Cardiotoxicity in Acute Myeloid Leukemia in Adults: A Scoping Study
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Epidemiology of AML
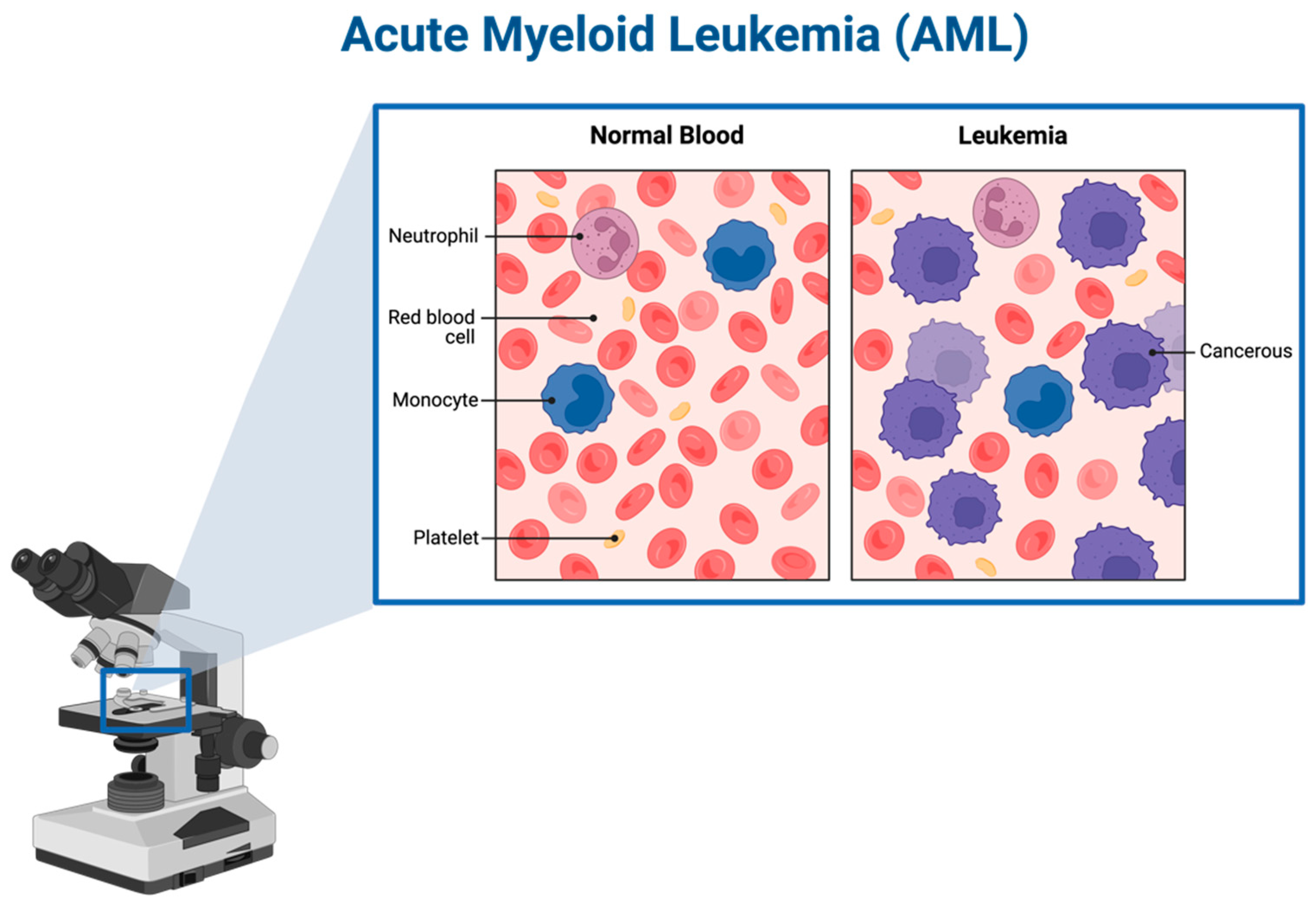
1.2. AML and Subtypes
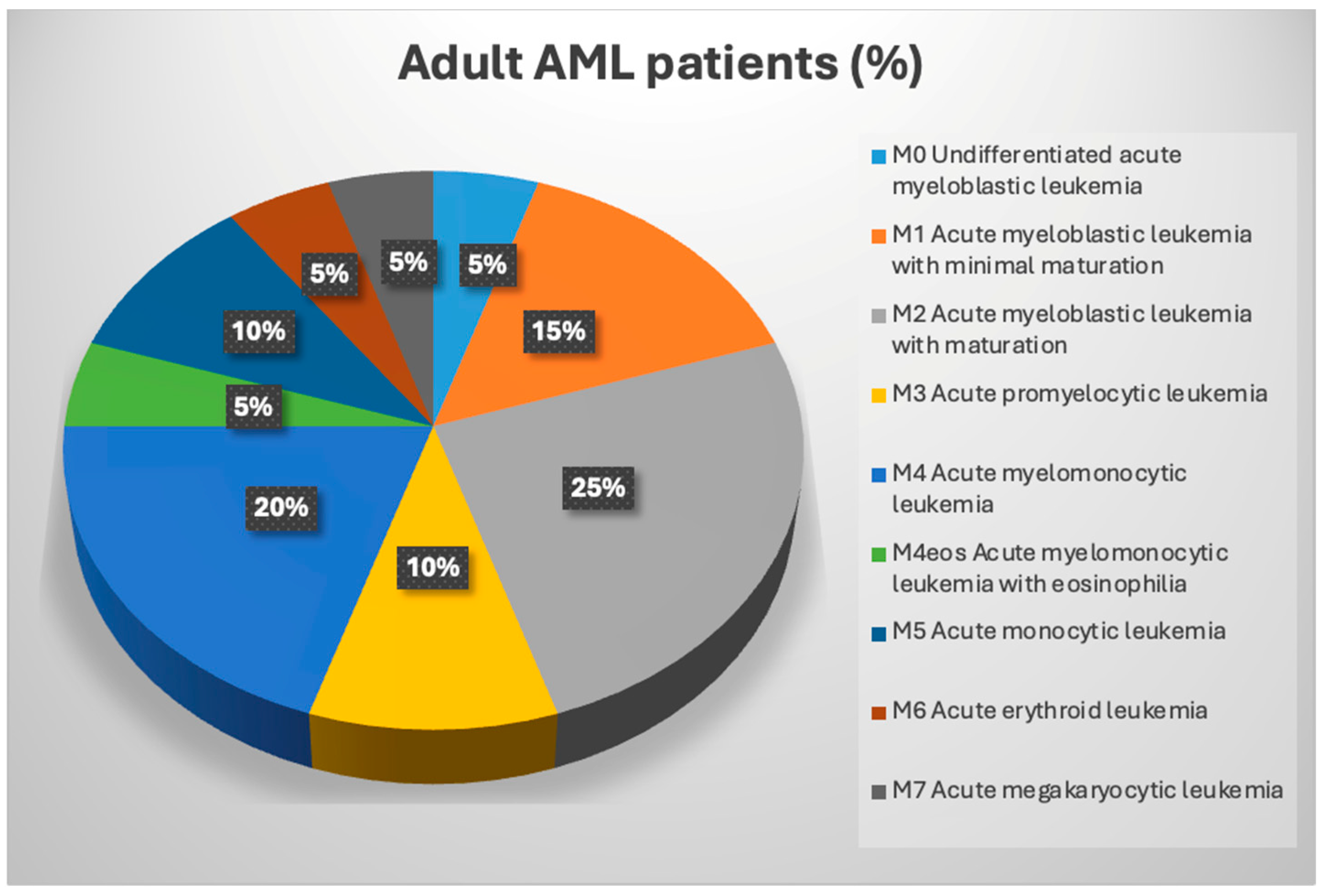
1.3. AML Classification
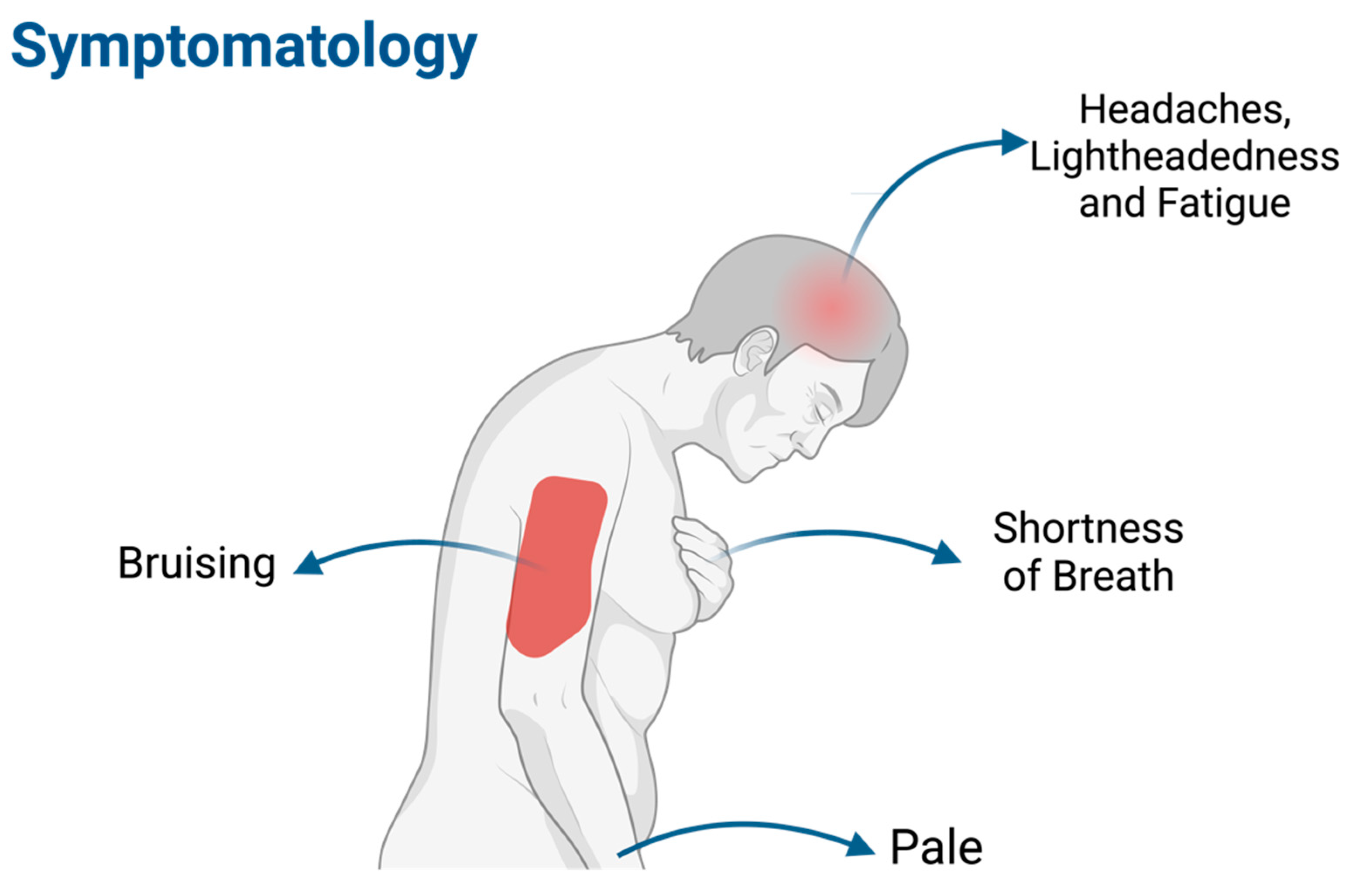
1.4. Symptomatology (Figure 3)
2. Methodology
3. Results
Treatment Regimens in AML in Adults
4. Discussion
4.1. Systemic Chemotherapy Drugs Used to Treat AML
4.1.1. Anthracyclines (Daunorubicin, Idarubicin, Mitoxantrone)
4.1.2. Cytarabine
4.1.3. Hypomethylating Agents (Azacitidine and Decitabine)
4.1.4. CPX-351 (Daunorubicin and Cytarabine)
4.2. Targeted Therapies Used to Treat AML
4.2.1. Gemtuzumab Ozogamicin
4.2.2. Midostaurin
4.2.3. Quizartinib
4.2.4. Enasidenib
4.2.5. Gilteritinib
4.2.6. Glasdegib
4.2.7. Ivosidenib
4.2.8. Venetoclax
4.2.9. Olutasidenib
4.3. Cardiovascular Complications in AML
4.3.1. Risk of CVDs in Patients with Newly Diagnosed Acute Leukemia in Adults
4.3.2. Myocarditis
4.3.3. Acute Coronary Syndromes (ACSs)
4.3.4. Leukostasis
4.4. Cardio-Oncology and Cardiotoxicity
4.4.1. Definitions
4.4.2. Cardiovascular Toxicity Risk Stratification Scores
4.4.3. Cumulative Incidence and Risk Factors of Cardiac Events in AML Patients
4.4.4. Pericardial Toxicity
4.4.5. Myocarditis [48]
4.5. Arrythmias
4.5.1. Atrial Fibrillation (AFib)
4.5.2. Sinus Bradycardia
4.5.3. QTc Prolongation
4.6. Anthracycline-Induced Cardiotoxicity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Definition of Acute Myeloid Leukemia—NCI Dictionary of Cancer Terms—NCI. 2011. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/acute-myeloid-leukemia (accessed on 12 May 2024).
- SEER. Acute Myeloid Leukemia—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 21 March 2024).
- Ou, Z.; Yu, D.; Liang, Y.; He, W.; Li, Y.; Zhang, M.; You, F.; He, H.; Chen, Q. Analysis of the Global Burden of Disease study highlights the trends in death and disability-adjusted life years of leukemia from 1990 to 2017. Cancer Commun. 2020, 40, 598–610. [Google Scholar] [CrossRef]
- Acute Myeloid Leukemia (AML) Staging: FAB and WHO Classifications for Acute Myeloid Leukemia. 27 October 2023. Available online: https://emedicine.medscape.com/article/2006750-overview?form=fpf (accessed on 12 May 2024).
- Mayo Clinic. Acute Myelogenous Leukemia—Symptoms and Causes. Available online: https://www.mayoclinic.org/diseases-conditions/acute-myelogenous-leukemia/symptoms-causes/syc-20369109 (accessed on 12 May 2024).
- Leukemia and Lymphoma Society, Acute Myeloid Leukemia in Adults. 2023. Available online: https://www.lls.org/sites/default/files/2023-06/PS32_Adult_AML_2023.pdf (accessed on 12 May 2024).
- Signs and Symptoms of Acute Myeloid Leukemia (AML). Available online: https://www.cancer.org/cancer/types/acute-myeloid-leukemia/detection-diagnosis-staging/signs-symptoms.html (accessed on 12 May 2024).
- Definition of Anthracycline—NCI Dictionary of Cancer Terms—NCI. 2011. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/anthracycline (accessed on 12 May 2024).
- Douedi, S.; Carson, M.P. Anthracycline Medications (Doxorubicin); StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551633/ (accessed on 8 April 2024).
- Cowgill, J.A.; Francis, S.A.; Sawyer, D.B. Anthracycline and Peripartum Cardiomyopathies. Circ Res. 2019, 124, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Neuendorff, N.R.; Loh, K.P.; Mims, A.S.; Christofyllakis, K.; Soo, W.-K.; Bölükbasi, B.; Oñoro-Algar, C.; Hundley, W.G.; Klepin, H.D. Anthracycline-related cardiotoxicity in older patients with acute myeloid leukemia: A Young SIOG review paper. Blood Adv. 2020, 4, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, A.; Tadi, P. Cytarabine; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557680/ (accessed on 8 April 2024).
- Derissen, E.J.B.; Beijnen, J.H.; Schellens, J.H.M. Concise Drug Review: Azacitidine and Decitabine. Oncologist. 2013, 18, 619–624. [Google Scholar] [CrossRef]
- Johnson, I.M.; Bezerra, E.D.; Farrukh, F.; McCullough, K.; Al-Kali, A.; Alkhateeb, H.B.; Begna, K.; Litzow, M.R.; Hogan, W.J.; Shah, M.V.; et al. Cardiac events in patients with acute myeloid leukemia treated with venetoclax combined with hypomethylating agents. Blood Adv. 2022, 6, 5227–5231. [Google Scholar] [CrossRef]
- Alfayez, M.; Kantarjian, H.; Kadia, T.; Ravandi-Kashani, F.; Daver, N. CPX-351 (vyxeos) in AML. Leuk Lymphoma. 2020, 61, 288–297. [Google Scholar] [CrossRef]
- Fortin, M.C.; LaCroix, A.S.; Grammatopoulos, T.N.; Tan, L.; Wang, Q.; Manca, D. Lower cardiotoxicity of CPX-351 relative to daunorubicin plus cytarabine free-drug combination in hiPSC-derived cardiomyocytes in vitro. Sci. Rep. 2023, 13, 21054. [Google Scholar] [CrossRef]
- Aronson, J.K. (Ed.) Gemtuzumab ozogamicin. In Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1488–1489. Available online: https://www.sciencedirect.com/science/article/pii/B0444510052008809 (accessed on 8 April 2024).
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.H.; Berger, M.S.; Feingoldr, J. Acute and Long-Term Toxicities Associated with Gemtuzumab Ozogamicin (Mylotarg®) Therapy of Acute Myeloid Leukemia. Clin. Lymphoma. 2002, 2, S29–S34. [Google Scholar] [CrossRef]
- Perry, R.; Penk, J.; Kapoor, N.; Shah, A.J. Gemtuzumab ozogamicin exposure and portal fibrosis. Pediatr. Blood Cancer. 2005, 45, 82–83. [Google Scholar] [CrossRef]
- McNerney, K.O.M.; Oranges, K.M.; Seif, A.E.; Oshrine, B.; Ky, B.M.; Lin, K.Y.; Getz, K.D.; Aplenc, R.M. Acute Left Ventricular Dysfunction Following Gemtuzumab Ozogamicin in Two Pediatric AML Patients. J. Pediatr. Hematol. Oncol. 2022, 44, e507–e511. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.; Joshi, S.K.; Traer, E. Profile of Quizartinib for the Treatment of Adult Patients with Relapsed/Refractory FLT3-ITD-Positive Acute Myeloid Leukemia: Evidence to Date. Cancer Manag. Res. 2020, 12, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.R.; Pemmaraju, N. How to Treat Adult Acute Myeloid Leukemia. JACC Cardio Oncol. 2021, 3, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Manouchehri, A.; Moslehi, J.; Salem, J.-E. Abstract 12978: Identification of Cardiovascular Adverse Effects Associated With Midostaurin—A WHO Pharmacovigilance Database Analysis. Circulation 2020, 142 (Suppl. 3), A12978. [Google Scholar] [CrossRef]
- Cremer, A.; Enssle, J.C.; Pfaff, S.; Kouidri, K.; Lang, F.; Brandts, C.; Zeiher, A.; Cremer, S.; Steffen, B.; Serve, H.; et al. Treatment with midostaurin and other FLT3 targeting inhibitors is associated with an increased risk of cardiovascular adverse events in patients who underwent allogeneic hematopoietic stem cell transplantation with FLT3-mutated AML. Ann. Hematol. 2023, 102, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Boluda, B.; Solana-Altabella, A.; Cano, I.; Martínez-Cuadrón, D.; Acuña-Cruz, E.; Torres-Miñana, L.; Rodríguez-Veiga, R.; Navarro-Vicente, I.; Martínez-Campuzano, D.; García-Ruiz, R.; et al. Incidence and Risk Factors for Development of Cardiac Toxicity in Adult Patients with Newly Diagnosed Acute Myeloid Leukemia. Cancers 2023, 15, 2267. [Google Scholar] [CrossRef]
- Belik, D.M.; Bernasconi, R.; Xu, L.; Della Verde, G.; Lorenz, V.; Grüterich, V.; Balzarolo, M.; Mochizuki, M.; Pfister, O.; Kuster, G.M. The Flt3-inhibitor quizartinib augments apoptosis and promotes maladaptive remodeling after myocardial infarction in mice. Apoptosis 2024, 29, 357–371. [Google Scholar] [CrossRef] [PubMed]
- de Botton, S.; Montesinos, P.; Schuh, A.C.; Papayannidis, C.; Vyas, P.; Wei, A.H.; Ommen, H.B.; Semochkin, S.; Kim, H.-J.; Larson, R.A.; et al. Enasidenib vs conventional care in older patients with late-stage mutant-IDH2 relapsed/refractory AML: A randomized phase 3 trial. Blood 2023, 141, 156–167. [Google Scholar] [CrossRef]
- Enasidenib. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK548492/ (accessed on 8 April 2024).
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Sarkaria, S.M.; Heaney, M.L. Glasdegib in newly diagnosed acute myeloid leukemia. Expert. Rev. Anticancer Ther. 2021, 21, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.G.; Stanchina, M.; Bradley, T.J.; Watts, J. Profile of Glasdegib for the Treatment of Newly Diagnosed Acute Myeloid Leukemia (AML): Evidence to Date. Cancer Manag. Res. 2022, 14, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.; Silverman, L.R.; Navada, S.C. Forsaken Pharmaceutical: Glasdegib in Acute Myeloid Leukemia and Myeloid Diseases. Clin. Lymphoma Myeloma Leuk. 2021, 21, e415–e422. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.P.; Martino, G.; Cardinali, V.; Falini, B.; Martinelli, G.; Cerchione, C. Enasidenib and ivosidenib in AML. Minerva Med. 2020, 111, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Thurlapati, A.; Thotamgari, S.; Grewal, U.S.; Sheth, A.R.; Gupta, D.; Beedupalli, K.; Dominic, P. Anti-cancer Drugs Associated Atrial Fibrillation—An Analysis of Real-World Pharmacovigilance Data. Front. Cardiovasc. Med. 2022, 9, 739044. [Google Scholar] [CrossRef] [PubMed]
- de Botton, S.; Fenaux, P.; Yee, K.W.; Récher, C.; Wei, A.H.; Montesinos, P.; Taussig, D.C.; Pigneux, A.; Braun, T.; Curti, A.; et al. Olutasidenib (FT-2102) induces durable complete remissions in patients with relapsed or refractory IDH1-mutated AML. Blood Adv. 2023, 7, 3117–3127. [Google Scholar] [CrossRef]
- Xiao, W.; Ma, L.; Shang, Y.; Yang, F.; Tan, Y.; Chen, G.; Wu, J.; Liang, Y.; Rouzi, T.; Wang, Q.; et al. Cardiac-Related Lesions in Newly Diagnosed Patients with Acute Leukemia: A Chinese Population-Based Real-World Study. Front. Med. 2022, 9, 844350. Available online: https://www.frontiersin.org/articles/10.3389/fmed.2022.844350 (accessed on 16 November 2023). [CrossRef] [PubMed]
- Acibuca, A.; Yeral, M.; Kocer, N.E.; Koc, Z.; Güllü, H. Relapsed acute myeloid leukemia presenting with myocardial hypertrophy and constrictive pericardial physiology. Anatol. J. Cardiol. 2019, 21, 287–289. [Google Scholar]
- Ferrel, M.N.; Ryan, J.J.; Han, F.T. Acute myeloid leukemia causing acute thrombosis of the coronary arteries: A case report. J. Med. Case Rep. 2022, 16, 149. [Google Scholar] [CrossRef]
- Jao, G.T.; Knovich, M.A.; Savage, R.W.; Sane, D.C. ST-elevation myocardial infarction and myelodysplastic syndrome with acute myeloid leukemia transformation. Tex. Heart Inst. J. 2014, 41, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Manogna, D.; Sham, R. Acute Myocardial Infarction as Initial Manifestation of Acute Myeloid Leukemia: A Rare Manifestation of Leukostasis. Cureus 2020, 12, e9551. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Lopez Candales, A. High Output Cardiac Failure in a Patient Presenting with Acute Myeloid Leukemia and Leukostasis. Bol. Asoc Medica P R. 2016, 108, 63–65. [Google Scholar]
- 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) | European Heart Journal |Oxford Academic. Available online: https://academic.oup.com/eurheartj/article/43/41/4229/6673995?login=false (accessed on 12 May 2024).
- Banerjee, P.; Qamar, I. Chapter 11—Novel Anticancer Drugs Related to Cardiotoxicity. In Cardiovascular Toxicity and Therapeutic Modalities Targeting Cardio-Oncology; Qamar, I., Maurya, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 195–213. Available online: https://www.sciencedirect.com/science/article/pii/B9780323904612000067 (accessed on 19 May 2024).
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [PubMed]
- Janus, S.E.; Heisler, A.C.; Al Jammal, M.; Chahine, N.; Chami, T.; Hajjari, J.; Mously, H.; Badhwar, A.; Arora, S.; Al-Juhaishi, T.; et al. Reported Pericardial Toxicities Associated with Acute Myelogenous Leukemia Treatments: A Pharmacovigilance Analysis of the FDA Adverse Reporting Database. Curr. Probl. Cardiol. 2022, 47, 101345. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Albsheer, K.; Fadul, A.; Khalafalla, A.; Abdalla, E.M.; Al-Dubai, H. Cytarabine-Induced Bradycardia: A Case Report. Cureus 2022, 14, e30624. [Google Scholar] [CrossRef] [PubMed]
- Kumagawa, M.; Suzuki, K.; Nagano, M.; Takamatsu, Y.; Suzumiya, J.; Tamura, K. High dose ara-C therapy induced bradycardia in an acute myeloid leukemia patient with inv (16)(p13q22). Rinsho Ketsueki. 2003, 44, 404–406. [Google Scholar]
- Romani, C.; Pettinau, M.; Murru, R.; Angelucci, E. Sinusal bradycardia after receiving intermediate or high dose cytarabine: Four cases from a single institution. Eur. J. Cancer Care 2009, 18, 320–321. [Google Scholar] [CrossRef]
| AML Categorisation | |
|---|---|
| By Genetic Abnormalities | By Differentiation |
| RUNX1::RUNX1T1 fusion | Minimal differentiation |
| CBFB::MYH11 fusion | Without maturation |
| DEK::NUP214 fusion | With maturation |
| RBM15::MRTFA fusion | Acute basophilic leukemia |
| BCR::ABL1 fusion | Acute myelomonocytic leukemia |
| K MT2A rearrangement | Acute monocytic leukemia |
| MECOM rearrangement | Acute erythroid leukemia |
| NUP98 rearrangement | Acute megakaryoblastic leukemia |
| NPM1 mutation | - |
| CEBPA mutation | - |
| myelodysplasia-related | - |
| Regimen | Mechanism of Action | Indication in AML Treatment | Adverse Effects (Common) | Cardiovascular Toxicity (Reported) |
|---|---|---|---|---|
| Systematic Chemotherapy Drugs | ||||
| “7 + 3” (cytarabine + anthracycline) | Induction therapy | Allergic reactions (anaphylaxis, rash) Fever Severe cough Photosensitivity Skin and nail hyperpigmentation Hoarseness of voice Bone marrow suppression, low blood cell counts Mucositis Fatigue Joint pain Persistent diarrhea | Anthracycline-induced cardiotoxicity (acute HF, arrhythmia, or myocarditis, and anthracycline-related left ventricular dysfunction [ARLVD]) | |
| Antracyclines (daunorubicin, idarubicin, mitoxantrone) | Inhibition of DNA synthesis and DNA topoisomerase II | |||
| Hypomethylating agents (HMA; azacitidine and decitabine) | Inhibition of DNA methylotransferase | Maintenance following intensive chemotherapy HSCT ineligible | Pancytopenia Hepatotoxicity Acute renal injury | Chest pain Atrial fibrillation (3–5%) Congestive heart failure (3–11%) Cardiomyopathy (<5%) Hyper/hypotension |
| Cytarabine | Antimetabolic agent and inhibition of DNA polymerase | Induction therapy | Gastrointestinal disturbances Stomatitis Conjunctivitis Reversible hepatic enzyme elevation Dermatitis | Anthracycline-associated cardiomyopathy, pericarditis, bradycardia (2.8%), angina pectoris |
| CPX-351 (daunorubicin and cytarabine) | Secondary AML Frontline | GI disturbances (constipation, diarrhea, nausea, vomiting) Cytopenias Allergic reactions Ototoxicity | Anthracycline-associated cardiomyopathy Edema Arrhythmia Hypo/hypertension Chest pain | |
| Targeted Therapy | ||||
| GO (gemtuzumab ozogamicin) | Anti-CD33 monoclonal antibody | Favorable/intermediate/unknown cytogenetics Patients with CD33+-expressing leukemic blasts Frontline with intensive chemotherapy | Neutropenia and thrombocytopenia Hepatotoxicity and portal fibrosis | Infusion-related hypotension (5%) Acute LV dysfunction |
| Midostaurin | Multi-kinase inhibitor against FLT3-ITD and FLT3-TKD | Newly diagnosed FLT3 ITD/TKD-mutated AML Frontline with intensive chemotherapy | Pancytopenia Infection, mucositis or stomatitis, pneumonitis or pulmonary infiltrates Diarrhea, nausea, increased alanine aminotransferase, hyperbilirubinemia Hypokalemia, hyponatremia, hypophosphatemia, hypocalcemia Pain, rash, fatigue | QTc prolongation, heart failure, atrial fibrillation, hypotension and pericardial disease |
| Quizartinib | FLT3-inhibitor | Relapsed/refractory FLT3-mutated AML (only in Japan) | Prolonged cytopenias | QTc prolongation (34%) |
| Enasidenib | Inhibition of mutant IDH2 enzyme | Mutant IDH2 R/R AML | Cytopenias GI symptoms (nausea, vomiting, decreased appetite) Blood bilirubin increased Differentiation syndrome | N/A |
| Gilteritinib | FLT3-inhibitor | Monotherapy for R/R FLT3-TKD or ITD AML | Pancytopenia Hepatotoxicity Pyrexia Diarrhea, constipation Vomiting Hypokalemia Pneumonia Peripheral edema | QTc prolongation (4.9%) Risk of heart failure development (4%) Edema Hypotension Pericardial effusion Myocarditis Pericarditis |
| Glasdegib | Inhibitor of the hedgehog signaling pathway by binding to the smoothened (SMO) receptor | Newly diagnosed AML in patients aged ≥75 years or those who have comorbidities that preclude use of intensive induction chemotherapy (+cytarabine) | Febrile neutropenia, nausea, decreased appetite, fatigue, constipation, dysgeusia, muscle spasms, dizziness, and vomiting | QTc prolongation Peripheral edema |
| Ivosidenib | Selective inhibitor of mutant IDH1 enzyme | Frontline monotherapy for ND IDH1-AML if ≥75 years or unfit for IC Frontline IVO/AZA for ND IDH1-AML if ≥75 years or unfit for IC Monotherapy for R/R IDH1-AML | Differentiation syndrome (DS) and leukocytosis | QTc prolongation (18–25%) Edema Hypotension Chest pain Ventricular fibrillation (<1%) |
| Olutasidenib | Inhibitor of mutant IDH1 enzyme | Monotherapy for IDH1-AML | Cytopenias Acute liver injury and failure (ALT, AST, gGT) GI side effects Pneumonia Hypokalemia DS Tumor lysis syndrome | QTc prolongation (8%) |
| Venetoclax | Anti-BCL2 monoclonal antibody | Elderly/unfit Frontline with azacitidine, decitabine | Pancytopenia (>10%) Nausea, constipation, diarrhea, vomiting (>30%) Hypokalemia Pneumonia (38%) Sepsis (6%) Tumor lysis syndrome (1%) | Atrial fibrillation (5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinidis, I.; Tsokkou, S.; Grigoriadis, S.; Lalayianni, C.; Gavriilaki, E. Cardiotoxicity in Acute Myeloid Leukemia in Adults: A Scoping Study. Cancers 2024, 16, 2474. https://doi.org/10.3390/cancers16132474
Konstantinidis I, Tsokkou S, Grigoriadis S, Lalayianni C, Gavriilaki E. Cardiotoxicity in Acute Myeloid Leukemia in Adults: A Scoping Study. Cancers. 2024; 16(13):2474. https://doi.org/10.3390/cancers16132474
Chicago/Turabian StyleKonstantinidis, Ioannis, Sophia Tsokkou, Savvas Grigoriadis, Chrysavgi Lalayianni, and Eleni Gavriilaki. 2024. "Cardiotoxicity in Acute Myeloid Leukemia in Adults: A Scoping Study" Cancers 16, no. 13: 2474. https://doi.org/10.3390/cancers16132474






