Real-Life Outcomes of Adjuvant Targeted Therapy and Anti-PD1 Agents in Stage III/IV Resected Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Effectiveness and Safety of Adjuvant Treatment
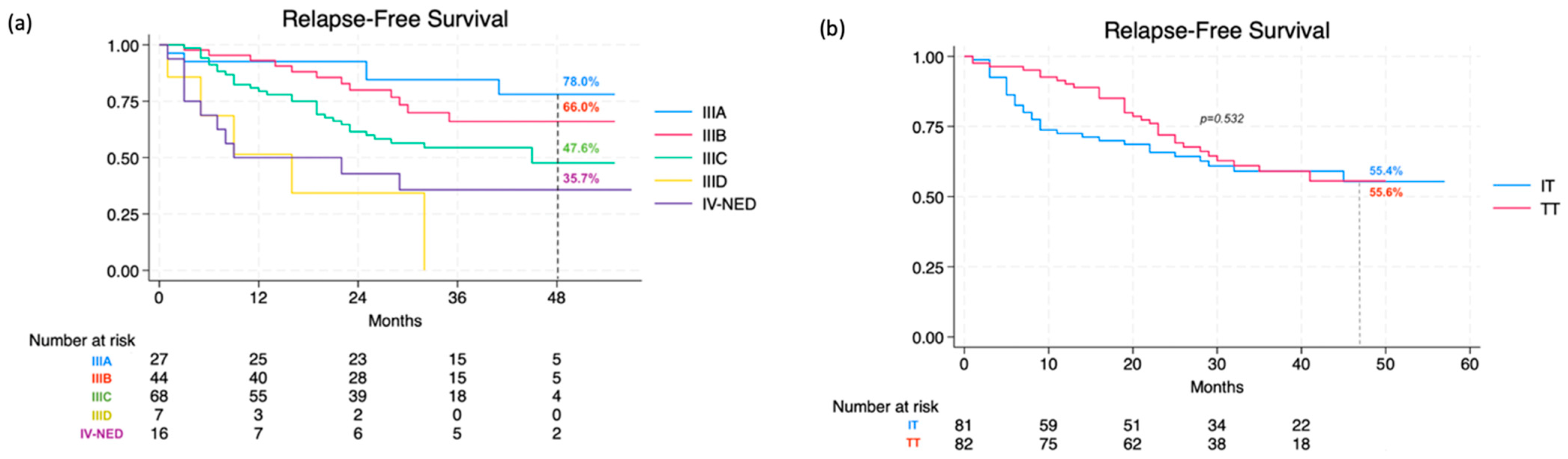
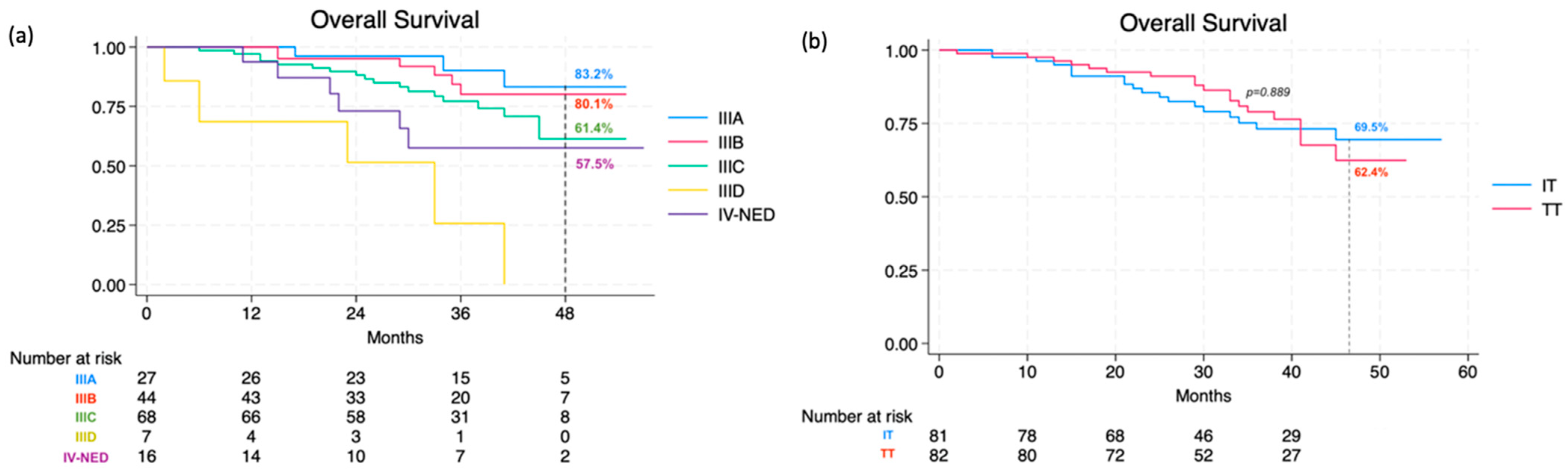
3.2. Patterns of Recurrence and Predictors of Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Amabile, S.; Roccuzzo, G.; Pala, V.; Tonella, L.; Rubatto, M.; Merli, M.; Fava, P.; Ribero, S.; Fierro, M.T.; Queirolo, P.; et al. Clinical Significance of Distant Metastasis-Free Survival (DMFS) in Melanoma: A Narrative Review from Adjuvant Clinical Trials. J. Clin. Med. 2021, 10, 5475. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Gershenwald, J.E. The Eighth Edition American Joint Committee on Cancer (AJCC) Melanoma Staging System: Implications for Melanoma Treatment and Care. Expert Rev. Anticancer Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Wada-Ohno, M.; Ito, T.; Furue, M. Adjuvant Therapy for Melanoma. Curr. Treat. Options Oncol. 2019, 20, 63. [Google Scholar] [CrossRef]
- Nepote, A.; Avallone, G.; Ribero, S.; Cavallo, F.; Roccuzzo, G.; Mastorino, L.; Conforti, C.; Paruzzo, L.; Poletto, S.; Schianca, F.C.; et al. Current Controversies and Challenges on BRAF V600K-Mutant Cutaneous Melanoma. J. Clin. Med. 2022, 11, 828. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage IIIB-C and Stage IV Melanoma (CheckMate 238): 4-Year Results from a Multicentre, Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma (EORTC 1325-MG/KEYNOTE-054): Distant Metastasis-Free Survival Results from a Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Hauschild, A.; Dummer, R.; Schadendorf, D.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Longer Follow-Up Confirms Relapse-Free Survival Benefit with Adjuvant Dabrafenib Plus Trametinib in Patients with Resected BRAFV600-Mutant Stage III Melanoma. J. Clin. Oncol. 2018, 36, 3441–3449. [Google Scholar] [CrossRef]
- Ng, G.; Xu, W.; Atkinson, V. Treatment Approaches for Melanomas That Relapse after Adjuvant or Neoadjuvant Therapy. Curr. Oncol. Rep. 2022, 24, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P.; et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- AIOM. Linee Guida AIOM 2023 Melanoma. Available online: https://www.aiom.it/linee-guida-aiom-2023-melanoma/ (accessed on 1 April 2024).
- Steyerberg, E.W. Clinical Prediction Models. In Statistics for Biology and Health; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Larkin, J.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Fernandez, A.M.A.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion- Sileni, V.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Clin. Cancer Res. 2023, 29, 3352–3361. [Google Scholar] [CrossRef]
- Bai, X.; Shaheen, A.; Grieco, C.; d’Arienzo, P.D.; Mina, F.; Czapla, J.A.; Lawless, A.R.; Bongiovanni, E.; Santaniello, U.; Zappi, H.; et al. Dabrafenib plus Trametinib versus Anti-PD-1 Monotherapy as Adjuvant Therapy in BRAF V600-Mutant Stage III Melanoma after Definitive Surgery: A Multicenter, Retrospective Cohort Study. EClinicalMedicine 2023, 65, 102290. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Di Giacomo, A.M.; Chiarion Sileni, V.; Queirolo, P.; Spagnolo, F.; De Galitiis, F.; Cognetti, F.; Mandalà, M.; Guidoboni, M.; Rinaldi, G.; et al. Italian Nivolumab Expanded Access Programme in Melanoma Adjuvant Setting: Patient Outcomes and Safety Profile. Eur. J. Cancer 2023, 191, 113246. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- De Falco, V.; Suarato, G.; Napolitano, R.; Argenziano, G.; Famiglietti, V.; Amato, A.; Servetto, A.; Bianco, R.; Formisano, L.; Terrano, V.; et al. Real-World Clinical Outcome and Safety of Adjuvant Therapy in Stage III Melanoma Patients: Data from Two Academic Italian Institutions. Int. J. Cancer 2023, 153, 133–140. [Google Scholar] [CrossRef]
- Schumann, K.; Mauch, C.; Klespe, K.C.; Loquai, C.; Nikfarjam, U.; Schlaak, M.; Akçetin, L.; Kölblinger, P.; Hoellwerth, M.; Meissner, M.; et al. Real-World Outcomes Using PD-1 Antibodies and BRAF + MEK Inhibitors for Adjuvant Melanoma Treatment from 39 Skin Cancer Centers in Germany, Austria and Switzerland. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 894–906. [Google Scholar] [CrossRef]
- Romano, E.; Scordo, M.; Dusza, S.W.; Coit, D.G.; Chapman, P.B. Site and Timing of First Relapse in Stage III Melanoma Patients: Implications for Follow-Up Guidelines. J. Clin. Oncol. 2010, 28, 3042–3047. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.N.; Shoushtari, A.N.; Chauhan, D.; Palmieri, D.J.; Lee, B.; Rohaan, M.W.; Mangana, J.; Atkinson, V.; Zaman, F.; Young, A.; et al. Management of Early Melanoma Recurrence despite Adjuvant Anti-PD-1 Antibody Therapy. Ann. Oncol. 2020, 31, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Bhave, P.; Pallan, L.; Long, G.V.; Menzies, A.M.; Atkinson, V.; Cohen, J.V.; Sullivan, R.J.; Chiarion-Sileni, V.; Nyakas, M.; Kahler, K.; et al. Melanoma Recurrence Patterns and Management after Adjuvant Targeted Therapy: A Multicentre Analysis. Br. J. Cancer 2021, 124, 574–580. [Google Scholar] [CrossRef]
- Villanueva, J.; Vultur, A.; Lee, J.T.; Somasundaram, R.; Fukunaga-Kalabis, M.; Cipolla, A.K.; Wubbenhorst, B.; Xu, X.; Gimotty, P.A.; Kee, D.; et al. Acquired Resistance to BRAF Inhibitors Mediated by a RAF Kinase Switch in Melanoma Can Be Overcome by Cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010, 18, 683–695. [Google Scholar] [CrossRef]
- Longo, C.; Pampena, R.; Lallas, A.; Kyrgidis, A.; Stratigos, A.; Peris, K.; Garbe, C.; Pellacani, G. Adjuvant Therapy for Cutaneous Melanoma: A Systematic Review and Network Meta-Analysis of New Therapies. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib Plus Trametinib in Patients with BRAFV600-Mutant Melanoma Brain Metastases (COMBI-MB): A Multicentre, Multicohort, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Mandalà, M.; Galli, F.; Cattaneo, L.; Merelli, B.; Rulli, E.; Ribero, S.; Quaglino, P.; De Giorgi, V.; Pigozzo, J.; Sileni, V.C.; et al. Mitotic Rate Correlates with Sentinel Lymph Node Status and Outcome in Cutaneous Melanoma Greater than 1 Millimeter in Thickness: A Multi-Institutional Study of 1524 Cases. J. Am. Acad. Dermatol. 2017, 76, 264–273.e2. [Google Scholar] [CrossRef]
- Quaglino, P.; Ribero, S.; Osella-Abate, S.; Macrì, L.; Grassi, M.; Caliendo, V.; Asioli, S.; Sapino, A.; Macripò, G.; Savoia, P.; et al. Clinico-Pathologic Features of Primary Melanoma and Sentinel Lymph Node Predictive for Non-Sentinel Lymph Node Involvement and Overall Survival in Melanoma Patients: A Single Centre Observational Cohort Study. Surg. Oncol. 2011, 20, 259–264. [Google Scholar] [CrossRef]
- Ribero, S.; Quaglino, P.; Roccuzzo, G. Predicting Progression in Very Thin Melanoma: The Challenge of the Next Decade? Br. J. Dermatol. 2023, 189, 362–363. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Moirano, G.; Fava, P.; Maule, M.; Ribero, S.; Quaglino, P. Obesity and Immune-Checkpoint Inhibitors in Advanced Melanoma: A Meta-Analysis of Survival Outcomes from Clinical Studies. Semin. Cancer Biol. 2023, 91, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Crystal, J.; Faries, M.B. Sentinel Lymph Node Biopsy: Indications and Technique. Surg. Oncol. Clin. N. Am. 2020, 29, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schulte, K.W.; Lehmann, P.; et al. Complete Lymph Node Dissection versus No Dissection in Patients with Sentinel Lymph Node Biopsy Positive Melanoma (DeCOG-SLT): A Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2016, 17, 757–767. [Google Scholar] [CrossRef]
- Lodde, G.C.; Hassel, J.; Wulfken, L.M.; Meier, F.; Mohr, P.; Kähler, K.; Hauschild, A.; Schilling, B.; Loquai, C.; Berking, C.; et al. Adjuvant Treatment and Outcome of Stage III Melanoma Patients: Results of a Multicenter Real-World German Dermatologic Cooperative Oncology Group (DeCOG) Study. Eur. J. Cancer 2023, 191, 112957. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, G.; Bongiovanni, E.; Tonella, L.; Pala, V.; Marchisio, S.; Ricci, A.; Senetta, R.; Bertero, L.; Ribero, S.; Berrino, E.; et al. Emerging Prognostic Biomarkers in Advanced Cutaneous Melanoma: A Literature Update. Expert. Rev. Mol. Diagn. 2024, 24, 49–66. [Google Scholar] [CrossRef]
- Krähenbühl, L.; Goldinger, S.M.; Mangana, J.; Kerl, K.; Chevolet, I.; Brochez, L.; Horak, C.; Levesque, M.; Dummer, R.; Cheng, P.F. A Longitudinal Analysis of IDO and PDL1 Expression during Immune- or Targeted Therapy in Advanced Melanoma. Neoplasia 2018, 20, 218–225. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Sarda, C.; Pala, V.; Ribero, S.; Quaglino, P. Prognostic Biomarkers in Melanoma: A 2023 Update from Clinical Trials in Different Therapeutic Scenarios. Expert Rev. Mol. Diagn. 2024, 24, 379–392. [Google Scholar] [CrossRef]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A Phase 1/2 Trial of an Immune-Modulatory Vaccine Against IDO/PD-L1 in Combination with Nivolumab in Metastatic Melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef]

| Global (n = 163) | Immunotherapy (n = 81) | Nivolumab (n = 63) | Pembrolizumab (n = 18) | Dabrafenib + Trametinib (n = 82) | p-Value | |
|---|---|---|---|---|---|---|
| Median age—years (range) | 55 (18–89) | 60 (20–85) | 60 (20–85) | 60.5 (33–78) | 51 (18–89) | 0.089 |
| Male sex—no (%) | 83 (50.9) | 40 (49.4) | 29 (46) | 11 (61.1) | 43 (52.4) | 0.696 |
| Female sex—no (%) | 80 (49.1) | 41 (50.6) | 34 (54) | 7 (38.9) | 39 (47.6) | 0.696 |
| Stage III—no (%) | 147 (90.2) | 65 (80.2) | 47 (74.6) | 18 (100) | 82 (100) | - |
| IIIA | 27 (16.6) | 8 (9.9) | 4 (6.4) | 4 (22.2) | 19 (19.5) | 0.022 |
| IIIB | 44 (26.9) | 25 (23.5) | 15 (23.8) | 10 (55.5) | 19 (23.2) | 0.713 |
| IIIC | 70 (43.0) | 29 (35.8) | 25 (39.7) | 3 (16.7) | 40 (48.8) | 0.054 |
| IIID | 7 (4.3) | 3 (3.7) | 2 (1.6) | 1 (5.6) | 4 (4.9) | 0.414 |
| Stage IV-NED—no (%) | 16 (9.8) | 16 (19.8) | 16 (25.4) | 0 (0.0) | 0 (0.0) | - |
| Brain | 4 (25) | 4 (25) | 4 (25) | 0 (0.0) | 0 (0.0) | - |
| Lung | 6 (37.5) | 6 (37.5) | 6 (37.5) | 0 (0.0) | 0 (0.0) | - |
| Liver | 1 (6.3) | 1 (6.3) | 1 (6.3) | 0 (0.0) | 0 (0.0) | - |
| Ileum | 2 (12.5) | 2 (12.5) | 2 (12.5) | 0 (0.0) | 0 (0.0) | - |
| Pancreas | 1 (6.3) | 1 (6.3) | 1 (6.3) | 0 (0.0) | 0 (0.0) | - |
| Lymph nodes | 2 (12.5) | 2 (12.5) | 2 (12.5) | 0 (0.0) | 0 (0.0) | - |
| In transit metastases—no (%) | 27 (16.6) | 16 (19.8) | 10 (15.9) | 6 (33.3) | 11 (13.4) | 0.276 |
| 1 metastasis | 21 (77.8) | 13 (81.3) | 8 (80) | 5 (83.3) | 8 (72.7) | 0.230 |
| ≥2 metastases | 6 (22.2) | 3 (18.7) | 2 (20) | 1 (16.7) | 3 (27.3) | 0.496 |
| Site of primary melanoma—no (%) | ||||||
| Back | 49 (30.1) | 25 (30.9) | 19 (30.2) | 6 (33.3) | 24 (29.3) | 0.824 |
| Lower limbs | 34 (20.1) | 13 (12.3) | 12 (19.0) | 1 (5.5) | 21 (25.6) | 0.133 |
| Abdomen | 17 (10.4) | 6 (7.4) | 5 (7.9) | 1 (5.5) | 11 (13.4) | 0.219 |
| Upper limbs | 17 (10.4) | 10 (12.3) | 10 (15.9) | 0 (0.0) | 7 (8.5) | 0.426 |
| Head–neck | 15 (9.2) | 8 (9.8) | 5 (7.9) | 3 (16.6) | 7 (8.5) | 0.767 |
| Thorax | 13 (7.9) | 4 (4.9) | 1 (1.6) | 3 (16.6) | 9 (10.9) | 0.154 |
| Occult melanoma | 11 (6.7) | 9 (11.1) | 8 (12.7) | 1 (5.5) | 2 (2.4) | 0.028 |
| Mucosal | 7 (4.3) | 6 (7.4) | 3 (4.7) | 3 (16.6) | 1 (1.2) | 0.050 |
| Histology—no (%) | ||||||
| SSM | 65 (39.9) | 28 (34.6) | 21 (33.3) | 7 (38.9) | 37 (45.1) | 0.168 |
| Nodular | 31 (19) | 13 (16) | 11 (17.5) | 2 (11.1) | 18 (22) | 0.337 |
| LMM | 2 (1.2) | 2 (2.5) | 1 (1.6) | 1 (5.6) | 0 (0.0) | 0.245 |
| Mucosal | 7 (4.3) | 6 (7.4) | 3 (4.8) | 3 (16.7) | 1 (1.2) | 0.050 |
| Occult melanoma | 11 (6.7) | 9 (11.1) | 8 (12.7) | 1 (5.6) | 2 (2.4) | 0.028 |
| Not otherwise specified | 29 (25) | 15 (32.1) | 14 (36.5) | 1 (16.7) | 16 (23.2) | 0.202 |
| Breslow thickness—no (%) | ||||||
| <1 mm | 11 (6.7) | 5 (6.1) | 4 (6.4) | 1 (5.6) | 6 (7.3) | 0.770 |
| 1–2 mm | 38 (23.3) | 16 (19.8) | 7 (11.1) | 9 (50) | 22 (26.8) | 0.285 |
| 2.1–4 mm | 43 (26.4) | 20 (24.7) | 19 (30.1) | 1 (5.5) | 23 (28) | 0.626 |
| >4 mm | 46 (28.2) | 23 (28.4) | 20 (31.8) | 3 (16.7) | 23 (28) | 0.960 |
| Not otherwise specified | 25 (15.3) | 17 (21) | 13 (20.6) | 4 (22.2) | 8 (9.8) | 0.076 |
| Lymphovascular invasion—no (%) | 21 (12.9) | 9 (11.1) | 7 (11.1) | 2 (11.1) | 12 (14.6) | 0.502 |
| Ulceration—no (%) | 77 (47.2) | 36 (44.4) | 27 (42.9) | 9 (50) | 41 (50) | 0.477 |
| Mitotic index—median (range) | 5 (0–27) | 5 (0–27) | 5 (1–17) | 4 (0–27) | 4 (0–20) | 0.850 |
| BRAF mutated, no (%) | 88 (53.9) | 6 (7.4) | 5 (8.0) | 1 (5.5) | 82 (100) | <0.001 |
| NRAS mutated—no (%) | 33 (20.2) | 32 (39.5) | 23 (36.5) | 9 (50) | 1 (1.2) | <0.001 |
| Lymphatic disease—no (%) | ||||||
| Macroscopic involvement | 35 (21.5) | 20 (24.7) | 17 (27) | 3 (16.7) | 15 (18.3) | 0.319 |
| Microscopic involvement | 99 (60.7) | 36 (44.4) | 26 (41.3) | 10 (55.6) | 63 (76.8) | <0.001 |
| Absent | 29 (17.8) | 25 (30.9) | 20 (31.7) | 5 (27.8) | 4 (4.9) | <0.001 |
| SLNB—no (%) | ||||||
| Performed | 123 (75.5) | 54 (66.7) | 42 (66.7) | 12 (66.7) | 69 (84.1) | 0.015 |
| Not performed | 40 (24.5) | 27 (33.3) | 21 (33.3) | 6 (33.3) | 13 (15.9) | - |
| SLNB positive | 103 (83.7) | 39 (72.2) | 29 (69) | 10 (83.3) | 64 (92.8) | 0.004 |
| SLNB negative | 20 (16.3) | 15 (27.8) | 13 (31) | 2 (16.7) | 5 (7.2) | - |
| 1 pos. lymph node | 73 (59.3) | 21 (38.9) | 15 (35.7) | 6 (50) | 52 (75.4) | <0.001 |
| 2 pos. lymph nodes | 23 (18.7) | 14 (25.9) | 10 (23.8) | 4 (33.3) | 9 (13) | 0.003 |
| 3–4 pos. lymph nodes | 7 (5.7) | 4 (7.5) | 4 (9.5) | 0 (0.0) | 3 (4.3) | 0.698 |
| Size of metastasis in sentinel lymph node—median (range) | 1.75 (0.10–18.0) | 1.8 (0.3–18.0) | 1.5 (0.3–8.0) | 4.0 (0.3–18.0) | 1.7 (0.10–12.0) | 0.850 |
| Lymphadenectomy– no (%) | ||||||
| Performed | 85 (52.1) | 39 (48.1) | 32 (50.8) | 7 (38.9) | 46 (56.1) | 0.309 |
| Not performed | 78 (47.9) | 42 (51.9) | 31 (49.2) | 11 (61.1) | 36 (43.9) | 0.309 |
| 0 pos. lymph nodes | 43 (50.6) | 18 (46.2) | 15 (46.9) | 3 (42.9) | 25 (54.3) | 0.451 |
| 1 pos. lymph node | 24 (28.2) | 14 (35.9) | 10 (31.3) | 4 (57.1) | 10 (21.7) | 0.148 |
| 2 pos. lymph nodes | 8 (9.4) | 2 (5.1) | 2 (6.3) | 0 (0.0) | 6 (13) | 0.279 |
| ≥3 pos. lymph nodes | 10 (11.9) | 5 (13.0) | 5 (15.5) | (0.0) | 5 (10.9) | 1 |
| Global | Immunotherapy (n = 81) | Targeted Therapy (n = 82) | p-Value | |
|---|---|---|---|---|
| Adjuvant treatment completed—no (%) | 123 (75.7) | 58 (47.1) | 65 (52.9) | 0.255 |
| Patients recurred—no (%) | 57 (35.0) | 28 (34.6) | 29 (35.4%) | 0.699 |
| Recurred ON therapy—no (%) | 26 (45.6) | 19 (67.9) | 7 (24.1) | 0.001 |
| Recurred OFF therapy—no (%) | 31 (54.4) | 9 (32.1) | 22 (75.9) | 0.001 |
| Site of recurrence—no | ||||
| 18 | 10 | 8 | 0.509 |
| 20 | 6 | 14 | 0.034 |
| 20 | 13 | 7 | 0.078 |
| 16 | 8 | 8 | 0.934 |
| 6 | 5 | 1 | 0.076 |
| 6 | 4 | 2 | 0.363 |
| 2 | 1 | 1 | 0.980 |
| 2 | 1 | 1 | 0.980 |
| Dose interruption due to adverse events *—no (%) | 67 (41.1) | 11 (13.6) | 56 (68.3) | <0.001 |
| Therapy discontinuation due to adverse event—no (%) | 21 (12.9) | 12 (14.8) | 9 (11.1) | 0.464 |
| End of therapy due to disease progression—no (%) | 17 (10.4) | 10 (58.8) | 7 (41.2) | 0.426 |
| Death—no (%) | 38 (23.3) | 19 (23.5) | 19 (23.2) | 0.973 |
| Univariate | Multivariate a | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Predictor | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| RFS | Age | 1.02 | 1.01–1.04 | 0.035 | - | ||
| Stage IIIA | 0.35 | 0.14–0.86 | 0.023 | - | |||
| Stage IIID | 3.75 | 1.50–9.41 | 0.005 | - | |||
| Stage IV-NED | 2.42 | 1.23–4.77 | 0.011 | - | |||
| Breslow thickness | 1.05 | 1.01–1.10 | 0.026 | - | |||
| Number of mitoses | 1.06 | 1.01–1.11 | 0.023 | 1.07 | 1.01–1.13 | 0.028 | |
| Lymphovascular Invasion | 2.30 | 1.14–4.63 | 0.020 | 2.37 | 1.17–4.79 | 0.017 | |
| Ulceration | 2.29 | 1.25–4.20 | 0.007 | 2.79 | 1.39–5.63 | 0.004 | |
| No. of positive sentinel LNs | 1.46 | 1.04–2.06 | 0.032 | 1.44 | 1.04–2.01 | 0.027 | |
| DMFS | Stage IIIA | 0.40 | 0.16–0.99 | 0.049 | - | ||
| Stage IIID | 4.32 | 1.34–13.87 | 0.014 | - | |||
| Stage IV-NED | 2.85 | 1.19–6.87 | 0.019 | - | |||
| Ulceration | 2.57 | 1.31–4.99 | 0.005 | - | |||
| OS | Age | 1.04 | 1.01–1.06 | 0.004 | 1.04 | 1.01–1.08 | 0.011 |
| Stage IIID | 6.89 | 2.67–17.81 | <0.001 | - | |||
| Ulceration | 2.80 | 1.26–6.22 | 0.011 | - | |||
| Lymphovascular invasion | 2.67 | 1.16–6.13 | 0.021 | 2.53 | 1.09–5.87 | 0.031 | |
| No. of positive sentinel LNs | 1.60 | 1.06–2.42 | 0.026 | - | |||
| Skin relapse | 0.21 | 0.07–0.61 | <0.001 | - | |||
| Distant Relapse | 43.72 | 10.51–181.68 | <0.001 | 40.40 | 9.68–168.57 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roccuzzo, G.; Fava, P.; Astrua, C.; Brizio, M.G.; Cavaliere, G.; Bongiovanni, E.; Santaniello, U.; Carpentieri, G.; Cangiolosi, L.; Brondino, C.; et al. Real-Life Outcomes of Adjuvant Targeted Therapy and Anti-PD1 Agents in Stage III/IV Resected Melanoma. Cancers 2024, 16, 3095. https://doi.org/10.3390/cancers16173095
Roccuzzo G, Fava P, Astrua C, Brizio MG, Cavaliere G, Bongiovanni E, Santaniello U, Carpentieri G, Cangiolosi L, Brondino C, et al. Real-Life Outcomes of Adjuvant Targeted Therapy and Anti-PD1 Agents in Stage III/IV Resected Melanoma. Cancers. 2024; 16(17):3095. https://doi.org/10.3390/cancers16173095
Chicago/Turabian StyleRoccuzzo, Gabriele, Paolo Fava, Chiara Astrua, Matteo Giovanni Brizio, Giovanni Cavaliere, Eleonora Bongiovanni, Umberto Santaniello, Giulia Carpentieri, Luca Cangiolosi, Camilla Brondino, and et al. 2024. "Real-Life Outcomes of Adjuvant Targeted Therapy and Anti-PD1 Agents in Stage III/IV Resected Melanoma" Cancers 16, no. 17: 3095. https://doi.org/10.3390/cancers16173095
APA StyleRoccuzzo, G., Fava, P., Astrua, C., Brizio, M. G., Cavaliere, G., Bongiovanni, E., Santaniello, U., Carpentieri, G., Cangiolosi, L., Brondino, C., Pala, V., Ribero, S., & Quaglino, P. (2024). Real-Life Outcomes of Adjuvant Targeted Therapy and Anti-PD1 Agents in Stage III/IV Resected Melanoma. Cancers, 16(17), 3095. https://doi.org/10.3390/cancers16173095







