-Glycoprotein in the Blood-Brain Barrier. Vascul. Pharmacol. 2002, 38, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.; Debinski, W. Challenges to Successful Implementation of the Immune Checkpoint Inhibitors for Treatment of Glioblastoma. Int. J. Mol. Sci. 2020, 21, 2759. [Google Scholar] [CrossRef]
- Kiyatkin, E.A.; Sharma, H.S. Permeability of the Blood-Brain Barrier Depends on Brain Temperature. Neuroscience 2009, 161, 926–939. [Google Scholar] [CrossRef]
- Hong, C.S.; Deng, D.; Vera, A.; Chiang, V.L. Laser-Interstitial Thermal Therapy Compared to Craniotomy for Treatment of Radiation Necrosis or Recurrent Tumor in Brain Metastases Failing Radiosurgery. J. Neurooncol. 2019, 142, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Schwalb, A.M.; Srinivasan, E.S.; Fecci, P.E. Commentary: Laser Interstitial Thermal Therapy for First-Line Treatment of Surgically Accessible Recurrent Glioblastoma: Outcomes Compared with a Surgical Cohort. Neurosurgery 2022, 91, e160–e163. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.D.; Barnett, G. The Value of Using a Brain Laser Interstitial Thermal Therapy (LITT) System in Patients Presenting with High Grade Gliomas Where Maximal Safe Resection May Not Be Feasible. Cost Eff. Resour. Alloc. 2016, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Kamath, A.A.; Leuthardt, E.C.; Kim, A.H. Management of Intracranial Metastatic Disease with Laser Interstitial Thermal Therapy. Front. Oncol. 2018, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.V.; Mian, M.; Stafford, R.J.; Nahed, B.V.; Willie, J.T.; Gross, R.E.; Danish, S.F. Laser Interstitial Thermal Therapy Technology, Physics of Magnetic Resonance Imaging Thermometry, and Technical Considerations for Proper Catheter Placement During Magnetic Resonance Imaging–Guided Laser Interstitial Thermal Therapy. Neurosurgery 2016, 79 (Suppl. S1), S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.A.; Friedman, D.D.; Akbari, S.H.A.; Kim, A.H.; Tao, Y.; Luo, J.; Leuthardt, E.C. Glioblastoma Treated with Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy: Safety, Efficacy, and Outcomes. Neurosurgery 2019, 84, 836–843. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Patel, B.; Kim, A.H. Laser Interstitial Thermal Therapy. Mo Med. 2020, 117, 50–55. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 18, StataCorp. LLC: College Station, TX, USA, 2023.
- Cai, S.; Zhou, J.; Pan, J. Estimating the Sample Mean and Standard Deviation from Order Statistics and Sample Size in Meta-Analysis. Stat. Methods Med. Res. 2021, 30, 2701–2719. [Google Scholar] [CrossRef]
- McGrath, S.; Katzenschlager, S.; Zimmer, A.J.; Seitel, A.; Steele, R.; Benedetti, A. Standard Error Estimation in Meta-Analysis of Studies Reporting Medians. Stat. Methods Med. Res. 2023, 32, 373–388. [Google Scholar] [CrossRef]
- Borghei-Razavi, H.; Koech, H.; Sharma, M.; Krivosheya, D.; Lee, B.S.; Barnett, G.H.; Mohammadi, A.M. Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: An Initial Experience. World Neurosurg. 2018, 117, e146–e153. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.W.; Han, R.H.; Smyth, M.D.; Leuthardt, E.C.; Kim, A.H. Laser Interstitial Thermal Therapy in Grade 2/3 IDH1/2 Mutant Gliomas: A Preliminary Report and Literature Review. Curr. Oncol. 2022, 29, 2550–2563. [Google Scholar] [CrossRef] [PubMed]
- Kahn, T.; Bettag, M.; Ulrich, F.; Schwarzmaier, H.J.; Schober, R.; Fürst, G.; Mödder, U. MRI-Guided Laser-Induced Interstitial Thermotherapy of Cerebral Neoplasms. J. Comput. Assist. Tomogr. 1994, 18, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Kunesch, E.; Classen, J.; Bettag, M.; Kahn, T.; Ulrich, F.; Bock, W.J.; Freund, H.J.; Seitz, R.J. Representational Cortical Plasticity Associated with Brain Tumours: Evidence from Laser-Induced Interstitial Thermotherapy. Acta Neurol. Scand. 2003, 108, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Murayi, R.; Borghei-Razavi, H.; Barnett, G.H.; Mohammadi, A.M. Laser Interstitial Thermal Therapy in the Treatment of Thalamic Brain Tumors: A Case Series. Oper. Neurosurg. 2020, 19, 641–650. [Google Scholar] [CrossRef]
- Beaumont, T.L.; Mohammadi, A.M.; Kim, A.H.; Barnett, G.H.; Leuthardt, E.C. Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy for Glioblastoma of the Corpus Callosum. Neurosurgery 2018, 83, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Dabecco, R.; Gigliotti, M.J.; Mao, G.; Myers, D.; Xu, L.; Lee, P.; Ranjan, T.; Aziz, K.; Yu, A. Laser Interstitial Thermal Therapy (LITT) for Intracranial Lesions: A Single-Institutional Series, Outcomes, and Review of the Literature. Br. J. Neurosurg. 2021, 38, 632–638. [Google Scholar] [CrossRef]
- Daggubati, L.C.; Ramos-Fresnedo, A.; Merenzon, M.A.; Bhatia, S.; Morell, A.A.; Berry, K.M.; Chandar, J.; Shah, A.H.; Komotar, R.J.; Ivan, M.E. Bilateral Laser Interstitial Thermal Therapy for Butterfly Gliomas Compared with Needle Biopsy: A Preliminary Survival Study. Oper. Neurosurg. 2023, 25, 435–440. [Google Scholar] [CrossRef]
- Di, L.; Wang, C.P.; Shah, A.H.; Eichberg, D.G.; Semonche, A.M.; Sanjurjo, A.D.; Luther, E.M.; Jermakowicz, W.J.; Komotar, R.J.; Ivan, M.E. A Cohort Study on Prognostic Factors for Laser Interstitial Thermal Therapy Success in Newly Diagnosed Glioblastoma. Neurosurgery 2021, 89, 496–503. [Google Scholar] [CrossRef]
- Hajtovic, S.; Mogilner, A.; Ard, J.; Gautreaux, J.E.; Britton, H.; Fatterpekar, G.; Young, M.G.; Placantonakis, D.G. Awake Laser Ablation for Patients with Tumors in Eloquent Brain Areas: Operative Technique and Case Series. Cureus 2020, 12, e12186. [Google Scholar] [CrossRef]
- Hawasli, A.H.; Bagade, S.; Shimony, J.S.; Miller-Thomas, M.; Leuthardt, E.C. Magnetic Resonance Imaging-Guided Focused Laser Interstitial Thermal Therapy for Intracranial Lesions: Single-Institution Series. Neurosurgery 2013, 73, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.M.; Eichberg, D.G.; Komotar, R.J.; Ivan, M. Safety Analysis of Bilateral Laser Interstitial Thermal Therapy for Treatment of Butterfly Glioma. World Neurosurg. 2020, 144, e156–e163. [Google Scholar] [CrossRef]
- Maraka, S.; Asmaro, K.; Walbert, T.; Lee, I. Cerebral Edema Induced by Laser Interstitial Thermal Therapy and Radiotherapy in Close Succession in Patients with Brain Tumor. Lasers Surg. Med. 2018, 50, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Merenzon, M.A.; Patel, N.V.; Morell, A.A.; Marcó Del Pont, F.; Moll, J.M.; Komotar, R.J.; Ivan, M.E. Newly Diagnosed Adult Basal Ganglia Gliomas Treated with Laser Interstitial Thermal Therapy: A Comparative Cohort with Needle Biopsy. Oper. Neurosurg. 2023, 24, 383–390. [Google Scholar] [CrossRef]
- Mohammadi, A.M.; Sharma, M.; Beaumont, T.L.; Juarez, K.O.; Kemeny, H.; Dechant, C.; Seas, A.; Sarmey, N.; Lee, B.S.; Jia, X.; et al. Upfront Magnetic Resonance Imaging-Guided Stereotactic Laser-Ablation in Newly Diagnosed Glioblastoma: A Multicenter Review of Survival Outcomes Compared to a Matched Cohort of Biopsy-Only Patients. Neurosurgery 2019, 85, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Muir, M.; Patel, R.; Traylor, J.I.; de Almeida Bastos, D.C.; Kamiya, C.; Li, J.; Rao, G.; Prabhu, S.S. Laser Interstitial Thermal Therapy for Newly Diagnosed Glioblastoma. Lasers Med. Sci. 2022, 37, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Rao, G.; Kew, Y.; Prabhu, S.S. Laser Interstitial Thermal Therapy for Newly Diagnosed and Recurrent Glioblastoma. Neurosurg. Focus 2016, 41, E12. [Google Scholar] [CrossRef] [PubMed]
- Viozzi, I.; Overduin, C.G.; Rijpma, A.; Rovers, M.M.; Laan, M.T. MR-Guided LITT Therapy in Patients with Primary Irresectable Glioblastoma: A Prospective, Controlled Pilot Study. J. Neurooncol. 2023, 164, 405–412. [Google Scholar] [CrossRef]
- Mirza, F.; Mitha, R.; Shamim, M. Current Role of Laser Interstitial Thermal Therapy in the Treatment of Intracranial Tumors. Asian J. Neurosurg. 2020, 15, 800–808. [Google Scholar] [CrossRef]
- Yu, P.; Yang, Y. Meta-Analysis of the Impact of Laser Interstitial Hyperthermia on Wound Healing Complications in Brain Tumors. Int. Wound J. 2024, 21, e14628. [Google Scholar] [CrossRef]
- Sabahi, M.; Bordes, S.J.; Najera, E.; Mohammadi, A.M.; Barnett, G.H.; Adada, B.; Borghei-Razavi, H. Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: A Systematic Review and Analysis of Multi-Institutional Outcomes. Cancers 2022, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Chugh, J.; Wright, C.H.; Alonso, F.; Hdeib, A.; Gittleman, H.; Barnholtz-Sloan, J.; Sloan, A.E. Laser Interstitial Thermal Therapy Followed by Minimal-Access Transsulcal Resection for the Treatment of Large and Difficult to Access Brain Tumors. Neurosurg. Focus 2016, 41, E14. [Google Scholar] [CrossRef] [PubMed]
- AbdelFatah, M.A.R.; Kotb, A.; Said, M.A.; Abouelmaaty, E.M.H. Impact of Extent of Resection of Newly Diagnosed Glioblastomas on Survival: A Meta-Analysis. Egypt. J. Neurosurg. 2022, 37, 3. [Google Scholar] [CrossRef]
- Kaisman-Elbaz, T.; Xiao, T.; Grabowski, M.M.; Barnett, G.H.; Mohammadi, A.M. The Impact of Extent of Ablation on Survival of Patients with Newly Diagnosed Glioblastoma Treated with Laser Interstitial Thermal Therapy: A Large Single-Institutional Cohort. Neurosurgery 2023, 93, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.L.; Pelloski, C.E.; Gilbert, M.R.; Colman, H.; De La Cruz, C.; Sulman, E.P.; Bekele, B.N.; Aldape, K.D. MGMT Promoter Methylation Is Predictive of Response to Radiotherapy and Prognostic in the Absence of Adjuvant Alkylating Chemotherapy for Glioblastoma. Neuro-Oncology 2010, 12, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.M.; Hawasli, A.H.; Rodriguez, A.; Schroeder, J.L.; Laxton, A.W.; Elson, P.; Tatter, S.B.; Barnett, G.H.; Leuthardt, E.C. The Role of Laser Interstitial Thermal Therapy in Enhancing Progression-Free Survival of Difficult-to-Access High-Grade Gliomas: A Multicenter Study. Cancer Med. 2014, 3, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Roa, W.; Kepka, L.; Kumar, N.; Sinaika, V.; Matiello, J.; Lomidze, D.; Hentati, D.; Guedes De Castro, D.; Dyttus-Cebulok, K.; Drodge, S.; et al. International Atomic Energy Agency Randomized Phase III Study of Radiation Therapy in Elderly and/or Frail Patients with Newly Diagnosed Glioblastoma Multiforme. J. Clin. Oncol. 2015, 33, 4145–4150. [Google Scholar] [CrossRef] [PubMed]
- Floyd, N.S.; Woo, S.Y.; Teh, B.S.; Prado, C.; Mai, W.-Y.; Trask, T.; Gildenberg, P.L.; Holoye, P.; Augspurger, M.E.; Carpenter, L.S.; et al. Hypofractionated Intensity-Modulated Radiotherapy for Primary Glioblastoma Multiforme. Int. J. Radiat. Oncol. 2004, 58, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Holste, K.G.; Orringer, D.A. Laser Interstitial Thermal Therapy. Neuro-Oncol. Adv. 2020, 2, vdz035. [Google Scholar] [CrossRef]
- Viozzi, I.; Guberinic, A.; Overduin, C.G.; Rovers, M.M.; Ter Laan, M. Laser Interstitial Thermal Therapy in Patients with Newly Diagnosed Glioblastoma: A Systematic Review. J. Clin. Med. 2021, 10, 355. [Google Scholar] [CrossRef]
- Zetterling, M.; Elf, K.; Semnic, R.; Latini, F.; Engström, E.R. Time Course of Neurological Deficits after Surgery for Primary Brain Tumours. Acta Neurochir. 2020, 162, 3005–3018. [Google Scholar] [CrossRef]
- Gempt, J.; Förschler, A.; Buchmann, N.; Pape, H.; Ryang, Y.-M.; Krieg, S.M.; Zimmer, C.; Meyer, B.; Ringel, F. Postoperative Ischemic Changes Following Resection of Newly Diagnosed and Recurrent Gliomas and Their Clinical Relevance: Clinical Article. J. Neurosurg. 2013, 118, 801–808. [Google Scholar] [CrossRef]
- Gulati, S.; Jakola, A.S.; Nerland, U.S.; Weber, C.; Solheim, O. The Risk of Getting Worse: Surgically Acquired Deficits, Perioperative Complications, and Functional Outcomes After Primary Resection of Glioblastoma. World Neurosurg. 2011, 76, 572–579. [Google Scholar] [CrossRef]
- Wang, W.-L.; Aru, N.; Liu, Z.; Shen, X.; Ding, Y.-M.; Wu, S.-J.; Qin, H.-H.; Jin, W.-Y. Prognosis of Patients with Newly Diagnosed Glioblastoma Treated with Molecularly Targeted Drugs Combined with Radiotherapy vs Temozolomide Monotherapy: A Meta-Analysis. Medicine 2019, 98, e17759. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing Percent Resection and Residual Volume Thresholds Affecting Survival and Recurrence for Patients with Newly Diagnosed Intracranial Glioblastoma. Neuro-Oncology 2014, 16, 113–122. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Arends, L.; Klimek, M.; Dirven, C.M.F.; Vincent, A.J.-P.E. Impact of Intraoperative Stimulation Mapping on High-Grade Glioma Surgery Outcome: A Meta-Analysis. Acta Neurochir. 2019, 161, 99–107. [Google Scholar] [CrossRef]
- Trinh, V.T.; Davies, J.M.; Berger, M.S. Surgery for Primary Supratentorial Brain Tumors in the United States, 2000–2009: Effect of Provider and Hospital Caseload on Complication Rates. J. Neurosurg. 2015, 122, 280–296. [Google Scholar] [CrossRef]
- Mao, H.; Li, X.; Mao, W. Advantages of Gross Total Resection in Patients with Astrocytoma: A Population-based Study. Oncol. Lett. 2020, 19, 3761–3774. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Sakai, T.; Fujishima, I.; Sugiyama, K.; Ryu, H.; Uemura, K. Interstitial Laserthermia in Neurosurgery. J. Clin. Laser Med. Surg. 1992, 10, 37–40. [Google Scholar] [CrossRef]
- Levy, A.S.; Merenzon, M.A.; Eatz, T.; Morell, A.A.; Eichberg, D.G.; Bloom, M.J.; Shah, A.H.; Komotar, R.J.; Ivan, M.E. Development of an Enhanced Recovery after Laser Ablation Surgery Protocol: A Preliminary Analysis. Neuro-Oncol. Pract. 2023, 10, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.; Gamble, A.; Black, K.; Schulder, M.; Mehta, A.D. Complication Avoidance in Laser Interstitial Thermal Therapy: Lessons Learned. J. Neurosurg. 2017, 126, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Johnson, C.D. Efficacy of Drug X in Treating Condition Y: A Randomized Controlled Trial. Clinical Trial. 15 January 2023. Available online: https://clinicaltrials.gov/Ct2/Show/NCT05318612 (accessed on 1 April 2024).
- Gonzalez, J.M.; Patel, R. Evaluation of Drug Z for the Treatment of Disease X: A Phase III Clinical Trial. Clinical Trial. 20 May 2021. Available online: https://clinicaltrials.gov/Ct2/Show/NCT02970448 (accessed on 1 April 2024).
- Chandar, J.S.; Bhatia, S.; Ingle, S.; Mendez Valdez, M.J.; Maric, D.; Seetharam, D.; Desgraves, J.F.; Govindarajan, V.; Daggubati, L.; Merenzon, M.; et al. Laser Interstitial Thermal Therapy Induces Robust Local Immune Response for Newly Diagnosed Glioblastoma with Long-Term Survival and Disease Control. J. Immunother. 2023, 46, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Campian, J.; Ghiaseddin, A.; Rahman, M.; Huang, J.; Ansstas, G.; Kim, A.; Leuthardt, E.; Tran, D. ATIM-45. Long Term Follow-Up of a Phase I/II Study Testing the Toxicities and Efficacy of Pembrolizumab in Combination with MRI-Guided Laser Interstitial Thermal Therapy (LITT) in Recurrent Malignant Gliomas. Neuro-Oncology 2019, 21 (Suppl. S6), vi11. [Google Scholar] [CrossRef]
- Luo, M.; Chen, S.-L.; Chen, J.; Yan, H.; Qiu, Z.; Chen, G.; Lu, L.; Zhang, F. Resection vs. Ablation for Lesions Characterized as Resectable-Ablative within the Colorectal Liver Oligometastases Criteria: A Propensity Score Matching from Retrospective Study. PeerJ 2020, 8, e8398. [Google Scholar] [CrossRef]
- Sloan, A.E.; Ahluwalia, M.S.; Valerio-Pascua, J.; Manjila, S.; Torchia, M.G.; Jones, S.E.; Sunshine, J.L.; Phillips, M.; Griswold, M.A.; Clampitt, M.; et al. Results of the NeuroBlate System First-in-Humans Phase I Clinical Trial for Recurrent Glioblastoma: Clinical Article. J. Neurosurg. 2013, 118, 1202–1219. [Google Scholar] [CrossRef]
- Wilson, H.; Chen, C. INNV-29. The Clearpoint Prism® Laser Ablation System: A New Platform for Laser Interstitial Thermal Therapy (LITT) in Neuro-Oncology. Neuro-Oncology 2023, 25 (Suppl. S5), v162–v163. [Google Scholar] [CrossRef]

| Study | Tumor Grade | Patients (N) | Mean Age (Years) | Males (%) | Mean KPS Pre-Op | Mean Tumor Volume (cc) |
|---|---|---|---|---|---|---|
| Astrocytoma | ||||||
| Borghei-Razavi 2018 [52] | 1 | 2 | 37 | 50 | - | 3 |
| Johnson 2022 [53] * | 3 | 2 | 37 | 100 | 90 | - |
| Johnson 2022 [53] * | 2 | 3 | 34 | 33 | 80 | - |
| Kahn 1994 [54] | 2 | 6 | 51 | - | - | - |
| Kunesch 2003 [55] | 2 | 5 | 35 | 60 | - | - |
| Murayi 2020 [56] † | 3 | 2 | 39 | 50 | 75 | 24 |
| Glioblastoma | ||||||
| Beaumont 2018 [57] | 4 | 9 | 55 | 78 | 80 | 23 |
| Dabecco 2021 [58] | 4 | 4 | 50 | - | - | - |
| Daggubati 2023 [59] | 4 | 9 | - | - | - | - |
| deGroot 2022 [16] | 4 | 29 | 63 | 69 | - | - |
| Di 2021 [60] | 4 | 20 | 63 | 60 | 85 | 41 |
| Hajtovic 2020 [61] | 4 | 2 | 67 | 100 | - | 11 |
| Hawasli 2013 [62] | 4 | 6 | 55 | 67 | - | 17 |
| Jamshidi 2020 [63] | 4 | 3 | 64 | 67 | 83 | 15 |
| Kamath 2019 [46] | 4 | 23 | - | - | - | - |
| Maraka 2018 [64] | 4 | 4 | - | - | - | - |
| Merenzon 2023 [65] | 4 | 5 | 66 | - | 78 | - |
| Mohammadi 2019 [66] | 4 | 24 | 54 | 50 | - | 9 |
| Muir 2022 [67] | 4 | 20 | - | 60 | 84 | 15 |
| Murayi 2020 [56] † | 4 | 9 | 55 | 56 | 77 | 11 |
| Thomas 2016 [68] | 4 | 8 | 61 | - | - | 22 |
| Viozzi 2023 [69] | 4 | 10 | 63 | 30 | 77 | 16 |
| Study ID | Follow-Up (Months) | Mean EOA (%) | Mean OS (Months) | Mean PFS (Months) | Mean KPS Post-Op |
|---|---|---|---|---|---|
| Astrocytoma | |||||
| Borghei-Razavi 2018 [52] | 14 | - | - | - | - |
| Johnson 2022 [53] * | 7 | 88 | - | - | - |
| Johnson 2022 [53] * | 36 | 82 | - | - | - |
| Kahn 1994 [54] | 17 | - | - | - | - |
| Kunesch 2003 [55] | 18 | - | - | - | - |
| Murayi 2020 [56] † | - | - | - | - | - |
| Glioblastoma | |||||
| Beaumont 2018 [57] | - | - | 10 | 4 | 66 |
| Dabecco 2021 [58] | - | 98 | - | - | - |
| Daggubati 2023 [59] | - | - | - | - | - |
| deGroot 2022 [16] | - | - | 10 | 6 | 93 |
| Di 2021 [60] | 10 | 88 | - | 8 | - |
| Hajtovic 2020 [61] | - | - | - | - | - |
| Hawasli 2013 [62] | 5 | - | - | - | - |
| Jamshidi 2020 [63] | 6 | 99 | - | 4 | 83 |
| Kamath 2019 [46] | 11 | - | 11 | 6 | - |
| Maraka 2018 [64] | - | - | - | - | - |
| Merenzon 2023 [65] | 31 | - | 9 | - | - |
| Mohammadi 2019 [66] | 14 | - | - | - | - |
| Muir 2022 [67] | 18 | - | - | - | - |
| Murayi 2020 [56] † | - | - | 7 | - | - |
| Thomas 2016 [68] | - | - | 8 | 2 | - |
| Viozzi 2023 [69] | 3 | - | - | - | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.; Chandla, A.; Mekonnen, M.; Hovis, G.E.A.; Teton, Z.E.; Patel, K.S.; Everson, R.G.; Wadehra, M.; Yang, I. Safety and Efficacy of Laser Interstitial Thermal Therapy as Upfront Therapy in Primary Glioblastoma and IDH-Mutant Astrocytoma: A Meta-Analysis. Cancers 2024, 16, 2131. https://doi.org/10.3390/cancers16112131
Pandey A, Chandla A, Mekonnen M, Hovis GEA, Teton ZE, Patel KS, Everson RG, Wadehra M, Yang I. Safety and Efficacy of Laser Interstitial Thermal Therapy as Upfront Therapy in Primary Glioblastoma and IDH-Mutant Astrocytoma: A Meta-Analysis. Cancers. 2024; 16(11):2131. https://doi.org/10.3390/cancers16112131
Chicago/Turabian StylePandey, Aryan, Anubhav Chandla, Mahlet Mekonnen, Gabrielle E. A. Hovis, Zoe E. Teton, Kunal S. Patel, Richard G. Everson, Madhuri Wadehra, and Isaac Yang. 2024. "Safety and Efficacy of Laser Interstitial Thermal Therapy as Upfront Therapy in Primary Glioblastoma and IDH-Mutant Astrocytoma: A Meta-Analysis" Cancers 16, no. 11: 2131. https://doi.org/10.3390/cancers16112131
APA StylePandey, A., Chandla, A., Mekonnen, M., Hovis, G. E. A., Teton, Z. E., Patel, K. S., Everson, R. G., Wadehra, M., & Yang, I. (2024). Safety and Efficacy of Laser Interstitial Thermal Therapy as Upfront Therapy in Primary Glioblastoma and IDH-Mutant Astrocytoma: A Meta-Analysis. Cancers, 16(11), 2131. https://doi.org/10.3390/cancers16112131






