Simple Summary
Lung cancer remains a leading cause of cancer-related mortality globally, requiring new diagnostic and therapeutic approaches. This research arises from the need to improve diagnostic accuracy and treatment effectiveness for patients with non-small cell lung cancer. The authors aim to systematically evaluate the potential of emerging biomarkers, including circulating tumor DNA, microRNAs and mutational load of blood tumors and their relationship with different treatments. The findings of this study could have a significant impact on the research community by providing a basis for integrating these biomarkers into clinical practice, thereby improving personalized treatment strategies and patient outcomes in non-small cell lung cancer.
Abstract
Non-small-cell lung cancer (NSCLC) comprises approximately 85% of all lung cancer cases, often diagnosed at advanced stages, which diminishes the effective treatment options and survival rates. This systematic review assesses the utility of emerging biomarkers—circulating tumor DNA (ctDNA), microRNAs (miRNAs), and the blood tumor mutational burden (bTMB)—enhanced by next-generation sequencing (NGS) to improve the diagnostic accuracy, prognostic evaluation, and treatment strategies in NSCLC. Analyzing data from 37 studies involving 10,332 patients from 2020 to 2024, the review highlights how biomarkers like ctDNA and PD-L1 expression critically inform the selection of personalized therapies, particularly beneficial in the advanced stages of NSCLC. These biomarkers are critical for prognostic assessments and in dynamically adapting treatment plans, where high PD-L1 expression and specific genetic mutations (e.g., ALK fusions, EGFR mutations) significantly guide the use of targeted therapies and immunotherapies. The findings recommend integrating these biomarkers into standardized clinical pathways to maximize their potential in enhancing the treatment precision, ultimately fostering significant advancements in oncology and improving patient outcomes and quality of life. This review substantiates the prognostic and predictive value of these biomarkers and emphasizes the need for ongoing innovation in biomarker research.
1. Introduction
Non-small-cell lung cancer (NSCLC) represents approximately 85% of all lung cancer cases and necessitates early detection to improve clinical outcomes [1,2,3]. Traditionally, NSCLC management includes surgery, chemotherapy, and radiation therapy, the effectiveness of which varies significantly based on the stage at diagnosis. Unfortunately, about 60% of patients are diagnosed in advanced stages [4], reducing the likelihood of curative treatment and increasing the mortality rate [5,6].
In 2020, lung cancer accounted for 2.2 million new cases and 1.8 million deaths globally, constituting 11.4% and 18% of all cancer cases and deaths, respectively. Approximately 65.33% of male cases were diagnosed at advanced stages (III or IV), contributing to the high mortality rates, particularly in regions with significant exposure to carcinogens like tobacco smoke in Central Europe and air pollution in China [7,8]. Notably, China experienced about 820,000 new cases and 715,000 deaths alone, indicating a rise in both incidence and mortality since 2015. In contrast, the U.S. reported 57% of cases at the metastatic stage, while the late-stage diagnosis rates were extraordinarily high in Mexico (98–99%), Brazil (70%), and Peru (85.5%), highlighting the need for early detection programs [9]. Colombia, dealing with environmental and occupational exposure, sees 6876 new cases yearly and considers lung cancer as its sixth most prevalent cancer, although recent trends suggest declining rates. Despite the common association with smoking, 12% of patients worldwide have never smoked, underscoring the influence of other risks, such as environmental pollutants [9].
NSCLC is a highly heterogeneous disease, exhibiting significant variability at both the molecular and clinical levels. This heterogeneity, which encompasses genetic mutations, histological subtypes, and tumor microenvironments, critically impacts treatment responses and overall prognosis [10,11]. Adenocarcinomas (ADCs) and squamous cell carcinomas (SCCs) are the predominant histological types of NSCLC, each associated with distinct genetic profiles. ADCs frequently harbor mutations in genes such as KRAS, EGFR, and ALK, while SCCs often exhibit alterations in DDR2, FGFR1, and the PI3K pathway [10,11,12]. The recognition of these genetic diversities has led to the development of targeted therapies, although their effectiveness is often short-lived due to acquired resistance. Additionally, the tumor microenvironment, comprising fibroblasts, immune cells, and extracellular matrix components, further complicates the disease landscape by influencing tumor progression and treatment responses [10,13,14,15]. The effective management of NSCLC requires well-documented diagnostic comparisons to tailor treatment strategies. Incorporating advanced biomarkers into diagnostic protocols, such as circulating tumor DNA (ctDNA), microRNAs (miRNA), and the blood tumor mutational burden (bTMB), is essential in identifying actionable mutations and predicting therapeutic responses [16,17].
ctDNA is crucial in detecting actionable mutations and monitoring tumor progression non-invasively, serving as both a predictive and prognostic biomarker [18,19]. PD-L1 is another relevant biomarker, as its expression in tumor cells can predict the response to immunotherapies, such as checkpoint inhibitors, making it a key predictive biomarker [20]. miRNAs play a vital role in NSCLC by acting as key regulators in cancer progression and as potential biomarkers for prognosis and treatment responses. Some, like miRNA-21, have been shown to be important predictors of the response to immunotherapy [21,22,23]. Finally, bTMB can be an important predictive indicator of the response to immunotherapies. Together, these biomarkers enable the more precise detection of NSCLC and the personalization of treatments, thereby improving the clinical outcomes.
The clinical outcomes for lung cancer are closely related to the stage at which the cancer is diagnosed. For example, patients diagnosed at stage I have a five-year survival rate of 68.4%, while those diagnosed at stage IV have a significantly lower survival rate of just 5.8% [24]. Unfortunately, most lung cancers are diagnosed at stage IV, associated with lower survival rates and a higher symptom burden. Early screening and detection significantly reduce lung cancer mortality [25]. The standard of care for NSCLC patients at stages I and II, and some at stage IIIA, is surgical resection, followed by adjuvant systemic therapy if needed. The introduction of osimertinib, a third-generation oral tyrosine kinase inhibitor, for adjuvant therapy in completely resected NSCLC at stages II and III has shown promising results [24]. Systemic treatment options for metastatic NSCLC include chemotherapy, targeted therapy, and immunotherapy, with specific tests for driver mutations in non-squamous tumors for more effective and less toxic treatment [24]. While the development and integration of osimertinib and other systemic treatments have marked a significant improvement in the care for patients with early and metastatic NSCLC, the role of molecular diagnostics has become increasingly central in the management of this disease [26].
Next-generation sequencing technologies have transformed NSCLC treatment by providing a comprehensive genetic snapshot of individual tumors. This advanced genomic profiling helps to identify actionable mutations and biomarkers, critical in customizing patient care [27,28]. By pinpointing specific genetic alterations, clinicians can select targeted therapies that are more likely to be effective, improving treatment outcomes and reducing unnecessary side effects [29,30].
Identifying specific genetic alterations, such as epidermal growth factor receptor (EGFR) mutations, NGS facilitates the development of targeted treatment strategies [31,32]. These strategies have significantly evolved to enhance the progression-free survival (PFS) and overall survival (OS) rates, particularly for NSCLC patients with EGFR mutations. EGFR mutations, primarily the exon 19 deletions (ex19del) and the L858R point mutation in exon 21, represent 85–90% of all EGFR mutations and are highly sensitive to EGFR tyrosine kinase inhibitors (EGFR-TKIs). However, the T790M mutation in exon 20, less common at diagnosis, is associated with about half of the cases of resistance to first- and second-generation EGFR-TKIs [31,33]. EGFR mutations, which occur in approximately 10% of cases in North America and Western Europe and between 30 and 50% in East Asia, are predictive of a good response to EGFR-TKIs. Clinical trials have demonstrated a 54% improvement in progression-free survival and a 6.8-month absolute benefit in overall survival with this medication [9,34].
Genes such as ALK, ROS1, BRAFV600E, and NTRK have also been identified as critical in developing targeted treatment strategies [35,36]. Immunotherapy with agents such as nivolumab, pembrolizumab, and atezolizumab, which inhibit PD-1 and PD-L1, has been shown to increase survival in NSCLC. However, resistance to these therapies persists, prompting ongoing research to develop combinations of targeted therapies and immunotherapies that address these resistance challenges [37,38].
ROS1 rearrangements, present in approximately 1–2% of NSCLC patients, are sensitive to the ROS1/MET inhibitor crizotinib, which has shown a response rate of 72% [9,39]. Lorlatinib has been effective against acquired resistance to crizotinib in NSCLC positive for ROS1, and entrectinib has been approved for these cases. KRAS mutations, the most common type of driver mutation in lung cancer, often found in individuals with a history of smoking, have recently been targeted by sotorasib, a new agent directed at KRAS G12C mutations, showing a response rate of 32% in clinical trials [9].
These advancements underscore the importance of biomarkers in personalizing NSCLC treatment, offering hope for significant improvements in patient outcomes through more targeted therapies. This review systematically examines recent studies on the identification and application of emerging biomarkers in NSCLC treatment, focusing on their utility in informing therapeutic decisions and improving survival outcomes. By contributing to the existing body of knowledge, this review emphasizes the impact of biomarkers on personalizing oncological treatment and enhancing the clinical outcomes in NSCLC.
2. Materials and Methods
2.1. Study Protocol
This systematic review was conducted in accordance with the guidelines of the Cochrane Collaboration Handbook and reported considering the recommendations for systematic reviews and meta-analyses of the PRISMA statement [40]. The research was formulated considering the PICO strategy (Population, Intervention, Comparison, Outcomes) [1].
2.2. Research Question
In patients with non-small-cell lung cancer (NSCLC) (P), how have emerging biomarkers (I), compared to non-specific or previously established biomarkers (C), impacted the diagnostic accuracy and prognostic value and informed treatment strategies, including predictive monitoring and treatment maintenance strategies, particularly in the context of immunotherapy and palliative care, over the last five years, in terms of reducing symptomatology or improving quality of life (O)?
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
The inclusion criteria were as follows:
- Manuscripts published between 2020 and 2024;
- Studies including predictive biomarkers;
- Studies including diagnostic biomarkers;
- Studies including monitoring biomarkers;
- Clinical follow-up studies with biomarkers for oncological treatments (predictive biomarkers);
- Full-access articles;
- Articles in English;
- Clinical trials.
2.3.2. Exclusion Criteria
- Articles in pre-print mode or letters to the editor;
- Studies with biomarkers not specific to NSCLC;
- Studies published >5 years ago;
- Studies with inconclusive experimental designs;
- Studies of selected population analysis;
- Quality of life studies;
- Studies on treatment maintenance strategies and palliative care management.
2.4. Data Sources and Search Strategy
The search was conducted in the following databases: PubMed, Cochrane Clinical Trials, SCOPUS, ScienceDirect, Biomed Central (BMC), Web of Science, Springer, and the Virtual Health Library (VHL). No language filters were applied, and the date range was set between 2020 and 2024. The search strategy was designed and executed from November 2023 to January 2024 by two researchers independently (D.M. and J.-C.R.L.). Terms (keywords) were combined using the Boolean operators AND and OR (see search details in Appendix A). The references of relevant articles were reviewed, and additional web searches were conducted to identify studies not evident through initial tracking. When necessary to confirm the clinical trial or expand the information of a study, access to ClinicalTrials.gov (https://clinicaltrials.gov/ (accessed on 18 September 2023)) was sought, if applicable. Data were stored using Zotero version 6.0 (accessed on 23 November 2023).
2.5. Selection and Data Extraction
The selection of potentially eligible studies was carried out independently by the two researchers, initially examining the title, abstract, and subsequently the full text. Studies whose relevance was unclear were discussed thoroughly, and the decision to include them in the review was reached by consensus. Two reviewers (J-C.R.L. and Y.L.) extracted information from the primary studies considering the details of the clinical trial (first author, year of publication), country, type of design, biological sample, number of participants, age of participants, sex of participants, biomarker evaluated, method of biomarker analysis, cut-off value, sensitivity and specificity of biomarkers, predictive and prognostic value, diagnostic performance, overall survival (OS) and progression-free survival (PFS), hazard ratios (HR) and 95% confidence intervals (CI) for tumor size, TNM stage, survival rates, treatment, statistical methods used for biomarker evaluation, fold change, p-value, and conclusions. Subsequently, a third and fourth reviewer (A.P. and D.M.G.) verified the integrity and accuracy of the recorded information.
2.6. Risk of Bias Assessment
The risk of bias in the primary studies was assessed by independent reviewers utilizing standardized instruments attuned to the core design elements of clinical trials. Data for this assessment were entered into Review Manager version 5.4® (RevMan, accessed on 23 January 2024), considering the following criteria: generation of random sequences, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, completeness of outcome data, and avoidance of selective reporting. The RCTs were judged to have a low or high risk of bias based on their conformity to pre-established guidelines.
For each domain, the risk was classified as “low”, “high”, or “unclear”, in line with the evaluative scales provided by the Cochrane Risk of Bias tool (accessed on 9 June 2023) for randomized trials and the ROBINS-I tool for non-randomized trials [41,42]. Any discrepancies found during the risk of bias assessment were addressed and resolved through discussions between the reviewers until a consensus was reached.
2.7. Ethical Considerations
During the development of this study, no interventions were made regarding the demographic and physiological variables of the participants. Therefore, this research work was considered of minimal risk according to Resolution No. 8430 of 1993 of the Colombian legislation and the Declaration of Helsinki.
3. Results
3.1. Characteristics of the Included Studies
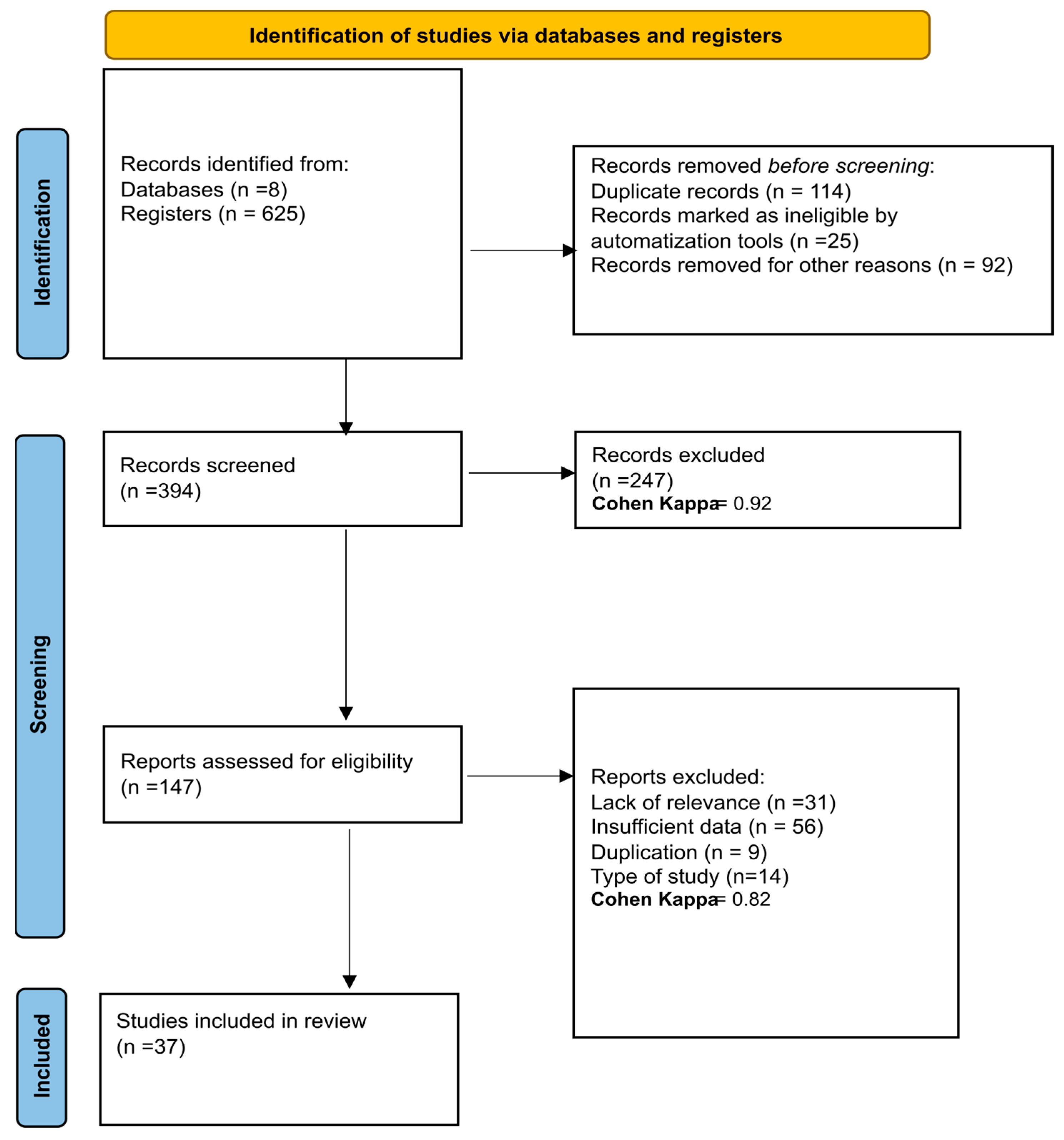
Following an extensive search across the referenced databases, a total of 625 articles were initially retrieved from eight databases. Of these, 114 were identified as duplicates and subsequently removed. The remaining articles underwent a title and abstract screening process, resulting in 394 articles being assessed. This screening led to the exclusion of 247 articles, and a Cohen’s kappa coefficient of 0.92 was calculated at this stage, suggesting a high level of agreement between the reviewers. Further evaluation was carried out on the full texts of the remaining 147 articles to determine their eligibility for inclusion in the review. During this phase, 31 articles were excluded due to their lack of relevance to the review topic, 54 were discarded due to having insufficient data, 9 were identified as additional duplicates within the dataset, and 14 were excluded as they did not match the required study type, and a Cohen’s Kappa coefficient of 0.823 was obtained, evidencing substantial agreement, slightly below that in the initial screening. Ultimately, 37 studies satisfied all inclusion criteria and were included in the systematic review. The details of the study selection process are depicted in the PRISMA [43] flowchart illustrated in Figure 1.
Figure 1.
PRISMA flow diagram with the search and study selection strategy. Cohen Kappa = 0.92 y Cohen Kappa = 0.82 shows a high level of agreement between the reviewers, which gives reliability and validity to the results.
3.2. Findings of the Studies
Table 1 summarizes the characteristics of the 37 studies reviewed, which included both randomized clinical trials (RCTs) (29 studies) and non-randomized trials (eight studies). These studies involved a total of 10,332 patients with NSCLC, examining a variety of emerging biomarkers through the analysis of peripheral blood samples for ctDNA and tumor biopsies. The studies predominantly focused on patients with advanced stages of NSCLC (IIIB-IV), ranging in age from 18 to 91 years, with a median age of 59 ± 20 years. The gender distribution was approximately 54.9% male and 45.1% female.
Most of the research focused on ctDNA as a biomarker, analyzing specific mutations such as ALK fusions, EGFR exon 20 insertions, bTMB, PD-L1 expression, and other genomic alterations, including mutations in EGFR, BRCA2, BRINP3, FBXW7, KIT, and RB1 and the EGFR T790M mutation [44,45,46,47]. Biomarkers related to the immune response were also evaluated, including the responses of circulating immune cells and gene expression profiles.

Table 1.
Synthesis of studies included in systematic review.
Table 1.
Synthesis of studies included in systematic review.
| Author and Publication Year | Country | Type of Design | Biological Sample | Number of Participants | Age of Participants | Sex of Participants | Biomarker Evaluated |
|---|---|---|---|---|---|---|---|
| Ren et al., 2022 [48] | China | Randomized Clinical Trial | Peripheral blood ctDNA samples | 389 | 18 to 75 years. | Men and women participated, but most patients were men | ctDNA dynamics |
| Yang et al., 2023 [49] | China | Randomized Clinical Trial | Peripheral blood ctDNA samples | 248 | Median age 54.0 years (brigatinib group), 53.0 years (alectinib group) | 54% female (brigatinib), 55% female (alectinib) | ALK fusions in plasma ctDNA |
| Riess et al., 2021 [50] | USA | Randomized Clinical Trial | Peripheral blood ctDNA samples | 11 | 60 (51–70) | 27% male, 73% female | EGFR exon 20 insertions |
| Lo Russo et al., 2022 [51] | USA | Randomized Clinical Trial | Peripheral blood ctDNA samples | 65 | Median age was 70.9 years (Q1–Q3: 63.7–77.1 years) | 21 (32.3%) were female and the rest were male | 36 immunobiomarkers like CD14, CD15, CD16, CD33, CD56, CD19, CD3, HLA-DR |
| Garon et al., 2023 [52] | USA, Japan | Randomized Clinical Trial | Peripheral blood ctDNA samples | 449 | NS | NS | The biomarkers evaluated were EGFR and co-occurring/treatment-emergent (TE) genomic alterations in ctDNA |
| Han et al., 2023 [53] | China | Non-Randomized Clinical Trial | Peripheral blood ctDNA samples | 40 | 18 to 75 years | NS | bTMB through ctDNA profiling |
| Si et al., 2021 [54] | USA | Randomized Clinical Trial | Peripheral blood ctDNA samples | 809 | NS | NS | bTMB |
| Jiang et al., 2021 [55] | China | Randomized Clinical Trial | Tumor biopsies for whole-exome and transcriptome sequencing and plasma samples for ctDNA analysis | 40 | 18 to 75 years old | Included both male (19, 47.5%) and female (21, 52.5%) patients | PD-L1, TMB, CD8+ TIL density, DSPP |
| Zhang et al., 2024 [56] | China | Randomized Clinical Trial | Peripheral blood ctDNA samples | 47 | Median age was 65 years (range: 52–76) | Mostly male (45/46, 97.8%); females (1/46, 2.2%) | ctDNA dynamics, BRCA2, BRINP3, FBXW7, KIT, RB1 |
| Tan et al., 2024 [44] | Australia | Non-Randomized Clinical Trial | Peripheral blood ctDNA samples | 47 | Median age was 60 years (range: 32–86) | 62% female, 38% male | EGFR T790M, EGFR exon 19 deletion, L858R mutation |
| Kim et al., 2022 [57] | USA | Randomized Clinical Trial | Peripheral blood ctDNA samples | 152 | NS | NS | bTMB |
| Peters et al., 2022 [58] | Multinational | Randomized Clinical Trial | Peripheral blood ctDNA samples | 471 | NS | NS | bTMB |
| Chaft et al., 2022 [59] | USA | Non-Randomized Clinical Trial | Tumor samples | 181 | Median age of 65 years (range: 37–83) | 93 females (51%) | Major pathological response (MPR), PD-L1 tumor proportion score (TPS) |
| Shi et al., 2022 [60] | China | Randomized Clinical Trial | Peripheral blood ctDNA samples | 290 | 18 to 75 years | Majority male (93.8% sintilimab, 90.4% docetaxel) | PD-L1, OVOL2, CTCF via tissue/blood sequencing |
| Papadimitrakopoulou et al., 2020 [45] | Multinational | Randomized Clinical Trial | Peripheral blood ctDNA samples | NS | NS | NS | EGFR mutations |
| Sakai et al., 2021a [46] | Japan | Randomized Clinical Trial | Formalin-fixed paraffin-embedded tumor tissue | 389 | NS | NS | Tumor mutation burden (TMB) |
| Park et al., 2023 [61] | South Korea | Non-Randomized Clinical Trial | Peripheral blood ctDNA samples | 100 | NS | 84 male, 16 female | bTMB, cfDNA concentration, hVAF, VAFSD |
| Park et al., 2021 [47] | South Korea | Randomized Clinical Trial | Peripheral blood ctDNA samples | 19 | Median age of 70 years (range: 32–84) | 13 females (68%) and 6 males (32%) | Activating EGFR mutations in ctDNA and tumor DNA |
| Gu et al., 2023 [62] | China | Randomized Clinical Trial | Peripheral blood ctDNA samples | 92 | Median age 65 years | 34% male and 66% female | EGFR mutations, ctDNA for minimal residual disease (MRD) |
| Han et al., 2022 [63] | China | Non-Randomized Clinical Trial | Peripheral blood ctDNA samples | 33 | Median age 56 years (range: 31–71) | 26 males (78.79%) and 7 females (21.21%) | ctDNA analysis with 448-gene panel for short-term dynamics |
| Zhong et al., 2023 [64] | China | Randomized Clinical Trial | Peripheral blood ctDNA samples | 69 | Median age was 58 years (range: 33–76 years) | 38 males (55.1%) and 31 females (44.9%) | EGFR mutation detection via ctDNA |
| García-Pardo et al., 2023 [65] | Canada | Non-Randomized Clinical Trial | Peripheral blood ctDNA samples | 150 | Median age at diagnosis 68 years (range: 33–91 years) | 80 men (53%), 70 women (47%) | ctDNA genotyping |
| Nomura et al., 2020 [66] | Japan | Randomized Clinical Trial | Peripheral blood ctDNA samples | 216 | NS | Both males and females (NS) | ctDNA (Guardant360® ancillary study) |
| Martini et al., 2022 [67] | Italy | Non-Randomized Clinical Trial | Basal fecal samples and peripheral blood samples for ctDNA analysis | 14 | NS | NS | Gut microbiota species and ctDNA RAS/BRAF WT MSS disease |
| Provencio et al., 2022 [68] | Spain | Non-Randomized Clinical Trial | Peripheral blood samples for ctDNA analysis and formalin-fixed paraffin-embedded tissue samples | 46 | Median age was 67.6 years | Majority male (66.5%) | ctDNA analysis for prognosis and predictive value |
| West et al., 2022 [69] | Multinational study | Randomized Clinical Trial | Peripheral blood ctDNA samples | 920 | NS | Both males and females included, exact distribution not provided | KRAS, STK11, KEAP1, TP53 mutations |
| Lo Russo et al., 2023 [70] | Italy | Randomized Clinical Trial | Blood and stool samples for circulating immune profiling and gut bacterial taxonomic abundance analysis | 65 | Median age was 70 years, with a range of 47–87 years | 44 men (68%) and 21 women (32%) | Immune circulating cell subsets and gene expression levels |
| Zhou et al., 2023 [71] | Multinational study | Randomized Clinical Trial | Formalin-fixed paraffin-embedded tumor tissue for PD-L1 expression assessment | NS | NS | Both males and females included, but the exact distribution is not provided | PD-L1 expression on tumor cells |
| Sakai et al., 2021b [72] | Japan | Randomized Clinical Trial | Peripheral blood ctDNA samples | 52 | Median age 67 (range: 37–82 years) | 17 male (32.7%), 35 female (67.3%) | EGFR genomic alterations including the T790M mutation |
| Redman et al., 2020 [73] | USA | Randomized Clinical Trial | Formalin-fixed paraffin-embedded tumor specimens for genomic DNA extraction | 1864 | NS | Both males and females included | Multiple biomarkers defined by the FoundationOne® NGS assay |
| Hirsch et al., 2022 [74] | USA | Randomized Clinical Trial | Formalin-fixed paraffin-embedded tumor specimens | 1313 | NS | Both males and females included | EGFR copy number and protein expression |
| Schuler et al., 2020 [75] | Multinational study | Randomized Clinical Trial | Tumor samples | 55 | Median age was 60 years | Both males (60%) and females (40%) | MET dysregulation |
| Gadgeel et al., 2022 [76] | Multinational study | Randomized Clinical Trial | Tumor samples | 577 | NS | Both males and females included | PD-L1 expression |
| Ramalingam et al., 2021 [77] | Multinational study | Randomized Clinical Trial | Formalin-fixed paraffin-embedded tumor samples | 970 | NS | Both males (82%) and females included | 52-gene expression histology classifier (LP52) |
| Song et al., 2022 [78] | China | Randomized Clinical Trial | Peripheral blood ctDNA samples | 78 | Median age 62 years | Both male (47.4%) and female (52.6%) participants | HER2 mutations |
| Anagnostou et al., 2023 [79] | Multinational study | Randomized Clinical Trial | Peripheral blood ctDNA samples | 50 | NS | Both males and females included | ctDNA dynamics |
| Park et al., 2021 [80] | South Korea | Randomized Clinical Trial | Peripheral blood ctDNA samples | 21 | Mean age of 68.5 years | 17 females and 4 males | EGFR exon 19 deletions, exon 21 point mutations (ctDNA) |
ctDNA: Circulating Tumor DNA; ALK: Anaplastic Lymphoma Kinase; EGFR: Epidermal Growth Factor Receptor; bTMB: Blood Tumor Mutational Burden; TMB: Tumor Mutational Burden; PD-L1: Programmed Death Ligand 1; MPR: Major Pathological Response; TPS: Tumor Proportion Score; MRD: Minimal Residual Disease; cfDNA: Circulating Free DNA; hVAF: High Variant Allele Frequency; VAFSD: Variant Allele Frequency Standard Deviation; NGS: Next-Generation Sequencing; IHC: Immunohistochemistry; HR: Hazard Ratio; CI: Confidence Interval; OS: Overall Survival; PFS: Progression-Free Survival; RECIST: Response Evaluation Criteria In Solid Tumors; ORR: Objective Response Rate; NSCLC: Non-Small-Cell Lung Cancer; Mb: Megabase; BRCA2: Breast Cancer 2, Early Onset; DSPP: Dentin Sialophosphoprotein; CTCF: CCCTC-Binding Factor; OVOL2: Ovo-Like Transcriptional Repressor 2; FFPE: Formalin-Fixed Paraffin-Embedded; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homolog; ABCP: Atezolizumab, Bevacizumab, Carboplatin, Paclitaxel; BCP: Bevacizumab, Carboplatin, Paclitaxel; KEAP1: Kelch-Like ECH-Associated Protein 1; STK11: Serine/Threonine Kinase 11; TP53: Tumor Protein p53; LASSO: Least Absolute Shrinkage and Selection Operator; ddPCR: Droplet Digital Polymerase Chain Reaction; miR: MicroRNA; LP52: 52-Gene Expression Histology Classifier; Mo: Month. NS: Not Specified.
The methodologies of these studies varied widely (see Table 2), employing techniques such as NGS, IHC, digital PCR, and specific assays like GuardantOMNI ctDNA and Foundation Medicine bTMB, reflecting a comprehensive approach to biomarker assessment through genetic and immunological analysis [37,54,56,61,74]. The differentiation between residual and non-residual ctDNA, along with the interpretation of PD-L1 expression and the density of infiltrating CD8+ T lymphocytes, highlights the importance of integrating genetic and immunological findings into NSCLC treatment [55,59,60]. Specifying the mutations per megabase to distinguish between high and low bTMB evidences the precision needed to assess the tumor’s mutational load, enhancing the predictive and prognostic value of these biomarkers [54,81].

Table 2.
Synthesis of studies included in systematic review by biomarker evaluated.
Basal activating alterations in EGFR, associated with shorter median PFS, served as prognostic markers; however, treatment with ramucirumab plus erlotinib improved the outcomes regardless of these alterations, indicating their predictive value. The reduction in bTMB was established as a predictive biomarker for treatment with sintilimab plus docetaxel, while a high bTMB indicated a clinical benefit with durvalumab plus tremelimumab compared to chemotherapy [52]. The DSPP mutation was identified as a predictive biomarker of longer PFS, as was the clearance of ctDNA, which was associated with more durable outcomes [55]. The decrease in and elimination of EGFRm and T790M in post-treatment ctDNA were linked to longer progression-free survival and overall survival, highlighting the efficacy of sequential therapies [44]. High levels of PD-L1 expression were related to a greater pathological response, and the levels of expression of OVOL2 and CTCF were associated with PFS outcomes, highlighting their potential as prognostic markers [60].
In the study conducted by Ren et al., in 2021 [48], it was observed that the combined treatment of camrelizumab and chemotherapy significantly extended the OS and PFS compared to a placebo combined with chemotherapy. The results indicated median PFS of 8.5 months versus 4.9 months, and median OS not reached versus 14.5 months, for the groups treated with camrelizumab–chemotherapy and placebo–chemotherapy, respectively. Similar findings were reported for treatments with brigatinib and alectinib, both with a median PFS of approximately 19 months [49]. Additionally, patients who presented detectable alterations of monoclonal antibody against epidermal growth factor (aEGFR) had a median PFS of 12.7 months, contrasting with the 22.0 months for those without these alterations, although specific OS data were not provided [52].
This systematic review also identified variations in PFS and OS associated with different molecular biomarkers, such as CD14, CD16, HLA-DR, CD3, CD56, and NKT, evidencing their impact on treatment outcomes. It was noted that different combinations of these markers were related to significant variations in OS and PFS [51].
In Han et al.’s 2022 [53] study, various therapies were compared, noting significant improvements in the median OS and PFS with sintilimab over docetaxel. Notably, for patients with mKRAS mutations and squamous cell carcinoma, targeted treatment regimens and the addition of cetuximab, respectively, showed significant enhancements in survival rates, regardless of the KRAS mutation status [69,74].
The analysis of the HR and 95% CI shed light on the OS and PFS across different scenarios. An HR of 0.55 for OS suggested a 45% reduction in mortality risk, while the HR for PFS of 0.97 between brigatinib and alectinib indicated no significant difference in disease progression between these treatments [49]. Detectable alterations of aEGFR with an HR of 1.87 suggested faster disease progression. Meanwhile, a positive molecular pathological response (MPR) status indicated a favorable prognosis, with the HRs showing significant improvements in disease-free survival (DFS) and OS [52]. A high tumor mutational burden (TMB) and low or undetectable levels of ctDNA following neoadjuvant treatment were associated with significant improvements in PFS and OS, highlighting their prognostic value [82,83].
For patients with a TMB of 12 mutations per megabase or higher, an HR of 0.477 for improved recurrence-free survival (RFS) underscores the TMB’s potential as a predictive biomarker for the treatment response [57]. The pre-treatment levels of ctDNA were found to be significant, with low levels correlating with improved PFS and OS outcomes, suggesting ctDNA’s prognostic importance after therapy [59].
The assessment of the biomarker-based treatment efficacy has unveiled a range of therapeutic approaches. These include monotherapy with immune checkpoint inhibitors (ICIs) such as pembrolizumab, as well as targeted therapy combinations with chemotherapy. The treatment regimens have spanned from specific targeted therapies, like ALK and EGFR inhibitors, to immunotherapies and their integration with chemotherapy [49,59,70].
The Kaplan–Meier methodology and the Cox proportional hazards model have been extensively used to analyze the OS and PFS across studies, comparing treatments such as camrelizumab with carboplatin and paclitaxel, brigatinib versus alectinib, and durvalumab with or without tremelimumab versus chemotherapy. These methods provide precise estimates of the survival time and assess the relative risks of events such as disease progression or death [46,47,48,62,67].
The Clopper–Pearson method for the estimation of objective response rates and disease control, and the use of two-sided tests at the 0.05 significance level, as in the study of docetaxel plus sintilimab, highlight the rigor in comparing treatments’ efficacy [63]. Furthermore, advanced techniques like cross-validation for the determination of the optimal cut-off for the bTMB and the minimum p-value approach reflect the ongoing evolution of statistical methods in oncological research [54].
In addition to these statistical analyses, some studies have incorporated innovative approaches such as multivariable Cox regression, competitive risk analysis, and corrections for multiple comparisons, showcasing the complexity and need for precision in interpreting data on survival and treatment responses [54,68].
In the context of immunotherapy, the studies by G. Lo Russo et al. (2022) [51] and Chaft et al. (2022) [59] suggest that the circulating immune biomarkers and innate immune cells in the peripheral blood before treatment can predict the pathological response following neoadjuvant atezolizumab, indicating a need for further validation studies.
Meanwhile, the research by Yang et al. (2023) [49] found that brigatinib did not show superiority over alectinib for PFS in patients with ALK-positive NSCLC previously treated with crizotinib, highlighting the consistent safety profiles of both drugs and reaffirming their positions as standard treatments post-crizotinib.
In 2022, Martini et al. [67] conducted a pivotal study exploring the impact of the gut microbiota on the antitumor efficacy of cetuximab and avelumab treatments in NSCLC and mCRC patients. Utilizing 16S rRNA sequencing, the research analyzed fecal samples to identify microbial compositions, particularly highlighting two butyrate-producing bacteria, Agathobacter M104/1 and Blautia SR1/5. Their findings revealed a significant correlation between the presence of these bacteria and extended PFS, suggesting these microbial species as promising biomarkers for the prediction of treatment success. The study illuminated the potential mechanism of action, where butyrate production by these bacteria modulates the immune response, thereby potentially enhancing the antitumor activity of the immunotherapy regimen.
In Table 3, the summarized findings are presented along with their clinical implications, the treatment response, the heterogeneity in NSCLC, and their clinical and demographic characteristics. For example, in the case of ctDNA, it is identified as a biomarker with detection correlated to the tumor burden and disease progression. A reduction in levels during treatment is indicative of better PFS and OS. Its clinical implication is highlighted as a non-invasive biomarker for the monitoring of the response and the adjustment of therapeutic strategies. The predictive response to treatment is noted, with ctDNA being predictive of the response to camrelizumab, carboplatin, paclitaxel, osimertinib, sintilimab, nab-paclitaxel, and gefitinib. The studies evaluated show that its heterogeneity varies with the mutation type (EGFR, ALK), tumor stage, distribution of histological subtypes, and frequency of co-alterations. The patient demographic is predominantly men with a history of heavy smoking, ECOG 1 status, and stage IV disease.

Table 3.
Summary of biomarker findings with heterogeneity in NSCLC.
PD-L1 is identified as a biomarker for tumor progression and prognosis. High levels are associated with better responses to ICIs and longer survival. Its clinical implication is its importance in selecting patients for immunotherapy. Regarding the treatment response, PD-L1 is predictive of the response to atezolizumab, pembrolizumab, and toripalimab. The heterogeneity of PD-L1 varies with the expression levels, tumor microenvironment, distribution of histological subtypes, and frequency of co-alterations. The patient demographic is predominantly men with a history of heavy smoking, ECOG 1 status, and stage IV disease.
miRNA is related to the response to immunotherapy and survival outcomes. miRNA-21 and miRNA-155 are correlated with the responses to immunotherapy and survival outcomes. Additionally, the plasma miR-32 levels correlate with the chemotherapy response and prognosis. The clinical implications of miRNA include patient stratification and treatment personalization based on molecular profiles. Xu et al., 2019 shows that it is predictive of the chemotherapy efficacy and prognosis with platinum-based chemotherapy. The heterogeneity of miRNA varies with the miRNA type, interaction with other molecular pathways, and frequency of co-alterations. The patient demographic includes those aged 45–78, predominantly men and 81.4% smokers, with ECOG 1–2 status and stage II–IV disease.
Finally, the bTMB is identified as a biomarker associated with tumor progression and prognosis. A high bTMB is associated with better outcomes in combined immunotherapy and chemotherapy. Accurate measurement predicts the immunotherapy efficacy and guides treatment selection. The studies by Han et al., 2023 [53] and Si et al., 2021 [54] highlight that it is predictive of the response to sintilimab + docetaxel and durvalumab + tremelimumab. The heterogeneity of the bTMB varies with the mutation burden, specific gene mutations, distribution of histological subtypes, and frequency of co-alterations. The patient demographic is predominantly men with a history of heavy smoking, ECOG 1 status, and stage IV disease.
3.3. Risk of Bias Assessment
Using the risk of bias graph created with RevMan 5.4® (accessed on 20 February 2024), the risk of bias assessment for the included studies is outlined as follows.
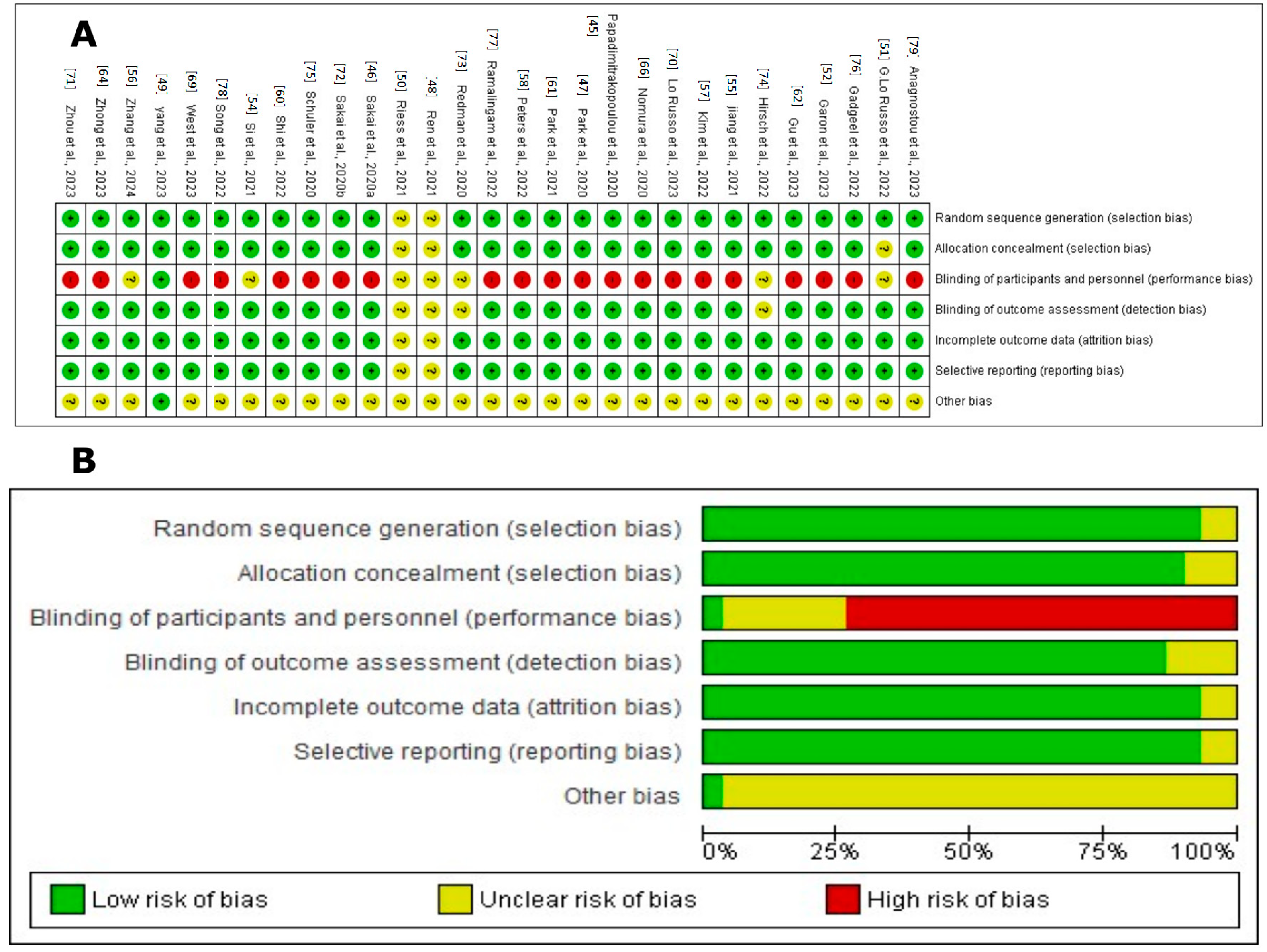
The assessment of the risk of bias in these studies was carried out using several criteria, as illustrated in Figure 2. Most of the studies, including those by Ren et al., 2021; Yang et al., 2023; G. Lo Russo et al., 2022; Garon et al., 2023; Si et al., 2021; Jiang et al., 2021; Zhang et al., 2024; Kim et al., 2022; Peters et al., 2022; Shi et al., 2022; Papadimitrakopoulou et al., 2020; Park et al., 2021; Gu et al., 2023; Zhong et al., 2023; Nomura et al., 2020; West et al., 2022; Lo Russo et al., 2023; Zhou et al., 2023; Sakai et al., 2020a; Redman et al., 2020; Hirsch et al., 2022; Schuler et al., 2020; Gadgeel et al., 2022; Ramalingam et al., 2022; Song et al., 2022; Anagnostou et al., 2023; and Park et al., 2020, were evaluated as having a low risk for random sequence generation (selection bias), suggesting thorough and well-documented randomization procedures [45,47,48,49,51,52,54,62,64,66,69,70,73,74,75,76,77,78,79].
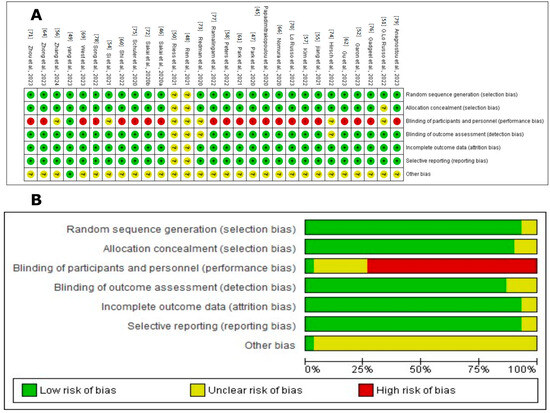
Figure 2.
Cochrane Risk of Bias assessment for randomized studies of interventions in the systematic review. (A). Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. The symbol “+” indicates a low risk of bias, the symbol “?” indicates an unclear risk of bias, and the symbol “−” indicates a high risk of bias. The colors used are green for low risk of bias, yellow for unclear risk of bias, and red for high risk of bias [45,47,48,49,51,52,54,62,64,66,69,70,73,74,75,76,77,78,79]. (B). Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies. Figure created by RevMan 5 (accessed on 20 February 2024).
Allocation concealment (selection bias) was primarily rated as low risk, indicating that the allocation process was sufficiently concealed to reduce selection bias in most studies. However, some studies, such as G. Lo Russo et al., 2022 [51], presented an unclear risk, indicating the need for more explicit documentation or reporting.
Regarding the blinding of participants and personnel (performance bias), many studies were evaluated as having a high risk due to the open nature of many clinical trials, which exposes them to possible performance bias. This includes the studies by Garon et al., 2023; Jiang et al., 2021; Kim et al., 2022; Peters et al., 2022; Shi et al., 2022; Papadimitrakopoulou et al., 2020; Park et al., 2021; Gu et al., 2023; Zhong et al., 2023; Nomura et al., 2020; West et al., 2022; Lo Russo et al., 2023; Zhou et al., 2023; Sakai et al., 2020b; Schuler et al., 2020; Gadgeel et al., 2022; Ramalingam et al., 2022; Song et al., 2022; Anagnostou et al., 2023; and Park et al., 2020 [45,47,48,49,51,52,54,62,64,66,69,70,73,74,75,76,77,78,79]. The concern around unblinding in open clinical trials underscores a significant issue, as both the researchers and participants are aware of the assigned treatments, potentially influencing the outcomes and adherence to treatment.
The blinding of the outcome assessment (detection bias) was mainly classified as low risk, suggesting that the outcome assessors were likely unaware of the intervention groups, thereby reducing the detection bias. This practice was consistent across most studies, reflecting a standard in the objective evaluation of clinical outcomes.
However, the domain concerning incomplete outcome data (attrition bias) exhibited a range of risks—low, unclear, and high—across the studies. A low risk indicates the transparent reporting of participant dropouts and proper handling of missing data. Conversely, studies with an unclear risk lacked detailed reporting, while a high risk indicated insufficient transparency, potentially undermining the study’s validity.
Selective reporting (reporting bias) varied, with most studies classified as low risk, implying that all predefined outcomes were likely reported and the study protocol was registered. However, some studies had an unclear risk due to insufficient information regarding the reporting of all expected outcomes.
Overall, the studies demonstrated a predominantly low risk of bias across most domains, indicating high methodological quality. Nevertheless, the variability in incomplete outcome data and selective reporting call for the cautious interpretation of the findings.
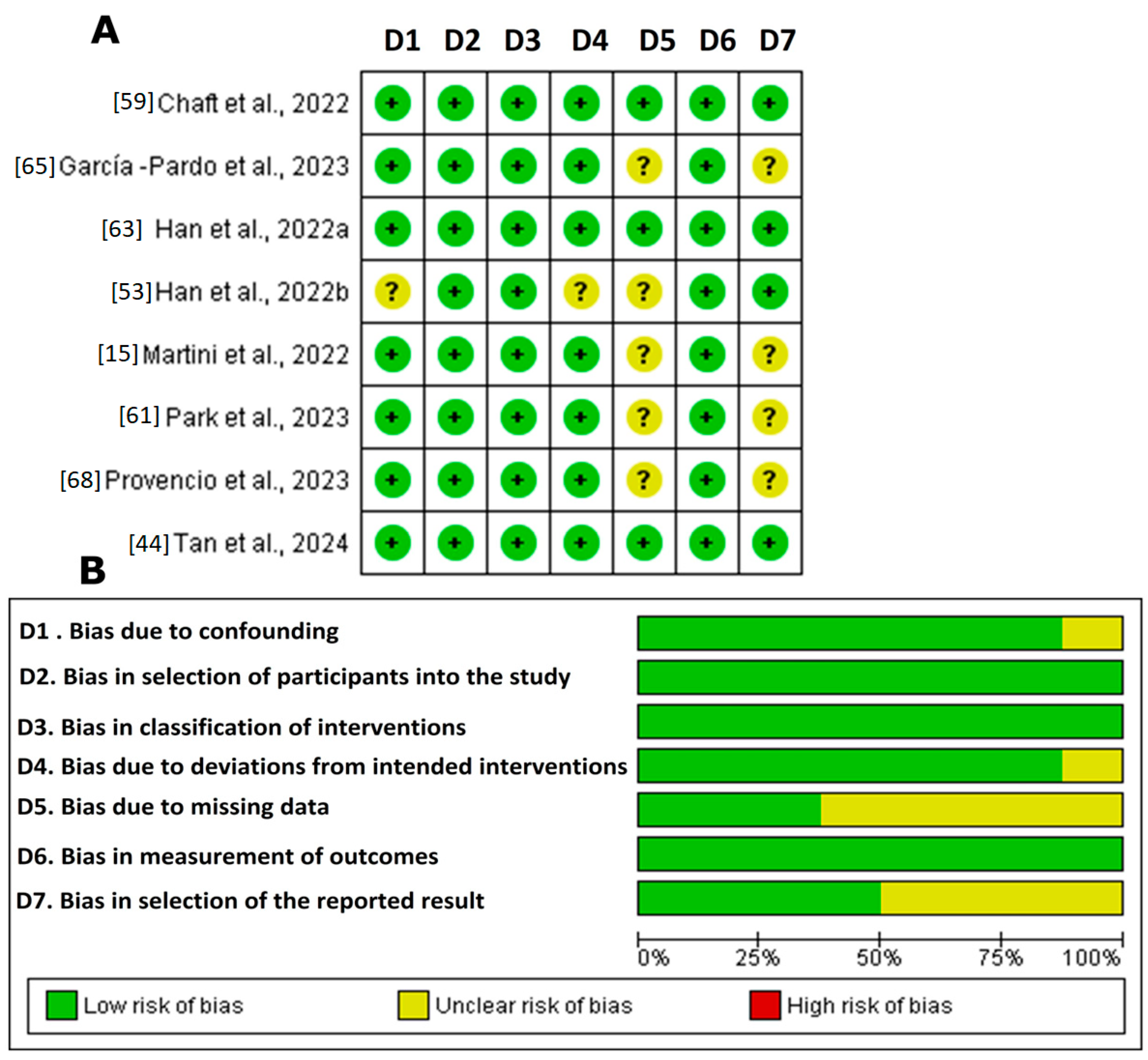
Figure 3 illustrates the bias risk analysis of the non-randomized clinical studies using the ROBINS-I tool (ROBINS-I tool | Cochrane Methods, accessed 18 September 2023) The results revealed that a significant proportion of the studies demonstrated high methodological quality. However, the studies by Han et al., 2022b [63]; Martini et al., 2022 [67]; Park et al., 2023 [61]; and Provencio et al., 2023 [68] showed an uncertain risk in domains 5 and 7 (bias due to missing data and bias in the selection of the reported result). This uncertainty underscores the need for the cautious interpretation of the findings. Although the general prevalence of a low risk reinforces the confidence in the accumulated evidence, the lack of transparency in these domains could influence the robustness of the final conclusions. Therefore, detailed assessment in future research is recommended to ensure robust clinical results.
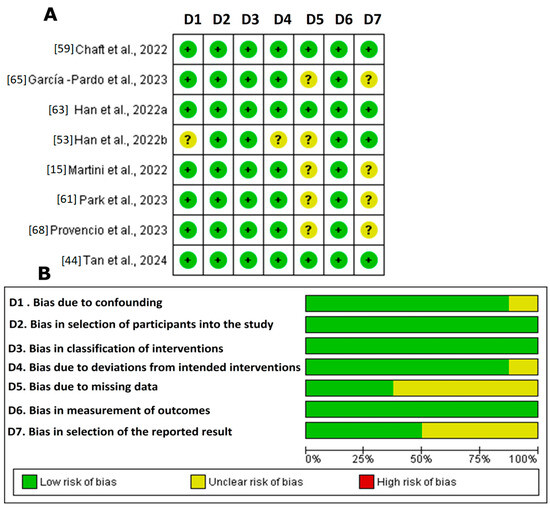
Figure 3.
ROBINS-I in the systematic review. (A). Summary of risk of bias: judgements of review authors regarding each risk of bias item for each included study [15,44,53,59,61,63,65,68]. The symbol “+” indicates a low risk of bias, the symbol “?” indicates an unclear risk of bias, and the symbol “−” indicates a high risk of bias. The colors used are green for low risk of bias, yellow for unclear risk of bias, and red for high risk of bias. (B). Graph of risk of bias: judgements of review authors on each risk of bias item, presented as percentages across all included studies. Figure created by RevMan 5 (accessed on 20 February 2024).
4. Discussion
4.1. Most Significant Findings
In this study, a systematic review was conducted focusing on the identification and application of emerging biomarkers for the treatment of NSCLC. The most significant findings include the identification of ctDNA, bTMB, miRNAs, and PD-L1 as valuable tools for early diagnosis, treatment response prediction, and disease monitoring.
It has been demonstrated that ctDNA, due to its non-invasive nature and high sensitivity, can accurately detect genetic alterations, providing an effective biomarker for early intervention and personalized treatment [44,48,49,52,56]. miRNAs, despite facing specificity challenges, have shown potential as independent prognostic factors [84]. The bTMB has emerged as a key predictor of the response to immunotherapy, although its utility depends on the standardization and cost of sequencing technologies [53,54,57,58]. Additionally, PD-L1 has been identified as essential in predicting the efficacy of immune checkpoint inhibitors, facilitating the selection of patients who may benefit most from immunotherapy [55,71,76].
The economic impact and accessibility of these biomarkers are crucial factors that greatly affect their use in clinical practice, particularly when evaluating their cost-effectiveness in various healthcare environments. Implementing widespread ctDNA screening, for example, involves assessing not only the direct costs associated with the screening technologies themselves but also the potential healthcare savings from early detection and personalized treatment strategies [85,86,87]. These savings can be substantial, as early intervention typically leads to better patient outcomes and reduced treatment costs over time. A comprehensive analysis of the cost-effectiveness of these biomarkers is essential. By understanding the economic benefits and constraints associated with ctDNA, bTMB, and miRNA technologies, healthcare providers can make informed decisions about incorporating these tools into practice. This not only optimizes health outcomes but also ensures that the benefits of precision medicine are accessible to a broader range of patients, thereby reducing disparities in care and advancing public health goals [88,89,90].
Table 4 provides a comparative analysis of these biomarkers in NSCLC. ctDNA, known for its non-invasiveness and high sensitivity, is proposed as an effective tool for early diagnosis and treatment monitoring. However, its high cost and the complexity of interpretation limit its widespread use. The study by Postel et al., 2017 [91] highlighted the significant role of ddPCR and optimized NGS in the detection and monitoring of ctDNA. It elucidates how these advanced methodologies have markedly enhanced the ability to identify ctDNA with high sensitivity and specificity.

Table 4.
Comparative analysis of biomarkers in NSCLC.
Ren et al., 2021 [48] showed that the dynamics of ctDNA during treatment could predict the efficacy of camrelizumab combined with chemotherapy in advanced squamous NSCLC, significantly improving the PFS and OS. Yang et al., 2023 [49] found that the detectability of ALK fusions in ctDNA was associated with the prognosis, with specific variants like EML4-ALK linked to poorer PFS. Garon et al., 2023 [52] reported that baseline alterations in ctDNA were prognostic indicators in patients with metastatic NSCLC harboring active EGFR mutations, with improvements in PFS observed when treated with ramucirumab plus erlotinib. Zhang et al., 2024 [56] highlighted that ctDNA clearance after two cycles of treatment was associated with longer PFS, emphasizing the predictive value of ctDNA in the combined treatment of sintilimab with nab-paclitaxel/carboplatin. Tan et al., 2024 [44] demonstrated that the reduction and clearance of ctDNA mutations, including EGFR and T790M, post-therapy were linked to longer PFS and OS in the alternating treatment of osimertinib and gefitinib. The studies by Goh et al., 2023 [101] and Cao et al., 2023 [102] have consistently demonstrated that reductions in ctDNA levels post-treatment are indicative of better PFS and OS. Tostes et al., 2023 [103] emphasized ctDNA as a dynamic biomarker reflecting changes in tumor burden and predicting therapy responses. Cao et al., 2023 [102] advocated for a multi-biomarker strategy, validated by the systematic review’s findings, where the integration of ctDNA with other biomarkers like the bTMB provided a more reliable prediction model for treatment responses. Comprehensive profiling aids in customizing therapy, as exemplified by Martini et al. (2022) [67], who found that specific gut microbiota species identified through ctDNA analysis were associated with longer PFS.
The significance of genetic mutations within ctDNA, particularly those in the EGFR or ALK genes, has been highlighted for their impact on clinical outcomes. Goh et al., 2023 [101] noted these mutations as crucial prognostic markers, a finding supported by Garon et al., 2023 [52], who observed that activating EGFR mutations correlated with shorter PFS.
These findings illustrate that ctDNA serves as a non-invasive biomarker for the monitoring of treatment responses and adjustment of therapeutic strategies in real time. It can also act as a predictive indicator of the treatment efficacy, particularly in targeted therapies and combinations of chemotherapy and immunotherapy [44,72,104].
The predictive efficacy of ctDNA varies according to the mutation type (e.g., EGFR, ALK), tumor stage, and histological subtype distribution. Additionally, the presence of co-alterations in genes and the clinical contexts of patients, such as their smoking history and ECOG performance status, can influence the outcomes. Elevated levels of ctDNA in serum are associated with a lower probability of a response to immunotherapy and generally poorer survival outcomes compared to patients with lower ctDNA levels, suggesting the potential of ctDNA as both a predictive and prognostic biomarker in treatment, including immunotherapy [18,105].
Regarding PD-L1, this biomarker is crucial in predicting the efficacy of checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 antibodies. Studies have shown that patients with high PD-L1 expression generally respond better to these treatments, leading to higher survival rates. This marker aids in the selection of patients who are likely to benefit most from immunotherapy, thus personalizing the therapeutic options and improving the clinical outcomes [20,106].
Several key studies illustrate the clinical utility of PD-L1. Jiang et al., 2021 [55] found that PD-L1 expression was not predictive of PFS in treatment with toripalimab plus chemotherapy in EGFR-mutant NSCLC. Zhou et al., 2023 [71] observed high concordance between the SP263 and 22C3 assays for PD-L1 expression, with both predicting the efficacy of adjuvant atezolizumab in early NSCLC. Gadgeel et al., 2022 [76] reported that atezolizumab showed better survival compared to docetaxel, regardless of the PD-L1 status, with a greater benefit observed in patients with high PD-L1 expression.
PD-L1 is crucial in selecting patients for immunotherapy, helping to identify those who may benefit most from ICIs. The accurate measurement of PD-L1 can guide therapeutic decisions and optimize clinical outcomes. However, the PD-L1 expression and its association with the treatment response vary with the tumor microenvironment and histological subtype distribution, influencing the treatment response and survival. The expression of PD-L1 as a predictive biomarker for immunotherapy is complicated by issues such as variable detection antibodies and differing immunohistochemistry (IHC) cut-offs. While PD-L1 protein detection by IHC can enrich the response to anti-PD-L1 blockade, it is not absolute [107,108,109].
Studies have shown that patients with NSCLC who overexpress PD-L1 tend to respond more favorably to immunotherapy and demonstrate higher survival rates compared to those whose tumors do not express or have low expression of PD-L1. This underscores the importance of evaluating PD-L1 expression as a predictive biomarker for the selection of candidates for immunotherapy [71,83,110]. However, its sensitivity varies depending on the dynamics of the immune and tumor microenvironments, which can change over time and with treatment. Despite its high specificity in identifying patients likely to respond to ICIs, PD-L1 expression alone is not always indicative of treatment success [103,110].
miRNAs have emerged as significant biomarkers in NSCLC. Particular miRNAs, like miRNA-21 and miRNA-155, are notable predictors of the response to immunotherapy and survival. Xu et al., 2019 [84] found that the plasma levels of miR-32 correlated with the chemotherapy response and prognosis in NSCLC patients treated with platinum-based chemotherapy. Similarly, Ren et al., 2021 [48] observed correlations between miRNA-21 and miRNA-155 and the immunotherapy response and survival outcomes. Li et al., 2017 [96] found that miR-21-5p and miR-30d-5p were independent prognostic factors for overall survival in NSCLC, while Liu et al., 2012 [94] indicated that elevated levels of serum miR-21 and tumor miR-200c were associated with a poor prognosis in NSCLC patients. Wu et al., 2014 [95] demonstrated that high miR-19b and low miR-146a expression in NSCLC tissue correlated with a higher TNM stage, lymph node metastasis, and decreased survival rates.
Their assessment provides valuable information for patient stratification and the optimization of treatment regimens based on specific molecular profiles. Consequently, miRNAs have the potential to serve as non-invasive and useful markers for the stratification of patients and prediction of outcomes in the context of immunotherapy [22,104,111,112]. However, the quantification of miRNAs presents challenges due to their low abundance and high susceptibility to degradation. This complexity is further compounded by the need to normalize their levels against stable reference miRNAs and the significant discrepancies caused by the different detection platforms, such as qRT-PCR and microarrays. Standardizing the protocols for the selection of reference miRNAs based on the tissue origin and pathological conditions is crucial to increase the reliability of these analyses [21,111,113,114].
The interaction between the microbiota, particularly the gut microbiota, and the patient’s immune system plays a critical role in modulating the responses to treatments such as immunotherapy [115,116]. Recent research has highlighted that the composition and diversity of the microbiota can directly influence therapeutic outcomes, affecting both the efficacy and toxicity of the administered treatments in patients with NSCLC [117,118]. In the analysis of the gut microbiome, miRNAs, and their interaction with NSCLC, an interesting perspective emerges on the potential of these biological entities as biomarkers and their modulatory effects on the treatment efficacy, especially in targeted therapies and immunotherapies [119,120].
For instance, Martini et al., 2022 [67] demonstrated that certain intestinal bacteria, such as butyrate producers (Agathobacter and Blautia), were associated with improved PFS in NSCLC patients treated with combined therapies including cetuximab and avelumab. This relationship suggests that the microbiota not only affects carcinogenesis but also modulates the immune response, which can enhance or inhibit the efficacy of treatments based on ICIs. Comparatively, Shah and Ng, 2023 [121] emphasize how the gut microbiota influences the response to ICIs in NSCLC, associating microbiota diversity with greater treatment efficacy. These findings support the notion that modifying the microbiota could increase the sensitivity of patients who are initially resistant to ICIs, thus opening new avenues for personalized therapies.
Additionally, the role of miRNAs in this microbiota–NSCLC dynamic is crucial. miRNAs play a significant role in modulating immunity, inflammation, and cellular stress responses—all processes that are dysregulated in NSCLC. Casciaro et al., 2020 [122] suggest that miRNAs can influence the composition of the microbiota and vice versa, where changes in the microbiota might alter the expression of host cell miRNAs, affecting cancer progression and treatment responses. For example, alterations in specific miRNAs could result in changes in intestinal permeability and antigen presentation capacity, directly influencing the efficacy of immunotherapy in NSCLC patients, as discussed by Martini et al., 2022 [67] and supported by Chrysostomou et al., 2023 [123], who explore how miRNAs derived from the microbiota can be crucial in regulating the immune response and tumor progression.
Understanding the interaction between miRNA, the microbiota, and NSCLC offers considerable potential to improve outcomes in NSCLC, particularly in personalized therapies. The use of probiotics, prebiotics, or dietary changes to alter the microbiota, along with interventions that modify the expression of specific miRNAs, could not only enhance the response to immunotherapy but also reduce the toxicity associated with conventional treatments [123,124]. The individual variability in the microbiota composition and miRNA profiles suggests that personalized treatments could be developed around these differences, as the same intervention might have varied effects on different patients [21,125]. To develop effective and personalized interventions, a deeper understanding of the interactions among the microbiota, miRNAs, and NSCLC is essential.
Casciaro et al., 2020 [122] and Shah and Ng, 2023 [121] recommend that future studies focus on identifying combined signatures of miRNAs and the microbial composition that could serve as predictive biomarkers and guides for personalized therapies. Allegra et al., 2020 [126] explore how the microbiota can impact miRNA expression (miR-21, miR-155, miR-146a, miR-223), which, in turn, regulates gene expression, which affects cancer progression and the response to treatment. The study highlights that miR-21 and miR-155 are often associated with promoting inflammation and cancer progression, making them targets for therapies aimed at reducing their expression to suppress tumor growth. Meanwhile, miR-146a and miR-223 typically act as tumor suppressors by regulating genes involved in inflammatory processes; thus, enhancing their activity could be beneficial in controlling NSCLC’s progression. Allegra et al. suggest that antagomirs (anti-miRNA oligonucleotides) could specifically inhibit these dysregulated miRNAs, providing a direct method to enhance the treatment responses, particularly in personalizing therapy based on individual microbiota and miRNA profiles.
Finally, the bTMB has emerged as a significant biomarker in predicting the efficacy of immunotherapy combined with chemotherapy in NSCLC. Several key studies have highlighted its clinical utility. Han et al., 2022 [63] found that a reduction in the bTMB at six weeks could serve as a potential predictive biomarker for the regimen of sintilimab plus docetaxel in advanced NSCLC. Si et al., 2021 [54] reported that a high bTMB (≥20 mutations/Mb) predicted clinical benefits with durvalumab plus tremelimumab compared to chemotherapy. Kim et al., 2022 [57] showed that a bTMB ≥ 16 was associated with a higher objective response rate (ORR) and, in exploratory analyses, greater overall survival (OS) compared to a bTMB < 16. Conversely, Peters et al., 2022 [58] noted that a primary endpoint benefit in PFS was not achieved with a bTMB ≥ 16 for atezolizumab versus chemotherapy.
The bTMB can predict the efficacy of combined immunotherapy and chemotherapy, aiding in the selection of patients for specific treatments [52]. However, the mutational burden and its impact on the treatment response vary depending on the mutation type, the frequency of co-alterations, and the histological subtype distribution. This heterogeneity in the bTMB can influence treatment response prediction and clinical outcomes. In patients treated with specific therapies, such as sintilimab plus docetaxel and durvalumab plus tremelimumab, the bTMB has been identified as a predictive and prognostic indicator. A high bTMB indicates a clinical benefit with durvalumab plus tremelimumab compared to conventional chemotherapy. Recent studies have highlighted the need for precision in measuring the bTMB, influenced by technical and sample factors, underlining the importance of standardizing and validating the assessment methods to ensure their clinical utility [53,54,63,81].
The precision of TMB assessment is crucial in predicting and prognosticating tumor behavior. Nie et al., 2020 [81] found that a low bTMB was associated with a survival advantage in NSCLC patients treated with docetaxel, indicating the potential of the bTMB as a prognostic and predictive biomarker. Si et al., 2020 [54] further validated this by identifying a bTMB cut-off of ≥20 mutations per megabase as predictive of a clinical benefit with durvalumab plus tremelimumab in first-line metastatic NSCLC treatment. Schuurbiers et al., 2022 [97] highlighted the challenges in assessing the bTMB, including the need for a minimally sized bTMB panel and the influence of technical and sample-related factors. Raiber-Moreau et al., 2023 [98] emphasized the importance of developing and validating bTMB reference standards to ensure the accuracy and reproducibility of bTMB measurement. These studies collectively underscore the need for precision in assessing the bTMB to enhance the predictive and prognostic value of this biomarker.
Harmonizing the gene panels used for the sequencing of the bTMB by standardizing the size and the genes included is suggested to decrease the results’ variability and refine the classification of mutations as either drivers or passengers.
4.2. Quality of Evidence
The quality of the evidence obtained from these studies was critical in validating the reliability of the biomarkers in a clinical setting. Most RCTs showed a low risk of bias in the generation of random sequences and concealment of allocation, indicating robust randomization processes that minimized selection bias. However, the risk of performance bias was significantly high due to the open nature of many trials, where the participants and investigators knew the treatment assignments, which could influence the outcomes. Although most studies ensured a low probability of bias in the outcome assessment, inconsistencies in terms of incomplete outcome data and selective reporting underline the need for more transparent reporting practices and rigorous methodologies to ensure the applicability and accuracy of these biomarkers in medical practice. This includes a strong focus on the validation of microRNAs and other emerging biomarkers, such as those related to the gut microbiota, which could offer new perspectives in the personalized treatment of NSCLC.
A critical issue is the inconsistency of biomarkers as measurable indicators used to distinguish precisely and objectively between a normal biological state, pathological condition, and response to a specific therapeutic intervention. The development of biomarkers presents a significant challenge in cancer due to its complexity, sensitivity, and therapy resistance. The complex interplay of molecular pathways makes it necessary to use multiple markers to create sensitive and specific biomarkers that can represent a type of cancer pathogenesis and predict treatment responses and outcomes [127].
Despite the potential of ctDNA to effectively monitor therapeutic responses and disease progression, many studies, like those in phase II clinical trials by Han et al., 2022 [63], are not yet concluded. These studies suggest that the state of ctDNA and the elimination of ctDNA mutations serve as predictive biomarkers for treatments combining sintilimab with docetaxel in patients with advanced, pre-treated NSCLC [60].
The heterogeneity of the study designs and evaluation methods, as seen in the research by Goh et al., 2023 [101], indicates that the responses are highly variable and influenced by individual tumor characteristics, such as PD-L1 expression (also known as the tumor proportion score; TPS), the tumor mutational burden (TMB), tumor-infiltrating lymphocytes (TIL), the host’s immune system, and molecular signatures [124]. Similarly, the studies by Cao et al., 2023 [102] found that patients with resected NSCLC and high levels of PD-L1 exhibited lower survival rates compared to those with low levels. Conversely, inhibitors of PD-L1 or PD-1 can substantially increase patient survival, and detectable perioperative ctDNA is also correlated with poorer survival outcomes. Elevated CEA levels in circulation before and after surgery predicted significantly reduced survival outcomes [102].
This diversity in the evaluation methods underscores the need for a more standardized approach in research. Defining and standardizing the key parameters of the most promising biomarkers is essential to enable all stakeholders to make meaningful observations and inferences about the efficacy of seemingly similar agents and combinations in various settings. Several studies have demonstrated that a higher TMB is associated with better outcomes in various types of tumors, including NSCLC, colorectal cancer, bladder cancer, and melanoma [128].
Predicting the response to immunotherapy is crucial to identify patients who are likely to benefit from costly therapy and to avoid subjecting those who do not benefit from ICIs to unnecessary adverse effects. However, the FDA has not approved any circulating biomarker to predict the response to immunotherapy. Patients with high sPD-L1, low PD1+ CD8+, and low NK cell counts were significantly associated with poorer PFS [101].
Researchers must strive for transparency and thoroughness in reporting their methodologies. Standard practices should include detailed descriptions of the randomization processes and blinding methods and the complete reporting of outcome data. Integrating these biomarkers into standard clinical pathways could maximize their potential in enhancing the treatment precision, thereby fostering significant advances in oncology and improving patient outcomes and quality of life.
4.3. Future Directions and Clinical Implications
Advances in biomarker research are fundamental in enhancing diagnostic procedures and treatment protocols in oncology, particularly for complex conditions such as NSCLC. Future research should focus on establishing standardized protocols for the use of biomarkers and their integration into clinical pathways, which promises to significantly elevate the accuracy of diagnostics and the personalization of treatment strategies, thereby improving patient outcomes [129].
These studies must encompass all stages of biomarker use, from detection methods and machine calibration to data interpretation and report formatting. Technologies such as digital PCR and NGS offer profound insights into genetic and epigenetic modifications; however, their results are only as reliable as the standards used to calibrate them. Developing universal calibration standards that are uniformly applied across different platforms and laboratories can minimize the variability and enhance the reproducibility of the results [129,130].
Moreover, integrating biomarkers into clinical pathways also requires the exploration of their systemic implications. For instance, understanding how the early detection of mutations through ctDNA impacts long-term survival rates and quality of life requires longitudinal studies that track these outcomes over extended periods. Future research should focus on standardizing analytical methods and evaluating ctDNA in various clinical contexts and treatment types to validate its utility as a universal biomarker [131]. Similarly, personalizing chemotherapy and immunotherapy regimens based on the bTMB and microRNA profiles could revolutionize treatment paradigms [97,112]. Additional studies are needed to refine the cut-off points for the bTMB and evaluate its predictive efficacy in different treatment combinations, with an emphasis on genetic heterogeneity and the impact on treatment responses [132,133]. Future research on miRNAs should focus on identifying specific profiles associated with the treatment response and disease progression, as well as developing targeted therapies that modulate miRNA levels to enhance the treatment efficacy [134,135]. Regarding PD-L1, further research is needed to understand the heterogeneity in PD-L1 expression and its impact on the treatment response, exploring therapeutic combinations that can overcome resistance in specific subgroups [99,110].
The incorporation of nanotechnology, biotechnology, and AI further enhances the potential of biomarkers in NSCLC. Nanotechnology can improve drug delivery systems, making them more effective by targeting specific cells and reducing side effects. For instance, nanoparticles can be designed to release drugs in response to specific biological signals or environmental conditions, thus improving the precision of drug delivery at tumor sites [129,136,137,138]. Meanwhile, biotechnology plays a crucial role in the development of biomarkers and therapeutic targets. Techniques like CRISPR and gene editing allow for the manipulation of genetic material in cells, aiding in the creation of more effective models for the study of cancer and development of targeted therapies. For example, gene editing could be used to modify immune cells to enhance their ability to recognize and destroy cancer cells, a method currently being explored with CAR-T cell therapies [139,140,141].
AI complements these technologies by providing advanced data analysis capabilities that can predict treatment outcomes, optimize treatment plans, and even identify new potential biomarkers from vast datasets [129]. AI algorithms are capable of analyzing complex patterns in data that would be impossible to efficiently process by humans. For instance, AI can analyze imaging data to distinguish between benign and malignant lesions with high precision or predict which patients are more likely to respond to certain therapies based on historical data [129,142].
These technologies not only refine but also expand the current landscape of NSCLC treatment by facilitating the development of new targets and therapeutic strategies. This harmonious integration of biomarkers with cutting-edge technologies promises to revolutionize oncological practice by improving the precision and personalization of care. Eventually, this will lead to more effective treatments, the better management of treatment side effects, and improved survival rates for patients. By combining detailed genetic, molecular, and imaging data with innovative treatment methods, clinicians can provide more targeted, efficient, and patient-friendly cancer care. This approach underscores the potential of modern oncology to transform the nature of cancer treatment, ensuring that patients receive the most effective interventions specifically tailored to their unique disease profiles.
5. Conclusions
This systematic review emphasizes the transformative role of ctDNA and other biomarkers in the management of NSCLC, integrating the findings from 37 diverse studies to substantiate their effectiveness in personalized medicine. It highlights ctDNA’s utility in prognostic evaluations and real-time therapy monitoring, allowing for adaptive treatment strategies, particularly with immunotherapies and targeted therapies, where PD-L1 expression and specific genetic alterations such as ALK fusions and EGFR mutations are pivotal. The review also points to emerging biomarkers like microRNA profiles and the gut microbiota, which broaden the scope of immunotherapy’s efficacy. Collectively, these biomarkers not only refine the diagnostic and therapeutic protocols but also underscore the necessity for their standardized integration into clinical practice to optimize outcomes and reduce therapeutic redundancies. This synthesis confirms their prognostic and predictive value, advocating for ongoing innovation in biomarker research to improve NSCLC management, patient survival, and quality of life.
Author Contributions
Conceptualization, Y.L. and J.C.R.; methodology, Y.L. and J.C.R.; validation, Y.L., D.M.G. and A.P.L.; formal analysis, Y.L. and J.C.R.; investigation, Y.L. and J.C.R.; resources, Y.L.; data curation, Y.L. and J.C.R.; writing—original draft preparation Y.L., J.F.M.P. and J.C.R.; writing—review and editing, Y.L., D.M.G., A.P.L. and J.F.M.P.; visualization J.C.R.; supervision Y.L.; project administration, Y.L.; funding acquisition Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Dirección General de Investigaciones de la Universidad Santiago de Cali, Convocatoria Interna No. 01-2024, Proyecto de fortalecimiento de grupo de investigación en salud integral, and grant number DGI-COCEIN No. 934-621121-3384.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Universidad Santiago de Cali for their financial and academic support in the preparation of this manuscript and give its consent for recognition in this study and publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| NSCLC | Non-Small-Cell Lung Cancer |
| SCLC | Small Cell Lung Cancer |
| ctDNA | Circulating Tumor DNA |
| miRNA | microRNA |
| bTMB | Blood Tumor Mutational Burden |
| NGS | Next-Generation Sequencing |
| EGFR | Epidermal Growth Factor Receptor |
| ALK | Anaplastic Lymphoma Kinase |
| ROS1 | ROS Proto-Oncogene 1, Receptor Tyrosine Kinase |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| PD-1 | Programmed Death-1 |
| PD-L1 | Programmed Death Ligand 1 |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| FISH | Fluorescence In Situ Hybridization |
| PCR | Polymerase Chain Reaction |
| IHC | Immunohistochemistry |
| TKIs | Tyrosine Kinase Inhibitors |
| EMT | Epithelial–Mesenchymal Transition |
| MET | Mesenchymal Epithelial Transition |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| VEGF | Vascular Endothelial Growth Factor |
| FDA | U.S. Food and Drug Administration |
| NIH | National Institutes of Health |
| ICBs | Immune Checkpoint Inhibitors |
| NTRK | Neurotrophic Tyrosine Kinase Receptor |
| TNM | Tumor, Node, Metastasis |
| TME | Tumor Microenvironment |
| CEA | Carcinoembryonic Antigen |
| CA125 | Carbohydrate Antigen 125 |
| STK11 | Serine/Threonine Kinase 11 |
| DDR2 | Discoidin Domain Receptor 2 |
| RET | Rearranged during Transfection |
| JAK-STAT | Janus Kinase Signal Transducer and Activator of Transcription |
| RAS/MAPK | Rat Sarcoma/Mitogen-Activated Protein Kinase |
| PI3K/AKT/mTOR | Phosphoinositide 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin |
| MEK/ERK | Mitogen-Activated Protein Kinase Kinase/Extracellular Signal-Regulated Kinase |
| BRAF | B-Raf Proto-Oncogene, Serine/Threonine Kinase |
| TUBB3 | Tubulin Beta-3 |
| TGF-β | Transforming Growth Factor Beta |
| LAG-3 | Lymphocyte Activation Gene-3 |
| MDS | Multidimensional Scaling |
| UMAP | Uniform Manifold Approximation and Projection |
| GEO | Gene Expression Omnibus |
| TCGA | The Cancer Genome Atlas |
| AMP | Association for Molecular Pathology |
| CAP | College of American Pathologists |
| IASLC | International Association for the Study of Lung Cancer |
| ASCO | American Society of Clinical Oncology |
| ESMO | European Society for Medical Oncology |
| NCCN | National Comprehensive Cancer Network |
| FGFR1 | Fibroblast Growth Factor Receptor 1 |
| TIM-3 | T-Cell Immunoglobulin and Mucin Domain Containing-3 |
| TMB | Tumor Mutational Burden |
| OPN | Osteopontin |
| DNA | Deoxyribonucleic Acid |
| SNPs | Single-Nucleotide Polymorphisms |
| FDA-NIH | FDA-NIH Biomarker Working Group |
| NKX2-1 | Thyroid Transcription Factor-1 |
| TTF-1 | Thyroid Transcription Factor-1 |
| NAPSA | Napsin A |
| CYFRA 21-1 | Cytokeratin 19 Fragment |
| SCCA | Squamous Cell Carcinoma Antigen |
| m6A | N6-Methyladenine |
| SFTA2 | Surfactant Protein A2 |
| KIAA1522 | KIAA1522 Protein |
| PFS | Progression-Free Survival |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| RCTs | Randomized Clinical Trials |
| MPR | Major Pathological Response |
| TPS | Tumor Proportion Score |
| MDR | Minimal Residual Disease |
| hVAF | High Variant Allele Frequency |
| VAFSD | Variant Allele Frequency Standard Deviation |
| RECIST | Response Evaluation Criteria In Solid Tumors |
| ORR | Objective Response Rate |
| BRCA2 | Breast Cancer 2, Early Onset |
| DSPP | Dentin Sialophosphoprotein |
| CTCF | CCCTC-Binding Factor |
| OVOL2 | Ovo-Like Transcriptional Repressor 2 |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| ABCP | Atezolizumab Bevacizumab Carboplatin Paclitaxel |
| BCP | Bevacizumab Carboplatin Paclitaxel |
| KEAP1 | Kelch-Like ECH-Associated Protein |
| TP53 | Tumor Protein p53 |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| ddPCR | Droplet Digital Polymerase Chain Reaction |
| LP52 | 52-Gene Expression Histology Classifier |
| BRAFV600E | BRAF Gene Mutation V600E |
| ASOs | Antisense Oligonucleotides |
| siRNAs | Small Interfering RNAs |
Appendix A
Reference search algorithms:
(Non small cell lung cancer) AND (Biomarker); ((Biomarker) AND (Predictive)) AND (Non small cell lung cancer); Non small cell lung cancer biomarker monitoring; ((Non small cell lung cancer) AND (biomarker)) AND (monitoring); (((non small cell lung cancer) AND (biomarkers)) AND (immunotherapy)) AND (chemotherapy).
References
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non–Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-Small-Cell Lung Cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and Prospects of Early Detection in Lung Cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Polanco, D.; Pinilla, L.; Gracia-Lavedan, E.; Mas, A.; Bertran, S.; Fierro, G.; Seminario, A.; Gómez, S.; Barbé, F. Prognostic Value of Symptoms at Lung Cancer Diagnosis: A Three-Year Observational Study. J. Thorac. Dis. 2021, 13, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-Small Cell Lung Cancer (NSCLC): A Review of Risk Factors, Diagnosis, and Treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Rizzo, S.; Attili, I.; Passaro, A.; Zilli, T.; Martucci, F.; Bonomo, L.; Del Grande, F.; Casiraghi, M.; De Marinis, F.; et al. Stage III Non-Small-Cell Lung Cancer: An Overview of Treatment Options. Curr. Oncol. 2023, 30, 3160–3175. [Google Scholar] [CrossRef] [PubMed]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, Y.; Wen, Y.; Zhou, C. Non-small Cell Lung Cancer in China. Cancer Commun. 2022, 42, 937–970. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.F.; Mejía, S.A.; Viola, L.; Chamorro, D.F.; Rojas, L.; Ruíz-Patiño, A.; Serna, A.; Martínez, S.; Muñoz, Á.; Rodríguez, J.; et al. Lung Cancer in Colombia. J. Thorac. Oncol. 2022, 17, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-Small-Cell Lung Cancers: A Heterogeneous Set of Diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Senosain, M.-F.; Massion, P.P. Intratumor Heterogeneity in Early Lung Adenocarcinoma. Front. Oncol. 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Flury, D.V.; Minervini, F.; Kocher, G.J. Heterogeneity of Stage IIIA Non-Small Cell Lung Cancer—Different Tumours, Different Nodal Status, Different Treatment, Different Prognosis: A Narrative Review. Curr. Chall Thorac. Surg. 2022, 4, 13. [Google Scholar] [CrossRef]
- Palumbo, A.; De Oliveira Meireles Da Costa, N.; Bonamino, M.H.; Ribeiro Pinto, L.F.; Nasciutti, L.E. Genetic Instability in the Tumor Microenvironment: A New Look at an Old Neighbor. Mol. Cancer 2015, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Siveke, J.T. Fibroblast-Activating Protein: Targeting the Roots of the Tumor Microenvironment. J. Nucl. Med. 2018, 59, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dai, Y. Tumor Microenvironment and Therapeutic Response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bevere, M.; Masetto, F.; Carazzolo, M.E.; Bettega, A.; Gkountakos, A.; Scarpa, A.; Simbolo, M. An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics 2023, 13, 2820. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D. Identification of Driver Mutations in Lung Cancer: First Step in Personalized Cancer. Targ. Oncol. 2013, 8, 3–14. [Google Scholar] [CrossRef]
- Zografos, E.; Dimitrakopoulos, F.-I.; Koutras, A. Prognostic Value of Circulating Tumor DNA (ctDNA) in Oncogene-Driven NSCLC: Current Knowledge and Future Perspectives. Cancers 2022, 14, 4954. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, F.; Rachiglio, A.M.; Iannaccone, A.; Chicchinelli, N.; Normanno, N. Circulating Tumor Cells and ctDNA in NSCLC. In Oncogenomics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 465–475. ISBN 978-0-12-811785-9. [Google Scholar]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Latini, A.; Borgiani, P.; Novelli, G.; Ciccacci, C. miRNAs in Drug Response Variability: Potential Utility as Biomarkers for Personalized Medicine. Pharmacogenomics 2019, 20, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, J.; Cui, Y.; Fan, K.; Zhou, X. Circulating microRNA-21 as Noninvasive Predictive Biomarker for Response in Cancer Immunotherapy. Med. Hypotheses 2013, 81, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Liu, C.; Zhang, X.; Wu, Y.; Diao, M.; Tan, S.; Huang, S.; Cheng, Y.; You, T. MicroRNA-21 as a Diagnostic and Prognostic Biomarker of Lung Cancer: A Systematic Review and Meta-Analysis. Biosci. Rep. 2022, 42, BSR20211653. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Yang, W.; Chen, Q.; Zhang, X.; Han, B. Screening for Early Stage Lung Cancer and Its Correlation with Lung Nodule Detection. J. Thorac. Dis. 2018, 10, S846–S859. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Buttitta, F.; D’Angelo, E. Adjuvant Osimertinib Treatment in Patients with Early Stage NSCLC (IB-IIIA): Pathological Pathway Adaptations. Oncotarget 2022, 13, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Sabari, J.K.; Santini, F.; Bergagnini, I.; Lai, W.V.; Arbour, K.C.; Drilon, A. Changing the Therapeutic Landscape in Non-Small Cell Lung Cancers: The Evolution of Comprehensive Molecular Profiling Improves Access to Therapy. Curr. Oncol. Rep. 2017, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Coco, S.; Truini, A.; Vanni, I.; Dal Bello, M.; Alama, A.; Rijavec, E.; Genova, C.; Barletta, G.; Sini, C.; Burrafato, G.; et al. Next Generation Sequencing in Non-Small Cell Lung Cancer: New Avenues Toward the Personalized Medicine. CDT 2015, 16, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Mariniello, A.; Righi, L.; Capozza, M.; Delcuratolo, M.D.; Terreno, E.; Farsad, M.; Volante, M.; Novello, S.; Deandreis, D. NSCLC Biomarkers to Predict Response to Immunotherapy with Checkpoint Inhibitors (ICI): From the Cells to In Vivo Images. Cancers 2021, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- Pretelli, G.; Spagnolo, C.C.; Ciappina, G.; Santarpia, M.; Pasello, G. Overview on Therapeutic Options in Uncommon EGFR Mutant Non-Small Cell Lung Cancer (NSCLC): New Lights for an Unmet Medical Need. Int. J. Mol. Sci. 2023, 24, 8878. [Google Scholar] [CrossRef] [PubMed]
- Simarro, J.; Pérez-Simó, G.; Mancheño, N.; Ansotegui, E.; Muñoz-Núñez, C.F.; Gómez-Codina, J.; Juan, Ó.; Palanca, S. Impact of Molecular Testing Using Next-Generation Sequencing in the Clinical Management of Patients with Non-Small Cell Lung Cancer in a Public Healthcare Hospital. Cancers 2023, 15, 1705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tam, K.Y. Combination Strategies Using EGFR-TKi in NSCLC Therapy: Learning from the Gap between Pre-Clinical Results and Clinical Outcomes. Int. J. Biol. Sci. 2018, 14, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Kambartel, K.; Häntschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2022, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent Advances in Non-Small Cell Lung Cancer Targeted Therapy; an Update Review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z. Non-Small Cell Lung Cancer Targeted Therapy: Drugs and Mechanisms of Drug Resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, J.; Qin, C.; Yan, H.; Liu, T.; Hu, H.; Tang, S.; Tang, S.; Zhou, H. Biomarker-Targeted Therapies in Non–Small Cell Lung Cancer: Current Status and Perspectives. Cells 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Saller, J.J.; Boyle, T.A. Molecular Pathology of Lung Cancer. Cold Spring Harb. Perspect. Med. 2022, 12, a037812. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Saifi, M.; Batra, U.; Suryavanshi, M.; Gupta, K. Incidence of ROS1-Rearranged Non-Small-Cell Lung Carcinoma in India and Efficacy of Crizotinib in Lung Adenocarcinoma Patients. LCTT 2020, 11, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS. Med. 2009, 15, 264–269. [Google Scholar] [CrossRef]
- Cochrane Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 9 June 2023).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Brown, C.; Mersiades, A.; Lee, C.K.; John, T.; Kao, S.; Newnham, G.; O’Byrne, K.; Parakh, S.; Bray, V.; et al. A Phase II Trial of Alternating Osimertinib and Gefitinib Therapy in Advanced EGFR-T790M Positive Non-Small Cell Lung Cancer: OSCILLATE. Nat. Commun. 2024, 15, 1823. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Han, J.; Ahn, M.; Ramalingam, S.S.; Delmonte, A.; Hsia, T.; Laskin, J.; Kim, S.; He, Y.; Tsai, C.; et al. Epidermal Growth Factor Receptor Mutation Analysis in Tissue and Plasma from the AURA3 Trial: Osimertinib versus Platinum-pemetrexed for T790M Mutation-positive Advanced Non–Small Cell Lung Cancer. Cancer 2020, 126, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Tsuboi, M.; Kenmotsu, H.; Yamanaka, T.; Takahashi, T.; Goto, K.; Daga, H.; Ohira, T.; Ueno, T.; Aoki, T.; et al. Tumor Mutation Burden as a Biomarker for Lung Cancer Patients Treated with Pemetrexed and Cisplatin (the JIPANG-TR). Cancer Sci. 2021, 112, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-K.; Cho, H.-J.; Choi, Y.-D.; Oh, I.-J.; Kim, Y.-C. A Phase II Trial of Osimertinib as the First-Line Treatment of Non–Small Cell Lung Cancer Harboring Activating EGFR Mutations in Circulating Tumor DNA: LiquidLung-O-Cohort 1. Cancer Res. Treat. 2021, 53, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, J.; Xu, X.; Jiang, T.; Cheng, Y.; Chen, G.; Pan, Y.; Fang, Y.; Wang, Q.; Huang, Y.; et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J. Thorac. Oncol. 2022, 17, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Liu, G.; Lu, S.; He, J.; Burotto, M.; Ahn, M.-J.; Kim, D.-W.; Liu, X.; Zhao, Y.; Vincent, S.; et al. Brigatinib Versus Alectinib in ALK-Positive NSCLC After Disease Progression on Crizotinib: Results of Phase 3 ALTA-3 Trial. J. Thorac. Oncol. 2023, 18, 1743–1755. [Google Scholar] [CrossRef]
- Riess, J.W.; Reckamp, K.L.; Frankel, P.; Longmate, J.; Kelly, K.A.; Gandara, D.R.; Weipert, C.M.; Raymond, V.M.; Keer, H.N.; Mack, P.C.; et al. Erlotinib and Onalespib Lactate Focused on EGFR Exon 20 Insertion Non-Small Cell Lung Cancer (NSCLC): A California Cancer Consortium Phase I/II Trial (NCI 9878). Clin. Lung Cancer 2021, 22, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo, G.; Sgambelluri, F.; Prelaj, A.; Galli, F.; Manglaviti, S.; Bottiglieri, A.; Di Mauro, R.M.; Ferrara, R.; Galli, G.; Signorelli, D.; et al. PEOPLE (NCT03447678), a First-Line Phase II Pembrolizumab Trial, in Negative and Low PD-L1 Advanced NSCLC: Clinical Outcomes and Association with Circulating Immune Biomarkers. ESMO Open 2022, 7, 100645. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Reck, M.; Nishio, K.; Heymach, J.V.; Nishio, M.; Novello, S.; Paz-Ares, L.; Popat, S.; Aix, S.P.; Graham, H.; et al. Ramucirumab plus Erlotinib versus Placebo plus Erlotinib in Previously Untreated EGFR-Mutated Metastatic Non-Small-Cell Lung Cancer (RELAY): Exploratory Analysis of next-Generation Sequencing Results. ESMO Open 2023, 8, 101580. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, J.; Tang, X.; Zhu, H.; Zhu, D.; Zhang, X.; Meng, X.; Hua, Y.; Wang, Z.; Zhang, Y.; et al. Efficacy and Safety of Sintilimab plus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study in China. J. Cancer Res. Clin. Oncol. 2023, 149, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Kuziora, M.; Quinn, K.J.; Helman, E.; Ye, J.; Liu, F.; Scheuring, U.; Peters, S.; Rizvi, N.A.; Brohawn, P.Z.; et al. A Blood-Based Assay for Assessment of Tumor Mutational Burden in First-Line Metastatic NSCLC Treatment: Results from the MYSTIC Study. Clin. Cancer Res. 2021, 27, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, P.; Zhang, J.; Zhao, Y.; Zhou, J.; Fan, Y.; Shu, Y.; Liu, X.; Zhang, H.; He, J.; et al. Toripalimab plus Chemotherapy as Second-Line Treatment in Previously EGFR-TKI Treated Patients with EGFR-Mutant-Advanced NSCLC: A Multicenter Phase-II Trial. Sig. Transduct. Target Ther. 2021, 6, 355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, G.; Niu, Y.; Zhang, G.; Ji, Y.; Yan, X.; Zhang, X.; Wang, Q.; Jing, X.; Wang, J.; et al. Sintilimab with Two Cycles of Chemotherapy for the Treatment of Advanced Squamous Non-Small Cell Lung Cancer: A Phase 2 Clinical Trial. Nat. Commun. 2024, 15, 1512. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Velcheti, V.; Mekhail, T.; Yun, C.; Shagan, S.M.; Hu, S.; Chae, Y.K.; Leal, T.A.; Dowell, J.E.; Tsai, M.L.; et al. Blood-Based Tumor Mutational Burden as a Biomarker for Atezolizumab in Non-Small Cell Lung Cancer: The Phase 2 B-F1RST Trial. Nat. Med. 2022, 28, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Dziadziuszko, R.; Morabito, A.; Felip, E.; Gadgeel, S.M.; Cheema, P.; Cobo, M.; Andric, Z.; Barrios, C.H.; Yamaguchi, M.; et al. Atezolizumab versus Chemotherapy in Advanced or Metastatic NSCLC with High Blood-Based Tumor Mutational Burden: Primary Analysis of BFAST Cohort C Randomized Phase 3 Trial. Nat. Med. 2022, 28, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Oezkan, F.; Kris, M.G.; Bunn, P.A.; Wistuba, I.I.; Kwiatkowski, D.J.; Owen, D.H.; Tang, Y.; Johnson, B.E.; Lee, J.M.; et al. Neoadjuvant Atezolizumab for Resectable Non-Small Cell Lung Cancer: An Open-Label, Single-Arm Phase II Trial. Nat. Med. 2022, 28, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, L.; Yu, X.; Xing, P.; Wang, Y.; Zhou, J.; Wang, A.; Shi, J.; Hu, Y.; Wang, Z.; et al. Sintilimab versus Docetaxel as Second-line Treatment in Advanced or Metastatic Squamous Non-small-cell Lung Cancer: An Open-label, Randomized Controlled Phase 3 Trial (ORIENT-3). Cancer Commun. 2022, 42, 1314–1330. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-K.; Jun, H.R.; Oh, H.-J.; Lee, J.-Y.; Cho, H.-J.; Kim, Y.-C.; Lee, J.E.; Yoon, S.H.; Choi, C.M.; Lee, J.C.; et al. Evaluation of Blood Tumor Mutation Burden for the Efficacy of Second-Line Atezolizumab Treatment in Non-Small Cell Lung Cancer: BUDDY Trial. Cells 2023, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, H.; Lu, Y.; Li, M.; Yang, S.; Liang, J.; Ye, Z.; Li, Z.; He, M.; Shi, X.; et al. EGFR-TKI Combined with Pemetrexed versus EGFR-TKI Monotherapy in Advanced EGFR-Mutated NSCLC: A Prospective, Randomized, Exploratory Study. Cancer Res. Treat. 2023, 55, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tang, X.; Zhu, H.; Zhu, D.; Zhang, X.; Meng, X.; Hua, Y.; Wang, Z.; Zhang, Y.; Huang, W.; et al. Short-Term Dynamics of Circulating Tumor DNA Predicting Efficacy of Sintilimab plus Docetaxel in Second-Line Treatment of Advanced NSCLC: Biomarker Analysis from a Single-Arm, Phase 2 Trial. J. Immunother. Cancer 2022, 10, e004952. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhang, X.; Tian, P.; Chu, T.; Guo, Q.; Yu, X.; Yu, Z.; Li, Y.; Chen, L.; Liu, J.; et al. Tislelizumab plus Chemotherapy for Patients with EGFR -Mutated Non-Squamous Non-Small Cell Lung Cancer Who Progressed on EGFR Tyrosine Kinase Inhibitor Therapy. J. Immunother. Cancer 2023, 11, e006887. [Google Scholar] [CrossRef]
- García-Pardo, M.; Czarnecka-Kujawa, K.; Law, J.H.; Salvarrey, A.M.; Fernandes, R.; Fan, Z.J.; Waddell, T.K.; Yasufuku, K.; Liu, G.; Donahoe, L.L.; et al. Association of Circulating Tumor DNA Testing Before Tissue Diagnosis with Time to Treatment Among Patients With Suspected Advanced Lung Cancer: The ACCELERATE Nonrandomized Clinical Trial. JAMA Netw. Open 2023, 6, e2325332. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Goto, Y.; Mizutani, T.; Kataoka, T.; Kawai, S.; Okuma, Y.; Murakami, H.; Tanaka, K.; Ohe, Y. A Randomized Phase III Study Comparing Continuation and Discontinuation of PD-1 Pathway Inhibitors for Patients with Advanced Non-Small-Cell Lung Cancer (JCOG1701, SAVE Study). Jpn. J. Clin. Oncol. 2020, 50, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Ciardiello, D.; Dallio, M.; Famiglietti, V.; Esposito, L.; Corte, C.M.D.; Napolitano, S.; Fasano, M.; Gravina, A.G.; Romano, M.; et al. Gut Microbiota Correlates with Antitumor Activity in Patients with mCRC and NSCLC Treated with Cetuximab plus Avelumab. Int. J. Cancer 2022, 151, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non–Small-Cell Lung Cancer (NADIM Phase II Trial). JCO 2022, 40, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- West, H.J.; McCleland, M.; Cappuzzo, F.; Reck, M.; Mok, T.S.; Jotte, R.M.; Nishio, M.; Kim, E.; Morris, S.; Zou, W.; et al. Clinical Efficacy of Atezolizumab plus Bevacizumab and Chemotherapy in KRAS- Mutated Non-Small Cell Lung Cancer with STK11, KEAP1, or TP53 Comutations: Subgroup Results from the Phase III IMpower150 Trial. J. Immunother. Cancer 2022, 10, e003027. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo, G.; Prelaj, A.; Dolezal, J.; Beninato, T.; Agnelli, L.; Triulzi, T.; Fabbri, A.; Lorenzini, D.; Ferrara, R.; Brambilla, M.; et al. PEOPLE (NTC03447678), a Phase II Trial to Test Pembrolizumab as First-Line Treatment in Patients with Advanced NSCLC with PD-L1 <50%: A Multiomics Analysis. J. Immunother. Cancer 2023, 11, e006833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Srivastava, M.K.; Xu, H.; Felip, E.; Wakelee, H.; Altorki, N.; Reck, M.; Liersch, R.; Kryzhanivska, A.; Oizumi, S.; et al. Comparison of SP263 and 22C3 Immunohistochemistry PD-L1 Assays for Clinical Efficacy of Adjuvant Atezolizumab in Non-Small Cell Lung Cancer: Results from the Randomized Phase III IMpower010 Trial. J. Immunother. Cancer 2023, 11, e007047. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Takahama, T.; Shimokawa, M.; Azuma, K.; Takeda, M.; Kato, T.; Daga, H.; Okamoto, I.; Akamatsu, H.; Teraoka, S.; et al. Predicting Osimertinib-treatment Outcomes through EGFR Mutant-fraction Monitoring in the Circulating Tumor DNA of EGFR T790M-positive Patients with Non-small Cell Lung Cancer (WJOG8815L). Mol. Oncol. 2021, 15, 126–137. [Google Scholar] [CrossRef]
- Redman, M.W.; Papadimitrakopoulou, V.A.; Minichiello, K.; Hirsch, F.R.; Mack, P.C.; Schwartz, L.H.; Vokes, E.; Ramalingam, S.; Leighl, N.; Bradley, J.; et al. Biomarker-Driven Therapies for Previously Treated Squamous Non-Small-Cell Lung Cancer (Lung-MAP SWOG S1400): A Biomarker-Driven Master Protocol. Lancet Oncol. 2020, 21, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Redman, M.W.; Moon, J.; Agustoni, F.; Herbst, R.S.; Semrad, T.J.; Varella-Garcia, M.; Rivard, C.J.; Kelly, K.; Gandara, D.R.; et al. EGFR High Copy Number Together With High EGFR Protein Expression Predicts Improved Outcome for Cetuximab-Based Therapy in Squamous Cell Lung Cancer: Analysis From SWOG S0819, a Phase III Trial of Chemotherapy With or Without Cetuximab in Advanced NSCLC. Clin. Lung Cancer 2022, 23, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Berardi, R.; Lim, W.-T.; De Jonge, M.; Bauer, T.M.; Azaro, A.; Gottfried, M.; Han, J.-Y.; Lee, D.H.; Wollner, M.; et al. Molecular Correlates of Response to Capmatinib in Advanced Non-Small-Cell Lung Cancer: Clinical and Biomarker Results from a Phase I Trial. Ann. Oncol. 2020, 31, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.; Hirsch, F.R.; Kerr, K.; Barlesi, F.; Park, K.; Rittmeyer, A.; Zou, W.; Bhatia, N.; Koeppen, H.; Paul, S.M.; et al. Comparison of SP142 and 22C3 Immunohistochemistry PD-L1 Assays for Clinical Efficacy of Atezolizumab in Non–Small Cell Lung Cancer: Results From the Randomized OAK Trial. Clin. Lung Cancer 2022, 23, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Novello, S.; Guclu, S.Z.; Bentsion, D.; Zvirbule, Z.; Szilasi, M.; Bernabe, R.; Syrigos, K.; Byers, L.A.; Clingan, P.; et al. Veliparib in Combination With Platinum-Based Chemotherapy for First-Line Treatment of Advanced Squamous Cell Lung Cancer: A Randomized, Multicenter Phase III Study. JCO 2021, 39, 3633–3644. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, Y.; Chen, S.; Ying, S.; Xu, S.; Huang, J.; Wu, D.; Lv, D.; Bei, T.; Liu, S.; et al. Efficacy and Safety of Pyrotinib in Advanced Lung Adenocarcinoma with HER2 Mutations: A Multicenter, Single-Arm, Phase II Trial. BMC Med. 2022, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Ho, C.; Nicholas, G.; Juergens, R.A.; Sacher, A.; Fung, A.S.; Wheatley-Price, P.; Laurie, S.A.; Levy, B.; Brahmer, J.R.; et al. ctDNA Response after Pembrolizumab in Non-Small Cell Lung Cancer: Phase 2 Adaptive Trial Results. Nat. Med. 2023, 29, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, S.; Lee, J.C.; Choi, C.; Lee, S.Y.; Jang, T.; Oh, I.; Kim, Y. Phase II OPEN-LABEL Multicenter Study to Assess the Antitumor Activity of Afatinib in Lung Cancer Patients with Activating Epidermal Growth Factor Receptor Mutation from Circulating Tumor DNA: LIQUID-LUNG-A. Thoracic. Cancer 2021, 12, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Qian, J.; Xu, M.-D.; Gu, K.; Qian, F.-F.; Lu, J.; Zhang, X.-Y.; Wang, H.-M.; Yan, B.; Zhang, B.; et al. Prognostic and Predictive Value of Blood Tumor Mutational Burden in Patients With Lung Cancer Treated With Docetaxel. J. Natl. Compr. Cancer Netw. 2020, 18, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.; Bauer, H.; Au, T.H.; Menon, J.; Unni, S.; Tran, D.; Rivers, Z.; Akerley, W.; Schabath, M.B.; Badin, F.; et al. Real-World Survival Analysis by Tumor Mutational Burden in Non-Small Cell Lung Cancer: A Multisite U.S. Study. Oncotarget 2022, 13, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yao, M.; Yang, Y. Higher Tumor Mutation Burden Was a Predictor for Better Outcome for NSCLC Patients Treated with PD-1 Antibodies: A Systematic Review and Meta-Analysis. SLAS Technol. 2021, 26, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, J.; Chen, L.; Guo, L.; Ye, M.; Wu, Y.; Ji, Q. Plasma miR-32 Levels in Non-Small Cell Lung Cancer Patients Receiving Platinum-Based Chemotherapy Can Predict the Effectiveness and Prognosis of Chemotherapy. Medicine 2019, 98, e17335. [Google Scholar] [CrossRef] [PubMed]
- Simeonidis, S.; Koutsilieri, S.; Vozikis, A.; Cooper, D.N.; Mitropoulou, C.; Patrinos, G.P. Application of Economic Evaluation to Assess Feasibility for Reimbursement of Genomic Testing as Part of Personalized Medicine Interventions. Front. Pharmacol. 2019, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Fagery, M.; Khorshidi, H.A.; Wong, S.Q.; Vu, M.; IJzerman, M. Health Economic Evidence and Modeling Challenges for Liquid Biopsy Assays in Cancer Management: A Systematic Literature Review. PharmacoEconomics 2023, 41, 1229–1248. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.M.; Leighl, N.B. Economic Impact of Tissue Testing and Treatments of Metastatic NSCLC in the Era of Personalized Medicine. Front. Oncol. 2014, 4, 258. [Google Scholar] [CrossRef] [PubMed]
- Hofmarcher, T.; Malmberg, C.; Lindgren, P. A Global Analysis of the Value of Precision Medicine in Oncology—The Case of Non-Small Cell Lung Cancer. Front. Med. 2023, 10, 1119506. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Anothaisintawee, T.; Butani, D.; Wang, Y.; Zemlyanska, Y.; Wong, C.B.N.; Virabhak, S.; Hrishikesh, M.A.; Teerawattananon, Y. Assessing the Cost-Effectiveness of Precision Medicine: Protocol for a Systematic Review and Meta-Analysis. BMJ Open 2022, 12, e057537. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Postel, M.; Roosen, A.; Laurent-Puig, P.; Taly, V.; Wang-Renault, S.-F. Droplet-Based Digital PCR and next Generation Sequencing for Monitoring Circulating Tumor DNA: A Cancer Diagnostic Perspective. Expert Rev. Mol. Diagn. 2018, 18, 7–17. [Google Scholar] [CrossRef]
- Valpione, S.; Campana, L. Detection of Circulating Tumor DNA (ctDNA) by Digital Droplet Polymerase Chain Reaction (Dd-PCR) in Liquid Biopsies. Methods Enzymol. 2019, 629, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Van Den Broek, D.; Denis, M.G.; Hofman, P.; Hubank, M.; Mouliere, F.; Paz-Ares, L.; Schuuring, E.; Sültmann, H.; Vainer, G.; et al. Recommendations for a Practical Implementation of Circulating Tumor DNA Mutation Testing in Metastatic Non-Small-Cell Lung Cancer. ESMO Open 2022, 7, 100399. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Zhu, W.-Y.; Huang, Y.-Y.; Ma, L.-N.; Zhou, S.-Q.; Wang, Y.-K.; Zeng, F.; Zhou, J.-H.; Zhang, Y.-K. High Expression of Serum miR-21 and Tumor miR-200c Associated with Poor Prognosis in Patients with Lung Cancer. Med. Oncol. 2012, 29, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cao, Y.; He, Z.; He, J.; Hu, C.; Duan, H.; Jiang, J. Serum Levels of miR-19b and miR-146a as Prognostic Biomarkers for Non-Small Cell Lung Cancer. Tohoku J. Exp. Med. 2014, 232, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, Y.; Liu, X.; Xi, X.; Xue, W.; Qu, Y. Non-Small Cell Lung Cancer Associated microRNA Expression Signature: Integrated Bioinformatics Analysis, Validation and Clinical Significance. Oncotarget 2017, 8, 24564–24578. [Google Scholar] [CrossRef] [PubMed]
- Schuurbiers, M.; Huang, Z.; Saelee, S.; Javey, M.; de Visser, L.; van den Broek, D.; van den Heuvel, M.; Lovejoy, A.F.; Monkhorst, K.; Klass, D. Biological and Technical Factors in the Assessment of Blood-Based Tumor Mutational Burden (bTMB) in Patients with NSCLC. J. Immunother. Cancer 2022, 10, e004064. [Google Scholar] [CrossRef] [PubMed]
- Raiber-Moreau, E.-A.; Portella, G.; Butler, M.G.; Clement, O.; Konigshofer, Y.; Hadfield, J. Development and Validation of Blood Tumor Mutational Burden Reference Standards. Genes Chromosomes Cancer 2023, 62, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, T.R.; Taube, J.M. PD-L1 and Emerging Biomarkers in Immune Checkpoint Blockade Therapy. Cancer J. 2018, 24, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Domblides, C.; Ruppert, A.-M.; Antoine, M.; Wislez, M. Immunotherapy’s New Challenge: Identification of Predictive Biomarkers for Tumor Response. Transl. Cancer Res. 2017, 6, S306–S308. [Google Scholar] [CrossRef]
- Goh, K.Y.; Cheng, T.Y.D.; Tham, S.C.; Lim, D.W.-T. Circulating Biomarkers for Prediction of Immunotherapy Response in NSCLC. Biomedicines 2023, 11, 508. [Google Scholar] [CrossRef]
- Cao, W.; Tang, Q.; Zeng, J.; Jin, X.; Zu, L.; Xu, S. A Review of Biomarkers and Their Clinical Impact in Resected Early-Stage Non-Small-Cell Lung Cancer. Cancers 2023, 15, 4561. [Google Scholar] [CrossRef]
- Tostes, K.; Siqueira, A.P.; Reis, R.M.; Leal, L.F.; Arantes, L.M.R.B. Biomarkers for Immune Checkpoint Inhibitor Response in NSCLC: Current Developments and Applicability. Int. J. Mol. Sci. 2023, 24, 11887. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, P.; Backes, C.; Blatt, M.; Keller, A.; Huwer, H.; Lepper, P.; Bals, R.; Meese, E. The Blood-Borne miRNA Signature of Lung Cancer Patients Is Independent of Histology but Influenced by Metastases. Mol. Cancer 2014, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; Van Der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, X.; Cheng, Y.; Zhang, Y.; Xia, L.; Xia, W.; Lu, S. Exosomal PD-L1 Predicts Response with Immunotherapy in NSCLC Patients. Clin. Exp. Immunol. 2022, 208, 316–322. [Google Scholar] [CrossRef]
- Massafra, M.; Passalacqua, M.I.; Gebbia, V.; Macrì, P.; Lazzari, C.; Gregorc, V.; Buda, C.; Altavilla, G.; Santarpia, M. Immunotherapeutic Advances for NSCLC. BTT 2021, 15, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Barraco, N.; Bono, M.; Corsini, L.R.; Galvano, A.; Gristina, V.; Listì, A.; Vieni, S.; et al. Programmed Death Ligand 1 (PD-L1) as a Predictive Biomarker for Pembrolizumab Therapy in Patients with Advanced Non-Small-Cell Lung Cancer (NSCLC). Adv. Ther. 2019, 36, 2600–2617. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Pulliam, S.; Karki, N.R.; Khan, J.; Jogezai, S.; Sultan, S.; Muhammad, L.; Khan, M.; Jamil, N.; Waheed, A.; et al. PD-L1 Over-Expression Varies in Different Subtypes of Lung Cancer: Will This Affect Future Therapies? Clin. Pract. 2022, 12, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Kwong, L.N. Diagnostic and Therapeutic Applications of miRNA-Based Strategies to Cancer Immunotherapy. Cancer Metastasis Rev. 2018, 37, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wang, Z.; Lu, Z.; Xia, J.; Xie, Z.; Jiao, M.; Liu, R.; Chu, Y. MicroRNAs: Immune Modulators in Cancer Immunotherapy. Immunother. Adv. 2021, 1, ltab006. [Google Scholar] [CrossRef]
- Sirker, M.; Liang, W.; Zhang, L.; Fimmers, R.; Rothschild, M.A.; Gomes, I.; Schneider, P.M. Impact of Using Validated or Standard Reference Genes for miRNA qPCR Data Normalization in Cell Type Identification. Forensic Sci. Int. Genet. Suppl. Ser. 2015, 5, e199–e201. [Google Scholar] [CrossRef]
- Nuzziello, N.; Liguori, M. Seeking a Standardized Normalization Method for the Quantification of microRNA Expression. Muscle Nerve 2019, 59, E39. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, F.E.C.; Guevara-Montoya, M.C.; Serna-Ramirez, V.; Liscano, Y. Neuroinflammation and Schizophrenia: New Therapeutic Strategies through Psychobiotics, Nanotechnology, and Artificial Intelligence (AI). JPM 2024, 14, 391. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, F.E.C.; Lizcano Martinez, S.; Liscano, Y. Effectiveness of Psychobiotics in the Treatment of Psychiatric and Cognitive Disorders: A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 1352. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome Is Associated with Favorable Responses to Anti–Programmed Death 1 Immunotherapy in Chinese Patients with NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ferrero, M.; Dong, N.; D’Auria, G.; Reyes-Prieto, M.; Herreros-Pomares, A.; Calabuig-Fariñas, S.; Duréndez, E.; Aparisi, F.; Blasco, A.; et al. Analysis of the Gut Microbiota: An Emerging Source of Biomarkers for Immune Checkpoint Blockade Therapy in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Iannone, L.F.; Bersanelli, M.; Mini, E.; Catalano, M. The Gut Microbiome and Efficacy of Cancer Immunotherapy. Pharmacol. Ther. 2022, 231, 107973. [Google Scholar] [CrossRef]
- Katayama, Y.; Yamada, T.; Shimamoto, T.; Iwasaku, M.; Kaneko, Y.; Uchino, J.; Takayama, K. The Role of the Gut Microbiome on the Efficacy of Immune Checkpoint Inhibitors in Japanese Responder Patients with Advanced Non-Small Cell Lung Cancer. Transl. Lung. Cancer Res. 2019, 8, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Ng, T.L. A Narrative Review from Gut to Lungs: Non-Small Cell Lung Cancer and the Gastrointestinal Microbiome. Transl. Lung. Cancer Res. 2023, 12, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, M.; Salvo, E.D.; Pioggia, G.; Gangemi, S. Microbiota and microRNAs in Lung Diseases: Mutual Influence and Role Insights. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 13000–13008. [Google Scholar] [PubMed]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology 2023, 164, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M. Training the Microbiota to Increase Immune Checkpoint Blockade and to Reduce Toxicity. Eur. J. Immunol. 2023, 53, 2250183. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Kim, H.; Koh, A. Approaching Precision Medicine by Tailoring the Microbiota. Mamm. Genome 2021, 32, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Musolino, C.; Tonacci, A.; Pioggia, G.; Gangemi, S. Interactions between the MicroRNAs and Microbiota in Cancer Development: Roles and Therapeutic Opportunities. Cancers 2020, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, K.; Dhar, R.; Pethusamy, K.; Srivastava, T.; Shankar, A.; Rath, G.K.; Karmakar, S. The Issues and Challenges with Cancer Biomarkers. J. Cancer Res. Ther. 2023, 19, S20–S35. [Google Scholar] [CrossRef]
- Truesdell, J.; Miller, V.A.; Fabrizio, D. Approach to Evaluating Tumor Mutational Burden in Routine Clinical Practice. Transl. Lung. Cancer Res. 2018, 7, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, J.C.; Dueñas, D.; Corredor, Z.; Liscano, Y. Advances in Genomic Data and Biomarkers: Revolutionizing NSCLC Diagnosis and Treatment. Cancers 2023, 15, 3474. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, D.; Hiltermann, T.J.N.; Biesma, B.; Dinjens, W.N.M.; ‘T Hart, N.A.; Hinrichs, J.W.J.; Leers, M.P.G.; Monkhorst, K.; Van Oosterhout, M.; Scharnhorst, V.; et al. Implementation of Novel Molecular Biomarkers for Non-Small Cell Lung Cancer in the Netherlands: How to Deal with Increasing Complexity. Front. Oncol. 2020, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. The Challenges of Evaluating Predictive Biomarkers Using Small Biopsy Tissue Samples and Liquid Biopsies from Non-Small Cell Lung Cancer Patients. J. Thorac. Dis. 2019, 11, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Das, B.; Shin, S.; Chen, A. Challenges and Future Directions in the Management of Tumor Mutational Burden-High (TMB-H) Advanced Solid Malignancies. Cancers 2023, 15, 5841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, J.; Wang, G.; He, X.; Mi, Y.; Cao, Y.; Yu, X. Predictive Efficacy of Blood-Based Tumor Mutation Burden Assay for Immune Checkpoint Inhibitors Therapy in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 795933. [Google Scholar] [CrossRef] [PubMed]
- Arghiani, N.; Shah, K. Modulating microRNAs in Cancer: Next-Generation Therapies. Cancer Biol. Med. 2021, 19, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, K.; Benhamou, R.I. Targeting MicroRNAs with Small Molecules. ncRNA 2024, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Pimienta, D.A.; Cruz Mosquera, F.E.; Palacios Velasco, I.; Giraldo Rodas, M.; Oñate-Garzón, J.; Liscano, Y. Specific Focus on Antifungal Peptides against Azole Resistant Aspergillus Fumigatus: Current Status, Challenges, and Future Perspectives. J. Fungi 2022, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wan, J.; Zhou, L.; Ma, W.; Yang, Y.; Luo, W.; Yu, Z.; Wang, H. Stimuli-responsive Nanotherapeutics for Precision Drug Delivery and Cancer Therapy. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1527. [Google Scholar] [CrossRef] [PubMed]
- Holder, J.E.; Ferguson, C.; Oliveira, E.; Lodeiro, C.; Trim, C.M.; Byrne, L.J.; Bertolo, E.; Wilson, C.M. The Use of Nanoparticles for Targeted Drug Delivery in Non-Small Cell Lung Cancer. Front. Oncol. 2023, 13, 1154318. [Google Scholar] [CrossRef] [PubMed]
- Macarrón Palacios, A.; Korus, P.; Wilkens, B.G.C.; Heshmatpour, N.; Patnaik, S.R. Revolutionizing in Vivo Therapy with CRISPR/Cas Genome Editing: Breakthroughs, Opportunities and Challenges. Front. Genome Ed. 2024, 6, 1342193. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Ma, Q.; Yin, W.; Ma, X.; He, Z. CRISPR/Cas9 Gene-Editing in Cancer Immunotherapy: Promoting the Present Revolution in Cancer Therapy and Exploring More. Front. Cell Dev. Biol. 2021, 9, 674467. [Google Scholar] [CrossRef] [PubMed]
- Yahya, S.; Abdelmenym Mohamed, S.I.; Yahya, S. Gene Editing: A Powerful Tool for Cancer Immunotherapy. Biointerface Res. Appl. Chem. 2022, 13, 98. [Google Scholar] [CrossRef]
- Mandair, D.; Reis-Filho, J.S.; Ashworth, A. Biological Insights and Novel Biomarker Discovery through Deep Learning Approaches in Breast Cancer Histopathology. NPJ. Breast. Cancer 2023, 9, 21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).