Short- and Long-Term Advantages of Laparoscopic Gastrectomy for Elderly Patients with Locally Advanced Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgery, Perioperative Management, and Follow-Up
2.3. Data Definition and Collection
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Primary Outcomes
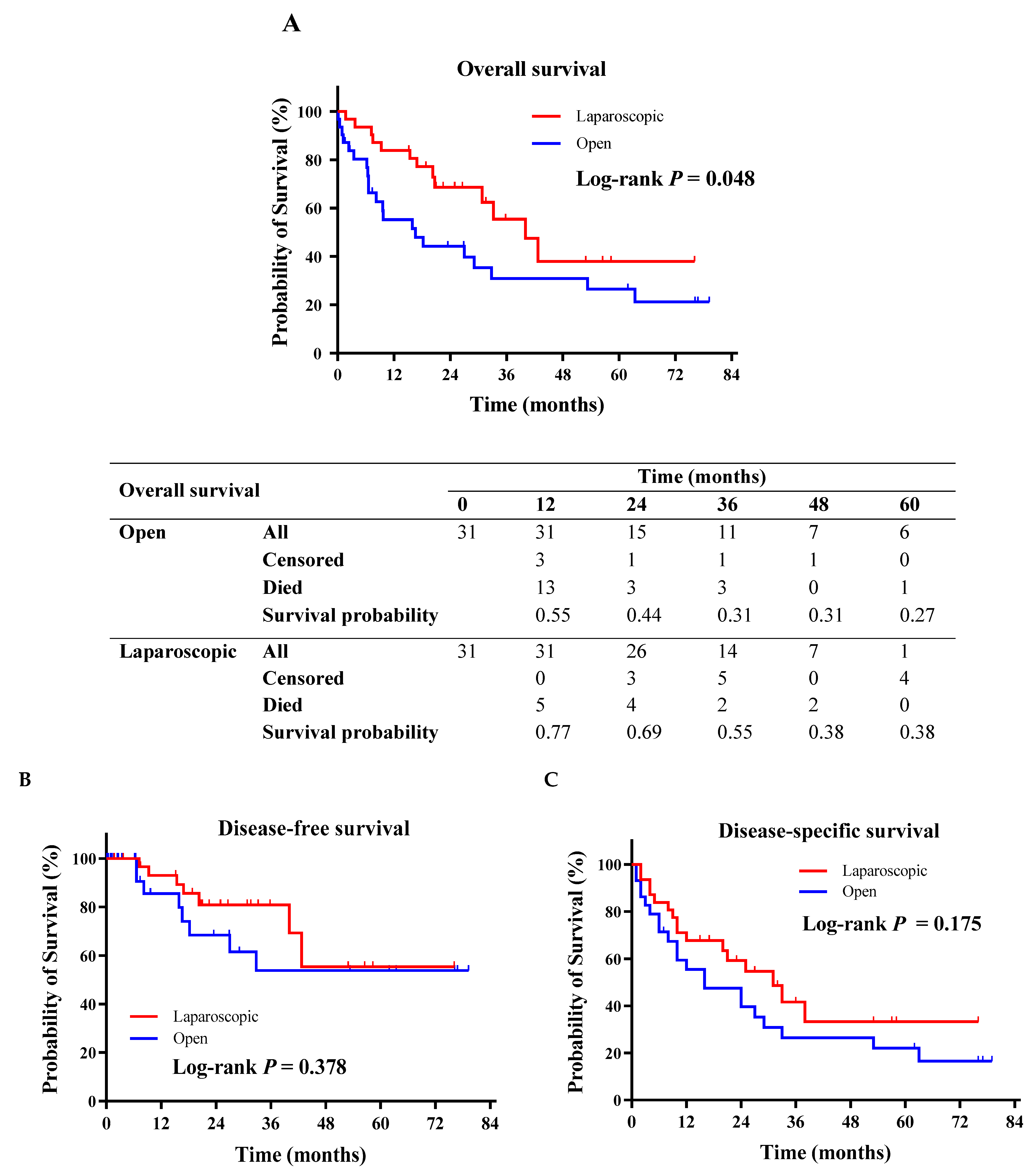
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Statistics Adapted from the American Cancer Society’s (ACS) Publication, Cancer Facts & Figures 2021, the ACS Website, and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 20 February 2021).
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Safiri, S.; Sepanlou, S.G.; Ikuta, K.; Bisignano, C.; Shakeri, R.; Amani, M.; Fitzmaurice, C.; Nixon, M.; Abbasi, N.; et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet. Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.D.; Best, L.; George, S.; Baughan, C.; Buchanan, R.; Davis, C.; Fentiman, I.; Gosney, M.; Northover, J.; Williams, C. Surgery for colorectal cancer in elderly patients: A systematic review. Lancet 2000, 356, 968–974. [Google Scholar] [CrossRef]
- Polanczyk, C.A.; Marcantonio, E.; Goldman, L.; Rohde, L.E.; Orav, J.; Mangione, C.M.; Lee, T.H. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann. Intern. Med. 2001, 134, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, D.; Koide, N.; Suzuki, A.; Ishizone, S.; Shimizu, F.; Tsuchiya, T.; Kumeda, S.; Miyagawa, S. Postoperative complications in elderly patients with gastric cancer. J. Surg. Res. 2015, 198, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mikami, J.; Kurokawa, Y.; Miyazaki, Y.; Takahashi, T.; Yamasaki, M.; Miyata, H.; Nakajima, K.; Takiguchi, S.; Mori, M.; Doki, Y. Postoperative gastrectomy outcomes in octogenarians with gastric cancer. Surg. Today 2015, 45, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.; Nilsson, M.; Slim, K.; Schafer, M.; Mariette, C.; Braga, M.; Carli, F.; Demartines, N.; Griffin, S.M.; Lassen, K.; et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Br. J. Surg. 2014, 101, 1209–1229. [Google Scholar] [CrossRef] [PubMed]
- Wee, I.J.Y.; Syn, N.L.; Shabbir, A.; Kim, G.; So, J.B.Y. Enhanced recovery versus conventional care in gastric cancer surgery: A meta-analysis of randomized and non-randomized controlled trials. Gastric Cancer 2019, 22, 423–434. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Li, J.; Hong, D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: A meta-analysis of randomized controlled trials. Surg. Oncol. 2020, 32, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Junttila, A.; Helminen, O.; Kairaluoma, V.; Mattila, A.; Sihvo, E.; Mrena, J. Implementation of Multimodality Therapy and Minimally Invasive Surgery: Short- and Long-term Outcomes of Gastric Cancer Surgery in Medium-Volume Center. J. Gastrointest. Surg. 2022, 26, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Iso, Y.; Moriyama, M.; Sugimachi, K. Laparoscopy-assisted Billroth I gastrectomy. Surg. Laparosc. Endosc. 1994, 4, 146–148. [Google Scholar] [PubMed]
- Japanese Gastric Cancer, A. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021, 6th ed.; Springer: Berlin, Germany, 2021. [Google Scholar]
- Hyung, W.J.; Yang, H.K.; Park, Y.K.; Lee, H.J.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; Hur, H.; et al. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J. Clin. Oncol. 2020, 38, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, H.; Hu, Y.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Wang, K.; Suo, J.; Tao, K.; et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg. 2022, 157, 9–17. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, A.; Brenkman, H.J.F.; Seesing, M.F.J.; Haverkamp, L.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; Stoot, J.; Tegels, J.J.W.; Wijnhoven, B.P.L.; Lagarde, S.M.; et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): A Multicenter Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 978–989. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, N.; Straatman, J.; Daams, F.; Rosati, R.; Parise, P.; Weitz, J.; Reissfelder, C.; Diez Del Val, I.; Loureiro, C.; Parada-González, P.; et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: Results of a European randomized trial. Gastric Cancer 2021, 24, 258–271. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; on behalf of theESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, F.; Cinelli, L.; Genova, L.; Battaglia, S.; Barbieri, L.A.; Treppiedi, E.; Cossu, A.; Elmore, U.; Rosati, R. Applicative Limitations of Indocyanine Green Fluorescence Assistance to Laparoscopic Lymph Node Dissection in Total Gastrectomy for Cancer. Ann. Surg. Oncol. 2022, 29, 5875–5882. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Kasumova, G.G.; Verheij, J.; Najarian, R.M.; Maggino, L.; de Pastena, M.; Malleo, G.; Marchegiani, G.; Salvia, R.; Ng, S.C.; et al. International Validation of the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients With Resected Pancreatic Cancer. JAMA Surg. 2018, 153, e183617. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology—A primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar] [PubMed]
- Morino, K.; Yamamoto, M.; Shimoike, N.; Iwasaki, Y.; Yamanaka, R.; Nakanishi, N.; Matsusue, R.; Machimoto, T. Safety and Limitations of Laparoscopic Total Gastrectomy for Gastric Cancer: A Comparative Analysis of Short and Long-term Outcomes With Open Surgery. Anticancer Res. 2024, 44, 1759–1766. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, M.S.; Lee, Y.T.; Lee, C.M.; Kim, J.H.; Park, S. Laparoscopic total gastrectomy as a valid procedure to treat gastric cancer option both in early and advanced stage: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, L.; Liu, L.; Zhu, Z.; Liu, S.; Hu, L.; He, Y.; Fang, Y.; Wan, X. Short-term outcomes and prognosis of laparoscopy-assisted total gastrectomy in elderly patients with stomach cancer. Surg. Endosc. 2020, 34, 5428–5438. [Google Scholar] [CrossRef]
- Haverkamp, L.; Weijs, T.J.; van der Sluis, P.C.; van der Tweel, I.; Ruurda, J.P.; van Hillegersberg, R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: A systematic review and meta-analysis. Surg. Endosc. 2013, 27, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Parise, P.; Cinelli, L.; Ferrari, C.; Cossu, A.; Puccetti, F.; Garutti, L.; Elmore, U.; Rosati, R. Early Red Flags Associated with Delayed Discharge in Patients Undergoing Gastrectomy: Analysis of Perioperative Variables and ERAS Protocol Items. World J. Surg. 2020, 44, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, Y.; Kurashima, Y.; Watanabe, Y.; Tanaka, K.; Matsui, A.; Nakanishi, Y.; Asano, T.; Noji, T.; Nakamura, T.; Murakami, S.; et al. Outcomes of laparoscopic total gastrectomy in elderly patients: A propensity score matching analysis. Langenbeck’s Arch. Surg. 2022, 407, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, H.; Kunisaki, C.; Miyamato, H.; Sato, K.; Sato, S.; Tanaka, Y.; Yukawa, N.; Rino, Y.; Kosaka, T.; Akiyama, H.; et al. Laparoscopic Total Gastrectomy for Gastric Cancer in Elderly Patients. In Vivo 2020, 34, 2933–2939. [Google Scholar] [CrossRef]
- Honda, M.; Kumamaru, H.; Etoh, T.; Miyata, H.; Yamashita, Y.; Yoshida, K.; Kodera, Y.; Kakeji, Y.; Inomata, M.; Konno, H.; et al. Surgical risk and benefits of laparoscopic surgery for elderly patients with gastric cancer: A multicenter prospective cohort study. Gastric Cancer 2019, 22, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Gao, C.; Li, X.L.; Li, Z.Y.; Ying, X.J.; Wang, Y.K.; Li, S.X.; Ji, X.; Ji, J.F. Short- and Long-Term Outcomes after Laparoscopic Versus Open Gastrectomy for Elderly Gastric Cancer Patients: A Systematic Review and Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. Part A 2020, 30, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, Y.; Kurokawa, Y.; Takahashi, T.; Saito, T.; Yamashita, K.; Tanaka, K.; Makino, T.; Yamasaki, M.; Nakajima, K.; Mori, M.; et al. Is Laparoscopic Gastrectomy More Advantageous for Elderly Patients Than for Young Patients with Resectable Advanced Gastric Cancer? World J. Surg. 2020, 44, 2332–2339. [Google Scholar] [CrossRef]
- Petrioli, R.; Francini, E.; Cherri, S.; Marrelli, D.; Rovello, F.; Fiaschi, A.I.; Miano, S.T.; Savelli, V.; Calomino, N.; Farsi, M.; et al. Feasibility of modified docetaxel, oxaliplatin, capecitabine followed by capecitabine as maintenance chemotherapy as first-line therapy for patients with metastatic gastric or gastroesophageal cancer. Anti-Cancer Drugs 2020, 31, 292–297. [Google Scholar] [CrossRef]
- Okholm, C.; Goetze, J.P.; Svendsen, L.B.; Achiam, M.P. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand. J. Gastroenterol. 2014, 49, 1027–1034. [Google Scholar] [CrossRef]
- Tsekrekos, A.; Vossen, L.E.; Lundell, L.; Jeremiasen, M.; Johnsson, E.; Hedberg, J.; Edholm, D.; Klevebro, F.; Nilsson, M.; Rouvelas, I. Improved survival after laparoscopic compared to open gastrectomy for advanced gastric cancer: A Swedish population-based cohort study. Gastric Cancer 2023, 26, 467–477. [Google Scholar] [CrossRef]
- Long, D.; Feng, Q.; Li, Z.S.; Zhao, Y.L.; Qian, F.; Tang, B.; Chen, J.; Li, P.A.; Shi, Y.; Yu, P.W. Laparoscopic versus open gastrectomy for serosa-invasive gastric cancer: A single-center retrospective cohort study. Surgery 2021, 169, 1486–1492. [Google Scholar] [CrossRef]
- Hallam, S.; Rickard, F.; Reeves, N.; Messenger, D.; Shabbir, J. Compliance with enhanced recovery protocols in elderly patients undergoing colorectal resection. Ann. R. Coll. Surg. Engl. 2018, 100, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Borghi, F.; Scatizzi, M.; Missana, G.; Guicciardi, M.A.; Bona, S.; Ficari, F.; Maspero, M.; Pecorelli, N.; PeriOperative Italian, S. Impact of laparoscopy on adherence to an enhanced recovery pathway and readiness for discharge in elective colorectal surgery: Results from the PeriOperative Italian Society registry. Surg. Endosc. 2017, 31, 4393–4399. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Yu, H.; Li, H.; Zhao, N.; Zhang, Y.; Li, J.; Cui, J.; Tang, D.; Li, Y.; Teng, Y.; et al. Hemodynamic changes of anesthesia, pneumoperitoneum, and head-down tilt during laparoscopic surgery in elderly patients. Ann. Transl. Med. 2021, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Bàllesta López, C.; Cid, J.A.; Poves, I.; Bettónica, C.; Villegas, L.; Memon, M.A. Laparoscopic surgery in the elderly patient. Surg. Endosc. 2003, 17, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nakamura, T.; Imanishi, T.; Kanaji, S.; Yamamoto, M.; Kanemitsu, K.; Yamashita, K.; Sumi, Y.; Tanaka, K.; Kuroda, D.; et al. Carbon dioxide pneumoperitoneum led to no severe morbidities for the elderly during laparoscopic-assisted distal gastrectomy. Ann. Surg. Oncol. 2015, 22, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Aratani, K.; Sakuramoto, S.; Chuman, M.; Kasuya, M.; Wakata, M.; Miyawaki, Y.; Gunji, H.; Sato, H.; Okamoto, K.; Yamaguchi, S.; et al. Laparoscopy-assisted Distal Gastrectomy for Gastric Cancer in Elderly Patients: Surgical Outcomes and Prognosis. Anticancer Res. 2018, 38, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, H.L.; Li, P.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.H.; et al. High preoperative modified frailty index has a negative impact on short- and long-term outcomes of octogenarians with gastric cancer after laparoscopic gastrectomy. Surg. Endosc. 2018, 32, 2193–2200. [Google Scholar] [CrossRef] [PubMed]

| Patients | Open 31 | Laparoscopic 31 | p-Value |
|---|---|---|---|
| Age, median (IQR) | 79 (77–83) | 80 (77–82) | 0.668 |
| Gender (Male/Female) | 19/12 | 18/13 | 0.796 |
| ASA, mean (±SD) | 3 (±1) | 3 (±1) | 0.117 |
| BMI, mean (±SD) | 25 (±6) | 24 (±6) | 0.314 |
| NRS, mean (±SD) | 3 (±1) | 3 (±1) | 0.102 |
| aa-CCI, mean (±SD) | 6 (±2) | 6 (±2) | 0.940 |
| COPD | 2 (6.5%) | 2 (6.5%) | 1.000 |
| CHF | 2 (6.5%) | 3 (9.7%) | 0.640 |
| Blood hypertension | 15 (48.4%) | 9 (29%) | 0.118 |
| Diabetes | 4 (12.9%) | 3 (9.7%) | 0.688 |
| Preoperative hemoglobin, mean (SD) | 11.2 (±2.4) | 11.7 (±2.6) | 0.260 |
| Preoperative serum creatinine, mean (SD) | 1.1 (±0.4) | 1 (±0.3) | 0.460 |
| Histotype | |||
| SRCC | 6 (19.4%) | 9 (29%) | 0.374 |
| non-SRCC | 25 (80.6%) | 22 (71%) | |
| Neoadjuvant treatment | |||
| Chemotherapy | 4 (12.9%) | 4 (12.9%) | 1.000 |
| Regimen: | |||
| 1/4 (25%) | 1/4 (25%) | 0.503 |
| 2/4 (50%) | 1/4 (25%) | |
| 1/4 (25%) | 0 | |
| 0 | 2/4 (50%) | |
| |||
| Completion rate | 3/4 (75%) | 3/4 (75%) | 1.000 |
| Surgery | |||
| Total gastrectomy | 13 (41.9%) | 16 (51.6%) | 0.268 |
| Distal gastrectomy | 18 (58.1%) | 15 (48.4%) | 0.346 |
| Conversion to open | 1 (3.2%) | ||
| TNM stage | |||
| LAGC | 31 (100%) | 31 (100%) | 1.000 |
| N > 0 | 24 (77.4%) | 19 (61.3%) | 0.168 |
| T > 3 | 11 (35.5%) | 10 (32.3%) | 0.788 |
| Patients | Open 31 | Laparoscopic 31 | p-Value |
|---|---|---|---|
| Intraoperative data | |||
| Operative time (minutes), mean (SD) | 190 (±65) | 216 (±60) | 0.058 |
| Lymph node retrieval, median (IQR) | 37 (25–45) | 37 (28–53) | 0.660 |
| Postoperative data | |||
| Complications: | |||
| 22 (71%) | 14 (45.2%) | 0.039 |
| 9 (29%) | 5 (16.1%) | 0.224 |
| 3 (9.7%) | 3 (9.7%) | 1.000 |
| 3 (9.7%) | 1 (3.2%) | 0.291 |
| 3 (9.7%) | 0 | 0.038 |
| 0 | 0 | - |
| 1 (3.2%) | 0 | 0.236 |
| 2 (6.5%) | 0 | 0.092 |
| Reoperation | 2 (6.5%) | 2 (6.5%) | 1.000 |
| Indication to reoperation: | |||
| 1 (3.2%) | 1 (3.2%) | 1.000 |
| 1 (3.2%) | 1 (3.2%) | 1.000 |
| 30-day mortality | 1 (3.2%) | 1 (3.2%) | 1.000 |
| 30-day readmission | 4 (12.9%) | 2 (6.5%) | 0.417 |
| Length of hospital stay, median (IQR) | 12 (9–18) | 8 (7–10) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccetti, F.; Cinelli, L.; Turi, S.; Socci, D.; Rosati, R.; Elmore, U.; on behalf of the OSR CCeR Collaborative Group. Short- and Long-Term Advantages of Laparoscopic Gastrectomy for Elderly Patients with Locally Advanced Cancer. Cancers 2024, 16, 2477. https://doi.org/10.3390/cancers16132477
Puccetti F, Cinelli L, Turi S, Socci D, Rosati R, Elmore U, on behalf of the OSR CCeR Collaborative Group. Short- and Long-Term Advantages of Laparoscopic Gastrectomy for Elderly Patients with Locally Advanced Cancer. Cancers. 2024; 16(13):2477. https://doi.org/10.3390/cancers16132477
Chicago/Turabian StylePuccetti, Francesco, Lorenzo Cinelli, Stefano Turi, Davide Socci, Riccardo Rosati, Ugo Elmore, and on behalf of the OSR CCeR Collaborative Group. 2024. "Short- and Long-Term Advantages of Laparoscopic Gastrectomy for Elderly Patients with Locally Advanced Cancer" Cancers 16, no. 13: 2477. https://doi.org/10.3390/cancers16132477





