Investigating the Role of SNAI1 and ZEB1 Expression in Prostate Cancer Progression and Immune Modulation of the Tumor Microenvironment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cohort
2.2. RNA Isolation
2.3. Transcription Analysis
2.4. Digital Cytometry Analysis
2.5. Statistical Analysis
3. Results
3.1. Identification of DEGs and Pathways Associated with BCR and Clinical Risk
3.2. Downstream Effects of ZEB1 and SNAI1 Expression
3.3. Impact of ZEB1 and SNAI1 Expression on the Immune TME
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Prim. 2021, 7, 9. [Google Scholar] [CrossRef]
- Baciarello, G.; Gizzi, M.; Fizazi, K. Advancing therapies in metastatic castration-resistant prostate cancer. Expert Opin. Pharmacother. 2018, 19, 1797–1804. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Wu, Y.M.; Cieślik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; Ning, Y.; Wang, L.; Kunju, L.P.; de Sarkar, N.; et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018, 173, 1770–1782.e14. [Google Scholar] [CrossRef]
- Melo, C.M.; Vidotto, T.; Chaves, L.P.; Lautert-Dutra, W.; Dos Reis, R.B.; Squire, J.A. The role of somatic mutations on the immune response of the tumor microenvironment in prostate cancer. Int. J. Mol. Sci. 2021, 22, 9550. [Google Scholar] [CrossRef]
- Stultz, J.; Fong, L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 697–717. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Nardone, V.; Botta, C.; Caraglia, M.; Martino, E.C.; Ambrosio, M.R.; Carfagno, T.; Tini, P.; Semeraro, L.; Misso, G.; Grimaldi, A.; et al. Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol. Ther. 2016, 17, 1213–1220. [Google Scholar] [CrossRef]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjöström, M.; Den, R.B.; Freedland, S.J.; Klein, E.A.; et al. The immune landscape of prostate cancer and nomination of PD-L2 as a potential therapeutic target. J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Cheng, L. Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance. NPJ Precis. Oncol. 2022, 6, 31. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Derynck, R.; Weinberg, R.A. EMT and Cancer: More Than Meets the Eye. Dev. Cell 2019, 49, 313–316. [Google Scholar] [CrossRef]
- Stemmler, M.P.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-redundant functions of EMT-TFs. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef]
- Orellana-Serradell, O.; Herrera, D.; Castellon, E.A.; Contreras, H.R. The transcription factor ZEB1 promotes an aggressive phenotype in prostate cancer cell lines. Asian J. Androl. 2018, 20, 294–299. [Google Scholar] [CrossRef]
- Stylianou, N.; Lehman, M.L.; Wang, C.; Fard, A.T.; Rockstroh, A.; Fazli, L.; Jovanovic, L.; Ward, M.; Sadowski, M.C.; Kashyap, A.S.; et al. A molecular portrait of epithelial–mesenchymal plasticity in prostate cancer associated with clinical outcome. Oncogene 2019, 38, 913–934. [Google Scholar] [CrossRef]
- Poblete, C.E.; Fulla, J.; Gallardo, M.; Muñoz, V.; Castellón, E.A.; Gallegos, I.; Contreras, H.R. Increased SNAIL expression and low syndecan levels are associated with high Gleason grade in prostate cancer. Int. J. Oncol. 2014, 44, 647–654. [Google Scholar] [CrossRef]
- Neal, C.L.; Henderson, V.; Smith, B.N.; McKeithen, D.; Graham, T.; Vo, B.T.; Odero-Marah, V.A. Snail transcription factor negatively regulates maspin tumor suppressor in human prostate cancer cells. BMC Cancer 2012, 12, 336. [Google Scholar] [CrossRef]
- Horn, L.A.; Fousek, K.; Palena, C. Tumor Plasticity and Resistance to Immunotherapy. Trends Cancer 2020, 6, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Abiko, K.; Ukita, M.; Murakami, R.; Yamanoi, K.; Yamaguchi, K.; Hamanishi, J.; Baba, T.; Matsumura, N.; Mandai, M. Tumor immune microenvironment during epithelial- mesenchymal transition. Clin. Cancer Res. 2021, 27, 4669–4679. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I. MicroRNA control of TGF-β signaling. Int. J. Mol. Sci. 2018, 19, 1901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, X.J.; Zhang, H.; Teng, Y.; Li, R.; Bai, F.; Elankumaran, S.; Xing, J. TGF-β-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci. Signal. 2014, 7, ra91. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Lautert-Dutra, W.; Melo, C.M.; Chaves, L.P.; Souza, F.C.; Crozier, C.; Sundby, A.E.; Woroszchuk, E.; Saggioro, F.P.; Avante, F.S.; dos Reis, R.B.; et al. Identification of tumor-agnostic biomarkers for predicting prostate cancer progression and biochemical recurrence. Front. Oncol. 2023, 13, 1280943. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Hilton, J.F.; Carroll, P.R. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer 2011, 22, 5039–5046. [Google Scholar] [CrossRef]
- Bayani, J.; Yao, C.Q.; Quintayo, M.A.; Yan, F.; Haider, S.; DCosta, A.; Brookes, C.L.; Van De Velde, C.J.H.; Hasenburg, A.; Kieback, D.G.; et al. Molecular stratification of early breast cancer identifies drug targets to drive stratified medicine. NPJ Breast Cancer 2017, 3, 3. [Google Scholar] [CrossRef]
- Patel, P.G.; Selvarajah, S.; Guérard, K.P.; Bartlett, J.M.S.; Lapointe, J.; Berman, D.M.; Okello, J.B.A.; Park, P.C. Reliability and performance of commercial RNA and DNA extraction kits for FFPE tissue cores. PLoS ONE 2017, 12, 0179732. [Google Scholar] [CrossRef]
- Goytain, A.; Ng, T. NanoString nCounter Technology: High-Throughput RNA Validation. In Chimeric RNA. Methods in Molecular Biology; Li, H., Elfman, J., Eds.; Humana: New York, NY, USA, 2020; pp. 125–139. ISBN 978-1-4939-9903-3. [Google Scholar]
- Olkhov-Mitsel, E.; Hodgson, A.; Liu, S.K.; Vesprini, D.; Bayani, J.; Bartlett, J.; Xu, B.; Downes, M.R. Immune gene expression profiles in high-grade urothelial carcinoma of the bladder: A NanoString study. J. Clin. Pathol. 2021, 74, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.M.; Spruill, L.; Jefferson, M.; Bethard, J.R.; Ball, L.E.; Hughes-Halbert, C.; Drake, R.R. Zonal regulation of collagen-type proteins and posttranslational modifications in prostatic benign and cancer tissues by imaging mass spectrometry. Prostate 2020, 80, 1071–1086. [Google Scholar] [CrossRef]
- Szabo, P.M.; Vajdi, A.; Kumar, N.; Tolstorukov, M.Y.; Chen, B.J.; Edwards, R.; Ligon, K.L.; Chasalow, S.D.; Chow, K.H.; Shetty, A.; et al. Cancer-associated fibroblasts are the main contributors to epithelial-to-mesenchymal signatures in the tumor microenvironment. Sci. Rep. 2023, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, I.; Gooding, R.J.; Doiron, R.C.; Day, A.; Selvarajah, S.; Davidson, C.; Berman, D.M.; Park, P.C. Molecular characterization of Gleason patterns 3 and 4 prostate cancer using reverse Warburg effect-associated genes. Cancer Metab. 2016, 4, 8. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, D.; Chen, F.; Qian, M.; Wei, H.; Chen, W.; Xu, J. SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene 2016, 35, 4321–4334. [Google Scholar] [CrossRef]
- van Loon, K.; Huijbers, E.J.M.; Griffioen, A.W. Secreted frizzled-related protein 2: A key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metast. Rev. 2021, 40, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, H.; Huo, W. THBS4 silencing regulates the cancer stem cell-like properties in prostate cancer via blocking the PI3K/Akt pathway. Prostate 2020, 80, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; De Menna, M.; Groenewoud, A.; Thalmann, G.N.; Kruithof-de Julio, M.; Snaar-Jagalska, B.E. A NF-ĸB-Activin A signaling axis enhances prostate cancer metastasis. Oncogene 2020, 39, 1634–1651. [Google Scholar] [CrossRef] [PubMed]
- Reader, K.L.; John-McHaffie, S.; Zellhuber-McMillan, S.; Jowett, T.; Mottershead, D.G.; Cunliffe, H.E.; Gold, E.J. Activin B and Activin C Have Opposing Effects on Prostate Cancer Progression and Cell Growth. Cancers 2023, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Hu, H.; Zhou, J. Knockdown of LncRNA SNHG7 inhibited epithelial-mesenchymal transition in prostate cancer though miR-324-3p/WNT2B axis in vitro. Pathol. Res. Pract. 2019, 215, 152537. [Google Scholar] [CrossRef] [PubMed]
- Yimamu, Y.; Yang, X.; Chen, J.; Luo, C.; Xiao, W.; Guan, H.; Wang, D. The Development of a Gleason Score-Related Gene Signature for Predicting the Prognosis of Prostate Cancer. J. Clin. Med. 2022, 11, 7164. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Wang, Y.; Hu, C.; Zhang, H.; Huang, Y.; Zhang, Z.; Ma, T.; Zhang, S.; Li, K. Evaluation of NOTCH family genes’ expression and prognostic value in prostate cancer. Transl. Androl. Urol. 2022, 11, 627–642. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Li, J.; Jin, H.; Jia, H.; Ge, X. Construction and Validation of a Robust Cancer Stem Cell-Associated Gene Set-Based Signature to Predict Early Biochemical Recurrence in Prostate Cancer. Dis. Markers 2020, 2020, 8860788. [Google Scholar] [CrossRef]
- Karanika, S.; Karantanos, T.; Li, L.; Wang, J.; Park, S.; Yang, G.; Zuo, X.; Song, J.H.; Maity, S.N.; Manyam, G.C.; et al. Targeting DNA Damage Response in Prostate Cancer by Inhibiting Androgen Receptor-CDC6-ATR-Chk1 Signaling. Cell Rep. 2017, 18, 1970–1981. [Google Scholar] [CrossRef]
- Bagherabadi, A.; Hooshmand, A.; Shekari, N.; Singh, P.; Zolghadri, S.; Stanek, A.; Dohare, R. Correlation of NTRK1 Downregulation with Low Levels of Tumor-Infiltrating Immune Cells and Poor Prognosis of Prostate Cancer Revealed by Gene Network Analysis. Genes 2022, 13, 840. [Google Scholar] [CrossRef]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, B.; Zhang, L.; Dang, T.; Rowley, D.; Ittmann, M.; Xin, L. Jagged1 upregulation in prostate epithelial cells promotes formation of reactive stroma in the Pten null mouse model for prostate cancer. Oncogene 2017, 36, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Ge, Y.; Xiao, J.; Wang, Y.; Li, L.; Ma, S.; Lan, L.; Liu, B.; Qin, B.; Luan, Y.; et al. GAS1RR, an immune-related enhancer RNA, is related to biochemical recurrence-free survival in prostate cancer. Exp. Biol. Med. 2023, 248, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, L.; Sun, W.; Zhu, P.; Cheng, S.; Yang, X.; Luo, C.; Yu, X.; Wu, X. Systematic Evaluation for the Influences of the SOX17/Notch Receptor Family Members on Reversing Enzalutamide Resistance in Castration-Resistant Prostate Cancer Cells. Front. Oncol. 2021, 11, 607291. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.C.; Duong, F.; Howard, L.E.; Wiggins, E.; Freedland, S.J.; Bhowmick, N.A.; Gong, J. Soluble endoglin (sCD105) as a novel biomarker for detecting aggressive prostate cancer. Anticancer Res. 2020, 40, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Lee, M.; Hu, Y.; Andreas, J.; Patel, S.J.; Zhang, S.; Chines, P.; Elkahloun, A.; Chandrasekharappa, S.; Gutkind, J.S.; et al. A Systems Genetics Approach Identifies CXCL14, ITGAX, and LPCAT2 as Novel Aggressive Prostate Cancer Susceptibility Genes. PLoS Genet. 2014, 10, e1004809. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wu, X.; Chen, X.; Yang, S.; Chen, W.; Wang, M.; Liu, Y.; Gu, D.; Zeng, G. A novel immune-related gene-based prognostic signature to predict biochemical recurrence in patients with prostate cancer after radical prostatectomy. Cancer Immunol. Immunother. 2021, 70, 3587–3602. [Google Scholar] [CrossRef] [PubMed]
- Ghotra, V.P.S.; He, S.; Van Der Horst, G.; Nijhoff, S.; De Bont, H.; Lekkerkerker, A.; Janssen, R.; Jenster, G.; Van Leenders, G.J.L.H.; Hoogland, A.M.M.; et al. SYK is a candidate kinase target for the treatment of advanced prostate cancer. Cancer Res. 2015, 75, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Wightman, S.C.; Uppal, A.; Pitroda, S.P.; Ganai, S.; Burnette, B.; Stack, M.; Oshima, G.; Khan, S.; Huang, X.; Posner, M.C.; et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br. J. Cancer 2015, 113, 327–335. [Google Scholar] [CrossRef]
- Long, X.; Wu, L.; Zeng, X.; Wu, Z.; Hu, X.; Jiang, H.; Lv, Z.; Yang, C.; Cai, Y.; Yang, K.; et al. Biomarkers in previous histologically negative prostate biopsies can be helpful in repeat biopsy decision-making processes. Cancer Med. 2020, 9, 7524–7536. [Google Scholar] [CrossRef]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- True, L.D.; Zhang, H.; Ye, M.; Huang, C.Y.; Nelson, P.S.; Von Haller, P.D.; Tjoelker, L.W.; Kim, J.S.; Qian, W.J.; Smith, R.D.; et al. CD90/THY1 is overexpressed in prostate cancer-associated fibroblasts and could serve as a cancer biomarker. Mod. Pathol. 2010, 23, 1346–1356. [Google Scholar] [CrossRef]
- Bauman, T.M.; Becka, A.J.; Sehgal, P.D.; Huang, W.; Ricke, W.A. SIGIRR/TIR8, an important regulator of TLR4 and IL-1R-mediated NF-κB activation, predicts biochemical recurrence after prostatectomy in low-grade prostate carcinomas. Hum. Pathol. 2015, 46, 1744–1751. [Google Scholar] [CrossRef]
- Tuerff, D.; Muller, D.J.; Lap, C.J.G.; Liu, S.; Diao, G.; Antonio, M.; Nava, V.; Jain, M.R. The association of HLA-DR and PD-L1 expression with clinical characteristics in prostate. J. Clin. Oncol. 2023, 41, 17017. [Google Scholar] [CrossRef]
- Tse, B.W.C.; Volpert, M.; Ratther, E.; Stylianou, N.; Nouri, M.; McGowan, K.; Lehman, M.L.; McPherson, S.J.; Roshan-Moniri, M.; Butler, M.S.; et al. Neuropilin-1 is upregulated in the adaptive response of prostate tumors to androgen-targeted therapies and is prognostic of metastatic progression and patient mortality. Oncogene 2017, 36, 3417–3427. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.P.; Melo, C.M.; Saggioro, F.P.; Borges, R.; Squire, J.A. Epithelial—Mesenchymal Transition Signaling and Prostate Precision Therapeutics. Genes 2021, 12, 1900. [Google Scholar] [CrossRef]
- Kitz, J.; Lefebvre, C.; Carlos, J.; Lowes, L.E.; Allan, A.L. Reduced zeb1 expression in prostate cancer cells leads to an aggressive partial-emt phenotype associated with altered global methylation patterns. Int. J. Mol. Sci. 2021, 22, 12840. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Meng, F.; Han, X.; Min, Z.; He, X.; Zhu, S. Prognostic signatures associated with high infiltration of Tregs in bone metastatic prostate cancer. Aging 2021, 13, 17442–17461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, C.; Li, Y.; Xing, C.; Wang, S.; Yu, Z.; Chen, L.; Li, P.; Dai, M. Data mining-based study of collagen type III alpha 1 (COL3A1) prognostic value and immune exploration in pan-cancer. Bioengineered 2021, 12, 3634–3646. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, A.S.; Dood, R.L.; Armaiz-Pena, G.; Kang, Y.; Wu, S.Y.; Allen, J.K.; Jennings, N.B.; Mangala, L.S.; Pradeep, S.; Lyons, Y.; et al. Adrenergic-mediated increases in INHBA drive CAF phenotype and collagens. JCI Insight 2017, 2, e93076. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, H.; Zhang, J.; Jiang, F.; Guo, Y.; Shi, Y.; Guo, Z.; Ao, L. A qualitative transcriptional signature for predicting the biochemical recurrence risk of prostate cancer patients after radical prostatectomy. Prostate 2020, 80, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Shak, S.; Lee, M.; Novotny, W.; Maddala, T.; Crager, M.; Cherbavaz, D.; Pelham, R.; Millward, C.L.; Knezevic, D. Gene Expression Profile Algorithm and Test for Determining Prognosis of Prostate Cancer. AU2018201688A1, 27 February 2020. [Google Scholar]
- Yin, W.; Zhu, H.; Tan, J.; Xin, Z.; Zhou, Q.; Cao, Y.; Wu, Z.; Wang, L.; Zhao, M.; Jiang, X.; et al. Identification of collagen genes related to immune infiltration and epithelial-mesenchymal transition in glioma. Cancer Cell Int. 2021, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Wang, J.; Yumoto, K.; Jung, Y.; Cackowski, F.C.; Decker, A.M.; Li, Y.; Franceschi, R.T.; Pienta, K.J.; Taichman, R.S. DNMT1 Regulates Epithelial-Mesenchymal Transition and Cancer Stem Cells, Which Promotes Prostate Cancer Metastasis. Neoplasia 2016, 18, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Renaude, E.; Kroemer, M.; Borg, C.; Peixoto, P.; Hervouet, E.; Loyon, R.; Adotévi, O. Epigenetic Reprogramming of CD4+ Helper T Cells as a Strategy to Improve Anticancer Immunotherapy. Front. Immunol. 2021, 12, 669992. [Google Scholar] [CrossRef]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.O.; Andrén, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; Dang, Q.; Chang, L.S.; Li, L. Infiltrating mast cells increase prostate cancer chemotherapy and radiotherapy resistances via modulation of p38/p53/p21 and ATM signals. Oncotarget 2016, 7, 1341–1353. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
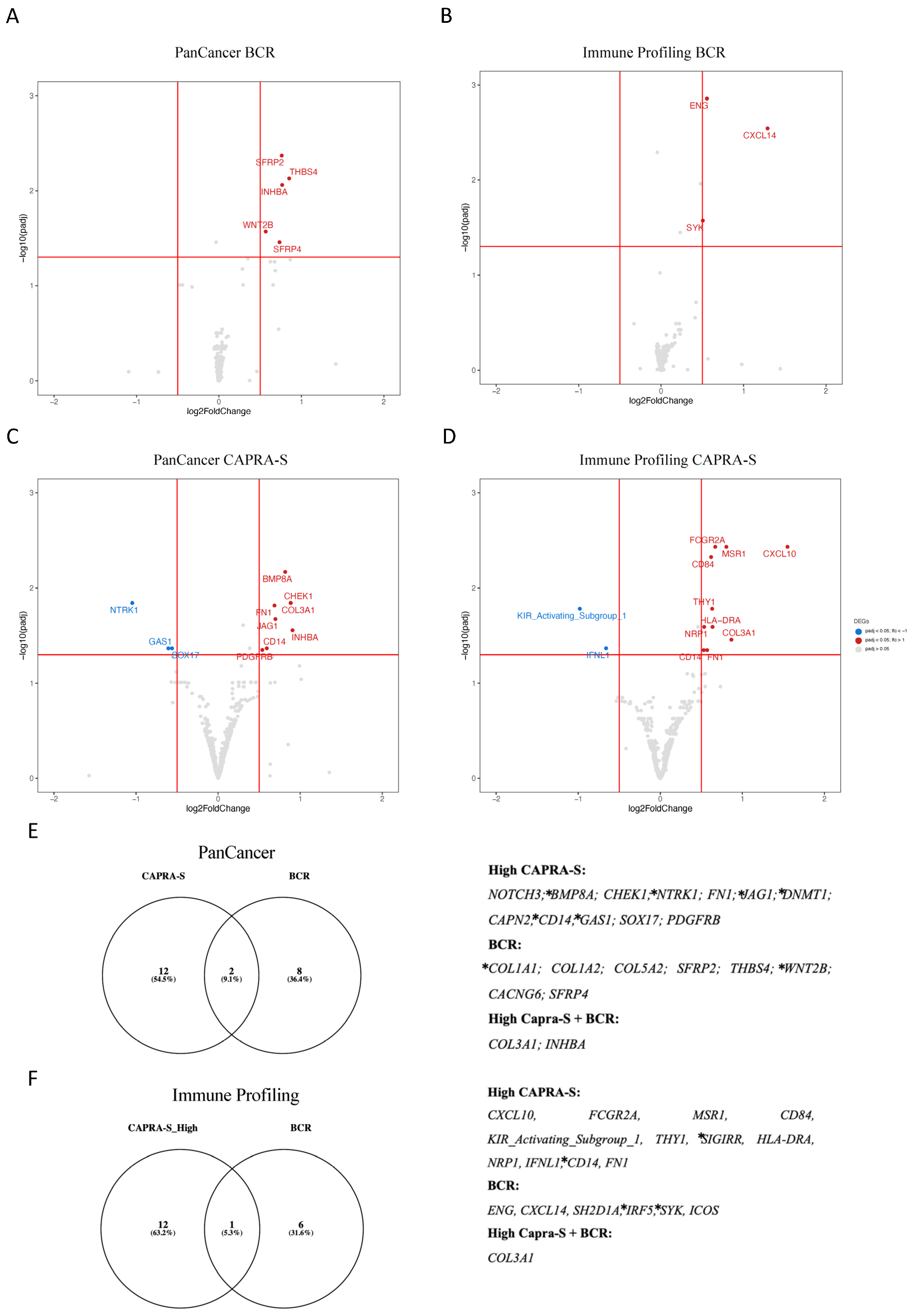
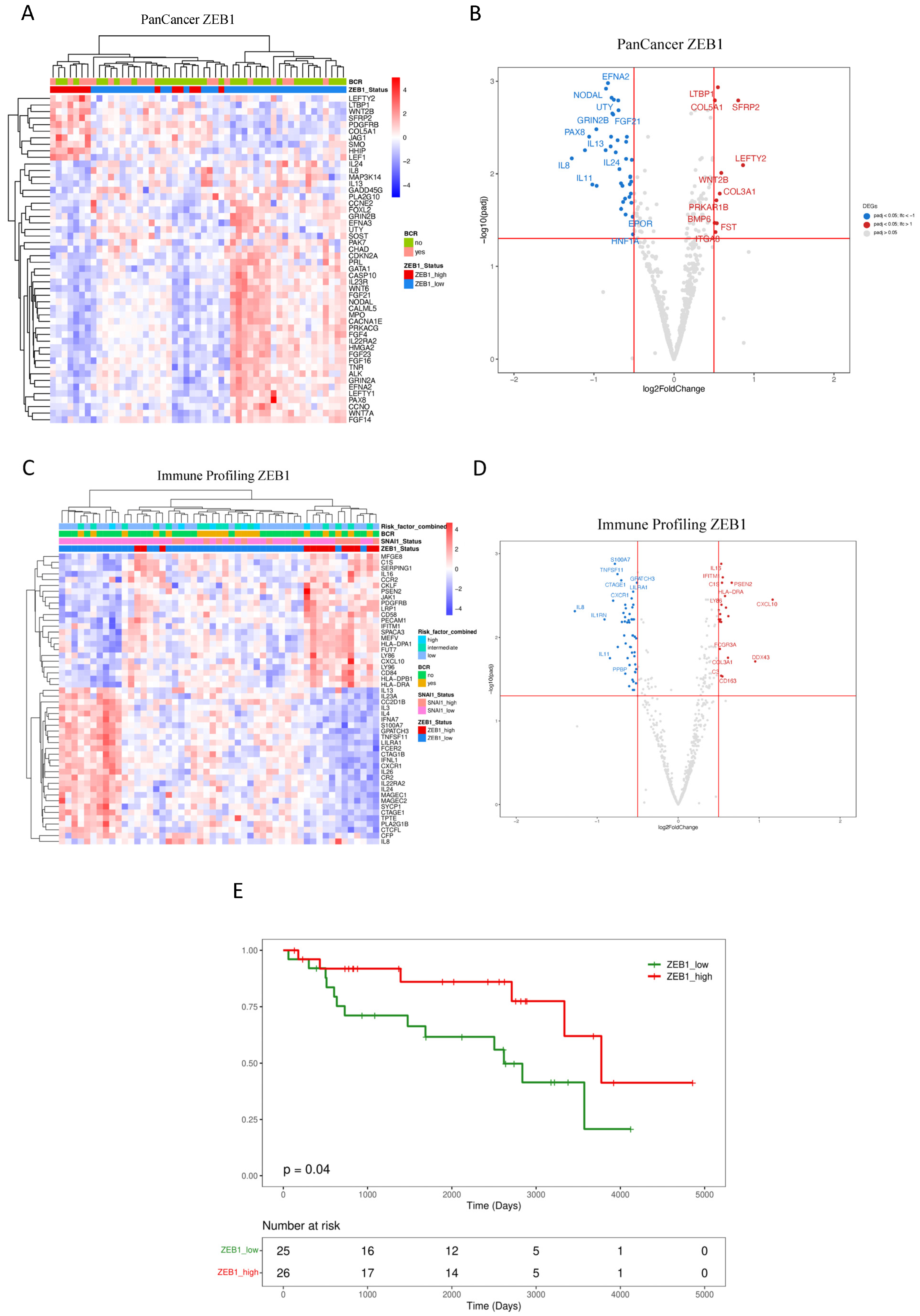
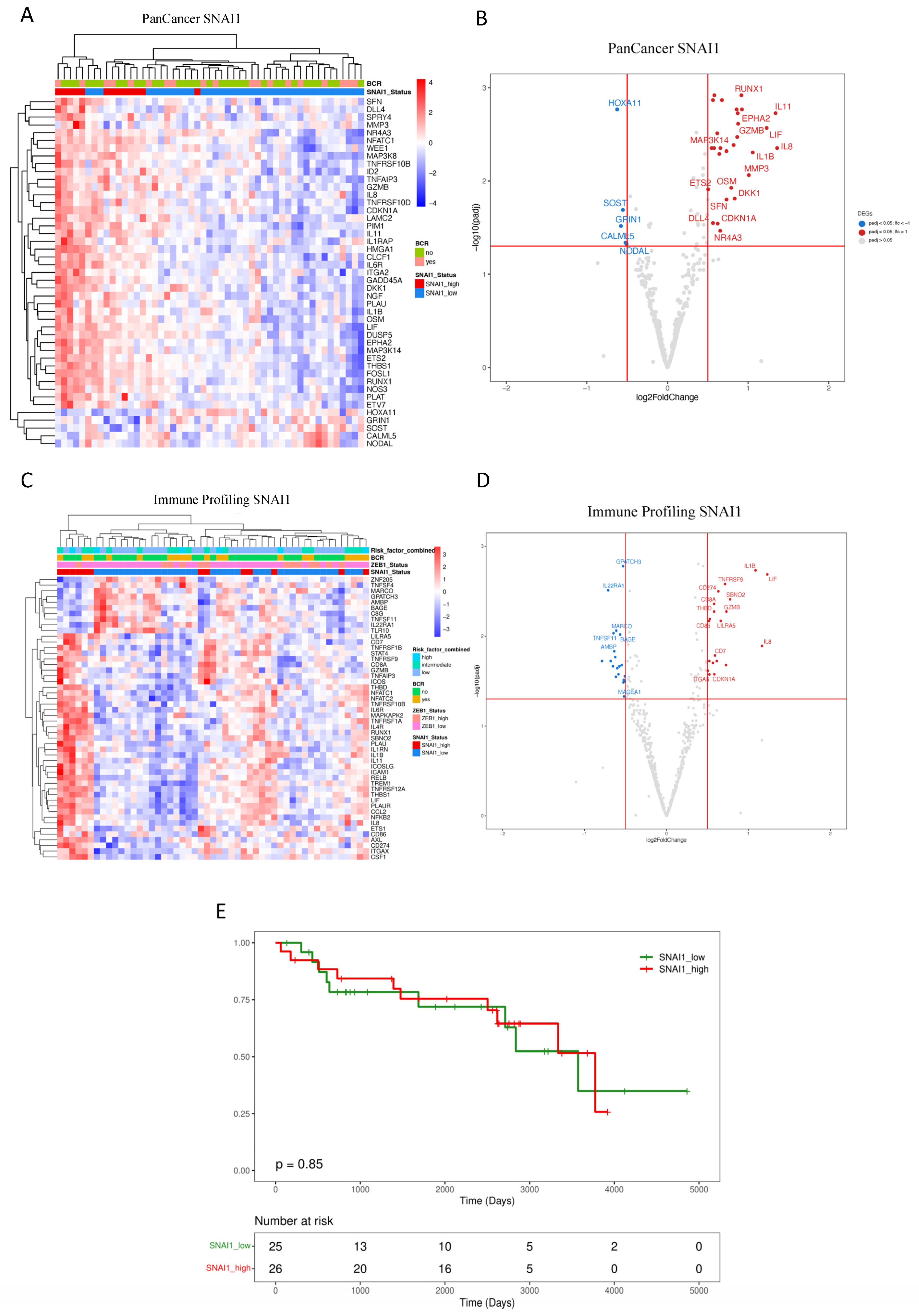
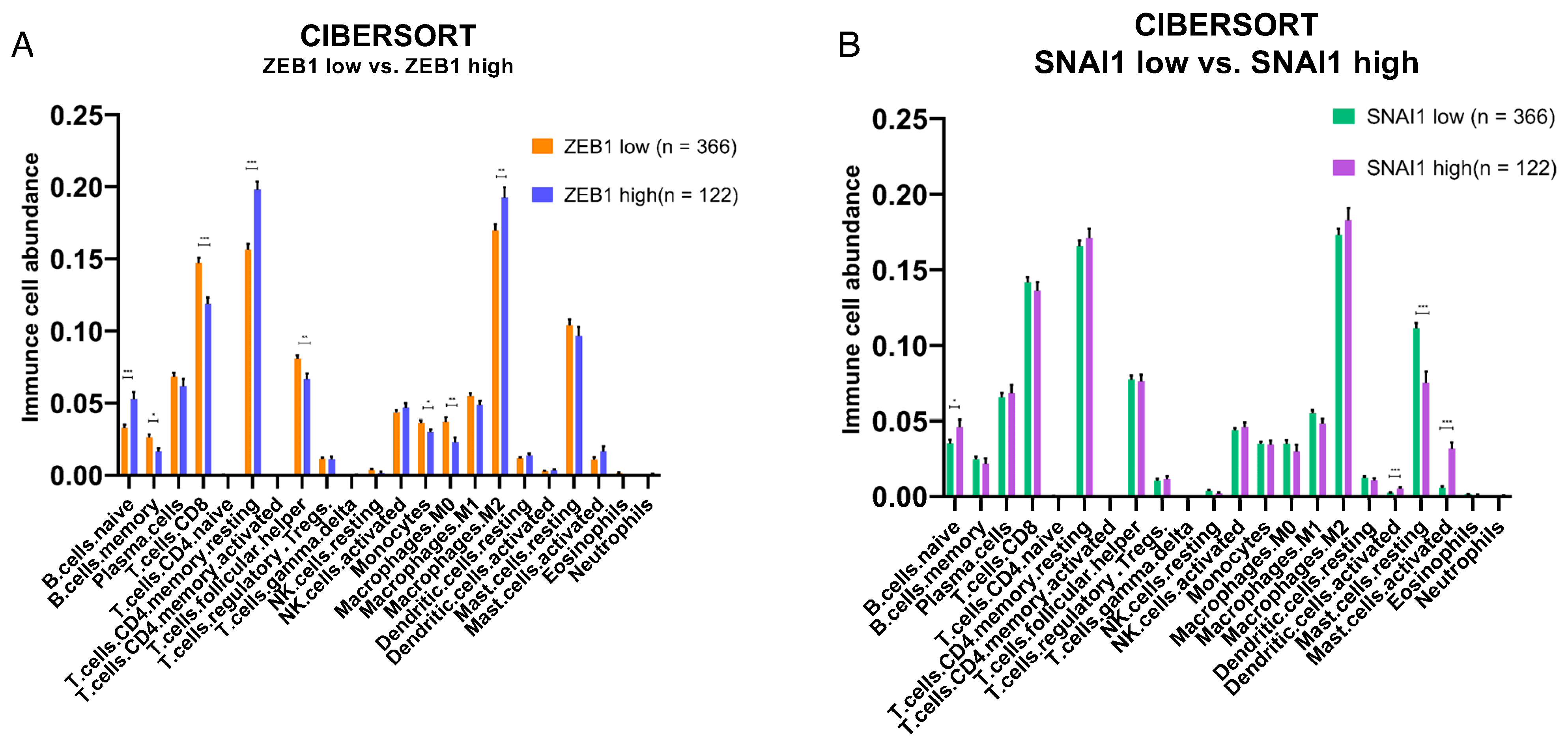
| Gene | Log2 FC | padj | Protein | Role in Progression and Biology of PCa | Citations | |
|---|---|---|---|---|---|---|
| BCR | COL1A1 | 0.876 | >0.001 | collagen type I alpha 1 chain | Collagens contribute to the ECM, which are the major structural components of the TME. COL1A1, COL1A2, and COL3A1 expression in CAFs have been associated with the EMT. COL1A1 expression is upregulated in PCa stromal cells and was associated with a worse prognosis in PCa. | [38,39] |
| COL3A1 | 0.879 | >0.001 | collagen type III alpha 1 chain | COL3A1 interacts with fibronectin. Increased expression of COL3A1 in PCa activates other pro-tumorigenic genes and pathways, such as the Wnt/beta-catenin. COL3A1 expression is associated with higher Gleason scores, higher PSA levels, and a higher likelihood of lymph node involvement. | [38] | |
| COL1A2 | 0.596 | >0.001 | collagen type I alpha 2 chain | COL1A2 expression is associated with higher Gleason scores. | [40] | |
| COL5A2 | 0.479 | >0.001 | collagen type V alpha 2 chain | COL5A2 expression has been associated with increased tumor cell invasion and resistance to androgen deprivation therapy. | [40] | |
| SFRP2 | 0.761 | 0.004 | secreted frizzled-related protein 2 | SFRP2 affects TME by regulating Wnt signaling and influencing tumor angiogenesis. | [41,42] | |
| THBS4 | 0.852 | 0.007 | thrombospondin 4 | THBS4 affects cancer stem cell-like properties in PCa by its regulation of the PI3K/Akt pathway. | [43] | |
| INHBA | 0.766 | 0.008 | inhibin beta A subunit | INHBA (Activin A) activates NF-κB and is associated with a higher Gleason score PCa. | [44,45] | |
| WNT2B | 0.567 | 0.02 | Wnt family member 2B | WNT2B is regulated by lncRNAs to influence the EMT in PCa. | [46] | |
| SFRP4 | 0.7349 | 0.03 | secreted frizzled-related protein 4 | SFRP4 predicts BCR in PCa and is associated with the EMT. | [47] | |
| CAPRA-S | NOTCH3 | 0.6882 | >0.001 | notch 3 | NOTCH1-4 expression was associated with disease progression, prognosis, and immune cell infiltration. | [48] |
| BMP8A | 0.8167 | 0.006 | bone morphogenetic protein 8a | BMPs are members of the TGF-beta family and are thought to be involved in PCa bone metastasis. | [49] | |
| CHEK1 | 0.8851 | 0.01 | checkpoint kinase 1 | CHEK1 (CHK1) is associated with DNA damage response and AR signaling. | [50] | |
| COL3A1 | 0.8822 | 0.01 | collagen type III alpha 1 chain | see above | ||
| NTRK1 | −1.045 | 0.01 | neurotrophic receptor tyrosine kinase 1 | NTRK1 downregulation is associated with reduced TILs in the TME of PCa and poor prognosis. | [51] | |
| FN1 | 0.686 | 0.01 | fibronectin 1 | FN1 is a key component of the ECM, and the TME is associated with collagens and CAFs. | [52] | |
| JAG1 | 0.697 | 0.02 | jagged 1 | JAG1 upregulation results in increased inflammatory foci in the TME of tumors in Pten-deficient mice. | [53] | |
| INHBA | 0.905 | 0.02 | inhibin beta A subunit | See above. | ||
| CD14 | 0.592 | 0.04 | CD14 molecule | CD14 is mostly expressed in macrophages. | -- | |
| GAS1 | −0.606 | 0.04 | growth arrest-specific 1 | GAS1RR (an immune-related enhancer RNA) represses GAS1 and is associated with BR-free survival in PCa. | [54] | |
| SOX17 | −0.561 | 0.04 | SRY-box 17 | SOX17 and Notch’s axis associated with enzalutamide resistance in CRPC models. | [55] |
| Gene | Log2 FC | padj | Protein | Role in Immune Oncology and PCa | Citations | |
|---|---|---|---|---|---|---|
| BCR | COL3A1 | 1.027 | >0.001 | collagen type III alpha 1 chain | See Table 1. | |
| ENG | 0.556 | 0.001 | endoglin | Endoglin (sCD105) in plasma associated with aggressive PCa. | [56] | |
| CXCL14 | 1.291 | 0.002 | C-X-C motif chemokine ligand 14 | CXAL14 expression associated with outcome in PCa. | [57] | |
| SH2D1A | −0.045 | 0.005 | SH2 domain containing 1A | Stimulation factor for T and B cells. | -- | |
| IRF5 | 0.478 | 0.01 | interferon regulatory factor 5 | IRF5 expression used for BCR prediction in PCa. | [58] | |
| SYK | 0.506 | 0.02 | spleen associated tyrosine kinase | Associated with metastatic PCa. | [59] | |
| ICOS | 0.231 | 0.03 | inducible T cell costimulator | ICOS + Treg cells exert immunosuppressive effects. | -- | |
| CAPRA-S | CXCL10 | 1.549 | 0.003 | C-X-C motif chemokine ligand 10 | CXCL10 co-expression with CXCR3 is a predictor of metastatic recurrence. | [60] |
| FCGR2A | 0.669 | 0.003 | Fc fragment of IgG receptor IIa | Expressed in macrophages, neutrophils, and other immune cells. | -- | |
| MSR1 | 0.804 | 0.003 | macrophage scavenger receptor 1 | Helpful as an additional diagnostic biomarker for PCa. | [61] | |
| CD84 | 0.618 | 0.004 | CD84 molecule | Expressed in numerous immune cell types. | -- | |
| KIR Activating Subgroup 1 | −0.98 | 0.01 | killer cell immunoglobulin-like receptors | KIRs expressed in NK and T cells. | [62] | |
| THY1 | 0.631 | 0.01 | Thy-1 cell surface antigen | THY1 overexpressed in PCa-associated fibroblasts. | [63] | |
| SIGIRR | 0.459 | 0.02 | single Ig and TIR domain containing | TLR4 and IL-1R-mediated NF-kB activation associated with BCR. | [64] | |
| HLA-DRA | 0.636 | 0.02 | major histocompatibility complex, class II, DR alpha | Antigen presentation in the TME. | [65] | |
| NRP1 | 0.534 | 0.02 | neuropilin 1 | Androgen-repressed gene upregulated by ADT in advanced PCa. | [66] | |
| COL3A1 | 0.865 | 0.03 | collagen type III alpha 1 chain | See Table 1. | ||
| IFNL1 | −0.66 | 0.04 | interferon lambda 1 | Interferon lambda 1 is involved in antiviral immune defense. | -- | |
| CD14 | 0.528 | 0.04 | CD14 molecule | CD14 is mostly expressed in macrophages. | -- | |
| FN1 | 0.570 | 0.04 | fibronectin 1 | See Table 1. |
| Term | Adjusted p-Value | Genes |
|---|---|---|
| Immune Profiling | ||
| TNF-alpha Signaling via NF-kB | >0.001 | EGR2; CDKN1A; CSF1; CD80; TNFRSF9; LIF; PLAUR; TNFAIP3; NFKB1; ICAM1; NFKB2; RELB; NFKBIA; BCL6; PLAU; IL1B; REL; CCL2; ICOSLG; CD44 |
| Inflammatory Response | >0.001 | CDKN1A; IL4R; CSF1; TNFRSF9; IL10RA; LIF; PLAUR; ICAM4; TNFRSF1B; NFKB1; ICAM1; NFKBIA; MARCO; IRAK2; AXL; IL1B; IRF7; CCL2; ITGA5; ICOSLG |
| Allograft Rejection | >0.001 | CD86; CCR1; IL11; IL4R; CSF1; CD80; LIF; GZMB; ETS1; ICAM1; NCR1; CD8A; IL1B; IL9; CD7; IRF7; STAT4; CCL2; IL12A; ICOSLG |
| Interferon-Gamma Response | >0.001 | CD86; CD274; CDKN1A; IL4R; VCAM1; IL10RA; TNFAIP3; NFKB1; ICAM1; NFKBIA; IRF7; STAT4; TXNIP; CCL2 |
| IL-2/STAT5 Signaling | >0.001 | CD86; IL4R; CSF1; TNFRSF9; IL10RA; LIF; ITGAE; TNFRSF1B; MAPKAPK2; TNFSF11; CTLA4; ICOS; CD44 |
| IL-6/JAK/STAT3 Signaling | >0.001 | CCR1; IL4R; CSF1; TNFRSF12A; IL1B; TNFRSF1B; CD44; TNFRSF1A |
| KRAS Signaling Up | >0.001 | PLAU; ITGA2; IL1B; IL10RA; LIF; PLAUR; TNFAIP3; TNFRSF1B; ETS1 |
| Coagulation | >0.001 | THBD; C8G; PLAU; ITGA2; C8A; THBS1; SH2B2 |
| Epithelial–Mesenchymal Transition | >0.001 | VCAM1; TNFRSF12A; ITGA2; PLAUR; TNFAIP3; ITGA5; THBS1; CD44 |
| Apical Junction | >0.001 | CD86; CD274; VCAM1; ITGA2; ICAM4; CD34; ICAM1 |
| Apoptosis | 0.005 | CDKN1A; TNFRSF12A; IL1B; TXNIP; CD44 |
| Interferon-Alpha Response | 0.005 | IL4R; CSF1; IRF7; TXNIP |
| PanCancer | ||
| TNF-alpha Signaling via NF-kB | >0.001 | DUSP5; CDKN1A; GADD45A; LIF; TNFAIP3; ETS2; FOSL1; NR4A3; PLAU; CLCF1; ID2; IL1B; MAP3K8 |
| Epithelial–Mesenchymal Transition | >0.001 | GADD45A; ITGA2; ID2; MMP3; TNFAIP3; LAMC2; THBS1; DKK1 |
| KRAS Signaling Up | >0.001 | PLAU; ITGA2; ID2; IL1B; LIF; TNFAIP3; PLAT; NGF |
| Coagulation | >0.001 | PLAU; ITGA2; MMP3; PLAT; THBS1 |
| Apoptosis | >0.001 | CDKN1A; WEE1; GADD45A; IL1B; PLAT |
| Complement | >0.001 | DUSP5; PIM1; TNFAIP3; GZMB; PLAT |
| p53 Pathway | >0.001 | CDKN1A; GADD45A; LIF; SFN; EPHA2 |
| IL-6/JAK/STAT3 Signaling | 0.002 | IL1B; PIM1; MAP3K8 |
| IL-2/STAT5 Signaling | 0.002 | SPRY4; PIM1; LIF; MAP3K8 |
| Inflammatory Response | 0.002 | CDKN1A; IL1B; LIF; OSM |
| Allograft Rejection | 0.002 | IL11; IL1B; LIF; GZMB |
| PI3K/AKT/mTOR Signaling | 0.003 | CDKN1A; SFN; NGF |
| TGF-beta Signaling | 0.01 | ID2; THBS1 |
| Hypoxia | 0.01 | CDKN1A; PIM1; TNFAIP3 |
| Estrogen Response Late | 0.01 | ID2; LAMC2; SFN |
| Interferon-Gamma Response | 0.01 | CDKN1A; PIM1; TNFAIP3 |
| E2F Targets | 0.01 | CDKN1A; WEE1; HMGA1 |
| Xenobiotic Metabolism | 0.01 | ID2; ETS2; EPHA2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lautert-Dutra, W.; Melo, C.M.; Chaves, L.P.; Sousa, F.C.; Crozier, C.; Dion, D.; Avante, F.S.; Saggioro, F.P.; dos Reis, R.B.; Archangelo, L.F.; et al. Investigating the Role of SNAI1 and ZEB1 Expression in Prostate Cancer Progression and Immune Modulation of the Tumor Microenvironment. Cancers 2024, 16, 1480. https://doi.org/10.3390/cancers16081480
Lautert-Dutra W, Melo CM, Chaves LP, Sousa FC, Crozier C, Dion D, Avante FS, Saggioro FP, dos Reis RB, Archangelo LF, et al. Investigating the Role of SNAI1 and ZEB1 Expression in Prostate Cancer Progression and Immune Modulation of the Tumor Microenvironment. Cancers. 2024; 16(8):1480. https://doi.org/10.3390/cancers16081480
Chicago/Turabian StyleLautert-Dutra, William, Camila Morais Melo, Luiz Paulo Chaves, Francisco Cesar Sousa, Cheryl Crozier, Dan Dion, Filipe S. Avante, Fabiano Pinto Saggioro, Rodolfo Borges dos Reis, Leticia Fröhlich Archangelo, and et al. 2024. "Investigating the Role of SNAI1 and ZEB1 Expression in Prostate Cancer Progression and Immune Modulation of the Tumor Microenvironment" Cancers 16, no. 8: 1480. https://doi.org/10.3390/cancers16081480





