Simple Summary
Pediatric brain tumors remain a leading cause of cancer-related death in children. Even with advances in the multimodality management of these malignancies, the outcome remains poor in many cases. The development of new drugs has been frustratingly slow, due to the cost and lengthy steps needed for new drug development. To expedite this, drug repurposing is an attractive approach, as it evaluates approved drugs, which have already gone through the safety, pharmacological, and regulatory validation prior to it being driven to clinics. This provides hope for these children who harbor such formidable tumors.
Abstract
Central nervous system (CNS) tumors are the leading cause of cancer-related mortality in children, with prognosis remaining dismal for some of these malignancies. Though the past two decades have seen advancements in surgery, radiation, and targeted therapy, major unresolved hurdles continue to undermine the therapeutic efficacy. These include challenges in suboptimal drug delivery through the blood–brain barrier (BBB), marked intra-tumoral molecular heterogeneity, and the elusive tumor microenvironment. Drug repurposing or re-tasking FDA-approved drugs with evidence of penetration into the CNS, using newer methods of intracranial drug delivery facilitating optimal drug exposure, has been an area of intense research. This could be a valuable tool, as most of these agents have already gone through the lengthy process of drug development and the evaluation of safety risks and the optimal pharmacokinetic profile. They can now be used and tested in clinics with an accelerated and different approach. Conclusions: The next-generation therapeutic strategy should prioritize repurposing oncologic and non-oncologic drugs that have been used for other indication, and have demonstrated robust preclinical activity against pediatric brain tumors. In combination with novel drug delivery techniques, these drugs could hold significant therapeutic promise in pediatric neurooncology.
1. Introduction
Tumors of the central nervous system (CNS) are the leading cause of cancer-related deaths among children and adolescents [1,2]. The past decade has yielded significant advances in the molecular genomics of these tumors, yet this knowledge has not translated into robust improved outcomes, with a marginal increase of ~1.0% in the overall survival (OS) from the 1970s to the early 2000s [3]. Though the rate of oncology drug growth is rapidly improving, pediatric-specific CNS tumor drug development has unfortunately remained slow, with only ten new Food-and-Drug-Administration (FDA)-approved drugs entering the market since 1953 [4].
Even with the advancements in the multimodal therapies for pediatric CNS tumors, which include safe maximal surgical resection, chemotherapy (including targeted therapy, stem cell transplant), and irradiation therapy, unpredictable relapses and elusive resistance mechanisms continue to undermine the therapeutic efficacy. These tumors are markedly more heterogeneous in their molecular, histological, epidemiological, and prognostic classification than their adult counterparts, making similar treatment inferences across all ages challenging [5]. Pediatric low-grade glioma (LGG) is a prime example of this, with treatment varying widely from the standard of care in adults.
LGGs are the most common CNS tumors in the pediatric population, treated with conventional chemotherapy, and irradiation is usually reserved for those tumors with inoperable or sub-totally resectable disease. Unfortunately, both of these strategies are fraught with toxicities. The last decade has witnessed significant advances in identifying novel molecular signatures and oncogenic drivers of these tumors, paving the way to develop targeted therapy against these malignancies. The most common molecular aberration in these tumors is activation of the mitogen-activated protein kinase (MAPK) pathway. This has led to promising studies targeting the key genomic alterations in MEK (mitogen-activated extracellular signal-regulated kinase) and BRAF in this pathway. However, paradoxical MAPK pathway activation with BRAF inhibitor monotherapy resulted in the development of early relapse. This led to the development of a combinatorial therapy with a repurposing approach, using previously tested drugs, the BRAF along with MEK inhibitors, Dabrafenib and Trametinib, respectively. A synergistic benefit with prolonged remission was noted with this therapy [6,7].
Various subtypes of pediatric HGG are now identified with continuing molecular revelations, as defined in the WHO 2021 classification of CNS tumors [8,9]. The poorest prognostic subtype is H3 K27M-altered tumors, in which the OS is dismal at <10% [10,11]. Conversely, infant-type hemispheric glioma, with NTRK, ROS1, ALK, or MET aberrations, have improved outcomes, with 5-year overall survival ranging from 25 to 53.8% [12].
Pediatric embryonal tumors harbor unique genetic and epigenetic signatures, resulting in the need for stratified treatment strategies. Current regimens depend on the age and biological subtype, with young children receiving irradiation-sparing therapy post-surgically and older children receiving craniospinal irradiation followed by maintenance chemotherapy. The OS is dependent on the molecular subgroup, with TP53-mutated sonic hedgehog (SHH) and Group 3 (non-SHH and non-WNT) bearing the worst outcomes and WN- mutated and TP53-wild-type SHH demonstrating near universal survival rates [13,14,15,16].
The heterogeneous tumor microenvironment (TME) in pediatric CNS tumors plays a crucial role in both disease development and progression precluding therapeutic efficacy. An improved understanding of the cross-talks between the tumor and its microenvironment, escape mechanisms of the tumor from the host-protective immune system, and underpinnings in the development of therapeutic resistance, have now helped in profiling effective therapies, bypassing these encumbrances.
Unlike adults, CNS tumors in children have a low mutational burden, providing fewer antigens to be attacked by the host immune system, hindering the development of targeted therapies. These tumors are immunologically “cold”, characterized by the low expression of immunogenic markers and a paucity of cytokine immune infiltrates, thus being poorly reactive to the host immune defense system. On the contrary, the increased presence of immune-regulatory cells, like myeloid-derived suppressor cells (MDSCs) and T regulatory cells (Tregs), undermines the desired inflammatory response to targeted therapy. Furthermore, immunosuppressive molecules in the TME, like tumor-associated macrophages (TAM) and TGFβ, hinder the immune response to treatment [17].
A large methylation study evaluated the immune phenotype of more than 6000 pediatric CNS samples, which showed three immune clusters that were statistically significant, correlating with molecular subgroups/subtypes, mutations, and prognosis [18]. Some of the clinically relevant insights are discussed below.
The number of immune infiltrates is higher in LGG compared to HGG, with most infiltrating cells being macrophages and T cells [19]. Pediatric LGG and HGG have mildly increased CD8+ T cell infiltration, while diffuse midline glioma (H3K27 altered) does not [17]. Though these higher-grade gliomas have increased immunosuppressive TAMs (macrophage type 2, also known as CD163 cells), this immune infiltration does not uniformly correlate with poor prognosis, unlike in adult glioblastoma [18,20]. Medulloblastoma (MB), the most common malignant pediatric brain tumor, also has a cold TME and the lowest immune infiltrates when compared to other pediatric CNS tumors [21]. The immunophenotyping analysis of a large cohort of pediatric MB patients revealed the predictive capacity of the immune cell infiltrate population towards survival outcomes in the molecular subtypes of MB [22,23]. Understanding these immunological phenotypes in different pediatric CNS tumors is now providing clues to define optimal therapeutic regimens.
Though these newer biological insights are promising, we still face significant hurdles in optimizing the drug concentration at tumor sites [24]. This is largely attributed to the BBB, a unique complex barrier consisting of a near impenetrable system, made of endothelial cells, astrocytes, pericytes, neurons, and an extracellular matrix [25]. While this tightly orchestrated interplay is critical for self-protection, it results in a markedly decreased permeability for drug delivery. The phenotypic heterogeneity of the BBB across molecular subtypes of primary pediatric CNS tumors makes it even more challenging to determine the best drug-delivery method.
Even with exciting research advances in the molecular basis of pediatric neuro-oncogenesis, current treatment outcomes have remained prognostically static, along with a miniscule pace of profiling new drugs against pediatric CNS tumors. A major challenge is the protracted time and enormous cost of developing a new drug. A newer expedited attractive approach is drug repositioning, using approved or investigational drugs with already established pharmacokinetics, pharmacodynamics, and safety profiles, facilitating quicker patient access to newer treatment options.
The following section provides updates on the repurposing of drugs (oncologic and non-oncologic) for pediatric brain tumors and reviews various methods of intracranial drug delivery, which mitigate the concerning systemic toxicity, augment the drug concentration into the tumors, and improve therapeutic efficacy.
2. Repurposing of Drugs for Brain Tumors
The last few decades have witnessed an outpouring of new biological data including druggable targets, in pediatric brain tumors. However, translational of new drugs to the clinics has been painstakingly slow [26,27]. Major obstacles of bringing a new drug from bench to bedside, are the protracted time of nearly 13 years, cost of 2–3 billion dollars and a low success rate of approximately 12% [28]. After a drug of interest is identified, it goes through pharmacokinetic (PK) and pharmacodynamic (PD) evaluation, involving in vitro and in vivo studies and assessment of toxicity. It then proceeds for regulatory approval, prior to moving into a phase I study in humans. This phase evaluates feasibility, safety, and toxicity profile, along with a tolerated dose level finding. If successful, it will move into a phase II and III study after IRB or FDA approval. Even with all these efforts, only one in 5000–10,000 prospective anticancer agents receive FDA approval, and only 5% of oncology drugs entering Phase I clinical trials are ultimately approved [29]. This is an arduous and lengthy process, resulting in significant delay in drug availability for cancer patients, with a recent trend in lack of interest by the pharmaceutical industry in developing a new drug. Thus, there is a dire need to develop a therapeutic innovation of a quicker, effective, and safer method of drug development for cancer patients, overcoming the decades of bottleneck of profiling new cancer therapeutics.
A promising next-generation therapeutic strategy of drug repurposing, also known as repositioning or redirecting, is now actively pursued in pediatric neurooncology. This novel concept allows drugs or investigational agents, which are FDA approved and used for other indications (oncologic or non-oncologic), including widely available generic medications, to be assessed for repurposing. The advantage of this approach is the drugs of interest have already gone through a rigorous process of preclinical studies, including its evaluation of PK, PD, drug interaction parameters, toxicity profile, and phase I studies, for a different noncancer morbidity or another type of malignancy. They can now be translated into clinical use without the need for structural or chemical modification. This concept proved successful as witnessed by the expedited approval of drugs during the 2020 COVID-19 pandemic [30,31]. Renewed interest by the industry in developing cancer drugs using this strategy has been kindled, with the potential of decreased time and cost along with need for much less regulatory barriers [32].
Similarities in cancer specific biological pathways or mechanisms of action (MOA), have enabled drugs used for different malignancy or non-oncologic conditions, to be expeditiously redirected for brain tumors. Other avenues for repurposing are identifying effective combinations of drugs with synergy targeting several signaling pathways, helping to overcome progression of adaptive therapy resistance. Another attractive method is redirecting drugs to the CNS, using novel drug delivery methods, undermining disconcerting toxicities seen with systemic therapy [33,34]. In addition, these repurposed drugs used alone or in combination, have the potential of targeting multiple unrelated oncogenic pathways driving tumorigenesis, including undruggable targets, a distinct advantage over single molecularly targeted therapy [35].
Identifying a suitable compound for repurposing in pediatric brain tumors, must have a strong biological and clinical rationale based on unmet need in this vulnerable population. Then the drug of interest will have to fulfill these criteria, before being approved for clinical use. They must have a favorable profile of BBB penetration, established safety in infants and children, proven efficacy in preclinical models of tumors of interest, and pharmacokinetic properties that allow therapeutically effective concentrations at the tumor site. If evaluated for a combinatorial therapy, synergy between the drugs must be demonstrated against the target tumor. Finally, FDA approval is needed, as the drug targets children with an orphan disease, prior to moving it into the clinical arena [2,36].
The initial idea of repurposing has been largely opportunistic and serendipitous, long known since World War II, when the deadly mustard gas was redirected, to develop nitrogen mustard as a chemotherapy for lymphoma. However, it was only recently that the concept of repurposing has been developed strategically with technological advances [37,38]. These include computational and experimental approaches, which help swiftly to identify a suitable medicament of interest for further evaluation. The current computational method based on molecular theory helps to pinpoint a drug by dissecting a large digital record of health information (diagnostic and pathophysiological data) and understanding it at the molecular level. This is complemented by a comprehensive analysis of experimental data, (which enumerates chemical, or protein structure, gene expression and omics profile) of the compound of interest [39]. Different platforms have been implemented for ideal candidate selection and its repurposing. These include the ReDO project, which is a database for candidate selection [40]. DRUGSURV, one of the largest repository of FDA approved and experimental drugs database [41], and Benevolent AI (a leader in advanced artificial intelligence, which generates machine learning based graphical data of drugs and their targets, aiding discovery of rare genomic aberration like the ACVR-mutant DIPG [42], are other pivotal approaches. The last few years have seen a dramatic rise in the use of AI in various steps of anti-cancer drug discovery, resulting in rapid turnover of drug identification, with perfect precision and minimized error of the pharmaceutical industry [43].
Though drug repurposing has gained significant momentum driving optimism in drug discovery and relevant translational research, challenges are concerning. The continuing spurt of biological data, and new molecular insights in various subtypes of pediatric brain tumors, far outpaces the emanating of appropriate preclinical studies including development of animal models to test drugs of interest. Ongoing collaboration between scientists, clinicians, industry and the regulatory teams are often fraught with challenges. Even so, the rapid pace of research and the implementation of repurposing compounds to the treatment of brain cancer, now ushers a new paradigm in cancer therapeutics. Finally, in the words of James Black, Nobel Laureate in physiology and medicine (1988), who once said “the most fruitful basis for the discovery of a new drug is to start with an old drug”, a reminder of the true essence of drug repurposing.
The following tables were generated using a search criterion of key words including childhood brain tumors and repurposed drugs for CNS tumors, retrieved from PubMed and ClinicalTrials.gov data bases. In Table 1, repurposed oncology drugs are presented, while Table 2 provides a comprehensive overview of non-oncologic repurposed drugs. Table 3 highlights outcomes for repurposed oncologic drugs, and Table 4 lists outcomes for repurposed non-oncologic drugs.

Table 1.
Repurposed Oncology Drugs.

Table 2.
Non-Oncologic Repurposed Drugs.

Table 3.
Outcomes for repurposed oncologic drugs.

Table 4.
Outcomes for repurposed non-oncologic drugs.
3. Non-Oncology Drugs Repurposed for Pediatric Brain Tumors
Landmark discoveries in antineoplastic properties of thalidomide in 1990 (which was initially used to treat nausea in pregnancy, and later discontinued for inducing fetal teratogenicity in the 1950’s) and mebendazole (used originally as an antiparasitic), paved the way of intense research evaluating non-oncologic drugs use in cancer [44,45]. To date more than 250 such therapeutics have shown promising anti-cancer properties and are being evaluated for various malignancies [46]. As pediatric brain tumors with their elusive biology continue to undermine therapeutic efficacy, newer approaches are profiled to overcome these impediments. Morbidities like headache, epilepsy, psychiatric illness, and neurodegenerative disease are seen in 20–30% of pediatric brain tumor patients. Drugs used for treating these ailments are now repurposed singly or in combination with conventional therapies, to improve clinical outcome of these malignancies [47,48]. These medications are appealing, as they have shown to cross the BBB in therapeutic concentrations, addressing a major challenge of optimal drug delivery in brain tumors. Moreover, they have antineoplastic properties targeting diverse signaling pathways or oncogenic drivers and would require much less resources and preclinical assessment prior to moving them into the clinical arena.
4. The Blood-Brain Barrier
The BBB is a critical structure that protects the brain from infection and neurotoxicants. It is composed of various cell types, most prominent of which include astrocytes, pericytes, and perivascular macrophages [49,50] (Figure 1). Together with endothelial cells and tight junctions, the BBB regulates the passage of compounds from the blood into the cerebrospinal fluid (CSF) [51,52,53,54]. Efflux pumps like P-glycoprotein (Pgp), breast cancer resistance protein (BCRP), and multidrug resistance-associated protein 4 (MRP4) actively remove substrates that can bypass the barrier. Nearly 60% of available drugs on the market are substrates of Pgp, making efflux pumps invaluable in preventing toxic accumulation of drugs in the CNS [55]. However, these efflux pumps may be upregulated in gliomas, impeding therapeutic drug delivery [56]. Prior studies have shown that an upregulation of P-gp and BRCP in GBM decreases the delivery of temozolomide (TMZ) [57]. Furthermore, these efflux pumps, specifically P-gp and BCRP, appear to remain active on tumor vasculature despite chemotherapeutic disruption of the BBB; as such, these efflux pumps may continue to decrease therapy efficacy, despite efforts to enhance initial delivery [58]. Previously conducted studies administering palbociclib, gefitinib, sunitinib to P-gp deficient mice have demonstrated markedly elevated levels of drug concentration within the brain as compared to wild-type mice [59,60,61]. However, clinical trials in humans investigating the co-administration of P-gp inhibitors such as elacridar and tariquidar with chemotherapy have been less fruitful, with significant uptake of chemotherapy yet to be demonstrated, along with concerns of toxicity [62,63,64].
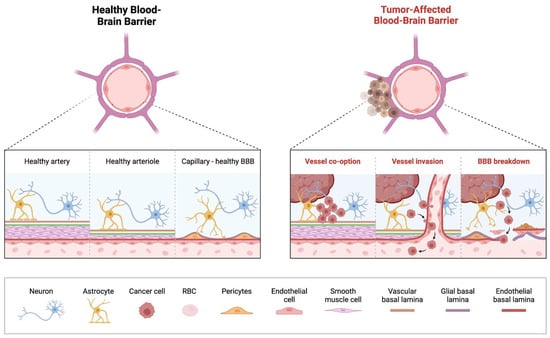
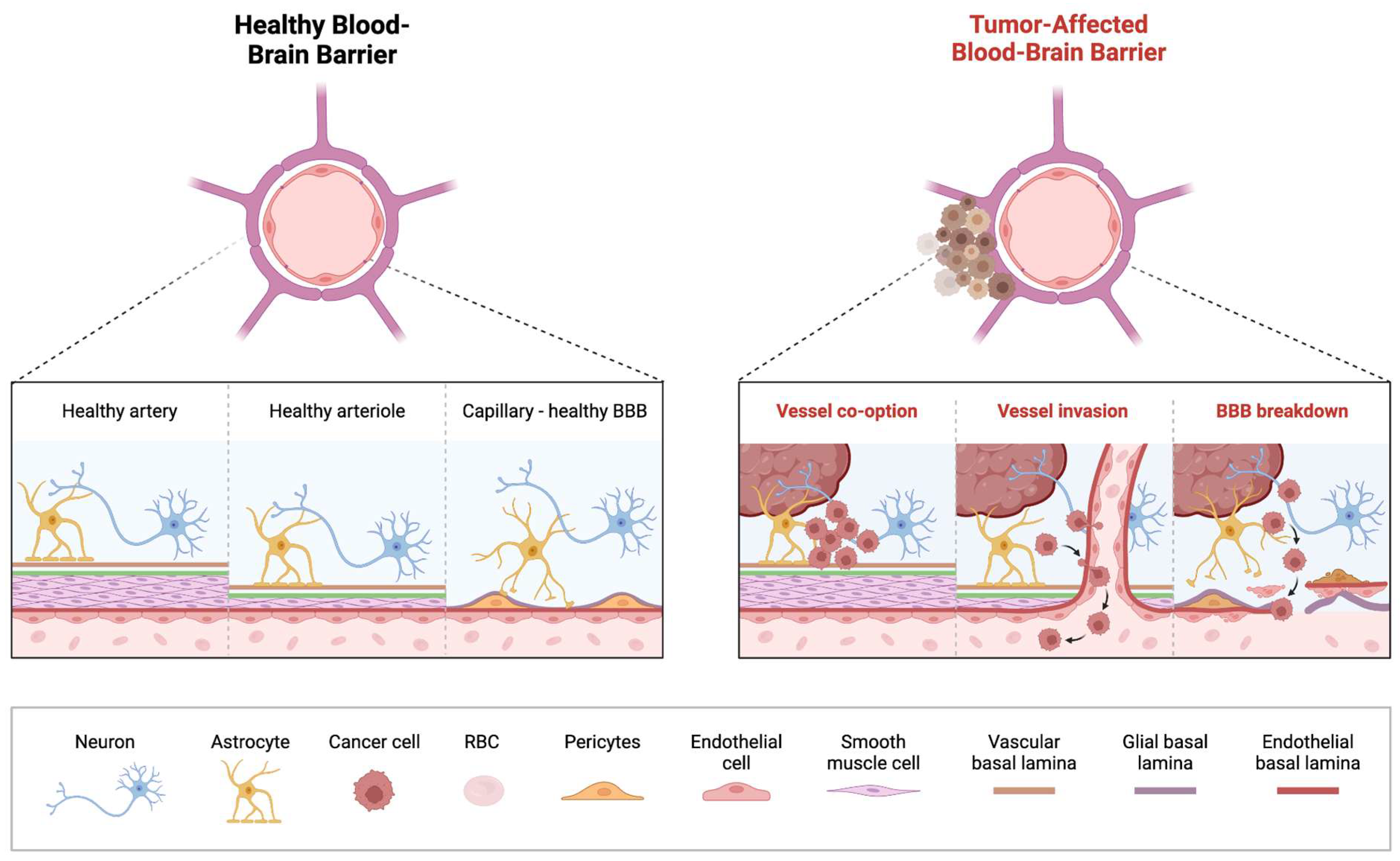
Figure 1.
Overview of Blood-Brain Barrier Alterations in Pediatric Brain Tumors. On the (left) is an illustration of the healthy BBB with correctly functioning arteries, arterioles and capillaries. On the (right) is an illustration of the breakdown of the BBB that takes place in a tumor-affected brain, with vessel co-option by the tumor and subsequent invasion of the vessel resulting in the breakdown of the previously healthy BBB.
Brain tumors contribute to a “leakier” BBB, as cancer cells interfere with the normal function and distribution of cells that compose the BBB [52,65,66,67]. This has been termed the blood-tumor barrier (BTB) wherein the integrity of BBB is compromised, characterized by heterogeneous permeability which can significantly impede delivery of therapeutic agents to the tumor cells [66,67]. Striking differences in the BTB are seen in some of the common malignant pediatric brain tumors, medulloblastoma (MB) and diffuse midline glioma (DMG). The WNT-activated molecular subgroup of MB, displays the most favorable prognosis amongst the four molecular subgroups, and generally responds well to chemotherapy [68,69]. It is believed that the aberrant vasculature network in the WNT-activated MB, enhances the “leaky” BBB, rendering them more susceptible to systemic chemotherapy, as also demonstrated in preclinical models. This contrasts with the formidable Sonic Hedgehog (SHH subgroup) which maintains BBB integrity, hindering access to systemic chemotherapy [68,70,71].
DMG are aggressive tumors, usually located in eloquent areas of the brain, precluding robust surgical intervention. They tend to maintain vascular integrity like the normal brain, preserving the intact nature of the BTB, explaining their mostly non-contrast neuroimaging features, limited drug penetration and poor clinical outcomes [11,71,72,73,74,75]. Histological and preclinical models of DMG showed uninterrupted expression of junctional proteins CLDN5 and CD31, normal expression of the transporter Glut1 and continuing coverage by pericytes, suggesting the maintenance of vascular integrity in presence of tumor cell infiltration [71].
In contrast, little is known about the phenotype of the BTB, BBB proteins and transporter in two other common pediatric malignant brain tumors, ependymoma and the atypical teratoid rhabdoid (ATRT) [24].
These diverse mechanisms impeding adequate BBB penetration of drugs, resulting in suboptimal tumor concentration, warrants the need to profile novel methods of drug delivery to circumvent these obstacles [49,51] (Figure 1).
5. Methods of Bypassing the Blood-Brain Barrier
5.1. Convection-Enhanced Delivery (CED)
Convection-enhanced Delivery (CED) is an advancement in drug delivery that bypasses the BBB via direct interstitial infusion into a tumor under a pressure gradient [76,77]. It is a direct, targeted delivery mechanism that limits off-target effects, while delivering optimal dosages. The ability to begin therapy with smaller initial drug load, while being able to have a controlled homogenous distribution with minimal systemic absorption, makes CED an attractive option in the pediatric population [77,78,79,80]. Despite challenges (Figure 2), as a growing area of research, CED has significant potential, especially when combined with various synergistic technologies like nanoparticles [81].
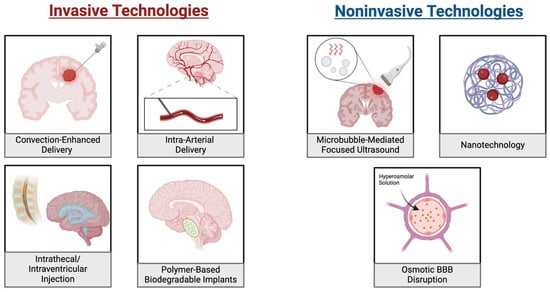
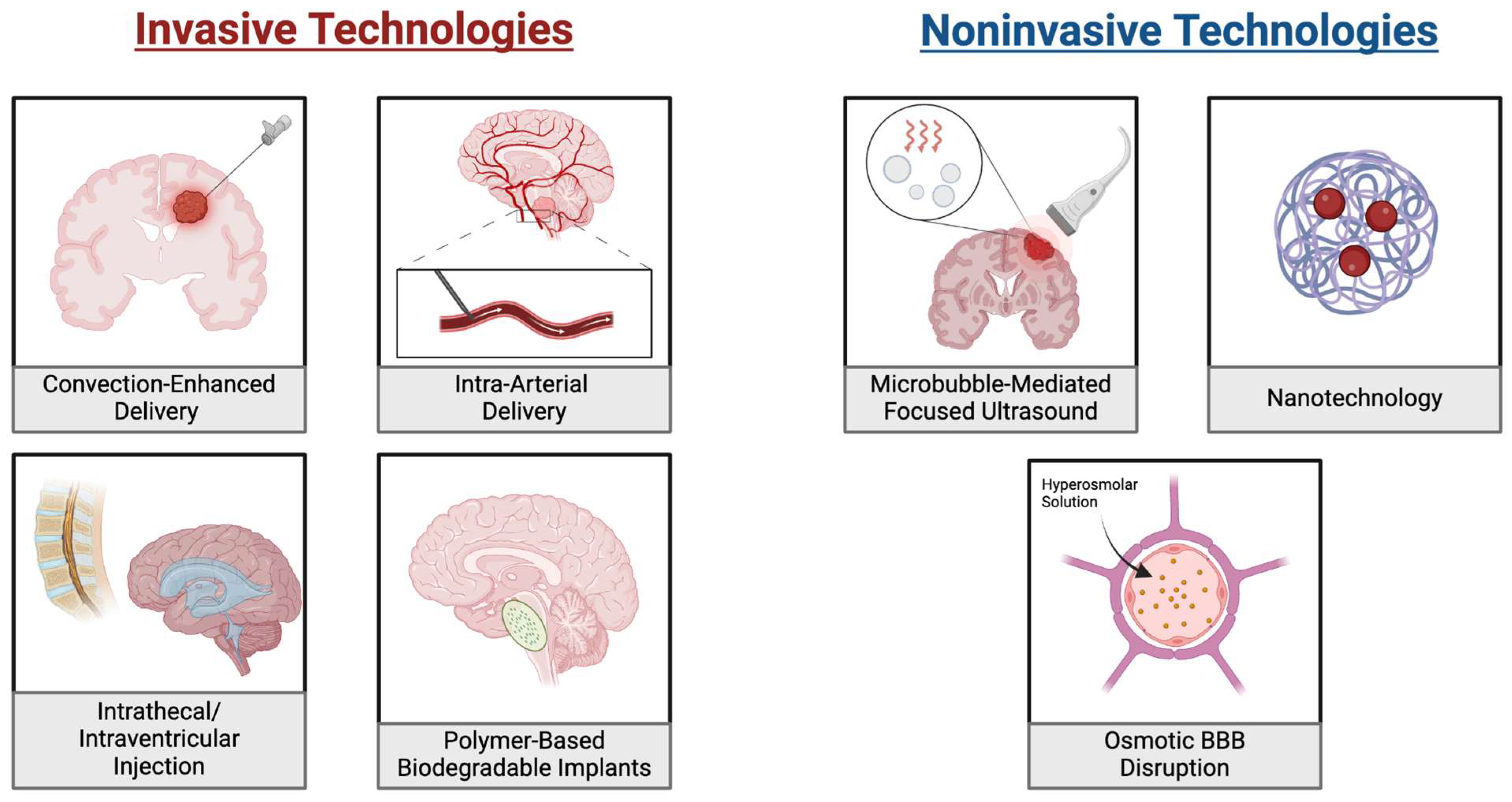
Figure 2.
Drug Delivery Approaches for Bypassing the Blood-Brain Barrier in Pediatric Brain Tumors. On the (left) are illustrations of four invasive approaches for bypassing the BBB. These include convection-enhanced, intra-arterial and intrathecal/intraventricular methods. Each of these distributes the drug directly at the tumor location or to a system that will reach it without passing the BBB. Additionally, the placement of biodegradable implants is shown where drug-loaded implants are placed near the tumor and gradually release the drug. On the (right) are three noninvasive approaches to delivering chemotherapies. These include microbubble mediated focused ultrasound, which uses ultrasound waves to enhance targeted drug delivery and nanotechnology which uses nanoparticles to carry drugs to the tumors. Additionally, osmotic BBB disruption utilizes a hyperosmolar solution to drive osmotic forces which disrupts the integrity of the BBB.
Over the last few years, CED has shown to be safe and efficacious in DIPG in vivo models and patients [82,83,84,85,86,87]. Carboplatin has been used systemically for various cancers including brain tumors with significant myelosuppressive toxicity. A study of repurposing carboplatin via intratumoral infusions in pontine lesion, using robot-guided implantation of catheter, demonstrated feasibility of accurately and safely delivering small-diameter catheters [88]. Based on encouraging preclinical data, panobinostat, a pan HDAC inhibitor, demonstrated safety when used orally in DIPG, with myelosuppression as the major toxicity in a phase I trial PBTC-047. Having also demonstrated good penetration through BBB in murine models of DIPG, this drug has now been repurposed using repeated CED infusions of MTX110, a water-soluble nanoparticle of panobinostat into the tumor (NCT03566199) [89].
Irinotecan, an alkylating agent, has been used alone or in combination with other drugs for recurrent HGG in children with variable efficacy and concerns of toxicity [90,91]. To undermine these side effects and increase drug delivery to tumor site, it is now repurposed using CED of a liposomal form of irinotecan injection in DIPG, as a part of ongoing phase I trial (NCT 03086616).
Dasatinib, an oral tyrosine kinase inhibitor, has been used in children with Philadelphia positive leukemia demonstrating improved survival. Recently, it has been used to target PDGFRα in pediatric HGG alone or along with mTOR inhibitors [92,93,94]. Though it demonstrated efficacy, adverse effects were concerning. Ongoing research is now exploring avenues to enhance delivery of dasatinib in brain tumors. ABC transporter inhibition along with dexamethasone, enhances its clinical efficacy when delivered by CED in pontine tumors. This approach demonstrated enhanced tumor cellular apoptosis and survival of the transgenic H3.3K27M mutant murine model of DIPG [82]. Trametinib, a MEK inhibitor, has been used in pediatric LGG and neurofibromatosis (NF) tumors, induced by the activation of the MAPK/ERK pathway, with varied success [95,96]. This drug has been repurposed using a combinatorial approach along with dabrafenib, a BRAF inhibitor, in similar tumors [97]. A promising study showed prolonged survival of intracranial genetic mouse models of DIPG when ZSTK474, a PI3K inhibitor, is used along with trametinib and delivered by CED, warranting further studies using this delivery technique [98].
Radiolabeled monoclonal antibodies (RMA) have been in use along with other drugs and radiation to treat pediatric tumors including leukemias, myelodysplastic syndrome and neuroblastoma [99,100]. Promising preclinical data has paved the way of RMA use through CED in pediatric brain tumors. A first-in-human study of 45 patients with DIPG received theranostic intratumoral CED of 123I-Omburtamab. This study demonstrated safety and was able to deliver high radiation doses to DIPG, with a wide safety margin and feasibility of real time tracking of the infused agent [101].
Over the last decade, oncolytic viral therapy (OVT) has garnered much attention as a therapeutic option in pediatric tumors, including brain tumor, osteosarcoma, retinoblastoma, and neuroblastoma. Though these efforts demonstrated safety, ongoing research continues to explore clinical outcomes in repositioning these viral constructs with newer genetic modifications and novel drug delivery methods which would enable less off-target toxicity. This approach is now further evaluated in combination with radiation therapy and immunotherapy to enhance clinical efficacy [102,103]. A recent study of DIPG patients who received intratumoral CED of oncolytic virus DNX-2401 along with radiation therapy, demonstrated safety with some response [86]. A phase 1b trial of twelve pediatric patients with recurrent HGG received intratumoral CED of lerapolturev, a recombinant polio-rhinovirus chimera, which demonstrated safety, affirming its evaluation in a larger cohort of patients (NCT03043391) [104].
Biodegradable implants in which polymers are loaded with various drugs, along with a controlled time release mechanism, can be embedded directly in the tumor bed after surgical resection [105,106]. This delivery mechanism has been explored in adults, but few studies have been done in pediatric brain tumors and they showed minimal efficacy.
Few case reports of efficacy in pediatric HGG were noted, with gliadel wafers used alone or in combination with temozolomide and etoposide [107,108]. Another study of gliadel wafers, used along with low dose oral etoposide, in children with anaplastic ependymoma demonstrated safety but did not show any clinical benefit [109]. The biological difference between adult and pediatric tumors likely limits the use of these in the pediatric population. These limitations include rapid degradation of wafers, unwanted effects of degradation products, short-term drug release kinetics and rapid drug hydrolysis of polymers [105,110]. Ways to undermine these limitations should be pursued in future studies, enabling development of optimal therapy of these implants, in combination with other drugs and radiation.
5.2. Intrathecal and Intraventricular Injections
Intrathecal (IT) and intraventricular (IV) injections are the most common ways to deliver drugs directly into the CSF. IT therapy has been used over many years in the treatment of primary and secondary CNS tumors [111,112].
These direct CSF injections have multiple benefits. They can bypass first-pass metabolism and thereby limit systemic toxicity. Being already in the CSF space, they can bypass barriers like BBB and achieve high concentration in the CNS [111,113]. Thus, IT and IV routes of drug delivery are therapeutically promising for lesions that occur in CSF or can spread through CSF such as ependymomas, medulloblastomas, and choroid plexus tumors.
Many drugs which have been evaluated for systemic use, are now being repurposed for delivery via IT or IV route to increase tumor access for increased clinical efficacy with limited side effects of systemic therapy. IT MTX resulted in a significant increase in 5-year PFS and OS and in pediatric patients with high-risk medulloblastoma [112]. Feasibility and safety were demonstrated with fourth ventricular infusions of methotrexate for the treatment of medulloblastoma and ependymoma [114]. Clinical trials of fourth ventricular infusions of methotrexate and etoposide for recurrent posterior fossa tumors in pediatric patients (NCT02905110) and MTX110 for recurrent medulloblastoma (NCT04315064) are ongoing. IT liposomal cytarabine (Ara-C) was well tolerated with clinical efficacy in children with high-risk recurrent cranial embryonal neoplasms [115]. A Phase I pilot study demonstrated safety with repeated fourth ventricular infusions of demethylating agent 5-azacitidine injections in posterior fossa ependymomas with no concerning neurological toxicity [116]. Topotecan, a topoisomerase inhibitor, has been widely used through intravenous or oral route for pediatric brain tumors with variable efficacy and concerning toxicity [117,118]. High-dose chemotherapy with stem cell rescue followed by intrathecal topotecan maintenance therapy could avoid whole brain radiation, showing efficacy in young children with aggressive atypical teratoid rhabdoid tumor (ATRT). This repurposing strategy, though promising, needs to be further evaluated in larger studies [119].
Nivolumab, a checkpoint inhibitor, and 5-azacytidine has been used via oral and systemic route respectively, in various pediatric malignancies. Clinical trials are now repurposing these drugs by IV routes, evaluating for increased tumor concentration, safety and toxicity profile. These studies include fourth ventricular infusions of nivolumab and 5-azacytidine or methotrexate for recurrent ependymoma and medulloblastoma (NCT0646679). A pilot study of 5-azacytidine and trastuzumab (an EZH2 inhibitor) infusions into the fourth ventricle or resection cavity in children and adults with recurrent posterior fossa ependymoma is also ongoing (NCT04958486). A phase 2 study for recurrent medulloblastoma and ependymoma is investigating whether the addition of IV RMA 131I-omburtamab to irinotecan, temozolomide, and bevacizumab can improve both the detection and treatment of these tumors. (NCT04743661). A clinical trial of 131I-omburtamab for treatment of CNS or leptomeningeal neoplasms in children and young adults is ongoing (NCT05064306).
Chimeric antigen receptor (CAR) T cell therapy has been evaluated against pediatric solid tumors based on promising results in pediatric leukemias. Though safe, it has limited antitumor efficacy due to fewer targetable antigens, hostile TME and suboptimal homing to tumor sites when delivered systemically [120]. Thus, IV or IT delivery of CART cells to brain tumors are now being evaluated for increased tumor concentration and clinical efficacy. Phase I studies in HGG demonstrated feasibility and safety when CAR-T therapy was delivered by IT or IV route [121,122,123,124]. H3K27M DMG express high levels of GD2, which led to a phase I study of initial intravenous infusions of this GD2-CAR T cells in these tumors. Those patients who exhibited clinical benefit, were eligible for subsequent GD2-CAR T cell infusions administered by IV route. Early results underscore the promise of this repositioning therapeutic approach for patients with these formidable tumors [125,126]. Based on increased expression of B7H3 in DIPG, a phase 1 clinical trial is underway to evaluate the safety and efficacy of IV delivery of CAR T cell with IL-7Ra signal, targeting B7H3 in children with DIPG (NCT06221553). A similar trial showed feasibility and safety of IV infusions of B7-H3 CAR T cells for patients with DIPG, with correlative evidence of immune activation, reaffirming efficacy of this novel delivery approach [126]. IV administration of autologous NK cells demonstrated safety and feasibility of its use in recurrent medulloblastoma and ependymoma [127].
5.3. Intra-Arterial Delivery (IA)
Cetuximab, an EGFR inhibitor, and bevacizumab, an angiogenesis inhibitor, has been used to treat adults and pediatric CNS tumors using the intravenous route. However, suboptimal tumor drug concentration has undermined clinical efficacy, and toxicity profile were concerning [128,129]. Repositioning these two drugs using super-selective intra-arterial cerebral infusion (SIACI) in pediatric patients with refractory HGG demonstrated safety in a phase 1 trial [130]. Based on this encouraging result, a phase I/II Trial of monthly dosing of SIACI is now enrolling patients with HGG under 22 years of age (NCT05956821).
Improving IA delivery to CNS lesions using transient cerebral hypoperfusion (TCH), has shown some promise in preclinical models, demonstrating enhanced tumor-specific uptake of peptide and liposome-based carrier molecules in glioma and metastatic brain cancer rodent models [131,132,133]. One such study reports glioma uptake increased four-fold with TCH-IA compared to IV with 2.41-fold greater tumor deposition. A recent study in choroid plexus carcinoma genetic mouse model, showed IA administration of melphalan (an alkylating agent) combined with elimusertib (inhibitor of Rad3-related kinase) led to a significant increase in survival and limited toxicity, emphasizing the potential of this delivery technique in pediatric CNS tumors. Both these drugs have been used orally in various cancers with significant toxicity.
Though IA delivery of therapeutics in pediatric CNS tumors has shown some clinical benefits, off-side effects are concerning. These include seizures, high-frequency hearing loss, and irreversible encephalopathy [134,135]. Other side effects mostly seen in adult patients include granulocytopenia, nephrotoxicity, intratumoral hemorrhage, and transient cerebral ischemia, raising concerns for its use in children [136].
5.4. Through the Blood Brain Barrier
5.4.1. Focused Ultrasound
Focused ultrasound (FUS) is an emerging technology used for the treatment of adult and pediatric brain tumors, due to its ability to induce diverse biological effects. These include thermal or mechanical tissue ablation, immunomodulation, radiosensitization and BBB disruption [137]. The therapy relies on using IV-administered microbubbles, which generate intravascular mechanical forces in sonicated regions, reflecting transmitted energy onto endothelial cells of local vasculature through oscillation, causing stable cavitation. This allows modulation of the BBB and induces transient separation of tight junctions, facilitating increased drug delivery [138,139,140]. These microbubbles and endothelial cells interactions also result in downregulation of efflux transportation and sonoporation, all of which contribute to enhanced drug delivery into the brain and improved accumulation of targeted agents in the sonicated region [137,141]. This anatomical disruption is transient, and the BBB permeability is restored in 6–24 h, making it an even more appropriate and attractive therapeutic option [141].
Carboplatin, doxorubicin and temozolomide have been used extensively by the intravenous or oral route in pediatric cancers. They are now being evaluated in preclinical models of brain tumors, repurposing their access to tumor site using FUS for increased tumoral concentration and clinical efficacy. Results of these studies are encouraging: a study with doxorubicin demonstrated mean concentration of 5366 ng/g with FUS compared to 251 ng/g while another study with temozolomide achieved 19 ng/mg compared to 6.983 ng/mg [142,143,144]. Based on these promising data, a clinical trial using low-intensity FUS (LIFS) with microbubbles to deliver doxorubicin in DIPG patients is open to accrual. Preliminary results are demonstrating safety (NCT03028246) [145].
DMG remains therapeutically challenging as the BBB remains largely intact and contributes to chemoresistance [146]. Radiotherapy remains the standard of care for these tumors. The potential for synergy of radiation with FUS to enhance clinical efficacy is being assessed in preclinical models [147,148]. A preclinical study using delivery of panobinostat using FUS with microbubbles in a DIPG mouse model demonstrated a three-fold increase in tumoral concentration of the drug (from 61.8 ng/g to 194.3 ng/g), with marked reduction of tumor size and increased survival [149]. These promising results led to an ongoing Phase I clinical study in progressive DMG, evaluating oral panobinostat along with FUS microbubbles and neuro-navigator-controlled sonication (NCT04804709).
A phase I/II trial in DIPG patients is evaluating the feasibility and safety of using an investigational agent, combined with the active ingredient aminolevulinic acid HCl using sonodynamic therapy (SDT) with LIFU. This approach has been shown to sensitize drugs of interest and improve its effect into target tissues. Preliminary results in 10 patients have demonstrated safety [145].
A phase I trial in patients with recurrent GBM is using a skull-implantable ultrasound device that transiently opens the BBB. Using this device with LIFU led to an increase in mean brain parenchymal concentrations of albumin-bound paclitaxel compared to non-sonicated brains from 37.3 nM to 138.6 nM [150]. FUS shows promise in enabling improved drug penetration to tumor sites, even with large therapeutics such as biologics and gene therapies [151,152].
5.4.2. Chemical BBB Disruption
Chemical reagents, such as vasoactive substances, can transiently and reversibly disrupt the tight junctions of endothelial cells lining the cerebral vasculature, improving drug delivery into CNS. These include bradykinin, histamine and arachidonic acid which have been used to increase BBB permeability [148,149,150,151,152,153]. Preclinical studies have shown a bradykinin analog, Lobradimil (RMP-7), induces BBB disruption and facilitates drug uptake into the brain, resulting in a 2.7-fold increase in drug concentration and 74% survival rate at 31 days compared to 37% in the control [154,155]. A phase II study was conducted, using intravenous Lobradimil along with carboplatin in pediatric brain tumors. Though safety was demonstrated, no favorable outcome was achieved, with a suboptimal AUC. A follow up study was planned with a higher dose [156]. In parallel, a phase I study confirmed safety and feasibility of giving this combination daily during radiotherapy to children with brainstem tumors [157].
5.4.3. Osmotic BBB Disruption
Studies have shown promising results of BBB disruptions using hyperosmolar agents like mannitol, lactamide and hypertonic saline. These agents split the tight junctions in the BBB, shrink endothelial cells, and increase the paracellular diffusion to improve delivery of both diagnostic and therapeutic agents to the CNS [158,159,160,161,162,163]. A phase I clinical trial using SIACI delivery of bevacizumab and cetuximab through BBB disruption with mannitol, in pediatric patients with HGG and DMG demonstrated safety and encouraging mean OS of 17.3 months in DIPG patients [130].
Though these modalities are encouraging, risks of seizures, pulmonary edema and renal failure are concerning [160,164,165]. Successful clinical translation to pediatric brain tumors requires understanding safety issues of repeated BBB opening and optimization of treatment that minimizes risk and maximize efficacy of BBB disruption and drug delivery.
5.4.4. Nanotechnology
Nanoparticles (NPs) are surfacing as a promising precision-based tool for diagnosis and a vehicle for drug delivery in brain tumors, due to their increased ability to cross the BBB. Their small size (ranging between 1–1000 nm), easily degradable phenotype and non-toxic profile holds promise in pediatric neurooncology. NPs fall into three major categories: organic (liposomes and polymers), inorganic (metals/metal oxides, ceramics, quantum dots) and carbon-based (fullerenes and nanotubes) [166,167]. They can absorb, entrap, or be modified with various pharmacological agents, facilitating its action as a non-invasive drug delivery vehicle for therapeutics in various cancers [168,169,170,171]. Encapsulating biological agents into various NPs has been exploited to alter the pharmacokinetics (PK) of drugs, improving its drug solubility, transportation through the BBB, biodistribution and delaying drug degradation to optimize clinical efficacy [172,173,174]. This provides an advantage to methods like IT and IV injections, alleviating barriers to use such as rapid clearance from CSF [175] and neurological toxicities including cytokine release syndromes [176].
Panobinostat has been used for the treatment of MB and DMG via oral or CED route. However, due to its poor water solubility and low BBB penetration, higher doses were used, resulting in toxicity [177]. Improved BBB penetration, tissue retention, and decreased efflux with improved survival was noted when panobinostat was delivered by nanotechnology methods [178]. Macrophage exosomes are nanosized vesicles, which can be used as a nanocarrier, for precision guided drug delivery [179]. PPM1D mutation is found in 9–25% of DIPG. This pathogenic gene leads to inactivation of DNA damage response, inducing decreased apoptosis and aggressive proliferation of these tumors. Macrophage exosomes loaded with panobinostat and PPM1D-siRNA were able to knock down the expression of PPM1D in DIPG cells, demonstrating greater killing effect of DIPG tumor cells and prolonging survival of orthotopic DIPG mice compared to controls inhibition [74,75,180]. Ongoing trials are using nanoparticle formulation of panobinostat (MTX110) administered by CED in H3K27M DMG patients (NCT03566199, NCT04264143).
Intranasal delivery of nanoliposomal SN-38, an active metabolite of irinotecan, prolonged survival in a mouse model of DIPG [181]. Based on this promising preclinical data, a phase I clinical trial of CED with irinotecan liposome injection in DIPG patients is ongoing (NCT03086616). Temozolomide, carboplatin, and doxorubicin which have been used systemically in brain tumors, are now being packaged into various forms of nanoparticles and delivered into the CNS tumors with promising results of increased tumor site concentration in preclinical models. These results now provide avenues to be tested in patients [174,182,183,184,185]. Use of gold Np (AuNP) in various cancers is gaining momentum [186]. A nanotechnology model using AuNPs loaded with an arginine-glycine-aspartic acid-like peptide, conjugated with doxorubicin, was used in preclinical models of GBM, which demonstrated lower systemic toxicities and high drug concentration in the tumor. The study demonstrated lower systemic toxicities and high drug concentration in the tumor, achieving an IC50 half that of 40 μg/mL for AuNP alone with a 3.7-fold higher accumulation in tumor cells.
Even though the last decade has witnessed a surge in use of nanotechnology to improve drug delivery of various therapeutics, this method remains challenging in pediatric tumors. To date FDA has approved nearly 20 nanoparticles pediatric cancers, but only a small improvement in survival and reduction of toxicities are seen [187]. Further research should continue to optimize efficacy, safety, and improve pharmacodynamic effects of the nanotechnologies in pediatric brain tumors.
5.4.5. BBB Peptide Shuttles
Specialized to facilitate the transport of therapeutic agents across the barrier, BBB peptide shuttles are yet another expanding area of research in treating brain tumors [188]. These shuttles are classified based on their transport mechanism, ranging from passive diffusion to receptor-mediated transcytosis (RMT).
RMT peptide shuttles mimic natural ligands of specific receptors on the BBB, enabling their transport across the barrier. Examples include those that target the transferrin receptor (TfR), low-density lipoprotein receptor (LDLR), or insulin receptor (InsR). Their transport mechanisms are highly specific which creates significant potential for therapeutics for pediatric populations [189,190]. Transferrin-conjugated carbon dots used to deliver doxorubicin showed that the conjugate had significantly higher uptake and cytotoxicity across multiple GBM cell lines, reducing viability at 10 nM by 14–45% compared to doxorubicin alone [191]. Similar results have been demonstrated with several 1,2,4-triazole derivatives via conjugation with peptide shuttles targeting αvβ6 integrin. More than 50% cytotoxicity against multiple GBM lines was achieved with 50 μg/mL, which was more effective than temozolomide, while also being nontoxic to healthy controls [192].
Drugs or drug delivery systems such as nanoparticles or liposomes can be modified with specific receptor ligands, enabling their internalization via endogenous transport mechanisms. RMT shuttles not only provide precise targeting but also allow the transport of large, polar molecules across the BBB that would otherwise have limited penetration [193]. CD276/B7-H3, highly expressed in medulloblastoma, is a promising target: a recent study demonstrated ligand concentration-dependent uptake of polymeric micelles into MB cell lines [194]. Targeting norepinephrine transporter (hNET) of neuroblastomas, a study confirmed the successful delivery of an ellipticine payload by coating ferritin-based nanovehicles with specific decapeptides, leading to higher levels of both early apoptosis (11% compared to non-coated nanovehicles at 7.3%) and late apoptosis (9.3% compared to 4.4%) [195].
These molecular vectors can be conjugated with a variety of drugs or incorporated with other technologies to enhance delivery and improve therapeutic efficacy while minimizing off-target effects [196,197]. However, more research is necessary to limit enzymatic degradation and avoid toxicological properties, among other shortcomings [198].
6. Conclusions
The last two decades have witnessed fervent research, driving the discovery of novel molecular signatures and therapeutic targets in pediatric brain tumors. However, its translation into clinical efficacy has remained markedly disappointing. New drug developments against these formidable neoplasms have remained painstakingly slow, expensive and fraught with regulatory challenges.
Repurposing of previously approved drugs (oncologic and non-oncologic) against pediatric brain tumors, has garnered significant therapeutic optimism, as it bypasses the expensive time-consuming preclinical and rigorous early phase safety studies needed in a new drug development. In parallel, repurposing of drugs using diverse novel drug delivery approaches circumventing the BBB, are now actively profiled to enhance drug concentration in the CNS tumors, mitigating the toxicities associated with systemic drug delivery.
Profiling newer targeted therapy by repurposing PDL-1 and MEK inhibitors (oncologic drugs), m-TOR inhibitors and autophagy inducers (non-oncology drugs) in combination with other standard chemotherapies, could improve therapeutic efficacy. Research should also continue to profile drug delivery to CNS tumors, using novel methods such as CED or FUS, which have shown promise in pediatric neurooncology.
7. Future Directions
Future endeavors need to further explore the use of non-oncologic drugs, as they have the unique potential to target multiple unrelated pathways and hit undruggable targets in tumors, not seen with the conventional molecularly targeted therapy. Research efforts should also focus on the use of these repurposed drugs, being optimally and safely redirected into the CNS, using appropriate drug delivery techniques.
Well-orchestrated efforts should continue to hasten and improve the identification of optimal candidate drug, using computational and experimental based approaches. In parallel, rapid development of preclinical models reflecting the molecular profile of tumors of interest, should be pursued. This would facilitate testing of the drug of interest in a timely fashion in vivo for efficacy, CNS penetrance, safety, and FDA approval, prior to it being driven into the clinical arena.
The ultimate success of drug repurposing in this orphan disease will ensue, with a relentless concerted systematic collaborative approach, between scientists, physicians, regulatory bodies, and the pharmaceutical industry. These much-needed efforts, though challenging, are worthwhile, as they usher a new horizon in pediatric CNS cancer therapeutics.
Author Contributions
Conceptualization, S.K.; data curation, S.K., J.S.R. and S.A.T.; writing—original draft preparation, S.K., J.S.R., S.A.T., P.N., S.B., H.B.Y., W.D.C. and N.K.; writing—review and editing, S.K., J.S.R., S.A.T. and P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ostrom, Q.T.; Price, M.; Ryan, K.; Edelson, J.; Neff, C.; Cioffi, G.; A Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncol. 2022, 24, iii1–iii38. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Elajami, M.K.; El Zarif, T.; Bou-Gharios, J.; Abou-Antoun, T.; Abou-Kheir, W. Drug repurposing towards targeting cancer stem cells in pediatric brain tumors. Cancer Metastasis Rev. 2020, 39, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K. Editorial Comment to Incidence and predictive factors of orgasmic dysfunction after robot-assisted radical prostatectomy: A cross-sectional, questionnaire-based study. Int. J. Urol. 2022, 29, 1309. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Pediatric Oncology Drug Approvals. Available online: https://www.fda.gov/about-fda/oncology-center-excellence/pediatric-oncology-drug-approvals (accessed on 15 January 2025).
- Partap, S.; Monje, M. Pediatric Brain Tumors. Contin. Lifelong Learn. Neurol. 2020, 26, 1553–1583. [Google Scholar] [CrossRef]
- Kilburn, L.B.; Kilburn, L.B.; Khuong-Quang, D.-A.; Khuong-Quang, D.-A.; Hansford, J.R.; Hansford, J.R.; Landi, D.; Landi, D.; van der Lugt, J.; van der Lugt, J.; et al. The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: The phase 2 FIREFLY-1 trial. Nat. Med. 2023, 30, 207–217. [Google Scholar] [CrossRef]
- Bouffet, E.; Geoerger, B.; Moertel, C.; Whitlock, J.A.; Aerts, I.; Hargrave, D.; Osterloh, L.; Tan, E.; Choi, J.; Russo, M.; et al. Efficacy and Safety of Trametinib Monotherapy or in Combination With Dabrafenib in Pediatric BRAF V600–Mutant Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 664–674. [Google Scholar] [CrossRef]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e5. [Google Scholar] [CrossRef]
- Hoffman, L.M.; van Zanten, S.E.V.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef]
- Stucklin, A.S.G.; Ryall, S.; Fukuoka, K.; Zapotocky, M.; Lassaletta, A.; Li, C.; Bridge, T.; Kim, B.; Arnoldo, A.; Kowalski, P.E.; et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat. Commun. 2019, 10, 4343. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Pfaff, E.; Shih, D.J.; Martin, D.C.; Castelo-Branco, P.; Baskin, B.; Ray, P.N.; Bouffet, E.; et al. Subgroup-Specific Prognostic Implications of TP53 Mutation in Medulloblastoma. J. Clin. Oncol. 2013, 31, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.W.; Kocak, M.; Dalton, J.; Megahed, H.; Lusher, M.E.; Ryan, S.L.; Zhao, W.; Nicholson, S.L.; Taylor, R.E.; Bailey, S.; et al. Definition of Disease-Risk Stratification Groups in Childhood Medulloblastoma Using Combined Clinical, Pathologic, and Molecular Variables. J. Clin. Oncol. 2011, 29, 1400–1407. [Google Scholar] [CrossRef]
- Ellison, D.W.; Onilude, O.E.; Lindsey, J.C.; Lusher, M.E.; Weston, C.L.; Taylor, R.E.; Pearson, A.D.; Clifford, S.C. β-Catenin Status Predicts a Favorable Outcome in Childhood Medulloblastoma: The United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J. Clin. Oncol. 2005, 23, 7951–7957. [Google Scholar] [CrossRef]
- Schwalbe, E.C.; Lindsey, J.C.; Nakjang, S.; Crosier, S.; Smith, A.J.; Hicks, D.; Rafiee, G.; Hill, R.M.; Iliasova, A.; Stone, T.; et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncol. 2017, 18, 958–971. [Google Scholar] [CrossRef]
- Schulz, M.; Sevenich, L. TAMs in Brain Metastasis: Molecular Signatures in Mouse and Man. Front. Immunol. 2021, 12, 716504. [Google Scholar] [CrossRef]
- Lieberman, N.A.P.; DeGolier, K.; Kovar, H.M.; Davis, A.; Hoglund, V.; Stevens, J.; Winter, C.; Deutsch, G.; Furlan, S.N.; A Vitanza, N.; et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: Implications for development of immunotherapy. Neuro-Oncol. 2018, 21, 83–94. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Gao, L.; Wang, Y.; Guo, Y.; Xing, B.; Ma, W. Classification of pediatric gliomas based on immunological profiling: Implications for immunotherapy strategies. Mol. Ther. - Oncolytics 2020, 20, 34–47. [Google Scholar] [CrossRef]
- Lu-Emerson, C.; Snuderl, M.; Kirkpatrick, N.D.; Goveia, J.; Davidson, C.; Huang, Y.; Riedemann, L.; Taylor, J.; Ivy, P.; Duda, D.G.; et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro-Oncol. 2013, 15, 1079–1087. [Google Scholar] [CrossRef]
- Nabbi, A.; Beck, P.; Delaidelli, A.; Oldridge, D.A.; Sudhaman, S.; Zhu, K.; Yang, S.Y.C.; Mulder, D.T.; Bruce, J.P.; Paulson, J.N.; et al. Transcriptional immunogenomic analysis reveals distinct immunological clusters in paediatric nervous system tumours. Genome Med. 2023, 15, 1–24. [Google Scholar] [CrossRef]
- Grabovska, Y.; Mackay, A.; O’hare, P.; Crosier, S.; Finetti, M.; Schwalbe, E.C.; Pickles, J.C.; Fairchild, A.R.; Avery, A.; Cockle, J.; et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 2020, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xie, W.; Ma, W.; Zhao, H.; Wang, J.; Liang, Z.; Tian, S.; Wang, B.; Ma, J. The unique immune ecosystems in pediatric brain tumors: Integrating single-cell and bulk RNA-sequencing. Front. Immunol. 2023, 14, 1238684. [Google Scholar] [CrossRef] [PubMed]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood–Brain Barrier for the Treatment of Primary Brain Tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Pui, C.-H.; Gajjar, A.J.; Kane, J.R.; Qaddoumi, I.A.; Pappo, A.S. Challenging issues in pediatric oncology. Nat. Rev. Clin. Oncol. 2011, 8, 540–549. [Google Scholar] [CrossRef]
- Eder, J.; Sedrani, R.; Wiesmann, C. The discovery of first-in-class drugs: Origins and evolution. Nat. Rev. Drug Discov. 2014, 13, 577–587. [Google Scholar] [CrossRef]
- Kurzrock, R.; Kantarjian, H.M.; Kesselheim, A.S.; Sigal, E.V. New drug approvals in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 140–146. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorganic Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daher, D.; Aljamal, A.A.; Elajami, M.K.; Oh, K.S.; Moreno, J.C.A.; Delgado, R.; Suarez, R.; Zaldivar, A.; Azimi, R.; et al. Repurposing of Anticancer Stem Cell Drugs in Brain Tumors. J. Histochem. Cytochem. 2021, 69, 749–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P. Scientific advice—Is drug repurposing missing a trick? Nat. Rev. Clin. Oncol. 2017, 14, 455–456. [Google Scholar] [CrossRef]
- Servidei, T.; Sgambato, A.; Lucchetti, D.; Navarra, P.; Ruggiero, A. Drug Repurposing in Pediatric Brain Tumors: Posterior Fossa Ependymoma and Diffuse Midline Glioma under the Looking Glass. Front. Biosci. 2023, 28, 77. [Google Scholar] [CrossRef]
- Moffat, J.G.; Rudolph, J.; Bailey, D. Phenotypic screening in cancer drug discovery—Past, present and future. Nat. Rev. Drug Discov. 2014, 13, 588–602. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Wang, Z.; Hulikova, A.; Swietach, P. Innovating cancer drug discovery with refined phenotypic screens. Trends Pharmacol. Sci. 2024, 45, 723–738. [Google Scholar] [CrossRef]
- Kowshik, A.V.; Manoj, M.; Sowmyanarayan, S.; Chatterjee, J. Drug repurposing: Databases and pipelines. CNS Spectr. 2023, 29, 6–9. [Google Scholar] [CrossRef]
- Amelio, I.; Gostev, M.; A Knight, R.; E Willis, A.; Melino, G.; Antonov, A.V. DRUGSURV: A resource for repositioning of approved and experimental drugs in oncology based on patient survival information. Cell Death Dis. 2014, 5, e1051. [Google Scholar] [CrossRef]
- Carvalho, D.M.; Richardson, P.J.; Olaciregui, N.; Stankunaite, R.; Lavarino, C.; Molinari, V.; Corley, E.A.; Smith, D.P.; Ruddle, R.; Donovan, A.; et al. Repurposing Vandetanib plus Everolimus for the Treatment of ACVR1-Mutant Diffuse Intrinsic Pontine Glioma. Cancer Discov. 2021, 12, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.H.; O’connor, D.; Cavaller-Bellaubi, M.; Fetro, C.; Gogou, M.; Hoen, P.A.C. ’.; de Kort, M.; Stone, H.; Valentine, N.; Pasmooij, A.M.G. Drug repurposing for rare: Progress and opportunities for the rare disease community. Front. Med. 2024, 11, 1352803. [Google Scholar] [CrossRef]
- Diggle, G. THALIDOMIDE: 40 YEARS ON. Int. J. Clin. Pract. 2001, 55, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.-Y.; Staedtke, V.; Aprhys, C.M.; Gallia, G.L.; Riggins, G.J. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro-Oncol. 2011, 13, 974–982. [Google Scholar] [CrossRef]
- Anticancer Fund. Drug Repurposing Database: ReDO DB. Available online: https://www.anticancerfund.org/en/redo-db (accessed on 28 March 2022).
- Robert-Boire, V.; Desnous, B.; Lortie, A.; Carmant, L.; Ellezam, B.; Weil, A.G.; Perreault, S. Seizures in Pediatric Patients With Primary Brain Tumors. Pediatr. Neurol. 2019, 97, 50–55. [Google Scholar] [CrossRef]
- Shah, S.S.; Dellarole, A.; Peterson, E.C.; Bregy, A.; Komotar, R.; Harvey, P.D.; Elhammady, M.S. Long-term psychiatric outcomes in pediatric brain tumor survivors. Child’s Nerv. Syst. 2015, 31, 653–663. [Google Scholar] [CrossRef]
- Harder, B.G.; Blomquist, M.R.; Wang, J.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A.; Loftus, J.C.; Tran, N.L. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018, 8, 462. [Google Scholar] [CrossRef]
- Jia, W.; Lu, R.; Martin, T.A.; Jiang, W.G. The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (Review). Mol. Med. Rep. 2013, 9, 779–785. [Google Scholar] [CrossRef]
- Power, E.A.; Rechberger, J.S.; Gupta, S.; Schwartz, J.D.; Daniels, D.J.; Khatua, S. Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors—An update. Adv. Drug Deliv. Rev. 2022, 185, 114303. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, E.R.; Viscusi, A.R. Blood–brain barrier: Mechanisms governing permeability and interaction with peripherally acting μ-opioid receptor antagonists. Reg. Anesth. Pain Med. 2020, 45, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Ntafoulis, I.; Koolen, S.L.W.; Leenstra, S.; Lamfers, M.L.M. Drug Repurposing, a Fast-Track Approach to Develop Effective Treatments for Glioblastoma. Cancers 2022, 14, 3705. [Google Scholar] [CrossRef] [PubMed]
- Bronger, H.; König, J.; Kopplow, K.; Steiner, H.-H.; Ahmadi, R.; Herold-Mende, C.; Keppler, D.; Nies, A.T. ABCC Drug Efflux Pumps and Organic Anion Uptake Transporters in Human Gliomas and the Blood-Tumor Barrier. Cancer Res. 2005, 65, 11419–11428. [Google Scholar] [CrossRef]
- Dréan, A.; Rosenberg, S.; Lejeune, F.-X.; Goli, L.; Nadaradjane, A.A.; Guehennec, J.; Schmitt, C.; Verreault, M.; Bielle, F.; Mokhtari, K.; et al. ATP binding cassette (ABC) transporters: Expression and clinical value in glioblastoma. J. Neuro-Oncol. 2018, 138, 479–486. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; Kemper, E.M.; Buil, L.C.; Çitirikkaya, C.H.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. ATP-binding cassette transporters restrict drug delivery and efficacy against brain tumors even when blood-brain barrier integrity is lost. Cell Rep. Med. 2021, 2, 100184. [Google Scholar] [CrossRef]
- Parrish, K.E.; Pokorny, J.; Mittapalli, R.K.; Bakken, K.; Sarkaria, J.N.; Elmquist, W.F. Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J. Pharmacol. Exp. Ther. 2015, 355, 264–271. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Gallardo, J.L.; Ohlfest, J.R.; Elmquist, W.F. Distribution of Gefitinib to the Brain Is Limited by P-glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2)-Mediated Active Efflux. J. Pharmacol. Exp. Ther. 2010, 334, 147–155. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Mittapalli, R.K.; Elmquist, W.F. Pharmacokinetic Assessment of Efflux Transport in Sunitinib Distribution to the Brain. J. Pharmacol. Exp. Ther. 2013, 347, 755–764. [Google Scholar] [CrossRef]
- Lai, J.-I.; Tseng, Y.-J.; Chen, M.-H.; Huang, C.-Y.F.; Chang, P.M.-H. Clinical Perspective of FDA Approved Drugs With P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Verheijen, R.; Yaqub, M.; Sawicki, E.; van Tellingen, O.; Lammertsma, A.; Nuijen, B.; Schellens, J.; Beijnen, J.; Huitema, A.; Hendrikse, N.; et al. Molecular imaging of PgP/BCRP inhibition at the blood brain barrier using elacridar and [11C]erlotinib PET. Ann. Oncol. 2016, 27, vi115. [Google Scholar] [CrossRef][Green Version]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Therapeutic Potential and Utility of Elacridar with Respect to P-glycoprotein Inhibition: An Insight from the Published In Vitro, Preclinical and Clinical Studies. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef]
- Steeg, P.S. The blood–tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2019, 20, 26–41. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, 11. [Google Scholar] [CrossRef]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef]
- Morris, E.K.; Daignault-Mill, S.; Stehbens, S.J.; Genovesi, L.A.; Lagendijk, A.K. Addressing blood-brain-tumor-barrier heterogeneity in pediatric brain tumors with innovative preclinical models. Front. Oncol. 2023, 13, 1101522. [Google Scholar] [CrossRef]
- Warren, K.E. Diffuse intrinsic pontine glioma: Poised for progress. Front. Oncol. 2012, 2, 205. [Google Scholar] [CrossRef]
- Warren, K.E. Beyond the Blood:Brain Barrier: The Importance of Central Nervous System (CNS) Pharmacokinetics for the Treatment of CNS Tumors, Including Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8, 239. [Google Scholar] [CrossRef]
- Hennika, T.; Becher, O.J. Diffuse Intrinsic Pontine Glioma. J. Child Neurol. 2016, 31, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.-J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-Oncol. 2016, 19, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sanche, L. Convection-Enhanced Delivery in Malignant Gliomas: A Review of Toxicity and Efficacy. J. Oncol. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Lonser, R.R.; Walbridge, S.; Garmestani, K.; Butman, J.A.; Walters, H.A.; Vortmeyer, A.O.; Morrison, P.F.; Brechbiel, M.W.; Oldfield, E.H. Successful and safe perfusion of the primate brainstem: In vivo magnetic resonance imaging of macromolecular distribution during infusion. J. Neurosurg. 2002, 97, 905–913. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Ozawa, T.; Drummond, D.C.; Kalra, A.; Fitzgerald, J.B.; Kirpotin, D.B.; Wei, K.-C.; Butowski, N.; Prados, M.D.; Berger, M.S.; et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro-Oncol. 2012, 15, 189–197. [Google Scholar] [CrossRef]
- Yun, J.; Rothrock, R.J.; Canoll, P.; Bruce, J.N. Convection-Enhanced Delivery for Targeted Delivery of Antiglioma Agents: The Translational Experience. J. Drug Deliv. 2013, 2013, 107573. [Google Scholar] [CrossRef][Green Version]
- Graham-Gurysh, E.; Moore, K.M.; Satterlee, A.B.; Sheets, K.T.; Lin, F.-C.; Bachelder, E.M.; Miller, C.R.; Hingtgen, S.D.; Ainslie, K.M. Sustained Delivery of Doxorubicin via Acetalated Dextran Scaffold Prevents Glioblastoma Recurrence after Surgical Resection. Mol. Pharm. 2018, 15, 1309–1318. [Google Scholar] [CrossRef]
- Tsvankin, V.; Hashizume, R.; Katagi, H.; E Herndon, J.; Lascola, C.; Venkatraman, T.N.; Picard, D.; Burrus, B.; Becher, O.J.; Thompson, E.M. ABC Transporter Inhibition Plus Dexamethasone Enhances the Efficacy of Convection Enhanced Delivery in H3.3K27M Mutant Diffuse Intrinsic Pontine Glioma. Neurosurgery 2020, 86, 742–751. [Google Scholar] [CrossRef]
- Singleton, W.; Collins, A.; Bienemann, A.; Killick-Cole, C.; Haynes, H.; Asby, D.; Butts, C.; Wyatt, M.; Barua, N.; Gill, S. Convection enhanced delivery of panobinostat (LBH589)-loaded pluronic nano-micelles prolongs survival in the F98 rat glioma model. Int. J. Nanomed. 2017, ume 12, 1385–1399. [Google Scholar] [CrossRef]
- Lin, G.L.; Wilson, K.M.; Ceribelli, M.; Stanton, B.Z.; Woo, P.J.; Kreimer, S.; Qin, E.Y.; Zhang, X.; Lennon, J.; Nagaraja, S.; et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci. Transl. Med. 2019, 11, eaaw0064. [Google Scholar] [CrossRef] [PubMed]
- Hennika, T.; Hu, G.; Olaciregui, N.G.; Barton, K.L.; Ehteda, A.; Chitranjan, A.; Chang, C.; Gifford, A.J.; Tsoli, M.; Ziegler, D.S.; et al. Pre-Clinical Study of Panobinostat in Xenograft and Genetically Engineered Murine Diffuse Intrinsic Pontine Glioma Models. PLoS ONE 2017, 12, e0169485. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Katagi, H.; Goldman, S.; Becher, O.J.; Hashizume, R. Convection-Enhanced Delivery of Enhancer of Zeste Homolog-2 (EZH2) Inhibitor for the Treatment of Diffuse Intrinsic Pontine Glioma. Neurosurgery 2020, 87, E680–E688. [Google Scholar] [CrossRef] [PubMed]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zhou, Z.; Haque, S.; Zanzonico, P.; A Carrasquillo, J.; Lyashchenko, S.K.; Thakur, S.B.; Donzelli, M.; et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018, 19, 1040–1050. [Google Scholar] [CrossRef]
- Barua, N.U.; Lowis, S.P.; Woolley, M.; O’sullivan, S.; Harrison, R.; Gill, S.S. Robot-guided convection-enhanced delivery of carboplatin for advanced brainstem glioma. Acta Neurochir. 2013, 155, 1459–1465. [Google Scholar] [CrossRef]
- Mueller, S.; Kline, C.; Stoller, S.; Lundy, S.; Christopher, L.; Reddy, A.T.; Banerjee, A.; Cooney, T.M.; Raber, S.; Hoffman, C.; et al. PNOC015: Repeated convection-enhanced delivery of MTX110 (aqueous panobinostat) in children with newly diagnosed diffuse intrinsic pontine glioma. Neuro-Oncol. 2023, 25, 2074–2086. [Google Scholar] [CrossRef]
- Hargrave, D.; Geoerger, B.; Frappaz, D.; Pietsch, T.; Gesner, L.; Cisar, L.; Breazna, A.; Dorman, A.; Cruz-Martinez, O.; Fuster, J.L.; et al. A phase II single-arm study of irinotecan in combination with temozolomide (TEMIRI) in children with newly diagnosed high grade glioma: A joint ITCC and SIOPE-brain tumour study. J. Neuro-Oncol. 2013, 113, 127–134. [Google Scholar] [CrossRef]
- Gajjar, A.; Chintagumpala, M.M.; Bowers, D.C.; Jones-Wallace, D.; Stewart, C.F.; Crews, K.R. Effect of intrapatient dosage escalation of irinotecan on its pharmacokinetics in pediatric patients who have high-grade gliomas and receive enzyme-inducing anticonvulsant therapy. Cancer 2003, 97, 2374–2380. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Cai, J.; Yu, J.; Gao, J.; Hu, S.; Zhai, X.; Liang, C.; Ju, X.; Jiang, H.; et al. Effect of dasatinib vs imatinib in the treatment of pediatric philadelphia chromosome-positive acute lymphoblastic leukemia: A randomized clinical trial. JAMA Oncol. 2020, 6, 358–366. [Google Scholar] [CrossRef]
- Miklja, Z.; Yadav, V.N.; Cartaxo, R.T.; Siada, R.; Thomas, C.C.; Cummings, J.R.; Mullan, B.; Stallard, S.; Paul, A.; Bruzek, A.K.; et al. Everolimus improves the efficacy of dasatinib in PDGFRα-driven glioma. J. Clin. Investig. 2020, 130, 5313–5325. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.L.; Starr, K.; Lockwood, C.M.; Leary, S.E.S. The “SEED” Study: The Feasibility of Selecting Patient-Specific Biologically Targeted Therapy with Sorafenib, Everolimus, Erlotinib or Dasatinib for Pediatric and Young Adult Patients with Recurrent or Refractory Brain Tumors. Front. Biosci. 2022, 27, 219. [Google Scholar] [CrossRef] [PubMed]
- Perreault, S.; Larouche, V.; Tabori, U.; Hawkin, C.; Lippé, S.; Ellezam, B.; Décarie, J.-C.; Théoret, Y.; Métras, M.; Sultan, S.; et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer 2019, 19, 1250. [Google Scholar] [CrossRef] [PubMed]
- Selt, F.; van Tilburg, C.M.; Bison, B.; Sievers, P.; Harting, I.; Ecker, J.; Pajtler, K.W.; Sahm, F.; Bahr, A.; Simon, M.; et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J. Neuro-Oncol. 2020, 149, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Schieffer, K.M.; Grischow, O.; Rodriguez, D.P.; Cottrell, C.E.; Leonard, J.R.; Finlay, J.L.; Mardis, E.R. Clinical response to dabrafenib plus trametinib in a pediatric ganglioglioma with BRAF p.T599dup mutation. Mol. Case Stud. 2021, 7, a006023. [Google Scholar] [CrossRef]
- Chang, R.; Tosi, U.; Voronina, J.; Adeuyan, O.; Wu, L.Y.; E Schweitzer, M.; Pisapia, D.J.; Becher, O.J.; Souweidane, M.M.; Maachani, U.B. Combined targeting of PI3K and MEK effector pathways via CED for DIPG therapy. Neuro-Oncol. Adv. 2019, 1, vdz004. [Google Scholar] [CrossRef]
- Nemecek, E.R.; Matthews, D.C. Use of radiolabeled antibodies in the treatment of childhood acute leukemia. Pediatr. Transplant. 2003, 7, 89–94. [Google Scholar] [CrossRef]
- Trautwein, N.F.; Schwenck, J.; Seitz, C.; Seith, F.; Calderón, E.; von Beschwitz, S.; Singer, S.; Reischl, G.; Handgretinger, R.; Schäfer, J.; et al. A novel approach to guide GD2-targeted therapy in pediatric tumors by PET and [64Cu]Cu-NOTA-ch14.18/CHO. Theranostics 2024, 14, 1212–1223. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Zanzonico, P.B.; Grkovski, M.; Donzelli, M.; Vietri, S.M.; Horan, C.; Serencsits, B.; Prasad, K.; Lyashchenko, S.; Kramer, K.; et al. Theranostic Intratumoral Convection-Enhanced Delivery of124I-Omburtamab in Patients with Diffuse Intrinsic Pontine Glioma: Pharmacokinetics and Lesion Dosimetry. J. Nucl. Med. 2024, 65, 1364–1370. [Google Scholar] [CrossRef]
- Gross, E.G.; A Hamo, M.; Estevez-Ordonez, D.; Laskay, N.M.; Atchley, T.J.; Johnston, J.M.; Markert, J.M. Oncolytic virotherapies for pediatric tumors. Expert Opin. Biol. Ther. 2023, 23, 987–1003. [Google Scholar] [CrossRef]
- Ghajar-Rahimi, G.; Kang, K.-D.; Totsch, S.K.; Gary, S.; Rocco, A.; Blitz, S.; Kachurak, K.; Chambers, M.; Li, R.; Beierle, E.A.; et al. Clinical advances in oncolytic virotherapy for pediatric brain tumors. Pharmacol. Ther. 2022, 239, 108193. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Landi, D.; Brown, M.C.; Friedman, H.S.; McLendon, R.; E Herndon, J.; Buckley, E.; Bolognesi, D.P.; Lipp, E.; Schroeder, K.; et al. Recombinant polio–rhinovirus immunotherapy for recurrent paediatric high-grade glioma: A phase 1b trial. Lancet Child Adolesc. Health 2023, 7, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Graham-Gurysh, E.G.; Murthy, A.B.; Moore, K.M.; Hingtgen, S.D.; Bachelder, E.M.; Ainslie, K.M. Synergistic drug combinations for a precision medicine approach to interstitial glioblastoma therapy. J. Control. Release 2020, 323, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Pizer, B.; Salehzadeh, A.; Brodbelt, A.; Mallucci, C. Prolonged survival associated with the use of intraoperative carmustine (Gliadel®) in a paediatric patient with recurrent Grade III astrocytoma. Br. J. Neurosurg. 2013, 27, 516–518. [Google Scholar] [CrossRef]
- Márquez-Rivas, J.; Ramirez, G.; Ollero-Ortiz, Á.; Giménez-Pando, J.; Emmerich, J.; Quiroga-Cantero, E.; Rivas, E.; Gómez-González, E. Initial Experience Involving Treatment and Retreatment With Carmustine Wafers in Combination With Oral Temozolomide: Long-term Survival in a Child With Relapsed Glioblastoma Multiforme. J. Pediatr. Hematol. 2010, 32, e202–e206. [Google Scholar] [CrossRef]
- Sardi, I.; Sanzo, M.; Giordano, F.; Sandri, A.; Mussa, F.; Donati, P.A.; Genitori, L. Intracavitary chemotherapy (Gliadel®) and oral low-dose etoposide for recurrent anaplastic ependymoma. Oncol. Rep. 2008, 19, 1219–1223. [Google Scholar] [CrossRef]
- Han, D.; Serra, R.; Gorelick, N.; Fatima, U.; Eberhart, C.G.; Brem, H.; Tyler, B.; Steckl, A.J. Multi-layered core-sheath fiber membranes for controlled drug release in the local treatment of brain tumor. Sci. Rep. 2019, 9, 17936. [Google Scholar] [CrossRef]
- Triarico, S.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Capozza, M.A.; Ruggiero, A. Improving the Brain Delivery of Chemotherapeutic Drugs in Childhood Brain Tumors. Cancers 2019, 11, 824. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wang, Y.; Zhong, X.-D.; Chang, J. Efficacy of intrathecal methotrexate in children with high-risk medulloblastoma over three years: A retrospective study from a single center. J. Neuro-Oncol. 2023, 164, 117–125. [Google Scholar] [CrossRef]
- Calias, P.; Banks, W.A.; Begley, D.; Scarpa, M.; Dickson, P. Intrathecal delivery of protein therapeutics to the brain: A critical reassessment. Pharmacol. Ther. 2014, 144, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.I.; Rytting, M.; Zaky, W.; Kerr, M.; Ketonen, L.; Kundu, U.; Moore, B.D.; Yang, G.; Hou, P.; Sitton, C.; et al. Methotrexate administration directly into the fourth ventricle in children with malignant fourth ventricular brain tumors: A pilot clinical trial. J. Neuro-Oncol. 2015, 125, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Partap, S.; Murphy, P.A.; Vogel, H.; Barnes, P.D.; Edwards, M.S.B.; Fisher, P.G. Liposomal cytarabine for central nervous system embryonal tumors in children and young adults. J. Neuro-Oncol. 2010, 103, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.I.; Yu, B.; Patel, R.; Hagan, J.; Miesner, E.; Sabin, J.; Smith, S.; Fletcher, S.; Shah, M.N.; Sirianni, R.W.; et al. Infusion of 5-Azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior Fossa Ependymoma: A pilot clinical trial. J. Neuro-Oncol. 2018, 141, 449–457. [Google Scholar] [CrossRef]
- Blaney, S.M.; Phillips, P.C.; Packer, R.J.; Heideman, R.L.; Berg, S.L.; Adamson, P.C.; Allen, J.C.; Sallan, S.E.; Jakacki, R.I.; Lange, B.J.; et al. Phase II evaluation of topotecan for pediatric central nervous system tumors. Cancer 1996, 78, 527–531. [Google Scholar] [CrossRef]
- Minturn, J.E.; Janss, A.J.; Fisher, P.G.; Allen, J.C.; Patti, R.; Phillips, P.C.; Belasco, J.B. A phase II study of metronomic oral topotecan for recurrent childhood brain tumors. Pediatr. Blood Cancer 2010, 56, 39–44. [Google Scholar] [CrossRef]
- Yamada, A.; Kinoshita, M.; Kamimura, S.; Jinnouchi, T.; Azuma, M.; Yamashita, S.; Yokogami, K.; Takeshima, H.; Moritake, H. Novel Strategy Involving High-Dose Chemotherapy with Stem Cell Rescue Followed by Intrathecal Topotecan Maintenance Therapy without Whole-Brain Irradiation for Atypical Teratoid/Rhabdoid Tumors. Pediatr. Hematol. Oncol. 2023, 40, 629–642. [Google Scholar] [CrossRef]
- DeRenzo, C.; Krenciute, G.; Gottschalk, S. The Landscape of CAR T Cells Beyond Acute Lymphoblastic Leukemia for Pediatric Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 830–837. [Google Scholar] [CrossRef]
- Brown, C.E.; Hibbard, J.C.; Alizadeh, D.; Blanchard, M.S.; Natri, H.M.; Wang, D.; Ostberg, J.R.; Aguilar, B.; Wagner, J.R.; Paul, J.A.; et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: A phase 1 trial. Nat. Med. 2024, 30, 1001–1012. [Google Scholar] [CrossRef]
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, E.; et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: Phase 1 trial interim results. Nat. Med. 2024, 30, 1320–1329. [Google Scholar] [CrossRef]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oill, A.T.; Blanchard, M.; Wu, M.; Hibbard, J.; Sepulveda, S.; Peter, M.; Kilpatrick, J.; Munoz, M.; Stiller, T.; et al. Expansion of endogenous T cells in CSF of pediatric CNS tumor patients undergoing locoregional delivery of IL13R〿2-targeting CAR T cells: An interim analysis. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Wilson, A.L.; Huang, W.; Seidel, K.; Brown, C.; Gustafson, J.A.; Yokoyama, J.K.; Johnson, A.J.; Baxter, B.A.; Koning, R.W.; et al. Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety. Cancer Discov. 2022, 13, 114–131. [Google Scholar] [CrossRef]
- Khatua, S.; Cooper, L.J.N.; I Sandberg, D.; Ketonen, L.; Johnson, J.M.; E Rytting, M.; Liu, D.D.; Meador, H.; Trikha, P.; Nakkula, R.J.; et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro-Oncol. 2020, 22, 1214–1225. [Google Scholar] [CrossRef]
- Fangusaro, J.; Gururangan, S.; Poussaint, T.Y.; McLendon, R.E.; Onar-Thomas, A.; Warren, K.E.; Wu, S.; Packer, R.J.; Banerjee, A.; Gilbertson, R.J.; et al. Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11). Cancer 2013, 119, 4180–4187. [Google Scholar] [CrossRef]
- Trippett, T.M.; Herzog, C.; Whitlock, J.A.; Wolff, J.; Kuttesch, J.; Bagatell, R.; Hunger, S.P.; Boklan, J.; Smith, A.A.; Arceci, R.J.; et al. Phase I and Pharmacokinetic Study of Cetuximab and Irinotecan in Children With Refractory Solid Tumors: A Study of the Pediatric Oncology Experimental Therapeutic Investigators’ Consortium. J. Clin. Oncol. 2009, 27, 5102–5108. [Google Scholar] [CrossRef]
- McCrea, H.J.; Ivanidze, J.; O’connor, A.; Hersh, E.H.; Boockvar, J.A.; Gobin, Y.P.; Knopman, J.; Greenfield, J.P. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: Results of a phase I trial. J. Neurosurg. Pediatr. 2021, 28, 371–379. [Google Scholar] [CrossRef]
- Ellis, J.A.; Cooke, J.; Singh-Moon, R.P.; Wang, M.; Bruce, J.N.; Emala, C.W.; Bigio, I.J.; Joshi, S. Safety, feasibility, and optimization of intra-arterial mitoxantrone delivery to gliomas. J. Neuro-Oncol. 2016, 130, 449–454. [Google Scholar] [CrossRef]
- Joshi, S.; Singh-Moon, R.P.; Ellis, J.A.; Chaudhuri, D.B.; Wang, M.; Reif, R.; Bruce, J.N.; Bigio, I.J.; Straubinger, R.M. Cerebral Hypoperfusion-Assisted Intra-arterial Deposition of Liposomes in Normal and Glioma-Bearing Rats. Neurosurgery 2015, 76, 92–100. [Google Scholar] [CrossRef]
- Joshi, S.; Cooke, J.R.N.; Ellis, J.A.; Emala, C.W.; Bruce, J.N. Targeting brain tumors by intra-arterial delivery of cell-penetrating peptides: A novel approach for primary and metastatic brain malignancy. J. Neuro-Oncol. 2017, 135, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Villemure, J.G.; Worthington, C.; Tyler, J.L.; Théron, J. Superselective intracerebral chemotherapy of malignant tumours with BCNU. Neuroradiology 1986, 28, 118–125. [Google Scholar] [CrossRef]
- Bashir, R.; Hochberg, F.H.; Linggood, R.M.; Hottleman, K. Pre-irradiation internal carotid artery BCNU in treatment of glioblastoma multiforme. J. Neurosurg. 1988, 68, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, F.H.; Miller, D.C. Primary central nervous system lymphoma. J. Neurosurg. 1988, 68, 835–853. [Google Scholar] [CrossRef]
- Meng, Y.; Pople, C.B.; Budiansky, D.; Li, D.; Suppiah, S.; Lim-Fat, M.J.; Perry, J.; Sahgal, A.; Lipsman, N. Current state of therapeutic focused ultrasound applications in neuro-oncology. J. Neuro-Oncol. 2021, 156, 49–59. [Google Scholar] [CrossRef]
- Timbie, K.F.; Mead, B.P.; Price, R.J. Drug and gene delivery across the blood–brain barrier with focused ultrasound. J. Control. Release 2015, 219, 61–75. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Targeted disruption of the blood–brain barrier with focused ultrasound: Association with cavitation activity. Phys. Med. Biol. 2006, 51, 793–807. [Google Scholar] [CrossRef]
- Bader, K.B.; Holland, C. Gauging the likelihood of stable cavitation from ultrasound contrast agents. Phys. Med. Biol. 2013, 58, 127–144. [Google Scholar] [CrossRef]
- Wu, S.-K.; Tsai, C.-L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2021, 13, 15. [Google Scholar] [CrossRef]
- McDannold, N.; Zhang, Y.; Supko, J.G.; Power, C.; Sun, T.; Peng, C.; Vykhodtseva, N.; Golby, A.J.; Reardon, D.A. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics 2019, 9, 6284–6299. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Huang, C.-Y.; Chen, J.-Y.; Wang, H.-Y.J.; Chen, P.-Y.; Wei, K.-C. Pharmacodynamic and Therapeutic Investigation of Focused Ultrasound-Induced Blood-Brain Barrier Opening for Enhanced Temozolomide Delivery in Glioma Treatment. PLoS ONE 2014, 9, e114311. [Google Scholar] [CrossRef] [PubMed]
- Chesney, K.M.; Keating, G.F.; Patel, N.; Kilburn, L.; Fonseca, A.; Wu, C.-C.; Nazarian, J.; Packer, R.J.; Donoho, D.A.; Oluigbo, C.; et al. The role of focused ultrasound for pediatric brain tumors: Current insights and future implications on treatment strategies. Child’s Nerv. Syst. 2024, 40, 2333–2344. [Google Scholar] [CrossRef]
- Alkins, R.; Burgess, A.; Ganguly, M.; Francia, G.; Kerbel, R.; Wels, W.S.; Hynynen, K. Focused Ultrasound Delivers Targeted Immune Cells to Metastatic Brain Tumors. Cancer Res. 2013, 73, 1892–1899. [Google Scholar] [CrossRef]
- Englander, Z.K.; Wei, H.-J.; Pouliopoulos, A.N.; Bendau, E.; Upadhyayula, P.; Jan, C.-I.; Spinazzi, E.F.; Yoh, N.; Tazhibi, M.; McQuillan, N.M.; et al. Focused ultrasound mediated blood–brain barrier opening is safe and feasible in a murine pontine glioma model. Sci. Rep. 2021, 11, 6521. [Google Scholar] [CrossRef]
- Ishida, J.; Alli, S.; Bondoc, A.; Golbourn, B.; Sabha, N.; Mikloska, K.; Krumholtz, S.; Srikanthan, D.; Fujita, N.; Luck, A.; et al. MRI-guided focused ultrasound enhances drug delivery in experimental diffuse intrinsic pontine glioma. J. Control. Release 2020, 330, 1034–1045. [Google Scholar] [CrossRef]
- Martinez, P.; Nault, G.; Steiner, J.; Wempe, M.F.; Pierce, A.; Brunt, B.; Slade, M.; Song, J.J.; Mongin, A.; Song, K.-H.; et al. MRI-guided focused ultrasound blood–brain barrier opening increases drug delivery and efficacy in a diffuse midline glioma mouse model. Neuro-Oncol. Adv. 2023, 5, vdad111. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; A Schmidt, K.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated blood–brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: A phase 1 trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef]
- Marty, B.; Larrat, B.; Van Landeghem, M.; Robic, C.; Robert, P.; Port, M.; Le Bihan, D.; Pernot, M.; Tanter, M.; Lethimonnier, F.; et al. Dynamic Study of Blood–Brain Barrier Closure after its Disruption using Ultrasound: A Quantitative Analysis. J. Cereb. Blood Flow Metab. 2012, 32, 1948–1958. [Google Scholar] [CrossRef]
- Hooge, M.N.L.; Kosterink, J.G.W.; Perik, P.J.; Nijnuis, H.; Tran, L.; Bart, J.; Suurmeijer, A.J.H.; de Jong, S.; Jager, P.L.; E de Vries, E.G. Preclinical characterisation of 111In-DTPA-trastuzumab. Br. J. Pharmacol. 2004, 143, 99–106. [Google Scholar] [CrossRef]
- Black, K.L.; Ningaraj, N.S. Modulation of Brain Tumor Capillaries for Enhanced Drug Delivery Selectively to Brain Tumor. Cancer Control. 2004, 11, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.; Elliott, P.; Hayward, N.; Dean, R.; McEwen, E.; Fisher, S. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: Evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology 1996, 33, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Matsukado, K.; Inamura, T.; Nakano, S.; Fukui, M.; Bartus, R.T.; Black, K.L. Enhanced Tumor Uptake of Carboplatin and Survival in Glioma-bearing Rats by Intracarotid Infusion of Bradykinin Analog, RMP-7. Neurosurgery 1996, 39, 125–134. [Google Scholar] [CrossRef]
- Warren, K.; Jakacki, R.; Widemann, B.; Aikin, A.; Libucha, M.; Packer, R.; Vezina, G.; Reaman, G.; Shaw, D.; Krailo, M.; et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: A report from the Children’s Oncology Group. Cancer Chemother. Pharmacol. 2006, 58, 343–347. [Google Scholar] [CrossRef]
- Packer, R.J.; Krailo, M.; Mehta, M.; Warren, K.; Allen, J.; Jakacki, R.; Villablanca, J.G.; Chiba, A.; Reaman, G. A Phase I study of concurrent RMP-7 and carboplatin with radiation therapy for children with newly diagnosed brainstem gliomas. Cancer 2005, 104, 1968–1974. [Google Scholar] [CrossRef]
- KrolI, R.A.; Neuwelt, E.A. Outwitting the Blood-Brain Barrier for Therapeutic Purposes: Osmotic Opening and Other Means. Neurosurgery 1998, 42, 1083–1099. [Google Scholar] [CrossRef]
- Rapoport, S.; Hori, M.; Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. Content 1972, 223, 323–331. [Google Scholar] [CrossRef]
- Cosolo, W.C.; Martinello, P.; Louis, W.J.; Christophidis, N.; Yu, A.S.; Hirayama, B.A.; Timbol, G.; Liu, J.; Diez-Sampedro, A.; Kepe, V.; et al. Blood-brain barrier disruption using mannitol: Time course and electron microscopy studies. Am. J. Physiol. Integr. Comp. Physiol. 1989, 256, R443–R447. [Google Scholar] [CrossRef]
- A Neuwelt, E. Reversible osmotic blood-brain barrier disruption in humans. Neurosurgery 1980, 7, 204. [Google Scholar] [CrossRef]
- Rapoport, S.I. Osmotic Opening of the Blood–Brain Barrier: Principles, Mechanism, and Therapeutic Applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Goldman, D.L.; Dahlborg, S.A.; Crossen, J.; Ramsey, F.; Roman-Goldstein, S.; Braziel, R.; Dana, B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: Prolonged survival and preservation of cognitive function. J. Clin. Oncol. 1991, 9, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Pappius, H.M.; Savaki, H.E.; Fieschi, C.; Rapoport, S.I.; Sokoloff, L. Osmotic opening of the blood-brain barrier and local cerebral glucose utilization. Ann. Neurol. 1979, 5, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-Promoting Effect of Blood–Brain Barrier Disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.-Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming Limitations in Nanoparticle Drug Delivery: Triggered, Intravascular Release to Improve Drug Penetration into Tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef]
- Poon, R.T.; Borys, N. Lyso-thermosensitive liposomal doxorubicin: A novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin. Pharmacother. 2009, 10, 333–343. [Google Scholar] [CrossRef]
- Chiannilkulchai, N.; Driouich, Z.; Benoit, J.; Parodi, A.; Couvreur, P. Doxorubicin-Loaded Nanoparticles: Increased Efficiency in Murine Hepatic Metastases. Sel. Cancer Ther. 1989, 5, 1–11. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Parodi, A.; Rudzińska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics 2019, 11, 245. [Google Scholar] [CrossRef]
- Mendes, M.; Sousa, J.J.; Pais, A.; Vitorino, C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics 2018, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Albertini, B.; Mathieu, V.; Iraci, N.; Van Woensel, M.; Schoubben, A.; Donnadio, A.; Greco, S.M.; Ricci, M.; Temperini, A.; Blasi, P.; et al. Tumor Targeting by Peptide-Decorated Gold Nanoparticles. Mol. Pharm. 2019, 16, 2430–2444. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef]
- Mahdi, J.; Dietrich, J.; Straathof, K.; Roddie, C.; Scott, B.J.; Davidson, T.B.; Prolo, L.M.; Batchelor, T.T.; Campen, C.J.; Davis, K.L.; et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 2023, 29, 803–810. [Google Scholar] [CrossRef]
- Singleton, W.G.B.; Bienemann, A.S.; Woolley, M.; Johnson, D.; Lewis, O.; Wyatt, M.J.; Damment, S.J.P.; Boulter, L.J.; Killick-Cole, C.L.; Asby, D.J.; et al. The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. J. Neurosurg. Pediatr. 2018, 22, 288–296. [Google Scholar] [CrossRef]
- Bredlau, A.L.; Dixit, S.; Chen, C.; Broome, A.-M. Nanotechnology Applications for Diffuse Intrinsic Pontine Glioma. Curr. Neuropharmacol. 2017, 15, 104–115. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Ma, C.; Liu, H.; Li, Z.; Yang, Y. Macrophage-Derived Exosomes as Advanced Therapeutics for Inflammation: Current Progress and Future Perspectives. Int. J. Nanomed. 2024, ume 19, 1597–1627. [Google Scholar] [CrossRef]
- Shan, S.; Chen, J.; Sun, Y.; Wang, Y.; Xia, B.; Tan, H.; Pan, C.; Gu, G.; Zhong, J.; Qing, G.; et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-siRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Adv. Sci. 2022, 9, e2200353. [Google Scholar] [CrossRef]
- Tziafas, D.; Kolokuris, I. Experimentally induced pulpitis in permanent teeth with open apices. Histological studies in dogs. Hell Stomatol. Chron. 1984, 28, 131–140. [Google Scholar]
- Zhu, Z.; Zhai, Y.; Hao, Y.; Wang, Q.; Han, F.; Zheng, W.; Hong, J.; Cui, L.; Jin, W.; Ma, S.; et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J. Extracell. Vesicles 2022, 11, e12255. [Google Scholar] [CrossRef]
- Rechberger, J.S.; Power, E.A.; Lu, V.M.; Zhang, L.; Sarkaria, J.N.; Daniels, D.J. Evaluating infusate parameters for direct drug delivery to the brainstem: A comparative study of convection-enhanced delivery versus osmotic pump delivery. Neurosurg. Focus 2020, 48, E2. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.S.; Athawale, R.B.; Bajaj, A.N.; Shrikhande, S.S.; Goel, P.N.; Nikam, Y.; Gude, R.P. Unraveling the cytotoxic potential of Temozolomide loaded into PLGA nanoparticles. DARU J. Pharm. Sci. 2014, 22, 18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2018, 16, 1–23. [Google Scholar] [CrossRef]
- Louis, N.; Liu, S.; He, X.; Drummond, D.C.; Noble, C.O.; Goldman, S.; Mueller, S.; Bankiewicz, K.; Gupta, N.; Hashizume, R. New therapeutic approaches for brainstem tumors: A comparison of delivery routes using nanoliposomal irinotecan in an animal model. J. Neuro-Oncol. 2017, 136, 475–484. [Google Scholar] [CrossRef]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. Blood–brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef]
- Sánchez-Navarro, M.; Teixidó, M.; Giralt, E. Jumping Hurdles: Peptides Able To Overcome Biological Barriers. Acc. Chem. Res. 2017, 50, 1847–1854. [Google Scholar] [CrossRef]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood–Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Li, S.; Amat, D.; Peng, Z.; Vanni, S.; Raskin, S.; De Angulo, G.; Othman, A.M.; Graham, R.M.; Leblanc, R.M. Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells. Nanoscale 2016, 8, 16662–16669. [Google Scholar] [CrossRef]
- Ajmal, M.; Yunus, U.; Graham, R.M.; Leblanc, R.M. Design, synthesis, and targeted delivery of fluorescent 1, 2, 4-triazole–peptide conjugates to pediatric brain tumor cells. ACS Omega 2019, 4, 22280–22291. [Google Scholar] [CrossRef]
- Baghirov, H. Receptor–mediated transcytosis of macromolecules across the blood–brain barrier. Expert Opin. Drug Deliv. 2023, 20, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Mizuno, H.L.; Norimatsu, J.; Obara, T.; Cabral, H.; Tsumoto, K.; Nakakido, M.; Kawauchi, D.; Anraku, Y. Ligand Installation to Polymeric Micelles for Pediatric Brain Tumor Targeting. Polymers 2023, 15, 1808. [Google Scholar] [CrossRef] [PubMed]
- Haddad, Y.; Charousova, M.; Zivotska, H.; Splichal, Z.; Rodrigo, M.A.M.; Michalkova, H.; Krizkova, S.; Tesarova, B.; Richtera, L.; Vitek, P.; et al. Norepinephrine transporter-derived homing peptides enable rapid endocytosis of drug delivery nanovehicles into neuroblastoma cells. J. Nanobiotechnol. 2020, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Culkins, C.; Adomanis, R.; Phan, N.; Robinson, B.; Slaton, E.; Lothrop, E.; Chen, Y.; Kimmel, B.R. Unlocking the Gates: Therapeutic Agents for Noninvasive Drug Delivery Across the Blood-Brain Barrier. Mol. Pharm. 2024, 21, 5430–5454. [Google Scholar] [CrossRef]
- Peng, X.; Liu, X.; Kim, J.Y.; Nguyen, A.; Leal, J.; Ghosh, D. Brain-Penetrating Peptide Shuttles across the Blood–Brain Barrier and Extracellular-like Space. Bioconjugate Chem. 2023, 34, 2319–2336. [Google Scholar] [CrossRef]
- Parrasia, S.; Szabò, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).