Is Cancer Metabolism an Atavism?
Abstract
:Simple Summary
Abstract
1. Introduction
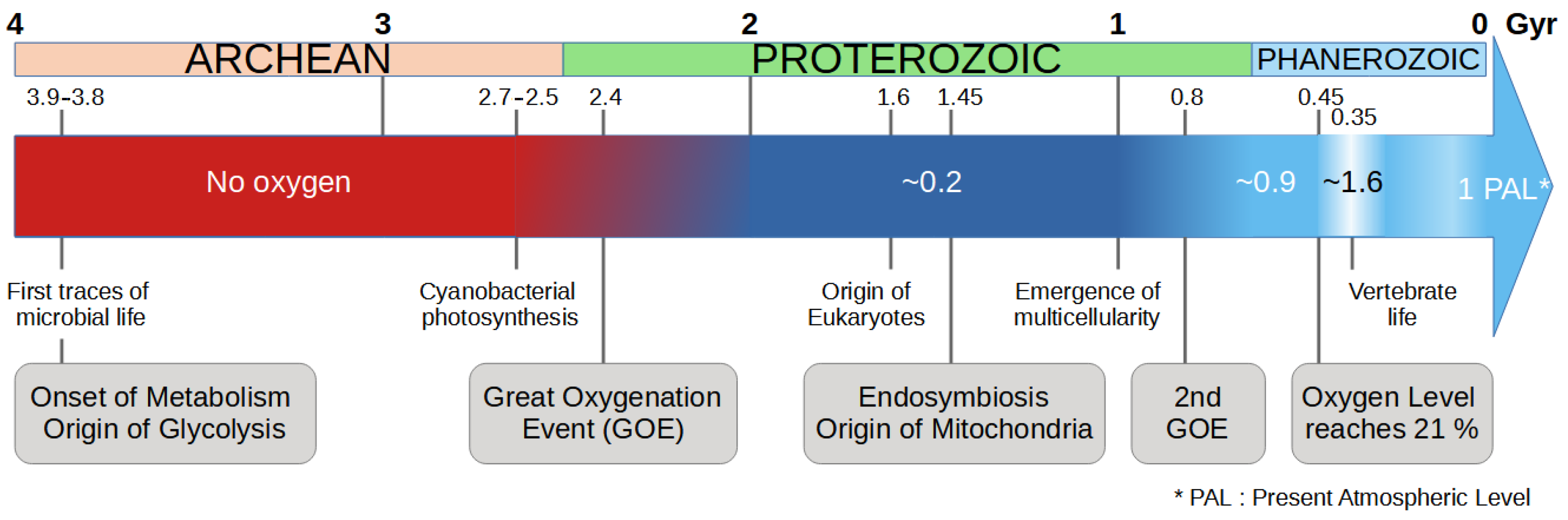
2. The What and the Why of the Atavistic Theory of Cancer
3. Update of the Theory
4. Where the Metabolism Comes into Play
5. Critique of the Serial Atavism Model
6. Discussion
6.1. About the Relevance of the SAM as a Conceptual Frame
6.2. Metabolic Plasticity versus Metabolic Rewiring
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAF | cancer-associated fibroblast |
| ETC | Electron Transport Chain |
| GOE | Great Oxygenation Event |
| OXPHOS | Oxydative Phosphorylation |
| ROS | Reactive Oxygen Species |
| SAM | Serial Atavism Model |
| SETOC | Systemic-Evolutionary Theory of the Origin of Cancer |
| SMT | Somatic Mutation Theory |
| TCA | TriCarboxylic Acid |
| TME | Tumor MicroEnvironment |
| TOFT | Tissue Organization Field Theory |
| UEME | Unicellular-Eukaryote-to-Multicellular Eukaryote |
| WE | Warburg effect |
References
- Davies, P.C.W.; Lineweaver, C.H. Cancer tumors as Metazoa 1.0: Tapping genes of ancient ancestors. Phys. Biol. 2011, 8, 015001. [Google Scholar] [CrossRef]
- Cipponi, A.; Thomas, D.M. Stress-induced cellular adaptive strategies: Ancient evolutionarily conserved programs as new anticancer therapeutic targets. BioEssays 2014, 36, 552–560. [Google Scholar] [CrossRef]
- Lineweaver, C.H.; Davies, P.C.W.; Vincent, M.D. Targeting cancer’s weaknesses (not its strengths): Therapeutic strategies suggested by the atavistic model. BioEssays 2014, 36, 827–835. [Google Scholar] [CrossRef]
- Vincent, M. Resistance to cancer chemotherapy as an atavism? (retrospective on DOI 10.1002/bies.201300170). BioEssays 2016, 38, 1065. [Google Scholar] [CrossRef]
- Snow, H. Cancers and the Cancer Process; J and A Churchill Publishers: London, UK, 1893. [Google Scholar]
- Boveri, T. Zur Frage der Entstehung maligner Tumoren. In The Origin of Malignant Tumors (1929); Boveri, M., Translator; Williams and Wilkins: Philadelphia, PA, USA, 1914. [Google Scholar]
- Roberts, M. Malignancy and Evolution; Grayson ans Grayson Publishers: London, UK, 1926. [Google Scholar]
- Israel, L. Tumour Progression: Random Mutations or an Integrated Survival Response to Cellular Stress Conserved from Unicellular Organisms? J. Theor. Biol. 1996, 178, 375–380. [Google Scholar] [CrossRef]
- Vincent, M. Cancer: A de-repression of a default survival program common to all cells?: A life-history perspective on the nature of cancer. BioEssays 2011, 34, 72–82. [Google Scholar] [CrossRef]
- Martin, W.F.; Neukirchen, S.; Zimorski, V.; Gould, S.B.; Sousa, F.L. Energy for two: New archaeal lineages and the origin of mitochondria. BioEssays 2016, 38, 850–856. [Google Scholar] [CrossRef]
- Trosko, J.E. A Conceptual Integration of Extra-, Intra- and Gap Junctional- Intercellular Communication in the Evolution of Multi-cellularity and Stem Cells: How Disrupted Cell-Cell Communication during Development can Affect Diseases later in Life. Int. J. Stem Cell Res. Ther. 2016, 3. [Google Scholar] [CrossRef]
- Duran-Nebreda, S.; Solé, R. Emergence of multicellularity in a model of cell growth, death and aggregation under size-dependent selection. J. R. Soc. Interface 2015, 12, 20140982. [Google Scholar] [CrossRef] [PubMed]
- Solé, R.; Ollé-Vila, A.; Vidiella, B.; Duran-Nebreda, S.; Conde-Pueyo, N. The road to synthetic multicellularity. Curr. Opin. Syst. Biol. 2018, 7, 60–67. [Google Scholar] [CrossRef]
- Chen, H.; Lin, F.; Xing, K.; He, X. The reverse evolution from multicellularity to unicellularity during carcinogenesis. Nat. Commun. 2015, 6, 6367. [Google Scholar] [CrossRef] [PubMed]
- Trigos, A.S.; Pearson, R.B.; Papenfuss, A.T.; Goode, D.L. Altered interactions between unicellular and multicellular genes drive hallmarks of transformation in a diverse range of solid tumors. Proc. Natl. Acad. Sci. USA 2017, 114, 6406–6411. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Gao, R.; Navin, N. Tumor evolution: Linear, branching, neutral or punctuated? Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yamada, Y. The causal relationship between epigenetic abnormality and cancer development: In vivo reprogramming and its future application. Proc. Jpn. Acad. Ser. B 2018, 94, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zimorski, V.; Mentel, M.; Tielens, A.G.M.; Martin, W.F. Energy metabolism in anaerobic eukaryotes and Earth’s late oxygenation. Free Radic. Biol. Med. 2019, 140, 279–294. [Google Scholar] [CrossRef]
- Aguadé-Gorgorió, G.; Costa, J.; Solé, R. An oncospace for human cancers. BioEssays 2023, 45, 2200215. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lineweaver, C.H.; Bussey, K.J.; Blackburn, A.C.; Davies, P.C.W. Cancer progression as a sequence of atavistic reversions. BioEssays 2021, 43, 2000305. [Google Scholar] [CrossRef]
- Mazzocca, A. The Systemic–Evolutionary Theory of the Origin of Cancer (SETOC): A New Interpretative Model of Cancer as a Complex Biological System. Int. J. Mol. Sci. 2019, 20, 4885. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.; Fais, S. New hypotheses for cancer generation and progression. Med. Hypotheses 2021, 152, 110614. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, D.; Nikolic, D.; Paparella, R.R.; Sabbà, C.; Mazzocca, A. Cellular Adaptation Takes Advantage of Atavistic Regression Programs during Carcinogenesis. Cancers 2023, 15, 3942. [Google Scholar] [CrossRef] [PubMed]
- Drochioiu, G. Multifactorial Distress, the Warburg Effect, and Respiratory and pH Imbalance in Cancer Development. Stresses 2023, 3, 500–528. [Google Scholar] [CrossRef]
- Epstein, T.; Xu, L.; Gillies, R.J.; Gatenby, R.A. Separation of metabolic supply and demand: Aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab. 2014, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Mentel, M. The Origin of Mitochondria. Nat. Educ. 2010, 3, 58. [Google Scholar]
- Zachar, I.; Szathmáry, E. Breath-giving cooperation: Critical review of origin of mitochondria hypotheses: Major unanswered questions point to the importance of early ecology. Biol. Direct 2017, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Myllymäki, H.; Kelly, L.; Elliot, A.M.; Carter, R.N.; Johansson, J.A.; Chang, K.Y.; Cholewa-Waclaw, J.; Morton, N.M.; Feng, Y. Preneoplastic cells switch to Warburg metabolism from their inception exposing multiple vulnerabilities for targeted elimination. Oncogenesis 2024, 13, 7. [Google Scholar] [CrossRef]
- Weinhouse, S. The Warburg hypothesis fifty years later. Z. Krebsforsch. Klin. Onkol. 1976, 87, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Segaran, A.; Lord, S. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. Semin. Cancer Biol. 2022, 86, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.; Lane, A.N.; Higashi, R.M.; Farag, M.A.; Gao, H.; Bousamra, M.; Miller, D.M. Altered regulation of metabolic pathways in human lung cancer discerned by 13C stable isotope-resolved metabolomics (SIRM). Mol. Cancer 2009, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Bartman, C.R.; Faubert, B.; Rabinowitz, J.D.; DeBerardinis, R.J. Metabolic pathway analysis using stable isotopes in patients with cancer. Nat. Rev. Cancer 2023, 23, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Iommarini, L.; Porcelli, A.M.; Gasparre, G.; Kurelac, I. Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer. Front. Oncol. 2017, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yokota, A.; Harada, H.; Huang, G. Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1α in cancer. Cancer Sci. 2019, 110, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ying, M.; Hu, X. Lactic acidosis switches cancer cells from aerobic glycolysis back to dominant oxidative phosphorylation. Oncotarget 2016, 7, 40621–40629. [Google Scholar] [CrossRef]
- Daverio, Z.; Kolkman, M.; Perrier, J.; Brunet, L.; Bendridi, N.; Sanglar, C.; Berger, M.A.; Panthu, B.; Rautureau, G.J.P. Warburg-associated acidification represses lactic fermentation independently of lactate, contribution from real-time NMR on cell-free systems. Sci. Rep. 2023, 13, 17733. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J., Jr.; Stryer, L. Biochemistry, 8th ed.; W. H. Freeman & Co. Ltd.: New York, NY, USA, 2015. [Google Scholar]
- Zeng, S.; Hu, X. Lactic acidosis switches cancer cells from dependence on glycolysis to OXPHOS and renders them highly sensitive to OXPHOS inhibitors. Biochem. Biophys. Res. Commun. 2023, 671, 46–57. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Jacquet, P.; Stéphanou, A. Metabolic Reprogramming, Questioning, and Implications for Cancer. Biology 2021, 10, 129. [Google Scholar] [CrossRef]
- Noell, W.K. The effect of iodoacetate on the vertebrate retina. J. Cell. Comp. Physiol. 1951, 37, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S. Glycolytic and oxidative metabolism in relation to retinal function. J. Gen. Physiol. 1981, 77, 667–692. [Google Scholar] [CrossRef]
- Ng, S.K.; Wood, J.P.; Chidlow, G.; Han, G.; Kittipassorn, T.; Peet, D.J.; Casson, R.J. Cancer-like metabolism of the mammalian retina. Clin. Exp. Ophthalmol. 2014, 43, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Haydinger, C.D.; Kittipassorn, T.; Peet, D.J. Power to see—Drivers of aerobic glycolysis in the mammalian retina: A review. Clin. Exp. Ophthalmol. 2020, 48, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, E.R.; Parent, M.J.; Souza, D.G.; Leuzy, A.; Lecrux, C.; Kim, H.I.; Gauthier, S.; Pellerin, L.; Hamel, E.; Rosa-Neto, P. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat. Neurosci. 2017, 20, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F.; Ruminot, I.; San Martín, A.; Lerchundi, R.; Fernández-Moncada, I.; Baeza-Lehnert, F. Aerobic Glycolysis in the Brain: Warburg and Crabtree Contra Pasteur. Neurochem. Res. 2020, 46, 15–22. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Oginuma, M.; Harima, Y.; Tarazona, O.A.; Diaz-Cuadros, M.; Michaut, A.; Ishitani, T.; Xiong, F.; Pourquié, O. Intracellular pH controls WNT downstream of glycolysis in amniote embryos. Nature 2020, 584, 98–101. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.M.; Lewis, N.E.; Jamshidi, N.; Mineta, K.; Gao, X.; Gojobori, T. The Emerging Facets of Non-Cancerous Warburg Effect. Front. Endocrinol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Penny, H.L.; Sieow, J.L.; Adriani, G.; Yeap, W.H.; See Chi Ee, P.; San Luis, B.; Lee, B.; Lee, T.; Mak, S.Y.; Ho, Y.S.; et al. Warburg metabolism in tumor-conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. OncoImmunology 2016, 5, e1191731. [Google Scholar] [CrossRef] [PubMed]
- Rabold, K.; Netea, M.G.; Adema, G.J.; Netea-Maier, R.T. Cellular metabolism of tumor-associated macrophages—Functional impact and consequences. FEBS Lett. 2017, 591, 3022–3041. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, C.; Ferrer, C.; Serra, M.; Choi, J.E.; Ducano, N.; Mira, A.; Shah, M.S.; Stopka, S.A.; Perciaccante, A.J.; Isella, C.; et al. A non-dividing cell population with high pyruvate dehydrogenase kinase activity regulates metabolic heterogeneity and tumorigenesis in the intestine. Nat. Commun. 2022, 13, 1503. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C. The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. BioEssays 2011, 33, 332–340. [Google Scholar] [CrossRef]
- Sonnenschein, C.; Soto, A.M. Carcinogenesis explained within the context of a theory of organisms. Prog. Biophys. Mol. Biol. 2016, 122, 70–76. [Google Scholar] [CrossRef]
- Soto, A.M.; Sonnenschein, C. The cancer puzzle: Welcome to organicism. Prog. Biophys. Mol. Biol. 2021, 165, 114–119. [Google Scholar] [CrossRef]
- Thomas, F.; Ujvari, B.; Renaud, F.; Vincent, M. Cancer adaptations: Atavism, de novo selection, or something in between? BioEssays 2017, 39, 1700039. [Google Scholar] [CrossRef] [PubMed]
- Catania, F.; Ujvari, B.; Roche, B.; Capp, J.P.; Thomas, F. Bridging Tumorigenesis and Therapy Resistance with a Non-Darwinian and Non-Lamarckian Mechanism of Adaptive Evolution. Front. Oncol. 2021, 11, 732081. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanchon, E.; Stéphanou, A. Is Cancer Metabolism an Atavism? Cancers 2024, 16, 2415. https://doi.org/10.3390/cancers16132415
Fanchon E, Stéphanou A. Is Cancer Metabolism an Atavism? Cancers. 2024; 16(13):2415. https://doi.org/10.3390/cancers16132415
Chicago/Turabian StyleFanchon, Eric, and Angélique Stéphanou. 2024. "Is Cancer Metabolism an Atavism?" Cancers 16, no. 13: 2415. https://doi.org/10.3390/cancers16132415




