Simple Summary
This study investigates the impact of resection margin (R) status on overall survival (OS) and disease-free survival (DFS) in patients undergoing pancreaticoduodenectomy (PD) for pancreatic ductal adenocarcinoma (PDAC). A retrospective analysis of 167 PD cases from 2012 to 2023 revealed that 62.8% achieved negative margins (R0), while 37.1% had positive margins (R1). Patients with R1 status had significantly lower OS (23 vs. 36 months, p = 0.003) and DFS (10 vs. 18 months, p = 0.004) compared to R0 patients. Multivariate analysis identified R1 status and positive lymph nodes (N+) as independent factors adversely affecting both OS and DFS. Specifically, among patients with N+ disease, R1 status was associated with a notably decreased DFS (10 vs. 16 months, p = 0.05). The study concludes that achieving R0 status during PD is crucial for improved long-term outcomes, emphasizing the importance of radical surgery, especially in patients with lymph node involvement.
Abstract
The influencing role of resection margin (R) status on long-term outcomes, namely overall (OS) and disease-free survival (DFS), after pancreaticoduodenectomy (PD) for pancreatic ductal adenocarcinoma (PDAC) is not still clear. The aim of this study is to evaluate the prognostic impact of R status after PD and to define tumor characteristics associated with a positive resection margin (R1). All PDs for PDAC performed between 2012 and 2023 were retrospectively enrolled. The effect of R status, patient clinico-demographic features, and tumor features on OS and DFS were assessed. One-hundred and sixty-seven patients who underwent PD for PDAC were included in the study. R0 was achieved in 105 cases (62.8%), while R1 was evidenced in 62 patients (37.1%). R1 was associated with a decreased OS (23 (13–38) months) as compared to R0 (36 (21–53) months) (p = 0.003). Similarly, DFS was shorter in R1 patients (10 (6–25) months) as compared to the R0 cohort (18 (9–70) months) (p = 0.004), with a consequent higher recurrence rate in cases of R1 (74.2% vs. 64.8% in the R0 group; p = 0.04). In the multivariate analysis, R1 and positive lymph nodes (N+) were the only independent influencing factors for OS (OR: 1.6; 95% CI: 1–2.5; p = 0.03 and OR: 1.7; 95% CI: 1–2.8; p = 0.04) and DFS (OR: 1.5; 95% CI: 1–2.1; p = 0.04 and OR: 1.8; 95% CI: 1.1–2.7; p = 0.009). Among 111 patients with N+ disease, R1 was associated with a significantly decreased DFS (10 (8–11) months) as compared to R0N+ patients (16 (11–21) months) (p = 0.05). In conclusion, the achievement of a negative resection margin is associated with survival benefits, particularly in cases of N1 disease. In addition, R0 was recognized as an independent prognostic feature for both OS and DFS. This further outlines the relevant role of radical surgery on long-term outcomes.
1. Introduction
Despite the recent advancement in the multimodal and surgical treatment of pancreatic ductal adenocarcinoma (PDAC), long-term outcomes still remain dismal, with a poor overall survival (OS) and disease-free survival (DFS) [1,2,3]. This is due to several factors, such as the presence of perineural and lymphovascular infiltration, tumor size, and metastatic lymph nodes, recognized as independent influencing features on survival outcomes after resection [4,5,6]. Furthermore, debates are still present in the literature on the influencing role of resection margin status (R) on long-term outcomes [3]. Some authors identified R status as an independent prognostic factor after pancreaticoduodenectomy (PD), reporting a 5-year OS of 26% in cases of tumor-free resection margins (R0) as compared to 8% in case of a microscopically positive margins (R1) [5,7,8]. Conversely, other authors did not recognize R status as independently affecting survival after resection [9,10,11,12]. Such a discrepancy may be explained by the nonconsensual definition of R status. Indeed, the Union for International Cancer Control (UICC) defines R1 as the presence of tumor cells on the resection margin (0 mm) [13], while the British Royal College of Pathologists defines R1 as the presence of tumor cells within 1 mm of the resection margin [14]. In addition, more recent reports demonstrated long-term advantages, namely OS and DFS, in cases of a resection margin clearance of at least 1.5–2 mm [15,16]. These contrasting definitions have inevitably resulted in a high variation in terms of positive resection margin rate, with reports in the literature between 17% and 85% after PD [17,18,19,20].
Apart from the contrasting definitions of R status, two additional hypotheses have been postulated to justify the discrepant oncological outcomes reported on the bases of the R value. Firstly, R1 is frequently concomitant to other prognostic features such as the presence of metastatic lymph nodes that may effectively have a more significant influence on OS and DFS than R status [21,22,23]. Secondly, it is likely that tumor recurrence may be more related to an aggressive tumor biology rather than the presence of tumor cells on the resection margin, as evidenced by locoregional recurrence (LRR) occurring in 10% to 25% of patients [9,24]. Based on these premises, the aim of this study is to evaluate the impact of R status on long-term outcomes, namely OS and DFS, and to define the clinico-demographic and oncological features that may be associated with R1 after PD for PDAC in a tertiary referral center.
2. Materials and Methods
All patients who underwent PD for a histologically proven diagnosis of PDAC at the Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS of Rome between January 2012 and December 2023 were retrospectively included in the study. Patients with other histopathological diagnoses, including malignant intrapapillary mucinous neoplasms, were excluded from enrollment. Moreover, given the potential influencing role of neoadjuvant treatment on long-term outcomes, patients who underwent neoadjuvant treatment (NAT) were excluded from the analysis. All data were retrospectively retrieved from prospectively maintained databases, and follow-up data were collected until April 2024.
The collected data included age; sex; tumor location; adjuvant therapy; and histopathological features, namely tumor diameter and grading, number of harvested and metastatic lymph nodes, evaluation of lymphovascular and angio-invasion and perineural infiltration, pTNM stage according to the 8th edition of the AJCC/UICC system [13], and R status.
From 2018 onwards, all cases were preoperatively discussed in a multidisciplinary tumor board in order to assess tumor resectability [25]. Contraindications to tumor resection were the preoperative detection of distant metastases, tumor infiltration of the celiac trunk, common hepatic artery or superior mesenteric artery, or encasement of the superior mesenteric vein/portal vein. Post-operatively, all cases were re-discussed at the multidisciplinary tumor board to determine the potential indication for adjuvant therapy. Since a consistent number of patients underwent adjuvant therapy at a site other than our institution, information on the type of adjuvant treatment (chemotherapy and/or radiotherapy), dose, and frequency was not available. Thus, the study included only if adjuvant therapy was recommended.
2.1. Surgical Technique
Details regarding the surgical procedure were previously reported [26,27]. All patients underwent a Whipple procedure with standard lymphadenectomy [28]. The division of the pancreatic head was followed by dissection of the retroportal lamina from the portal/superior mesenteric vein and along the right/anterior aspect of the superior mesenteric artery. The retroportal lamina margin was always macroscopically assessed and inked with methylene blue along its length for an appropriate pathological assessment.
2.2. Post-Operative Follow-Up and Long-Term Outcomes Analysis
Follow-up information was obtained from the electronical medical records of our institution, primary care physician, or through a direct telephonic contact with the patient or relatives. When performed at our institution, post-operative follow-up included physical examination, laboratory tests (including measurement of carbohydrate antigen 19.9 and carcinoembryonic antigen levels), and transabdominal ultrasound every 3 months for the first two years after PD. Computed tomography (CT) was prescribed every 6 months after surgery. A whole-body positron emission tomography (PET) scan with 18-fluoro-2-desoxy-glucose (FDG) was indicated in cases of an inconclusive diagnosis at the CT scan.
The evaluation of long-term outcomes included tumor recurrence, OS, and DFS. Local recurrence (LR) was defined as tumor relapse in the retroperitoneum at the pancreatic remnant, regional lymph nodes, and around the mesenteric vessels and/or celiac trunk, while tumor recurrence at any other site, such as the liver, lung, para-aortic lymph nodes, and peritoneal cavity, were defined as distant metastases. OS was defined as the time from surgery to the last follow-up, while DFS was defined as the time between surgery and the detection of tumor recurrence or death.
2.3. Pathological Assessment
Macroscopic and microscopic evaluation of the surgical specimens as well as the definition of the resection margins were conducted according to the recommendation of Verbeke et al. [17,29]. Specifically, resection margins identified and evaluated were the anterior and posterior pancreatic surfaces, medial (defined as the superior mesenteric vein root), retroportal lamina (the closest margin to the superior mesenteric artery), common bile duct, and pancreatic neck. R status was defined according to the Royal College of Pathologists [14]. Specifically, the microscopic resection margin was considered to be positive (R1) when tumor cells were detected within 1 mm of the transection margin. All the histopathological slides were retrospectively reviewed by a dedicated pathologist in order to reassess the R status [14] and reclassify the tumor staging according to the 8th edition of the AJCC/UICC TNM system 2018.
2.4. Study Outcomes
The primary aim of the study was to evaluate the impact of R status on the long-term outcomes after PD in terms of local recurrence, OS, and DFS. The secondary aim was a further definition of the clinico-demographic and oncological features related to R1 and their impact on long-term survival and recurrence.
2.5. Statistical Analysis
Continuous variables were reported as medians and quartile ranks (QRs) and categorical variables as numbers and percentages. Student’s t test, Mann–Whitney U test, Fisher’s test, and the χ2 test were used for the univariate analysis. A p value ≤ 0.05 was considered statistically significant. OS and DFS were calculated using the Kaplan–Meier method, and the log-rank test was employed for the evaluation of differences in recurrence and survival between groups. Features significantly related to recurrence or survival were included in a Cox proportional hazards regression analysis. Results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Statistical significance was reached for a two-tailed p value < 0.05. SPSS version 25 for Windows (SPSS Inc., Chicago, IL, USA) was used to perform all tests.
3. Results
Between January 2012 and December 2023, 221 patients underwent PD for a histologically proven diagnosis of PDAC at the Pancreatic Surgery Unit of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS of Rome. Of these, 39 (17.6%) underwent NAT, 6 (2.7%) died during hospitalization, and 9 (4%) were lost to follow-up and were excluded from the analysis. As a whole, 167 (75.6%) patients constituted the study cohort (Figure 1), with a nearly equal distribution between males (78–46.7%) and females (89–53.3%). The median age was 69 (60.5–75) years. The majority of the lesions were located in the pancreatic head (146–87.4%), 17 (10.2%) in the uncinate process and 4 (2.4%) in the isthmus. Margin negativity (R0 group) was reached in 105 patients (62.8%), while 62 patients (37.1%) presented at least one positive margin and, thus, constituted the R1 group. Table 1 reports the clinico-demographic characteristics of the study cohort.
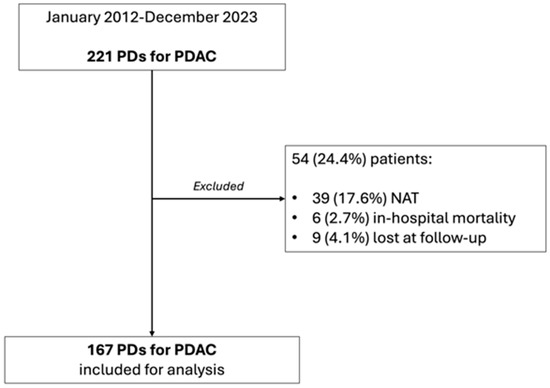
Figure 1.
Study population flowchart.

Table 1.
Clinico-demographic characteristics of the study cohort and according to resection margin status.
3.1. Comparison between R0 and R1 Patients (Table 1)
Clinico-demographic features were comparable between the R0 and R1 cohorts. Specifically, no difference was documented in terms of tumor location (p = 0.48), grading (p = 0.32), and dimension (p = 0.54), while a more advanced T staging was evidenced in the R1 cohort (p = 0.004). A tangential venous resection was needed in 9 cases (5.4%) for an intraoperative suspicion of vascular invasion, with no difference between the R0 and R1 groups (p = 0.2). The median number of harvested lymph nodes was comparable between the two groups (p = 0.28). However, the R1 cohort more frequently presented metastatic lymph nodes (48–77.4%) as compared to R0 patients (63–60%) (p = 0.02). Moreover, perineural infiltration was more frequently encountered in cases of R1 resection (60–96.8% vs. 92–87.6% in cases of R0; p = 0.05). Similarly, the R1 group presented a higher rate of angio/lymphovascular invasion than the R0 cohort (p = 0.03). Adjuvant therapy was administered equally to R0 and R1 patients (p = 0.24). In this last regard, 6 R1 patients (9.7%) did not undergo adjuvant treatment due to patient refusal (4 cases) and poor post-operative performance status (2 cases). The site of margin positivity is reported in Supplementary Table S1.
3.2. Impact of R Status on Long-Term Outcomes
As a whole, tumor recurrence was evidenced in 114 (68.3%) patients after a median time of 10 (6–18) months: 21 (12.6%) had a local recurrence, 61 (36.5%) presented distant metastases, and 32 (19.2%) had local and distant recurrence. Notably, the R1 cohort presented a higher recurrence rate (74.2%–46 patients) as compared to the R0 cohort (64.8%–68 patients) (p = 0.04) with no difference in terms of median recurrence time between the R1 (9 (5–13) months)) and R0 groups (10 (6–18) months) (p = 0.18). No difference was noted between the two cohorts in terms of local, distant, and local and distant recurrence rate (p = 0.15), although a slightly higher rate of local recurrence was noted in the R1 population. Data on tumor relapse are reported in Table 2.

Table 2.
Patterns of recurrence.
The median OS of the whole study population was 30 (17–46) months with a significantly longer survival in cases of R0 (36 (21–53) months) in comparison to R1 (23 (13–38) months) (p = 0.003). Similarly, the overall median DFS was 14 (7–33) months, with a significantly worse outcome in cases of R1 (10 (6–25) months) as compared to R0 (18 (9–70) months) (p = 0.004) (Figure 2A,B).
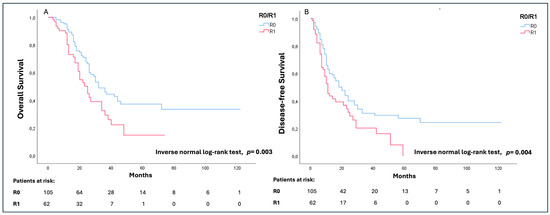
Figure 2.
Overall survival (OS) (A) and disease-free survival (DFS) (B) analysis according to the R status.
For OS, in the univariate analysis, R1 status (OR: 1.8; 95% CI: 1.1–2.7; p = 0.009), T staging (OR: 2.1; 95% CI: 1.2–3.8; p = 0.01), and N+ (OR: 2; 95% CI: 1.3–3.3; p = 0.004) resulted in negative prognostic factors. The multivariate analysis confirmed R1 status (OR: 1.6; 95% CI: 1–2.5; p = 0.03) and N+ (OR: 1.7; 95% CI: 1–2.8; p = 0.04) as factors independently associated with a worse OS (Table 3).

Table 3.
Univariate and multivariate analysis for OS and DFS.
In the univariate analysis, R1 (OR: 1.7; 95% CI: 1.2–2.4; p = 0.006), T staging (OR: 1.7; 95% CI: 1–3.1; p = 0.02), and N+ (OR: 2; 95% CI: 1.3–3; p = 0.001) resulted in negative prognostic factors for DFS. As for OS, in the multivariate analysis, only R1 (OR: 1.5; 95% CI: 1–2.1; p = 0.04) and N+ status (OR: 1.8; 95% CI: 1.1–2.7; p = 0.009) were confirmed as factors independently affecting DFS (Table 3).
3.3. Correlation Analysis of N and R Status with Oncological Outcomes
A further analysis was conducted to assess the impact of margin and nodal status, combined or alone, on long-term outcomes, namely OS and DFS. Patients were grouped according to R status into lymph node negative (R0N0, 42 patients—25.1% and R1N0 14 patients −8.4%) and node positive (R0N+, 61 patients −36.5% and R1N+, 50 patients—29.9%).
Tumor characteristics according to the R and N status are reported in Table 4.

Table 4.
Histopathological characteristics according to R and N status.
The R0N0 cohort presented a longer OS (37 (19–54) months) as compared to the R0N+ (27 (23–30) months; p = 0.005), R1N0 (34 (16–51) months; p = 0.01), and R1N+ (23 (17–28) months; p< 0.0001) groups (Figure 3A). Similarly, the R0N0 group had a significantly longer DFS (24 (16–32) months) as compared to the R0N+ (16 (11–21) months; p = 0.008) and R1N+ (10 (10–17) months; p < 0.0001) groups, while no difference was noted in comparison to the R1N0 cohort (20 (10–37) months; p = 0.27). Interestingly, R0N+ patients presented a longer DFS than R1N+ (16 months, (11–21) vs. 10 (8–11) months, respectively; p = 0.05) (Figure 3B).
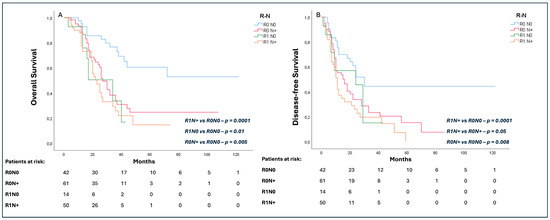
Figure 3.
Subgroup analysis of effect of R and N status on overall survival (OS) (A) and disease-free survival (DFS) (B).
4. Discussion
This study shows that a margin clearance of ≤1 mm significantly affects long-term survival after PD for PDAC as compared to a negative resection margin. Moreover, R1 was significantly related to a higher rate of tumor recurrence as compared to R0 patients, although no difference was noted in terms of median time of disease relapse. These findings give further support to the “1 mm rule”, according to which oncological radicality in PD for PDAC is guaranteed when at least 1 mm of clearance margin is reached. Although this recommendation is in line with several other reports in the literature [30,31,32], other authors did not recognize R status as an independent predictor of a poor prognosis [9,10,11,12]. These contrasting data may be explained by the different R status definitions currently used in the literature [13,14,15,16] and, above all, in the frequent concomitant presence of other confounding prognostic factors associated with R1, such as the tumor size, tumor location, and the presence of positive lymph nodes [21,22,23]. According to this last hypothesis, the concomitance of these other validated prognostic features would make the R status a consequence of a more advanced disease rather than a surrogate marker of tumor biology [17,33,34]. It is, thus, undeniable that the effect of positive resection margins on PDAC outcomes still remains controversial. In order to give our contribution to this ongoing debate, we performed a detailed analysis of tumor characteristics and outcomes according to the R status, along with a subcategorization of patients according to the concomitant presence of further features significantly associated, at the multivariate analysis, with a worse OS and DFS, namely N status. This additional analysis was aimed at effectively demonstrating the independent impact of margin and N status on both survival and recurrence.
Reviewing our data, R1 patients effectively presented a more advanced disease, with a higher tumor stage, a more frequent detection of metastastic lymph nodes, and perineural and angio/lymphovascular infiltration, probably reflecting a more aggressive tumor biology. These results are similar to those reported by Tummers et al. [7] and Kimbrough et al. [35], making the R1 status representative of more aggressive disease. As a consequence, our R1 population presented a poor overall survival of 23 (13–38) months as compared to 36 (21–53) months in cases of R0 (p = 0.003). However, independent of the coexistence of additional prognostic factors, R1 itself was an independent risk factor for a worse OS in the multivariate analysis (OR: 1.6; 95% CI: 1–2.5; p = 0.03), supporting the independent influencing role of R status on long-term outcomes. This inevitably underlines the key role of curative resection (R0) on OS after PD for PDAC [36,37,38] and makes patient selection for resection an essential step to guarantee an adequate long-term outcome. In this context, the benefits of systemic therapy for pancreatic tumors in terms of OS and DFS are highly recognized. Indeed, the impressive survival advantages of FOLFIRINOX chemotherapy are increasingly emphasized, and the clear benefits in terms of tumor downstaging and improvement in resection rates in borderline lesions are currently paving the way for its application to even resectable PDACs, although contrasting results are currently present in the literature on this last topic [39,40,41].
With regards to the correlation between R1 and disease recurrence, our analysis outlined a higher rate of tumor relapse (more than 70%) and a consequent poorer DFS (10 (6–25) months) in R1 patients as compared to the R0 cohort. Notably, although not statistically significant (probably due to the small sample size of the study cohorts), R1 was related to a higher rate of local recurrence (12 cases (19.4%) vs. 9 cases (8.6%) in the R0 population). Although these data are in line with the majority of evidence [7,35], some authors [9,42,43] did not recognize the resection margin status as affecting disease relapse. The postulation that a positive margin may be predictive of a higher risk of tumor recurrence makes sense, since the microscopic tumor residual may trigger a disease relapse. However, recent reports outlined a sort of selective correlation between the type of margin involved and a higher risk of tumor recurrence. Specifically, the “vascular” margin seems to be the most relevant [17,33,44,45] in comparison to the posterior margin and anterior surface involvement [34]. Similarly, Pingpank et al. [44], documented a significantly lower DFS in patients with vascular margin positivity than those with posterior or pancreatic margin involvement. However, the limited sample size of our study population did not permit a further subanalysis according to the type of positive margin encountered, although the majority of our R1 patients presented vascular margin positivity (superior mesenteric vein margin).
Lymph node involvement and the number of positive lymph nodes are well-known prognostic factors after PD [21,23] related to a worse OS and DFS. These findings are further confirmed in our cohort of analysis, according to which the detection of positive lymph nodes was directly correlated with a lower OS and a higher recurrence rate. More interestingly, the prognostic role of N+ was additionally evaluated in relation to the R status, evidencing worse long-term outcomes independent of the resection margin positivity or negativity. Similarly, a further confirmation of the independent prognostic role of R1 was documented in the comparison of DFS between R0N+ and R1N+ patients, highlighting a worse long-term outcome in cases of R1 and positive lymph nodes as compared to R0 and metastatic lymph nodes.
This study presents several limitations. Firstly, the retrospective study design may have introduced a selection bias in the study population. Secondly, for the analysis of margin clearance, the resection margins were grouped together. This significantly limited the specific evaluation of the influencing role of the different margins that may differently affect long-term outcomes, as evidenced by previous studies [33,34,46]. Furthermore, the limited sample size of the study population significantly reduced the number of patients grouped according to the R and N status, making the statistical comparison not fully reliable. Finally, data on adjuvant treatment were not available. This inevitably limited the evaluation of the impact of type and duration of the post-operative treatment on long-term outcomes.
5. Conclusions
Despite the above-mentioned limitations, this work substantially contributes to the ongoing debate on the prognostic role of R status after PD. Our analysis further supports survival benefits for patients with a margin clearance of at least 1 mm, independent of the concomitance of additional prognostic features. This inevitably highlights the relevant role that radical surgery has on long-term outcomes, making a tumor-free resection essential for patients. Nevertheless, there is an undeniable need for additional studies conducted in a prospective manner and according to predefined protocols for the definition of R status and for a uniform pathological analysis to further and definitively support our findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16132347/s1, Table S1: Site of margin positivity.
Author Contributions
Conceptualization, G.Q. and D.D.S.; methodology, C.F., C.L. and F.T.; validation, S.A., F.R. and V.T.; formal analysis, V.L., T.M. and E.P.; investigation, B.B., L.L. and G.S.; data curation, G.M. and R.M.; writing—original draft preparation, G.Q. and D.D.S.; writing—review and editing, S.A. and V.T.; visualization, S.A.; supervision, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective study design and the absence of procedures out of the normal clinical practice.
Informed Consent Statement
Patient consent was waived due to the retrospective study design and the absence of procedures out of the normal clinical practice.
Data Availability Statement
Data are available upon reasonable request at davide.desio01@gmail.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic Cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, N.; Halloran, C.; Raraty, M.; Ghaneh, P.; Sutton, R.; Neoptolemos, J.P. Current Standards of Surgery for Pancreatic Cancer. Br. J. Surg. 2004, 91, 1410–1427. [Google Scholar] [CrossRef]
- Ethun, C.G.; Kooby, D.A. The Importance of Surgical Margins in Pancreatic Cancer. J. Surg. Oncol. 2016, 113, 283–288. [Google Scholar] [CrossRef]
- Bilici, A. Prognostic Factors Related with Survival in Patients with Pancreatic Adenocarcinoma. World J. Gastroenterol. 2014, 20, 10802–10812. [Google Scholar] [CrossRef]
- Baldwin, S.; Kukar, M.; Gabriel, E.; Attwood, K.; Wilkinson, N.; Hochwald, S.N.; Kuvshinoff, B. Pancreatic Cancer Metastatic to a Limited Number of Lymph Nodes Has No Impact on Outcome. HPB 2016, 18, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Gravante, G.; Kosmin, M.; Riaz, A.; Al-Bahrani, A. A Systematic Review of the Prognostic Value of Lymph Node Ratio, Number of Positive Nodes and Total Nodes Examined in Pancreatic Ductal Adenocarcinoma. Ann. R. Coll. Surg. Engl. 2017, 99, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Groen, J.V.; Sibinga Mulder, B.G.; Farina-Sarasqueta, A.; Morreau, J.; Putter, H.; van de Velde, C.J.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.; et al. Impact of Resection Margin Status on Recurrence and Survival in Pancreatic Cancer Surgery. Br. J. Surg. 2019, 106, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Kasumova, G.G.; Tabatabaie, O.; Ng, S.C.; van Rijssen, L.B.; Verheij, J.; Najarian, R.M.; van Gulik, T.M.; Besselink, M.G.; Busch, O.R.; et al. Pathological Margin Clearance and Survival After Pancreaticoduodenectomy in a US and European Pancreatic Center. Ann. Surg. Oncol. 2018, 25, 1760–1767. [Google Scholar] [CrossRef]
- Raut, C.P.; Tseng, J.F.; Sun, C.C.; Wang, H.; Wolff, R.A.; Crane, C.H.; Hwang, R.; Vauthey, J.-N.; Abdalla, E.K.; Lee, J.E.; et al. Impact of Resection Status on Pattern of Failure and Survival after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. Ann. Surg. 2007, 246, 52–60. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant Chemotherapy with Fluorouracil plus Folinic Acid vs Gemcitabine Following Pancreatic Cancer Resection: A Randomized Controlled Trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef]
- Kato, K.; Yamada, S.; Sugimoto, H.; Kanazumi, N.; Nomoto, S.; Takeda, S.; Kodera, Y.; Morita, S.; Nakao, A. Prognostic Factors for Survival after Extended Pancreatectomy for Pancreatic Head Cancer: Influence of Resection Margin Status on Survival. Pancreas 2009, 38, 605–612. [Google Scholar] [CrossRef]
- Daamen, L.A.; van Goor, I.W.J.M.; Schouten, T.J.; Dorland, G.; van Roessel, S.R.; Besselink, M.G.; Bonsing, B.A.; Bosscha, K.; Brosens, L.A.A.; Busch, O.R.; et al. Microscopic Resection Margin Status in Pancreatic Ductal Adenocarcinoma—A Nationwide Analysis. Eur. J. Surg. Oncol. 2021, 47, 708–716. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM Classification of Malignant Tumours-towards Common Understanding and Reasonable Expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline Resectable Pancreatic Cancer: A Consensus Statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef]
- Chang, D.K.; Johns, A.L.; Merrett, N.D.; Gill, A.J.; Colvin, E.K.; Scarlett, C.J.; Nguyen, N.Q.; Leong, R.W.L.; Cosman, P.H.; Kelly, M.I.; et al. Margin Clearance and Outcome in Resected Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 2855–2862. [Google Scholar] [CrossRef]
- Gebauer, F.; Tachezy, M.; Vashist, Y.K.; Marx, A.H.; Yekebas, E.; Izbicki, J.R.; Bockhorn, M. Resection Margin Clearance in Pancreatic Cancer after Implementation of the Leeds Pathology Protocol (LEEPP): Clinically Relevant or Just Academic? World J. Surg. 2015, 39, 493–499. [Google Scholar] [CrossRef]
- Verbeke, C.S.; Leitch, D.; Menon, K.V.; McMahon, M.J.; Guillou, P.J.; Anthoney, A. Redefining the R1 Resection in Pancreatic Cancer. Br. J. Surg. 2006, 93, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Millikan, K.W.; Deziel, D.J.; Silverstein, J.C.; Kanjo, T.M.; Christein, J.D.; Doolas, A.; Prinz, R.A. Prognostic Factors Associated with Resectable Adenocarcinoma of the Head of the Pancreas. Am. Surg. 1999, 65, 618–623; discussion 623–624. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected Adenocarcinoma of the Pancreas-616 Patients: Results, Outcomes, and Prognostic Indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Piccoli, A.; Pedrazzoli, S. Survival after Resection for Ductal Adenocarcinoma of the Pancreas. Br. J. Surg. 1996, 83, 625–631. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Cameron, J.L.; Winter, J.M.; Assumpcao, L.; Lillemoe, K.D.; Wolfgang, C.; Hruban, R.H.; Schulick, R.D.; Yeo, C.J.; et al. Prognostic Relevance of Lymph Node Ratio Following Pancreaticoduodenectomy for Pancreatic Cancer. Surgery 2007, 141, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Riediger, H.; Keck, T.; Wellner, U.; zur Hausen, A.; Adam, U.; Hopt, U.T.; Makowiec, F. The Lymph Node Ratio Is the Strongest Prognostic Factor after Resection of Pancreatic Cancer. J. Gastrointest. Surg. 2009, 13, 1337–1344. [Google Scholar] [CrossRef]
- Strobel, O.; Hinz, U.; Gluth, A.; Hank, T.; Hackert, T.; Bergmann, F.; Werner, J.; Büchler, M.W. Pancreatic Adenocarcinoma: Number of Positive Nodes Allows to Distinguish Several N Categories. Ann. Surg. 2015, 261, 961–969. [Google Scholar] [CrossRef]
- Van den Broeck, A.; Sergeant, G.; Ectors, N.; Van Steenbergen, W.; Aerts, R.; Topal, B. Patterns of Recurrence after Curative Resection of Pancreatic Ductal Adenocarcinoma. Eur. J. Surg. Oncol. 2009, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Salvatore, L.; Fiorillo, C.; Bagalà, C.; Menghi, R.; Maria, B.; Cina, C.; Laterza, V.; Di Stefano, B.; Maratta, M.G.; et al. The Impact of the Multidisciplinary Tumor Board (MDTB) on the Management of Pancreatic Diseases in a Tertiary Referral Center. ESMO Open 2021, 6, 100010. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Fiorillo, C.; Menghi, R.; Cina, C.; Galiandro, F.; Longo, F.; Sofo, F.; Rosa, F.; Tortorelli, A.P.; Giustiniani, M.C.; et al. Total Mesopancreas Excision for Periampullary Malignancy: A Single-Center Propensity Score-Matched Comparison of Long-Term Outcomes. Langenbecks Arch. Surg. 2020, 405, 303–312. [Google Scholar] [CrossRef]
- Quero, G.; Fiorillo, C.; De Sio, D.; Laterza, V.; Menghi, R.; Cina, C.; Schena, C.A.; Rosa, F.; Galiandro, F.; Alfieri, S. The Role of Mesopancreas Excision for Ampullary Carcinomas: A Single Center Propensity-Score Matched Analysis. HPB 2021, 23, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.A.M.G.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a Standard Lymphadenectomy in Surgery for Pancreatic Ductal Adenocarcinoma: A Consensus Statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S. Resection Margins in Pancreatic Cancer. Pathologe 2013, 34 (Suppl. 2), 241–247. [Google Scholar] [CrossRef]
- Niesen, W.; Hank, T.; Büchler, M.; Strobel, O. Local Radicality and Survival Outcome of Pancreatic Cancer Surgery. Ann. Gastroenterol. Surg. 2019, 3, 464–475. [Google Scholar] [CrossRef]
- Wittekind, C.; Compton, C.; Quirke, P.; Nagtegaal, I.; Merkel, S.; Hermanek, P.; Sobin, L.H. A Uniform Residual Tumor (R) Classification: Integration of the R Classification and the Circumferential Margin Status. Cancer 2009, 115, 3483–3488. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Warshaw, A.L.; Allen, J.N.; Blaszkowsky, L.S.; Castillo, C.F.-D.; Deshpande, V.; Hong, T.S.; Kwak, E.L.; Lauwers, G.Y.; Ryan, D.P.; et al. Pancreatic Ductal Adenocarcinoma: Is There a Survival Difference for R1 Resections versus Locally Advanced Unresectable Tumors? What Is a “True” R0 Resection? Ann. Surg. 2013, 257, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Gnerlich, J.L.; Luka, S.R.; Deshpande, A.D.; Dubray, B.J.; Weir, J.S.; Carpenter, D.H.; Brunt, E.M.; Strasberg, S.M.; Hawkins, W.G.; Linehan, D.C. Microscopic Margins and Patterns of Treatment Failure in Resected Pancreatic Adenocarcinoma. Arch. Surg. 2012, 147, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, N.B.; Foulis, A.K.; Oien, K.A.; Going, J.J.; Glen, P.; Dickson, E.J.; Imrie, C.W.; McKay, C.J.; Carter, R. Positive Mobilization Margins Alone Do Not Influence Survival Following Pancreatico-Duodenectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2010, 251, 1003–1010. [Google Scholar] [CrossRef]
- Kimbrough, C.W.; St Hill, C.R.; Martin, R.C.G.; McMasters, K.M.; Scoggins, C.R. Tumor-Positive Resection Margins Reflect an Aggressive Tumor Biology in Pancreatic Cancer. J. Surg. Oncol. 2013, 107, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Shaib, Y.; Davila, J.; Naumann, C.; El-Serag, H. The Impact of Curative Intent Surgery on the Survival of Pancreatic Cancer Patients: A U.S. Population-Based Study. Am. J. Gastroenterol. 2007, 102, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Dunn, J.A.; Almond, J.; Beger, H.G.; Pederzoli, P.; Bassi, C.; Dervenis, C.; Fernandez-Cruz, L.; Lacaine, F.; et al. Influence of Resection Margins on Survival for Patients with Pancreatic Cancer Treated by Adjuvant Chemoradiation and/or Chemotherapy in the ESPAC-1 Randomized Controlled Trial. Ann. Surg. 2001, 234, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant Chemotherapy with Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer: A Randomized Controlled Trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1129. [Google Scholar] [CrossRef]
- Labori, K.J.; Bratlie, S.O.; Andersson, B.; Angelsen, J.-H.; Biörserud, C.; Björnsson, B.; Bringeland, E.A.; Elander, N.; Garresori, H.; Grønbech, J.E.; et al. Neoadjuvant FOLFIRINOX versus Upfront Surgery for Resectable Pancreatic Head Cancer (NORPACT-1): A Multicentre, Randomised, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2024, 9, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Delpero, J.R.; Bachellier, P.; Regenet, N.; Le Treut, Y.P.; Paye, F.; Carrere, N.; Sauvanet, A.; Autret, A.; Turrini, O.; Monges-Ranchin, G.; et al. Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma: A French Multicentre Prospective Evaluation of Resection Margins in 150 Evaluable Specimens. HPB 2014, 16, 20–33. [Google Scholar] [CrossRef]
- Tseng, J.F.; Raut, C.P.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.-N.; Abdalla, E.K.; Gomez, H.F.; Sun, C.C.; Crane, C.H.; Wolff, R.A.; et al. Pancreaticoduodenectomy with Vascular Resection: Margin Status and Survival Duration. J. Gastrointest. Surg. 2004, 8, 935–949; discussion 949–950. [Google Scholar] [CrossRef] [PubMed]
- Pingpank, J.F.; Hoffman, J.P.; Ross, E.A.; Cooper, H.S.; Meropol, N.J.; Freedman, G.; Pinover, W.H.; LeVoyer, T.E.; Sasson, A.R.; Eisenberg, B.L. Effect of Preoperative Chemoradiotherapy on Surgical Margin Status of Resected Adenocarcinoma of the Head of the Pancreas. J. Gastrointest. Surg. 2001, 5, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most Pancreatic Cancer Resections Are R1 Resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Zhang, Y.; Frampton, A.E.; Cohen, P.; Kyriakides, C.; Bong, J.J.; Habib, N.A.; Spalding, D.R.C.; Ahmad, R.; Jiao, L.R. Tumor Infiltration in the Medial Resection Margin Predicts Survival after Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1875–1882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).