Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma including Acral and Mucosal Subtypes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients
2.3. Procedure
2.4. Assessment
2.5. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Three-Year TTR in Subtypes of Melanoma
3.3. Three-Year OS in Subtypes of Melanoma
3.4. Multivariate Analysis of the Prognostic Factors in Both TTR and OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Ascierto, P.A.; Khattak, M.A.; de la Cruz Merino, L.; Del Vecchio, M.; Rutkowski, P.; Spagnolo, F.; Mackiewicz, J.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Pembrolizumab Versus Placebo as Adjuvant Therapy in Resected Stage IIB or IIC Melanoma: Final Analysis of Distant Metastasis-Free Survival in the Phase III KEYNOTE-716 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, 42, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in Acral and Mucosal Melanoma: Current Status and Future Directions. Front. Immunol. 2021, 12, 680407. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Fukushima, S.; Ito, T.; Maekawa, T.; Shoichiro, I.; Uchi, H.; Matsushita, S.; Yamamoto, Y.; et al. Postoperative adjuvant therapy for 120 patients with melanoma, including acral and mucosal subtypes: A multicentre, observational study of 2-year follow-up results. Br. J. Dermatol. 2023, 189, 476–478. [Google Scholar] [CrossRef]
- Dugan, M.M.; Perez, M.C.; Karapetyan, L.; Zager, J.S. Management of acral lentiginous melanoma: Current updates and future directions. Front. Oncol. 2024, 14, 1323933. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.A.; Amir, E. HYPE or HOPE: The prognostic value of infiltrating immune cells in cancer. Br. J. Cancer 2017, 117, 451–460. [Google Scholar] [CrossRef]
- Maibach, F.; Sadozai, H.; Seyed Jafari, S.M.; Hunger, R.E.; Schenk, M. Tumor-Infiltrating Lymphocytes and Their Prognostic Value in Cutaneous Melanoma. Front. Immunol. 2020, 11, 2105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Smalley, I.; Chen, Z.; Wu, J.Y.; Phadke, M.S.; Teer, J.K.; Nguyen, T.; Karreth, F.A.; Koomen, J.M.; Sarnaik, A.A.; et al. Single-cell Characterization of the Cellular Landscape of Acral Melanoma Identifies Novel Targets for Immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 2131–2146. [Google Scholar] [CrossRef]
- Topham, N.J.; Hewitt, E.W. Natural killer cell cytotoxicity: How do they pull the trigger? Immunology 2009, 128, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lawless, A.R.; Czapla, J.A.; Gerstberger, S.C.; Park, B.C.; Jung, S.; Johnson, R.; Yamazaki, N.; Ogata, D.; Umeda, Y.; et al. Benefit, recurrence pattern, and toxicity to adjuvant anti-PD-1 monotherapy varies by ethnicity and melanoma subtype: An international multicenter cohort study. JAAD Int. 2024, 15, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.K.; McKeown, J.; Grover, P.; Johnson, D.B.; Zaremba, A.; Dimitriou, F.; Weiser, R.; Farid, M.; Namikawa, K.; Sullivan, R.J.; et al. Outcomes of patients with resected stage III/IV acral or mucosal melanoma, treated with adjuvant anti-PD-1 based therapy. Eur. J. Cancer 2024, 199, 113563. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Sun, W.; Hu, T.; Wang, C.; Yan, W.; Luo, Z.; Liu, X.; Xu, Y.; Chen, Y. Comparative analysis of adjuvant therapy for stage III BRAF-mut melanoma: A real-world retrospective study from single center in China. Cancer Med. 2023, 12, 11475–11482. [Google Scholar] [CrossRef] [PubMed]
- Bloem, M.; van Not, O.J.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; Blank, C.U.; Blokx, W.A.M.; Boers-Sonderen, M.J.; Bonenkamp, J.J.; de Groot, J.B.; Haanen, J.B.; et al. Adjuvant treatment with anti-PD-1 in acral melanoma: A nationwide study. Int. J. Cancer, 2024; in press. [Google Scholar] [CrossRef]
- Mori, T.; Izumi, T.; Doi, R.; Kamimura, A.; Takai, S.; Teramoto, Y.; Nakamura, Y. Immune checkpoint inhibitor-based therapy for advanced acral and mucosal melanoma. Exp. Dermatol. 2023, 32, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, K.; Ito, T.; Yoshikawa, S.; Yoshino, K.; Kiniwa, Y.; Ohe, S.; Isei, T.; Takenouchi, T.; Kato, H.; Mizuhashi, S.; et al. Systemic therapy for Asian patients with advanced BRAF V600-mutant melanoma in a real-world setting: A multi-center retrospective study in Japan (B-CHECK-RWD study). Cancer Med. 2023, 12, 17967–17980. [Google Scholar] [CrossRef] [PubMed]
- Kanaki, T.; Stang, A.; Gutzmer, R.; Zimmer, L.; Chorti, E.; Sucker, A.; Ugurel, S.; Hadaschik, E.; Gräger, N.S.; Satzger, I.; et al. Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. Eur. J. Cancer 2019, 119, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Yoshikawa, S.; Minagawa, A.; Takenouchi, T.; Yokota, K.; Uchi, H.; Noma, N.; Nakamura, Y.; Asai, J.; Kato, J.; et al. Classification of 3097 patients from the Japanese melanoma study database using the American joint committee on cancer eighth edition cancer staging system. J. Dermatol. Sci. 2019, 94, 284–289. [Google Scholar] [CrossRef] [PubMed]
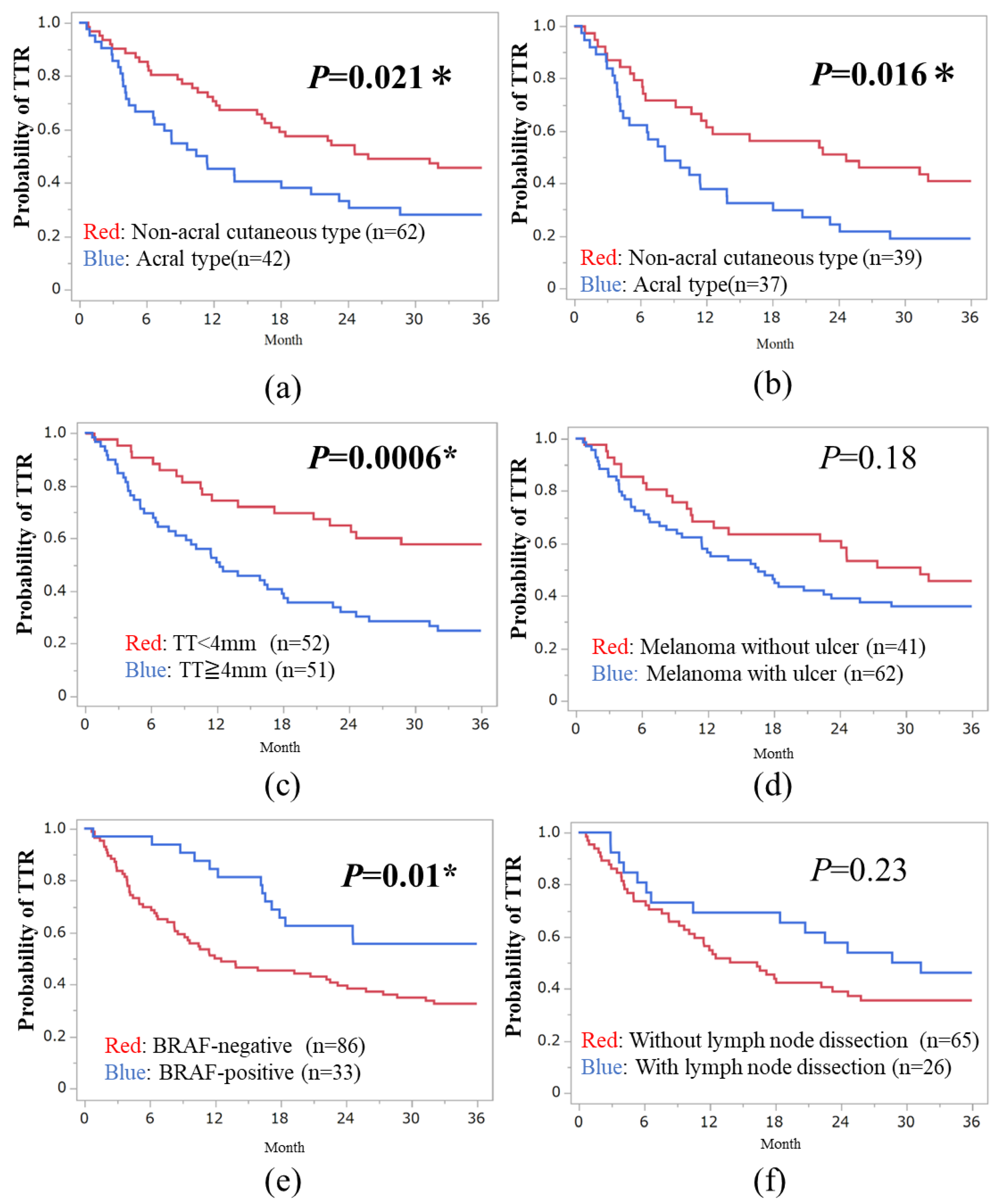
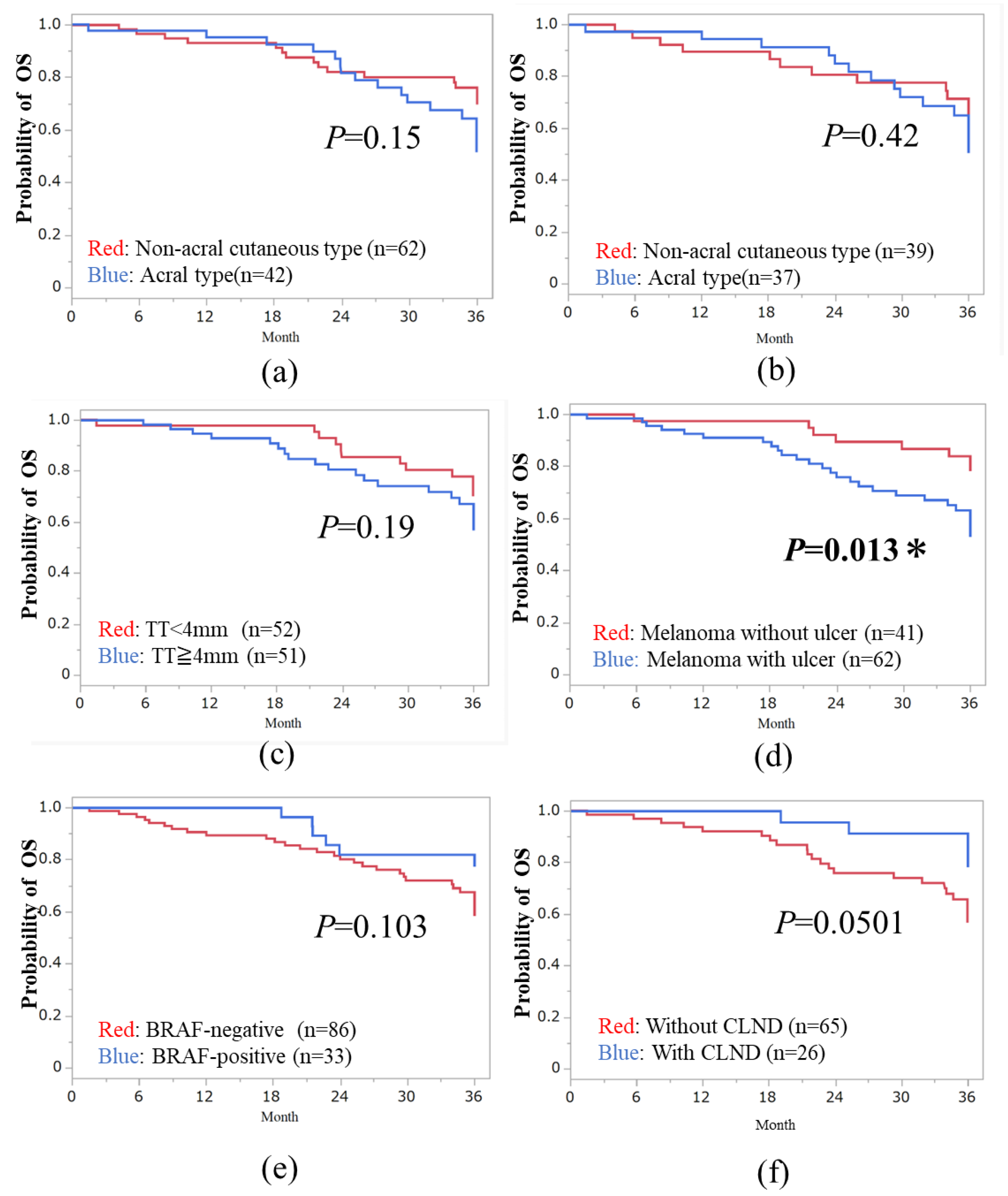
| Parameters | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Histological characteristics | ||
| Non-acral cutaneous vs. acral types | 0.28 (0.14–0.59) | 0.0007 * |
| Tumor thickness | 0.32 (0.16–0.66) | 0.0020 * |
| Tumor ulceration | 0.83 (0.40–1.7) | 0.61 |
| BRAF wild vs. mutated type | 0.44 (0.12–1.6) | 0.21 |
| Baseline characteristics | ||
| Sex | 0.65 (0.34–1.3) | 0.2 |
| With or Without lymph node dissection | 0.77 (0.40–1.5) | 0.45 |
| Parameters | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Histological characteristics | ||
| Non-acral cutaneous vs. acral types | 0.95 (0.38–2.33) | 0.9 |
| Tumor thickness | 0.83 (0.32–2.2) | 0.71 |
| Tumor ulceration | 0.30 (0.095–0.97) | 0.044 * |
| BRAF wild vs. mutated type | NA | 0.99 |
| Baseline characteristics | ||
| Sex | 0.72 (0.28–1.9) | 0.5 |
| With or Without lymph node dissection | 0.77 (0.40–1.5) | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muto, Y.; Kambayashi, Y.; Kato, H.; Mizuhashi, S.; Ito, T.; Maekawa, T.; Ishizuki, S.; Uchi, H.; Matsushita, S.; Yamamoto, Y.; et al. Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma including Acral and Mucosal Subtypes. Cancers 2024, 16, 2755. https://doi.org/10.3390/cancers16152755
Muto Y, Kambayashi Y, Kato H, Mizuhashi S, Ito T, Maekawa T, Ishizuki S, Uchi H, Matsushita S, Yamamoto Y, et al. Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma including Acral and Mucosal Subtypes. Cancers. 2024; 16(15):2755. https://doi.org/10.3390/cancers16152755
Chicago/Turabian StyleMuto, Yusuke, Yumi Kambayashi, Hiroshi Kato, Satoru Mizuhashi, Takamichi Ito, Takeo Maekawa, Shoichiro Ishizuki, Hiroshi Uchi, Shigeto Matsushita, Yuki Yamamoto, and et al. 2024. "Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma including Acral and Mucosal Subtypes" Cancers 16, no. 15: 2755. https://doi.org/10.3390/cancers16152755
APA StyleMuto, Y., Kambayashi, Y., Kato, H., Mizuhashi, S., Ito, T., Maekawa, T., Ishizuki, S., Uchi, H., Matsushita, S., Yamamoto, Y., Yoshino, K., Fujisawa, Y., Amagai, R., Ohuchi, K., Hashimoto, A., Fukushima, S., Asano, Y., & Fujimura, T. (2024). Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma including Acral and Mucosal Subtypes. Cancers, 16(15), 2755. https://doi.org/10.3390/cancers16152755







