Quantitative Multi-Parametric MRI of the Prostate Reveals Racial Differences
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
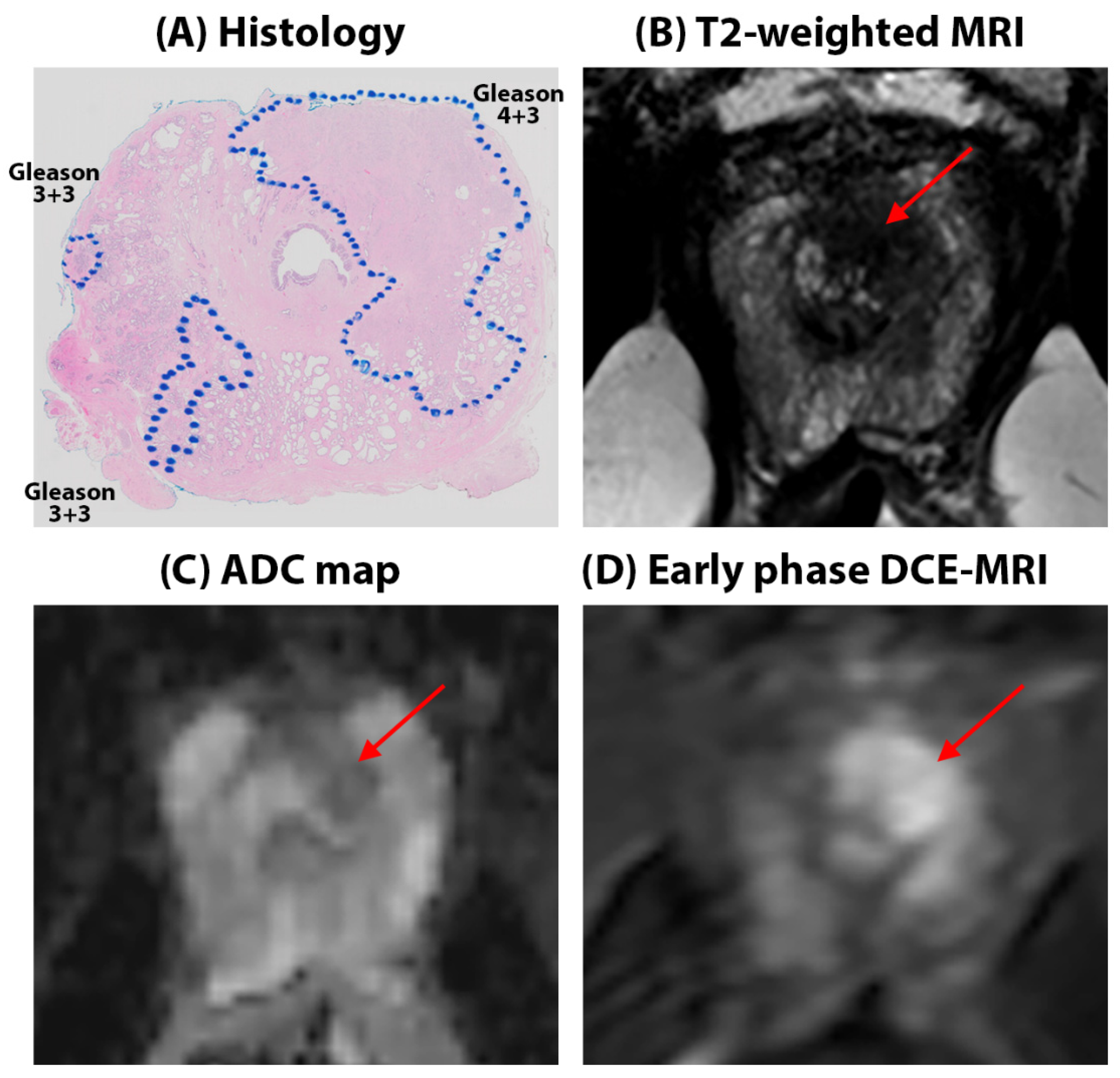
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Prostate Cancer. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html (accessed on 9 April 2024).
- Mahal, B.A.; Alshalalfa, M.; Spratt, D.E.; Davicioni, E.; Zhao, S.G.; Feng, F.Y.; Rebbeck, T.R.; Nguyen, P.L.; Huang, F.W. Prostate Cancer Genomic-risk Differences Between African-American and White Men Across Gleason Scores. Eur. Urol. 2019, 75, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Berman, R.A.; Taplin, M.-E.; Huang, F.W. Prostate Cancer–Specific Mortality Across Gleason Scores in Black vs Nonblack MenProstate Cancer–Specific Mortality Across Gleason Scores in Black and Nonblack MenLetters. JAMA 2018, 320, 2479–2481. [Google Scholar] [CrossRef] [PubMed]
- US Centers for Disease Control and Prevention (CDC). Prostate Cancer Risk Factors. Available online: https://www.cdc.gov/prostate-cancer/risk-factors/index.html (accessed on 9 May 2024).
- Lillard, J.W.; Moses, K.A., Jr.; Mahal, B.A.; George, D.J. Racial disparities in Black men with prostate cancer: A literature review. Cancer 2022, 128, 3787–3795. [Google Scholar] [CrossRef]
- Krimphove, M.J.; Cole, A.P.; Fletcher, S.A.; Harmouch, S.S.; Berg, S.; Lipsitz, S.R.; Sun, M.; Nabi, J.; Nguyen, P.L.; Hu, J.C.; et al. Evaluation of the contribution of demographics, access to health care, treatment, and tumor characteristics to racial differences in survival of advanced prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 125–136. [Google Scholar] [CrossRef]
- Sundi, D.; Ross, A.E.; Humphreys, E.B.; Han, M.; Partin, A.W.; Carter, H.B.; Schaeffer, E.M. African american men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: Should active surveillance still be an option for them? J. Clin. Oncol. 2013, 31, 2991–2997. [Google Scholar] [CrossRef]
- Tolcher, A.; Moinpour, C.M.; Tangen, C.M.; Crawford, E.D.; Thompson, I.M.; Eisenberger, M. Association of African-American Ethnic Background With Survival in Men With Metastatic Prostate Cancer. JNCI J. Natl. Cancer Inst. 2001, 93, 219–225. [Google Scholar]
- Rebbeck, T.R. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin. Radiat. Oncol. 2017, 27, 3–10. [Google Scholar] [CrossRef]
- Bonekamp, D.; Jacobs, M.A.; El-Khouli, R.; Stoianovici, D.; Macura, K.J. Advancements in MR Imaging of the Prostate: From Diagnosis to Interventions. RadioGraphics 2011, 31, 677–703. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.S.; Shankar, P.R. Biparametric Prostate MRI Influencing Care Patterns in a Caribbean Population. Radiol Imaging Cancer 2020, 2, e200096. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J. Epidemiology and pathophysiology of prostate cancer in African-American men. J. Urol. 2007, 177, 444–449. [Google Scholar] [CrossRef]
- Chung, B.H.; Horie, S.; Chiong, E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Abashidze, N.; Stecher, C.; Rosenkrantz, A.B.; Duszak, R.; Hughes, D.R., Jr. Racial and Ethnic Disparities in the Use of Prostate Magnetic Resonance Imaging Following an Elevated Prostate-Specific Antigen Test. JAMA Netw. Open 2021, 4, 32388. [Google Scholar] [CrossRef]
- Mahran, A.; Mishra, K.; Bukavina, L.; Schumacher, F.; Quian, A.; Buzzy, C.; Nguyen, C.T.; Gulani, V.; Ponsky, L.E. Observed racial disparity in the negative predictive value of multi-parametric MRI for the diagnosis for prostate cancer. Int. Urol. Nephrol. 2019, 51, 1343–1348. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, Y.; Antic, T.; Sethi, I.; Schmid-Tannwald, C.; Eggener, S.; Oto, A. Apparent Diffusion Coefficient for Prostate Cancer Imaging: Impact of b Values. Am. J. Roentgenol. 2014, 202, W247–W253. [Google Scholar] [CrossRef]
- Feng, Z.; Min, X.; Wang, L.; Yan, X.; Li, B.; Ke, Z.; Zhang, P.; You, H. Effects of Echo Time on IVIM Quantification of the Normal Prostate. Sci. Rep. 2018, 8, 2572. [Google Scholar] [CrossRef]
- Chatterjee, A.; Nolan, P.; Sun, C.; Mathew, M.; Dwivedi, D.; Yousuf, A.; Antic, T.; Karczmar, G.S.; Oto, A. Effect of Echo Times on Prostate Cancer Detection on T2-Weighted Images. Acad. Radiol. 2020, 27, 1555–1563. [Google Scholar] [CrossRef]
- Chatterjee, A.; Tokdemir, S.; Gallan, A.J.; Yousuf, A.; Antic, T.; Karczmar, G.S.; Oto, A. Multiparametric MRI Features and Pathologic Outcome of Wedge-Shaped Lesions in the Peripheral Zone on T2-Weighted Images of the Prostate. Am. J. Roentgenol. 2019, 212, 124–129. [Google Scholar] [CrossRef]
- Fan, X.; Medved, M.; River, J.N.; Zamora, M.; Corot, C.; Robert, P.; Bourrinet, P.; Lipton, M.; Culp, R.M.; Karczmar, G.S. New model for analysis of dynamic contrast-enhanced MRI data distinguishes metastatic from nonmetastatic transplanted rodent prostate tumors. Magn. Reson. Med. 2004, 51, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Gallan, A.; Fan, X.; Medved, M.; Akurati, P.; Bourne, R.M.; Antic, T.; Karczmar, G.S.; Oto, A. Prostate Cancers Invisible on Multiparametric MRI: Pathologic Features in Correlation with Whole-Mount Prostatectomy. Cancers 2023, 15, 5825. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Mercado, C.; Bourne, R.; Yousuf, A.; Hess, B.; Antic, T.; Eggener, S.; Oto, A.; Karczmar, G.S. Validation of prostate tissue composition using Hybrid Multidimensional MRI: Correlation with histology. Radiology 2022, 302, 368–377. [Google Scholar] [CrossRef]
- Hötker, A.M.; Dappa, E.; Mazaheri, Y.; Ehdaie, B.; Zheng, J.; Capanu, M.; Hricak, H.; Akin, O. The Influence of Background Signal Intensity Changes on Cancer Detection in Prostate MRI. Am. J. Roentgenol. 2019, 212, 823–829. [Google Scholar] [CrossRef]
- Lowder, D.; Rizwan, K.; McColl, C.; Paparella, A.; Ittmann, M.; Mitsiades, N.; Kaochar, S. Racial disparities in prostate cancer: A complex interplay between socioeconomic inequities and genomics. Cancer Lett. 2022, 531, 71–82. [Google Scholar] [CrossRef]
- Rourke, E.; Sunnapwar, A.; Mais, D.; Kukkar, V.; DiGiovanni, J.; Kaushik, D.; Liss, M.A. Inflammation appears as high Prostate Imaging-Reporting and Data System scores on prostate magnetic resonance imaging (MRI) leading to false positive MRI fusion biopsy. Investig. Clin. Urol. 2019, 60, 388–395. [Google Scholar] [CrossRef]
- Chatterjee, A.; He, D.; Fan, X.; Wang, S.; Szasz, T.; Yousuf, A.; Pineda, F.; Antic, T.; Mathew, M.; Karczmar, G.S.; et al. Performance of ultrafast DCE-MRI for diagnosis of prostate cancer. Acad. Radiol. 2018, 25, 349–358. [Google Scholar] [CrossRef]
- Clemente, A.; Selva, G.; Berks, M.; Morrone, F.; Morrone, A.A.; Aulisa, M.D.C.; Bliakharskaia, E.; De Nicola, A.; Tartaro, A.; Summers, P.E. Comparison of Early Contrast Enhancement Models in Ultrafast Dynamic Contrast-Enhanced Magnetic Resonance Imaging of Prostate Cancer. Diagnostics 2024, 14, 870. [Google Scholar] [CrossRef]
- Goswami, S.; Sarkar, C.; Singh, S.; Singh, A.P.; Chakroborty, D. Racial differences in prostate tumor microenvironment: Implications for disparate clinical outcomes and potential opportunities. Cancer Health Disparities 2022, 6, 31. [Google Scholar]
- Gillard, M.; Javier, R.; Ji, Y.; Zheng, S.L.; Xu, J.; Brendler, C.B.; Crawford, S.E.; Pierce, B.L.; Griend, D.J.V.; Franco, O.E. Elevation of Stromal-Derived Mediators of Inflammation Promote Prostate Cancer Progression in African-American Men. Cancer Res. 2018, 78, 6134–6145. [Google Scholar] [CrossRef]
- Powell, I.J.; Bock, C.H.; Ruterbusch, J.J.; Sakr, W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J. Urol. 2010, 183, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Nisha, Y.; Hadziomerovic, A.R.; Prabhakar, S.; Flood, T.A.; Breau, R.H.; McGrath, T.A.; Ramsay, T.; Morash, C.; Goh, V. Comparison of Positive Predictive Values of Biparametric MRI and Multiparametric MRI–directed Transrectal US–guided Targeted Prostate Biopsy. Radiology 2024, 311, e231383. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.W.; Koller, C.R.; Casado, C.; Triche, B.L.; Krane, L.S. A narrative review of biparametric MRI (bpMRI) implementation on screening, detection, and the overall accuracy for prostate cancer. Ther. Adv. Urol. 2022, 14, 17562872221096377. [Google Scholar] [CrossRef]
- Chatterjee, A.; Dwivedi, D.K. MRI-based virtual pathology of the prostate. Magn. Reson. Mater. Phys. Biol. Med. 2024, 37, 709–720. [Google Scholar] [CrossRef]
- Saha, A.; Bosma, J.S.; Twilt, J.J.; van Ginneken, B.; Bjartell, A.; Padhani, A.R.; Bonekamp, D.; Villeirs, G.; Salomon, G.; Giannarini, G. Artificial intelligence and radiologists in prostate cancer detection on MRI (PI-CAI): An international, paired, non-inferiority, confirmatory study. Lancet Oncol. 2024, 25, 879–887. [Google Scholar] [CrossRef]
- Singanamalli, A.; Rusu, M.; Sparks, R.E.; Shih, N.N.; Ziober, A.; Wang, L.P.; Tomaszewski, J.; Rosen, M.; Feldman, M.; Madabhushi, A. Identifying in vivo DCE MRI markers associated with microvessel architecture and gleason grades of prostate cancer. J. Magn. Reson. Imaging 2016, 43, 149–158. [Google Scholar] [CrossRef]
- Epstein, J.I.; Feng, Z.; Trock, B.J.; Pierorazio, P.M. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: Incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur. Urol. 2012, 61, 1019–1024. [Google Scholar] [CrossRef]

| Imaging Sequence | Pulse Sequence | FOV (mm) | Scan Matrix Size | In-Plane Resolution (mm) | TE (ms) | TR (ms) | Slice Thickness (mm) | Flip Angle (°) |
|---|---|---|---|---|---|---|---|---|
| Axial T2W | SE-TSE | 180 × 180 | 450 × 450 | 0.4 × 0.4 | 115 or 150 | 4800 | 3 | 90 |
| Multi-echo T2W (T2 mapping) | SE-TSE | 160 × 160 | 212 × 212 | 0.75 × 0.75 | 30, 60, 90, 120, 150, 180, 210, 240, 270 | 7850 | 3 | 90 |
| DWI a | SE-EPI | 180 × 180 | 120 × 120 | 1.5 × 1.5 | 80 | 5000 | 3 | 90 |
| DCE-MRI b | T1-FFE | 220 × 260 | 148 × 171 | 1.5 × 1.5 | 1.5 | 3.1 | 3 | 10 |
| African Americans | Caucasian Americans | p | ||
|---|---|---|---|---|
| Age (years) | 60 ± 7 | 58 ± 8 | 0.29 | |
| PSA (ng/mL) | 8.5 ± 5.9 | 8.6 ± 9.9 | 0.94 | |
| Patients | Biopsy | 25 (53%) | 51 (52%) | 0.89 |
| Prostatectomy | 22 (47%) | 47 (48%) | ||
| Total | 47 | 98 | - | |
| ROIs | Cancer | 45 (32%) | 99 (34%) | 0.81 |
| Benign | 94 (68%) | 196 (66%) | ||
| Total | 139 | 295 | - | |
| ISUP grade group (Gleason score) | 1 (Gleason 3 + 3) | 13 (29%) | 29 (29%) | 0.01 |
| 2 (Gleason 3 + 4) | 16 (36%) | 56 (57%) | ||
| 3 (Gleason 4 + 3) | 10 (22%) | 11 (11%) | ||
| 4 (Gleason 4 + 4) | 5 (11%) | 1 (1%) | ||
| 5 (Gleason 4 + 5) | 1 (2%) | 2 (2%) |
| African Americans | Caucasian Americans | p-Value | ||
|---|---|---|---|---|
| ADC (µm2/ms) | Benign | 1.53 ± 0.37 | 1.62 ± 0.37 | 0.12 |
| Cancer | 1.03 ± 0.32 | 1.07 ± 0.34 | 0.75 | |
| Gleason 3 + 3 | 1.23 ± 0.27 | 1.22 ± 0.34 | 0.88 | |
| Gleason 3 + 4 | 1.12 ± 0.29 | 1.06 ± 0.33 | 0.55 | |
| Gleason ≥ 4 + 3 | 0.85 ± 0.28 | 0.81 ± 0.27 | 0.62 | |
| T2 (ms) | Benign | 151.9 ± 96.5 | 159.1 ± 73.2 | 0.70 |
| Cancer | 107.5 ± 55.8 | 99.8 ± 27.5 | 0.25 | |
| Gleason 3 + 3 | 110.8 ± 40.2 | 112.7 ± 31.2 | 0.89 | |
| Gleason 3 + 4 | 108.0 ± 26.5 | 96.9 ± 26.6 | 0.31 | |
| Gleason ≥ 4 + 3 | 104.9 ± 74.5 | 85.7 ± 12.8 | 0.35 | |
| DCE Signal enhancement amplitude or A (%) | Benign | 157.6 ± 82.6 | 126.1 ± 51.2 | 0.01 |
| Cancer | 181.1 ± 44.8 | 120.2 ± 47.4 | <0.001 | |
| Gleason 3 + 3 | 200.9 ± 64.1 | 114.8 ± 28.44 | <0.001 | |
| Gleason 3 + 4 | 158.5 ± 28.2 | 123.3 ± 55.6 | 0.18 | |
| Gleason ≥ 4 + 3 | 179.1 ± 35.3 | 125.4 ± 35.6 | 0.03 | |
| DCE Signal enhancement rate (s−1) | Benign | 5.1 ± 4.6 | 4.9 ± 2.9 | 0.81 |
| Cancer | 13.3 ± 9.3 | 6.1 ± 4.7 | <0.001 | |
| Gleason 3 + 3 | 10.3 ± 4.4 | 4.9 ± 2.9 | 0.002 | |
| Gleason 3 + 4 | 11.7 ± 6.5 | 6.7 ± 5.6 | 0.04 | |
| Gleason ≥ 4 + 3 | 15.6 ± 11.7 | 6.2 ± 2.9 | 0.02 | |
| DCE Signal washout rate (s−1) | Benign | 0.01 ± 0.09 | 0.07 ± 0.07 | <0.001 |
| Cancer | 0.12 ± 0.07 | 0.07 ± 0.08 | 0.02 | |
| Gleason 3 + 3 | 0.10 ± 0.09 | 0.04 ± 0.08 | 0.12 | |
| Gleason 3 + 4 | 0.11 ± 0.07 | 0.09 ± 0.08 | 0.62 | |
| Gleason ≥ 4 + 3 | 0.13 ± 0.07 | 0.08 ± 0.03 | 0.13 |
| African Americans | Caucasian Americans | p-Value + | |
|---|---|---|---|
| ADC | AUC = 0.79 95% CI = [0.69, 0.88] Cutoff = 1.00 µm2/ms Sensitivity = 93% Specificity = 56% | AUC = 0.87 95% CI = [0.81, 0.92] Cutoff = 1.23 µm2/ms Sensitivity = 89% Specificity = 74% | 0.06 * |
| T2 | AUC = 0.68 95% CI = [0.56, 0.78] Cutoff = 136.2 ms Sensitivity = 45% Specificity = 88% | AUC = 0.79 95% CI = [0.72, 0.86] Cutoff = 116.9 ms Sensitivity = 67% Specificity = 80% | 0.10 * |
| DCE Signal enhancement rate | AUC = 0.88 95% CI = [0.81, 0.96] Cutoff = 6.0 s−1 Sensitivity = 96% Specificity = 73% | AUC = 0.58* 95% CI = [0.48, 0.69] Cutoff = 6.3 s−1 Sensitivity = 38% Specificity = 79% | <0.001 |
| DCE Signal washout rate | AUC = 0.81 95% CI = [0.73, 0.91] Cutoff = 0.02 s−1 Sensitivity = 96% Specificity = 52% | AUC = 0.50* 95% CI = [0.39, 0.60] Cutoff = 0.10 s−1 Sensitivity = 38% Specificity = 69% | <0.001 |
| African Americans | Caucasian Americans | p-Value | ||
|---|---|---|---|---|
| Stroma | Benign | 42.3 ± 10.2 | 43.1 ± 12.1 | 0.44 |
| Cancer | 39.1 ± 11.5 | 38.6 ± 12.4 | 0.46 | |
| Epithelium | Benign | 28.7 ± 9.0 | 29.6 ± 9.2 | 0.41 |
| Cancer | 44.7 ± 12.8 | 50.9 ± 12.3 | 0.04 | |
| Lumen | Benign | 28.8 ± 13.3 | 27.4 ± 11.1 | 0.38 |
| Cancer | 16.2 ± 6.8 | 10.5 ± 6.9 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, A.; Fan, X.; Slear, J.; Asare, G.; Yousuf, A.N.; Medved, M.; Antic, T.; Eggener, S.; Karczmar, G.S.; Oto, A. Quantitative Multi-Parametric MRI of the Prostate Reveals Racial Differences. Cancers 2024, 16, 3499. https://doi.org/10.3390/cancers16203499
Chatterjee A, Fan X, Slear J, Asare G, Yousuf AN, Medved M, Antic T, Eggener S, Karczmar GS, Oto A. Quantitative Multi-Parametric MRI of the Prostate Reveals Racial Differences. Cancers. 2024; 16(20):3499. https://doi.org/10.3390/cancers16203499
Chicago/Turabian StyleChatterjee, Aritrick, Xiaobing Fan, Jessica Slear, Gregory Asare, Ambereen N. Yousuf, Milica Medved, Tatjana Antic, Scott Eggener, Gregory S. Karczmar, and Aytekin Oto. 2024. "Quantitative Multi-Parametric MRI of the Prostate Reveals Racial Differences" Cancers 16, no. 20: 3499. https://doi.org/10.3390/cancers16203499
APA StyleChatterjee, A., Fan, X., Slear, J., Asare, G., Yousuf, A. N., Medved, M., Antic, T., Eggener, S., Karczmar, G. S., & Oto, A. (2024). Quantitative Multi-Parametric MRI of the Prostate Reveals Racial Differences. Cancers, 16(20), 3499. https://doi.org/10.3390/cancers16203499








