Hepatic Iron Overload and Hepatocellular Carcinoma: New Insights into Pathophysiological Mechanisms and Therapeutic Approaches
Simple Summary
Abstract
1. Introduction
2. Iron Metabolism and Iron Overload: An Overview of Pathophysiological Mechanisms and the Significance of Liver Involvement
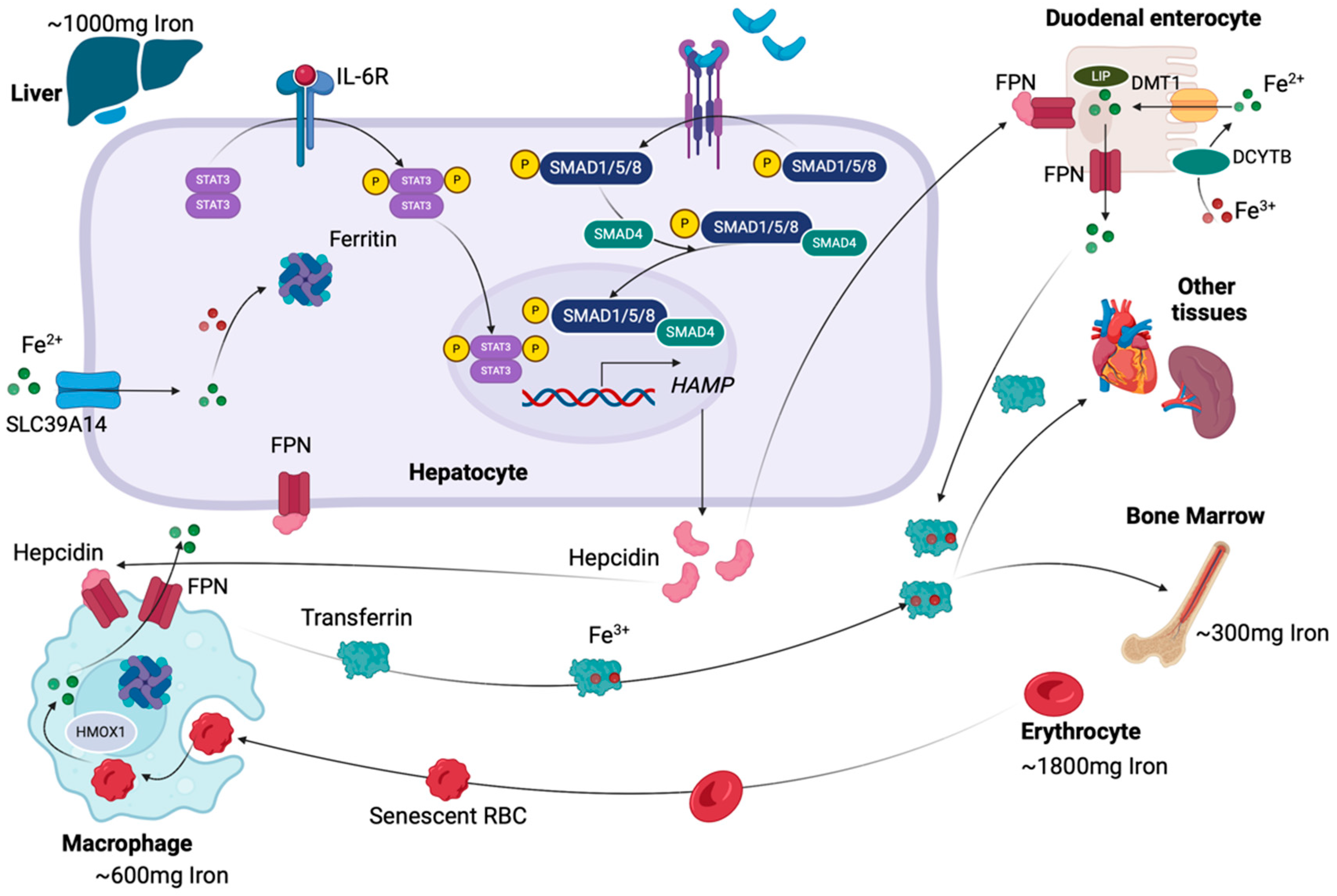
2.1. A Brief Summary of Iron Metabolism
2.2. Iron Overload and Its Role in Liver Damage
3. The Role of IO-Induced Oxidative Stress in Hepatocarcinogenesis
3.1. Oxidative Stress: Correlation with Iron Metabolism and Associated Liver Disease Pathogenesis
3.2. OS-Associated Hepatocarcinogenesis: The Four Major Aspects
4. Dysregulated Glucose and Lipid Metabolism in Iron Overload States
4.1. Iron Overload-Induced Insulin Resistance and Its Association with Fibrotic Changes: From MASLD to HCC
4.2. Interactions Between Liver Iron and Lipid Metabolism and Their Implication in MASLD and HCC
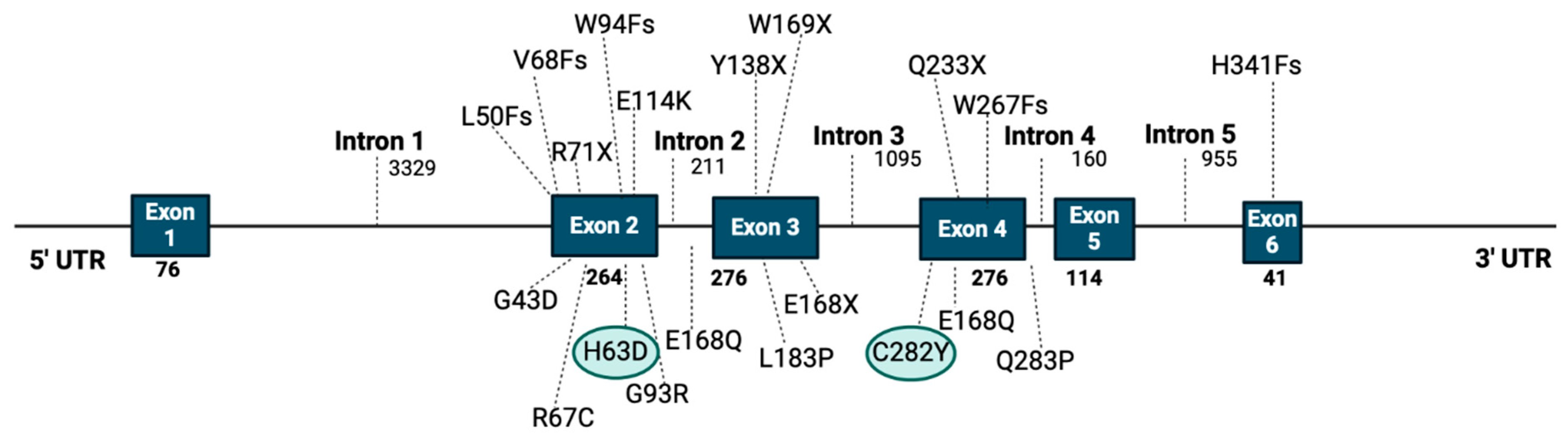
5. The Risk of HCC Development in Patients with HFE Mutations
6. Hepcidin Expression in MASLD and HCC: Pathophysiological Correlation and Raising Enigmas
6.1. Hepcidin Expression in MASLD
6.2. Hepcidin Expression and Regulation in HCC
6.2.1. Hepcidin Expression Patterns in HCC
6.2.2. Hepcidin Regulatory Mechanisms in HCC
7. The Promising Role of IO-Induced Cell Death in HCC Pathogenesis and Risk Stratification
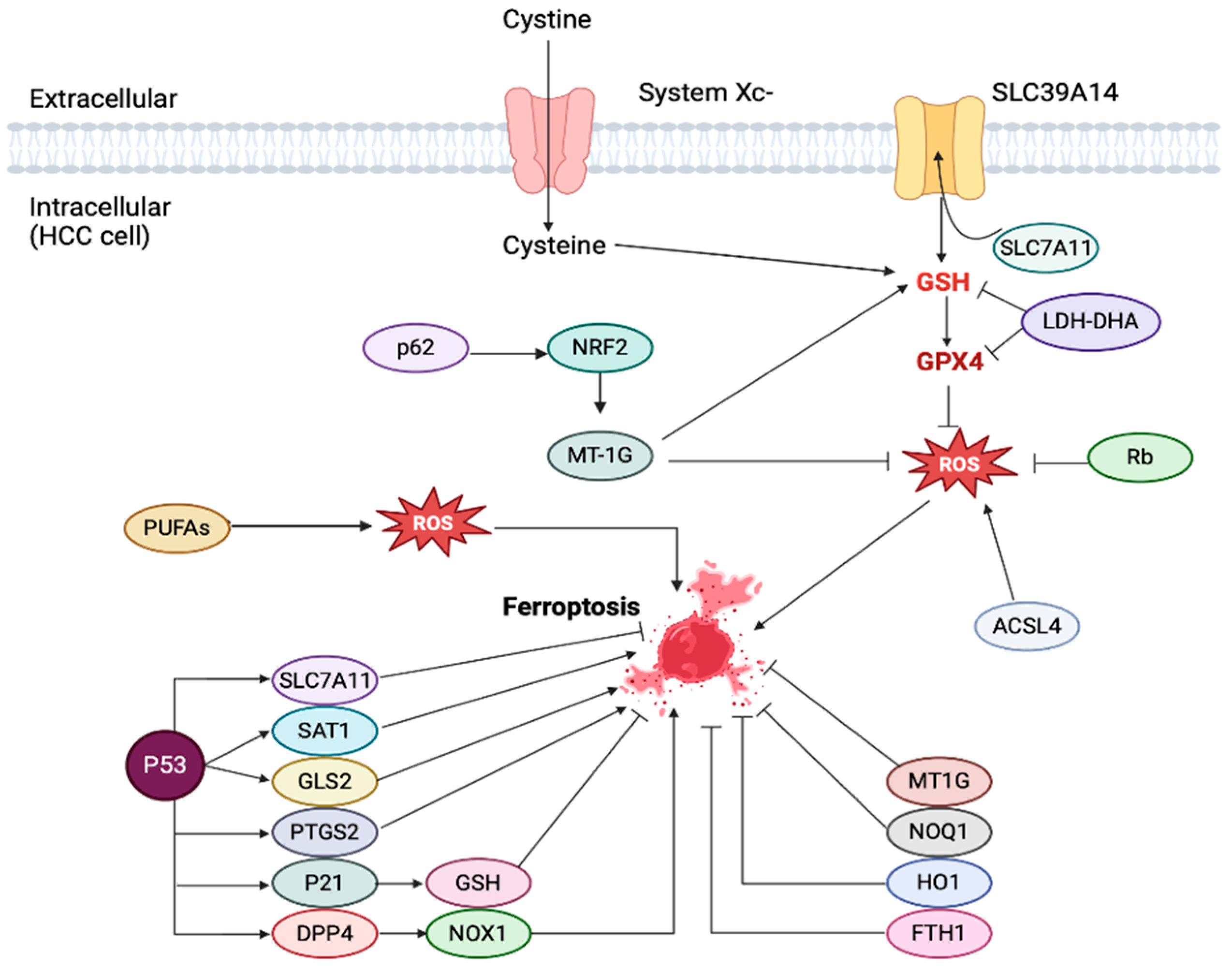
7.1. Ferroptosis: A Brief Overview of Pathophysiology and Its Association with Liver Disease
7.2. Ferroptosis in HCC: Its Role in Tumorigenesis, Prognostic Utilization, and Novel Risk Stratification Scoring Systems
8. Iron Metabolism as a Novel Therapeutic Target for HCC
9. Discussion and Future Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chacko, S.; Samanta, S. Hepatocellular Carcinoma: A Life-Threatening Disease. Biomed. Pharmacother. 2016, 84, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.A.; Schmittdiel, J.A.; Liu, L.; Macdonald, B.A.; Balasubramanian, S.; Chai, K.P.; Seo, S.I.; Mukhtar, N.; Levin, T.R.; Saxena, V. Hepatocellular Carcinoma in Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Netw. Open 2024, 7, 2421019. [Google Scholar] [CrossRef]
- Guo, Y.; Chow, P.K.H. A Windfall Year for HCC: The Most Impactful Clinical Papers in 2023. Hepatol. Commun. 2024, 8, 9–12. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Papadakos, S.P.; Vakadaris, G.; Chatzikalil, E.; Stergiou, I.E.; Kalopitas, G.; Theocharis, S.; Germanidis, G. Shedding Light on the Role of LAG-3 in Hepatocellular Carcinoma: Unraveling Immunomodulatory Pathways. Hepatoma Res. 2024, 10, 20. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Chatzikalil, E.; Vakadaris, G.; Reppas, L.; Arvanitakis, K.; Koufakis, T.; Siakavellas, S.I.; Manolakopoulos, S.; Germanidis, G.; Theocharis, S. Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma. Cancers 2024, 16, 2609. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Chatzikalil, E.; Arvanitakis, K.; Vakadaris, G.; Stergiou, I.E.; Koutsompina, M.-L.; Argyrou, A.; Lekakis, V.; Konstantinidis, I.; Germanidis, G.; et al. Understanding the Role of Connexins in Hepatocellular Carcinoma: Molecular and Prognostic Implications. Cancers 2024, 16, 1533. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and Surveillance for Hepatocellular Carcinoma: New Trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Zhu, B.; Wei, Y.; Zhang, M.; Yang, S.; Tong, R.; Li, W.; Long, E. Metabolic Dysfunction-Associated Steatotic Liver Disease: Ferroptosis Related Mechanisms and Potential Drugs. Front. Pharmacol. 2023, 14, 1286449. [Google Scholar] [CrossRef]
- Shah, P.A.; Patil, R.; Harrison, S.A. NAFLD-related Hepatocellular Carcinoma: The Growing Challenge. Hepatology 2023, 77, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Trad, D.; Bibani, N.; Sabbah, M.; Elloumi, H.; Gargouri, D.; Ouakaa, A.; Kharrat, J. Known, New and Emerging Risk Factors of Hepatocellular Carcinoma (Review). Presse Med. 2017, 46, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.M.; Graubard, B.I.; Quraishi, S.; Zeuzem, S.; Davila, J.A.; El-Serag, H.B.; McGlynn, K.A. Population-Attributable Fractions of Risk Factors for Hepatocellular Carcinoma in the United States. Off. J. Am. Coll. Gastroenterol. ACG 2013, 108, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Bechmann, L.P.; Sowa, J.-P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735. [Google Scholar] [CrossRef]
- Bianco, C.; Jamialahmadi, O.; Pelusi, S.; Baselli, G.; Dongiovanni, P.; Zanoni, I.; Santoro, L.; Maier, S.; Liguori, A.; Meroni, M. Non-Invasive Stratification of Hepatocellular Carcinoma Risk in Non-Alcoholic Fatty Liver Using Polygenic Risk Scores. J. Hepatol. 2021, 74, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Kang, D.; Cho, S.J.; Paik, S.W.; Guallar, E.; Cho, J.; Gwak, G.-Y. Risk of Hepatocellular Carcinoma in Individuals without Traditional Risk Factors: Development and Validation of a Novel Risk Score. Int. J. Epidemiol. 2020, 49, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Caviglia, G.P.; Govaere, O.; Rosso, C.; Armandi, A.; Sanavia, T.; Pennisi, G.; Liguori, A.; Francione, P.; Gallego-Durán, R. Long-Term Outcomes and Predictive Ability of Non-Invasive Scoring Systems in Patients with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2021, 75, 786–794. [Google Scholar] [CrossRef]
- Brown, R.A.M.; Richardson, K.L.; Kabir, T.D.; Trinder, D.; Ganss, R.; Leedman, P.J. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front. Oncol. 2020, 10, 476. [Google Scholar] [CrossRef]
- Song, A.; Eo, W.; Kim, S.; Shim, B.; Lee, S. Significance of Serum Ferritin as a Prognostic Factor in Advanced Hepatobiliary Cancer Patients Treated with Korean Medicine: A Retrospective Cohort Study. BMC Complement. Altern. Med. 2018, 18, 176. [Google Scholar] [CrossRef]
- Salomao, M.A. Pathology of Hepatic Iron Overload. Clin. Liver Dis. 2021, 17, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, Q.; Mao, M.; Han, R.; Liu, Y.; Wang, X. Novel Prognostic Scores Based on Serum Ferritin/Globulin Ratio in Patients with Hepatocellular Carcinoma. Transl. Cancer Res. 2020, 9, 5925–5939. [Google Scholar] [CrossRef]
- Yu, Y.C.; Luu, H.N.; Wang, R.; Thomas, C.E.; Glynn, N.W.; Youk, A.O.; Behari, J.; Yuan, J.M. Serum Biomarkers of Iron Status and Risk of Hepatocellular Carcinoma Development in Patients with Nonalcoholic Fatty Liver Disease. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 230–235. [Google Scholar] [CrossRef]
- Wu, S.-J.; Zhang, Z.-Z.; Cheng, N.-S.; Xiong, X.-Z.; Yang, L. Preoperative Serum Ferritin Is an Independent Prognostic Factor for Liver Cancer after Hepatectomy. Surg. Oncol. 2019, 29, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of Iron Metabolism. Part 1: Molecular Basis of Iron Homoeostasis. J. Clin. Pathol. 2011, 64, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of Cellular Iron Metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, E.A.; Georgieff, M.K. Chronic Inflammation and Iron Metabolism HHS Public Access. J. Pediatr. 2015, 166, 1351–1357. [Google Scholar] [CrossRef]
- Anderson, E.R.; Shah, Y.M. Iron Homeostasis in the Liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar] [CrossRef]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and Regulatory Aspects of Intestinal Iron Absorption. Am. J. Physiol. Liver Physiol. 2014, 307, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, Ó.; Ramos, A.S.; Gomes, L.T.S.; Gomes, M.S.; Moreira, A.C. New Perspectives on Circulating Ferritin: Its Role in Health and Disease. Molecules 2023, 28, 7707. [Google Scholar] [CrossRef]
- Scaramellini, N.; Fischer, D.; Agarvas, A.R.; Motta, I.; Muckenthaler, M.U.; Mertens, C. Interpreting Iron Homeostasis in Congenital and Acquired Disorders. Pharmaceuticals 2023, 16, 329. [Google Scholar] [CrossRef]
- Katsarou, A.; Pantopoulos, K. Hepcidin Therapeutics. Pharmaceuticals 2018, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Core, A.B.; Canali, S.; Babitt, J.L. Hemojuvelin and Bone Morphogenetic Protein (BMP) Signaling in Iron Homeostasis. Front. Pharmacol. 2014, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, A.-S.; Enns, C.A. Iron Regulation by Hepcidin. J. Clin. Investig. 2013, 123, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Iron Load Toxicity in Medicine: From Molecular and Cellular Aspects to Clinical Implications. Int. J. Mol. Sci. 2023, 24, 12928. [Google Scholar] [CrossRef] [PubMed]
- Milic, S.; Mikolasevic, I.; Orlic, L.; Devcic, E.; Starcevic-Cizmarevic, N.; Stimac, D.; Kapovic, M.; Ristic, S. The Role of Iron and Iron Overload in Chronic Liver Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V. Iron, Hemochromatosis, and Hepatocellular Carcinoma. Gastroenterology 2004, 127, 79–86. [Google Scholar] [CrossRef]
- Kawabata, H. The Mechanisms of Systemic Iron Homeostasis and Etiology, Diagnosis, and Treatment of Hereditary Hemochromatosis. Int. J. Hematol. 2018, 107, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Pinyopornpanish, K.; Tantiworawit, A.; Leerapun, A.; Soontornpun, A.; Thongsawat, S. Secondary Iron Overload and the Liver: A Comprehensive Review. J. Clin. Transl. Hepatol. 2023, 11, 932–941. [Google Scholar] [CrossRef]
- Soto, A.; Spongberg, C.; Martinino, A.; Giovinazzo, F. Exploring the Multifaceted Landscape of MASLD: A Comprehensive Synthesis of Recent Studies, from Pathophysiology to Organoids and Beyond. Biomedicines 2024, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Takei, Y. Iron Overload in Nonalcoholic Steatohepatitis. Adv. Clin. Chem. 2011, 55, 105–132. [Google Scholar] [CrossRef]
- Bloomer, S.A.; Brown, K.E. Hepcidin and Iron Metabolism in Experimental Liver Injury. Am. J. Pathol. 2021, 191, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, J.; Lu, Y.; Wu, L. Association of Metabolic-Dysfunction Associated Steatotic Liver Disease with Polycystic Ovary Syndrome. iScience 2024, 27, 108783. [Google Scholar] [CrossRef] [PubMed]
- Delaporta, P.; Chatzikalil, E.; Kyriakopoulou, D.; Berdalli, S.; Chouliara, V.; Hatzieleftheriou, M.-I.; Mylona, S.; Kattamis, A. Abrupt Increases in Ferritin Levels May Indicate a Malignant Process and Not Changes in Iron Overload in Thalassemic Patients. Blood 2024, 144, 3860. [Google Scholar] [CrossRef]
- Koyama, S.; Fujisawa, S.; Watanabe, R.; Itabashi, M.; Ishibashi, D.; Ishii, Y.; Hattori, Y.; Nakajima, Y.; Motohashi, K.; Takasaki, H.; et al. Serum Ferritin Level Is a Prognostic Marker in Patients with Peripheral T-Cell Lymphoma. Int. J. Lab. Hematol. 2017, 39, 112–117. [Google Scholar] [CrossRef]
- Moroz, V.; Machin, D.; Hero, B.; Ladenstein, R.; Berthold, F.; Kao, P.; Obeng, Y.; Pearson, A.D.J.; Cohn, S.L.; London, W.B. The Prognostic Strength of Serum LDH and Serum Ferritin in Children with Neuroblastoma: A Report from the International Neuroblastoma Risk Group (INRG) Project. Pediatr. Blood Cancer 2020, 67, 28359. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-B.; Li, X.; Jiang, J.; Zhao, W.-Q.; Ji, M.; Wu, C.-P. Serum Ferritin Is Elevated in Advanced Non-Small Cell Lung Cancer Patients and Is Associated with Efficacy of Platinum-Based Chemotherapy. J. Cancer Res. Ther. 2014, 10, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Niu, T.; Lin, B.; Gao, F.; Tan, B.; Du, X. Prognostic Value of Preoperative Serum Ferritin in Hepatocellular Carcinoma Patients Undergoing Transarterial Chemoembolization. Mol. Clin. Oncol. 2024, 20, 22. [Google Scholar] [CrossRef]
- Puntarulo, S. Iron, Oxidative Stress and Human Health. Mol. Aspects Med. 2005, 26, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Ye, Y.; Xie, L.; Li, W. Oxidative Stress and Liver Cancer: Etiology and Therapeutic Targets. Oxid. Med. Cell. Longev. 2016, 2016, 7891574. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, K.; Xing, W.; Dong, M.; Yi, M.; Zhang, H. Role of Iron-Related Oxidative Stress and Mitochondrial Dysfunction in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 5124553. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Yang, L.; Wang, R. Insights into the Role of Oxidative Stress in Hepatocellular Carcinoma Development. Front. Biosci. 2023, 28, 286. [Google Scholar] [CrossRef] [PubMed]
- Trasolini, R.; Cox, B.; Galts, C.; Yoshida, E.M.; Marquez, V. Elevated Serum Ferritin in Non-Alcoholic Fatty Liver Disease Is Not Predictive of Fibrosis. Can. Liver J. 2022, 5, 152–159. [Google Scholar] [CrossRef]
- Manne, V.; Handa, P.; Kowdley, K. V Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Datz, C.; Müller, E.; Aigner, E. Iron Overload and Non-Alcoholic Fatty Liver Disease. Minerva Endocrinol. 2016, 42, 173–183. [Google Scholar] [CrossRef]
- Fernandez, M.; Lokan, J.; Leung, C.; Grigg, A. A Critical Evaluation of the Role of Iron Overload in Fatty Liver Disease. J. Gastroenterol. Hepatol. 2022, 37, 1873–1883. [Google Scholar] [CrossRef]
- Okada, K.; Warabi, E.; Sugimoto, H.; Horie, M.; Tokushige, K.; Ueda, T.; Harada, N.; Taguchi, K.; Hashimoto, E.; Itoh, K. Nrf2 Inhibits Hepatic Iron Accumulation and Counteracts Oxidative Stress-Induced Liver Injury in Nutritional Steatohepatitis. J. Gastroenterol. 2012, 47, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Sinha, R.A. Current Treatment Paradigms and Emerging Therapies for NAFLD/NASH. Front. Biosci. 2021, 26, 206. [Google Scholar] [CrossRef]
- Bottani, M.; Banfi, G.; Lombardi, G. Circulating miRNAs as Diagnostic and Prognostic Biomarkers in Common Solid Tumors: Focus on Lung, Breast, Prostate Cancers, and Osteosarcoma. J. Clin. Med. 2019, 8, 1661. [Google Scholar] [CrossRef]
- Grattagliano, I.; Russmann, S.; Diogo, C.; Bonfrate, L.J.; Oliveira, P.Q.-H.; Wang, D.; Portincasa, P. Mitochondria in Chronic Liver Disease. Curr. Drug Targets 2011, 12, 879–893. [Google Scholar] [CrossRef]
- Shetty, S.; Anushree, U.; Kumar, R.; Bharati, S. Mitochondria-Targeted Antioxidant, Mito-TEMPO Mitigates Initiation Phase of N-Nitrosodiethylamine-Induced Hepatocarcinogenesis. Mitochondrion 2021, 58, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Kumar, R.; Bharati, S. Mito-TEMPO, a Mitochondria-Targeted Antioxidant, Prevents N-Nitrosodiethylamine-Induced Hepatocarcinogenesis in Mice. Free Radic. Biol. Med. 2019, 136, 76–86. [Google Scholar] [CrossRef]
- Xie, W.; Ding, J.; Xie, X.; Yang, X.; Wu, X.-F.; Chen, Z.; Guo, Q.; Gao, W.; Wang, X.; Li, D. Hepatitis B Virus X Protein Promotes Liver Cell Pyroptosis under Oxidative Stress through NLRP3 Inflammasome Activation. Inflamm. Res. 2020, 69, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Nishina, S.; Sasaki, K.; Hara, Y. Mitochondrial Damage and Iron Metabolic Dysregulation in Hepatitis C Virus Infection. Free Radic. Biol. Med. 2019, 133, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Smirnova, O.A.; Petrushanko, I.Y.; Ivanova, O.N.; Karpenko, I.L.; Alekseeva, E.; Sominskaya, I.; Makarov, A.A.; Bartosch, B.; Kochetkov, S.N. HCV Core Protein Uses Multiple Mechanisms to Induce Oxidative Stress in Human Hepatoma Huh7 Cells. Viruses 2015, 7, 2745–2770. [Google Scholar] [CrossRef]
- Lim, S.-O.; Gu, J.-M.; Kim, M.S.; Kim, H.-S.; Park, Y.N.; Park, C.K.; Cho, J.W.; Park, Y.M.; Jung, G. Epigenetic Changes Induced by Reactive Oxygen Species in Hepatocellular Carcinoma: Methylation of the E-Cadherin Promoter. Gastroenterology 2008, 135, 2128–2140. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, W.; He, Q.; Li, D.; Wang, Z.; Feng, Y.; Liu, D.; Zhang, T.; Wang, Y.; Xie, M. FOXC1 Promotes HCC Proliferation and Metastasis by Upregulating DNMT3B to Induce DNA Hypermethylation of CTH Promoter. J. Exp. Clin. Cancer Res. 2021, 40, 50. [Google Scholar] [CrossRef]
- Sartorius, K.; Sartorius, B.; Winkler, C.; Chuturgoon, A.; Makarova, J. The Biological and Diagnostic Role of MiRNA’s in Hepatocellular Carcinoma. Front. Biosci. 2018, 23, 1701–1720. [Google Scholar] [CrossRef] [PubMed]
- Klieser, E.; Mayr, C.; Kiesslich, T.; Wissniowski, T.; Di Fazio, P.; Neureiter, D.; Ocker, M. The Crosstalk of MiRNA and Oxidative Stress in the Liver: From Physiology to Pathology and Clinical Implications. Int. J. Mol. Sci. 2019, 20, 5266. [Google Scholar] [CrossRef]
- Cui, H.; Guo, D.; Zhang, X.; Zhu, Y.; Wang, Z.; Jin, Y.; Guo, W.; Zhang, S. ENO3 Inhibits Growth and Metastasis of Hepatocellular Carcinoma via Wnt/β-Catenin Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 797102. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, M.; Wang, M.; Feng, M.; Yang, F.; Li, L.; Zhao, J.; Chang, C.; Dong, H.; Xie, T. Dysregulation of Wnt/Β-catenin Signaling by Protein Kinases in Hepatocellular Carcinoma and Its Therapeutic Application. Cancer Sci. 2021, 112, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, D.; Wang, Z.; Tian, W.; Li, X.; Duan, J.; Wang, Y.; Yang, H.; You, L.; Cheng, Y. Combretastatin A-1 Phosphate, a Microtubule Inhibitor, Acts on Both Hepatocellular Carcinoma Cells and Tumor-Associated Macrophages by Inhibiting the Wnt/β-Catenin Pathway. Cancer Lett. 2016, 380, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K. Review of PIP2 in Cellular Signaling, Functions and Diseases. Int. J. Mol. Sci. 2020, 21, 8342. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, Y.; Chen, W.; Merlino, G.; Yu, Y. PTEN Dual Lipid- and Protein-Phosphatase Function in Tumor Progression. Cancers 2022, 14, 3666. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and Metabolic Functions of MTORC1 and MTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Duda, D.G.; Zhu, Y.; Pei, S.; Wang, X.; Huang, Y.; Yi, P.; Huang, Z.; Peng, F.; Hu, X. VCP Interaction with HMGB1 Promotes Hepatocellular Carcinoma Progression by Activating the PI3K/AKT/MTOR Pathway. J. Transl. Med. 2022, 20, 212. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, L.; Ma, L.; Ahmad Farooqi, A.; Qiao, G.; Zhang, Y.; Ye, H.; Liu, M.; Huang, J.; Yang, X. Alnustone Inhibits the Growth of Hepatocellular Carcinoma via ROS-mediated PI3K/Akt/MTOR/P70S6K Axis. Phyther. Res. 2022, 36, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, M.; Wang, J.; Sui, Y.; Liu, S.; Shi, D. NAC Selectively Inhibit Cancer Telomerase Activity: A Higher Redox Homeostasis Threshold Exists in Cancer Cells. Redox Biol. 2016, 8, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen Peroxide Sensing, Signaling and Regulation of Transcription Factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, J.; Ji, X.; Cao, H.; Zhu, J.; Chen, Y.; Yang, J.; Zhao, Z.; Ren, T.; Xing, J. MCUR1 Facilitates Epithelial-Mesenchymal Transition and Metastasis via the Mitochondrial Calcium Dependent ROS/Nrf2/Notch Pathway in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 136. [Google Scholar] [CrossRef]
- Zhdanovskaya, N.; Firrincieli, M.; Lazzari, S.; Pace, E.; Scribani Rossi, P.; Felli, M.P.; Talora, C.; Screpanti, I.; Palermo, R. Targeting Notch to Maximize Chemotherapeutic Benefits: Rationale, Advanced Strategies, and Future Perspectives. Cancers 2021, 13, 5106. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Hartmann, D.; Braren, R.; Gupta, A.; Wang, B.; Wang, Y.; Mogler, C.; Cheng, Z.; Wirth, T.; Friess, H. Oncogenic Akt-FOXO3 Loop Favors Tumor-Promoting Modes and Enhances Oxidative Damage-Associated Hepatocellular Carcinogenesis. BMC Cancer 2019, 19, 887. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of Reactive Oxygen Species: An Emerging Approach for Cancer Therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Aolymat, I.; Hatmal, M.M.; Olaimat, A.N. The Emerging Role of Heat Shock Factor 1 (HSF1) and Heat Shock Proteins (HSPs) in Ferroptosis. Pathophysiology 2023, 30, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Sigmond, T.; Hotzi, B.; Bohár, B.; Fazekas, D.; Deák, V.; Vellai, T.; Barna, J. HSF1Base: A Comprehensive Database of HSF1 (Heat Shock Factor 1) Target Genes. Int. J. Mol. Sci. 2019, 20, 5815. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Huang, D.A.; Li, M.M.; Liu, H.D.; Guo, K. HSF1: A Mediator in Metabolic Alteration of Hepatocellular Carcinoma Cells in Cross-Talking with Tumor-Associated Macrophages. Am. J. Transl. Res. 2019, 11, 5054. [Google Scholar]
- Moreira, A.J.; Rodrigues, G.R.; Bona, S.; Fratta, L.X.S.; Weber, G.R.; Picada, J.N.; Dos Santos, J.L.; Cerski, C.T.; Marroni, C.A.; Marroni, N.P. Ductular Reaction, Cytokeratin 7 Positivity, and Gamma-Glutamyl Transferase in Multistage Hepatocarcinogenesis in Rats. Protoplasma 2017, 254, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. The Diverse and Complex Roles of NF-ΚB Subunits in Cancer. Nat. Rev. Cancer 2012, 12, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, J.; Qu, K.; Wan, Y.; Liu, S.; Zheng, W.; Zhang, Z.; Liu, C. CRIF1 Overexpression Facilitates Tumor Growth and Metastasis through Inducing ROS/NFκB Pathway in Hepatocellular Carcinoma. Cell Death Dis. 2020, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tan, K.; Wang, H.; Zhang, X. Pterostilbene Inhibits Hepatocellular Carcinoma through P53/SOD2/ROS-Mediated Mitochondrial Apoptosis. Oncol. Rep. 2016, 36, 3233–3240. [Google Scholar] [CrossRef]
- Liang, W.; Ferrara, N. Iron Metabolism in the Tumor Microenvironment: Contributions of Innate Immune Cells. Front. Immunol. 2021, 11, 626812. [Google Scholar] [CrossRef]
- Mastrogeorgiou, M.; Chatzikalil, E.; Theocharis, S.; Papoudou-Bai, A.; Péoc’h, M.; Mobarki, M.; Karpathiou, G. The Immune Microenvironment of Cancer of the Uterine Cervix. Histol. Histopathol. 2024, 39, 1245–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.-G. ROS Play a Critical Role in the Differentiation of Alternatively Activated Macrophages and the Occurrence of Tumor-Associated Macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Mora, J.; Ören, B.; Grein, S.; Winslow, S.; Scholich, K.; Weigert, A.; Malmström, P.; Forsare, C.; Fernö, M.; et al. Macrophage-Derived Lipocalin-2 Transports Iron in the Tumor Microenvironment. Oncoimmunology 2018, 7, e1408751. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, A.; Matikainen, S.; Miettinen, M.; Julkunen, I. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)-Induced STAT5 Activation and Target-Gene Expression during Human Monocyte/Macrophage Differentiation. J. Leukoc. Biol. 2002, 71, 511–519. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, R.; Kong, X.; You, Y.; He, K.; Sun, X.; Huang, Y.; Chen, W.; Duan, L. Elevated Neutrophil Extracellular Traps by HBV-mediated S100A9-TLR4/RAGE-ROS Cascade Facilitate the Growth and Metastasis of Hepatocellular Carcinoma. Cancer Commun. 2023, 43, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Chatzikalil, E.; Kalopitas, G.; Patoulias, D.; Popovic, D.S.; Metallidis, S.; Kotsa, K.; Germanidis, G.; Koufakis, T. Metabolic Dysfunction-Associated Steatotic Liver Disease and Polycystic Ovary Syndrome: A Complex Interplay. J. Clin. Med. 2024, 13, 4243. [Google Scholar] [CrossRef]
- Fillebeen, C.; Lam, N.H.; Chow, S.; Botta, A.; Sweeney, G.; Pantopoulos, K. Regulatory Connections between Iron and Glucose Metabolism. Int. J. Mol. Sci. 2020, 21, 7773. [Google Scholar] [CrossRef] [PubMed]
- Klisic, A.; Kavaric, N.; Kotur, J.; Ninic, A. Serum Soluble Transferrin Receptor Levels Are Independently Associated with Homeostasis Model Assessment of Insulin Resistance in Adolescent Girls. Arch. Med. Sci. AMS 2023, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cai, J.; Wang, Y.; Liu, J.; Fu, S. Non-Enzymatic Glycation of Transferrin and Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2021, 2021, 2539–2548. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Yang, Y.; Ma, L. Iron Metabolism and Type 2 Diabetes Mellitus: A Meta-analysis and Systematic Review. J. Diabetes Investig. 2020, 11, 946–955. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and Disorders of Iron Metabolism. Annu. Rev. Med. 2011, 62, 347–360. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in Fatty Liver and in the Metabolic Syndrome: A Promising Therapeutic Target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Gabrielsen, J.S.; Simcox, J.A.; Lee, S.; Jones, D.; Cooksey, B.; Stoddard, G.; Cefalu, W.T.; McClain, D.A. Adipocyte Iron Regulates Leptin and Food Intake. J. Clin. Investig. 2015, 125, 3681–3691. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Gao, Y.; Simcox, J.A.; Huang, J.; Thorup, D.; Jones, D.; Cooksey, R.C.; Gabrielsen, D.; Adams, T.D.; Hunt, S.C. Adipocyte Iron Regulates Adiponectin and Insulin Sensitivity. J. Clin. Investig. 2012, 122, 3529–3540. [Google Scholar] [CrossRef]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative Stress, β-Cell Apoptosis, and Decreased Insulin Secretory Capacity in Mouse Models of Hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef]
- Hansen, J.B.; Tonnesen, M.F.; Madsen, A.N.; Hagedorn, P.H.; Friberg, J.; Grunnet, L.G.; Heller, R.S.; Nielsen, A.Ø.; Størling, J.; Baeyens, L. Divalent Metal Transporter 1 Regulates Iron-Mediated ROS and Pancreatic β Cell Fate in Response to Cytokines. Cell Metab. 2012, 16, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Latham, P.S.; Oates, P.S. Interactions between Hepatic Iron and Lipid Metabolism with Possible Relevance to Steatohepatitis. World J. Gastroenterol. 2012, 18, 4651–4658. [Google Scholar] [CrossRef]
- Brunet, S.; Thibault, L.; Delvin, E.; Yotov, W.V.; Bendayan, M.; Levy, E. Dietary Iron Overload and Induced Lipid Peroxidation Are Associated with Impaired Plasma Lipid Transport and Hepatic Sterol Metabolism in Rats. Hepatology 1999, 29, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Gong, Z.; Fernando, T.; Zhang, L.; Zhu, X.; Shi, Y. The Lipid Profiles in Different Characteristics of Women with PCOS and the Interaction Between Dyslipidemia and Metabolic Disorder States: A Retrospective Study in Chinese Population. Front. Endocrinol. 2022, 13, 892125. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, M.; Ferraro, M.G.; Iazzetti, F.; Santamaria, R.; Irace, C. Insight into Iron, Oxidative Stress and Ferroptosis: Therapy Targets for Approaching Anticancer Strategies. Cancers 2024, 16, 1220. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, P.; Chanderbhan, R.F. Effect of Increasing Iron Supplementation on Blood Lipids in Rats. Br. J. Nutr. 2001, 86, 587–592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirsch, R.; Sijtsema, H.P.; Tlali, M.; Marais, A.D.; Hall, P. de la M. Effects of Iron Overload in a Rat Nutritional Model of Non-Alcoholic Fatty Liver Disease. Liver Int. 2006, 26, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.; Pereira, C.F.; Rodrigues, P.; Cardoso, E.M.; Arosa, F.A. Altered Expression of CD1d Molecules and Lipid Accumulation in the Human Hepatoma Cell Line HepG2 after Iron Loading. FEBS J. 2005, 272, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Bort, A.; Sánchez, B.G.; de Miguel, I.; Mateos-Gómez, P.A.; Diaz-Laviada, I. Dysregulated Lipid Metabolism in Hepatocellular Carcinoma Cancer Stem Cells. Mol. Biol. Rep. 2020, 47, 2635–2647. [Google Scholar] [CrossRef]
- Tomeno, W.; Imajo, K.; Takayanagi, T.; Ebisawa, Y.; Seita, K.; Takimoto, T.; Honda, K.; Kobayashi, T.; Nogami, A.; Kato, T.; et al. Complications of Non-Alcoholic Fatty Liver Disease in Extrahepatic Organs. Diagnostics 2020, 10, 912. [Google Scholar] [CrossRef]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef]
- Fisher, E.A. The Degradation of Apolipoprotein B100: Multiple Opportunities to Regulate VLDL Triglyceride Production by Different Proteolytic Pathways. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 778–781. [Google Scholar] [CrossRef]
- Paiva, A.A.; Raposo, H.F.; Wanschel, A.C.B.A.; Nardelli, T.R.; Oliveira, H.C.F. Apolipoprotein CIII Overexpression-Induced Hypertriglyceridemia Increases Nonalcoholic Fatty Liver Disease in Association with Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2017, 2017, 1838679. [Google Scholar] [CrossRef]
- Duran, E.K.; Pradhan, A.D. Triglyceride-Rich Lipoprotein Remnants and Cardiovascular Disease. Clin. Chem. 2021, 67, 183–196. [Google Scholar] [CrossRef]
- Hinds, T.D.; Hosick, P.A.; Chen, S.; Tukey, R.H.; Hankins, M.W.; Nestor-Kalinoski, A.; Stec, D.E. Mice with Hyperbilirubinemia Due to Gilbert’s Syndrome Polymorphism Are Resistant to Hepatic Steatosis by Decreased Serine 73 Phosphorylation of PPARα. Am. J. Physiol. Metab. 2017, 312, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Stingl, H.; Chandramouli, V.; Schumann, W.C.; Hofer, A.; Landau, B.R.; Nowotny, P.; Waldhäusl, W.; Shulman, G.I. Effects of Free Fatty Acid Elevation on Postabsorptive Endogenous Glucose Production and Gluconeogenesis in Humans. Diabetes 2000, 49, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.-H.; Brickey, W.J.; Ting, J.P.-Y. Fatty Acid–Induced NLRP3-ASC Inflammasome Activation Interferes with Insulin Signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Szczerbinska, A.; Kasztelan-Szczerbinska, B.; Rycyk-Bojarzynska, A.; Kocki, J.; Cichoz-Lach, H. Hemochromatosis—How Not to Overlook and Properly Manage “Iron People”—A Review. J. Clin. Med. 2024, 13, 3360. [Google Scholar] [CrossRef] [PubMed]
- Onuigwe, F.; Odeh, R.; Uchechukwu, N.; Obeagu, E. Iron Chelators in The Management of Hereditary Hemochromatosis. Elite J. Haematol. 2024, 2, 1–19. [Google Scholar]
- Delatycki, M.B.; Allen, K.J. Population Screening for Hereditary Haemochromatosis—Should It Be Carried Out, and If So, How? Genes 2024, 15, 967. [Google Scholar] [CrossRef]
- Barton, J.C.; McLaren, C.E.; Chen, W.; Ramm, G.A.; Anderson, G.J.; Powell, L.W.; Subramaniam, V.N.; Adams, P.C.; Phatak, P.D.; Gurrin, L.C. Cirrhosis in Hemochromatosis: Independent Risk Factors in 368 HFE p. C282Y Homozygotes. Ann. Hepatol. 2018, 17, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Anderson, R.A.; Kuzuya, T.; Kitaura, Y.; Shimomura, Y. Multiple Factors and Pathways Involved in Hepatic Very Low Density Lipoprotein-ApoB100 Overproduction in Otsuka Long-Evans Tokushima Fatty Rats. Atherosclerosis 2012, 222, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C.; Barton, J.C. How I Treat Hemochromatosis. Blood 2010, 116, 317–325. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron. Int. J. Hematol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Jin, F.; Qu, L.S.; Shen, X.Z. Association between C282Y and H63D Mutations of the HFE Gene with Hepatocellular Carcinoma in European Populations: A Meta-Analysis. J. Exp. Clin. Cancer Res. 2010, 29, 18. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bacon, B.R. Relation of Hemochromatosis with Hepatocellular Carcinoma: Epidemiology, Natural History, Pathophysiology, Screening, Treatment, and Prevention. Med. Clin. N. Am. 2005, 89, 391–409. [Google Scholar] [CrossRef]
- Ellervik, C.; Birgens, H.; Tybjaerg-Hansen, A.; Nordestgaard, B. Hemochromatosis Genotypes and Risk of 31 Disease Endpoints: Meta-Analyses Including 66,000 Cases and 226,000 Controls. Hepatology 2007, 46, 1071–1080. [Google Scholar] [CrossRef]
- Yang, Q.; McDonnell, S.M.; Khoury, M.J.; Cono, J.; Parrish, R.G. Hemochromatosis-Associated Mortality in the United States from 1979 to 1992: An Analysis of Multiple-Cause Mortality Data. Ann. Intern. Med. 1998, 129, 946–953. [Google Scholar] [CrossRef]
- Elmberg, M.; Hultcrantz, R.; Ekbom, A.; Brandt, L.; Olsson, S.; Olsson, R.; Lindgren, S.; Lööf, L.; Stål, P.; Wallerstedt, S.; et al. Cancer Risk in Patients with Hereditary Hemochromatosis and in Their First-Degree Relatives. Gastroenterology 2003, 125, 1733–1741. [Google Scholar] [CrossRef]
- Pascale, R.M.; Calvisi, D.F.; Feo, F.; Simile, M.M. Genetic Predisposition to Hepatocellular Carcinoma. Metabolites 2023, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Luna, S.A.; Brown, K.E. Clinical Burden of Liver Disease from Hemochromatosis at an Academic Medical Center. Hepatol. Commun. 2017, 1, 453–459. [Google Scholar] [CrossRef]
- Ye, Q.; Qian, B.X.; Yin, W.L.; Wang, F.M.; Han, T. Association between the HFE C282Y, H63D Polymorphisms and the Risks of Non-Alcoholic Fatty Liver Disease, Liver Cirrhosis and Hepatocellular Carcinoma: An Updated Systematic Review and Meta-Analysis of 5,758 Cases and 14,741 Controls. PLoS ONE 2016, 11, e0163423. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, A.; Shrestha, R.; Bridle, K.R.; Crawford, D.H.G. Association between Hereditary Hemochromatosis and Hepatocellular Carcinoma: A Comprehensive Review. Hepatoma Res. 2020, 6, 8. [Google Scholar] [CrossRef]
- Sandnes, M.; Ulvik, R.J.; Vorland, M.; Reikvam, H. Hyperferritinemia—A Clinical Overview. J. Clin. Med. 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, T.; Trotter, J.F.; Kam, I. Hepatocellular Carcinoma in a Noncirrhotic Patient With Hereditary Hemochromatosis. Am. J. Med. Sci. 2007, 334, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Mossanda, K.S.; Kew, M.C.; Paterson, A.C.; Kahler-Venter, C.P.; Siziba, K. Hepatocellular Carcinoma Caused by Iron Overload: A Possible Mechanism of Direct Hepatocarcinogenicity. Toxicology 2006, 219, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Giger, R.S.; Krayenbuehl, P.A. Higher Age at Diagnosis of Hemochromatosis Is the Strongest Predictor of the Occurrence of Hepatocellular Carcinoma in the Swiss Hemochromatosis Cohort A Prospective Longitudinal Observational Study. Medicine 2018, 97, 12886. [Google Scholar] [CrossRef] [PubMed]
- Hellerbrand, C.; Pöppl, A.; Hartmann, A.; Schölmerich, J.; Lock, G. HFE C282Y Heterozygosity in Hepatocellular Carcinoma: Evidence for an Increased Prevalence. Clin. Gastroenterol. Hepatol. 2003, 1, 279–284. [Google Scholar] [CrossRef]
- Arosio, P. New Advances in Iron Metabolism, Ferritin and Hepcidin Research. Int. J. Mol. Sci. 2022, 23, 14700. [Google Scholar] [CrossRef]
- Varga, E.; Pap, R.; Jánosa, G.; Sipos, K.; Pandur, E. IL-6 Regulates Hepcidin Expression Via the BMP/SMAD Pathway by Altering BMP6, TMPRSS6 and TfR2 Expressions at Normal and Inflammatory Conditions in BV2 Microglia. Neurochem. Res. 2021, 46, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, W.; Yan, X.; Huang, T.; Yang, A. Overexpression of Hepcidin Alleviates Steatohepatitis and Fibrosis in a Diet-Induced Nonalcoholic Steatohepatitis. J. Clin. Transl. Hepatol. 2022, 10, 577–588. [Google Scholar] [CrossRef]
- Aigner, E.; Theurl, I.; Theurl, M.; Lederer, D.; Haufe, H.; Dietze, O.; Strasser, M.; Datz, C.; Weiss, G. Pathways Underlying Iron Accumulation in Human Nonalcoholic Fatty Liver Disease1. Am. J. Clin. Nutr. 2008, 87, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini–Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased Adipose Tissue Expression of Hepcidin in Severe Obesity Is Independent from Diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, N.; Nishimata, S.; Shimura, M.; Kashiwagi, Y.; Kawashima, H. Hepcidin Levels and Pathological Characteristics in Children with Fatty Liver Disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 295. [Google Scholar] [CrossRef]
- Sorrentino, P.; D’Angelo, S.; Ferbo, U.; Micheli, P.; Bracigliano, A.; Vecchione, R. Liver Iron Excess in Patients with Hepatocellular Carcinoma Developed on Non-Alcoholic Steato-Hepatitis. J. Hepatol. 2009, 50, 351–357. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron Metabolism and Iron Disorders Revisited in the Hepcidin Era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Mehta, K.J.; Je Farnaud, S.; Sharp, P.A. Iron and Liver Fibrosis: Mechanistic and Clinical Aspects. World J. Gastroenterol. 2019, 25, 521–538. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. The Role of Hepcidin in Iron Metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Wang, J.Y.; Liu, P.Y.; Sun, J.; Wang, X.M.; Wu, R.H.; He, X.T.; Tu, Z.K.; Wang, C.G.; Xu, H.Q.; et al. Iron Metabolism Disorders in Patients with Hepatitis B-Related Liver Diseases. World J. Clin. Cases 2018, 6, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Maegdefrau, U.; Arndt, S.; Kivorski, G.; Hellerbrand, C.; Bosserhoff, A.-K. Downregulation of Hemojuvelin Prevents Inhibitory Effects of Bone Morphogenetic Proteins on Iron Metabolism in Hepatocellular Carcinoma. Lab. Investig. 2011, 91, 1615–1623. [Google Scholar] [CrossRef][Green Version]
- Abd Elmonem, E.; Tharwa, E.-S.; Farag, M.A.; Fawzy, A.; El Shinnawy, S.F.; Suliman, S. Hepcidin MRNA Level as a Parameter of Disease Progression in Chronic Hepatitis C and Hepatocellular Carcinoma. J. Egypt. Natl. Canc. Inst. 2009, 21, 333–342. [Google Scholar]
- Hawula, Z.J.; Wallace, D.F.; Subramaniam, V.N.; Rishi, G. Therapeutic Advances in Regulating the Hepcidin/Ferroportin Axis. Pharmaceuticals 2019, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yang, Y.; Wu, K.; Zhao, T.; Shi, Y.; Song, M.; Li, J. The Effects of Dandelion Polysaccharides on Iron Metabolism by Regulating Hepcidin via JAK/STAT Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 7184760. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, A.; Liu, G.; Anderson, G.J.; Hu, T.Y.; Shi, J.; Hu, Y.; Nie, G. Correlation of Serum Hepcidin Levels with Disease Progression in Hepatitis B Virus-Related Disease Assessed by Nanopore Film Based Assay. Sci. Rep. 2016, 6, 34252. [Google Scholar] [CrossRef]
- Kijima, H.; Sawada, T.; Tomosugi, N.; Kubota, K. Expression of Hepcidin MRNA Is Uniformly Suppressed in Hepatocellular Carcinoma. BMC Cancer 2008, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Udali, S.; Castagna, A.; Corbella, M.; Ruzzenente, A.; Moruzzi, S.; Mazzi, F.; Campagnaro, T.; De Santis, D.; Franceschi, A.; Pattini, P.; et al. Hepcidin and DNA Promoter Methylation in Hepatocellular Carcinoma. Eur. J. Clin. Investig. 2018, 48, 12870. [Google Scholar] [CrossRef]
- Wang, L.F.; Fokas, E.; Juricko, J.; You, A.; Rose, F.; Pagenstecher, A.; Engenhart-Cabillic, R.; An, H.X. Increased Expression of EphA7 Correlates with Adverse Outcome in Primary and Recurrent Glioblastoma Multiforme Patients. BMC Cancer 2008, 8, 79. [Google Scholar] [CrossRef]
- Tan, M.G.K.; Kumarasinghe, M.P.; Wang, S.M.; Ooi, L.L.P.J.; Aw, S.E.; Hui, K.M. Modulation of Iron-Regulatory Genes in Human Hepatocellular Carcinoma and Its Physiological Consequences. Exp. Biol. Med. 2009, 234, 693–702. [Google Scholar] [CrossRef]
- Saleem, M.; Adhami, V.M.; Zhong, W.; Longley, B.J.; Lin, C.-Y.; Dickson, R.B.; Reagan-Shaw, S.; Jarrard, D.F.; Mukhtar, H. A Novel Biomarker for Staging Human Prostate Adenocarcinoma: Overexpression of Matriptase with Concomitant Loss of Its Inhibitor, Hepatocyte Growth Factor Activator Inhibitor-1. Cancer Epidemiol. Biomark. Prev. 2006, 15, 217–227. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Chao, Y.-C.; Lee, W.-H.; Chen, A.; Sheu, L.-F.; Jin, J.-S. Increasing EMMPRIN and Matriptase Expression in Hepatocellular Carcinoma: Tissue Microarray Analysis of Immunohistochemical Scores with Clinicopathological Parameters. Histopathology 2006, 49, 388–395. [Google Scholar] [CrossRef]
- El-Mahdy, R.I.; Zakhary, M.M.; Maximous, D.W.; Mokhtar, A.A.; El Dosoky, M.I. Circulating Osteocyte-related Biomarkers (Vitamin D, Sclerostin, Dickkopf-1), Hepcidin, and Oxidative Stress Markers in Early Breast Cancer: Their Impact in Disease Progression and Outcome. J. Steroid Biochem. Mol. Biol. 2020, 204, 105773. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, J.-M.; Lee, Y.-S.; Li, S.; Lee, S.-J.; Bae, S.-C.; Jung, H.-S. Runx3 Regulates Iron Metabolism via Modulation of BMP Signalling. Cell Prolif. 2021, 54, 13138. [Google Scholar] [CrossRef]
- Link, T.; Iwakuma, T. Roles of P53 in Extrinsic Factor-Induced Liver Carcinogenesis. Hepatoma Res. 2017, 3, 95. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Liu, X.; Chi, X.; Gong, Q.; Gao, L.; Niu, Y.; Chi, X.; Cheng, M.; Si, Y.; Wang, M.; Zhong, J.; et al. Association of Serum Level of Growth Differentiation Factor 15 with Liver Cirrhosis and Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0127518. [Google Scholar] [CrossRef]
- Wu, M.; Sun, T.; Xing, L. Circ_0004913 Inhibits Cell Growth, Metastasis, and Glycolysis by Absorbing MiR-184 to Regulate HAMP in Hepatocellular Carcinoma. Cancer Biother. Radiopharm. 2020, 38, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Nahon, P.; Nuraldeen, R.; Rufat, P.; Sutton, A.; Trautwein, C.; Strnad, P. In Alcoholic Cirrhosis, Low-Serum Hepcidin Levels Associate with Poor Long-Term Survival. Liver Int. 2016, 36, 185–188. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Li, J.C.; Li, M.; Li, B.; Zhu, R. Hepcidin Downregulation Correlates with Disease Aggressiveness and Immune Infiltration in Liver Cancers. Front. Oncol. 2021, 11, 714756. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Koo, J.H.; Kim, S.H.; Gardenghi, S.; Rivella, S.; Strnad, P.; Hwang, S.J.; Kim, S.G. Hepcidin Inhibits Smad3 Phosphorylation in Hepatic Stellate Cells by Impeding Ferroportin-Mediated Regulation of Akt. Nat. Commun. 2016, 7, 13817. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Yagoda, N.; Von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J. RAS–RAF–MEK-Dependent Oxidative Cell Death Involving Voltage-Dependent Anion Channels. Nature 2007, 447, 865–869. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.W.; Sviderskiy, V.O.; Terzi, E.M.; Papagiannakopoulos, T.; Moreira, A.L.; Adams, S.; Sabatini, D.M.; Birsoy, K.; Possemato, R. NFS1 Undergoes Positive Selection in Lung Tumours and Protects Cells from Ferroptosis. Nature 2017, 551, 639–643. [Google Scholar] [CrossRef]
- Capelletti, M.M.; Manceau, H.; Puy, H.; Peoc’h, K. Ferroptosis in Liver Diseases: An Overview. Int. J. Mol. Sci. 2020, 21, 4908. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, S.; Tsuchiya, Y.; Koumura, T.; Nakasone, M.; Sakamoto, T.; Matsuoka, M.; Imai, H.; Yuet-Yin Kok, C.; Okochi, H.; Nakano, H. Hepatic Ferroptosis Plays an Important Role as the Trigger for Initiating Inflammation in Nonalcoholic Steatohepatitis. Cell Death Dis. 2019, 10, 449. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Huang, X.; Li, Y.; Sun, T.; Zang, S.; Guan, K.; Xiong, Y.; Liu, J.; Yuan, H. Targeting Ferroptosis Alleviates Methionine-choline Deficient (MCD)-diet Induced NASH by Suppressing Liver Lipotoxicity. Liver Int. 2020, 40, 1378–1394. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The Multifaceted Role of Ferroptosis in Liver Disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef]
- Liao, H.; Shi, J.; Wen, K.; Lin, J.; Liu, Q.; Shi, B.; Yan, Y.; Xiao, Z. Molecular Targets of Ferroptosis in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 2021, 985–996. [Google Scholar] [CrossRef]
- Recalcati, S.; Correnti, M.; Gammella, E.; Raggi, C.; Invernizzi, P.; Cairo, G. Iron Metabolism in Liver Cancer Stem Cells. Front. Oncol. 2019, 9, 149. [Google Scholar] [CrossRef]
- Liu, J.; Dai, E.; Kang, R.; Kroemer, G.; Tang, D. The Dark Side of Ferroptosis in Pancreatic Cancer. Oncoimmunology 2021, 10, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, C.; Chen, Y.; Hu, W.; Lu, Y.; Wu, W.; Zhang, Y.; Yang, B.; Wu, H.; Peng, C.; et al. ACSL4 Promotes Hepatocellular Carcinoma Progression via C-Myc Stability Mediated by ERK/FBW7/c-Myc Axis. Oncogenesis 2020, 9, 42. [Google Scholar] [CrossRef]
- Fu, Y.; Silverstein, S.; Mccutcheon, J.N.; Dyba, M.; Nath, R.G.; Aggarwal, M.; Coia, H.; Bai, A.; Pan, J.; Jiang, J.; et al. HHS Public Access. Hepatology 2019, 67, 159–170. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Y.; Zhang, Z.; Tan, N.; Zhao, F.; Ge, C.; Liang, L.; Jia, D.; Chen, T.; Yao, M.; et al. Disruption of XCT Inhibits Cell Growth via the ROS/Autophagy Pathway in Hepatocellular Carcinoma. Cancer Lett. 2011, 312, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, Y.; Pan, Z.; Glandorff, C.; Sun, M. Ferroptosis: A New Hunter of Hepatocellular Carcinoma. Cell Death Discov. 2024, 10, 136. [Google Scholar] [CrossRef]
- Lim, K.; Han, C.; Dai, Y.; Shen, M.; Wu, T. Omega-3 Polyunsaturated Fatty Acids Inhibit Hepatocellular Carcinoma Cell Growth through Blocking β-Catenin and Cyclooxygenase-2. Mol. Cancer Ther. 2009, 8, 3046–3055. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Krause, L.F.; Gomolka, B.; Chiu, C.-Y.; Bilal, S.; Nadolny, A.; Waechter, S.F.; Fischer, A.; Rothe, M.; Kang, J.X. Suppressed Liver Tumorigenesis in Fat-1 Mice with Elevated Omega-3 Fatty Acids Is Associated with Increased Omega-3 Derived Lipid Mediators and Reduced TNF-α. Carcinogenesis 2011, 32, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Bekric, D.; Ocker, M.; Mayr, C.; Stintzing, S.; Ritter, M.; Kiesslich, T.; Neureiter, D. Ferroptosis in Hepatocellular Carcinoma: Mechanisms, Drug Targets and Approaches to Clinical Translation. Cancers 2022, 14, 1826. [Google Scholar] [CrossRef]
- Ren, X.; Wang, X.; Yan, Y.; Chen, X.; Cai, Y.; Liang, Q.; Peng, B.; Xu, Z.; He, Q.; Kang, F.; et al. Integrative Bioinformatics and Experimental Analysis Revealed TEAD as Novel Prognostic Target for Hepatocellular Carcinoma and Its Roles in Ferroptosis Regulation. Aging 2022, 14, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yao, X.; Xiang, J.; Huang, F.; Luo, H. Identification of Ferroptosis-Related Genes for Overall Survival Prediction in Hepatocellular Carcinoma. Sci. Rep. 2022, 12, 10007. [Google Scholar] [CrossRef]
- Wang, W.; Pan, F.; Lin, X.; Yuan, J.; Tao, C.; Wang, R. Ferroptosis-Related Hub Genes in Hepatocellular Carcinoma: Prognostic Signature, Immune-Related, and Drug Resistance Analysis. Front. Genet. 2022, 13, 907331. [Google Scholar] [CrossRef]
- Lin, C.L.; Kao, J.H. Development of Hepatocellular Carcinoma in Treat- Ed and Untreated Patients with Chronic Hepatitis B Virus Infection. Clin. Mol. Hepatol. 2023, 29, 605–622. [Google Scholar] [CrossRef]
- Miyanishi, K.; Tanaka, S.; Sakamoto, H.; Kato, J. The Role of Iron in Hepatic Inflammation and Hepatocellular Carcinoma. Free Radic. Biol. Med. 2019, 133, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Yonal, O.; Akyuz, F.; Demir, K.; Ciftci, S.; Keskin, F.; Pinarbasi, B.; Uyanikoglu, A.; Issever, H.; Ozdil, S.; Boztas, G.; et al. Decreased Prohepcidin Levels in Patients with HBV-Related Liver Disease: Relation with Ferritin Levels. Dig. Dis. Sci. 2010, 55, 3548–3551. [Google Scholar] [CrossRef]
- Gaboriau, F.; Leray, A.-M.; Ropert, M.; Gouffier, L.; Cannie, I.; Troadec, M.-B.; Loréal, O.; Brissot, P.; Lescoat, G. Effects of Deferasirox and Deferiprone on Cellular Iron Load in the Human Hepatoma Cell Line HepaRG. Biometals 2010, 23, 231–245. [Google Scholar] [CrossRef]
- Lui, G.Y.L.; Obeidy, P.; Ford, S.J.; Tselepis, C.; Sharp, D.M.; Jansson, P.J.; Kalinowski, D.S.; Kovacevic, Z.; Lovejoy, D.B.; Richardson, D.R. The Iron Chelator, Deferasirox, as a Novel Strategy for Cancer Treatment: Oral Activity against Human Lung Tumor Xenografts and Molecular Mechanism of Action. Mol. Pharmacol. 2013, 83, 179–190. [Google Scholar] [CrossRef]
- Ba, Q.; Hao, M.; Huang, H.; Hou, J.; Ge, S.; Zhang, Z.; Yin, J.; Chu, R.; Jiang, H.; Wang, F. Iron Deprivation Suppresses Hepatocellular Carcinoma Growth in Experimental Studies. Clin. Cancer Res. 2011, 17, 7625–7633. [Google Scholar] [CrossRef]
- Saeki, I.; Yamamoto, N.; Yamasaki, T.; Takami, T.; Maeda, M.; Fujisawa, K.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T. Effects of an Oral Iron Chelator, Deferasirox, on Advanced Hepatocellular Carcinoma. World J. Gastroenterol. 2016, 22, 8967. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Wang, H.; Xia, J.; Yang, Y.; Jin, Z.; Xu, H.; Shi, J.; De Domenico, I.; Tricot, G.; Zhan, F. Decreased Ferroportin Promotes Myeloma Cell Growth and Osteoclast Differentiation. Cancer Res. 2015, 75, 2211–2221. [Google Scholar] [CrossRef]
- Poli, M.; Girelli, D.; Campostrini, N.; Maccarinelli, F.; Finazzi, D.; Luscieti, S.; Nai, A.; Arosio, P. Heparin: A Potent Inhibitor of Hepcidin Expression in Vitro and in Vivo. Blood 2011, 117, 997–1004. [Google Scholar] [CrossRef]
- Tisi, M.C.; Bozzoli, V.; Giachelia, M.; Massini, G.; Ricerca, B.M.; Maiolo, E.; D’Alo’, F.; Larocca, L.M.; Piciocchi, A.; Tjalsma, H. Anemia in Diffuse Large B-Cell Non-Hodgkin Lymphoma: The Role of Interleukin-6, Hepcidin and Erythropoietin. Leuk. Lymphoma 2014, 55, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.; Asperti, M.; Ruzzenenti, P.; Regoni, M.; Arosio, P. Hepcidin Antagonists for Potential Treatments of Disorders with Hepcidin Excess. Front. Pharmacol. 2014, 5, 86. [Google Scholar] [CrossRef]
- Nishizawa, S.; Araki, H.; Ishikawa, Y.; Kitazawa, S.; Hata, A.; Soga, T.; Hara, T. Low Tumor Glutathione Level as a Sensitivity Marker for Glutamate-cysteine Ligase Inhibitors. Oncol. Lett. 2018, 15, 8735–8743. [Google Scholar] [CrossRef]
- Guo, J.; Xu, B.; Han, Q.; Zhou, H.; Xia, Y.; Gong, C.; Dai, X.; Li, Z.; Wu, G. Ferroptosis: A Novel Anti-Tumor Action for Cisplatin. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Reliene, R.; Schiestl, R.H. Glutathione Depletion by Buthionine Sulfoximine Induces DNA Deletions in Mice. Carcinogenesis 2006, 27, 240–244. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H. The Role of Ferroptosis in Ionizing Radiation-Induced Cell Death and Tumor Suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cao, W.; Zhou, H.; Qian, H.; Wang, H. CLTRN, Regulated by NRF1/RAN/DLD Protein Complex, Enhances Radiation Sensitivity of Hepatocellular Carcinoma Cells through Ferroptosis Pathway. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 859–871. [Google Scholar] [CrossRef]
- Liu, H.-T.; Cheng, S.-B.; Huang, Y.-C.; Huang, Y.-T.; Lin, P.-T. Coenzyme Q10 and Oxidative Stress: Inflammation Status in Hepatocellular Carcinoma Patients after Surgery. Nutrients 2017, 9, 29. [Google Scholar] [CrossRef]
- Scarpellini, C.; Klejborowska, G.; Lanthier, C.; Hassannia, B.; Berghe, T.V.; Augustyns, K. Beyond Ferrostatin-1: A Comprehensive Review of Ferroptosis Inhibitors. Trends Pharmacol. Sci. 2023, 44, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Lian, P.; Lv, Q.; Liu, F. Silencing LncRNA HCG18 Regulates GPX4-Inhibited Ferroptosis by Adsorbing MiR-450b-5p to Avert Sorafenib Resistance in Hepatocellular Carcinoma. Hum. Exp. Toxicol. 2023, 42, 09603271221142818. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Q.; Cheng, J.; Shen, X.; Li, J.; Chen, M.; Zhou, C.; Zhou, J. LncRNA SNHG1 Upregulates FANCD2 and G6PD to Suppress Ferroptosis by Sponging MiR-199a-5p/3p in Hepatocellular Carcinoma. Drug Discov. Ther. 2023, 17, 248–256. [Google Scholar] [CrossRef]
- Tu, S.; Zou, Y.; Yang, M.; Zhou, X.; Zheng, X.; Jiang, Y.; Wang, H.; Chen, B.; Qian, Q.; Dou, X.; et al. Ferroptosis in Hepatocellular Carcinoma: Mechanisms and Therapeutic Implications. Biomed. Pharmacother. 2025, 182, 117769. [Google Scholar] [CrossRef]
- Chen, Y.; Shang, H.; Wang, C.; Zeng, J.; Zhang, S.; Wu, B.; Cheng, W. RNA-Seq Explores the Mechanism of Oxygen-Boosted Sonodynamic Therapy Based on All-in-One Nanobubbles to Enhance Ferroptosis for the Treatment of HCC. Int. J. Nanomed. 2022, 2022, 105–123. [Google Scholar] [CrossRef]
- YoungáKim, W.; SeungáKim, J. Biotin-Guided Anticancer Drug Delivery with Acidity-Triggered Drug Release. Chem. Commun. 2015, 51, 9343–9345. [Google Scholar] [CrossRef]
- Wang, L.; Tong, L.; Xiong, Z.; Chen, Y.; Zhang, P.; Gao, Y.; Liu, J.; Yang, L.; Huang, C.; Ye, G. Ferroptosis-Inducing Nanomedicine and Targeted Short Peptide for Synergistic Treatment of Hepatocellular Carcinoma. J. Nanobiotechnol. 2024, 22, 533. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, X.; Xu, H.; Yang, J.; Wang, C.; Zhang, C.; Deng, Y.; Lu, A.; Zheng, C. Carrier-Free Self-Assembled Nanomedicine Based on Celastrol and Galactose for Targeting Therapy of Hepatocellular Carcinoma via Inducing Ferroptosis. Eur. J. Med. Chem. 2024, 267, 116183. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, X.; Qi, X.; Meng, X.; Xu, K. Co-Administration of IRGD with Sorafenib-Loaded Iron-Based Metal-Organic Framework as a Targeted Ferroptosis Agent for Liver Cancer Therapy. Int. J. Nanomed. 2021, 2021, 1037–1050. [Google Scholar] [CrossRef]
- Yang, F.; Dong, Q.; Chen, Z.; Gao, B.; Zheng, D.; Wang, R.; Qin, S.; Peng, F.; Luo, M.; Yang, J. A PH-Responsive Drug-Delivery System Based on Apatinib-Loaded Metal-Organic Frameworks for Ferroptosis-Targeted Synergistic Anti-Tumor Therapy. Int. J. Nanomed. 2024, 2024, 9055–9070. [Google Scholar] [CrossRef] [PubMed]
- Delaporta, P.; Chatzikalil, E.; Ladis, V.; Moraki, M.; Kattamis, A. Evolving Changes in the Characteristics of Death in Transfusion Dependent Thalassemia in Greece. Blood 2023, 142, 1103. [Google Scholar] [CrossRef]
- Leyh, C.; Coombes, J.D.; Schmidt, H.H.; Canbay, A.; Manka, P.P.; Best, J. MASLD-Related HCC—Update on Pathogenesis and Current Treatment Options. J. Pers. Med. 2024, 14, 370. [Google Scholar] [CrossRef] [PubMed]
- Chatzikalil, E.; Stergiou, I.E.; Papadakos, S.P.; Konstantinidis, I.; Theocharis, S. The Clinical Relevance of the EPH/Ephrin Signaling Pathway in Pediatric Solid and Hematologic Malignancies. Int. J. Mol. Sci. 2024, 25, 3834. [Google Scholar] [CrossRef]
- Zheng, S.; Chan, S.W.; Liu, F.; Liu, J.; Chow, P.K.H.; Toh, H.C.; Hong, W. Hepatocellular Carcinoma: Current Drug Therapeutic Status, Advances and Challenges. Cancers 2024, 16, 1582. [Google Scholar] [CrossRef]
- Cunha, G.M.; Hosseini, M.; Furlan, A.; Fowler, K.J. Hepatocellular Carcinoma Staging: Differences Between Radiologic and Pathologic Systems and Relevance to Patient Selection and Outcomes in Liver Transplantation. Am. J. Roentgenol. 2021, 218, 77–86. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Botta, C.; Aversa, I.; Mancuso, S.; Costanzo, F.; Biamonte, F. Iron Metabolism in the Tumor Microenvironment—Implications for Anti-Cancer Immune Response. Cells 2021, 10, 303. [Google Scholar] [CrossRef]
- Natarajan, Y.; Patel, P.; Chu, J.; Yu, X.; Hernaez, R.; El-Serag, H.; Kanwal, F. Risk of Hepatocellular Carcinoma in Patients with Various HFE Genotypes. Dig. Dis. Sci. 2023, 68, 312–322. [Google Scholar] [CrossRef]
- Salgia, R.J.; Brown, K. Diagnosis and Management of Hereditary Hemochromatosis. Clin. Liver Dis. 2015, 19, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]



| REF | Serum | Tissue | HBV-HCC | Non-Viral HCC | Median | Range/SD | Control | Hepcidin Level (Increased/Decreased) |
|---|---|---|---|---|---|---|---|---|
| [168] | + | + | 4.62 nm | 3.28–6.51 nm | 4.33 to 8.41 nm | Normal | ||
| [161] | + | + | 200 ng/mL | n/a | 600 ng/mL | Decreased | ||
| [162] | + | + | 175 ng/mL | ±175 ng/mL | 250–550 ng/mL | Decreased | ||
| [163] | + | + | 2351 copies/mL | ±505 copies/mL | 16,308 ± 2194 copies/mL | Decreased | ||
| [169] | + | + | 9.3 ng/mL | ±4.9 ng/mL | 4.8 ± 2.0 ng/ml | Increased | ||
| [167] | + | + | (1) 42.6 (2) 15.5 | (1) 35.6–75.0 (2) 1.2–28.5 | 22.2 ± 12.3 ng/ml | Increased (1)/Normal (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzikalil, E.; Arvanitakis, K.; Kalopitas, G.; Florentin, M.; Germanidis, G.; Koufakis, T.; Solomou, E.E. Hepatic Iron Overload and Hepatocellular Carcinoma: New Insights into Pathophysiological Mechanisms and Therapeutic Approaches. Cancers 2025, 17, 392. https://doi.org/10.3390/cancers17030392
Chatzikalil E, Arvanitakis K, Kalopitas G, Florentin M, Germanidis G, Koufakis T, Solomou EE. Hepatic Iron Overload and Hepatocellular Carcinoma: New Insights into Pathophysiological Mechanisms and Therapeutic Approaches. Cancers. 2025; 17(3):392. https://doi.org/10.3390/cancers17030392
Chicago/Turabian StyleChatzikalil, Elena, Konstantinos Arvanitakis, Georgios Kalopitas, Matilda Florentin, Georgios Germanidis, Theocharis Koufakis, and Elena E. Solomou. 2025. "Hepatic Iron Overload and Hepatocellular Carcinoma: New Insights into Pathophysiological Mechanisms and Therapeutic Approaches" Cancers 17, no. 3: 392. https://doi.org/10.3390/cancers17030392
APA StyleChatzikalil, E., Arvanitakis, K., Kalopitas, G., Florentin, M., Germanidis, G., Koufakis, T., & Solomou, E. E. (2025). Hepatic Iron Overload and Hepatocellular Carcinoma: New Insights into Pathophysiological Mechanisms and Therapeutic Approaches. Cancers, 17(3), 392. https://doi.org/10.3390/cancers17030392







