The Use of Apparent Diffusion Coefficient Values for Differentiating Bevacizumab-Related Cytotoxicity from Tumor Recurrence and Radiation Necrosis in Glioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Parameters and Postprocessing
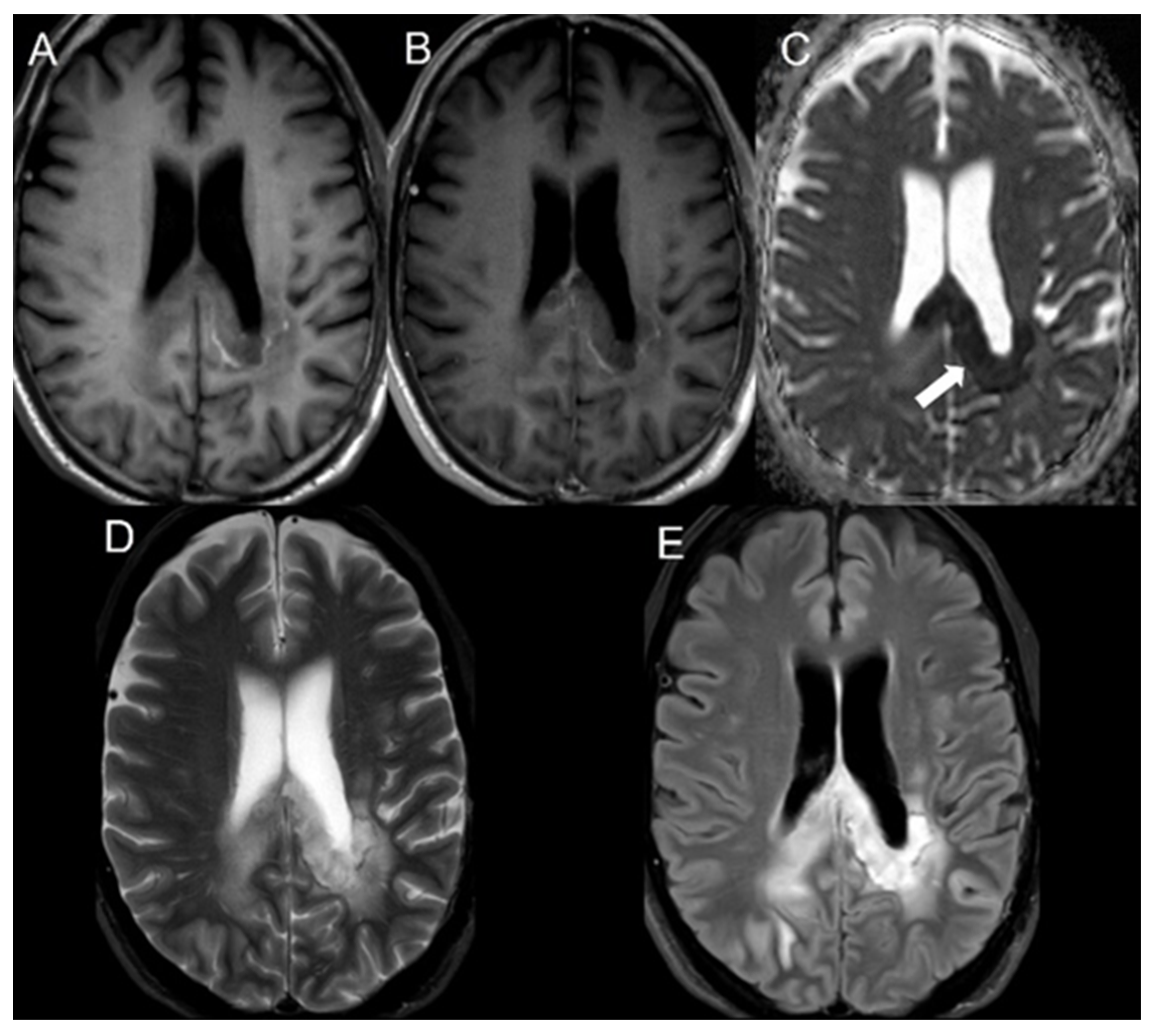
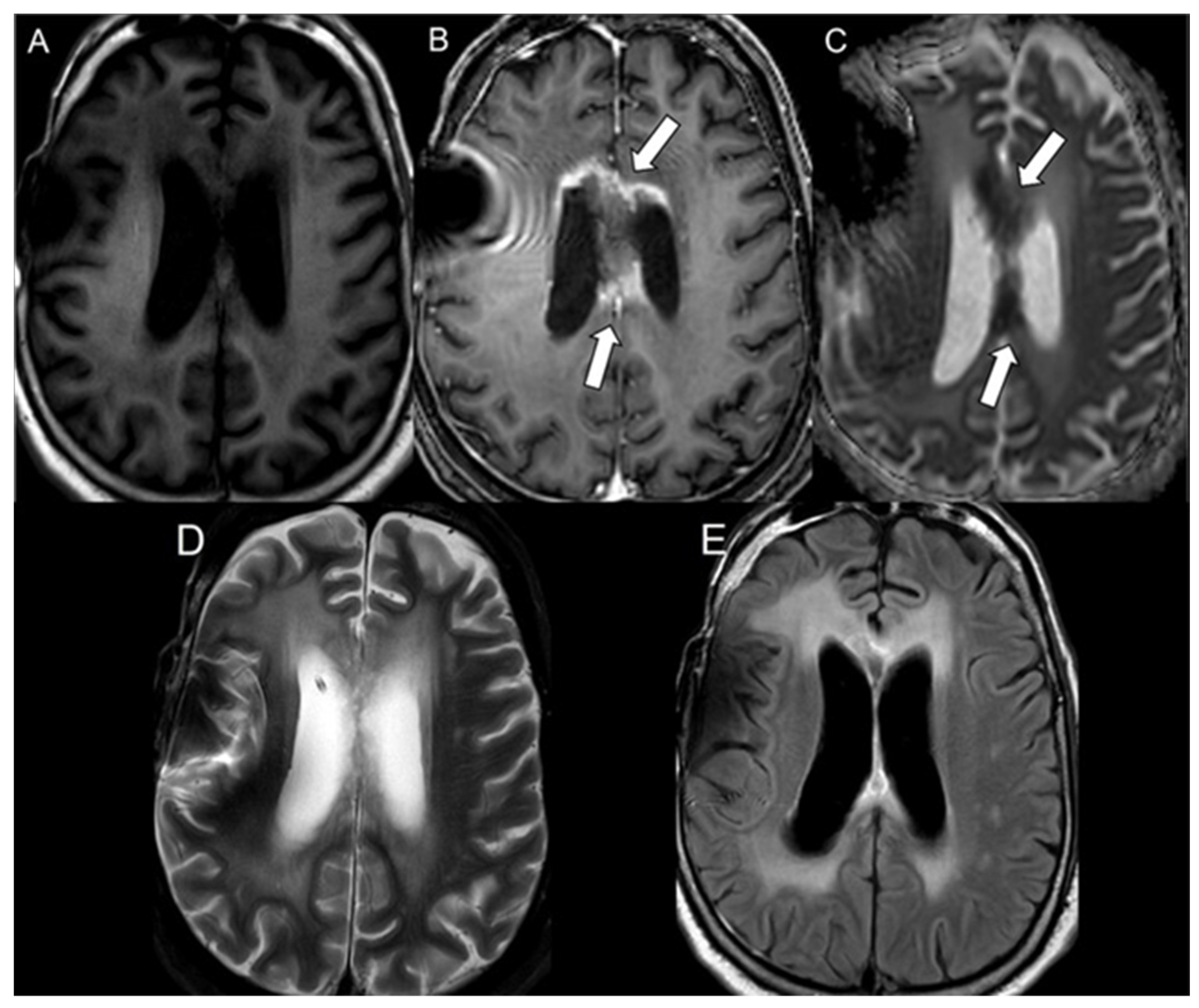

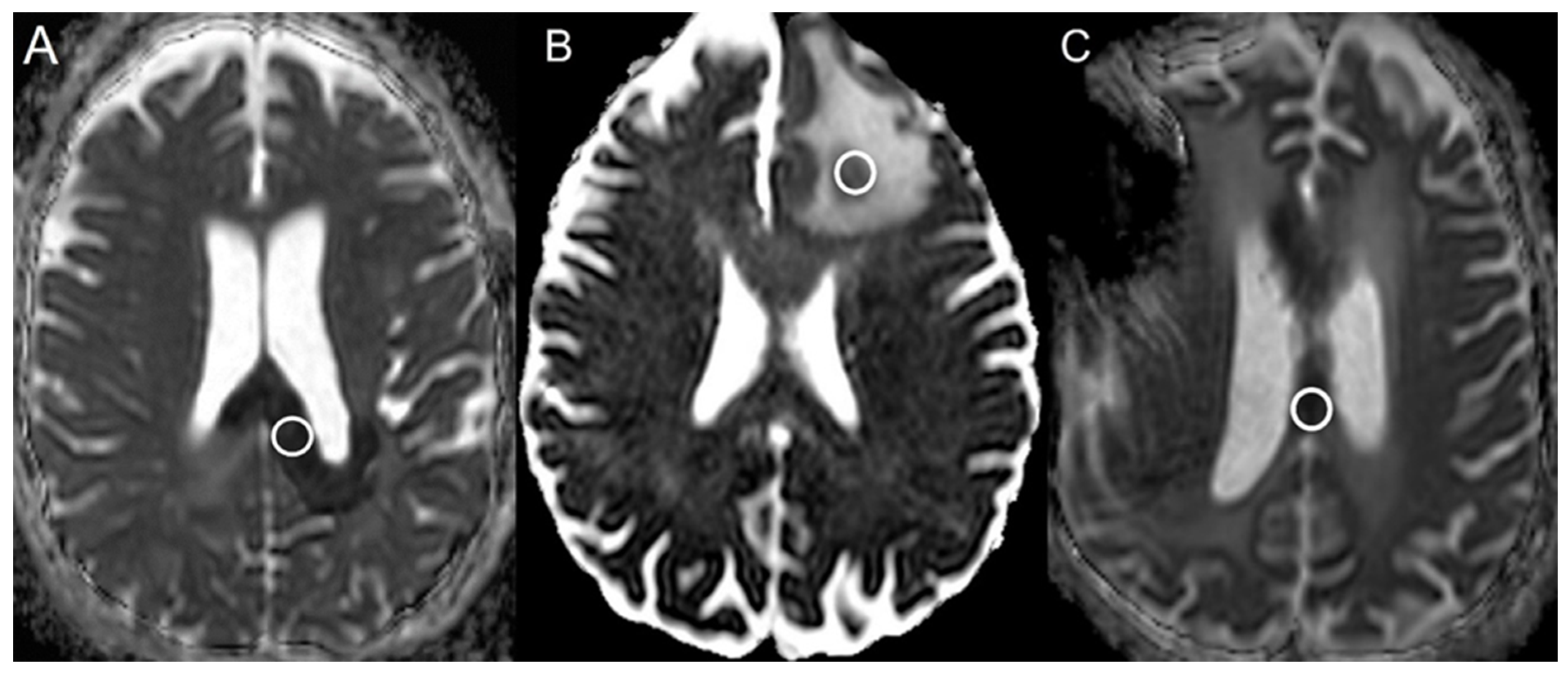
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Kaufmann, T.J.; Smits, M.; Boxerman, J.; Huang, R.; Barboriak, D.P.; Weller, M.; Chung, C.; Tsien, C.; Brown, P.D.; Shankar, L.; et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro-Oncology 2020, 22, 757–772. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Surgical oncology for gliomas: The state of the art. Nat. Rev. Clin. Oncol. 2018, 15, 112–125. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro-Oncology 2023, 25, 940–954. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Iwamoto, F.M.; Abrey, L.E.; Beal, K.; Gutin, P.H.; Rosenblum, M.K.; Reuter, V.E.; DeAngelis, L.M.; Lassman, A.B. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 2009, 73, 1200–1206. [Google Scholar] [CrossRef]
- Iwamoto, F.M.; Fine, H.A. Bevacizumab for Malignant Gliomas. Arch. Neurol. 2010, 67, 285–288. [Google Scholar] [CrossRef]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E.; Dowell, J.M.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Wagner, M.; et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res. 2007, 13, 1253–1259. [Google Scholar] [CrossRef]
- Zuniga, R.M.; Torcuator, R.; Jain, R.; Anderson, J.; Doyle, T.; Ellika, S.; Schultz, L.; Mikkelsen, T. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J. Neuro-Oncol. 2009, 91, 329–336. [Google Scholar] [CrossRef]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.A.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef]
- Nguyen, H.; Milbach, N.; Hurrell, S.; Cochran, E.; Connelly, J.; Bovi, J.; Schultz, C.; Mueller, W.; Rand, S.; Schmainda, K.; et al. Progressing Bevacizumab-Induced Diffusion Restriction Is Associated with Coagulative Necrosis Surrounded by Viable Tumor and Decreased Overall Survival in Patients with Recurrent Glioblastoma. Am. J. Neuroradiol. 2016, 37, 2201–2208. [Google Scholar] [CrossRef]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 1999, 9, 53–60. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Cloughesy, T.F.; Lai, A.; Mischel, P.S.; Nghiemphu, P.L.; Lalezari, S.; Schmainda, K.M.; Pope, W.B. Graded functional diffusion map-defined characteristics of apparent diffusion coefficients predict overall survival in recurrent glioblastoma treated with bevacizumab. Neuro-Oncology 2011, 13, 1151–1161. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Kim, E.; Woodworth, D.C.; Marques, H.; Boxerman, J.L.; Safriel, Y.; McKINSTRY, R.C.; Bokstein, F.; Jain, R.; Chi, T.L.; et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: A multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int. J. Oncol. 2015, 46, 1883–1892. [Google Scholar] [CrossRef]
- Hamstra, D.A.; Galbán, C.J.; Meyer, C.R.; Johnson, T.D.; Sundgren, P.C.; Tsien, C.; Lawrence, T.S.; Junck, L.; Ross, D.J.; Rehemtulla, A.; et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: Correlation with conventional radiologic response and overall survival. J. Clin. Oncol. 2008, 26, 3387–3394. [Google Scholar] [CrossRef]
- Moffat, B.A.; Chenevert, T.L.; Lawrence, T.S.; Meyer, C.R.; Johnson, T.D.; Dong, Q.; Tsien, C.; Mukherji, S.; Quint, D.J.; Gebarski, S.S.; et al. Functional diffusion map: A noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc. Natl. Acad. Sci. USA 2005, 102, 5524–5529. [Google Scholar] [CrossRef]
- Kamali, A.; Gandhi, A.; Nunez, L.C.; Lugo, A.E.; Arevalo-Espejo, O.; Zhu, J.-J.; Esquenazi-Levy, Y.; Zhang, X.; Riascos, R.F. The Role of Apparent Diffusion Coefficient Values in Glioblastoma: Differentiating Tumor Progression Versus Treatment-Related Changes. J. Comput. Assist. Tomogr. 2022, 46, 923–928. [Google Scholar] [CrossRef]
- Mong, S.; Ellingson, B.; Nghiemphu, P.; Kim, H.; Mirsadraei, L.; Lai, A.; Yong, W.; Zaw, T.; Cloughesy, T.; Pope, W. Persistent diffusion-restricted lesions in bevacizumab-treated malignant gliomas are associated with improved survival compared with matched controls. Am. J. Neuroradiol. 2012, 33, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, O.D.; Soto, C.; Rabiei, P.; Kamali, A.; Ballester, L.Y.; Esquenazi, Y.; Zhu, J.-J.; Riascos, R.F. Assessment of Glioblastoma Response in the Era of Bevacizumab: Longstanding and Emergent Challenges in the Imaging Evaluation of Pseudoresponse. Front. Neurol. 2019, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Farid, N.; Almeida-Freitas, D.B.; White, N.S.; McDonald, C.R.; Muller, K.A.; VandenBerg, S.R.; Kesari, S.; Dale, A.M. Restriction-Spectrum Imaging of Bevacizumab-Related Necrosis in a Patient with GBM. Front. Oncol. 2013, 3, 64288. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Young, R.; Karimi, S.; Sood, S.; Zhang, Z.; Mo, Q.; Gutin, P.; Holodny, A.; Lassman, A. Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. Am. J. Neuroradiol. 2011, 32, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.; Bähr, O.; Müller, K.; Franz, K.; Steinbach, J.; Hattingen, E. Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J. Neuro-Oncol. 2010, 99, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gulotta, B.; Thomas, A.; Kaley, T.; Karimi, S.; Gavrilovic, I.; Woo, K.M.; Zhang, Z.; Arevalo-Perez, J.; Holodny, A.I.; et al. Large-volume low apparent diffusion coefficient lesions predict poor survival in bevacizumab-treated glioblastoma patients. Neuro-Oncology 2016, 18, 735–743. [Google Scholar] [CrossRef]
- Coleman, J.F. Robbins and Cotran’s Pathologic Basis of Disease; LWW: New York, NY, USA, 2010. [Google Scholar]
- Brandsma, D.; Bent, M.J.v.D. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 2009, 22, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Jeyaretna, D.S.; Curry, W.T.; Batchelor, T.T.; Stemmer-Rachamimov, A.; Plotkin, S.R. Exacerbation of cerebral radiation necrosis by bevacizumab. J. Clin. Oncol. 2011, 29, e159–e162. [Google Scholar] [CrossRef] [PubMed]
- van den Elshout, R.; Scheenen, T.W.J.; Driessen, C.M.L.; Smeenk, R.J.; Meijer, F.J.A.; Henssen, D. Diffusion imaging could aid to differentiate between glioma progression and treatment-related abnormalities: A meta-analysis. Insights Imaging 2022, 13, 158. [Google Scholar] [CrossRef]
- Gerstner, E.R.; Frosch, M.P.; Batchelor, T.T. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J. Clin. Oncol. 2010, 28, e91–e93. [Google Scholar] [CrossRef]
- Pope, W.B.; Kim, H.J.; Huo, J.; Alger, J.; Brown, M.S.; Gjertson, D.; Sai, V.; Young, J.R.; Tekchandani, L.; Cloughesy, T.; et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 2009, 252, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Hamdan, A.; Zweifler, R.; Jiang, H.; Norden, A.D.; Reardon, D.A.; Mukundan, S.; Wen, P.Y.; Huang, R.Y. Histogram analysis of apparent diffusion coefficient within enhancing and nonenhancing tumor volumes in recurrent glioblastoma patients treated with bevacizumab. J. Neuro-Oncol. 2014, 119, 149–158. [Google Scholar] [CrossRef] [PubMed]
- LaViolette, P.S.; Mickevicius, N.J.; Cochran, E.J.; Rand, S.D.; Connelly, J.; Bovi, J.A.; Malkin, M.G.; Mueller, W.M.; Schmainda, K.M. Precise ex vivo histological validation of heightened cellularity and diffusion-restricted necrosis in regions of dark apparent diffusion coefficient in 7 cases of high-grade glioma. Neuro-Oncology 2014, 16, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Vinay, K.; Jon, A.K.A.; Aster, C. Robbins and Cotran Pathologic Basis of Disease, 10th ed.; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Wang, Y.; Zhang, F.; Xiong, N.; Xu, H.; Chai, S.; Wang, H.; Wang, J.; Zhao, H.; Jiang, X.; Fu, P.; et al. Remodelling and Treatment of the Blood-Brain Barrier in Glioma. Cancer Manag. Res. 2021, 13, 4217–4232. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Frenkel, E.P.; Neuwelt, E.A. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology 2011, 76, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar] [CrossRef]
- Maghsoudinia, F.; Tavakoli, M.B.; Samani, R.K.; Motaghi, H.; Hejazi, S.H.; Mehrgardi, M.A. Bevacizumab and folic acid dual-targeted gadolinium-carbon dots for fluorescence/magnetic resonance imaging of hepatocellular carcinoma. J. Drug Deliv. Sci. Technol. 2020, 61, 102288. [Google Scholar] [CrossRef]
| Patient No. | Age at Death | Tumor Type | Surgery | XRT | TMZ | Location of Focal Region of Diffusion Restriction | Bevacizumab (Day) before Death | Bevacizumab (Day) before Focal Region Appears | Days between Focal Region and Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Still alive | GBM | + | + | + | CC | N/A | 181 | N/A |
| 2 | 32 | GBM | + | + | + | CC | 90 | 74 | 16 |
| 3 | 45 | GBM | + | + | + | CC | 574 | 61 | 513 |
| 4 | 77 | GBM | + | + | + | CR | 424 | 58 | 366 |
| 5 | 57 | GBM | + | + | + | CC | 647 | 339 | 308 |
| 6 | Still alive | GBM | + | + | + | CC | N/A | 258 | N/A |
| 7 | 48 | GBM | + | + | + | PV | 283 | 16 | 267 |
| 8 | 68 | GBM | + | + | + | CR | 92 | 34 | 58 |
| 9 | 67 | GBM | + | + | + | CR | 573 | 150 | 423 |
| 10 | 64 | GBM | + | + | + | PV | 589 | 25 | 564 |
| 11 | 57 | GBM | + | + | + | CC | 281 | 57 | 224 |
| 12 | 67 | GBM | + | + | + | CC | 75 | 41 | 34 |
| 13 | 65 | GBM | + | + | + | PV | 272 | 90 | 182 |
| 14 | 48 | GBM | + | + | + | PV | 717 | 371 | 346 |
| 15 | 51 | GBM | + | + | + | CC | 229 | 41 | 188 |
| 16 | 72 | GBM | + | + | + | PV | 878 | 49 | 829 |
| 17 | 52 | GBM | + | + | + | CC | 575 | 140 | 435 |
| 18 | 54 | GBM | + | + | + | CC | 651 | 355 | 296 |
| 19 | 83 | GBM | + | + | + | PV | 168 | 36 | 132 |
| 20 | 57 | GBM | + | + | + | PV and CC | 359 | 41 | 318 |
| 21 | 47 | GBM | + | + | + | Parasagittal frontal lobe, cingulate gyrus, CC | 295 | 99 | 196 |
| Group | N | Lesion | Normal | p Value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Bevacizumab | 21 | 248.1 ± 67.2 | 647.2 ± 94.6 | <0.001 |
| Progressive glioblastoma | 49 | 752.8 ± 132.5 | 709.2 ± 63.5 | 0.08 |
| Radiation necrosis | 58 | 479.0 ± 105.2 | 723.3 ± 64.0 | <0.001 |
| Group | AUC | 95% CI |
|---|---|---|
| Bevacizumab | 1.00 | N/A |
| Progressive glioblastoma | 0.59 | 0.41–0.70 |
| Radiation necrosis | 0.98 | 0.95–1.00 |
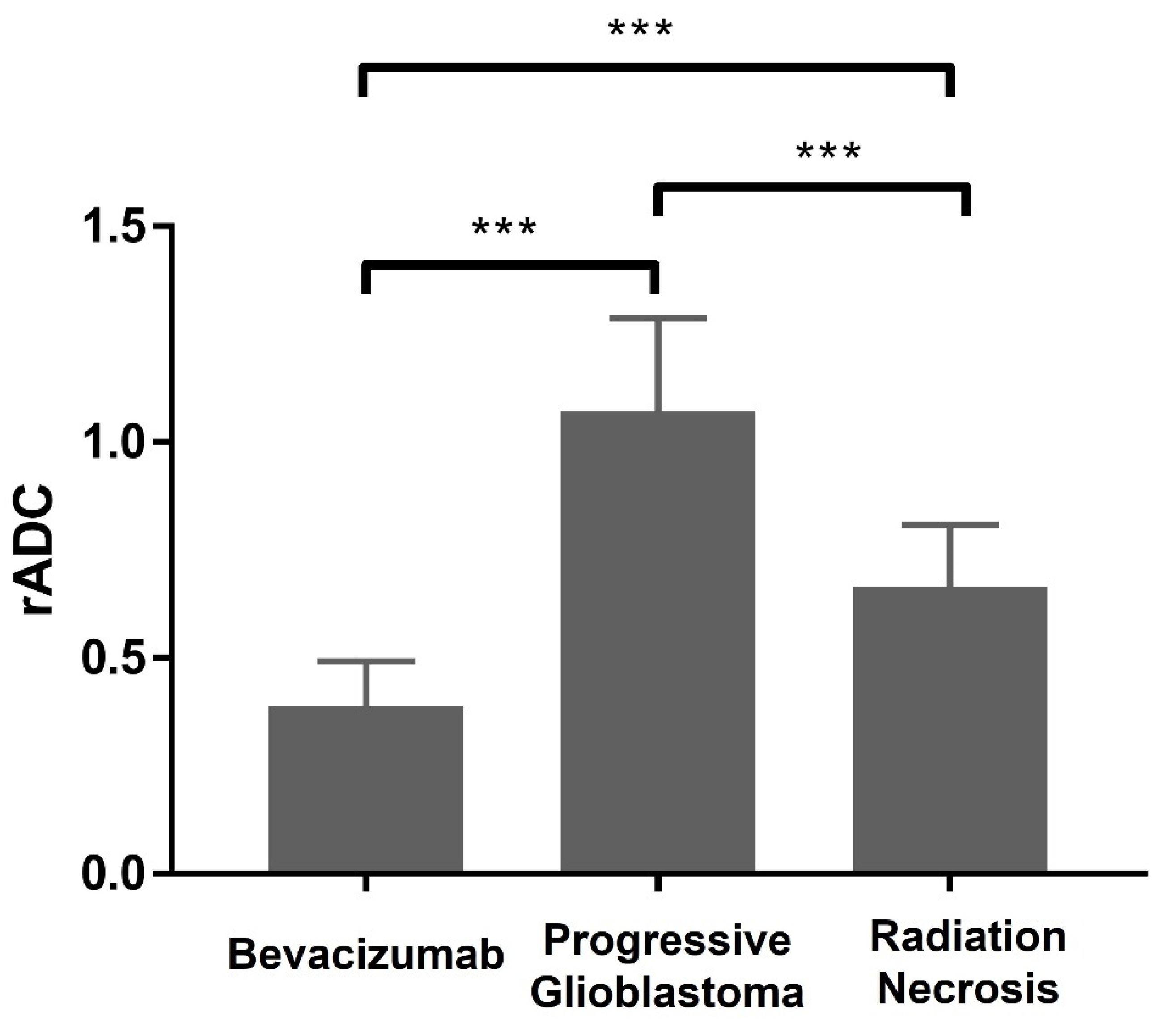
| Group | N | Mean ± SD |
|---|---|---|
| Bevacizumab | 21 | 0.39 ± 0.10 |
| Progressive glioblastoma | 49 | 1.07 ± 0.22 |
| Radiation necrosis | 58 | 0.66 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaj, K.; Jacobs, M.A.; Zhu, J.-J.; Esquenazi, Y.; Hsu, S.; Tandon, N.; Akhbardeh, A.; Zhang, X.; Riascos, R.; Kamali, A. The Use of Apparent Diffusion Coefficient Values for Differentiating Bevacizumab-Related Cytotoxicity from Tumor Recurrence and Radiation Necrosis in Glioblastoma. Cancers 2024, 16, 2440. https://doi.org/10.3390/cancers16132440
Khalaj K, Jacobs MA, Zhu J-J, Esquenazi Y, Hsu S, Tandon N, Akhbardeh A, Zhang X, Riascos R, Kamali A. The Use of Apparent Diffusion Coefficient Values for Differentiating Bevacizumab-Related Cytotoxicity from Tumor Recurrence and Radiation Necrosis in Glioblastoma. Cancers. 2024; 16(13):2440. https://doi.org/10.3390/cancers16132440
Chicago/Turabian StyleKhalaj, Kamand, Michael A. Jacobs, Jay-Jiguang Zhu, Yoshua Esquenazi, Sigmund Hsu, Nitin Tandon, Alireza Akhbardeh, Xu Zhang, Roy Riascos, and Arash Kamali. 2024. "The Use of Apparent Diffusion Coefficient Values for Differentiating Bevacizumab-Related Cytotoxicity from Tumor Recurrence and Radiation Necrosis in Glioblastoma" Cancers 16, no. 13: 2440. https://doi.org/10.3390/cancers16132440





