Dichloroacetate and Quercetin Prevent Cell Proliferation, Induce Cell Death and Slow Tumor Growth in a Mouse Model of HPV-Positive Head and Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell Culture
2.2. Colony Formation Assay
2.3. Sytox Green Cytotoxicity Assay
2.4. Cell Proliferation Assay
2.5. Western Blotting
2.6. Mitochondrial ROS Staining
2.7. Lactate Assay
2.8. In Vivo Tumor Growth Assay
2.9. In Vitro and In Vivo pH Measurements
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. DCA and Quercetin Inhibit mTOR and Have Synergistic Inhibitory Effects on Cell Proliferation
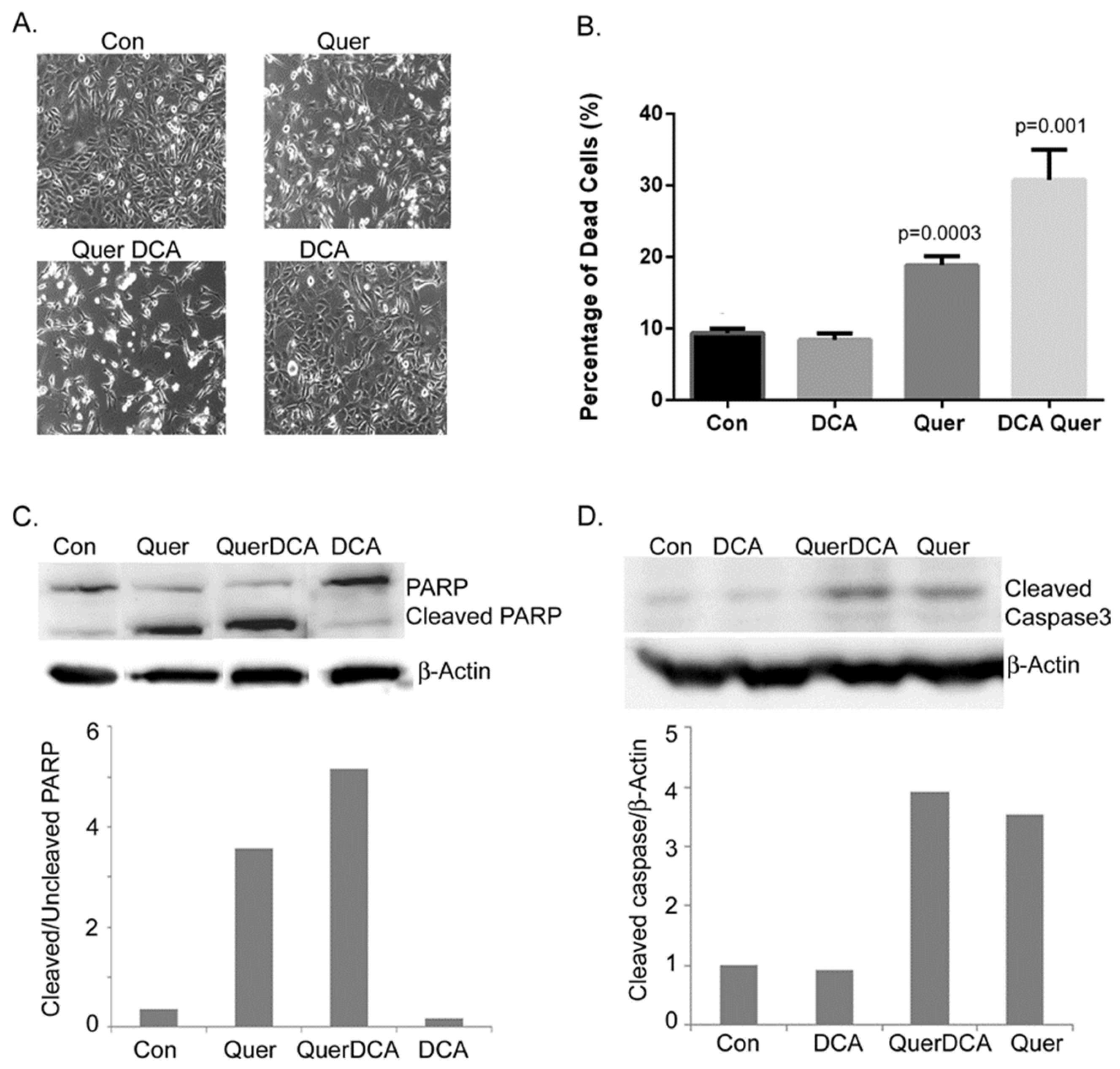
3.2. DCA and Quercetin Induce Apoptosis
3.3. DCA and Quercetin Increase DNA Damage through Enhanced ROS Production
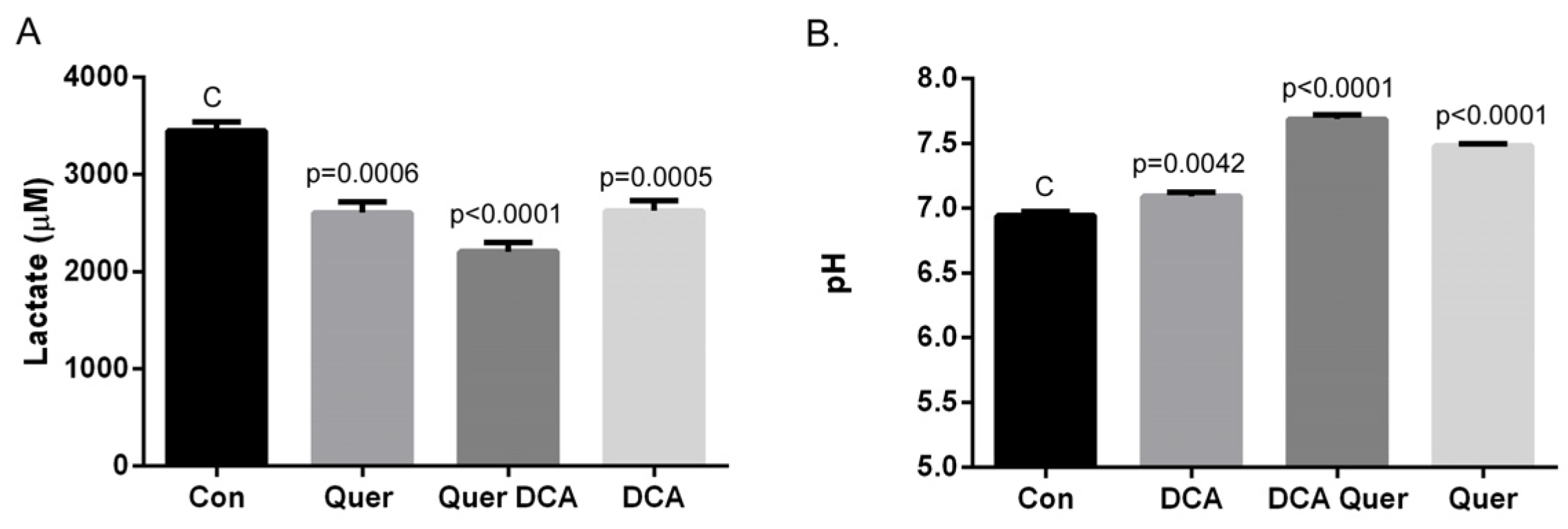
3.4. DCA and Quercetin Prevent Extracellular Acidification
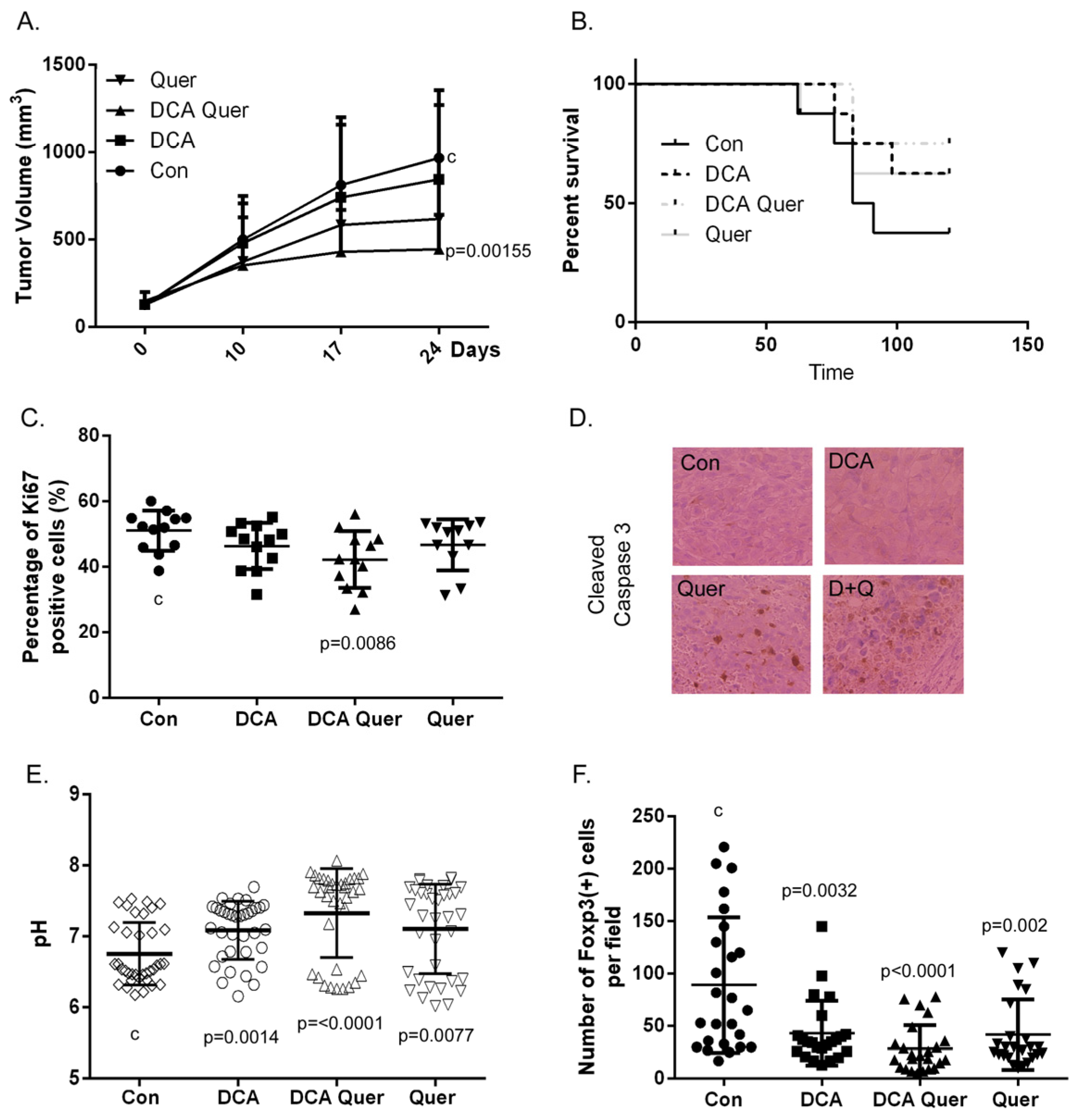
3.5. DCA and Quercetin Inhibit Tumor Growth and Enhance Clearance in an Immune-Competent HPV+ HNSCC Mouse Model and This Is Associated with Decreased Percentage of Ki67 Positive Cells and Treg (+) Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sadri, G.; Mahjub, H. Tobacco smoking and oral cancer: A meta-analysis. J. Res. Health Sci. 2007, 7, 18–23. [Google Scholar] [PubMed]
- Lubin, J.H.; Purdue, M.; Kelsey, K.; Zhang, Z.F.; Winn, D.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; Sturgis, E.M.; Smith, E.; et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: A pooled analysis of case-control studies. Am. J. Epidemiol. 2009, 170, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Dufour, X.; Beby-Defaux, A.; Agius, G.; St Guily, J.L. HPV and head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Palme, C.E.; Morgan, G.J.; Gebski, V.; Wang, A.Y.; Veness, M.J. Predictors of outcome in patients with metastatic cutaneous head and neck squamous cell carcinoma involving cervical lymph nodes: Improved survival with the addition of adjuvant radiotherapy. Head Neck 2012, 34, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Garaud, P.; Bardet, E.; Alfonsi, M.; Sire, C.; Germain, T.; Bergerot, P.; Rhein, B.; Tortochaux, J.; Calais, G. Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J. Clin. Oncol. 2003, 22, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Spanos, W.C.; Nowicki, P.; Lee, D.W.; Hoover, A.; Hostager, B.; Gupta, A.; Anderson, M.E.; Lee, J.H. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.B.; Chu, P.Y.; Liu, J.C.; Lan, M.C.; Chang, S.Y.; Tsai, T.L.; Huang, J.L.; Wang, Y.F.; Tai, S.K. Role of Chest Computed Tomography in Head and Neck Cancer. Otolaryngol. Head Neck Surg. 2008, 134, 1050–1054. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Glycolysis in cancer: A potential target for therapy. Int. J. Biochem. Cell Biol. 2007, 39, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Sattler, U.G.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.L.; Hu, X.; Tam, K.Y. Targeting Tumor Metabolism for Cancer Treatment: Is Pyruvate Dehydrogenase Kinases (PDKs) a Viable Anticancer Target? Int. J. Biol. Sci. 2015, 11, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Sivridis, E.; Fiska, A.; Koukourakis, M.I. Hypoxia-inducible factor-2 alpha (HIF-2 alpha) induces angiogenesis in breast carcinomas. Appl. Immunohistochem. Mol. Morphol. 2006, 14, 78–82. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Tsirigos, A.; Whitaker-Menezes, D.; Pavlides, S.; Pestell, R.G.; Chiavarina, B.; Frank, P.G.; Flomenberg, N.; Howell, A.; Martinez-Outschoorn, U.E.; et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010, 9, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Schroeder, T.; Scher, R.L.; Walenta, S.; Clough, R.W.; Dewhirst, M.W.; Mueller-Klieser, W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Coppock, J.D.; Wieking, B.G.; Molinolo, A.A.; Gutkind, J.S.; Miskimins, W.K.; Lee, J.H. Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition. Neoplasia 2013, 15, 620–630. [Google Scholar] [CrossRef]
- Durrbach, A.; Francois, H. Intracellular lactate flux: A new regulator of the allogenic immune response. Transpl. Int. 2013, 26, 20–21. [Google Scholar] [CrossRef]
- Grotius, J.; Dittfeld, C.; Huether, M.; Mueller-Klieser, W.; Baumann, M.; Kunz-Schughart, L.A. Impact of exogenous lactate on survival and radioresponse of carcinoma cells in vitro. Int. J. Radiat. Biol. 2009, 85, 989–1001. [Google Scholar] [CrossRef]
- Xiao, D.; Powolny, A.A.; Moura, M.B.; Kelley, E.E.; Bommareddy, A.; Kim, S.H.; Hahm, E.R.; Normolle, D.; Van Houten, B.; Singh, S.V. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J. Biol. Chem. 2010, 285, 26558–26569. [Google Scholar] [CrossRef]
- Ayyanathan, K.; Kesaraju, S.; Dawson-Scully, K.; Weissbach, H. Combination of sulindac and dichloroacetate kills cancer cells via oxidative damage. PLoS ONE 2012, 7, e39949. [Google Scholar] [CrossRef] [PubMed]
- Robey, I.F.; Martin, N.K. Bicarbonate and dichloroacetate: Evaluating pH altering therapies in a mouse model for metastatic breast cancer. BMC Cancer 2011, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.F.; Mazurczak, M.; Dib, E.G.; Bleeker, J.S.; Geeraerts, L.H.; Tinguely, M.; Lohr, M.M.; McGraw, S.C.; Jensen, A.W.; Ellison, C.A.; et al. Phase II study of dichloroacetate, an inhibitor of pyruvate dehydrogenase, in combination with chemoradiotherapy for unresected, locally advanced head and neck squamous cell carcinoma. Investig. New Drugs 2022, 40, 622–633. [Google Scholar]
- Vermeer, D.W.; Coppock, J.D.; Zeng, E.; Lee, K.M.; Spanos, W.C.; Onken, M.D.; Uppaluri, R.; Lee, J.H.; Vermeer, P.D. Metastatic model of HPV+ oropharyngeal squamous cell carcinoma demonstrates heterogeneity in tumor metastasis. Oncotarget 2016, 7, 24194–24207. [Google Scholar] [CrossRef] [PubMed]
- Boly, R.; Gras, T.; Lamkami, T.; Guissou, P.; Serteyn, D.; Kiss, R.; Dubois, J. Quercetin inhibits a large panel of kinases implicated in cancer cell biology. Int. J. Oncol. 2011, 38, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Arunkumar, R.; Elumalai, P.; Sharmila, G.; Gunadharini, D.N.; Banudevi, S.; Krishnamoorthy, G.; Benson, C.S.; Arunakaran, J. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem. Funct. 2011, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration, and epithelial-mesenchymal transition. J. Mol. Signal 2010, 5, 14. [Google Scholar] [CrossRef]
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Venkataraman, P.; Krishnamoorthy, G.; Arunakaran, J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol. Cell Biochem. 2006, 287, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Martini, C.; Bulzomi, P.; Leone, S.; Bolli, A.; Pallottini, V.; Marino, M. Quercetin-induced apoptotic cascade in cancer cells: Antioxidant versus estrogen receptor alpha-dependent mechanisms. Mol. Nutr. Food Res. 2009, 53, 699–708. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Alfieri, A.A.; Young, C.W. Quercetin, an inhibitor of lactate transport and a hyperthermic sensitizer of HeLa cells. Cancer Res. 1984, 44, 102–106. [Google Scholar] [PubMed]
- Belt, J.A.; Thomas, J.A.; Buchsbaum, R.N.; Racker, E. Inhibition of lactate transport and glycolysis in Ehrlich ascites tumor cells by bioflavonoids. Biochemistry 1979, 18, 3506–3511. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sadhukhan, R.; Mondal, J.; Khuda-Bukhsh, A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 2013, 46, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ou, Y.X.; Da, W.M.; Kang, J.H. Coadjustment of quercetin and hydrogen peroxide: The role of ROS in the cytotoxicity of quercetin. Pharmazie 2004, 59, 155–158. [Google Scholar] [PubMed]
- Shen, G.X. Mitochondrial dysfunction, oxidative stress, and diabetic cardiovascular disorders. Cardiovasc. Hematol. Disord. Drug Targets 2012, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Hoover, A.C.; Spanos, W.C.; Harris, G.F.; Anderson, M.E.; Klingelhutz, A.J.; Lee, J.H. The role of human papillomavirus 16 E6 in anchorage-independent and invasive growth of mouse tonsil epithelium. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Anticancer properties of the flavone wogonin. Toxicology 2013, 314, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, M.; Bilotto, S.; Tedesco, I.; Laratta, B.; Russo, G.L. Dietary polyphenols in cancer prevention: The example of the flavonoid quercetin in leukemia. Ann. N. Y. Acad. Sci. 2012, 1259, 95–103. [Google Scholar] [CrossRef]
- Klappan, A.K.; Hones, S.; Mylonas, I.; Brüning, A. Proteasome inhibition by quercetin triggers macroautophagy and blocks mTOR activity. Histochem. Cell Biol. 2012, 137, 25–36. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Coppock, J.D.; Haugrud, A.B.; Lee, J.H.; Messerli, S.M.; Miskimins, W.K. Dichloroacetate and Quercetin Prevent Cell Proliferation, Induce Cell Death and Slow Tumor Growth in a Mouse Model of HPV-Positive Head and Neck Cancer. Cancers 2024, 16, 1525. https://doi.org/10.3390/cancers16081525
Zhuang Y, Coppock JD, Haugrud AB, Lee JH, Messerli SM, Miskimins WK. Dichloroacetate and Quercetin Prevent Cell Proliferation, Induce Cell Death and Slow Tumor Growth in a Mouse Model of HPV-Positive Head and Neck Cancer. Cancers. 2024; 16(8):1525. https://doi.org/10.3390/cancers16081525
Chicago/Turabian StyleZhuang, Yongxian, Joseph D. Coppock, Allison B. Haugrud, John H. Lee, Shanta M. Messerli, and W. Keith Miskimins. 2024. "Dichloroacetate and Quercetin Prevent Cell Proliferation, Induce Cell Death and Slow Tumor Growth in a Mouse Model of HPV-Positive Head and Neck Cancer" Cancers 16, no. 8: 1525. https://doi.org/10.3390/cancers16081525
APA StyleZhuang, Y., Coppock, J. D., Haugrud, A. B., Lee, J. H., Messerli, S. M., & Miskimins, W. K. (2024). Dichloroacetate and Quercetin Prevent Cell Proliferation, Induce Cell Death and Slow Tumor Growth in a Mouse Model of HPV-Positive Head and Neck Cancer. Cancers, 16(8), 1525. https://doi.org/10.3390/cancers16081525




