Personalized Medicine in Pancreatic Cancer: The Promise of Biomarkers and Molecular Targeting with Dr. Michael J. Pishvaian
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Pancreatic Cancer Epidemiology
1.2. Current Standards of Care
1.3. Biomarkers for Pancreatic Cancer
1.4. Cancer Survival Rates
2. Present and Future of Precision Medicine in Pancreatic Cancer
2.1. Molecular Classification and Precision Medicine
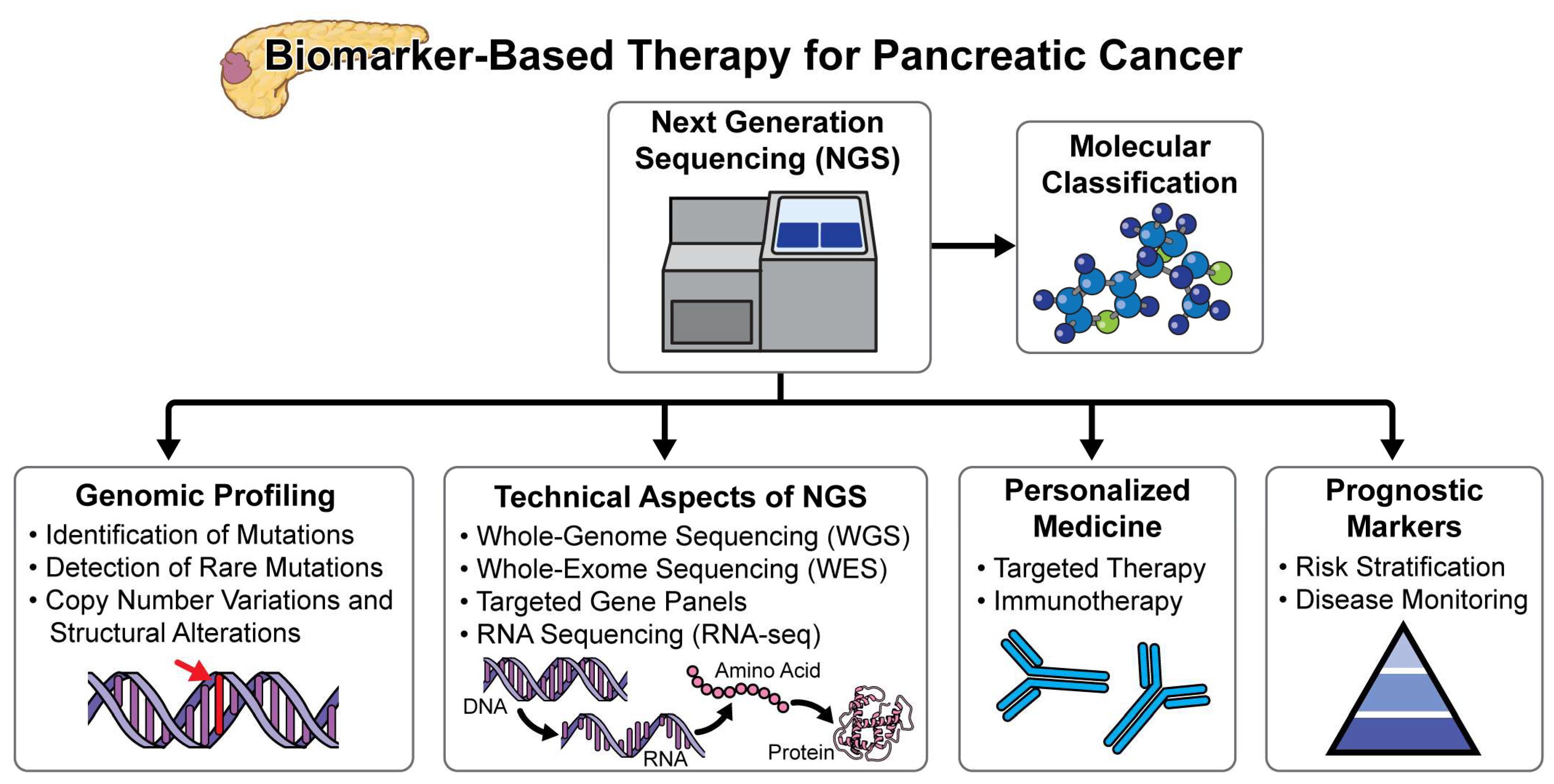
2.2. Next-Generation Sequencing Efforts in Pancreatic Cancer
2.3. Remarkaable Benefit
- Improved Survival with Surgery: Patients with pancreatic cancer who are eligible for surgery to remove the tumor can expect a significantly longer survival. Experts consider surgical resection as potentially curative, particularly when the tumor remains localized and has not spread beyond the pancreas [46]. According to recent studies, the median survival for patients undergoing successful surgical resection can be extended to approximately 20–23 months compared to a median of 6–11 months with non-surgical management. Moreover, advancements in surgical techniques and perioperative care have further improved outcomes, reducing postoperative complications and enhancing recovery rates [47].
- Tailoring Therapies: While the majority of pancreatic cancer patients do not have germline mutations, using all available tools, including genetic testing, can help select therapies. Personalized treatment may not achieve a cure but can improve quality of life and extend survival [48,49]. For example, patients with BRCA mutations may benefit from PARP inhibitors, which have shown promise in prolonging progression-free survival. Additionally, tailored chemotherapeutic regimens based on genetic profiling can lead to better management outcomes. The implementation of molecular profiling in routine clinical practice allows for more precise targeting of therapies, potentially leading to better responses and fewer side effects compared to conventional treatments. The implementation of molecular profiling in routine clinical practice allows for more precise targeting of therapies, potentially leading to better responses and fewer side effects compared to conventional treatments. Precision medicine approaches, guided by biomarker or genetic testing, have demonstrated significant benefits in patient outcomes. Specifically, therapies matched to biomarkers or genetic profiles have been associated with extended survival rates (Pancreatic Cancer Action Network [PanCAN], 2024). PanCAN advocates for the adoption of genetic testing for inherited mutations at the time of diagnosis and recommends biomarker testing of tumor tissue for all patients, unless medically contraindicated. They provide comprehensive resources, including the Know Your Tumor® precision medicine service, to facilitate informed treatment decisions and enhance patient care. Next-generation sequencing (NGS) is now widely utilized to detect diagnostic, prognostic, and predictive mutations across various cancers, contributing significantly to enhanced treatment efficacy [45,50,51]. In pancreatic cancer (PC), genomic profiling data have demonstrated potential benefits in guiding treatment decisions and improving patient survival [52,53]. Precision medicine, which tailors therapies based on molecular profiling of gene expressions and mutations, is anticipated to play a crucial role, especially in cases of unresectable pancreatic cancer. These mutations often involve genes such as KRAS, TP53, CDKN2A, and SMAD4, among others, which are known to play critical roles in pancreatic cancer pathogenesis and progression. The identification of these mutations through NGS holds promise for personalized treatment approaches, including targeted therapies and enrollment in clinical trials aimed at exploiting specific molecular vulnerabilities in pancreatic cancer cells. Despite challenges such as tumor heterogeneity and the complex genomic landscape of pancreatic cancer, NGS continues to emerge as a valuable tool in oncology.
- Challenges: Regrettably, only approximately 10% of patients with pancreatic cancer receive an early diagnosis, thereby qualifying them for potentially curative surgical resection [50]. Most cases receive a diagnosis at a more advanced stage, which restricts the available treatment options. This highlights the need for improved early detection methods and public awareness to increase the rate of early diagnosis and intervention. Furthermore, the development of more effective systemic therapies for advanced-stage pancreatic cancer remains a critical area of ongoing research. Recent efforts in biomarker discovery and the utilization of liquid biopsies are promising steps toward earlier detection and better monitoring of treatment response.
2.4. Future Perspectives in Precision Medicine
3. Advancing Pancreatic Cancer Treatment: The Power of Targeted Therapies and Genetic Insights
4. Emerging Therapeutic Targets in Metastatic Pancreatic Cancer: MDM2, CLAUDIN 18.2, and MTAP Deletion
5. Ongoing Advancements in Pancreatic Cancer Management through Biomarker-Based Clinical Trials and Precision Medicine
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pancreatic Cancer—Symptoms and Causes—Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421 (accessed on 3 May 2024).
- A-Kader, H.H.; Ghishan, F.K. The Pancreas. In Textbook of Clinical Pediatrics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1925–1936. [Google Scholar] [CrossRef]
- Pancreatic Cancer Types. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/pancreatic-cancer/pancreatic-cancer-types (accessed on 3 May 2024).
- Rossiaky, D. What Are the Different Types of Pancreatic Cancer? Available online: https://www.healthline.com/health/pancreatic-cancer/types-of-pancreatic-cancer (accessed on 3 May 2024).
- Magi, L.; Marasco, M.; Rinzivillo, M.; Faggiano, A.; Panzuto, F. Management of Functional Pancreatic Neuroendocrine Neoplasms. Curr. Treat. Options Oncol. 2023, 24, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Symptoms of Pancreatic Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/pancreatic-cancer/symptoms (accessed on 3 May 2024).
- Khalaf, N.; El-Serag, H.B.; Abrams, H.R.; Thrift, A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2021, 19, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics for Pancreatic Cancer. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/about/key-statistics.html#:~:text=About%2066%2C440%20people%20%2834%2C530%20men%20and%2031%2C910%20women%29,men%20and%20about%201%20in%2060%20in%20women (accessed on 3 May 2024).
- Ecancer Pancreatic Cancer Projected to Become Second Leading Cause of Cancer-Related Death in the United States by 2030. Available online: https://ecancer.org/en/news/5660-pancreatic-cancer-projected-to-become-second-leading-cause-of-cancer-related-death-in-the-united-states-by-2030#:~:text=An%20analysis%20projects%20pancreatic%20and%20liver%20cancers%20to,journal%20of%20the%20American%20Association%20for%20Cancer%20Research (accessed on 3 May 2024).
- Chun, J.W.; Lee, S.H.; Kim, J.S.; Park, N.; Huh, G.; Cho, I.R.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T. Comparison between FOLFIRINOX and Gemcitabine plus Nab-Paclitaxel Including Sequential Treatment for Metastatic Pancreatic Cancer: A Propensity Score Matching Approach. BMC Cancer 2021, 21, 537. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Melisi, D.; Macarulla, T.; Cid, R.P.; Chandana, S.R.; De La Fouchardière, C.; Dean, A.; Kiss, I.; Lee, W.J.; Goetze, T.O.; et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): A randomised, open-label, phase 3 trial. Lancet 2023, 402, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Herbst, B.; Zheng, L. Precision Medicine in Pancreatic Cancer: Treating Every Patient as an Exception. Lancet Gastroenterol. Hepatol. 2019, 4, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Perkhofer, L.; Golan, T.; Cuyle, P.-J.; Matysiak-Budnik, T.; Van Laethem, J.-L.; Macarulla, T.; Cauchin, E.; Kleger, A.; Beutel, A.K.; Gout, J.; et al. Targeting DNA Damage Repair Mechanisms in Pancreas Cancer. Cancers 2021, 13, 4259. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shin, K.; Kim, I.-H.; Hong, T.; Kim, Y.; Suh, J.; Lee, M. Clinicopathological Features and Prognosis of Resected Pancreatic Ductal Adenocarcinoma Patients with Claudin-18 Overexpression. J. Clin. Med. 2023, 12, 5394. [Google Scholar] [CrossRef]
- Oliner, J.D.; Saiki, A.Y.; Caenepeel, S. The Role of MDM2 Amplification and Overexpression in Tumorigenesis. Cold Spring Harb. Perspect. Med. 2016, 6, a026336. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.C.; Grimmond, S.M.; McArthur, G.A.; Sheppard, K.E. PRMT5: An Emerging Target for Pancreatic Adenocarcinoma. Cancers 2021, 13, 5136. [Google Scholar] [CrossRef]
- Ansari, D.; Andersson, R. Biomarkers in Pancreatic Cancer. In Textbook of Pancreatic Cancer; Springer: Cham, Switzerland, 2021; pp. 467–487. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Xu, W.; Ji, S.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Diagnostic and Prognostic Value of Carcinoembryonic Antigen in Pancreatic Cancer: A Systematic Review and Meta-Analysis. OncoTargets Ther. 2017, 10, 4591–4598. [Google Scholar] [CrossRef]
- Seladi-Schulman, J. Pancreatic Cancer (Tumor) Markers: Uses and Accuracy. Available online: https://www.healthline.com/health/pancreatic-cancer/pancreatic-cancer-markers (accessed on 3 May 2024).
- Khomiak, A.; Brunner, M.; Kordes, M.; Lindblad, S.; Miksch, R.C.; Öhlund, D.; Regel, I. Recent Discoveries of Diagnostic, Prognostic and Predictive Biomarkers for Pancreatic Cancer. Cancers 2020, 12, 3234. [Google Scholar] [CrossRef] [PubMed]
- Madadjim, R.; An, T.; Cui, J. MicroRNAs in Pancreatic Cancer: Advances in Biomarker Discovery and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 3914. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, C.-Y. Diagnostic Biomarkers for Pancreatic Cancer: An Update. World J. Gastroenterol. 2021, 27, 7862–7865. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ou, S.; Zhang, H.; Huang, R.; Yu, S.; Zhao, M.; Tai, S. Advances in Biomarkers and Techniques for Pancreatic Cancer Diagnosis. Cancer Cell Int. 2022, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Survival Rates for Pancreatic Cancer. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 3 May 2024).
- Pishvaian, M. Updates in Biomarker-Based Therapy for Pancreatic Cancer. Presented at the MedNews Week, Online. 27 February 2023. [Google Scholar]
- Seppälä, T.T.; Zimmerman, J.W.; Suri, R.; Zlomke, H.; Ivey, G.D.; Szabolcs, A.; Shubert, C.R.; Cameron, J.L.; Burns, W.R.; Lafaro, K.J.; et al. Precision Medicine in Pancreatic Cancer: Patient-Derived Organoid Pharmacotyping Is a Predictive Biomarker of Clinical Treatment Response. Clin. Cancer Res. 2022, 28, 3296–3307. [Google Scholar] [CrossRef] [PubMed]
- George, B. Precision Medicine and Pancreatic Cancer. Surg. Oncol. Clin. N. Am. 2021, 30, 693–708. [Google Scholar] [CrossRef]
- Victor Navigating the Future of Precision Medicine: The Role of Genetic Testing. Available online: https://www.novo-dx.com/post/navigating-the-future-of-precision-medicine-the-role-of-genetic-testing#:~:text=Genetic%20testing%20lies%20at%20the%20core%20of%20precision,disease%20risk%20factors%2C%20and%20potential%20responses%20to%20treatment (accessed on 3 May 2024).
- Zhang, Q.; Fu, Q.; Bai, X.; Liang, T. Molecular Profiling–Based Precision Medicine in Cancer: A Review of Current Evidence and Challenges. Front. Oncol. 2020, 10, 532403. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.-H.; Ludwig, W. Molecular Taxonomy: Classification and Identification. In Bacterial Diversity and Systematics; Springer: Boston, MA, USA, 1994; pp. 1–15. [Google Scholar] [CrossRef]
- Libretexts 10.3: Classification and Detection of Molecular Markers. Available online: https://bio.libretexts.org/Bookshelves/Genetics/Online_Open_Genetics_%28Nickle_and_Barrette-Ng%29/10%3A__Molecular_Markers_and_Quantitative_Traits/10.03%3A_Classification_and_Detection_of_Molecular_Markers (accessed on 3 May 2024).
- Precision Medicine. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine (accessed on 3 May 2024).
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision Medicine: Concept and Tools. Med. J. Armed Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Brody, J.R. Molecular Profiling and Precision Medicine for Pancreatic Cancer. In Pancreatic Cancer: A Multidisciplinary Approach; Springer: Cham, Switzerland, 2022; pp. 255–267. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Nakagawa, H.; Koike, K. Molecular and Phenotypic Profiling for Precision Medicine in Pancreatic Cancer: Current Advances and Future Perspectives. Front. Oncol. 2021, 11, 682872. [Google Scholar] [CrossRef]
- Shen, G.-Q.; Aleassa, E.M.; Walsh, R.M.; Morris-Stiff, G. Next-Generation Sequencing in Pancreatic Cancer. Pancreas 2019, 48, 739–748. [Google Scholar] [CrossRef]
- Jung, K.; Lee, S.; Na, H.Y.; Kim, J.-W.; Lee, J.-C.; Hwang, J.-H.; Kim, J.W.; Kim, J. NGS-Based Targeted Gene Mutational Profiles in Korean Patients with Pancreatic Cancer. Sci. Rep. 2022, 12, 20937. [Google Scholar] [CrossRef] [PubMed]
- Stover, E.H.; Konstantinopoulos, P.A.; Matulonis, U.A.; Swisher, E.M. Biomarkers of Response and Resistance to DNA Repair Targeted Therapies. Clin. Cancer Res. 2016, 22, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Rahnamay Farnood, P.; Danesh Pazhooh, R.; Asemi, Z.; Yousefi, B. DNA Damage Response and Repair in Pancreatic Cancer Development and Therapy. DNA Repair 2021, 103, 103116. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, M.; Lampis, A.; Mirchev, M.B.; Okuducu, A.F.; Ratti, M.; Valeri, N.; Hahne, J.C. Immune-Based Therapies and the Role of Microsatellite Instability in Pancreatic Cancer. Genes 2020, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Sudhesh Dev, S.; Zainal Abidin, S.A.; Farghadani, R.; Othman, I.; Naidu, R. Receptor Tyrosine Kinases and Their Signaling Pathways as Therapeutic Targets of Curcumin in Cancer. Front. Pharmacol. 2021, 12, 772510. [Google Scholar] [CrossRef] [PubMed]
- Daoud, A.Z.; Mulholland, E.J.; Cole, G.; McCarthy, H.O. MicroRNAs in Pancreatic Cancer: Biomarkers, Prognostic, and Therapeutic Modulators. BMC Cancer 2019, 19, 1130. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Heider, D.; Hauschild, A.-C. Integrative Analysis of Next-Generation Sequencing for Next-Generation Cancer Research toward Artificial Intelligence. Cancers 2021, 13, 3148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Abboud, Y.; Liang, J.; Larson, B.; Osipov, A.; Gong, J.; Hendifar, A.E.; Atkins, K.; Liu, Q.; Nissen, N.N.; et al. The Disproportionate Rise in Pancreatic Cancer in Younger Women Is Due to a Rise in Adenocarcinoma and Not Neuroendocrine Tumors: A Nationwide Time-Trend Analysis Using 2001–2018 United States Cancer Statistics Databases. Cancers 2024, 16, 971. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.I.; Shia, J.; Stadler, Z.K.; Varghese, A.M.; Capanu, M.; Salo-Mullen, E.; Lowery, M.A.; Diaz, L.A.; Mandelker, D.; Yu, K.H.; et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin. Cancer Res. 2018, 24, 1326–1336. [Google Scholar] [CrossRef]
- Corey, C. Long-Term Pancreatic Cancer Survivors Report Excellent Post-Surgery Quality of Life. Available online: https://newsnetwork.mayoclinic.org/discussion/long-term-pancreatic-cancer-survivors-report-excellent-post-surgery-quality-of-life/ (accessed on 3 May 2024).
- Pancreatic Cancer Survival Rate—Pancreatic Cancer Action Network. Available online: https://pancan.org/facing-pancreatic-cancer/about-pancreatic-cancer/survival-rate/ (accessed on 3 May 2024).
- Ferrara, N. People with Pancreatic Cancer Are Living Longer, Thanks to Improved Approaches—Mayo Clinic Comprehensive Cancer Center Blog. Available online: https://cancerblog.mayoclinic.org/2022/11/15/people-with-pancreatic-cancer-are-living-longer-thanks-to-improved-approaches/ (accessed on 3 May 2024).
- Lee, M.S.; Pant, S. Targeted Therapies for Pancreatic Cancer. In Pancreatic Cancer; Springer: Cham, Switzerland, 2023; pp. 67–95. [Google Scholar] [CrossRef]
- Gheorghe, G.; Bungau, S.; Ilie, M.; Behl, T.; Vesa, C.M.; Brisc, C.; Bacalbasa, N.; Turi, V.; Costache, R.S.; Diaconu, C.C. Early Diagnosis of Pancreatic Cancer: The Key for Survival. Diagnostics 2020, 10, 869. [Google Scholar] [CrossRef]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.N.; Goodwin, R.A.; Vickers, M.M. BRCA mutated pancreatic cancer: A change is coming. World J. Gastroenterol. 2021, 27, 1943–1958. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.K. Numerous Clinical Trials Investigate Next Generation of PARP Inhibitors. Available online: https://www.pharmacytimes.com/view/numerous-clinical-trials-investigate-next-generation-of-parp-inhibitors (accessed on 5 May 2024).
- Taylor, A.K.; Kosoff, D.; Emamekhoo, H.; Lang, J.M.; Kyriakopoulos, C.E. PARP inhibitors in metastatic prostate cancer. Front. Oncol. 2023, 13, 1159557. [Google Scholar] [CrossRef] [PubMed]
- Slootbeek, P.H.J.; Kloots, I.S.H.; van Oort, I.M.; Kroeze, L.I.; Schalken, J.A.; Bloemendal, H.J.; Mehra, N. Cross-Resistance between Platinum-Based Chemotherapy and PARP Inhibitors in Castration-Resistant Prostate Cancer. Cancers 2023, 15, 2814. [Google Scholar] [CrossRef] [PubMed]
- Iannantuono, G.M.; Chandran, E.; Floudas, C.S.; Choo-Wosoba, H.; Butera, G.; Roselli, M.; Gulley, J.L.; Karzai, F. Efficacy and safety of PARP inhibitors in metastatic castration-resistant prostate cancer: A systematic review and meta-analysis of clinical trials. Cancer Treat Rev. 2023, 120, 102623. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Philip, P.A.; Almhanna, K.; Beck, F.W.; Sarkar, F.H.; Mohammad, R.M. MDM2 Inhibitors for Pancreatic Cancer Therapy. Mini-Rev. Med. Chem, 2010; 10, 518–526. [Google Scholar] [CrossRef]
- Azmi, A.S.; Philip, P.A.; Aboukameel, A.; Wang, Z.; Banerjee, S.; Zafar, S.F.; Goustin, A.S.; Almhanna, K.; Yang, D.; Sarkar, F.H.; et al. Reactivation of p53 by novel MDM2 inhibitors: Implications for pancreatic cancer therapy. Curr. Cancer Drug Targets. 2010, 10, 319–331. [Google Scholar] [CrossRef]
- Traweek, R.S.; Cope, B.M.; Roland, C.L.; Keung, E.Z.; Nassif, E.F.; Erstad, D.J. Targeting the MDM2-p53 pathway in dedifferentiated liposarcoma. Front. Oncol. 2022, 12, 1006959. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Lordick, F.; Cutsem, E.V.; Plazas, J.G.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Wang, W.; Albadari, N.; Du, Y.; Fowler, J.F.; Sang, H.T.; Xian, W.; McKeon, F.; Li, W.; Zhou, J.; Zhang, R. MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future. Pharmacol. Rev. 2024, 76, 414–453. [Google Scholar] [CrossRef]
- Hou, H.; Sun, D.; Zhang, X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jia, C.; Ou, Y.; Zeng, C.; Jia, Y. Dark horse target Claudin18.2 opens new battlefield for pancreatic cancer. Front. Oncol. 2024, 14, 1371421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Flodby, P.; Luo, J.; Castillo, D.R.; Liu, Y.; Yu, F.X.; Mcconnell, A.; Varghese, B.; Li, G.; Chimge, N.-O.; et al. Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. J. Clin. Investig. 2018, 128, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Shimobaba, S.; Taga, S.; Akizuki, R.; Hichino, A.; Endo, S.; Matsunaga, T.; Watanabe, R.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; et al. Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of PDK1 and Akt in human lung adenocarcinoma A549 cells. Biochim. Biophys. Acta 2016, 1863, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chimge, N.O.; Zhou, B.; Flodby, P.; Castaldi, A.; Firth, A.L.; Liu, Y.; Wang, H.; Yang, C.; Marconett, C.N.; et al. CLDN18.1 attenuates Malignancy and related signaling pathways of lung adenocarcinoma in vivo and in vitro. Int. J. Cancer 2018, 143, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Context-Dependent Roles of Claudins in Tumorigenesis. Front. Oncol. 2021, 11, 676781. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Qin, Y.; Ji, S.; Shi, X.; Dai, W.; Fan, G.; Li, S.; Xu, W.; Liu, W.; Liu, M.; et al. MTAP Deficiency–Induced Metabolic Reprogramming Creates a Vulnerability to Cotargeting De Novo Purine Synthesis and Glycolysis in Pancreatic Cancer. Metab. Chem. Biol. 2021, 81, 4964–4980. [Google Scholar] [CrossRef] [PubMed]
- Bray, C.; Balcells, C.; McNeish, I.A.; Keun, H.C. The potential and challenges of targeting MTAP-negative cancers beyond synthetic lethality. Front. Oncol. 2023, 13, 1264785. [Google Scholar] [CrossRef] [PubMed]
- Casolino, R.; Braconi, C.; Malleo, G.; Paiella, S.; Bassi, C.; Milella, M.; Dreyer, S.B.; Froeling, F.E.M.; Chang, D.K.; Biankin, A.V.; et al. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine. Ann. Oncol. 2021, 32, 183–196. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Stephenson, J.J., Jr.; Rosen, P.; Loesch, D.M.; Borad, M.J.; Anthony, S.; Jameson, G.; Brown, S.; Cantafio, N.; Richards, D.A.; et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 2010, 28, 4877–4883. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. NCI-MATCH Trial. Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match (accessed on 6 May 2024).
- American Society of Clinical Oncology. TAPUR Study. Available online: https://www.tapur.org (accessed on 6 May 2024).
- Pancreatic Cancer Canada. COMPASS Trial. Available online: https://pancreaticcancercanada.ca/research/clinical-trials/compass-trial/ (accessed on 6 May 2024).
- IMPaCT Trial. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03337087 (accessed on 6 May 2024).
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Giacomo, A.M.D.; Jesus-Acosta, A.D.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Rybkin, I.I.; Spira, A.I.; Riley, G.J.; Papadopoulos, K.P.; Sabari, J.K.; Johnson, M.L.; Heist, R.S.; Bazhenova, L.; Barve, M.; et al. KRYSTAL-1: Adagrasib (MRTX849) in non-small cell lung cancer harboring a KRAS G12C mutation. Eur. J. Cancer 2020, 138, S1–S2. [Google Scholar] [CrossRef]
- Schram, A.M.; Odintsov, I.; Espinosa-Cotton, M.; Khodos, I.; Sisso, W.J.; Mattar, M.S.; Lui, A.J.W.; Vojnic, M.; Shameem, S.H.; Chauhan, T.; et al. Zenocutuzumab in ERBB2-amplified and NRG1-rearranged cancers. Cancer Discov. 2022, 12, 1233–1247. [Google Scholar] [CrossRef]
- Sacher, A.; LoRusso, P.; Patel, M.R.; Miller, W.H., Jr.; Garralda, E.; Forster, M.D.; Santoro, A.; Falcon, A.; Kim, T.W.; Paz-Ares, L.; et al. Single-Agent Divarasib (GDC-6036) in Solid Tumors with a KRAS G12C Mutation. N. Engl. J. Med. 2023, 389, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, L.; Gu, Y.; Calles, A.; Wu, L.; Ba, Y.; Li, Z.H.; Bai, C.; Yao, Y.; Hubert, A.; et al. Preliminary activity and safety results of KRAS G12C inhibitor glecirasib (JAB-21822) in patients with pancreatic cancer and other solid tumors. J. Clin. Oncol. 2024, 42, 604. [Google Scholar] [CrossRef]
- National Cancer Institute. NCI-Supported Research Programs. Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/pancreatic-cancer (accessed on 8 May 2024).
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of precision medicine in diverse cancers: A meta-analysis of phase II clinical trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Farris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klomp, J.E.; Diehl, J.N.; Klomp, J.A.; Edwards, A.C.; Yang, R.; Morales, A.J.; Taylor, K.E.; Drizyte-Miller, K.; Bryant, K.L.; Schaefer, A.; et al. Determining the ERK-regulated phosphoproteome driving KRAS-mutant cancer. Science 2024, 384, eadk0850. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Lyons, E.; DeArbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sahai, V.; Sohal, D.P.S.; et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020, 21, 508–518. [Google Scholar] [CrossRef]
- Mahadevan, K.K.; LeBleu, V.S.; Ramirez, E.V.; Chen, Y.; Li, B.; Sockwell, A.M.; Gagea, M.; Sugimoto, H.; Sthanam, L.K.; Tampe, D.; et al. Elimination of oncogenic KRAS in genetic mouse models eradicates pancreatic cancer by inducing FAS-dependent apoptosis by CD8+ T cells. Dev. Cell. 2023, 58, 1562–1577.e8. [Google Scholar] [CrossRef]
- Mahadevan, K.K.; McAndrews, K.M.; LeBleu, V.S.; Yang, S.; Lyu, H.; Li, B.; Sockwell, A.M.; Kirtley, M.L.; Morse, S.J.; Moreno Diaz, B.A.; et al. KRASG12D inhibition reprograms the microenvironment of early and advanced pancreatic cancer to promote FAS-mediated killing by CD8+ T cells. Cancer Cell 2023, 41, 1606–1620. [Google Scholar] [CrossRef]
- Brozos-Vázquez, E.; Toledano-Fonseca, M.; Costa-Fraga, N.; Garcia-Ortiz, M.V.; Diaz-Lagares, A.; Rodriguez-Ariza, A.; Aranda, E.; Lopez-Lopez, R. Pancreatic cancer biomarkers: A pathway to advance in personalized treatment selection. Cancer Treat. Rev. 2024, 125, 102719. [Google Scholar] [CrossRef]
- Lawlor, R.T.; Mattiolo, P.; Mafficini, A.; Hong, S.-M.; Piredda, M.L.; Taormina, S.V.; Malleo, G.; Marchegiani, G.; Pea, A.; Salvia, R.; et al. Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Pancreatic Cancer: Systematic Review and Still-Open Questions. Cancers 2021, 13, 3119. [Google Scholar] [CrossRef]
- Koay, E.J.; Zaid, M.; Aliru, M.; Bagereka, P.; Wieren, A.V.; Rodriguez, M.J.; Jacobson, G.; Wolff, R.A.; Overman, M.; Varadhachary, G.; et al. Nab-Paclitaxel, Capecitabine, and Radiation Therapy After Induction Chemotherapy in Treating Patients With Locally Advanced and Borderline Resectable Pancreatic Cancer: Phase 1 Trial and Imaging-based Biomarker Validation. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 444–453. [Google Scholar] [CrossRef]
- Pant, S.; Martin, L.K.; Geyer, S.; Wei, L.; Loon, K.V.; Sommovilla, N.; Zalupski, M.; Iyer, R.; Fogelman, D.; Ko, A.H.; et al. Baseline serum albumin is a predictive biomarker for patients with advanced pancreatic cancer treated with bevacizumab: A pooled analysis of 7 prospective trials of gemcitabine-based therapy with or without bevacizumab. Cancer 2014, 120, 1780–1786. [Google Scholar] [CrossRef]
- Abe, T.; Koi, C.; Kohi, S.; Song, K.-B.; Tamura, K.; Macgregor-Das, A.; Kitaoka, N.; Chuidian, M.; Ford, M.; Dbouk, M.; et al. Gene Variants That Affect Levels of Circulating Tumor Markers Increase Identification of Patients With Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 1161–1169. [Google Scholar] [CrossRef]
- Agnihotri, N.; Ambavane, A.; Fan, L.; Li, W.; Yoo, H.; Joo, S.; Muston, D. Modeling health outcomes associated with BRCA testing and treatment strategies for patients with metastatic pancreatic cancer. Pancreatology 2024, 24, 271–278. [Google Scholar] [CrossRef]
- Mukherji, R.; Debnath, D.; Hartley, M.L.; Noel, M.S. The Role of Immunotherapy in Pancreatic Cancer. Curr. Oncol. 2022, 29, 6864–6892. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jung, Y.-J.; Moon, S.-H. Immunotherapy for Pancreatic Cancer. World J. Clin. Cases 2021, 9, 2969–2982. [Google Scholar] [CrossRef]
- Puccini, A.; Battaglin, F.; Iaia, M.L.; Lenz, H.-J.; Salem, M.E. Overcoming Resistance to Anti-PD1 and Anti-PD-L1 Treatment in Gastrointestinal Malignancies. J. Immunother. Cancer 2020, 8, e000404. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Xue, B.; Luo, X.; Liu, Y.; Ye, S.; Zhou, L.; Li, S.; Li, P.; Liang, A. CAR T-Cell Therapy Combined with PD-1 Inhibitors Significantly Improve the Efficacy and Prognosis of r/r DLBCL with TP53 Alterations. Blood 2023, 142, 3515. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination Strategies with PD-1/PD-L1 Blockade: Current Advances and Future Directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Farhangnia, P.; Khorramdelazad, H.; Nickho, H.; Delbandi, A.-A. Current and Future Immunotherapeutic Approaches in Pancreatic Cancer Treatment. J. Hematol. Oncol. 2024, 17, 40. [Google Scholar] [CrossRef]
- Maitra, A.; Topol, E.J. Early Detection of Pancreatic Cancer and AI Risk Partitioning. Lancet 2024, 403, 1438. [Google Scholar] [CrossRef]
- Bhat, A.A.; Nisar, S.; Mukherjee, S.; Saha, N.; Yarravarapu, N.; Lone, S.N.; Masoodi, T.; Chauhan, R.; Maacha, S.; Bagga, P.; et al. Integration of CRISPR/Cas9 with Artificial Intelligence for Improved Cancer Therapeutics. J. Transl. Med. 2022, 20, 534. [Google Scholar] [CrossRef]
- Qin, Q.; Yu, R.; Eriksson, J.E.; Tsai, H.-I.; Zhu, H. Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma Therapy: Challenges and Opportunities. Cancer Lett. 2024, 591, 216859. [Google Scholar] [CrossRef]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat. Rev. Drug Discov. 2018, 18, 99–115. [Google Scholar] [CrossRef]
- Maia, A.; Wiemann, S. Cancer-Associated Fibroblasts: Implications for Cancer Therapy. Cancers 2021, 13, 3526. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Qian, H.; Zhuang, Q. Cancer-Associated Fibroblasts: From Basic Science to Anticancer Therapy. Exp. Mol. Med. 2023, 55, 1322–1332. [Google Scholar] [CrossRef]

| Clinical Trial Name | Aim and Description |
|---|---|
| NCI-MATCH (Molecular Analysis for Therapy Choice) [74] | Targets therapy based on tumor mutations rather than their cancer type. |
| TAPUR (Targeted Agent and Profiling Utilization Registry) [75] | Evaluates the safety and efficacy of FDA-approved targeted therapies in patients with advanced cancer harboring specific genetic alterations. |
| COMPASS (Comprehensive Molecular Characterization of Advanced Pancreatic Ductal Adenocarcinoma for Better Treatment Selection) [76] | Uses molecular profiling for advanced pancreatic cancer treatment. |
| IMPaCT (Integrative Molecular Profiling of Pancreatic Cancer Therapy) [77] | Matches patients with therapies based on tumor molecular characteristics. |
| KEYNOTE-158 (Phase II) [78] | Pembrolizumab in MSI-H/dMMR tumors: 18.2% RR, 2.1m PFS, 4 m OS. |
| CODEBREAK-100 (Phase Ib/II) [79] | Sotorasib in KRAS G12C mutations: 21% RR, 4 m PFS, 6.9 m OS. |
| KRYSTAL-1 (Phase II) [80] | Adagrasib in KRAS G12C mutations: 33.3% RR, 5.4 m PFS, 8 m OS. |
| Schram’s 2021 Phase II [81] | Zenocutuzumab in NRG1 fusions: 40% RR. |
| Sacher’s 2023 Phase I [82] | Diravasib in KRAS G12C mutations: 36% RR. |
| Li’s 2024 Phase I/II [83] | Olomorasib in KRAS G12C mutations: 33% RR. |
| Hollebecque’s 2024 Phase I/II [84] | Glecirasib in KRAS G12C mutations: 46.4% RR, 5.5 m PFS.I |
| Pancreatic Cancer Cohort Consortium [76] | Studies cause and natural history of pancreatic cancer (includes PanScan). |
| Pancreatic Cancer Detection Consortium (PCDC) [76] | Develops/tests early detection biomarkers. |
| Pancreatic Ductal Adenocarcinoma (PDAC) Stromal Reprogramming Consortium (PSRC) | Studies tumor microenvironment in PDAC progression and therapy response. |
| Pancreatic Specialized Programs of Research Excellence (Pancreatic SPOREs) | Translates basic research into clinical settings. |
| RAS Initiative [87] | Develops therapies for RAS mutations in pancreatic cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortiana, V.; Abbas, R.H.; Chorya, H.; Gambill, J.; Mahendru, D.; Park, C.H.; Leyfman, Y. Personalized Medicine in Pancreatic Cancer: The Promise of Biomarkers and Molecular Targeting with Dr. Michael J. Pishvaian. Cancers 2024, 16, 2329. https://doi.org/10.3390/cancers16132329
Cortiana V, Abbas RH, Chorya H, Gambill J, Mahendru D, Park CH, Leyfman Y. Personalized Medicine in Pancreatic Cancer: The Promise of Biomarkers and Molecular Targeting with Dr. Michael J. Pishvaian. Cancers. 2024; 16(13):2329. https://doi.org/10.3390/cancers16132329
Chicago/Turabian StyleCortiana, Viviana, Rabab Hunaid Abbas, Harshal Chorya, Jade Gambill, Diksha Mahendru, Chandler H. Park, and Yan Leyfman. 2024. "Personalized Medicine in Pancreatic Cancer: The Promise of Biomarkers and Molecular Targeting with Dr. Michael J. Pishvaian" Cancers 16, no. 13: 2329. https://doi.org/10.3390/cancers16132329
APA StyleCortiana, V., Abbas, R. H., Chorya, H., Gambill, J., Mahendru, D., Park, C. H., & Leyfman, Y. (2024). Personalized Medicine in Pancreatic Cancer: The Promise of Biomarkers and Molecular Targeting with Dr. Michael J. Pishvaian. Cancers, 16(13), 2329. https://doi.org/10.3390/cancers16132329







