Multiparametric Characterization of the DSL-6A/C1 Pancreatic Cancer Model in Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Line and Culture
2.3. Tumor Induction and Growth Measurement
2.4. Euthanasia, Tissue Collection, and Histology
2.5. Magnetic Resonance Imaging
2.6. MRI Data Image Processing and Analysis
2.7. Synthesis and Quality Control of 68Ga-FAPI
2.8. Positron Emission Tomography
2.9. Isolation of Tumor-Infiltrating Lymphocytes from Rat Tissue and Flow Cytometry
3. Results
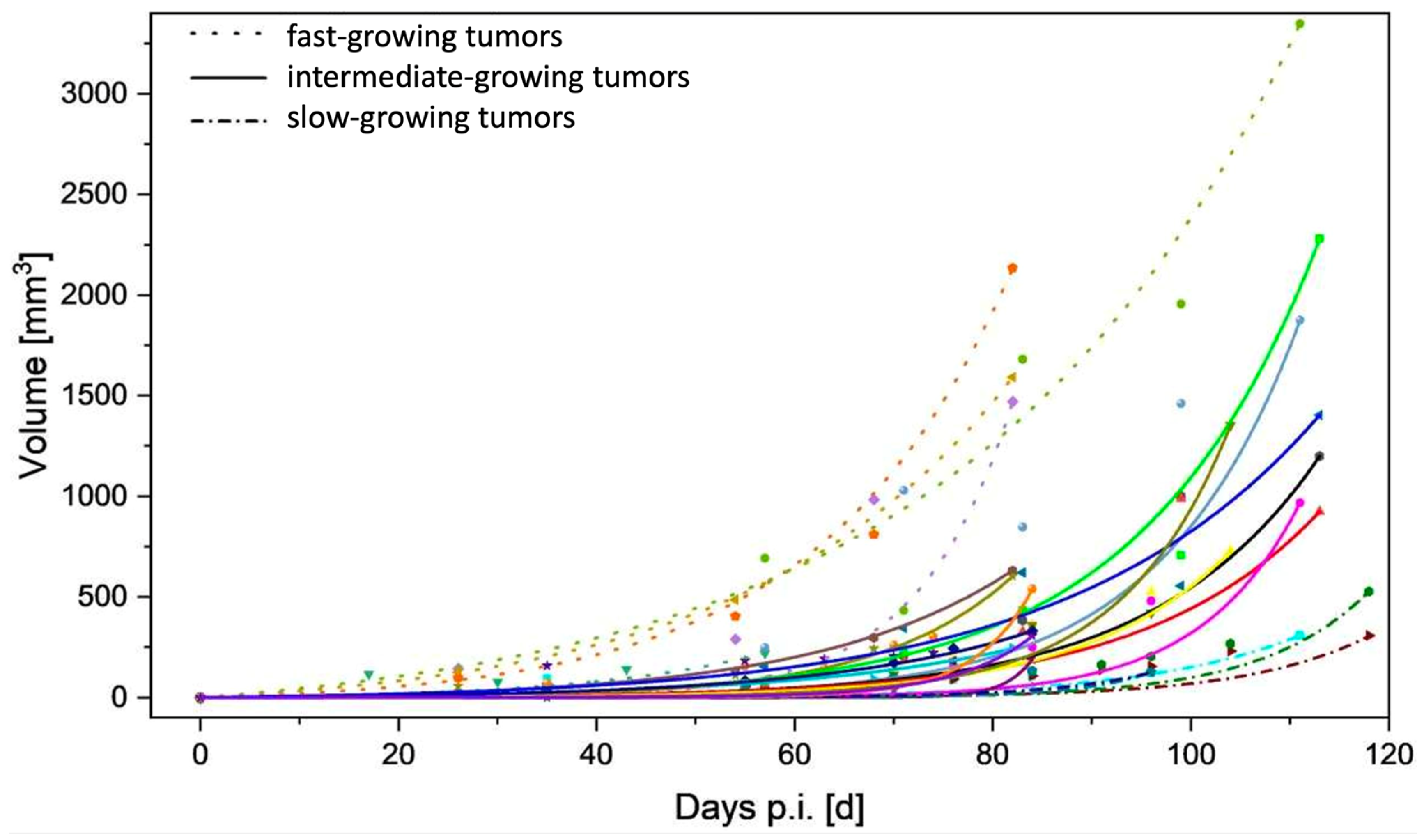
3.1. Tumor Take-up Rate and Growth
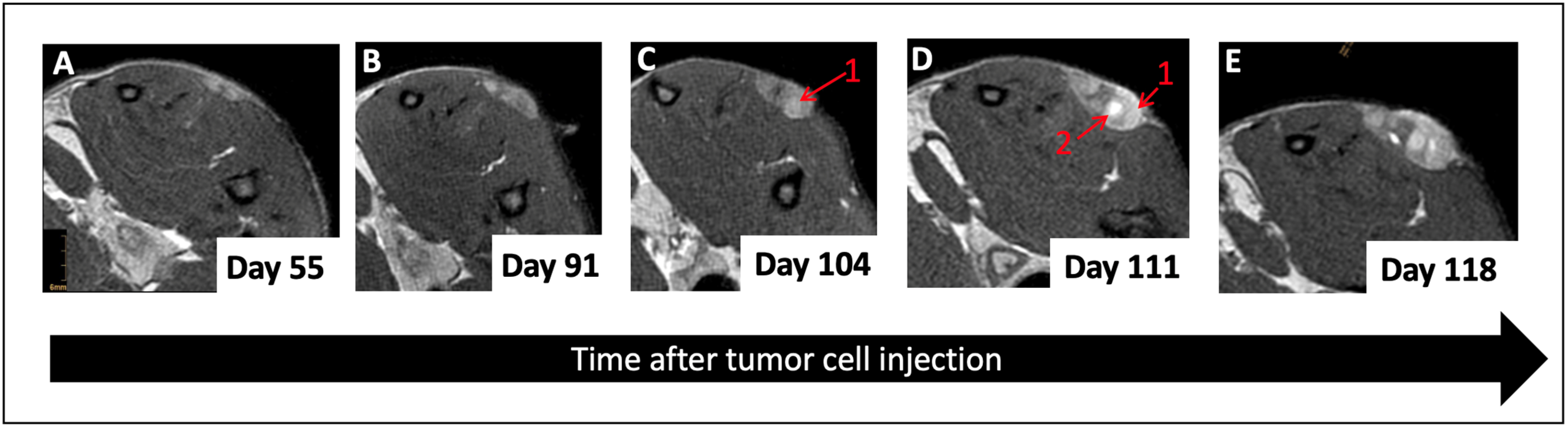
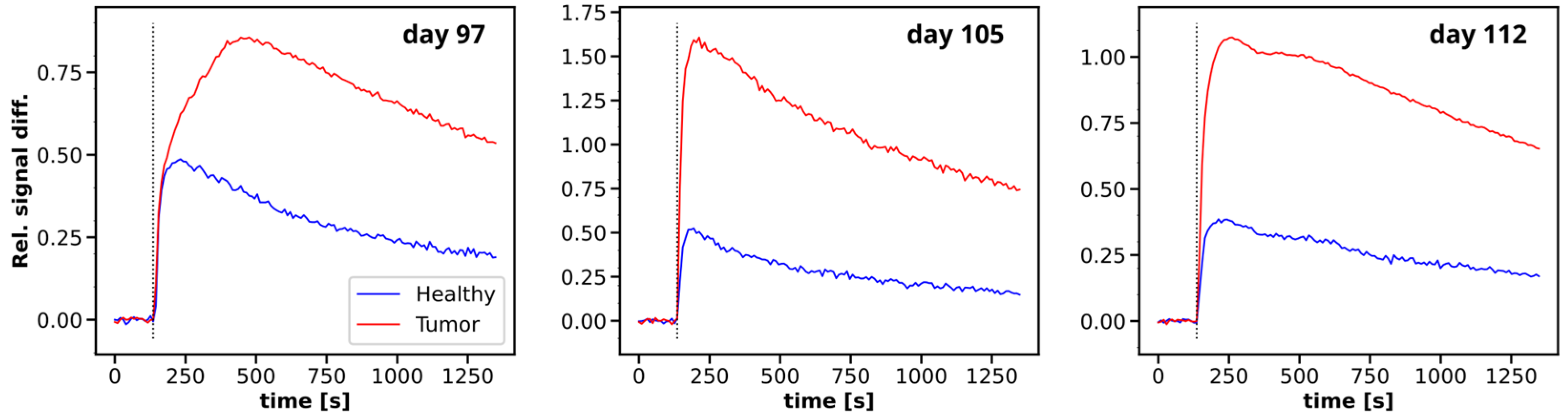
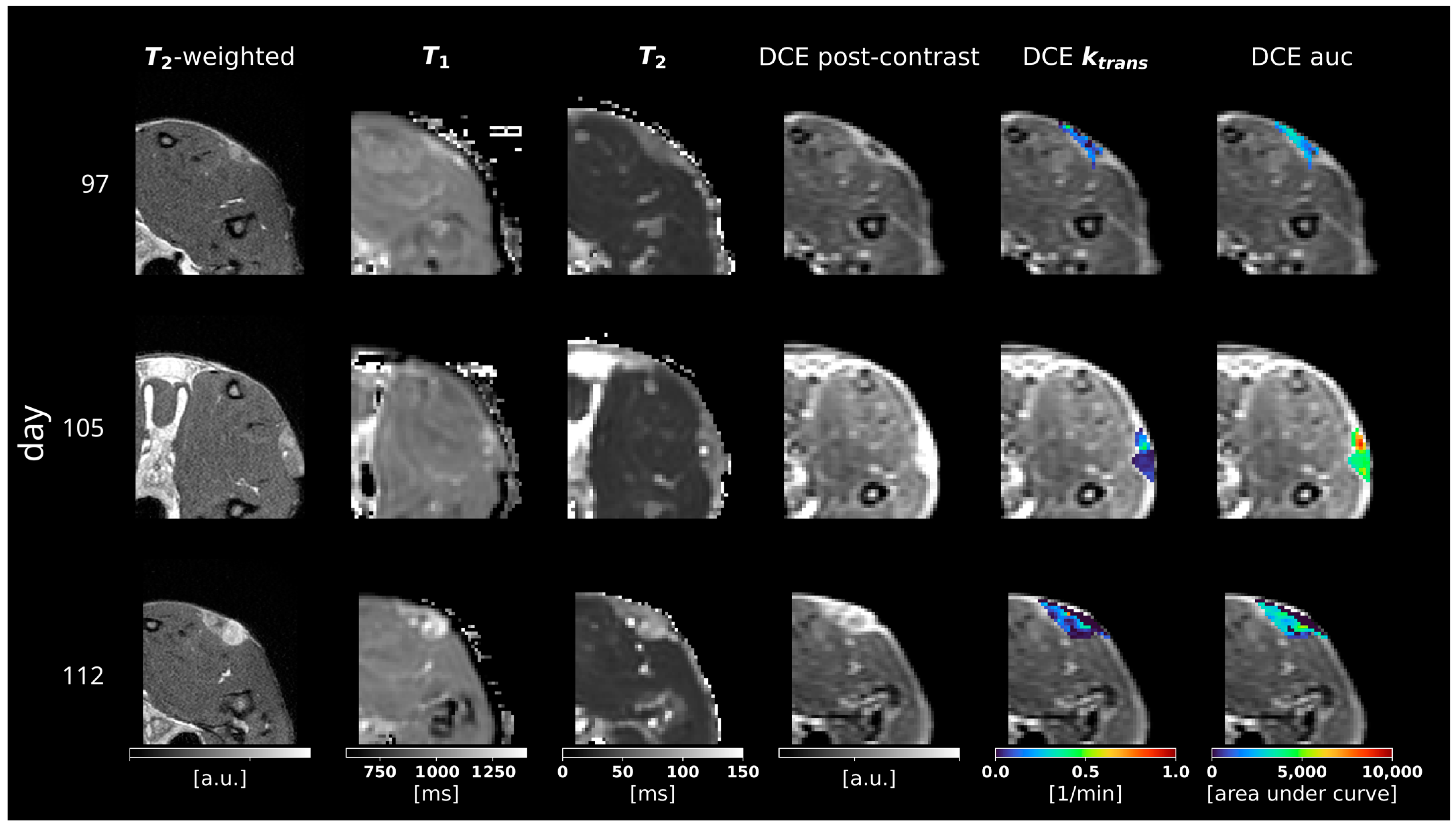
3.2. Multiparametric MRI
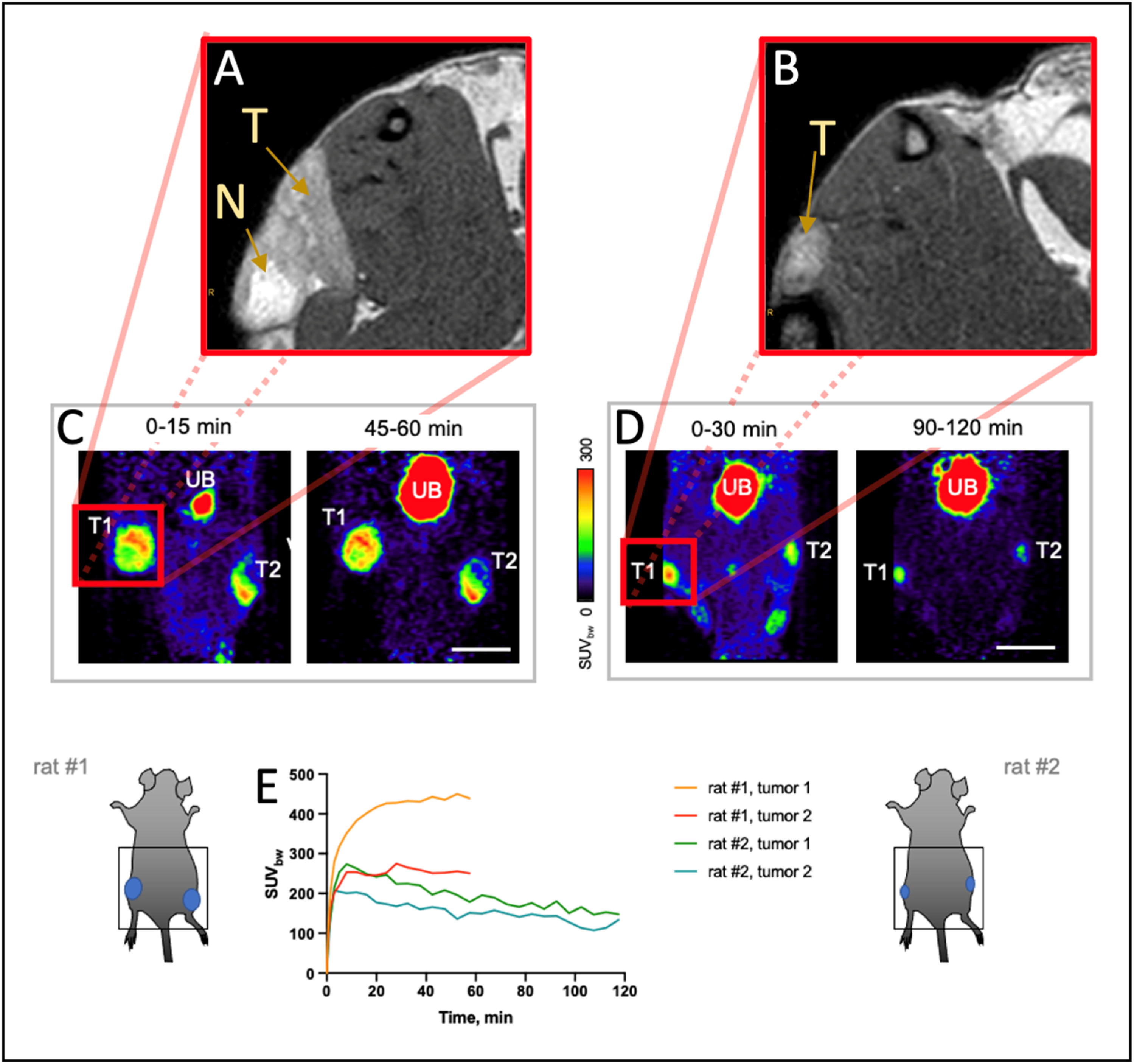
3.3. 68Ga-FAPI-46 PET Imaging
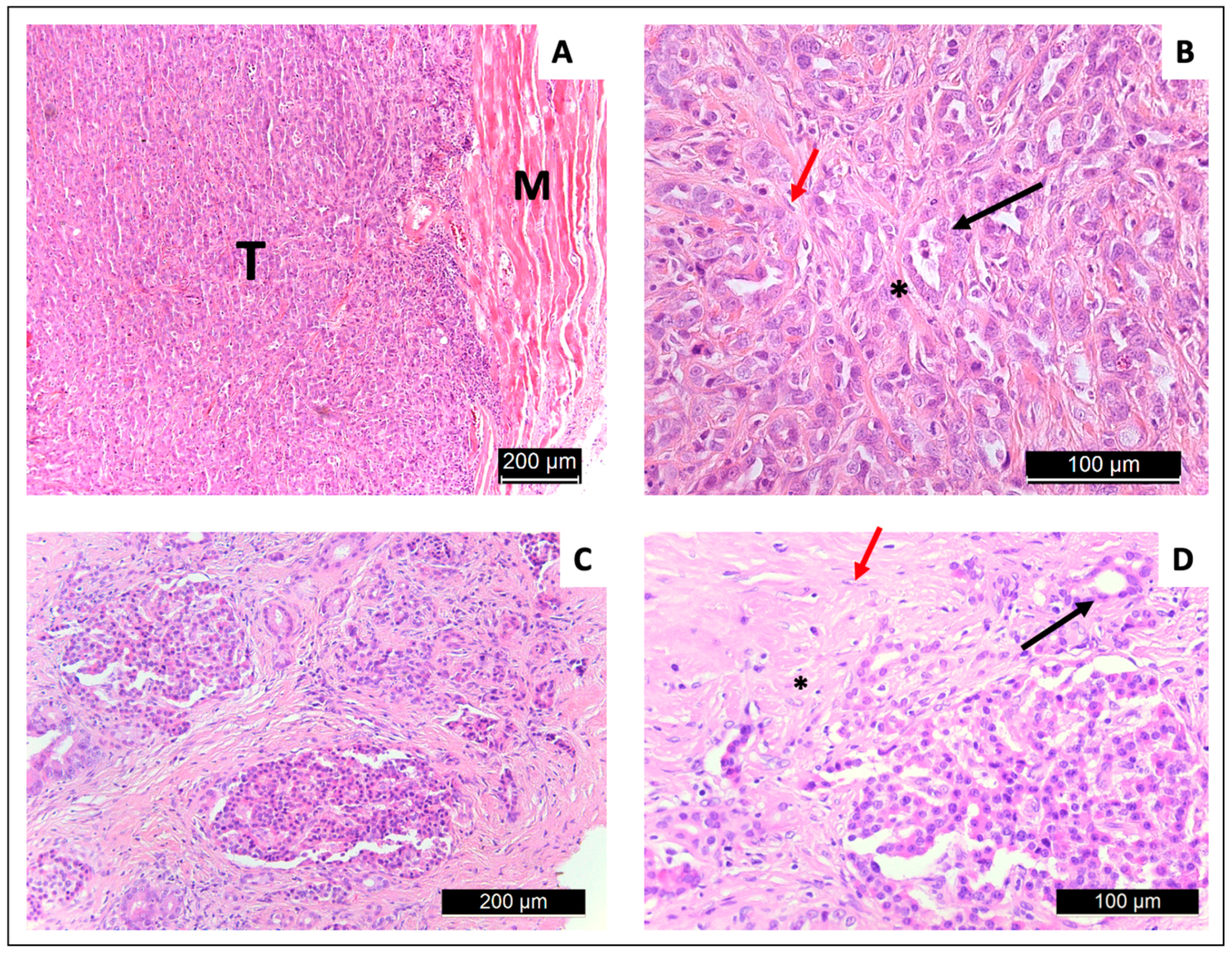
3.4. Tissue Extraction and Histology
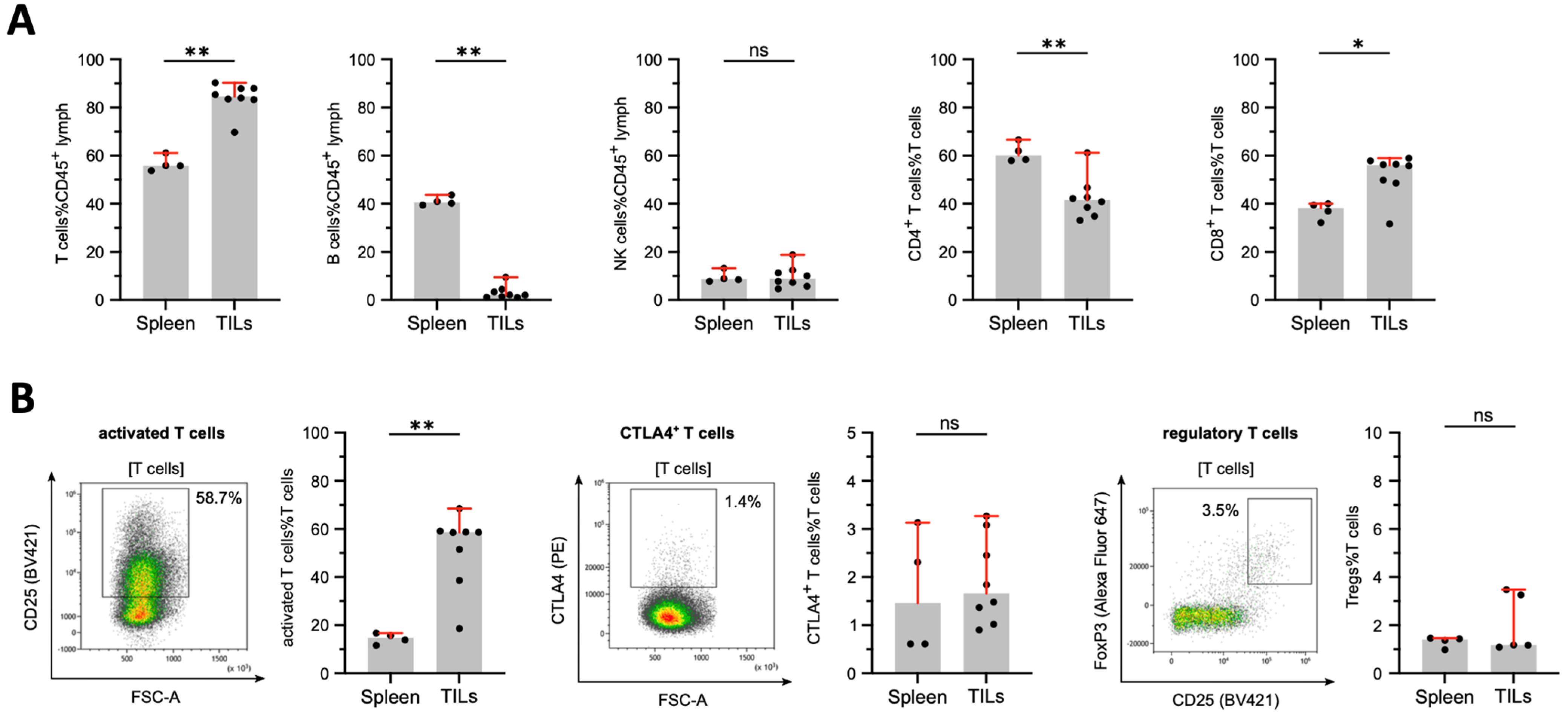
3.5. Tumor-Infiltrating Lymphocytes in DSL-6A/C1 Pancreatic Ductal Adenocarcinoma Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Antibody | Fluorochrome | Clone | Host | Company | Catalogue |
|---|---|---|---|---|---|
| Zombie Aqua | Biolegend | 423102 | |||
| CD38 | FITC | REA683 | Human cell line | Miltenyi | 130-110-277 |
| CD3 | FITC | REA223 | Human cell line | Miltenyi | 130-102-678 |
| IgG | PerCp Cy5.5 | Poly4054 | Goat | Biolegend | 405424 |
| CD8a | PerCP-Vio 700 | REA437 | Human cell line | Miltenyi | 130-132-092 |
| CD54 | PE | 1A29 | Mouse | Biolegend | 202405 |
| CD11a | PE | REA596 | Human cell line | Miltenyi | 130-109-171 |
| CD152 (CTLA4) | PE | WKH203 | Mouse | Biolegend | 203007 |
| CD27 | PE Dazzle 594 | LG.3A10 | Armenian Hamster | Biolegend | 124228 |
| CD45RA | PE-Cy7 | OX-33 | Mouse | Biolegend | 202316 |
| CD161 | PE-Vio 770 | REA227 | Human cell line | Miltenyi | 130-102-714 |
| CD86 | APC | 24F | Mouse | Miltenyi | 130-109-130 |
| CD314 (NKG2D) | APC | REA471 | Human cell line | Miltenyi | 130-106-992 |
| FoxP3 | Alexa Fluor 647 | 150D | Mouse | Biolegend | 320014 |
| CD45 | Alexa Fluor 700 | OX-1 | Mouse | Biolegend | 202218 |
| CD4 | APC Cy7 | W3/25 | Mouse | Biolegend | 201518 |
| CD80 | BV421 | 3H5 | Mouse | BD | 743863 |
| CD25 | BV421 | OX-39 | Mouse | BD | 565608 |
| CD134 (OX40) | BV421 | OX-40 | Mouse | BD | 744815 |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Coleman, M.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J.; et al. Cancer Survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An Analysis of Population-Based Cancer Registry Data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in Diagnosis of Pancreatic Cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic Cancer Stroma: An Update on Therapeutic Targeting Strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Schima, W.; Böhm, G.; Rösch, C.S.; Klaus, A.; Függer, R.; Kopf, H. Mass-Forming Pancreatitis versus Pancreatic Ductal Adenocarcinoma: CT and MR Imaging for Differentiation. Cancer Imaging 2020, 20, 52. [Google Scholar] [CrossRef]
- Klapman, J.; Malafa, M.P. Early Detection of Pancreatic Cancer: Why, Who, and How to Screen. Cancer Control 2008, 15, 280–287. [Google Scholar] [CrossRef]
- Bronstein, Y.L.; Loyer, E.M.; Kaur, H.; Choi, H.; David, C.; DuBrow, R.A.; Broemeling, L.D.; Cleary, K.R.; Charnsangavej, C. Detection of Small Pancreatic Tumors with Multiphasic Helical CT. Am. J. Roentgenol. 2004, 182, 619–623. [Google Scholar] [CrossRef]
- Prokesch, R.W.; Chow, L.C.; Beaulieu, C.F.; Bammer, R.; Jr, R.B.J. Isoattenuating Pancreatic Adenocarcinoma at Multi–Detector Row CT: Secondary Signs. Radiology 2002, 224, 764–768. [Google Scholar] [CrossRef]
- Kayal, N.E.; Lennartz, S.; Ekdawi, S.; Holz, J.; Slebocki, K.; Haneder, S.; Wybranski, C.; Mohallel, A.; Eid, M.; Grüll, H.; et al. Value of Spectral Detector Computed Tomography for Assessment of Pancreatic Lesions. Eur. J. Radiol. 2019, 118, 215–222. [Google Scholar] [CrossRef]
- Beall, P.T.; Asch, B.B.; Chang, D.C.; Medina, D.; Hazlewood, C.F. Distinction of Normal, Preneoplastic, and Neoplastic Mouse Mammary Primary Cell Cultures by Water Nuclear Magnetic Resonance Relaxation Times. J. Natl. Cancer Inst. 1980, 64, 335–338. [Google Scholar] [CrossRef]
- Gambarota, G.; Veltien, A.; van Laarhoven, H.; Philippens, M.; Jonker, A.; Mook, O.R.; Frederiks, W.M.; Heerschap, A. Measurements of T1 and T2 Relaxation Times of Colon Cancer Metastases in Rat Liver at 7 T. Magn. Reson. Mater. Phys. Biol. Med. 2004, 17, 281–287. [Google Scholar] [CrossRef]
- Ravoori, M.K.; Nishimura, M.; Singh, S.P.; Lu, C.; Han, L.; Hobbs, B.P.; Pradeep, S.; Choi, H.J.; Bankson, J.A.; Sood, A.K.; et al. Tumor T1 Relaxation Time for Assessing Response to Bevacizumab Anti-Angiogenic Therapy in a Mouse Ovarian Cancer Model. PLoS ONE 2015, 10, e0131095. [Google Scholar] [CrossRef]
- Muraoka, N.; Uematsu, H.; Kimura, H.; Imamura, Y.; Fujiwara, Y.; Murakami, M.; Yamaguchi, A.; Itoh, H. Apparent Diffusion Coefficient in Pancreatic Cancer: Characterization and Histopathological Correlations. J. Magn. Reson. Imaging 2008, 27, 1302–1308. [Google Scholar] [CrossRef]
- Karaosmanoglu, A.D.; Onur, M.R.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Magnetic Resonance Imaging of Liver Metastasis. Semin. Ultrasound Ct Mri 2016, 37, 533–548. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Jia, G.; Shah, Z.K.; Pohar, K.; Mortazavi, A.; Zynger, D.L.; Wei, L.; Yang, X.; Clark, D.; Knopp, M.V. Prediction of Chemotherapeutic Response in Bladder Cancer Using K-means Clustering of Dynamic Contrast-enhanced (DCE)-MRI Pharmacokinetic Parameters. J. Magn. Reson. Imaging 2015, 41, 1374–1382. [Google Scholar] [CrossRef]
- Hectors, S.J.C.G.; Jacobs, I.; Moonen, C.T.W.; Strijkers, G.J.; Nicolay, K. MRI Methods for the Evaluation of High Intensity Focused Ultrasound Tumor Treatment: Current Status and Future Needs. Magn. Reson. Med. 2016, 75, 302–317. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and Therapeutic Relevance of Cancer-Associated Fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Röhrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.L.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of 68Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2021, 62, 779–786. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, G.; Pan, G.; Cao, K.; Yang, Q.; Meng, H.; Yang, J.; Zhang, L.; Wang, T.; Cheng, C.; et al. Comparison of the Diagnostic Efficacy of 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT in Patients with Pancreatic Cancer. Eur. J. Nucl. Med. Mol. Iamging 2022, 49, 2877–2888. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D Cooperate to Promote Chromosomal Instability and Widely Metastatic Pancreatic Ductal Adenocarcinoma in Mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef]
- Pham, T.N.D.; Shields, M.A.; Spaulding, C.; Principe, D.R.; Li, B.; Underwood, P.W.; Trevino, J.G.; Bentrem, D.J.; Munshi, H.G. Preclinical Models of Pancreatic Ductal Adenocarcinoma and Their Utility in Immunotherapy Studies. Cancers 2021, 13, 440. [Google Scholar] [CrossRef]
- Lee, J.W.; Komar, C.A.; Bengsch, F.; Graham, K.; Beatty, G.L. Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery. Curr. Protoc. Pharmacol. 2016, 73, 14.39.1–14.39.20. [Google Scholar] [CrossRef]
- Suklabaidya, S.; Dash, P.; Das, B.; Suresh, V.; Sasmal, P.K.; Senapati, S. Experimental Models of Pancreatic Cancer Desmoplasia. Lab. Investig. 2018, 98, 27–40. [Google Scholar] [CrossRef]
- Rubio-Viqueira, B.; Jimeno, A.; Cusatis, G.; Zhang, X.; Iacobuzio-Donahue, C.; Karikari, C.; Shi, C.; Danenberg, K.; Danenberg, P.V.; Kuramochi, H.; et al. An In Vivo Platform for Translational Drug Development in Pancreatic Cancer. Clin. Cancer Res. 2006, 12, 4652–4661. [Google Scholar] [CrossRef]
- Garrido-Laguna, I.; Uson, M.; Rajeshkumar, N.V.; Tan, A.C.; de Oliveira, E.; Karikari, C.; Villaroel, M.C.; Salomon, A.; Taylor, G.; Sharma, R.; et al. Tumor Engraftment in Nude Mice and Enrichment in Stroma- Related Gene Pathways Predict Poor Survival and Resistance to Gemcitabine in Patients with Pancreatic Cancer. Clin. Cancer Res. 2011, 17, 5793–5800. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Partecke, L.I.; Sendler, M.; Kaeding, A.; Weiss, F.U.; Mayerle, J.; Dummer, A.; Nguyen, T.D.; Albers, N.; Speerforck, S.; Lerch, M.M.; et al. A Syngeneic Orthotopic Murine Model of Pancreatic Adenocarcinoma in the C57/BL6 Mouse Using the Panc02 and 6606PDA Cell Lines. Eur. Surg. Res. 2011, 47, 98–107. [Google Scholar] [CrossRef]
- Pour, P.; Wallcave, L.; Gingell, R.; Nagel, D.; Lawson, T.; Salmasi, S.; Tines, S. Carcinogenic Effect of N-Nitroso(2-Hydroxypropyl)(2-Oxopropyl)Amine, a Postulated Proximate Pancreatic Carcinogen in Syrian Hamsters. Cancer Res. 1979, 39, 3828–3833. [Google Scholar]
- Pettengill, O.S.; Faris, R.A.; Bell, R.H.; Kuhlmann, E.T.; Longnecker, D.S. Derivation of Ductlike Cell Lines from a Transplantable Acinar Cell Carcinoma of the Rat Pancreas. Am. J. Pathol. 1993, 143, 292–303. [Google Scholar]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Look, D.C.; Locker, D.R. Time Saving in Measurement of NMR and EPR Relaxation Times. Rev. Sci. Instrum. 1970, 41, 250–251. [Google Scholar] [CrossRef]
- Deichmann, R.; Haase, A. Quantification of T1 Values by SNAPSHOT-FLASH NMR Imaging. J. Magnetic Reson. (1969) 1992, 96, 608–612. [Google Scholar] [CrossRef]
- Barnes, S.R.; Ng, T.S.C.; Santa-Maria, N.; Montagne, A.; Zlokovic, B.V.; Jacobs, R.E. ROCKETSHIP: A Flexible and Modular Software Tool for the Planning, Processing and Analysis of Dynamic MRI Studies. BMC Med. Imaging 2015, 15, 19. [Google Scholar] [CrossRef]
- Mäkinen, K.; Loimas, S.; Kosma, V.-M.; Wahlfors, J.; Pettengill, O.S.; Longnecker, D.S.; Johansson, R.; Alhava, E. Azaserine-Induced Rat Pancreas Tumor Model with Transplantable Cultured Cells. Pancreas 1998, 16, 160–164. [Google Scholar] [CrossRef]
- Vohra, R.; Park, J.; Wang, Y.-N.; Gravelle, K.; Whang, S.; Hwang, J.-H.; Lee, D. Evaluation of Pancreatic Tumor Development in KPC Mice Using Multi-Parametric MRI. Cancer Imaging 2018, 18, 41. [Google Scholar] [CrossRef]
- Maloney, E.; Wang, Y.-N.; Vohra, R.; Son, H.; Whang, S.; Khokhlova, T.; Park, J.; Gravelle, K.; Totten, S.; Hwang, J.H.; et al. Magnetic Resonance Imaging Biomarkers for Pulsed Focused Ultrasound Treatment of Pancreatic Ductal Adenocarcinoma. World J. Gastroenterol. 2020, 26, 904–917. [Google Scholar] [CrossRef]
- Wegner, C.S.; Hauge, A.; Gaustad, J.-V.; Andersen, L.M.K.; Simonsen, T.G.; Galappathi, K.; Rofstad, E.K. Dynamic Contrast-Enhanced MRI of the Microenvironment of Pancreatic Adenocarcinoma Xenografts. Acta Oncol. 2017, 56, 1754–1762. [Google Scholar] [CrossRef]
- Cao, J.; Pickup, S.; Clendenin, C.; Blouw, B.; Choi, H.; Kang, D.; Rosen, M.; O’Dwyer, P.J.; Zhou, R. Dynamic Contrast-Enhanced MRI Detects Responses to Stroma-Directed Therapy in Mouse Models of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 2314–2322. [Google Scholar] [CrossRef]
- Kim, H.; Samuel, S.; Totenhagen, J.W.; Warren, M.; Sellers, J.C.; Buchsbaum, D.J. Dynamic Contrast Enhanced Magnetic Resonance Imaging of an Orthotopic Pancreatic Cancer Mouse Model. J. Vis. Exp. 2015, 98, e52641. [Google Scholar] [CrossRef]
- Foley, J.F.; Phadke, D.P.; Hardy, O.; Hardy, S.; Miller, V.; Madan, A.; Howard, K.; Kruse, K.; Lord, C.; Ramaiahgari, S.; et al. Whole Exome Sequencing in the Rat. BMC Genom. 2018, 19, 487. [Google Scholar] [CrossRef]
- Majumder, K.; Arora, N.; Modi, S.; Chugh, R.; Nomura, A.; Giri, B.; Dawra, R.; Ramakrishnan, S.; Banerjee, S.; Saluja, A.; et al. A Novel Immunocompetent Mouse Model of Pancreatic Cancer with Robust Stroma: A Valuable Tool for Preclinical Evaluation of New Therapies. J. Gastrointest. Surg. 2016, 20, 53–65. [Google Scholar] [CrossRef]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune Cell Infiltration as an Indicator of the Immune Microenvironment of Pancreatic Cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef]
- Goulart, M.R.; Stasinos, K.; Fincham, R.E.A.; Delvecchio, F.R.; Kocher, H.M. T Cells in Pancreatic Cancer Stroma. World J. Gastroenterol. 2021, 27, 7956–7968. [Google Scholar] [CrossRef]
- Foucher, E.D.; Ghigo, C.; Chouaib, S.; Galon, J.; Iovanna, J.; Olive, D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front. Immunol. 2018, 9, 1044. [Google Scholar] [CrossRef]
- Anandappa, A.J.; Wu, C.J.; Ott, P.A. Directing Traffic: How to Effectively Drive T Cells into Tumors. Cancer Discov. 2020, 10, 185–197. [Google Scholar] [CrossRef]
- Picard, F.S.R.; Lutz, V.; Brichkina, A.; Neuhaus, F.; Ruckenbrod, T.; Hupfer, A.; Raifer, H.; Klein, M.; Bopp, T.; Pfefferle, P.I.; et al. IL-17A-Producing CD8+ T Cells Promote PDAC via Induction of Inflammatory Cancer-Associated Fibroblasts. Gut 2023, 72, 1510–1522. [Google Scholar] [CrossRef]
- Muller, M.; Haghnejad, V.; Schaefer, M.; Gauchotte, G.; Caron, B.; Peyrin-Biroulet, L.; Bronowicki, J.-P.; Neuzillet, C.; Lopez, A. The Immune Landscape of Human Pancreatic Ductal Carcinoma: Key Players, Clinical Implications, and Challenges. Cancers 2022, 14, 995. [Google Scholar] [CrossRef]
- Hu, Z.I.; O’Reilly, E.M. Therapeutic Developments in Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 7–24. [Google Scholar] [CrossRef]
- Yin, T.; Peeters, R.; Feng, Y.; Liu, Y.; Yu, J.; Dymarkowski, S.; Himmelreich, U.; Oyen, R.; Ni, Y. Characterization of a Rat Orthotopic Pancreatic Head Tumor Model Using Three-dimensional and Quantitative Multi-parametric MRI. NMR Biomed. 2017, 30, e3676. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, L.; Li, W.; Gordon, A.C.; Huan, Y.; Shangguan, J.; Procissi, D.; Bentrem, D.J.; Larson, A.C. Quantitative Functional MRI in a Clinical Orthotopic Model of Pancreatic Cancer in Immunocompetent Lewis Rats. Am. J. Transl. Res. 2015, 7, 1475–1486. [Google Scholar]
- Klaassen, R.; Steins, A.; Gurney-Champion, O.J.; Bijlsma, M.F.; Tienhoven, G.; Engelbrecht, M.R.W.; Eijck, C.H.J.; Suker, M.; Wilmink, J.W.; Besselink, M.G.; et al. Pathological Validation and Prognostic Potential of Quantitative MRI in the Characterization of Pancreas Cancer: Preliminary Experience. Mol. Oncol. 2020, 14, 2176–2189. [Google Scholar] [CrossRef]
- Antonova, D.V.; Gnatenko, D.A.; Kotova, E.S.; Pleshkan, V.V.; Kuzmich, A.I.; Didych, D.A.; Sverdlov, E.D.; Alekseenko, I.V. Cell-Specific Expression of the FAP Gene Is Regulated by Enhancer Elements. Front. Mol. Biosci. 2023, 10, 1111511. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; Yu, C.; Yang, X.; Zhang, R. Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 11, 648187. [Google Scholar] [CrossRef]
- Shi, M.; Yu, D.-H.; Chen, Y.; Zhao, C.-Y.; Zhang, J.; Liu, Q.-H.; Ni, C.-R.; Zhu, M.-H. Expression of Fibroblast Activation Protein in Human Pancreatic Adenocarcinoma and Its Clinicopathological Significance. World J. Gastroenterol. 2012, 18, 840–846. [Google Scholar] [CrossRef]
- Qi, N.; Wang, H.; Wang, H.; Ren, S.; You, Z.; Chen, X.; Guan, Y.; Xie, F.; Hua, F.; Zhao, J. Non-Tumoral Uptake of 68Ga-FAPI-04 PET: A Retrospective Study. Front. Oncol. 2022, 12, 989595. [Google Scholar] [CrossRef]
- Guberina, N.; Kessler, L.; Pöttgen, C.; Guberina, M.; Metzenmacher, M.; Herrmann, K.; Mucha, M.; Rischpler, C.; Indenkämpen, F.; Siveke, J.T.; et al. [68Ga]FAPI-PET/CT for Radiation Therapy Planning in Biliary Tract, Pancreatic Ductal Adeno-, and Adenoidcystic Carcinomas. Sci. Rep. 2022, 12, 16261. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between Cancer-Associated Fibroblasts and Immune Cells in the Tumor Microenvironment: New Findings and Future Perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The Role of Stromal Cancer-Associated Fibroblasts in Pancreatic Cancer. J. Hematol. Oncol. 2017, 10, 76. [Google Scholar] [CrossRef]
- Hendricks-Wenger, A.; Sereno, J.; Gannon, J.; Zeher, A.; Brock, R.M.; Beitel-White, N.; Simon, A.; Davalos, R.V.; Coutermarsh-Ott, S.; Vlaisavljevich, E.; et al. Histotripsy Ablation Alters the Tumor Microenvironment and Promotes Immune System Activation in a Subcutaneous Model of Pancreatic Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2987–3000. [Google Scholar] [CrossRef]

| Protocol | TSE2D | FFE2D | T2MAP | T1MAP | DCE |
|---|---|---|---|---|---|
| Sequence Type | 2D TSE | 2D FFE | 2D TSE | IR Look–Locker | 3D FFE |
| TE (ms) | 30 | 4.8 | 6.25 + n × 25 | 3.7 | 2.8 |
| TR (ms) | 12117 | 230 | 11970 | 8000 | 6 |
| Field of View (mm3) | ~90 × 60 × 50 | ~90 × 60 × 66 | 60 × 60 × 21 | 60 × 60 × 21 | 60 × 60 × 20 |
| Reconstruction voxel size (mm3) | 0.2 × 0.2 × 1.0 | 0.28 × 0.28 × 1.00 | 0.63 × 0.63 × 2.1 | 0.63 × 0.63 × 2.1 | 0.62 × 0.62 × 0.7 |
| Acquisition voxel size (mm3) | 0.3 × 0.3 × 1 | 0.3 × 0.3 × 1 | 0.94 × 0.94 × 2.1 | 0.94 × 1.00 × 2.1 | 0.7 × 0.7 × 0.7 |
| NSA | 1 | 2 | |||
| Flip Angle (°) | 90 | 60 | 90 | 5 | 15 |
| No. of frames (Slices) | 40 | 60 | 10 | 10 | 29 |
| Fat saturation | no | no | SPIR | SPIR | no |
| Other parameters | TI = 22 + n × 100 ms | RF-spoiled, dyn. scan time = 9.7 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, P.; Lindemeyer, J.; Raut, P.; Schütz, M.; Saniternik, S.; Jönsson, J.; Endepols, H.; Fischer, T.; Quaas, A.; Schlößer, H.A.; et al. Multiparametric Characterization of the DSL-6A/C1 Pancreatic Cancer Model in Rats. Cancers 2024, 16, 1535. https://doi.org/10.3390/cancers16081535
Schmidt P, Lindemeyer J, Raut P, Schütz M, Saniternik S, Jönsson J, Endepols H, Fischer T, Quaas A, Schlößer HA, et al. Multiparametric Characterization of the DSL-6A/C1 Pancreatic Cancer Model in Rats. Cancers. 2024; 16(8):1535. https://doi.org/10.3390/cancers16081535
Chicago/Turabian StyleSchmidt, Patrick, Johannes Lindemeyer, Pranali Raut, Markus Schütz, Sven Saniternik, Jannika Jönsson, Heike Endepols, Thomas Fischer, Alexander Quaas, Hans Anton Schlößer, and et al. 2024. "Multiparametric Characterization of the DSL-6A/C1 Pancreatic Cancer Model in Rats" Cancers 16, no. 8: 1535. https://doi.org/10.3390/cancers16081535
APA StyleSchmidt, P., Lindemeyer, J., Raut, P., Schütz, M., Saniternik, S., Jönsson, J., Endepols, H., Fischer, T., Quaas, A., Schlößer, H. A., Thelen, M., & Grüll, H. (2024). Multiparametric Characterization of the DSL-6A/C1 Pancreatic Cancer Model in Rats. Cancers, 16(8), 1535. https://doi.org/10.3390/cancers16081535








