Sargramostim for Prophylactic Management of Gastrointestinal Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy for Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Gastrointestinal Immune-Related Adverse Events
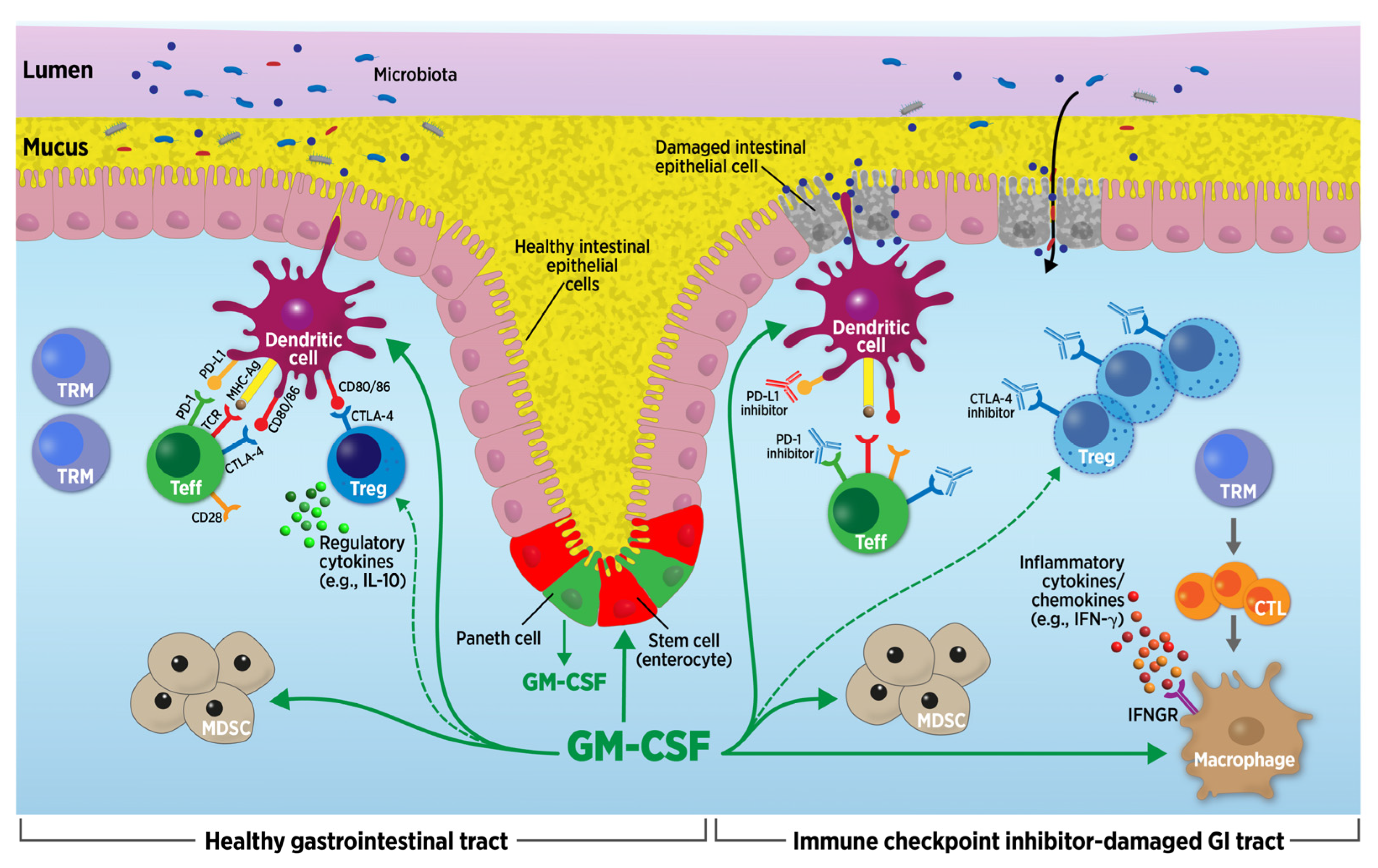
2.1. Mechanisms Underlying GI irAEs
2.2. Lessons Learned about GI irAEs from Inflammatory Bowel Disease
2.3. Treatment of GI irAEs
3. Development and Biologic Effects of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
3.1. Recombinant GM-CSF Expression Systems
3.2. Gastrointestinal Therapeutic Uses of Recombinant GM-CSF
3.2.1. Pre-Clinical Studies
3.2.2. Clinical Studies
4. Clinical Studies of Sargramostim (rhu GM-CSF) with Immune Checkpoint Inhibitors
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Topalian, S.L.; Weiner, G.J.; Pardoll, D.M. Cancer immunotherapy comes of age. J. Clin. Oncol. 2011, 29, 4828–4836. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Dougan, M.; Rose, N.R.; Lichtman, A.H.; Sharpe, A.H. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019, 40, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Helander, H.F.; Fandriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- McDermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M. Checkpoint Blockade Toxicity and Immune Homeostasis in the Gastrointestinal Tract. Front. Immunol. 2017, 8, 1547. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.S.; Molina, G.E.; Chen, S.T.; Zheng, H.; Deshpande, V.; Fadden, R.; Sullivan, R.J.; Dougan, M. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Badran, Y.R.; Shih, A.; Leet, D.; Mooradian, M.J.; Coromilas, A.; Chen, J.; Kem, M.; Zheng, H.; Borowsky, J.; Misdraji, J.; et al. Immune checkpoint inhibitor-associated celiac disease. J. Immunother. Cancer 2020, 8, e000958. [Google Scholar] [CrossRef]
- Mooradian, M.J.; Wang, D.Y.; Coromilas, A.; Lumish, M.; Chen, T.; Giobbie-Hurder, A.; Johnson, D.B.; Sullivan, R.J.; Dougan, M. Mucosal inflammation predicts response to systemic steroids in immune checkpoint inhibitor colitis. J. Immunother. Cancer 2020, 8, e000451. [Google Scholar] [CrossRef]
- Dougan, M.; Wang, Y.; Rubio-Tapia, A.; Lim, J.K. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160, 1384–1393. [Google Scholar] [CrossRef]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Qiao, W.; Trinh, V.A.; Zobniw, C.; Johnson, D.H.; Samdani, R.; Lum, P.; et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24, 1695–1705. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671. [Google Scholar] [CrossRef]
- Sasson, S.C.; Slevin, S.M.; Cheung, V.T.F.; Nassiri, I.; Olsson-Brown, A.; Fryer, E.; Ferreira, R.C.; Trzupek, D.; Gupta, T.; Al-Hillawi, L.; et al. Interferon-Gamma-Producing CD8(+) Tissue Resident Memory T Cells Are a Targetable Hallmark of Immune Checkpoint Inhibitor-Colitis. Gastroenterology 2021, 161, 1229–1244 e1229. [Google Scholar] [CrossRef]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.F.; Bourdier de Beauregard, M.; et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J. Crohn’s Colitis 2016, 10, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, J.; Lin, N.; Zhou, Y.; He, W.; Liu, J.; Ma, X. Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management. Front. Immunol. 2021, 12, 800879. [Google Scholar] [CrossRef] [PubMed]
- Siakavellas, S.I.; Bamias, G. Checkpoint inhibitor colitis: A new model of inflammatory bowel disease? Curr. Opin. Gastroenterol. 2018, 34, 377–383. [Google Scholar] [CrossRef]
- Bellaguarda, E.; Hanauer, S. Checkpoint Inhibitor-Induced Colitis. Am. J. Gastroenterol. 2020, 115, 202–210. [Google Scholar] [CrossRef]
- Dabritz, J. Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G455–G465. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Faleck, D.M.; Ricciuti, B.; Mendelsohn, R.B.; Naqash, A.R.; Cohen, J.V.; Sellers, M.C.; Balaji, A.; Ben-Betzalel, G.; Hajir, I.; et al. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. J. Clin. Oncol. 2020, 38, 576–583. [Google Scholar] [CrossRef]
- Zou, F.; Faleck, D.; Thomas, A.; Harris, J.; Satish, D.; Wang, X.; Charabaty, A.; Ernstoff, M.S.; Glitza Oliva, I.C.; Hanauer, S.; et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: A two-center observational study. J. Immunother. Cancer 2021, 9, e003277. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Alsaadi, D.; Jennings, J.; Luo, W.; Gong, Z.; Richards, D.M.; Charabaty, A.; Wang, Y. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: A multi-center study. J. Immunother. Cancer 2018, 6, 142. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; Committee, A.G.A.I.C.G. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P.; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- Bishu, S.; Melia, J.; Sharfman, W.; Lao, C.D.; Fecher, L.A.; Higgins, P.D.R. Efficacy and Outcome of Tofacitinib in Immune checkpoint Inhibitor Colitis. Gastroenterology 2021, 160, 932–934.e933. [Google Scholar] [CrossRef] [PubMed]
- Shirwaikar Thomas, A.; Lee, S.E.; Shatila, M.; De Toni, E.N.; Torok, H.P.; Ben Khaled, N.; Powell, N.; Weight, R.; Faleck, D.M.; Wang, Y. IL12/23 Blockade for Refractory Immune-Mediated Colitis: 2-Center Experience. Am. J. Gastroenterol. 2023, 118, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Thompson, J.A.; Hamid, O.; Minor, D.; Amin, A.; Ron, I.; Ridolfi, R.; Assi, H.; Maraveyas, A.; Berman, D.; et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 2009, 15, 5591–5598. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; Ragsdale, C.E.; Gale, R.P.; Lyman, G.H. Sargramostim (rhu GM-CSF) as Cancer Therapy (Systematic Review) and An Immunomodulator. A Drug Before Its Time? Front. Immunol. 2021, 12, 706186. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.G.; Witek, J.S.; Temple, P.A.; Wilkens, K.M.; Leary, A.C.; Luxenberg, D.P.; Jones, S.S.; Brown, E.L.; Kay, R.M.; Orr, E.C.; et al. Human GM-CSF: Molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science 1985, 228, 810–815. [Google Scholar] [CrossRef]
- Bradley, T.R.; Metcalf, D. The growth of mouse bone marrow cells in vitro. Aust. J. Exp. Biol. Med. Sci. 1966, 44, 287–299. [Google Scholar] [CrossRef]
- Gough, N.M.; Gough, J.; Metcalf, D.; Kelso, A.; Grail, D.; Nicola, N.A.; Burgess, A.W.; Dunn, A.R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. Nature 1984, 309, 763–767. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Ruby, C.E.; Hughes, T.; Slingluff, C.L., Jr. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef]
- Damiani, G.; McCormick, T.S.; Leal, L.O.; Ghannoum, M.A. Recombinant human granulocyte macrophage-colony stimulating factor expressed in yeast (sargramostim): A potential ally to combat serious infections. Clin. Immunol. 2020, 210, 108292. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; Pitts, K.; Wang, T.; Lee, E.; Buchbinder, E.; Dougan, M.; Armstrong, D.G.; Paine, R., 3rd; Ragsdale, C.E.; Boyd, T.; et al. Recombinant GM-CSF for diseases of GM-CSF insufficiency: Correcting dysfunctional mononuclear phagocyte disorders. Front. Immunol. 2022, 13, 1069444. [Google Scholar] [CrossRef] [PubMed]
- Hercus, T.R.; Thomas, D.; Guthridge, M.A.; Ekert, P.G.; King-Scott, J.; Parker, M.W.; Lopez, A.F. The granulocyte-macrophage colony-stimulating factor receptor: Linking its structure to cell signaling and its role in disease. Blood 2009, 114, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Hossain, M.S.; Giver, C.R.; Desai, S.; Haroun, Y.; Kauser, T.; Ejaz, K.; Quyyumi, A.A.; Waller, E.K. Intermittent sargramostim administration expands proliferating naïve T cells, Tregs, HLA-DR+ PD-L1+ monocytes and myeloid-derived suppressor cells: Results from a randomized placebo-controlled clinical trial of GM-CSF in patients with peripheral artery disease. Blood 2023, 142 (Suppl. S1), 5355. [Google Scholar]
- Tarhini, A.A.; Joshi, I.; Garner, F. Sargramostim and immune checkpoint inhibitors: Combinatorial therapeutic studies in metastatic melanoma. Immunotherapy 2021, 13, 1011–1029. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Liu, K.; Li, H.; Wang, F.S.; Xu, R. Granulocyte-macrophage colony-stimulating factor: An immunotarget for sepsis and COVID-19. Cell. Mol. Immunol. 2021, 18, 2057–2058. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Budnick, I.; Singh, M.; Thiruppathi, M.; Alharshawi, K.; Elshabrawy, H.; Holterman, M.J.; Prabhakar, B.S. Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: Implications for immune therapy. J. Interferon Cytokine Res. 2015, 35, 585–599. [Google Scholar] [CrossRef]
- Zhang, J.; Roberts, A.I.; Liu, C.; Ren, G.; Xu, G.; Zhang, L.; Devadas, S.; Shi, Y. A novel subset of helper T cells promotes immune responses by secreting GM-CSF. Cell Death Differ. 2013, 20, 1731–1741. [Google Scholar] [CrossRef]
- Mach, N.; Gillessen, S.; Wilson, S.B.; Sheehan, C.; Mihm, M.; Dranoff, G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000, 60, 3239–3246. [Google Scholar]
- Dranoff, G.; Crawford, A.D.; Sadelain, M.; Ream, B.; Rashid, A.; Bronson, R.T.; Dickersin, G.R.; Bachurski, C.J.; Mark, E.L.; Whitsett, J.A.; et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994, 264, 713–716. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From growth factor to central mediator of tissue inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W. G-CSF: A key regulator of neutrophil production, but that’s not all! Growth Factors 2005, 23, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, B.C.; Nakata, K.; Bonella, F.; Campo, I.; Griese, M.; Hamilton, J.; Wang, T.; Morgan, C.; Cottin, V.; McCarthy, C. Pulmonary alveolar proteinosis. Nat. Rev. Dis. Primers 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Ataya, A.; Knight, V.; Carey, B.C.; Lee, E.; Tarling, E.J.; Wang, T. The Role of GM-CSF Autoantibodies in Infection and Autoimmune Pulmonary Alveolar Proteinosis: A Concise Review. Front. Immunol. 2021, 12, 752856. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Carey, B.C.; Trapnell, B.C. Autoimmune Pulmonary Alveolar Proteinosis. Am. J. Respir. Crit. Care Med. 2022, 205, 1016–1035. [Google Scholar] [CrossRef]
- Geertsma, M.F.; Van Furth, R.; Nibbering, P.H. Monocytes incubated with surfactant: A model for human alveolar macrophages? J. Leukoc. Biol. 1997, 62, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Rosler, B.; Herold, S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy? Mol. Cell. Pediatr. 2016, 3, 29. [Google Scholar] [CrossRef]
- Casacuberta-Serra, S.; Pares, M.; Golbano, A.; Coves, E.; Espejo, C.; Barquinero, J. Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol. Cell Biol. 2017, 95, 538–548. [Google Scholar] [CrossRef]
- Hirata, Y.; Egea, L.; Dann, S.M.; Eckmann, L.; Kagnoff, M.F. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe 2010, 7, 151–163. [Google Scholar] [CrossRef]
- Coon, C.; Beagley, K.W.; Bao, S. The role of granulocyte macrophage-colony stimulating factor in gastrointestinal immunity to salmonellosis. Scand. J. Immunol. 2009, 70, 106–115. [Google Scholar] [CrossRef]
- Fukuzawa, H.; Sawada, M.; Kayahara, T.; Morita-Fujisawa, Y.; Suzuki, K.; Seno, H.; Takaishi, S.; Chiba, T. Identification of GM-CSF in Paneth cells using single-cell RT-PCR. Biochem. Biophys. Res. Commun. 2003, 312, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- del Rio, M.L.; Bernhardt, G.; Rodriguez-Barbosa, J.I.; Forster, R. Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 2010, 234, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Granulocyte-macrophage colony stimulating factor and inflammatory bowel disease: Establishing a connection. Gastroenterology 2011, 141, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Enzler, T.; Gillessen, S.; Dougan, M.; Allison, J.P.; Neuberg, D.; Oble, D.A.; Mihm, M.; Dranoff, G. Functional deficiencies of granulocyte-macrophage colony stimulating factor and interleukin-3 contribute to insulitis and destruction of beta cells. Blood 2007, 110, 954–961. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef]
- Dabritz, J. GM-CSF and the role of myeloid regulatory cells in the pathogenesis and treatment of Crohn’s disease. Mol. Cell. Pediatr. 2015, 2, 12. [Google Scholar] [CrossRef]
- Egea, L.; Hirata, Y.; Kagnoff, M.F. GM-CSF: A role in immune and inflammatory reactions in the intestine. Expert. Rev. Gastroenterol. Hepatol. 2010, 4, 723–731. [Google Scholar] [CrossRef]
- Dranoff, G.; Jaffee, E.; Lazenby, A.; Golumbek, P.; Levitsky, H.; Brose, K.; Jackson, V.; Hamada, H.; Pardoll, D.; Mulligan, R.C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA 1993, 90, 3539–3543. [Google Scholar] [CrossRef]
- Si, Z.; Hersey, P.; Coates, A.S. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996, 6, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kurbacher, C.M.; Kurbacher, J.A.; Cramer, E.M.; Rhiem, K.; Mallman, P.K.; Reichelt, R.; Reinhold, U.; Stier, U.; Cree, I.A. Continuous low-dose GM-CSF as salvage therapy in refractory recurrent breast or female genital tract carcinoma. Oncology Williston Park 2005, 19, 23–26. [Google Scholar] [PubMed]
- Grotz, T.E.; Kottschade, L.; Pavey, E.S.; Markovic, S.N.; Jakub, J.W. Adjuvant GM-CSF improves survival in high-risk stage IIIC melanoma: A single-center Study. Am. J. Clin. Oncol. 2014, 37, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Kumar, R.; Yang, X.; Fidler, I.J. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 1997, 88, 801–810. [Google Scholar] [CrossRef]
- Mashima, H.; Zhang, R.; Kobayashi, T.; Hagiya, Y.; Tsukamoto, H.; Liu, T.; Iwama, T.; Yamamoto, M.; Lin, C.; Nakatsuka, R.; et al. Generation of GM-CSF-producing antigen-presenting cells that induce a cytotoxic T cell-mediated antitumor response. Oncoimmunology 2020, 9, 1814620. [Google Scholar] [CrossRef]
- Dorr, R.T. Clinical properties of yeast-derived versus Escherichia coli-derived granulocyte-macrophage colony-stimulating factor. Clin. Ther. 1993, 15, 19–29. [Google Scholar]
- Sola, R.J.; Griebenow, K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. [Google Scholar] [CrossRef]
- Sola, R.J.; Griebenow, K. Glycosylation of therapeutic proteins: An effective strategy to optimize efficacy. BioDrugs 2010, 24, 9–21. [Google Scholar] [CrossRef]
- Sainathan, S.K.; Hanna, E.M.; Gong, Q.; Bishnupuri, K.S.; Luo, Q.; Colonna, M.; White, F.V.; Croze, E.; Houchen, C.; Anant, S.; et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm. Bowel Dis. 2008, 14, 88–99. [Google Scholar] [CrossRef]
- Xu, Y.; Hunt, N.H.; Bao, S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008, 18, 1220–1229. [Google Scholar] [CrossRef]
- Sennikov, S.V.; Temchura, V.V.; Kozlov, V.A.; Trufakin, V.A. The influence of conditioned medium from mouse intestinal epithelial cells on the proliferative activity of crypt cells: Role of granulocyte-macrophage colony-stimulating factor. J. Gastroenterol. 2002, 37, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gilbert, S.; Groschwitz, K.; Hogan, S.; Jurickova, I.; Trapnell, B.; Samson, C.; Gully, J. Loss of GM-CSF signalling in non-haematopoietic cells increases NSAID ileal injury. Gut 2010, 59, 1066–1078. [Google Scholar] [CrossRef]
- Egea, L.; McAllister, C.S.; Lakhdari, O.; Minev, I.; Shenouda, S.; Kagnoff, M.F. GM-CSF produced by nonhematopoietic cells is required for early epithelial cell proliferation and repair of injured colonic mucosa. J. Immunol. 2013, 190, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, E.; Favre, L.; Maillard, M.H.; Bachmann, D.; Pythoud, C.; Bouzourene, H.; Croze, E.; Velichko, S.; Parkinson, J.; Michetti, P.; et al. Granulocyte-macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm. Bowel Dis. 2010, 16, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Pilar, A.; Gupta, M.; Ghosh Laskar, S.; Laskar, S. Intraoperative radiotherapy: Review of techniques and results. Ecancermedicalscience 2017, 11, 750. [Google Scholar] [CrossRef]
- Erdem, E.; Dinc, S.; Erdem, D.; Ustun, H.; Caydere, M.; Alagol, H. Effects of intraperitoneal chemotherapy and GM-CSF on anastomotic healing: An experimental study in rats. J. Surg. Res. 2002, 108, 1–6. [Google Scholar] [CrossRef]
- Cetinkaya, K.; Dinc, S.; Gulcelik, M.A.; Renda, N.; Ustun, H.; Caydere, M.; Alagol, H. Granulocyte macrophage-colony stimulating factor improves impaired anastomotic wound healing in rats treated with intraperitoneal mitomycin-C. Surg. Today 2005, 35, 290–294. [Google Scholar] [CrossRef]
- Dinc, S.; Ozbirecikli, B.; Gulcelik, M.A.; Ergeneci, D.; Kuru, B.; Erdem, E.; Caydere, M.; Alagol, H. The effects of locally injected granulocyte macrophage-colony stimulating factor on the healing of intraoperatively irradiated intestinal anastomoses in rats. J. Exp. Clin. Cancer Res. 2004, 23, 77–82. [Google Scholar]
- Hu, X.; Sun, H.; Han, C.; Wang, X.; Yu, W. Topically applied rhGM-CSF for the wound healing: A systematic review. Burns 2011, 37, 729–741. [Google Scholar] [CrossRef]
- Brem, H.; Howell, R.; Criscitelli, T.; Senderowicz, A.; Siegart, N.; Gorenstein, S.; Gillette, B. Practical Application of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) in Patients with Wounds. Surg. Technol. Int. 2018, 32, 61–66. [Google Scholar]
- Brubaker, J.; Zhang, X.; Bourgeois, A.L.; Harro, C.; Sack, D.A.; Chakraborty, S. Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. coli infection and impact on intestinal colonization and ETEC specific immune responses in an experimental human challenge model. Gut Microbes 2021, 13, 1891852. [Google Scholar] [CrossRef] [PubMed]
- Buskirk, A.D.; Ndungo, E.; Shimanovich, A.A.; Lam, D.; Blackwelder, W.C.; Ikumapayi, U.N.; Ma, B.; Powell, H.; Antonio, M.; Nataro, J.P.; et al. Mucosal Immune Profiles Associated with Diarrheal Disease Severity in Shigella- and Enteropathogenic Escherichia coli-Infected Children Enrolled in the Global Enteric Multicenter Study. mBio 2022, 13, e0053822. [Google Scholar] [CrossRef] [PubMed]
- Korzenik, J.R.; Dieckgraefe, B.K.; Valentine, J.F.; Hausman, D.F.; Gilbert, M.J.; Sargramostim in Crohn’s Disease Study Group. Sargramostim for active Crohn’s disease. N. Engl. J. Med. 2005, 352, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Meropol, N.J.; Rustum, Y.M.; Creaven, P.J.; Blumenson, L.E.; Frank, C. Phase I and pharmacokinetic study of weekly 5-fluorouracil administered with granulocyte-macrophage colony-stimulating factor and high-dose leucovorin: A potential role for growth factor as mucosal protectant. Cancer Investig. 1999, 17, 1–9. [Google Scholar] [CrossRef]
- Melichar, B.; Kohout, P.; Bratova, M.; Solichova, D.; Kralickova, P.; Zadak, Z. Intestinal permeability in patients with chemotherapy-induced stomatitis. J. Cancer Res. Clin. Oncol. 2001, 127, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Chachoua, A.; Oratz, R.; Hoogmoed, R.; Caron, D.; Peace, D.; Liebes, L.; Blum, R.H.; Vilcek, J. Monocyte activation following systemic administration of granulocyte-macrophage colony-stimulating factor. J. Immunother. Emphasis Tumor Immunol. 1994, 15, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Naidoo, J.; Zhong, Q.; Xiong, Y.; Mammen, J.; de Flores, M.V.; Cappelli, L.; Balaji, A.; Palmer, T.; Forde, P.M.; et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Investig. 2019, 129, 4305–4315. [Google Scholar] [CrossRef]
- van Elsas, A.; Hurwitz, A.A.; Allison, J.P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999, 190, 355–366. [Google Scholar] [CrossRef]
- Hodi, F.S.; Lee, S.; McDermott, D.F.; Rao, U.N.; Butterfield, L.H.; Tarhini, A.A.; Leming, P.; Puzanov, I.; Shin, D.; Kirkwood, J.M. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 2014, 312, 1744–1753. [Google Scholar] [CrossRef]
- Kwek, S.S.; Kahn, J.; Greaney, S.K.; Lewis, J.; Cha, E.; Zhang, L.; Weber, R.W.; Leonard, L.; Markovic, S.N.; Fong, L.; et al. GM-CSF and ipilimumab therapy in metastatic melanoma: Clinical outcomes and immunologic responses. Oncoimmunology 2016, 5, e1101204. [Google Scholar] [CrossRef]
- Luke, J.J.; Donahue, H.; Nishino, M.; Giobbie-Hurder, A.; Davis, M.; Bailey, N.; Ott, P.A.; Hodi, F.S. Single institution experience of ipilimumab 3 mg/kg with sargramostim (GM-CSF) in metastatic melanoma. Cancer Immunol. Res. 2015, 3, 986–991. [Google Scholar] [CrossRef]
- ClinicalTrials.gov: A Phase II/III Trial of Nivolumab, Ipilimumab, and GM-CSF in Patients with Advanced Melanoma. ClinicalTrials.gov Identifier: NCT02339571. Available online: https://clinicaltrials.gov/study/NCT02339571 (accessed on 4 January 2024).
- Kelley, R.K.; Bracci, P.M.; Keenan, B.; Behr, S.; Ibrahim, F.; Pollak, M.; Gordan, J.; Ko, A.H.; Van Loon, K.; Atreya, C.E.; et al. Pembrolizumab (PEM) plus granulocyte macrophage colony stimulating factor (GM-CSF) in advanced biliary cancers (ABC): Final outcomes of a phase 2 trial. J. Clin. Oncol. 2022, 40 (Suppl. S4), A444. [Google Scholar] [CrossRef]
- Kwek, S.S.; Lewis, J.; Zhang, L.; Weinberg, V.; Greaney, S.K.; Harzstark, A.L.; Lin, A.M.; Ryan, C.J.; Small, E.J.; Fong, L. Preexisting Levels of CD4 T Cells Expressing PD-1 Are Related to Overall Survival in Prostate Cancer Patients Treated with Ipilimumab. Cancer Immunol. Res. 2015, 3, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov: A Study of Sargramostim Plus Pembrolizumab with or without Pemetrexed in Patients with Advance NON-small Cell Lung Cancer after Completion of Chemoimmunotherapy. ClinicalTrials.gov Identifier: NCT04856176. Available online: https://clinicaltrials.gov/study/NCT04856176 (accessed on 4 January 2024).
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Zheng, Y.; Lee, S.J.; Zhou, J.; Giobbie-Hurder, A.; Butterfield, L.H.; Dranoff, G.; Hodi, F.S. Granulocyte-Macrophage Colony-Stimulating Factor Influence on Soluble and Membrane-Bound ICOS in Combination with Immune Checkpoint Blockade. Cancer Immunol. Res. 2023, 11, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Carthon, B.C.; Wolchok, J.D.; Yuan, J.; Kamat, A.; Ng Tang, D.S.; Sun, J.; Ku, G.; Troncoso, P.; Logothetis, C.J.; Allison, J.P.; et al. Preoperative CTLA-4 blockade: Tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 2010, 16, 2861–2871. [Google Scholar] [CrossRef]
- Machado, A.P.; Ratliff, H.; Abdelwahab, A.; Vohra, M.H.; Kuang, A.; Shatila, M.; Khan, M.A.; Shafi, M.A.; Thomas, A.S.; Philpott, J.; et al. The Safety of Immunosuppressants Used in the Treatment of Immune-Related Adverse Events due to Immune Checkpoint Inhibitors: A Systematic Review. J. Cancer 2023, 14, 2956–2963. [Google Scholar] [CrossRef]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef]



| Grading | Symptoms | CTLA-4 Inhibitor Frequency (%) | PD-1/PD-L1 Inhibitor Frequency (%) |
|---|---|---|---|
| Mild | Diarrhea, nausea, vomiting, decreased appetite, reflux | 20–40 | 7–20 |
| Moderate/Severe | Diarrhea (commonly watery, can be bloody), nausea, vomiting, decreased appetite, reflux, cramping, urgency, fever | 15–20 | 2–5 |
| Features | ICI GI irAEs | Ulcerative Colitis | Crohn’s Disease |
|---|---|---|---|
| Symptoms |
|
|
|
| Location |
|
|
|
| Morphological Changes |
|
|
|
| Biopsy Results |
|
|
|
| Stage of Rescue Therapy | Mild | Moderate/Severe |
|---|---|---|
| First-line | Systemic glucocorticosteroid (oral; IV if refractory) | Systemic glucocorticosteroid (IV) ± TNF-alpha inhibitor (e.g., infliximab) OR integrin inhibitor (e.g., vedolizumab) |
| Second-line | TNF-alpha inhibitor (e.g., infliximab) OR integrin inhibitor (e.g., vedolizumab) | If not responding to TNF-alpha inhibitor (e.g., infliximab) OR integrin inhibitor (e.g., vedolizumab), switch treatment class |
| Third-line | IL-23p40 inhibitor (e.g., ustekinumab) OR JAK inhibitor (e.g., tofacitinib) | |
| Citation | Patient Population and Study Design | Treatment | Efficacy | Adverse Events | Comments |
|---|---|---|---|---|---|
| Melanoma | |||||
| Hodi 2014 (ECOG 1608) [99] | Melanoma (unresectable stage III/IV; N = 245); Phase 2, randomized | Ipilimumab 10 mg/kg IV d1 + sargramostim 250 μg SC d1-14 of 21 d cycles × 4, then maintenance vs. ipilimumab alone | Ipilimumab + sargramostim vs. ipilimumab alone:
| Ipilimumab + sargramostim vs. ipilimumab alone:
|
sargramostim
|
| Kwek 2016 [100] | Melanoma (unresectable stage III/IV; N = 22); Phase 2, single-arm | Ipilimumab 10 mg/kg IV d1 + sargramostim 125 µg/m2 SC d1-14 of 21 d cycles × 4, then sargramostim × 3 m, then ipilimumab + sargramostim maintenance |
|
|
|
| Luke 2015 [101] | Melanoma (metastatic (41% with CNS metastases); N = 32); Retrospective | Ipilimumab 3 mg/kg IV d1 + sargramostim 250 µg SC d1-14 of 21 d cycles × 4 |
|
|
|
| EA6141/NCT02339571 (estimated completion 2033) [102] | Melanoma (unresectable stage III/IV); Phase 2, randomized | Ipilimumab 3 mg/kg IV d1 + nivolumab 1 mg/kg IV d1 + sargramostim 250 µg SC d1-14 of 21 d cycles × 4, then maintenance nivolumab + sargramostim vs. ipilimumab + nivolumab, then nivolumab alone | Ongoing | Ongoing |
|
| Biliary Cancer | |||||
| Kelley 2022 (abstract only available) [103] | Advanced biliary cancer (N = 42); Phase 2, single-arm | Pembrolizumab 200 mg IV d1 + sargramostim 250 µg SC d1-14 × 2 of 3 21 d cycles |
|
|
|
| Prostate Cancer | |||||
| Kwek 2015 [104] | Prostate cancer (metastatic, castration-resistant; N = 42); Phase 1b, dose-escalation study | Ipilimumab escalating doses 0.5–10 mg/kg IV d1 + sargramostim 250 µg/m2 SC d1-14 of 28 d cycles × 4 |
|
|
|
| Non-Small Cell Lung Cancer | |||||
| NCT04856176 (estimated completion 2024) [105] | Non-small cell lung cancer (stage III/IV); Phase 2, single-arm | Chemo-immunotherapy × 4 cycles, then pembrolizumab + sargramostim ± pemetrexed | Ongoing | Ongoing | Ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dougan, M.; Nguyen, L.H.; Buchbinder, E.I.; Lazarus, H.M. Sargramostim for Prophylactic Management of Gastrointestinal Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy for Cancer. Cancers 2024, 16, 501. https://doi.org/10.3390/cancers16030501
Dougan M, Nguyen LH, Buchbinder EI, Lazarus HM. Sargramostim for Prophylactic Management of Gastrointestinal Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy for Cancer. Cancers. 2024; 16(3):501. https://doi.org/10.3390/cancers16030501
Chicago/Turabian StyleDougan, Michael, Long H. Nguyen, Elizabeth I. Buchbinder, and Hillard M. Lazarus. 2024. "Sargramostim for Prophylactic Management of Gastrointestinal Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy for Cancer" Cancers 16, no. 3: 501. https://doi.org/10.3390/cancers16030501
APA StyleDougan, M., Nguyen, L. H., Buchbinder, E. I., & Lazarus, H. M. (2024). Sargramostim for Prophylactic Management of Gastrointestinal Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy for Cancer. Cancers, 16(3), 501. https://doi.org/10.3390/cancers16030501






