Optimizing Osimertinib for NSCLC: Targeting Resistance and Exploring Combination Therapeutics
Simple Summary
Abstract
1. Background
2. The Mechanisms of NSCLC Osimertinib Resistance
3. On-Target (EGFR-Dependent) Mechanisms and Corresponding Therapeutic Strategies
3.1. Tertiary Mutations at C797
3.2. Other EGFR Mutations
| Mechanism Category | Approximate Prevalence | Therapeutic Strategies | References |
|---|---|---|---|
| Tertiary mutations at C797 (e.g., C797S, C797G) | 11–29% (real-world); ~15% (AURA3); ~7% (FLAURA) |
| [32,40,50,57,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,96,97,98] |
| Other EGFR mutations L792 (L792H/Y/F), L718 (L718Q/V), G724S, G796 (G796R/S/H/D) | Rare (varies by cohort, typically <5%) |
| [39,59,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115] |
| Exon 20 insertions (EGFR20ins) | 10–12% of EGFR-driven NSCLC (all lines) |
| [116,117,118,119,120] |
3.3. Exon 20 Insertions
4. Off-Target (EGFR-Independent) Resistance Mechanisms and Corresponding Therapeutic Strategies
4.1. MET Amplification
4.2. HER2 Amplification and HER3 Upregulation
4.3. Abnormalities in Cell Proliferation and Apoptosis-Related Factors
4.4. Abnormal Activation of Downstream Proliferative Signaling Pathways
4.5. Histologic Transformation and Epithelial-to-Mesenchymal Transition (EMT)
| Mechanism Category | Approximate Prevalence | Therapeutic Strategies | References | |
|---|---|---|---|---|
| MET amplification | Up to 8–19% of resistance cases; more frequent in first-line osimertinib |
| [32,40,72,74,96,104,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150] | |
| HER2 amplification and HER3 upregulation | HER2 amplification/mutation | ~2–5% in post-osimertinib setting (higher in second-line) |
| [32,40,119,120,151,152,153] |
| HER3 upregulation | Frequently seen in resistant tumors; exact incidence unclear |
| [128,154,155,156,157,158,159,160,161,162,163,164,165,166] | |
| Alterations in proliferation and apoptosis | FGFR1 amplification | Rare; exact rates vary |
| [167,168,169,170] |
| IGF1R activation | Rare; data mostly preclinical |
| [171,172,173,174] | |
| AXL overexpression | Frequently upregulated in resistant clones |
| [15,168,170,175,176,177,178,179,180,181,182,183,184] | |
| BCL-2 family dysregulation | BIM deletion polymorphism in ~21% of East Asians |
| [185,186,187,188,189] | |
| Cell cycle pathway alterations | Common in relapse; often correlated with shorter PFS |
| [190,191,192,193,194,195,196] | |
| Abnormal activation of downstream proliferative signaling pathways | PI3K/AKT/mTOR-driven | Mutations in PIK3CA in ~2–5%; PTEN loss also reported |
| [92,197,198,199,200,201,202] |
| RAS/RAF/MEK/ERK (MAPK)-driven | KRAS/NRAS/BRAF mutations in ~1–3% post-osimertinib |
| [40,50,96,104,113,168,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229] | |
| Histologic transformation and epithelial-to-mesenchymal transition (EMT) | Histologic transformation | 2–15% of resistance cases vary with line of therapy |
| [78,85,91,230,231,232,233,234,235,236,237,238,239,240,241] |
| Epithelial-to-mesenchymal transition (EMT) | Common in resistant tumors, the exact incidence varies |
| [242,243,244,245,246,247,248,249,250,251,252,253,254] |
5. Osimertinib-Based Combination Therapies: Exploring Synergistic Approaches
5.1. Chemotherapy
5.2. VEGF Inhibitor-Based Therapy
5.3. Radiotherapy
5.4. MET-Inhibitor Based Therapy
5.5. Immunotherapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| AXL | anexelekto |
| ABCB1 | ATP-binding cassette subfamily B member 1 |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| BCL-2 | B-cell lymphoma 2 |
| BIM | Bcl-2 Interacting Mediator of Cell Death |
| C797S | cysteine 797 to serine (mutation in EGFR) |
| CDK4/6 | cyclin-dependent kinases 4 and 6 |
| CEP7 | chromosome enumeration probe 7 |
| CNS | central nervous system |
| CTNNB1 | catenin beta 1 |
| CAFs | cancer-associated fibroblasts |
| cGAS–STING | cyclic GMP-AMP synthase–stimulator of interferon genes |
| c-MET | mesenchymal–epithelial transition factor |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial–mesenchymal transition |
| ERK | extracellular signal-regulated kinase |
| EMT-TFs | epithelial–mesenchymal transition transcription factors |
| FGFR1 | fibroblast growth factor receptor 1 |
| FOXP | forkhead box P |
| GSK-3β | glycogen synthase kinase 3 beta |
| HER2 | human epidermal growth factor receptor 2 |
| HER3 | human epidermal growth factor receptor 3 |
| HGF | hepatocyte growth factor |
| HER3-DXd | HER3-directed DXd conjugate |
| IGF1R | insulin-like growth factor 1 receptor |
| IL-6 | interleukin 6 |
| IL-12 | interleukin 12 |
| JAK | Janus kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homologue |
| L792H | leucine 792 to histidine (mutation in EGFR) |
| MAPK | mitogen-activated protein kinase |
| MERTK | proto-oncogene tyrosine-protein kinase MER |
| MET | mesenchymal–epithelial transition factor |
| MCL1 | myeloid cell leukemia 1 |
| NSCLC | non-small-cell lung cancer |
| NRAS | neuroblastoma RAS viral oncogene homologue |
| NOX | NADPH oxidase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| ORR | overall response rate |
| OS | overall survival |
| PD-L1 | programmed death-ligand 1 |
| PFS | progression-free survival |
| PI3K | phosphoinositide 3-kinase |
| PLK1 | polo-like kinase 1 |
| PTEN | phosphatase and tensin homologue |
| PARP | poly(ADP-ribose) polymerase |
| PD-1 | programmed cell death protein 1 |
| RAF | rapidly accelerated fibrosarcoma |
| RAS | rat sarcoma viral oncogene |
| RB1 | retinoblastoma 1 |
| RTK | receptor tyrosine kinase |
| RT | radiation therapy |
| SMAD2 | mothers against decapentaplegic homologue 2 |
| STAT | signal transducer and activator of transcription |
| STAT3 | signal transducer and activator of transcription 3 |
| T790M | threonine 790 to methionine (mutation in EGFR) |
| TGFβ | transforming growth factor beta |
| TME | tumor microenvironment |
| TKI | tyrosine kinase inhibitor |
| TAM | tumor-associated macrophage |
| TARO3 | tyrosine-protein kinase receptor 3 |
| T-DM1 | trastuzumab emtansine |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| ZEB | zinc finger E-box binding homeobox |
References
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic Non-Small Cell Lung Cancer: Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29 (Suppl. S4), iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Kauffmann-Guerrero, D.; Kahnert, K.; Huber, R.M. Treatment Sequencing for Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer. Drugs 2021, 81, 87–100. [Google Scholar] [CrossRef]
- Wang, Y.T.; Yang, P.C.; Zhang, J.Y.; Sun, J.F. Synthetic Routes and Clinical Application of Representative Small-Molecule Egfr Inhibitors for Cancer Therapy. Molecules 2024, 29, 1448. [Google Scholar] [CrossRef]
- Dong, R.F.; Zhu, M.L.; Liu, M.M.; Xu, Y.T.; Yuan, L.L.; Bian, J.; Xia, Y.Z.; Kong, L.Y. Egfr Mutation Mediates Resistance to Egfr Tyrosine Kinase Inhibitors in Nsclc: From Molecular Mechanisms to Clinical Research. Pharmacol. Res. 2021, 167, 105583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Zhu, Y.; Dong, T.; Liu, Z. Understanding the Treatment Response and Resistance to Targeted Therapies in Non-Small Cell Lung Cancer: Clinical Insights and Perspectives. Front. Oncol. 2024, 14, 1387345. [Google Scholar] [CrossRef]
- Melosky, B.; Vincent, M.D.; McGuire, A.L.; Brade, A.M.; Chu, Q.; Cheema, P.; Martins, I.; Spicer, J.D.; Snow, S.; Juergens, R.A. Modern Era Systemic Therapies: Expanding Concepts of Cure in Early and Locally Advanced Non-Small Cell Lung Cancer. Int. J. Cancer 2024, 155, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Wang, P.; Zhou, L.; Li, Y. Strategies and Influencing Factors for the Treatment of Advanced Non-Small Cell Lung Cancer Based on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: A Narrative Review. Transl. Cancer Res. 2024, 13, 5123–5140. [Google Scholar] [CrossRef]
- Ciardiello, F.; Hirsch, F.R.; Pirker, R.; Felip, E.; Valencia, C.; Smit, E.F. The Role of Anti-Egfr Therapies in Egfr-Tki-Resistant Advanced Non-Small Cell Lung Cancer. Cancer Treat. Rev. 2024, 122, 102664. [Google Scholar] [CrossRef]
- Ward, R.A.; Anderton, M.J.; Ashton, S.; Bethel, P.A.; Box, M.; Butterworth, S.; Colclough, N.; Chorley, C.G.; Chuaqui, C.; Cross, D.A.; et al. Structure- and Reactivity-Based Development of Covalent Inhibitors of the Activating and Gatekeeper Mutant Forms of the Epidermal Growth Factor Receptor (Egfr). J. Med. Chem. 2013, 56, 7025–7048. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to Egfr-Tki Therapy in 155 Patients with Egfr-Mutant Lung Cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Finlay, M.R.; Anderton, M.; Ashton, S.; Ballard, P.; Bethel, P.A.; Box, M.R.; Bradbury, R.H.; Brown, S.J.; Butterworth, S.; Campbell, A.; et al. Discovery of a Potent and Selective Egfr Inhibitor (Azd9291) of Both Sensitizing and T790m Resistance Mutations That Spares the Wild Type Form of the Receptor. J. Med. Chem. 2014, 57, 8249–8267. [Google Scholar] [CrossRef]
- Wu, S.G.; Liu, Y.N.; Tsai, M.F.; Chang, Y.L.; Yu, C.J.; Yang, P.C.; Yang, J.C.; Wen, Y.F.; Shih, J.Y. The Mechanism of Acquired Resistance to Irreversible Egfr Tyrosine Kinase Inhibitor-Afatinib in Lung Adenocarcinoma Patients. Oncotarget 2016, 7, 12404–12413. [Google Scholar] [CrossRef]
- Mu, Y.; Hao, X.; Xing, P.; Hu, X.; Wang, Y.; Li, T.; Zhang, J.; Xu, Z.; Li, J. Acquired Resistance to Osimertinib in Patients with Non-Small-Cell Lung Cancer: Mechanisms and Clinical Outcomes. J. Cancer Res. Clin. Oncol. 2020, 146, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Kashima, Y.; Shibahara, D.; Suzuki, A.; Muto, K.; Kobayashi, I.S.; Plotnick, D.; Udagawa, H.; Izumi, H.; Shibata, Y.; Tanaka, K.; et al. Single-Cell Analyses Reveal Diverse Mechanisms of Resistance to Egfr Tyrosine Kinase Inhibitors in Lung Cancer. Cancer Res. 2021, 81, 4835–4848. [Google Scholar] [CrossRef]
- Șandor, A.; Ionuț, I.; Marc, G.; Oniga, I.; Eniu, D.; Oniga, O. Structure-Activity Relationship Studies Based on Quinazoline Derivatives as Egfr Kinase Inhibitors (2017-Present). Pharmaceuticals 2023, 16, 534. [Google Scholar] [CrossRef] [PubMed]
- Laface, C.; Maselli, F.M.; Santoro, A.N.; Iaia, M.L.; Ambrogio, F.; Laterza, M.; Guarini, C.; De Santis, P.; Perrone, M.; Fedele, P. The Resistance to Egfr-Tkis in Non-Small Cell Lung Cancer: From Molecular Mechanisms to Clinical Application of New Therapeutic Strategies. Pharmaceutics 2023, 15, 1604. [Google Scholar] [CrossRef]
- Cross, D.A.; Ashton, S.E.; Ghiorghiu, S.; Eberlein, C.; Nebhan, C.A.; Spitzler, P.J.; Orme, J.P.; Finlay, M.R.; Ward, R.A.; Mellor, M.J.; et al. Azd9291, an Irreversible Egfr Tki, Overcomes T790m-Mediated Resistance to Egfr Inhibitors in Lung Cancer. Cancer Discov. 2014, 4, 1046–1061. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Yang, J.C.; Kim, D.W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.J.; Kim, S.W.; Su, W.C.; Horn, L.; et al. Azd9291 in Egfr Inhibitor-Resistant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Planchard, D. Azd9291 in Egfr-Mutant Advanced Non-Small-Cell Lung Cancer Patients. Future Oncol. 2015, 11, 3069–3081. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Steuer, C.E.; Ramalingam, S.S.; Felip, E. Osimertinib and Other Third-Generation Egfr Tki in Egfr-Mutant Nsclc Patients. Ann. Oncol. 2018, 29, i20–i27. [Google Scholar] [CrossRef]
- Bollinger, M.K.; Agnew, A.S.; Mascara, G.P. Osimertinib: A Third-Generation Tyrosine Kinase Inhibitor for Treatment of Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer with the Acquired Thr790met Mutation. J. Oncol. Pharm. Pract. 2018, 24, 379–388. [Google Scholar] [CrossRef]
- Lin, C.C.; Shih, J.Y.; Yu, C.J.; Ho, C.C.; Liao, W.Y.; Lee, J.H.; Tsai, T.H.; Su, K.Y.; Hsieh, M.S.; Chang, Y.L.; et al. Outcomes in Patients with Non-Small-Cell Lung Cancer and Acquired Thr790met Mutation Treated with Osimertinib: A Genomic Study. Lancet Respir. Med. 2018, 6, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated Egfr-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected Egfr-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Vaid, A.K.; Gupta, A.; Momi, G. Overall Survival in Stage iv Egfr Mutation-Positive Nsclc: Comparing First-, Second- and Third-Generation Egfr-Tkis (Review). Int. J. Oncol. 2021, 58, 171–184. [Google Scholar] [CrossRef]
- Bertoli, E.; De Carlo, E.; Del Conte, A.; Stanzione, B.; Revelant, A.; Fassetta, K.; Spina, M.; Bearz, A. Acquired Resistance to Osimertinib in Egfr-Mutated Non-Small Cell Lung Cancer: How Do We Overcome It? Int. J. Mol. Sci. 2022, 23, 6936. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Yang, J.C.; Lee, C.K.; Kurata, T.; Kim, D.W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenkov, Y.; et al. Osimertinib as First-Line Treatment of Egfr Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 841–849. [Google Scholar] [CrossRef]
- Goss, G.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Lee, J.S.; Chang, G.C.; Crino, L.; Satouchi, M.; Chu, Q.; Hida, T.; et al. Osimertinib for Pretreated Egfr Thr790met-Positive Advanced Non-Small-Cell Lung Cancer (Aura2): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2016, 17, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Ahn, M.J.; Kim, D.W.; Kim, S.W.; Planchard, D.; Ramalingam, S.S.; Frewer, P.; Cantarini, M.; Ghiorghiu, S.; Yang, J.H. A Phase I Study of Azd9291 in Patients with Egfr-Tki-Resistant Advanced Nsclc—Updated Progression Free Survival and Duration of Response Data. Ann. Oncol. 2015, 26, i57. [Google Scholar]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in Egfr T790m-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Wu, Y.L.; Han, J.Y.; Ahn, M.J.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.H.; Bulusu, K.C.; Laus, G.; et al. Analysis of Resistance Mechanisms to Osimertinib in Patients with Egfr T790m Advanced Nsclc from the Aura3 Study. Ann. Oncol. 2018, 29, viii741. [Google Scholar] [CrossRef]
- Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Han, J.Y.; Katakami, N.; Kim, H.R.; Hodge, R.; Kaur, P.; Brown, A.P.; Ghiorghiu, D.; et al. Cns Efficacy of Osimertinib in Patients with T790m-Positive Advanced Non-Small-Cell Lung Cancer: Data from a Randomized Phase Iii Trial (Aura3). J. Clin. Oncol. 2018, 36, 2702–2709. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V.A.; Mok, T.S.; Han, J.Y.; Ahn, M.J.; Delmonte, A.; Ramalingam, S.S.; Kim, S.W.; Shepherd, F.A.; Laskin, J.; He, Y.; et al. Osimertinib Versus Platinum-Pemetrexed for Patients with Egfr T790m Advanced Nsclc and Progression on a Prior Egfr-Tyrosine Kinase Inhibitor: Aura3 Overall Survival Analysis. Ann. Oncol. 2020, 31, 1536–1544. [Google Scholar] [CrossRef]
- Chmielecki, J.; Mok, T.; Wu, Y.L.; Han, J.Y.; Ahn, M.J.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.; Shepherd, F.A.; et al. Analysis of Acquired Resistance Mechanisms to Osimertinib in Patients with Egfr-Mutated Advanced Non-Small Cell Lung Cancer from the Aura3 Trial. Nat. Commun. 2023, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Han, J.Y.; Ahn, M.J.; Ramalingam, S.S.; Delmonte, A.; Hsia, T.C.; Laskin, J.; Kim, S.W.; He, Y.; Tsai, C.M.; et al. Epidermal Growth Factor Receptor Mutation Analysis in Tissue and Plasma from the Aura3 Trial: Osimertinib Versus Platinum-Pemetrexed for T790m Mutation-Positive Advanced Non-Small Cell Lung Cancer. Cancer 2020, 126, 373–380. [Google Scholar] [CrossRef]
- Gray, J.E.; Ahn, M.J.; Oxnard, G.R.; Shepherd, F.A.; Imamura, F.; Cheng, Y.; Okamoto, I.; Cho, B.C.; Lin, M.C.; Wu, Y.L.; et al. Early Clearance of Plasma Epidermal Growth Factor Receptor Mutations as a Predictor of Outcome on Osimertinib in Advanced Non-Small Cell Lung Cancer, Exploratory Analysis from Aura3 and Flaura. Clin. Cancer Res. 2023, 29, 3340–3351. [Google Scholar] [CrossRef] [PubMed]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. Cns Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients with Untreated Egfr-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Suda, K.; Kobayashi, Y.; Ohara, S.; Fujino, T.; Koga, T.; Chiba, M.; Shimoji, M.; Tomizawa, K.; Takemoto, T.; et al. Effects of Secondary Egfr Mutations on Resistance against Upfront Osimertinib in Cells with Egfr-Activating Mutations in Vitro. Lung Cancer 2018, 126, 149–155. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Cheng, Y.; Zhou, C.; Ohe, Y.; Imamura, F.; Cho, B.C.; Lin, M.C.; Majem, M.; Shah, R.; Rukazenkov, Y.; et al. Mechanisms of Acquired Resistance to First-Line Osimertinib: Preliminary Data from the Phase Iii Flaura Study. Ann. Oncol. 2018, 29, viii740. [Google Scholar] [CrossRef]
- Planchard, D.; Feng, P.H.; Karaseva, N.; Kim, S.W.; Kim, T.M.; Lee, C.K.; Poltoratskiy, A.; Yanagitani, N.; Marshall, R.; Huang, X.; et al. Osimertinib Plus Platinum-Pemetrexed in Newly Diagnosed Epidermal Growth Factor Receptor Mutation-Positive Advanced/Metastatic Non-Small-Cell Lung Cancer: Safety Run-in Results from the Flaura2 Study. ESMO Open 2021, 6, 100271. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Planchard, D.; Kobayashi, K.; Cheng, Y.; Lee, C.K.; Valdiviezo, N.; Laktionov, K.; Yang, T.Y.; Yu, Y.; Kato, T.; et al. Cns Efficacy of Osimertinib with or without Chemotherapy in Epidermal Growth Factor Receptor-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Asahina, H.; Tanaka, K.; Morita, S.; Maemondo, M.; Seike, M.; Okamoto, I.; Oizumi, S.; Kagamu, H.; Takahashi, K.; Kikuchi, T.; et al. A Phase Ii Study of Osimertinib Combined with Platinum Plus Pemetrexed in Patients with Egfr-Mutated Advanced Non-Small-Cell Lung Cancer: The Opal Study (Nej032c/Logik1801). Clin. Lung Cancer 2021, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Viray, H.; Piper-Vallillo, A.J.; Widick, P.; Academia, E.; Shea, M.; Rangachari, D.; VanderLaan, P.A.; Kobayashi, S.S.; Costa, D.B. A Real-World Study of Patient Characteristics and Clinical Outcomes in Egfr Mutated Lung Cancer Treated with First-Line Osimertinib: Expanding the Flaura Trial Results into Routine Clinical Practice. Cancers 2024, 16, 1079. [Google Scholar] [CrossRef]
- Lu, S.; Kato, T.; Dong, X.; Ahn, M.J.; Quang, L.V.; Soparattanapaisarn, N.; Inoue, T.; Wang, C.L.; Huang, M.; Yang, J.C.; et al. Osimertinib after Chemoradiotherapy in Stage Iii Egfr-Mutated Nsclc. N. Engl. J. Med. 2024, 391, 585–597. [Google Scholar] [CrossRef]
- Herbst, R.S.; Wu, Y.L.; John, T.; Grohe, C.; Majem, M.; Wang, J.; Kato, T.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; et al. Adjuvant Osimertinib for Resected Egfr-Mutated Stage Ib-Iiia Non-Small-Cell Lung Cancer: Updated Results from the Phase Iii Randomized Adaura Trial. J. Clin. Oncol. 2023, 41, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.C.; et al. Overall Survival with Osimertinib in Resected Egfr-Mutated Nsclc. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Lakkunarajah, S.; Truong, P.T.; Bone, J.N.; Hughesman, C.; Yip, S.; Alex, D.; Hart, J.; Pollock, P.; Egli, S.; Clarkson, M.; et al. First-Line Osimertinib for Patients with Egfr-Mutated Advanced Non-Small Cell Lung Cancer: Efficacy and Safety During the Covid-19 Pandemic. Transl. Lung Cancer Res. 2023, 12, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, S.; Park, T.; Hwang, H.; Park, S.; Kim, J.; Pyun, J.C.; Lee, M. Reduction of Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2) Variant Infection by Blocking the Epidermal Growth Factor Receptor (Egfr) Pathway. Microbiol. Spectr. 2024, 12, e0158324. [Google Scholar] [CrossRef]
- Enrico, D.H.; Lacroix, L.; Rouleau, E.; Scoazec, J.Y.; Loriot, Y.; Tselikas, L.; Jovelet, C.; Planchard, D.; Gazzah, A.; Mezquita, L.; et al. 1526p—Multiple Synchronous Mechanisms May Contribute to Osimertinib Resistance in Non-Small Cell Lung Cancer (Nsclc) Patients: Insights of the Match-R Study. Ann. Oncol. 2019, 30, v627. [Google Scholar] [CrossRef]
- Xie, L.; Nagpal, S.; Wakelee, H.A.; Li, G.; Soltys, S.G.; Neal, J.W. Osimertinib for Egfr-Mutant Lung Cancer with Brain Metastases: Results from a Single-Center Retrospective Study. Oncologist 2019, 24, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Marrett, E.; Kwong, W.J.; Xie, J.; Manceur, A.M.; Sendhil, S.R.; Wu, E.; Ionescu-Ittu, R.; Subramanian, J. Treatment Patterns and Adverse Event-Related Hospitalization among Patients with Epidermal Growth Factor Receptor (Egfr)-Mutated Metastatic Non-Small Cell Lung Cancer after Treatment with Egfr Tyrosine Kinase Inhibitor and Platinum-Based Chemotherapy Regimens. Drugs Real. World Outcomes 2023, 10, 531–544. [Google Scholar]
- Watanabe, K.; Saito, R.; Miyauchi, E.; Nagashima, H.; Nakamura, A.; Sugawara, S.; Tanaka, N.; Terasaki, H.; Fukuhara, T.; Maemondo, M. Monitoring of Plasma Egfr Mutations During Osimertinib Treatment for Nsclc Patients with Acquired T790m Mutation. Cancers 2023, 15, 4231. [Google Scholar] [CrossRef]
- Watanabe, K.; Hosomi, Y.; Naoki, K.; Nakahara, Y.; Tsukita, Y.; Matsumoto, H.; Yoh, K.; Fujisaka, Y.; Takahashi, S.; Takata, S.; et al. The Whole Picture of First-Line Osimertinib for Egfr Mutation-Positive Advanced Nsclc: Real-World Efficacy, Safety, Progression Pattern, and Posttreatment Therapy (Reiwa Study). JTO Clin. Res. Rep. 2024, 5, 100720. [Google Scholar] [CrossRef] [PubMed]
- Uy, N.F.; Tratt, M.; Eaton, K.D.; Santana-Davila, R.; Baik, C.S. Osimertinib Rechallenge in Advanced Egfr Non-Small Cell Lung Cancer Patients. J. Clin. Oncol. 2023, 41, e21196. [Google Scholar] [CrossRef]
- Wang, R.H.; Chang, C.J.; Chen, C.H.; Liu, K.K.; Chao, J.I. Osimertinib Induces the Opposite Effect of Proliferation and Migration in the Drug Resistance of Egfr-T790m Non-Small Cell Lung Cancer Cells. Anticancer. Agents Med. Chem. 2023, 23, 1309–1319. [Google Scholar] [CrossRef]
- Chamorro, D.F.; Cardona, A.F.; Rodríguez, J.; Ruiz-Patiño, A.; Arrieta, O.; Moreno-Pérez, D.A.; Rojas, L.; Zatarain-Barrón, Z.L.; Ardila, D.V.; Viola, L.; et al. Genomic Landscape of Primary Resistance to Osimertinib among Hispanic Patients with Egfr-Mutant Non-Small Cell Lung Cancer (Nsclc): Results of an Observational Longitudinal Cohort Study. Target. Oncol. 2023, 18, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, L.; Han, C. Expert Consensus on the Use of Third-Generation Egfr-Tkis in Egfr-Mutated Advanced Non-Small Cell Lung Cancer with Various T790m Mutations Post-Resistance to First-/Second-Generation Egfr-Tkis. Ther. Adv. Med. Oncol. 2024, 16, 17588359241289648. [Google Scholar] [CrossRef]
- Floc’h, N.; Lim, S.; Bickerton, S.; Ahmed, A.; Orme, J.; Urosevic, J.; Martin, M.J.; Cross, D.A.E.; Cho, B.C.; Smith, P.D. Osimertinib, an Irreversible Next-Generation Egfr Tyrosine Kinase Inhibitor, Exerts Antitumor Activity in Various Preclinical Nsclc Models Harboring the Uncommon Egfr Mutations G719x or L861q or S768i. Mol. Cancer Ther. 2020, 19, 2298–2307. [Google Scholar] [CrossRef]
- Rybarczyk-Kasiuchnicz, A.; Ramlau, R.; Stencel, K. Treatment of Brain Metastases of Non-Small Cell Lung Carcinoma. Int. J. Mol. Sci. 2021, 22, 593. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Tsuboi, M.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; Vu, H.V.; et al. A Plain Language Summary of Results from the Adaura Study: Osimertinib after Surgery for Patients Who Have Early-Stage Egfr-Mutated Non-Small Cell Lung Cancer. Future Oncol. 2021, 17, 4827–4835. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.L.; Jiang, L.Y.; Shi, Y.K.; Chen, Y.; Yu, J.M.; Zhou, C.C.; He, Y.; Hu, Y.P.; Liang, Z.A.; et al. Real-World Evidence of Osimertinib in Chinese Patients with Egfr T790m-Positive Non-Small Cell Lung Cancer: A Subgroup Analysis from Astris Study. J. Cancer Res. Clin. Oncol. 2023, 149, 10771–10780. [Google Scholar] [CrossRef]
- Ohara, S.; Suda, K.; Mitsudomi, T. Cell Line Models for Acquired Resistance to First-Line Osimertinib in Lung Cancers-Applications and Limitations. Cells 2021, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nie, W.; Guo, J.; Xiong, A.; Zhong, H.; Chu, T.; Zhong, R.; Xu, J.; Lu, J.; Zheng, X.; et al. Osimertinib Alone as Second-Line Treatment for Brain Metastases (Bm) Control May Be More Limited Than for Non-Bm in Advanced Nsclc Patients with an Acquired Egfr T790m Mutation. Respir. Res. 2021, 22, 145. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Hu, Y.; Mileham, K.F.; Husain, H.; Costa, D.B.; Tracy, P.; Feeney, N.; Sholl, L.M.; Dahlberg, S.E.; Redig, A.J.; et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients with Egfr T790m-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018, 4, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Zhu, V.W.; Lim, S.M.; Greco, M.; Wu, F.; Ou, S.I. Beyond Osimertinib: The Development of Third-Generation Egfr Tyrosine Kinase Inhibitors for Advanced Egfr+ Nsclc. J. Thorac. Oncol. 2021, 16, 740–763. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Chen, J.; Xue, H.; He, Q.; Zhong, D.; Diao, X. Covalent Binding Mechanism of Furmonertinib and Osimertinib with Human Serum Albumin. Drug Metab. Dispos. 2023, 51, 8–16. [Google Scholar] [CrossRef]
- Skribek, M.; Bozoky, B.; Tsakonas, G. Osimertinib-Induced Syndrome of Inappropriate Secretion of Antidiuretic Hormone in Oncogene-Addicted Lung Adenocarcinoma: A Case Report. Lung Cancer 2022, 166, 132–134. [Google Scholar] [CrossRef]
- Takeyasu, Y.; Yoshida, T.; Masuda, K.; Matsumoto, Y.; Shinno, Y.; Okuma, Y.; Goto, Y.; Horinouchi, H.; Yamamoto, N.; Ohe, Y. Distinct Progression and Efficacy of First-Line Osimertinib Treatment According to Mutation Subtypes in Metastatic Nsclc Harboring Egfr Mutations. JTO Clin. Res. Rep. 2024, 5, 100636. [Google Scholar] [CrossRef]
- Attili, I.; Corvaja, C.; Spitaleri, G.; Aliaga, P.T.; Del Signore, E.; Passaro, A.; de Marinis, F. Post-Progression Analysis of Egfr-Mutant Nsclc Following Osimertinib Therapy in Real-World Settings. Cancers 2024, 16, 2589. [Google Scholar] [CrossRef]
- Gibson, A.J.W.; Dean, M.L.; Litt, I.; Box, A.; Cheung, W.Y.; Navani, V. Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for Egfr Mutation-Positive Patients Treated with First-Line Osimertinib. Curr. Oncol. 2024, 31, 2427–2440. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.S.; Lee, B.; Kim, H.K.; Sun, J.M.; Ahn, J.S.; Ahn, M.J.; Park, K.; Lee, S.H. Genomic Landscape of Acquired Resistance to Third-Generation Egfr Tyrosine Kinase Inhibitors in Egfr T790m-Mutant Non-Small Cell Lung Cancer. Cancer 2020, 126, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yun, C.H.; Park, E.; Ercan, D.; Manuia, M.; Juarez, J.; Xu, C.; Rhee, K.; Chen, T.; Zhang, H.; et al. Overcoming Egfr(T790m) and Egfr(C797s) Resistance with Mutant-Selective Allosteric Inhibitors. Nature 2016, 534, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Niederst, M.J.; Hu, H.; Mulvey, H.E.; Lockerman, E.L.; Garcia, A.R.; Piotrowska, Z.; Sequist, L.V.; Engelman, J.A. The Allelic Context of the C797s Mutation Acquired Upon Treatment with Third-Generation Egfr Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin. Cancer Res. 2015, 21, 3924–3933. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.J.; Huang, J.; Ye, J.Y.; Zhang, X.C.; Tu, H.Y.; Han-Zhang, H.; Wu, Y.L. Lung Adenocarcinoma Harboring Egfr T790m and In trans C797s Responds to Combination Therapy of First- and Third-Generation Egfr Tkis and Shifts Allelic Configuration at Resistance. J. Thorac. Oncol. 2017, 12, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsui, S.T.; Liu, C.; Song, Y.; Liu, D. Egfr C797s Mutation Mediates Resistance to Third-Generation Inhibitors in T790m-Positive Non-Small Cell Lung Cancer. J. Hematol. Oncol. 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Bordi, P.; Del Re, M.; Minari, R.; Rofi, E.; Buti, S.; Restante, G.; Squadrilli, A.; Crucitta, S.; Casartelli, C.; Gnetti, L.; et al. From the Beginning to Resistance: Study of Plasma Monitoring and Resistance Mechanisms in a Cohort of Patients Treated with Osimertinib for Advanced T790m-Positive Nsclc. Lung Cancer 2019, 131, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Akli, A.; Girard, N.; Fallet, V.; Rousseau-Bussac, G.; Gounant, V.; Friard, S.; Trédaniel, J.; Dujon, C.; Wislez, M.; Duchemann, B.; et al. Histomolecular Resistance Mechanisms to First-Line Osimertinib in Egfr-Mutated Advanced Non-Small Cell Lung Cancer: A Multicentric Retrospective French Study. Target. Oncol. 2022, 17, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Jiang, H.; Zhang, C.; Geng, C.; Xu, X.; Zhang, L.; Zhang, H.; Zhang, Z.; Lan, K.; Ji, Y. Mutational Profiling of Non-Small-Cell Lung Cancer Resistant to Osimertinib Using Next-Generation Sequencing in Chinese Patients. Biomed. Res. Int. 2018, 2018, 9010353. [Google Scholar] [CrossRef]
- Ou, S.I.; Cui, J.; Schrock, A.B.; Goldberg, M.E.; Zhu, V.W.; Albacker, L.; Stephens, P.J.; Miller, V.A.; Ali, S.M. Emergence of Novel and Dominant Acquired Egfr Solvent-Front Mutations at Gly796 (G796s/R) Together with C797s/R and L792f/H Mutations in One Egfr (L858r/T790m) Nsclc Patient Who Progressed on Osimertinib. Lung Cancer 2017, 108, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Rangachari, D.; To, C.; Shpilsky, J.E.; VanderLaan, P.A.; Kobayashi, S.S.; Mushajiang, M.; Lau, C.J.; Paweletz, C.P.; Oxnard, G.R.; Jänne, P.A.; et al. Egfr-Mutated Lung Cancers Resistant to Osimertinib through Egfr C797s Respond to First-Generation Reversible Egfr Inhibitors but Eventually Acquire Egfr T790m/C797s in Preclinical Models and Clinical Samples. J. Thorac. Oncol. 2019, 14, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, D.; Yoon, S.; Lee, D.H.; Kim, S.W. Exploring the Resistance Mechanisms of Second-Line Osimertinib and Their Prognostic Implications Using Next-Generation Sequencing in Patients with Non-Small-Cell Lung Cancer. Eur. J. Cancer 2021, 148, 202–210. [Google Scholar] [CrossRef]
- Suryavanshi, M.; Jaipuria, J.; Mattoo, S.; Dhandha, S.; Khatri, M. Audit of Molecular Mechanisms of Primary and Secondary Resistance to Various Generations of Tyrosine Kinase Inhibitors in Known Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Patients in a Tertiary Centre. Clin. Oncol. 2022, 34, e451–e462. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; Tang, M.; Li, H.; Zhao, C.; Zhao, M.; Zhang, Y.; Li, X.; Mi, J.; Shen, H.; et al. Effective Clinical Response of Lung Adenocarcinoma Harboring Egfr 19del/T790m/in Cis-C797s Osimertinib to Osimertinib and Gefitinib Combination Therapy. Quant. Imaging Med. Surg. 2023, 13, 5362–5368. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, Z.; Han, L.; Gong, Y.; Xie, C. Mechanisms and Management of 3rd-Generation Egfr-Tki Resistance in Advanced Non-Small Cell Lung Cancer (Review). Int. J. Oncol. 2021, 59, 90. [Google Scholar] [CrossRef]
- Park, S.; Ku, B.M.; Jung, H.A.; Sun, J.M.; Ahn, J.S.; Lee, S.H.; Park, K.; Ahn, M.J. Egfr C797s as a Resistance Mechanism of Lazertinib in Non-Small Cell Lung Cancer with Egfr T790m Mutation. Cancer Res. Treat. 2020, 52, 1288–1290. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Chen, Z.H.; Zhang, X.C.; Xu, C.R.; Yan, H.H.; Xie, Z.; Chuai, S.K.; Ye, J.Y.; Han-Zhang, H.; Zhang, Z.; et al. Analysis of Resistance Mechanisms to Abivertinib, a Third-Generation Egfr Tyrosine Kinase Inhibitor, in Patients with Egfr T790m-Positive Non-Small Cell Lung Cancer from a Phase I Trial. EBioMedicine 2019, 43, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Thress, K.S.; Brant, R.; Carr, T.H.; Dearden, S.; Jenkins, S.; Brown, H.; Hammett, T.; Cantarini, M.; Barrett, J.C. Egfr Mutation Detection in Ctdna from Nsclc Patient Plasma: A Cross-Platform Comparison of Leading Technologies to Support the Clinical Development of Azd9291. Lung Cancer 2015, 90, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Chabon, J.J.; Simmons, A.D.; Lovejoy, A.F.; Esfahani, M.S.; Newman, A.M.; Haringsma, H.J.; Kurtz, D.M.; Stehr, H.; Scherer, F.; Karlovich, C.A.; et al. Circulating Tumour DNA Profiling Reveals Heterogeneity of Egfr Inhibitor Resistance Mechanisms in Lung Cancer Patients. Nat. Commun. 2016, 7, 11815. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.N.; Becker, T.; Bray, V.; Chua, W.; Ma, Y.; Xu, B.; Lynch, D.; de Souza, P.; Roberts, T. Plasma Next Generation Sequencing and Droplet Digital Pcr-Based Detection of Epidermal Growth Factor Receptor (Egfr) Mutations in Patients with Advanced Lung Cancer Treated with Subsequent-Line Osimertinib. Thorac. Cancer 2019, 10, 1879–1884. [Google Scholar] [CrossRef]
- Vendrell, J.A.; Quantin, X.; Serre, I.; Solassol, J. Combination of Tissue and Liquid Biopsy Molecular Profiling to Detect Transformation to Small Cell Lung Carcinoma During Osimertinib Treatment. Ther. Adv. Med. Oncol. 2020, 12, 1758835920974192. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Xi, L.; Cultraro, C.M.; Wei, F.; Jones, G.; Cheng, J.; Shafiei, A.; Pham, T.H.; Roper, N.; Akoth, E.; et al. Longitudinal Circulating Tumor DNA Analysis in Blood and Saliva for Prediction of Response to Osimertinib and Disease Progression in Egfr-Mutant Lung Adenocarcinoma. Cancers 2021, 13, 3342. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wan, X.; D’Aiello, A.; Sun, H.; Gu, W.; Li, Y.; Zhou, C.; Xie, B.; Deng, Q.; Cheng, H.; et al. Temporal Genomic Heterogeneity Guiding Individualized Therapy in Recurrent Non-Small Cell Lung Cancer. Front. Oncol. 2023, 13, 1116809. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.F.; de Azevedo, J.C., Jr.; Teixeira, E.B.; Casseb, S.M.M.; Moreira, F.C.; de Assumpção, P.P.; Santos, S.E.B.D.; Calcagno, D.Q. From Haystack to High Precision: Advanced Sequencing Methods to Unraveling Circulating Tumor DNA Mutations. Front. Mol. Biosci. 2024, 11, 1423470. [Google Scholar] [CrossRef]
- Ntzifa, A.; Marras, T.; Kallergi, G.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. Comprehensive Liquid Biopsy Analysis for Monitoring Nsclc Patients under Second-Line Osimertinib Treatment. Front. Oncol. 2024, 14, 1435537. [Google Scholar] [CrossRef] [PubMed]
- Mehlman, C.; Cadranel, J.; Rousseau-Bussac, G.; Lacave, R.; Pujals, A.; Girard, N.; Callens, C.; Gounant, V.; Théou-Anton, N.; Friard, S.; et al. Resistance Mechanisms to Osimertinib in Egfr-Mutated Advanced Non-Small-Cell Lung Cancer: A Multicentric Retrospective French Study. Lung Cancer 2019, 137, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Nie, N.; Li, J.; Zhang, J.; Dai, J.; Liu, Z.; Ding, Z.; Wang, Y.; Zhu, M.; Hu, C.; Han, R.; et al. First-Line Osimertinib in Patients with Egfr-Mutated Non-Small Cell Lung Cancer: Effectiveness, Resistance Mechanisms, and Prognosis of Different Subsequent Treatments. Clin. Med. Insights Oncol. 2022, 16, 11795549221134735. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, N.; Ou, Q.; Xiang, Y.; Jiang, T.; Wu, X.; Bao, H.; Tong, X.; Wang, X.; Shao, Y.W.; et al. Investigating Novel Resistance Mechanisms to Third-Generation Egfr Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, P.; Xia, L.; Li, L.; Han, R.; Zhu, M.; Lizaso, A.; Qin, T.; Li, M.; Yu, B.; et al. The Clinical Efficacy of Combinatorial Therapy of Egfr-Tki and Crizotinib in Overcoming Met Amplification-Mediated Resistance from Prior Egfr-Tki Therapy. Lung Cancer 2020, 146, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Rotow, J.K.; Lee, J.K.; Madison, R.W.; Oxnard, G.R.; Jänne, P.A.; Schrock, A.B. Real-World Genomic Profile of Egfr Second-Site Mutations and Other Osimertinib Resistance Mechanisms and Clinical Landscape of Nsclc Post-Osimertinib. J. Thorac. Oncol. 2024, 19, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Hu, M.; Bai, Y.; Zhu, X.; Lu, X.; Wu, C.; Wang, J.; Liu, L.; Wang, Z.; Ni, J.; et al. Egfr G796d Mutation Mediates Resistance to Osimertinib. Oncotarget 2017, 8, 49671–49679. [Google Scholar] [CrossRef]
- Li, Y.; Mao, T.; Wang, J.; Zheng, H.; Hu, Z.; Cao, P.; Yang, S.; Zhu, L.; Guo, S.; Zhao, X.; et al. Toward the Next Generation Egfr Inhibitors: An Overview of Osimertinib Resistance Mediated by Egfr Mutations in Non-Small Cell Lung Cancer. Cell Commun. Signal. 2023, 21, 71. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, F.; Shen, W.; Jiang, T.; Wu, X.; Tong, X.; Shao, Y.W.; Qin, S.; Zhou, C. Novel Mutations on Egfr Leu792 Potentially Correlate to Acquired Resistance to Osimertinib In advanced Nsclc. J. Thorac. Oncol. 2017, 12, e65–e68. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, R.; Lu, J.; Xin, B.; Wang, C.; Zhu, K.; Zhang, H.; Chen, X. The Potential Role of Next-Generation Sequencing in Identifying Met Amplification and Disclosing Resistance Mechanisms in Nsclc Patients with Osimertinib Resistance. Front. Oncol. 2024, 14, 1470827. [Google Scholar] [CrossRef]
- Fukuda, S.; Suda, K.; Hamada, A.; Oiki, H.; Ohara, S.; Ito, M.; Soh, J.; Mitsudomi, T.; Tsutani, Y. Potential Utility of a 4th-Generation Egfr-Tki and Exploration of Resistance Mechanisms-an in Vitro Study. Biomedicines 2024, 12, 1412. [Google Scholar] [CrossRef]
- Song, Z.; Ren, G.; Wang, X.; Du, H.; Sun, Y.; Hu, L. Durable Clinical Benefit from Afatinib in a Lung Adenocarcinoma Patient with Acquired Egfr L718v Mutation-Mediated Resistance Towards Osimertinib: A Case Report and Literature Review. Ann. Palliat. Med. 2022, 11, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Callegari, D.; Ranaghan, K.E.; Woods, C.J.; Minari, R.; Tiseo, M.; Mor, M.; Mulholland, A.J.; Lodola, A. L718q Mutant Egfr Escapes Covalent Inhibition by Stabilizing a Non-Reactive Conformation of the Lung Cancer Drug Osimertinib. Chem. Sci. 2018, 9, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Huang, Y.; Gan, J.; Zheng, Q.; Zhang, L. Emergence of Egfr G724s after Progression on Osimertinib Responded to Afatinib Monotherapy. J. Thorac. Oncol. 2020, 15, e36–e37. [Google Scholar] [CrossRef] [PubMed]
- Minari, R.; Leonetti, A.; Gnetti, L.; Zielli, T.; Ventura, L.; Bottarelli, L.; Lagrasta, C.; La Monica, S.; Petronini, P.G.; Alfieri, R.; et al. Afatinib Therapy in Case of Egfr G724s Emergence as Resistance Mechanism to Osimertinib. Anticancer Drugs 2021, 32, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Ercan, D.; Choi, H.G.; Yun, C.H.; Capelletti, M.; Xie, T.; Eck, M.J.; Gray, N.S.; Jänne, P.A. Egfr Mutations and Resistance to Irreversible Pyrimidine-Based Egfr Inhibitors. Clin. Cancer Res. 2015, 21, 3913–3923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, B.; Zhou, D.; Li, M.; Hu, C. Newly Emergent Acquired Egfr Exon 18 G724s Mutation after Resistance of a T790m Specific Egfr Inhibitor Osimertinib in Non-Small-Cell Lung Cancer: A Case Report. Onco Targets Ther. 2019, 12, 51–56. [Google Scholar] [CrossRef]
- Brown, B.P.; Zhang, Y.K.; Westover, D.; Yan, Y.; Qiao, H.; Huang, V.; Du, Z.; Smith, J.A.; Ross, J.S.; Miller, V.A.; et al. On-Target Resistance to the Mutant-Selective Egfr Inhibitor Osimertinib Can Develop in an Allele-Specific Manner Dependent on the Original Egfr-Activating Mutation. Clin. Cancer Res. 2019, 25, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, M.; Zhou, Y.; Liu, X.; Tian, P.; Mei, F. Afatinib Overcoming Resistance to Icotinib and Osimertinib in Nsclc with Leptomeningeal Metastasis in Patients with Acquired Egfr L858r/T790m or L858r/S768i Mutations: Two Case Reports. Heliyon 2023, 9, e20690. [Google Scholar] [CrossRef]
- Schrock, A.B.; Zhu, V.W.; Hsieh, W.S.; Madison, R.; Creelan, B.; Silberberg, J.; Costin, D.; Bharne, A.; Bonta, I.; Bosemani, T.; et al. Receptor Tyrosine Kinase Fusions and Braf Kinase Fusions Are Rare but Actionable Resistance Mechanisms to Egfr Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2018, 13, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.M.; Aisner, J.; Burley, S.K.; Vallat, B.; Yu, H.A.; Pine, S.R.; Ganesan, S. A Novel Acquired Exon 20 Egfr M766q Mutation in Lung Adenocarcinoma Mediates Osimertinib Resistance but Is Sensitive to Neratinib and Poziotinib. J. Thorac. Oncol. 2019, 14, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, J.P.; Le, X.; Vijayan, R.S.K.; Hicks, J.K.; Heeke, S.; Elamin, Y.Y.; Lin, H.Y.; Udagawa, H.; Skoulidis, F.; Tran, H.; et al. Structure-Based Classification Predicts Drug Response in Egfr-Mutant Nsclc. Nature 2021, 597, 732–737. [Google Scholar] [CrossRef]
- Vyse, S.; Huang, P.H. Targeting Egfr Exon 20 Insertion Mutations in Non-Small Cell Lung Cancer. Signal Transduct. Target. Ther. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Riess, J.W.; Gandara, D.R.; Frampton, G.M.; Madison, R.; Peled, N.; Bufill, J.A.; Dy, G.K.; Ou, S.I.; Stephens, P.J.; McPherson, J.D.; et al. Diverse Egfr Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of Nsclc. J. Thorac. Oncol. 2018, 13, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Meador, C.B.; Sequist, L.V.; Piotrowska, Z. Targeting Egfr Exon 20 Insertions in Non-Small Cell Lung Cancer: Recent Advances and Clinical Updates. Cancer Discov. 2021, 11, 2145–2157. [Google Scholar] [CrossRef]
- Friedlaender, A.; Subbiah, V.; Russo, A.; Banna, G.L.; Malapelle, U.; Rolfo, C.; Addeo, A. Egfr and Her2 Exon 20 Insertions in Solid Tumours: From Biology to Treatment. Nat. Rev. Clin. Oncol. 2022, 19, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Aredo, J.V.; Starrett, J.H.; Stockhammer, P.; van Alderwerelt van Rosenburgh, I.K.; Wurtz, A.; Piper-Valillo, A.J.; Piotrowska, Z.; Falcon, C.; Yu, H.A.; et al. Efficacy of Osimertinib in Patients with Lung Cancer Positive for Uncommon Egfr Exon 19 Deletion Mutations. Clin. Cancer Res. 2023, 29, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Chan, J.M.; Kubota, D.; Sato, H.; Rizvi, H.; Daneshbod, Y.; Chang, J.C.; Paik, P.K.; Offin, M.; Arcila, M.E.; et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations as Early Resistance Mechanisms to First-Line Osimertinib in Egfr-Mutant Lung Cancer. Clin. Cancer Res. 2020, 26, 2654–2663. [Google Scholar] [CrossRef]
- Le, X.; Puri, S.; Negrao, M.V.; Nilsson, M.B.; Robichaux, J.; Boyle, T.; Hicks, J.K.; Lovinger, K.L.; Roarty, E.; Rinsurongkawong, W.; et al. Landscape of Egfr-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in Egfr-Mutant Nsclc. Clin. Cancer Res. 2018, 24, 6195–6203. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Kerr, K.; Rolfo, C.D.; Fang, J.; Finocchiaro, G.; Wong, K.-H.; Veillon, R.; Kato, T.; Yang, J.C.-H.; Nadal, E.; et al. Detection of Met Amplification (Metamp) in Patients with Egfr Mutant (M) Nsclc after First-Line (1l) Osimertinib. J. Clin. Oncol. 2023, 41, 9074. [Google Scholar] [CrossRef]
- Chmielecki, J.; Gray, J.E.; Cheng, Y.; Ohe, Y.; Imamura, F.; Cho, B.C.; Lin, M.C.; Majem, M.; Shah, R.; Rukazenkov, Y.; et al. Candidate Mechanisms of Acquired Resistance to First-Line Osimertinib in Egfr-Mutated Advanced Non-Small Cell Lung Cancer. Nat. Commun. 2023, 14, 1070. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Marra, A.; Sui, J.S.Y.; Flynn, J.; Yang, S.R.; Falcon, C.J.; Selenica, P.; Schoenfeld, A.J.; Rekhtman, N.; Gomez, D.; et al. Molecular Biomarkers of Disease Outcomes and Mechanisms of Acquired Resistance to First-Line Osimertinib in Advanced Egfr-Mutant Lung Cancers. J. Thorac. Oncol. 2023, 18, 463–475. [Google Scholar] [CrossRef]
- Cardona, A.F.; Ruiz-Patiño, A.; Recondo, G.; Martín, C.; Raez, L.; Samtani, S.; Minata, J.N.; Blaquier, J.B.; Enrico, D.; Burotto, M.; et al. Mechanisms of Resistance to First-Line Osimertinib in Hispanic Patients with Egfr Mutant Non-Small Cell Lung Cancer (Freston-Clicap). Clin. Lung Cancer 2022, 23, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Yonesaka, K.; Tanizaki, J.; Maenishi, O.; Haratani, K.; Kawakami, H.; Tanaka, K.; Hayashi, H.; Sakai, K.; Chiba, Y.; Tsuya, A.; et al. Her3 Augmentation Via Blockade of Egfr/Akt Signaling Enhances Anticancer Activity of Her3-Targeting Patritumab Deruxtecan in Egfr-Mutated Non-Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Yonesaka, K.; Takegawa, N.; Watanabe, S.; Haratani, K.; Kawakami, H.; Sakai, K.; Chiba, Y.; Maeda, N.; Kagari, T.; Hirotani, K.; et al. An Her3-Targeting Antibody-Drug Conjugate Incorporating a DNA Topoisomerase I Inhibitor U3-1402 Conquers Egfr Tyrosine Kinase Inhibitor-Resistant Nsclc. Oncogene 2019, 38, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Z.; Shi, P.; Fan, S.; He, Y.; Wang, Q.; Li, Y.; Ramalingam, S.S.; Owonikoko, T.K.; Sun, S.Y. Downregulation of Death Receptor 4 Is Tightly Associated with Positive Response of Egfr Mutant Lung Cancer to Egfr-Targeted Therapy and Improved Prognosis. Theranostics 2021, 11, 3964–3980. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, N.; Zhang, Y. Gopc-Ros1 Rearrangement as an Acquired Resistance Mechanism to Osimertinib and Responding to Crizotinib Combined Treatments in Lung Adenocarcinoma. J. Thorac. Oncol. 2018, 13, e114–e116. [Google Scholar] [CrossRef]
- Yano, S.; Wang, W.; Li, Q.; Matsumoto, K.; Sakurama, H.; Nakamura, T.; Ogino, H.; Kakiuchi, S.; Hanibuchi, M.; Nishioka, Y.; et al. Hepatocyte Growth Factor Induces Gefitinib Resistance of Lung Adenocarcinoma with Epidermal Growth Factor Receptor-Activating Mutations. Cancer Res. 2008, 68, 9479–9487. [Google Scholar] [CrossRef]
- Kim, J.; Lee, T.S.; Lee, M.H.; Cho, I.R.; Ryu, J.K.; Kim, Y.T.; Lee, S.H.; Paik, W.H. Pancreatic Cancer Treatment Targeting the Hgf/C-Met Pathway: The Mek Inhibitor Trametinib. Cancers 2024, 16, 1056. [Google Scholar] [CrossRef]
- Turke, A.B.; Zejnullahu, K.; Wu, Y.L.; Song, Y.; Dias-Santagata, D.; Lifshits, E.; Toschi, L.; Rogers, A.; Mok, T.; Sequist, L.; et al. Preexistence and Clonal Selection of Met Amplification in Egfr Mutant Nsclc. Cancer Cell 2010, 17, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Yadav, V.K.; Cheng, W.H.; Wang, C.H.; Hsieh, M.S.; Huang, T.Y.; Lin, S.F.; Yeh, C.T.; Kuo, K.T. The Mek/Erk/Mir-21 Signaling Is Critical in Osimertinib Resistance in Egfr-Mutant Non-Small Cell Lung Cancer Cells. Cancers 2021, 13, 6005. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Piotrowska, Z.; Hare, P.J.; Chen, H.; Mulvey, H.E.; Mayfield, A.; Noeen, S.; Kattermann, K.; Greenberg, M.; Williams, A.; et al. Three Subtypes of Lung Cancer Fibroblasts Define Distinct Therapeutic Paradigms. Cancer Cell 2021, 39, 1531–1547.e10. [Google Scholar] [CrossRef]
- Sun, B.; Qiu, T.; Zeng, X.; Duan, J.; Bai, H.; Xu, J.; Li, J.; Li, J.; Hao, X.; Liu, Y.; et al. Detection of Met Polysomy by Next-Generation Sequencing and Its Clinical Relevance for Met Inhibitors. Cancer Res. Commun. 2023, 3, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond Epidermal Growth Factor Receptor: Met Amplification as a General Resistance Driver to Targeted Therapy in Oncogene-Driven Non-Small-Cell Lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef] [PubMed]
- Noonan, S.A.; Berry, L.; Lu, X.; Gao, D.; Barón, A.E.; Chesnut, P.; Sheren, J.; Aisner, D.L.; Merrick, D.; Doebele, R.C.; et al. Identifying the Appropriate Fish Criteria for Defining Met Copy Number-Driven Lung Adenocarcinoma through Oncogene Overlap Analysis. J. Thorac. Oncol. 2016, 11, 1293–1304. [Google Scholar] [CrossRef]
- Sakamoto, M.; Patil, T. Met Alterations in Advanced Non-Small Cell Lung Cancer. Lung Cancer 2023, 178, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-X.; Jie, G.-L.; Li, A.-N.; Liu, S.-Y.; Sun, H.; Zheng, M.-M.; Zhou, J.-Y.; Zhang, J.-T.; Zhang, X.-C.; Zhou, Q.; et al. Met Amplification Identified by Next-Generation Sequencing and Its Clinical Relevance for Met Inhibitors. Exp. Hematol. Oncol. 2021, 10, 52. [Google Scholar] [CrossRef]
- Xiang, C.; Lv, X.; Chen, K.; Guo, L.; Zhao, R.; Teng, H.; Ye, M.; Kuang, T.; Hou, T.; Liu, C.; et al. Unraveling the Significance of Met Focal Amplification in Lung Cancer: Integrative Ngs, Fish, and Ihc Investigation. Mod. Pathol. 2024, 37, 100451. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Cheng, J.; Tang, Z.; Toruner, G.; Hu, S.; Guo, M.; Robinson, M.; Medeiros, L.J.; Tang, G. MET amplification (MET/CEP7 ratio≥ 1.8) is an independent poor prognostic marker in patients with treatment-naive non–small-cell lung cancer. Clin. Lung Cancer 2021, 22, e512–e518. [Google Scholar] [CrossRef]
- Guo, R.; Luo, J.; Chang, J.; Rekhtman, N.; Arcila, M.; Drilon, A. Met-Dependent Solid Tumours—Molecular Diagnosis and Targeted Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 569–587. [Google Scholar] [CrossRef]
- Mu, Y.; Hao, X.; Yang, K.; Ma, D.; Wang, S.; Xu, Z.; Li, J.; Xing, P. Clinical Modality of Resistance and Subsequent Management of Patients with Advanced Non-Small Cell Lung Cancer Failing Treatment with Osimertinib. Target. Oncol. 2019, 14, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Blakely, C.M.; Watkins, T.B.K.; Wu, W.; Gini, B.; Chabon, J.J.; McCoach, C.E.; McGranahan, N.; Wilson, G.A.; Birkbak, N.J.; Olivas, V.R.; et al. Evolution and Clinical Impact of Co-Occurring Genetic Alterations in Advanced-Stage Egfr-Mutant Lung Cancers. Nat. Genet. 2017, 49, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Marti, A.; Felip, E.; Matito, J.; Mereu, E.; Navarro, A.; Cedrés, S.; Pardo, N.; de Castro, A.M.; Remon, J.; Miquel, J.M.; et al. Dual Met and Erbb Inhibition Overcomes Intratumor Plasticity in Osimertinib-Resistant-Advanced Non-Small-Cell Lung Cancer (Nsclc). Ann. Oncol. 2017, 28, 2451–2457. [Google Scholar] [CrossRef]
- Suzawa, K.; Offin, M.; Schoenfeld, A.J.; Plodkowski, A.J.; Odintsov, I.; Lu, D.; Lockwood, W.W.; Arcila, M.E.; Rudin, C.M.; Drilon, A.; et al. Acquired Met Exon 14 Alteration Drives Secondary Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in Egfr-Mutated Lung Cancer. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Hsu, R. Anti-Met Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions. Antibodies 2024, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Lv, Z.; Xiong, J.; Zheng, H.; Zhang, S.; Jin, H.; Yu, L.; Li, Z.; Zhang, J.; Li, C.; et al. Met Inhibitor, Capmatinib Overcomes Osimertinib Resistance Via Suppression of Met/Akt/Snail Signaling in Non-Small Cell Lung Cancer and Decreased Generation of Cancer-Associated Fibroblasts. Aging 2021, 13, 6890–6903. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Liao, B.C.; Liao, W.Y.; Markovets, A.; Stetson, D.; Thress, K.; Yang, J.C. Exon 16-Skipping Her2 as a Novel Mechanism of Osimertinib Resistance in Egfr L858r/T790m-Positive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2020, 15, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Singh, V.; Baca, Y.; Sukari, A.; Kim, C.; Mamdani, H.; Spira, A.I.; Uprety, D.; Bepler, G.; Kim, E.S.; et al. The Effects of Her2 Alterations in Egfr Mutant Non-Small Cell Lung Cancer. Clin. Lung Cancer 2022, 23, 52–59. [Google Scholar] [CrossRef]
- Jebbink, M.; de Langen, A.J.; Monkhorst, K.; Boelens, M.C.; van den Broek, D.; van der Noort, V.; de Gooijer, C.J.; Mahn, M.; van der Wekken, A.J.; Hendriks, L.; et al. Trastuzumab-Emtansine and Osimertinib Combination Therapy to Target Her2 Bypass Track Resistance in Egfr Mutation-Positive Nsclc. JTO Clin. Res. Rep. 2023, 4, 100481. [Google Scholar] [CrossRef] [PubMed]
- Sergina, N.V.; Rausch, M.; Wang, D.; Blair, J.; Hann, B.; Shokat, K.M.; Moasser, M.M. Escape from Her-Family Tyrosine Kinase Inhibitor Therapy by the Kinase-Inactive Her3. Nature 2007, 445, 437–441. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, P.; Ohlson, C.; Xu, E.; Symonds, L.; Isabella, A.; Muller, W.J.; Lin, N.U.; Krop, I.E.; Roberts, T.M.; et al. Pik3ca(H1047r)- and Her2-Initiated Mammary Tumors Escape Pi3k Dependency by Compensatory Activation of Mek-Erk Signaling. Oncogene 2016, 35, 2961–2970. [Google Scholar] [CrossRef]
- Lee, S.; Greenlee, E.B.; Amick, J.R.; Ligon, G.F.; Lillquist, J.S.; Natoli, E.J., Jr.; Hadari, Y.; Alvarado, D.; Schlessinger, J. Inhibition of Erbb3 by a Monoclonal Antibody That Locks the Extracellular Domain in an Inactive Configuration. Proc. Natl. Acad. Sci. USA 2015, 112, 13225–13230. [Google Scholar] [CrossRef]
- Romaniello, D.; Marrocco, I.; Nataraj, N.B.; Ferrer, I.; Drago-Garcia, D.; Vaknin, I.; Oren, R.; Lindzen, M.; Ghosh, S.; Kreitman, M.; et al. Targeting Her3, a Catalytically Defective Receptor Tyrosine Kinase, Prevents Resistance of Lung Cancer to a Third-Generation Egfr Kinase Inhibitor. Cancers 2020, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, G.; Zhang, X.; Ma, W. Targeting Her3 to Overcome Egfr Tki Resistance in Nsclc. Front. Immunol. 2023, 14, 1332057. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Evans, R.; Green, R.; An, Z.; Deng, J.; Treacy, C.; Mustapha, R.; Monypenny, J.; Costoya, C.; Lawler, K.; et al. Osimertinib and Anti-Her3 Combination Therapy Engages Immune Dependent Tumor Toxicity Via Sting Activation in Trans. Cell Death Dis. 2022, 13, 274. [Google Scholar] [CrossRef]
- Bermúdez-Abreut, E.; Báez, G.B.; Pestano, M.M.; Attanasio, G.; Castillo, C.Y.G.; Fernández, D.R.H.; Alvarez-Arzola, R.; Alimonti, A.; Sánchez-Ramírez, B. Antitumor Activity of Pabs Generated by Immunization with a Novel Her3-Targeting Protein-Based Vaccine Candidate in Preclinical Models. Front. Oncol. 2024, 14, 1472607. [Google Scholar] [CrossRef]
- Larsen, M.E.; Lyu, H.; Liu, B. Her3-Targeted Therapeutic Antibodies and Antibody-Drug Conjugates in Non-Small Cell Lung Cancer Refractory to Egfr-Tyrosine Kinase Inhibitors. Chin. Med. J. Pulm. Crit. Care Med. 2023, 1, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qian, G.; Zhang, H.; Magliocca, K.R.; Nannapaneni, S.; Amin, A.R.; Rossi, M.; Patel, M.; El-Deiry, M.; Wadsworth, J.T.; et al. Her3 Targeting Sensitizes Hnscc to Cetuximab by Reducing Her3 Activity and Her2/Her3 Dimerization: Evidence from Cell Line and Patient-Derived Xenograft Models. Clin. Cancer Res. 2017, 23, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Han, A.; Polsdofer, E.; Liu, S.; Liu, B. Understanding the Biology of Her3 Receptor as a Therapeutic Target in Human Cancer. Acta Pharm. Sin. B 2018, 8, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Yang, J.C.; Hayashi, H.; Goto, Y.; Felip, E.; Reck, M.; Vigliotti, M.; Dong, Q.; Cantero, F.; Fan, P.D.; et al. Herthena-Lung01: A Phase Ii Study of Patritumab Deruxtecan (Her3-Dxd) in Previously Treated Metastatic Egfr-Mutated Nsclc. Future Oncol. 2023, 19, 1319–1329. [Google Scholar] [CrossRef]
- Yu, H.A.; Goto, Y.; Hayashi, H.; Felip, E.; Yang, J.C.-H.; Reck, M.; Yoh, K.; Lee, S.H.; Paz-Ares, L.; Besse, B.; et al. Herthena-Lung01, a Phase Ii Trial of Patritumab Deruxtecan (Her3-Dxd) in Epidermal Growth Factor Receptor-Mutated Non-Small-Cell Lung Cancer after Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J. Clin. Oncol. 2023, 41, 5363–5375. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Jänne, P.A.; Nishio, M.; Novello, S.; Reck, M.; Steuer, C.; Wu, Y.L.; Fougeray, R.; Fan, P.D.; Meng, J.; et al. Herthena-Lung02: Phase Iii Study of Patritumab Deruxtecan in Advanced Egfr-Mutated Nsclc after a Third-Generation Egfr Tki. Future Oncol. 2024, 20, 969–980. [Google Scholar] [CrossRef]
- Raoof, S.; Mulford, I.J.; Frisco-Cabanos, H.; Nangia, V.; Timonina, D.; Labrot, E.; Hafeez, N.; Bilton, S.J.; Drier, Y.; Ji, F.; et al. Targeting Fgfr Overcomes Emt-Mediated Resistance in Egfr Mutant Non-Small Cell Lung Cancer. Oncogene 2019, 38, 6399–6413. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Oeck, S.; Zhang, G.J.; Schramm, A.; Glazer, P.M. Hypoxia Induces Resistance to Egfr Inhibitors in Lung Cancer Cells Via Upregulation of Fgfr1 and the Mapk Pathway. Cancer Res. 2020, 80, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Terp, M.G.; Jacobsen, K.; Molina, M.A.; Karachaliou, N.; Beck, H.C.; Bertran-Alamillo, J.; Giménez-Capitán, A.; Cardona, A.F.; Rosell, R.; Ditzel, H.J. Combined Fgfr and Akt Pathway Inhibition Abrogates Growth of Fgfr1 Overexpressing Egfr-Tki-Resistant Nsclc Cells. NPJ Precis. Oncol. 2021, 5, 65. [Google Scholar] [CrossRef]
- Nakamura, R.; Yamada, T.; Tokuda, S.; Morimoto, K.; Katayama, Y.; Matsui, Y.; Hirai, S.; Ishida, M.; Kawachi, H.; Sawada, R.; et al. Triple Combination Therapy Comprising Osimertinib, an Axl Inhibitor, and an Fgfr Inhibitor Improves the Efficacy of Egfr-Mutated Non-Small Cell Lung Cancer. Cancer Lett. 2024, 598, 217124. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Yasuda, H.; Terai, H.; Kagiwada, H.; Hamamoto, J.; Ebisudani, T.; Kobayashi, K.; Masuzawa, K.; Ikemura, S.; Kawada, I.; et al. Igf2 Autocrine-Mediated Igf1r Activation Is a Clinically Relevant Mechanism of Osimertinib Resistance in Lung Cancer. Mol. Cancer Res. 2020, 18, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yamada, T.; Kita, K.; Taniguchi, H.; Arai, S.; Fukuda, K.; Terashima, M.; Ishimura, A.; Nishiyama, A.; Tanimoto, A.; et al. Transient Igf-1r Inhibition Combined with Osimertinib Eradicates Axl-Low Expressing Egfr Mutated Lung Cancer. Nat. Commun. 2020, 11, 4607. [Google Scholar] [CrossRef]
- Tang, Y.F.; Liu, Z.H.; Zhang, L.Y.; Shi, S.H.; Xu, S.; Ma, J.A.; Hu, C.H.; Zou, F.W. Circ_Ppapdc1a Promotes Osimertinib Resistance by Sponging the Mir-30a-3p/ Igf1r Pathway in Non-Small Cell Lung Cancer (Nsclc). Mol. Cancer 2024, 23, 91. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Z.; Xiao, P.; Li, X.; Chen, Y.; Tang, H.; Chai, Y.; Liu, Y.; Zhu, Z.; Xie, Q.; et al. Hsa_Circ_0005576 Promotes Osimertinib Resistance through the Mir-512-5p/Igf1r Axis in Lung Adenocarcinoma Cells. Cancer Sci. 2022, 113, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Yamada, T.; Wang, R.; Tanimura, K.; Adachi, Y.; Nishiyama, A.; Tanimoto, A.; Takeuchi, S.; Araujo, L.H.; Boroni, M.; et al. Axl Confers Intrinsic Resistance to Osimertinib and Advances the Emergence of Tolerant Cells. Nat. Commun. 2019, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Bach, D.H.; Fan, Y.H.; Luu, T.T.; Hong, J.Y.; Park, H.J.; Lee, S.K. Axl Degradation in Combination with Egfr-Tki Can Delay and Overcome Acquired Resistance in Human Non-Small Cell Lung Cancer Cells. Cell Death Dis. 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wei, Y.; Wei, X. Axl Receptor Tyrosine Kinase as a Promising Anti-Cancer Approach: Functions, Molecular Mechanisms and Clinical Applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef]
- Jung, S.; Kim, D.H.; Choi, Y.J.; Kim, S.Y.; Park, H.; Lee, H.; Choi, C.M.; Sung, Y.H.; Lee, J.C.; Rho, J.K. Contribution of P53 in Sensitivity to Egfr Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. Sci. Rep. 2021, 11, 19667. [Google Scholar] [CrossRef] [PubMed]
- Kariolis, M.S.; Miao, Y.R.; Diep, A.; Nash, S.E.; Olcina, M.M.; Jiang, D.; Jones, D.S.; Kapur, S.; Mathews, I.I.; Koong, A.C.; et al. Inhibition of the Gas6/Axl Pathway Augments the Efficacy of Chemotherapies. J. Clin. Investig. 2017, 127, 183–198. [Google Scholar] [CrossRef]
- Codony-Servat, J.; García-Roman, S.; Molina-Vila, M.; Bertran-Alamillo, J.; Giménez-Capitán, A.; Viteri, S.; Cardona, A.F.; D’Hondt, E.; Karachaliou, N.; Rosell, R. Anti-Epidermal Growth Factor Vaccine Antibodies Enhance the Efficacy of Tyrosine Kinase Inhibitors and Delay the Emergence of Resistance in Egfr Mutant Lung Cancer Cells. J. Thorac. Oncol. 2018, 13, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Xu, T.; Zhang, N.; Zou, X.; Kong, Z.; Wei, C.; Wang, Z. Anlotinib Combined with Osimertinib Reverses Acquired Osimertinib Resistance in Nsclc by Targeting the C-Met/Myc/Axl Axis. Pharmacol. Res. 2023, 188, 106668. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakagami, H.; Kaneko, N.; Konagai, S.; Yamamoto, H.; Matsuya, T.; Yuri, M.; Yamanaka, Y.; Mori, M.; Takeuchi, M.; et al. Mutant-Selective Irreversible Egfr Inhibitor, Naquotinib, Inhibits Tumor Growth in Nsclc Models with Egfr-Activating Mutations, T790m Mutation, and Axl Overexpression. Mol. Cancer Ther. 2019, 18, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Koopman, L.A.; Terp, M.G.; Zom, G.G.; Janmaat, M.L.; Jacobsen, K.; den Heuvel, E.G.-V.; Brandhorst, M.; Forssmann, U.; de Bree, F.; Pencheva, N.; et al. Enapotamab Vedotin, an Axl-Specific Antibody-Drug Conjugate, Shows Preclinical Antitumor Activity in Non-Small Cell Lung Cancer. JCI Insight 2019, 4, e128199. [Google Scholar] [CrossRef] [PubMed]
- Okura, N.; Nishioka, N.; Yamada, T.; Taniguchi, H.; Tanimura, K.; Katayama, Y.; Yoshimura, A.; Watanabe, S.; Kikuchi, T.; Shiotsu, S.; et al. Ono-7475, a Novel Axl Inhibitor, Suppresses the Adaptive Resistance to Initial Egfr-Tki Treatment in Egfr-Mutated Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Li, B.; Zou, B.; Wang, S.; Fan, B.; Li, W.; Yu, J.; Wang, L. Clinical Implications of Germline Bcl2l11 Deletion Polymorphism in Pretreated Advanced Nsclc Patients with Osimertinib Therapy. Lung Cancer 2021, 151, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Li, B.; Wang, Z.; Shang, S.; Shao, Y.; Sun, X.; Wang, L. Bim Deletion Polymorphism Confers Resistance to Osimertinib in Egfr T790m Lung Cancer: A Case Report and Literature Review. Target. Oncol. 2018, 13, 517–523. [Google Scholar] [CrossRef]
- Nakagawa, T.; Takeuchi, S.; Yamada, T.; Ebi, H.; Sano, T.; Nanjo, S.; Ishikawa, D.; Sato, M.; Hasegawa, Y.; Sekido, Y.; et al. Egfr-Tki Resistance Due to Bim Polymorphism Can Be Circumvented in Combination with Hdac Inhibition. Cancer Res. 2013, 73, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, A.; Takeuchi, S.; Arai, S.; Fukuda, K.; Yamada, T.; Roca, X.; Ong, S.T.; Yano, S. Histone Deacetylase 3 Inhibition Overcomes Bim Deletion Polymorphism-Mediated Osimertinib Resistance in Egfr-Mutant Lung Cancer. Clin. Cancer Res. 2017, 23, 3139–3149. [Google Scholar] [CrossRef]
- Bertino, E.M.; Gentzler, R.D.; Clifford, S.; Kolesar, J.; Muzikansky, A.; Haura, E.B.; Piotrowska, Z.; Camidge, D.R.; Stinchcombe, T.E.; Hann, C.; et al. Phase Ib Study of Osimertinib in Combination with Navitoclax in Egfr-Mutant Nsclc Following Resistance to Initial Egfr Therapy (Etctn 9903). Clin Cancer Res 2021, 27, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Luo, W.; Zhou, Y.; Zhang, Z.; Jiang, Z. Anoikis-Related Gene Cdkn2a Predicts Prognosis and Immune Response and Mediates Proliferation and Migration in Thyroid Carcinoma. Transl. Oncol. 2024, 40, 101873. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. Cdk4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, S.; Yang, H.W. Non-Canonical Pathway for Rb Inactivation and External Signaling Coordinate Cell-Cycle Entry without Cdk4/6 Activity. Nat. Commun. 2023, 14, 7847. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ng, D.S.-C.; Yam, J.C.; Chen, L.J.; Tham, C.C.; Pang, C.P.; Chu, W.K. Post-Translational Modifications on the Retinoblastoma Protein. J. Biomed. Sci. 2022, 29, 33. [Google Scholar] [CrossRef]
- Dai, X.; Liu, X.; Ge, F.; Zhu, H.; Zheng, C.; Yan, F.; Yang, B. The Effect of Plk1 Inhibitor in Osimertinib Resistant Non-Small Cell Lung Carcinoma Cells. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023, 52, 558–566. [Google Scholar] [CrossRef]
- La Monica, S.; Fumarola, C.; Cretella, D.; Bonelli, M.; Minari, R.; Cavazzoni, A.; Digiacomo, G.; Galetti, M.; Volta, F.; Mancini, M.; et al. Efficacy of the Cdk4/6 Dual Inhibitor Abemaciclib in Egfr-Mutated Nsclc Cell Lines with Different Resistance Mechanisms to Osimertinib. Cancers 2020, 13, 6. [Google Scholar] [CrossRef]
- Qin, Q.; Li, X.; Liang, X.; Zeng, L.; Wang, J.; Sun, L.; Zhong, D. Cdk4/6 Inhibitor Palbociclib Overcomes Acquired Resistance to Third-Generation Egfr Inhibitor Osimertinib in Non-Small Cell Lung Cancer (Nsclc). Thorac. Cancer 2020, 11, 2389–2397. [Google Scholar] [CrossRef]
- Ntzifa, A.; Marras, T.; Georgoulias, V.; Lianidou, E. Liquid Biopsy for the Management of Nsclc Patients under Osimertinib Treatment. Crit. Rev. Clin. Lab. Sci. 2024, 61, 347–369. [Google Scholar] [CrossRef]
- Wang, C.; Fei, K.; Liu, L.; Duan, J.; Wang, Z.; Li, S.; Xu, J.; Zhang, X.; Tian, Y.; Qu, Y.; et al. Abnormal Activation of Nf-Κb and Mapk Signaling Pathways Affect Osimertinib Resistance and Influence the Recruitment of Myeloid-Derived Suppressor Cells to Shape the Immunosuppressive Tumor Immune Microenvironment. Thorac. Cancer 2023, 14, 1843–1856. [Google Scholar] [CrossRef]
- Kato, R.; Hayashi, H.; Sakai, K.; Suzuki, S.; Haratani, K.; Takahama, T.; Tanizaki, J.; Nonagase, Y.; Tanaka, K.; Yoshida, T.; et al. Capp-Seq Analysis of Circulating Tumor DNA from Patients with Egfr T790m-Positive Lung Cancer after Osimertinib. Int. J. Clin. Oncol. 2021, 26, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guha, U.; Kim, C.; Ye, L.; Cheng, J.; Li, F.; Chia, D.; Wei, F.; Wong, D.T.W. Longitudinal Monitoring of Egfr and Pik3ca Mutations by Saliva-Based Efirm in Advanced Nsclc Patients with Local Ablative Therapy and Osimertinib Treatment: Two Case Reports. Front. Oncol. 2020, 10, 1240. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shim, J.H.; Lee, B.; Cho, I.; Park, W.Y.; Kim, Y.; Lee, S.H.; Choi, Y.; Han, J.; Ahn, J.S.; et al. Paired Genomic Analysis of Squamous Cell Carcinoma Transformed from Egfr-Mutated Lung Adenocarcinoma. Lung Cancer 2019, 134, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Khosroshahi, E.M.; Asadi, S.; Tanha, M.; Mohseni, F.G.; Sagha, R.A.; Taheri, E.; Vazayefi, P.; Shekarriz, H.; Habibi, F.; et al. Emerging Roles of Non-Coding Rnas in Modulating the Pi3k/Akt Pathway in Cancer. Noncoding RNA Res. 2025, 10, 1–15. [Google Scholar] [CrossRef]
- Dimou, A.; Nasarre, C.; Peterson, Y.K.; Pagano, R.; Gooz, M.; Nasarre, P.; Drabkin, H.A.; Armeson, K.E.; Gibney, B.C.; Gemmill, R.M.; et al. Neuropilin-2b Facilitates Resistance to Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2021, 162, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.E.; Caenepeel, S.; Wu, L.C. Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol. 2016, 37, 462–476. [Google Scholar] [CrossRef]
- Lu, T.; Zhou, L.; Chu, Z.; Song, Y.; Wang, Q.; Zhao, M.; Dai, C.; Chen, L.; Cheng, G.; Wang, J.; et al. Cordyceps Sinensis Relieves Non-Small Cell Lung Cancer by Inhibiting the Mapk Pathway. Chin. Med. 2024, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.M.; Ciaramella, V.; Cardone, C.; La Monica, S.; Alfieri, R.; Petronini, P.G.; Malapelle, U.; Vigliar, E.; Pepe, F.; Troncone, G.; et al. Antitumor Efficacy of Dual Blockade of Egfr Signaling by Osimertinib in Combination with Selumetinib or Cetuximab in Activated Egfr Human Nclc Tumor Models. J. Thorac. Oncol. 2018, 13, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, Z.; Qiu, F.; Li, X.; Xu, X. Circdonson Regulates the Proliferation, Invasion and Migration of Non-Small Cell Lung Cancer Cells through the Mapk Signaling Pathway. Genes Dis. 2025, 12, 101217. [Google Scholar] [CrossRef]
- Ohashi, K.; Sequist, L.V.; Arcila, M.E.; Moran, T.; Chmielecki, J.; Lin, Y.L.; Pan, Y.; Wang, L.; de Stanchina, E.; Shien, K.; et al. Lung Cancers with Acquired Resistance to Egfr Inhibitors Occasionally Harbor Braf Gene Mutations but Lack Mutations in Kras, Nras, or Mek1. Proc. Natl. Acad. Sci. USA 2012, 109, E2127–E2133. [Google Scholar] [CrossRef]
- Minari, R.; Bordi, P.; La Monica, S.; Squadrilli, A.; Leonetti, A.; Bottarelli, L.; Azzoni, C.; Lagrasta, C.A.M.; Gnetti, L.; Campanini, N.; et al. Concurrent Acquired Braf V600e Mutation and Met Amplification as Resistance Mechanism of First-Line Osimertinib Treatment in a Patient with Egfr-Mutated Nsclc. J. Thorac. Oncol. 2018, 13, e89–e91. [Google Scholar] [CrossRef]
- Blair, H.A. Sotorasib: First Approval. Drugs 2021, 81, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Chauhan, S.C.; Kashyap, V.K.; Roy, K.K. Structural Insights into Small-Molecule Kras Inhibitors for Targeting Kras Mutant Cancers. Eur. J. Med. Chem. 2024, 277, 116771. [Google Scholar] [CrossRef]
- Mezquita, L.; Oulhen, M.; Aberlenc, A.; Deloger, M.; Aldea, M.; Honore, A.; Lecluse, Y.; Howarth, K.; Friboulet, L.; Besse, B.; et al. Resistance to Braf Inhibition Explored through Single Circulating Tumour Cell Molecular Profiling in Braf-Mutant Non-Small-Cell Lung Cancer. Br. J. Cancer 2024, 130, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Chimbangu, C.T.; Ya, Z.; Xi, L.; Jiayue, Z.; Xiao, M.; Ying, W.; Xingxu, Y.; Liu, X. Promising Response of Dabrafenib, Trametinib, and Osimertinib Combination Therapy for Concomitant Braf and Egfr-Tki Resistance Mutations. Anticancer Drugs 2024, 35, 109–115. [Google Scholar] [CrossRef] [PubMed]
- La Monica, S.; Minari, R.; Cretella, D.; Bonelli, M.; Fumarola, C.; Cavazzoni, A.; Galetti, M.; Digiacomo, G.; Riccardi, F.; Petronini, P.G.; et al. Acquired Braf G469a Mutation as a Resistance Mechanism to First-Line Osimertinib Treatment in Nsclc Cell Lines Harboring an Egfr Exon 19 Deletion. Target. Oncol. 2019, 14, 619–626. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Encorafenib: A Review in Metastatic Colorectal Cancer with a Braf V600e Mutation. Drugs 2021, 81, 849–856. [Google Scholar] [CrossRef]
- Xie, Z.; Gu, Y.; Xie, X.; Lin, X.; Ouyang, M.; Qin, Y.; Zhang, J.; Lizaso, A.; Chen, S.; Zhou, C. Lung Adenocarcinoma Harboring Concomitant Egfr Mutations and Braf V600e Responds to a Combination of Osimertinib and Vemurafenib to Overcome Osimertinib Resistance. Clin. Lung Cancer 2021, 22, e390–e394. [Google Scholar] [CrossRef]
- Meng, P.; Koopman, B.; Kok, K.; Elst, A.T.; Schuuring, E.; van Kempen, L.C.; Timens, W.; Hiltermann, T.J.N.; Groen, H.J.M.; van den Berg, A.; et al. Combined Osimertinib, Dabrafenib and Trametinib Treatment for Advanced Non-Small-Cell Lung Cancer Patients with an Osimertinib-Induced Braf V600e Mutation. Lung Cancer 2020, 146, 358–361. [Google Scholar] [CrossRef]
- Huang, Y.; Gan, J.; Guo, K.; Deng, Y.; Fang, W. Acquired Braf V600e Mutation Mediated Resistance to Osimertinib and Responded to Osimertinib, Dabrafenib, and Trametinib Combination Therapy. J. Thorac. Oncol. 2019, 14, e236–e237. [Google Scholar] [CrossRef]
- Gu, J.; Yao, W.; Shi, P.; Zhang, G.; Owonikoko, T.K.; Ramalingam, S.S.; Sun, S.Y. Mek or Erk Inhibition Effectively Abrogates Emergence of Acquired Osimertinib Resistance in the Treatment of Epidermal Growth Factor Receptor-Mutant Lung Cancers. Cancer 2020, 126, 3788–3799. [Google Scholar] [CrossRef] [PubMed]
- Tsamis, I.; Gomatou, G.; Chachali, S.P.; Trontzas, I.P.; Patriarcheas, V.; Panagiotou, E.; Kotteas, E. Braf/Mek Inhibition in Nsclc: Mechanisms of Resistance and How to Overcome It. Clin. Transl. Oncol. 2023, 25, 10–20. [Google Scholar] [CrossRef]
- Shi, P.; Oh, Y.T.; Zhang, G.; Yao, W.; Yue, P.; Li, Y.; Kanteti, R.; Riehm, J.; Salgia, R.; Owonikoko, T.K.; et al. Met Gene Amplification and Protein Hyperactivation Is a Mechanism of Resistance to Both First and Third Generation Egfr Inhibitors in Lung Cancer Treatment. Cancer Lett. 2016, 380, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Oh, Y.T.; Deng, L.; Zhang, G.; Qian, G.; Zhang, S.; Ren, H.; Wu, G.; Legendre, B., Jr.; Anderson, E.; et al. Overcoming Acquired Resistance to Azd9291, a Third-Generation Egfr Inhibitor, through Modulation of Mek/Erk-Dependent Bim and Mcl-1 Degradation. Clin. Cancer Res. 2017, 23, 6567–6579. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.J.H.; Lazaratos, A.M.; Maritan, S.M.; Quaiattini, A.; Zeng, Z.; Zhu, Z.; Sener, U.; Malani, R.; Kim, Y.J.; Ichihara, E.; et al. Osimertinib Is Associated with Improved Outcomes in Pre-Treated Non-Small Cell Lung Cancer Leptomeningeal Metastases: A Systematic Review and Meta-Analysiss. Heliyon 2024, 10, e29668. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Otani, S.; Takeuchi, S.; Arai, S.; Nanjo, S.; Tanimoto, A.; Nishiyama, A.; Naoki, K.; Yano, S. Trametinib Overcomes Kras-G12v-Induced Osimertinib Resistance in a Leptomeningeal Carcinomatosis Model of Egfr-Mutant Lung Cancer. Cancer Sci. 2021, 112, 3784–3795. [Google Scholar] [CrossRef]
- Chen, W.; Yu, D.; Sun, S.Y.; Li, F. Nanoparticles for Co-Delivery of Osimertinib and Selumetinib to Overcome Osimertinib-Acquired Resistance in Non-Small Cell Lung Cancer. Acta Biomater. 2021, 129, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, J.; Wu, A.; Zhang, M.; Zhao, Y.; Tang, Y.; Wang, B.; Chen, T.; Li, F.; Zhao, Q.; et al. Biomimetic Codelivery Overcomes Osimertinib-Resistant Nsclc and Brain Metastasis Via Macrophage-Mediated Innate Immunity. J. Control Release 2021, 329, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.M.; Malapelle, U.; Vigliar, E.; Pepe, F.; Troncone, G.; Ciaramella, V.; Troiani, T.; Martinelli, E.; Belli, V.; Ciardiello, F.; et al. Efficacy of Continuous Egfr-Inhibition and Role of Hedgehog in Egfr Acquired Resistance in Human Lung Cancer Cells with Activating Mutation of Egfr. Oncotarget 2017, 8, 23020–23032. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Tang, Y.; Zang, H.; Luo, J.; Zhou, H.; Zhan, Y.; Zou, Y.; Wen, Q.; Ma, J.; Fan, S. Itraconazole Reversing Acquired Resistance to Osimertinib in Nsclc by Inhibiting the Shh/Dusp13b/P-Stat3 Axis. Adv. Sci. 2024, e2409416. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Yang, J.C.; Yu, H.; Kim, S.W.; Saka, H.; Horn, L.; Goto, K.; Ohe, Y.; Mann, H.; Thress, K.S.; et al. Tatton: A Multi-Arm, Phase Ib Trial of Osimertinib Combined with Selumetinib, Savolitinib, or Durvalumab in Egfr-Mutant Lung Cancer. Ann. Oncol. 2020, 31, 507–516. [Google Scholar] [CrossRef]
- Lee, P.H.; Huang, Y.H.; Lin, H.; Hsu, K.H.; Chen, K.C.; Tseng, J.S.; Chang, G.C.; Yang, T.Y. Histological Transformation after Acquired Resistance to the Third-Generation Egfr-Tki in Patients with Advanced Egfr-Mutant Lung Adenocarcinoma. Medicina 2022, 58, 908. [Google Scholar] [CrossRef]
- Chakraborty, S.; Coleman, C.; Manoj, P.; Demircioglu, D.; Shah, N.; de Stanchina, E.; Rudin, C.M.; Hasson, D.; Sen, T. De Novo and Histologically Transformed Small-Cell Lung Cancer Is Sensitive to Lurbinectedin Treatment through the Modulation of Emt and Notch Signaling Pathways. Clin. Cancer Res. 2023, 29, 3526–3540. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, J.; Li, L.; Pan, Z.; Wang, L. Reuse of Osimertinib after Small Cell Lung Cancer Transformation in Lung Adenocarcinoma with De-Novo Epidermal Growth Factor Receptor T790m Mutation: Case Report. Anticancer Drugs 2023, 34, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Sato, Y.; Sato, Y.; Hirabayashi, R.; Hara, S.; Takahashi, Y.; Tomii, K. Long-Term Efficacy of Immune Checkpoint Inhibitor for Squamous Cell Carcinoma Lesion Transformed from Egfr-Mutated Adenocarcinoma after Osimertinib Treatment: A Case Report. JTO Clin. Res. Rep. 2024, 5, 100639. [Google Scholar] [CrossRef]
- Stegelmeier, P.; Dawson, J.A.; Wallace, M.; Esebua, M. Osimertinib Resistance Via Histologic Transformation from Non-Small Cell Lung Carcinoma to Carcinosarcoma. Cureus 2024, 16, e59293. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Kitazono, M.; Takamori, M.; Kiriu, T. Combined Small and Squamous Transformation in Egfr-Mutated Lung Adenocarcinoma. Intern. Med. 2020, 59, 1291–1294. [Google Scholar] [CrossRef]
- Lee, P.H.; Chang, G.C. Transformations First into Squamous-Cell Carcinoma and Later into Sarcomatoid Carcinoma after Acquired Resistance to Osimertinib in a Patient with Egfr-Mutant Lung Adenocarcinoma: Case Report. Clin. Lung Cancer 2021, 22, e536–e541. [Google Scholar] [CrossRef]
- Jin, C.B.; Yang, L. Histological Transformation of Non-Small Cell Lung Cancer: Clinical Analysis of Nine Cases. World J. Clin. Cases 2021, 9, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Quintanal-Villalonga, A.; Taniguchi, H.; Zhan, Y.A.; Hasan, M.M.; Chavan, S.S.; Meng, F.; Uddin, F.; Allaj, V.; Manoj, P.; Shah, N.S.; et al. Comprehensive Molecular Characterization of Lung Tumors Implicates Akt and Myc Signaling in Adenocarcinoma to Squamous Cell Transdifferentiation. J. Hematol. Oncol. 2021, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, J.; Kim, S.; Kim, S.; Youk, J.; Park, S.; An, Y.; Keam, B.; Kim, D.W.; Heo, D.S.; et al. Clonal History and Genetic Predictors of Transformation into Small-Cell Carcinomas from Lung Adenocarcinomas. J. Clin. Oncol. 2017, 35, 3065–3074. [Google Scholar] [CrossRef]
- Offin, M.; Chan, J.M.; Tenet, M.; Rizvi, H.A.; Shen, R.; Riely, G.J.; Rekhtman, N.; Daneshbod, Y.; Quintanal-Villalonga, A.; Penson, A.; et al. Concurrent Rb1 and Tp53 Alterations Define A subset of Egfr-Mutant Lung Cancers at Risk For histologic Transformation and Inferior Clinical Outcomes. J. Thorac. Oncol. 2019, 14, 1784–1793. [Google Scholar] [CrossRef]
- Cao, P.; Li, Y.; Shi, R.; Yuan, Y.; Gong, H.; Zhu, G.; Zhang, Z.; Chen, C.; Zhang, H.; Liu, M.; et al. Combining Egfr-Tki with Saha Overcomes Egfr-Tki-Acquired Resistance by Reducing the Protective Autophagy in Non-Small Cell Lung Cancer. Front. Chem. 2022, 10, 837987. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Li, X.; Liang, X.; Zeng, L.; Wang, J.; Sun, L.; Zhong, D. Targeting the Emt Transcription Factor Snail Overcomes Resistance to Osimertinib in Egfr-Mutant Non-Small Cell Lung Cancer. Thorac. Cancer 2021, 12, 1708–1715. [Google Scholar] [CrossRef]
- Dong, B.; Li, S.; Zhu, S.; Yi, M.; Luo, S.; Wu, K. MiRNA-Mediated Emt and Cscs in Cancer Chemoresistance. Exp. Hematol. Oncol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Castosa, R.; Martinez-Iglesias, O.; Roca-Lema, D.; Casas-Pais, A.; Díaz-Díaz, A.; Iglesias, P.; Santamarina, I.; Graña, B.; Calvo, L.; Valladares-Ayerbes, M.; et al. Hakai Overexpression Effectively Induces Tumour Progression and Metastasis in Vivo. Sci. Rep. 2018, 8, 3466. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Chen, L.Y.; Lin, Y.C.; Shih, J.Y.; Lin, Y.C.; Tseng, R.Y.; Chiu, A.C.; Yeh, Y.H.; Liu, C.; Lin, Y.T.; et al. Epithelial-Mesenchymal Transition (Emt) Beyond Egfr Mutations Per Se Is a Common Mechanism for Acquired Resistance to Egfr Tki. Oncogene 2019, 38, 455–468. [Google Scholar] [CrossRef] [PubMed]
- De Craene, B.; Berx, G. Regulatory Networks Defining Emt During Cancer Initiation and Progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Baek, K.E.; Saika, S.; Jeong, M.J.; Yoo, J. Snail Is Required for Transforming Growth Factor-Beta-Induced Epithelial-Mesenchymal Transition by Activating Pi3 Kinase/Akt Signal Pathway. Biochem. Biophys. Res. Commun. 2007, 353, 337–343. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. Tgf-Beta-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Hisakane, K.; Seike, M.; Sugano, T.; Yoshikawa, A.; Matsuda, K.; Takano, N.; Takahashi, S.; Noro, R.; Gemma, A. Exosome-Derived Mir-210 Involved in Resistance to Osimertinib and Epithelial-Mesenchymal Transition in Egfr Mutant Non-Small Cell Lung Cancer Cells. Thorac. Cancer 2021, 12, 1690–1698. [Google Scholar] [CrossRef]
- Han, R.; Guo, H.; Shi, J.; Wang, H.; Zhao, S.; Jia, Y.; Liu, X.; Li, J.; Cheng, L.; Zhao, C.; et al. Tumour Microenvironment Changes after Osimertinib Treatment Resistance in Non-Small Cell Lung Cancer. Eur. J. Cancer 2023, 189, 112919. [Google Scholar] [CrossRef]
- Yochum, Z.A.; Cades, J.; Wang, H.; Chatterjee, S.; Simons, B.W.; O’Brien, J.P.; Khetarpal, S.K.; Lemtiri-Chlieh, G.; Myers, K.V.; Huang, E.H.; et al. Targeting the Emt Transcription Factor Twist1 Overcomes Resistance to Egfr Inhibitors in Egfr-Mutant Non-Small-Cell Lung Cancer. Oncogene 2019, 38, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, X.; Wu, L.; Chen, S.; Fang, N.; Cai, L.; Jia, J. Id1 Mediates Resistance to Osimertinib in Egfr T790m-Positive Non-Small Cell Lung Cancer through Epithelial-Mesenchymal Transition. BMC Pulm. Med. 2021, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.M.; Xu, Y.L.; Yuan, L.W.; Zhang, L.L.; Huang, M.Y.; Ye, Z.H.; Su, M.X.; Chen, X.P.; Zhu, H.; Ye, R.D.; et al. Tgfβ2-Mediated Epithelial-Mesenchymal Transition and Nf-Κb Pathway Activation Contribute to Osimertinib Resistance. Acta Pharmacol. Sin. 2021, 42, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, Y.; Yang, G.; Tian, L. The Interplay between Tgf-Β/Smad and Bmp/Smad Signaling Pathways in the Epithelial Mesenchymal Transition of A549 Cells Induced by Silica. Toxicol. Mech. Methods 2018, 28, 286–292. [Google Scholar] [CrossRef] [PubMed]
- La Monica, S.; Minari, R.; Cretella, D.; Flammini, L.; Fumarola, C.; Bonelli, M.; Cavazzoni, A.; Digiacomo, G.; Galetti, M.; Madeddu, D.; et al. Third Generation Egfr Inhibitor Osimertinib Combined with Pemetrexed or Cisplatin Exerts Long-Lasting Anti-Tumor Effect in Egfr-Mutated Pre-Clinical Models of Nsclc. J. Exp. Clin. Cancer Res. 2019, 38, 222. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.H.; Lu, Y.J.; Li, Y.Q.; Huang, Y.H.; Hsieh, C.H.; Wu, C.P. Osimertinib (Azd9291) Attenuates the Function of Multidrug Resistance-Linked Atp-Binding Cassette Transporter Abcb1 in Vitro. Mol. Pharm. 2016, 13, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, W.; Wang, Z.; Li, X.; Xiong, Y.; Lu, H.; Wang, X.; Yin, H.; Cao, X.; Xin, H. Enhanced Anti-Brain Metastasis from Non-Small Cell Lung Cancer of Osimertinib and Doxorubicin Co-Delivery Targeted Nanocarrier. Int. J. Nanomed. 2020, 15, 5491–5501. [Google Scholar] [CrossRef]
- Tanaka, K.; Asahina, H.; Kishimoto, J.; Miyata, Y.; Uchida, T.; Watanabe, K.; Hamai, K.; Harada, T.; Tsubata, Y.; Sugawara, S.; et al. Osimertinib Versus Osimertinib Plus Chemotherapy for Non-Small Cell Lung Cancer with Egfr (T790m)-Associated Resistance to Initial Egfr Inhibitor Treatment: An Open-Label, Randomised Phase 2 Clinical Trial. Eur. J. Cancer 2021, 149, 14–22. [Google Scholar] [CrossRef]
- Sequist, L.V.; Peled, N.; Tufman, A.; Servidio, L.; Li, J.; Taylor, R.; Zhao, J. P47.11 Compel: Chemotherapy with/without Osimertinib in Patients with Egfrm Advanced Nsclc and Progression on First-Line Osimertinib. J. Thorac. Oncol. 2021, 16, S1101. [Google Scholar] [CrossRef]
- Okuma, Y.; Nomura, S.; Ninomiya, K.; Yamaguchi, H.; Murakami, S.; Kogure, Y.; Harada, D.; Okishio, K.; Okamoto, H.; Goto, Y. 1186tip Epona, Efficacy of Osimertinib with Platinum and Pemetrexed in Egfr Mutant Non-Small Cell Lung Cancer Patients Bearing Cns Metastasis, and Have Systemic Progression but Stable Intracranial Disease on Osimertinib Resistance (Torg 1938). Ann. Oncol. 2022, 33, S1090–S1091. [Google Scholar] [CrossRef]
- Saito, R.; Sugawara, S.; Ko, R.; Azuma, K.; Morita, R.; Maemondo, M.; Oizumi, S.; Takahashi, K.; Kagamu, H.; Tsubata, Y.; et al. Phase 2 Study of Osimertinib in Combination with Platinum and Pemetrexed in Patients with Previously Untreated Egfr-Mutated Advanced Non-Squamous Non-Small Cell Lung Cancer: The Opal Study. Eur. J. Cancer 2023, 185, 83–93. [Google Scholar] [CrossRef]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in Egfr-Mutated Advanced Nsclc. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Kanda, S.; Niho, S.; Kurata, T.; Nomura, S.; Kawashima, Y.; Yoneshima, Y.; Yokoyama, T.; Watanabe, Y.; Tanaka, H.; Fujiwara, Y.; et al. A Phase Iii Study Comparing Egfr Tyrosine Kinase Inhibitor (Egfr-Tki) Monotherapy and Egfr-Tki with Inserted Cisplatin (Cddp) Plus Pemetrexed (Pem) as a First-Line Treatment in Patients (Pts) with Advanced Non-Squamous Non–Small-Cell Lung Cancer (Nsqnsclc) Harboring Egfr Activating Mutation (Egfr-Nsqnsclc): Jcog1404/Wjog8214l, Again Study. J. Clin. Oncol. 2023, 41, LBA9009. [Google Scholar]
- Tabernero, J. The Role of Vegf and Egfr Inhibition: Implications for Combining Anti-Vegf and Anti-Egfr Agents. Mol. Cancer Res. 2007, 5, 203–220. [Google Scholar] [CrossRef]
- Seto, T.; Kato, T.; Nishio, M.; Goto, K.; Atagi, S.; Hosomi, Y.; Yamamoto, N.; Hida, T.; Maemondo, M.; Nakagawa, K.; et al. Erlotinib Alone or with Bevacizumab as First-Line Therapy in Patients with Advanced Non-Squamous Non-Small-Cell Lung Cancer Harbouring Egfr Mutations (Jo25567): An Open-Label, Randomised, Multicentre, Phase 2 Study. Lancet Oncol. 2014, 15, 1236–1244. [Google Scholar] [CrossRef]
- Saito, H.; Fukuhara, T.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, M.; Yoshimori, K.; et al. Erlotinib Plus Bevacizumab Versus Erlotinib Alone in Patients with Egfr-Positive Advanced Non-Squamous Non-Small-Cell Lung Cancer (Nej026): Interim Analysis of an Open-Label, Randomised, Multicentre, Phase 3 Trial. Lancet Oncol. 2019, 20, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, C.R.; Cheng, Y.; Liu, Y.P.; Chen, G.Y.; Cui, J.W.; Yang, N.; Song, Y.; Li, X.L.; Lu, S.; et al. Bevacizumab Plus Erlotinib in Chinese Patients with Untreated, Egfr-Mutated, Advanced Nsclc (Artemis-Ctong1509): A Multicenter Phase 3 Study. Cancer Cell 2021, 39, 1279–1291.e3. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhang, C.; Li, D.; Xu, L.; Yang, X. Efficacy and Safety of Osimertinib Plus Bevacizumab Versus Osimertinib Alone for Advanced Non-Small-Cell Lung Cancer with Egfr Mutations: A Meta-Analysis of Randomized Controlled Trials. Medicine 2024, 103, e40320. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Schoenfeld, A.J.; Makhnin, A.; Kim, R.; Rizvi, H.; Tsui, D.; Falcon, C.; Houck-Loomis, B.; Meng, F.; Yang, J.L.; et al. Effect of Osimertinib and Bevacizumab on Progression-Free Survival for Patients with Metastatic Egfr-Mutant Lung Cancers: A Phase 1/2 Single-Group Open-Label Trial. JAMA Oncol. 2020, 6, 1048–1054. [Google Scholar] [CrossRef]
- Kenmotsu, H.; Wakuda, K.; Mori, K.; Kato, T.; Sugawara, S.; Kirita, K.; Yoneshima, Y.; Azuma, K.; Nishino, K.; Teraoka, S.; et al. Randomized Phase 2 Study of Osimertinib Plus Bevacizumab Versus Osimertinib for Untreated Patients with Nonsquamous Nsclc Harboring Egfr Mutations: Wjog9717l Study. J. Thorac. Oncol. 2022, 17, 1098–1108. [Google Scholar] [CrossRef]
- Soo, R.A.; Han, J.Y.; Dafni, U.; Cho, B.C.; Yeo, C.M.; Nadal, E.; Carcereny, E.; de Castro, J.; Sala, M.A.; Bernabé, R.; et al. A Randomised Phase Ii Study of Osimertinib and Bevacizumab Versus Osimertinib Alone as Second-Line Targeted Treatment in Advanced Nsclc with Confirmed Egfr and Acquired T790m Mutations: The European Thoracic Oncology Platform (Etop 10-16) Booster Trial. Ann. Oncol. 2022, 33, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, J.; Cang, S.D.; Lin, J.X.; Tu, H.Y.; Du, Y.; Qin, J.W.; Liang, X.H.; Yu, Y.; Lan, H.T.; et al. Flair: A Phase Ii, Open Label, Randomized Study of Osimertinib Plus Bevacizumab Versus Osimertinib in Recurrent or Metastatic Treatment-Naïve Nsclc Patients Harboring Egfr 21l858r Mutation. Clin. Lung Cancer 2024, in press. [Google Scholar] [CrossRef]
- Nishio, M.; Paz-Ares, L.; Reck, M.; Nakagawa, K.; Garon, E.B.; Popat, S.; Ceccarelli, M.; Graham, H.T.; Visseren-Grul, C.; Novello, S. Relay, Ramucirumab Plus Erlotinib (Ram+Erl) in Untreated Metastatic Egfr-Mutant Nsclc (Egfr+ Nsclc): Association between Tp53 Status and Clinical Outcome. Clin. Lung Cancer 2023, 24, 415–428. [Google Scholar] [CrossRef]
- Le, X.; Patel, J.D.; Shum, E.; Baik, C.; Sanborn, R.E.; Shu, C.A.; Kim, C.; Fidler, M.J.; Hall, R.; Elamin, Y.Y.; et al. A Multicenter Open-Label Randomized Phase Ii Study of Osimertinib with and without Ramucirumab in Tyrosine Kinase Inhibitor-Naïve Egfr-Mutant Metastatic Non-Small Cell Lung Cancer (Ramose Trial). J. Clin. Oncol. 2024, Jco2400533. [Google Scholar] [CrossRef] [PubMed]
- Daum, S.; Hagen, H.; Naismith, E.; Wolf, D.; Pircher, A. The Role of Anti-Angiogenesis in the Treatment Landscape of Non-Small Cell Lung Cancer—New Combinational Approaches and Strategies of Neovessel Inhibition. Front. Cell Dev. Biol. 2020, 8, 610903. [Google Scholar] [CrossRef]
- Han, R.; Guo, H.; Shi, J.; Zhao, S.; Jia, Y.; Liu, X.; Liu, Y.; Cheng, L.; Zhao, C.; Li, X.; et al. Osimertinib in Combination with Anti-Angiogenesis Therapy Presents a Promising Option for Osimertinib-Resistant Non-Small Cell Lung Cancer. BMC Med. 2024, 22, 174. [Google Scholar]
- Jin, X.; Pan, Y.; Cheng, C.; Shen, H.; Zhai, C.; Yin, K.; Zhu, X.; Pan, H.; You, L. Optimizing First-Line Tki Treatment Efficacy in Pd-L1-Positive Egfr-Mutated Nsclc: The Impact of Antiangiogenic Agents. Front. Pharmacol. 2024, 15, 1391972. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Imai, H.; Mouri, A.; Hashimoto, K.; Miura, Y.; Shiono, A.; Yamaguchi, O.; Kobayashi, K.; Kawasaki, T.; Yasuda, M.; et al. Clinicopathological Impact of Vegfr2 and Vegf-C in Patients with Egfr-Major Mutant Nsclc Receiving Osimertinib. Thorac. Cancer 2023, 14, 2950–2961. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wu, D.; Liu, J.; Yang, Y. Efficacy of Adjuvant First-Generation Tkis Versus Chemotherapy in Patients with Completely Resected Egfr-Mutant Non-Small Cell Lung Cancer: A Meta-Analysis. Cancer Investig. 2024, 42, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xia, B.; Xie, R.; Xu, X.; Zhang, M.; Wu, K.; Wang, B.; Ma, S. Timing in Combination with Radiotherapy and Patterns of Disease Progression in Non-Small Cell Lung Cancer Treated with Egfr-Tki. Lung Cancer 2020, 140, 65–70. [Google Scholar] [CrossRef]
- Sanchis-Borja, M.; Parrot, A.; Sroussi, D.; Del Campo, E.R.; Fallet, V.; Cadranel, J. Dramatic Radiation Recall Pneumonitis Induced by Osimertinib after Palliative Thoracic Radiotherapy for Lung Cancer. J. Thorac. Oncol. 2019, 14, e224–e226. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Guo, H.; Jing, W.; Jing, X.; Li, J.; Wang, M.; Yu, J.; Zhu, H. An Especially High Rate of Radiation Pneumonitis Observed in Patients Treated with Thoracic Radiotherapy and Simultaneous Osimertinib. Radiother. Oncol. 2020, 152, 96–100. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Meng, X.; Wang, J.; Zhu, L.; Liu, C.; Li, S.; Zheng, L.; Yang, Z.; Xing, L.; et al. Osimertinib (Azd9291) Increases Radio-Sensitivity in Egfr T790m Non-Small Cell Lung Cancer. Oncol. Rep. 2019, 41, 77–86. [Google Scholar] [CrossRef]
- Liu, B.; Liu, H.; Ma, Y.; Ding, Q.; Zhang, M.; Liu, X.; Liu, M. Egfr-Mutated Stage Iv Non-Small Cell Lung Cancer: What Is the Role of Radiotherapy Combined with Tki? Cancer Med. 2021, 10, 6167–6188. [Google Scholar] [CrossRef]
- Saw, S.P.L.; Low, Y.F.; Lai, G.G.Y.; Chan, L.L.; Wong, W.K.Y.; Tsui, G.; Chen, O.H.; Seet, A.O.L.; Tan, W.C.; Tan, A.C.; et al. Real-World Outcomes of Pemetrexed-Platinum Chemotherapy Plus Osimertinib after Progression on First-Line Osimertinib in Advanced Egfr-Mutated Nsclc. Lung Cancer 2024, 193, 107856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, L.; Liang, F.; Chu, L.; Chu, X.; Yang, X.; Zhang, J.; Guo, T.; Jiang, S.; Pang, Y.; et al. Safety and Efficacy of Consolidative Stereotactic Radiotherapy for Oligo-Residual Egfr-Mutant Non-Small Cell Lung Cancer after First-Line Third-Generation Egfr-Tyrosine Kinase Inhibitors: A Single-Arm, Phase 2 Trial. eClinicalMedicine 2024, 76, 102853. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.J. 20 Phase Ii Randomized Study of Osimertinib (Osi) with or without Local Consolidative Therapy (Lct) for Metastatic Egfr Mutant Non-Small Cell Lung Cancer (Nsclc): Analysis of Adverse Events (Aes). J. Thorac. Oncol. 2023, 18, S36. [Google Scholar] [CrossRef]
- Peng, P.; Gong, J.; Zhang, Y.; Zhou, S.; Li, Y.; Han, G.; Meng, R.; Chen, Y.; Yang, M.; Shen, Q.; et al. Egfr-Tkis Plus Stereotactic Body Radiation Therapy (Sbrt) for Stage Iv Non-Small Cell Lung Cancer (Nsclc): A Prospective, Multicenter, Randomized, Controlled Phase Ii Study. Radiother. Oncol. 2023, 184, 109681. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, S.; Sampath, S.; Iyengar, P.; Dowell, J.; Ahn, C.; Westover, K.D.; Zhang, Y.; Cole, S.; Massarelli, E.; Amini, A.; et al. Safety and Efficacy of Osimertinib Plus Consolidative Stereotactic Ablative Radiation (Sabr) in Advanced Egfr Mutant Non-Small Cell Lung Cancer (Nsclc): Results from a Multi-Center Phase Ii Trial. J. Clin. Oncol. 2024, 42, 8518. [Google Scholar] [CrossRef]
- Deng, L.; Kiedrowski, L.A.; Ravera, E.; Cheng, H.; Halmos, B. Response to Dual Crizotinib and Osimertinib Treatment in a Lung Cancer Patient with Met Amplification Detected by Liquid Biopsy Who Acquired Secondary Resistance to Egfr Tyrosine Kinase Inhibition. J. Thorac. Oncol. 2018, 13, e169–e172. [Google Scholar] [CrossRef] [PubMed]
- Wilgucki, M.; Yeung, V.; Ho, G.; Montenegro, G.L.B.; Jones, G.; Reuss, J.E.; Liu, S.V.; Kim, C. Osimertinib and Capmatinib Combination Therapy to Overcome Met Y1003n-Mediated Resistance in Egfr-Mutant Nsclc: A Case Report. JTO Clin. Res. Rep. 2022, 3, 100396. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Sun, K.D.; Gu, J.; Chen, Z.; Owonikoko, T.K.; Ramalingam, S.S.; Sun, S.Y. The Novel Met Inhibitor, Hqp8361, Possesses Single Agent Activity and Enhances Therapeutic Efficacy of Azd9291 (Osimertinib) against Azd9291-Resistant Nsclc Cells with Activated Met. Am. J. Cancer Res. 2020, 10, 3316–3327. [Google Scholar] [PubMed]
- Chen, Z.; Vallega, K.A.; Chen, H.; Zhou, J.; Ramalingam, S.S.; Sun, S.Y. The Natural Product Berberine Synergizes with Osimertinib Preferentially against Met-Amplified Osimertinib-Resistant Lung Cancer Via Direct Met Inhibition. Pharmacol. Res. 2022, 175, 105998. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, L.; Peng, J.; Ward, R.; Hao, P.; Wang, J.; Zhang, N.; Yang, Y.; Guo, X.; Xiang, C.; et al. Dictamnine, a Novel C-Met Inhibitor, Suppresses the Proliferation of Lung Cancer Cells by Downregulating the Pi3k/Akt/Mtor and Mapk Signaling Pathways. Biochem. Pharmacol. 2022, 195, 114864. [Google Scholar] [CrossRef]
- Yun, J.; Lee, S.H.; Kim, S.Y.; Jeong, S.Y.; Kim, J.H.; Pyo, K.H.; Park, C.W.; Heo, S.G.; Yun, M.R.; Lim, S.; et al. Antitumor Activity of Amivantamab (Jnj-61186372), an Egfr-Met Bispecific Antibody, in Diverse Models of Egfr Exon 20 Insertion-Driven Nsclc. Cancer Discov. 2020, 10, 1194–1209. [Google Scholar] [CrossRef]
- Wang, K.; Du, R.; Myall, N.J.; Lewis, W.E.; Uy, N.; Hong, L.; Skoulidis, F.; Byers, L.A.; Tsao, A.; Cascone, T.; et al. Real-World Efficacy and Safety of Amivantamab for Egfr-Mutant Nsclc. J. Thorac. Oncol. 2024, 19, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.Y.; Kim, S.W.; Lee, C.K.; et al. Amivantamab in Egfr Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results from the Chrysalis Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Balmanoukian, A.S.; Madison, R.; Zhang, S.S.; Klempner, S.J.; Ou, S.I. Amivantamab (Jnj-61186372) Induces Clinical, Biochemical, Molecular, and Radiographic Response in a Treatment-Refractory Nsclc Patient Harboring Amplified Triple Egfr Mutations (L858r/ T790m/G796s) in Cis. Lung Cancer 2022, 164, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Felip, E.; Hayashi, H.; Thomas, M.; Lu, S.; Besse, B.; Sun, T.; Martinez, M.; Sethi, S.N.; Shreeve, S.M.; et al. Mariposa: Phase 3 Study of First-Line Amivantamab + lazertinib Versus Osimertinib in Egfr-Mutant Non-Small-Cell Lung Cancer. Future Oncol. 2022, 18, 639–647. [Google Scholar] [CrossRef]
- Cho, B.C.; Kim, D.W.; Spira, A.I.; Gomez, J.E.; Haura, E.B.; Kim, S.W.; Sanborn, R.E.; Cho, E.K.; Lee, K.H.; Minchom, A.; et al. Amivantamab Plus Lazertinib in Osimertinib-Relapsed Egfr-Mutant Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial. Nat. Med. 2023, 29, 2577–2585. [Google Scholar] [CrossRef]
- Felip, E.; Cho, B.C.; Gutiérrez, V.; Alip, A.; Besse, B.; Lu, S.; Spira, A.I.; Girard, N.; Califano, R.; Gadgeel, S.M.; et al. Amivantamab Plus Lazertinib Versus Osimertinib in First-Line Egfr-Mutant Advanced Non-Small-Cell Lung Cancer with Biomarkers of High-Risk Disease: A secondary Analysis from Mariposa. Ann. Oncol. 2024, 35, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Emdal, K.B.; Dittmann, A.; Reddy, R.J.; Lescarbeau, R.S.; Moores, S.L.; Laquerre, S.; White, F.M. Characterization of in Vivo Resistance to Osimertinib and Jnj-61186372, an Egfr/Met Bispecific Antibody, Reveals Unique and Consensus Mechanisms of Resistance. Mol. Cancer Ther. 2017, 16, 2572–2585. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.H.; Melosky, B.; Shih, J.Y.; Wang, J.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; et al. Amivantamab Plus Chemotherapy with and without Lazertinib in Egfr-Mutant Advanced Nsclc after Disease Progression on Osimertinib: Primary Results from the Phase Iii Mariposa-2 Study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Gao, M.; Xu, Y.; Fu, L.; Li, Y.; Bao, X.; Fu, H.; Quan, H.; Lou, L. Shr-A1403, a Novel C-Mesenchymal-Epithelial Transition Factor (C-Met) Antibody-Drug Conjugate, Overcomes Azd9291 Resistance in Non-Small Cell Lung Cancer Cells Overexpressing C-Met. Cancer Sci. 2019, 110, 3584–3594. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Han, J.Y.; Ahn, M.J.; Cho, B.C.; Yu, H.; Kim, S.W.; Yang, J.C.; Lee, J.S.; Su, W.C.; Kowalski, D.; et al. Osimertinib Plus Savolitinib in Patients with Egfr Mutation-Positive, Met-Amplified, Non-Small-Cell Lung Cancer after Progression on Egfr Tyrosine Kinase Inhibitors: Interim Results from a Multicentre, Open-Label, Phase 1b Study. Lancet Oncol. 2020, 21, 373–386. [Google Scholar] [CrossRef]
- Yoh, K.; Hirashima, T.; Saka, H.; Kurata, T.; Ohe, Y.; Hida, T.; Mellemgaard, A.; Verheijen, R.B.; Ou, X.; Ahmed, G.F.; et al. Savolitinib ± Osimertinib in Japanese Patients with Advanced Solid Malignancies or Egfrm Nsclc: Ph1b Tatton Part C. Target. Oncol. 2021, 16, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.J.; De Marinis, F.; Bonanno, L.; Cho, B.C.; Kim, T.M.; Cheng, S.; Novello, S.; Proto, C.; Kim, S.W.; Lee, J.S.; et al. Ep08.02-140 Met Biomarker-Based Preliminary Efficacy Analysis in Savannah: Savolitinib+Osimertinib in Egfrm Nsclc Post-Osimertinib. J. Thorac. Oncol. 2022, 17, S469–S470. [Google Scholar] [CrossRef]
- Wu, Y.L.; Guarneri, V.; Voon, P.J.; Lim, B.K.; Yang, J.J.; Wislez, M.; Huang, C.; Liam, C.K.; Mazieres, J.; Tho, L.M.; et al. Tepotinib Plus Osimertinib in Patients with Egfr-Mutated Non-Small-Cell Lung Cancer with Met Amplification Following Progression on First-Line Osimertinib (Insight 2): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2024, 25, 989–1002. [Google Scholar] [CrossRef]
- Cheema, P.K.; Banerji, S.O.; Blais, N.; Chu, Q.S.; Desmeules, P.; Juergens, R.A.; Leighl, N.B.; Sheffield, B.S.; Wheatley-Price, P.F.; Melosky, B.L. Canadian Consensus Recommendations on the Management of Met-Altered Nsclc. Curr. Oncol. 2021, 28, 4552–4576. [Google Scholar] [CrossRef]
- Leighl, N.B.; Akamatsu, H.; Lim, S.M.; Cheng, Y.; Minchom, A.R.; Marmarelis, M.E.; Sanborn, R.E.; Yang, J.C.-H.; Liu, B.; John, T.; et al. Subcutaneous Versus Intravenous Amivantamab, Both in Combination with Lazertinib, in Refractory Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer: Primary Results from the Phase Iii Paloma-3 Study. J. Clin. Oncol. 2024, 42, 3593–3605. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Lu, S.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.S.; Lee, S.H.; Ostapenko, Y.; Danchaivijitr, P.; Liu, B.; et al. Amivantamab Plus Lazertinib in Previously Untreated Egfr-Mutated Advanced Nsclc. N. Engl. J. Med. 2024, 391, 1486–1498. [Google Scholar] [CrossRef]
- Li, X.; Lian, Z.; Wang, S.; Xing, L.; Yu, J. Interactions between Egfr and Pd-1/Pd-L1 Pathway: Implications for Treatment of Nsclc. Cancer Lett. 2018, 418, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Tanimoto, T.; Yuji, K.; Tojo, A. Egfr-Tki-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients with Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Guo, X.; Li, T.; Hu, W.; Zhang, L.; Wu, C.; Ye, F. Efficacy of Immune Checkpoint Inhibitors in Egfr-Mutant Nsclc Patients with Egfr-Tki Resistance: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 926890. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, X.; Zhao, K.; Meng, X. Case Report: Long Progression-Free Survival of Immunotherapy for Lung Adenocarcinoma with Epidermal Growth Factor Receptor Mutation. Front. Oncol. 2021, 11, 731429. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Y.; Wang, W.; Cai, Q.; Ge, F.; Chen, Z.; Zheng, J.; Zhang, Y.; Deng, H.; Chen, Y.; et al. Efficacy and Safety of Immune Checkpoint Inhibitors for Individuals with Advanced Egfr-Mutated Non-Small-Cell Lung Cancer Who Progressed on Egfr Tyrosine-Kinase Inhibitors: A Systematic Review, Meta-Analysis, and Network Meta-Analysis. Lancet Oncol. 2024, 25, 1347–1356. [Google Scholar] [CrossRef]
- Thress, K.S.; Jacobs, V.; Angell, H.K.; Yang, J.C.; Sequist, L.V.; Blackhall, F.; Su, W.C.; Schuler, M.; Wolf, J.; Gold, K.A.; et al. Modulation of Biomarker Expression by Osimertinib: Results of the Paired Tumor Biopsy Cohorts of the Aura Phase I Trial. J. Thorac. Oncol. 2017, 12, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wu, Y.; Shen, F.; MuLaTiAize, Y.; Xinhua, N. Osimertinib Improves the Immune Microenvironment of Lung Cancer by Downregulating Pd-L1 Expression of Vascular Endothelial Cells and Enhances the Antitumor Effect of Bevacizumab. J. Oncol. 2022, 2022, 1531353. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Shepherd, F.A.; Kim, D.W.; Lee, G.W.; Lee, J.S.; Chang, G.C.; Lee, S.S.; Wei, Y.F.; Lee, Y.G.; Laus, G.; et al. Osimertinib Plus Durvalumab Versus Osimertinib Monotherapy in Egfr T790m-Positive Nsclc Following Previous Egfr Tki Therapy: Caural Brief Report. J. Thorac. Oncol. 2019, 14, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Bronte, G.; Delmonte, A.; Burgio, M.A.; Andrikou, K.; Monti, M.; Menna, C.; Frassineti, G.L.; Crinò, L. Case Report: Stevens-Johnson Syndrome and Hepatotoxicity Induced by Osimertinib Sequential to Pembrolizumab in a Patient with Egfr-Mutated Lung Adenocarcinoma. Front. Pharmacol. 2021, 12, 672233. [Google Scholar] [CrossRef]
- Lopez, M.; Hagopian, G.; Doan, L.; Lee, B.J.; Rojek, N.W.; Smith, J.; Ou, S.I.; Demirdag, Y.Y.; Nagasaka, M. Osimertinib Tolerance in a Patient with Stevens Johnson Syndrome During Osimertinib Therapy after Treatment with Pembrolizumab. Allergy Asthma Clin. Immunol. 2023, 19, 93. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, L.; Zhou, F.M.; Liu, S.W.; Wang, W.; Zhao, E.J.; Yao, Q.J.; Li, W.; Zhao, Y.Q.; Shi, Z.; et al. Elevated Nox4 Promotes Tumorigenesis and Acquired Egfr-Tkis Resistance Via Enhancing Il-8/Pd-L1 Signaling in Nsclc. Drug Resist. Updat. 2023, 70, 100987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Meng, Y.; Shen, M.; Yang, F.; Ren, X. Osimertinib Exacerbates Immune Checkpoint Inhibitor-Related Severe Adverse Events by Activating the Il-6/Jak/Stat3 Pathway in Macrophages. Cancer Biol. Med. 2024, 21, 20240269. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Arbour, K.C.; Rizvi, H.; Iqbal, A.N.; Gadgeel, S.M.; Girshman, J.; Kris, M.G.; Riely, G.J.; Yu, H.A.; Hellmann, M.D. Severe Immune-Related Adverse Events Are Common with Sequential Pd-(L)1 Blockade and Osimertinib. Ann. Oncol. 2019, 30, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.; Liu, L.; Peng, Q.; Chen, J.; Zhu, Y.D. The Relationship between Blood-Based Tumor Mutation Burden Level and Efficacy of Pd-1/Pd-L1 Inhibitors in Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2021, 21, 1220. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non-Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I Keynote-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Hsu, K.H.; Tseng, J.S.; Yang, T.Y.; Chen, K.C.; Su, K.Y.; Yu, S.L.; Chen, J.J.W.; Huang, Y.H.; Chang, G.C. Pd-L1 Strong Expressions Affect the Clinical Outcomes of Osimertinib in Treatment Naïve Advanced Egfr-Mutant Non-Small Cell Lung Cancer Patients. Sci. Rep. 2022, 12, 9753. [Google Scholar] [CrossRef] [PubMed]
- Papazyan, T.; Denis, M.G.; Sagan, C.; Raimbourg, J.; Herbreteau, G.; Pons-Tostivint, E. Impact of Pd-L1 Expression on the Overall Survival of Caucasian Patients with Advanced Egfr-Mutant Nsclc Treated with Frontline Osimertinib. Target. Oncol. 2024, 19, 611–621. [Google Scholar] [CrossRef]
- Jiang, X.M.; Xu, Y.L.; Huang, M.Y.; Zhang, L.L.; Su, M.X.; Chen, X.; Lu, J.J. Osimertinib (Azd9291) Decreases Programmed Death Ligand-1 in Egfr-Mutated Non-Small Cell Lung Cancer Cells. Acta Pharmacol. Sin. 2017, 38, 1512–1520. [Google Scholar] [CrossRef]
- Shiozawa, T.; Numata, T.; Tamura, T.; Endo, T.; Kaburagi, T.; Yamamoto, Y.; Yamada, H.; Kikuchi, N.; Saito, K.; Inagaki, M.; et al. Prognostic Implication of Pd-L1 Expression on Osimertinib Treatment for Egfr-Mutated Non-Small Cell Lung Cancer. Anticancer Res. 2022, 42, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Kulendran, L.; Chia, P.L.; Samol, J.; Chang, A.Y. Combination of Osimertinib and Pembrolizumab Successfully Overcome Dual Resistances in a Patient with Advanced Adenocarcinoma of the Lung, a Case Report. Curr. Probl. Cancer Case Rep. 2022, 5, 100132. [Google Scholar] [CrossRef]
- Song, Z.; Ren, G.; Hu, L.; Wang, X.; Song, J.; Jia, Y.; Zhao, G.; Zang, A.; Du, H.; Sun, Y.; et al. Two Case Reports of Non-Small Cell Lung Cancer Patients Harboring Acquired Egfr T790m-Cis-C797s Benefit from Immune Checkpoint Inhibitor Combined with Platinum-Based Doublet Chemotherapy. Ann. Transl. Med. 2022, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Champiat, S.; Ferrara, R.; Massard, C.; Besse, B.; Marabelle, A.; Soria, J.C.; Ferté, C. Hyperprogressive Disease: Recognizing a Novel Pattern to Improve Patient Management. Nat. Rev. Clin. Oncol. 2018, 15, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Kim, K.H.; Pyo, K.H.; Xin, C.F.; Hong, M.H.; Ahn, B.C.; Kim, Y.; Choi, S.J.; Yoon, H.I.; Lee, J.G.; et al. Hyperprogressive Disease During Pd-1/Pd-L1 Blockade in Patients with Non-Small-Cell Lung Cancer. Ann. Oncol. 2019, 30, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, L.; Qin, H.; Fu, W.; Wang, Y.; Wu, M.; Wang, J.; Han, Y. Synergistic Anti-Tumor Efficacy Achieved by Reversing Drug Resistance through the Regulation of the Tumor Immune Microenvironment with Il-12 and Osimertinib Combination Therapy. J. Cancer 2024, 15, 4534–4550. [Google Scholar] [CrossRef]
- Baglivo, S.; Mandarano, M.; Bellezza, G.; Minotti, V.; Bonaiti, A.; Fischer, M.J.; Birocchi, I.; Roila, F.; Metelli, N.; Ludovini, V.; et al. Inflamed Tumor Phenotype as Predictor of Long-Term Response to Pembrolizumab in an Egfr-Mutated Non-Small Cell Lung Cancer (Nsclc) Patient with Acquired Resistance to Afatinib: A Case Report and Review of the Literature. Oncol. Ther. 2022, 10, 291–300. [Google Scholar] [CrossRef]
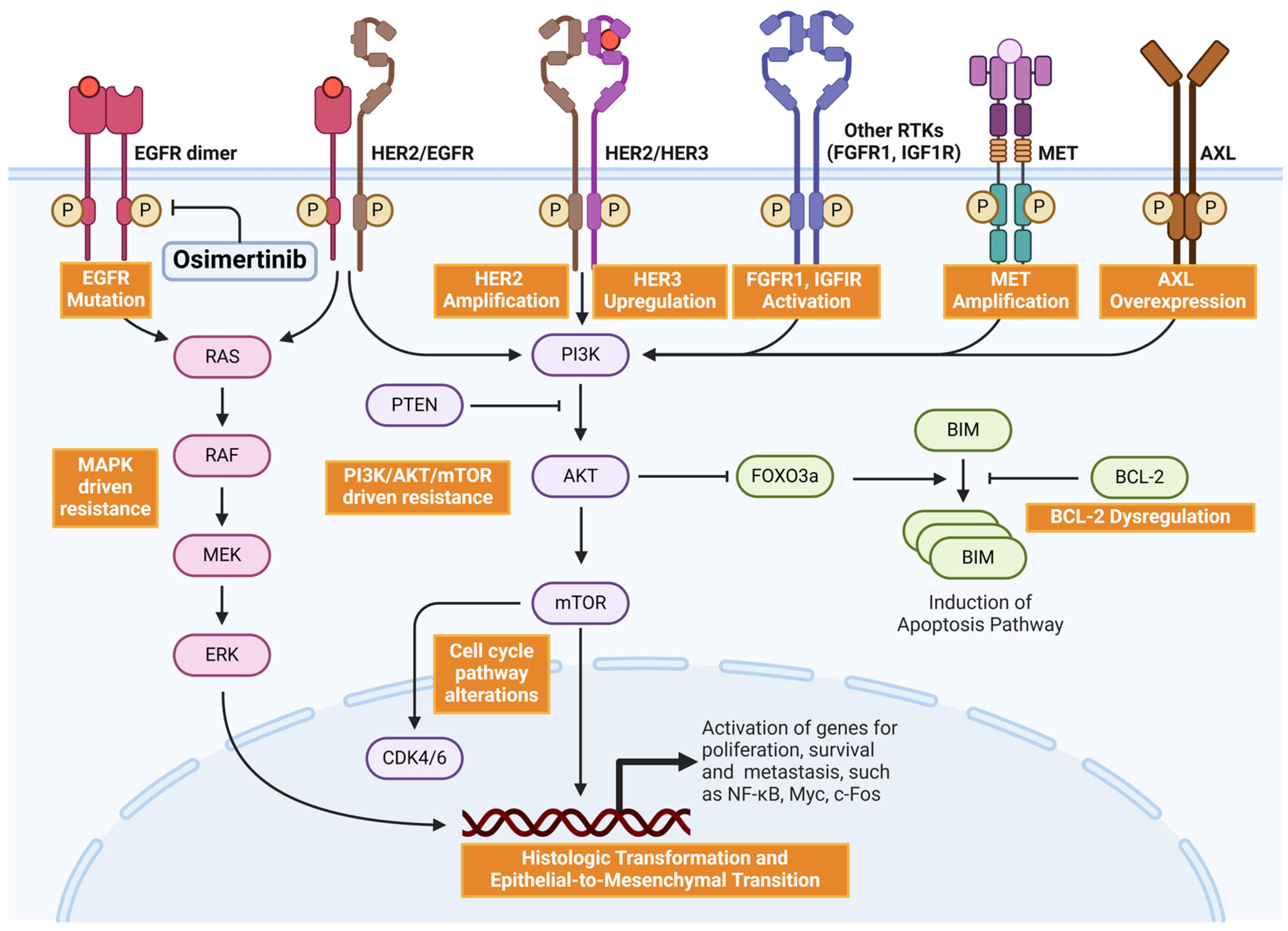
| Combination | Phase | Status | Clinical Trials Number | |
|---|---|---|---|---|
| Radiotherapy | Stereotactic body radiation therapy (SBRT) | II | Active, not recruiting | NCT03667820 |
| SBRT | N/A | Not yet recruiting | NCT05583409 | |
| SBRT | N/A | Recruiting | NCT05033691 | |
| SBRT | N/A | Not yet recruiting | NCT05998993 | |
| Chemotherapy | carboplatin and pemetrexed | III | Recruiting | NCT04695925 |
| carboplatin and pemetrexed | II | Recruiting | NCT04410796 | |
| VEGF inhibitor | Bevacizumab | III | Recruiting | NCT04181060 |
| Bevacizumab | II | Not yet recruiting | NCT04988607 | |
| Ramucirumab | III | Active, not recruiting | NCT02411448 | |
| MET inhibitor | Tepotinib | II | Active, not recruiting | NCT03940703 |
| Capmatinib, Nazartinib, and Gefitinib | II | Recruiting | NCT03040973 | |
| Savolitinib | III | Recruiting | NCT05261399 | |
| Savolitinib | III | Recruiting | NCT05015608 | |
| Savolitinib | II | Active, not recruiting | NCT03778229 | |
| Savolitinib | II | Active, not recruiting | NCT05163249 | |
| Savolitinib | II | Active, not recruiting | NCT04606771 | |
| Others | Abemaciclib (CDK4/6 inhibitor) | II | Active, not recruiting | NCT04545710 |
| Itacitinib (JAK1 inhibitor) | I/II | Active, not recruiting | NCT02917993 | |
| Selumetinib (MEK inhibitor) | II | Active, not recruiting | NCT03392246 | |
| Sapanisertib (mTOR inhibitor) | I | Active, not recruiting | NCT02503722 | |
| Multi-drugs | AZD6094, Selumetinib | I | Active, not recruiting | NCT02143466 |
| AZD4547, Vistusertib, Palbociclib, Crizotinib, Selumetinib, Docetaxel, AZD5363, Durvalumab, Sitravatinib, AZD6738 | II | Active, not recruiting | NCT02664935 | |
| Savolitinib, Gefitinib, Necitumumab, Durvalumab, Carboplatin, Pemetrexed, Alectinib, Selpercatinib, Selumetinib, Etoposide, Cisplatin, Datopotamab deruxtecan | II | Active, not recruiting | NCT03944772 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.-Y.; Tsai, C.-L.; Huang, H.-P. Optimizing Osimertinib for NSCLC: Targeting Resistance and Exploring Combination Therapeutics. Cancers 2025, 17, 459. https://doi.org/10.3390/cancers17030459
Liao Y-Y, Tsai C-L, Huang H-P. Optimizing Osimertinib for NSCLC: Targeting Resistance and Exploring Combination Therapeutics. Cancers. 2025; 17(3):459. https://doi.org/10.3390/cancers17030459
Chicago/Turabian StyleLiao, Yan-You, Chia-Luen Tsai, and Hsiang-Po Huang. 2025. "Optimizing Osimertinib for NSCLC: Targeting Resistance and Exploring Combination Therapeutics" Cancers 17, no. 3: 459. https://doi.org/10.3390/cancers17030459
APA StyleLiao, Y.-Y., Tsai, C.-L., & Huang, H.-P. (2025). Optimizing Osimertinib for NSCLC: Targeting Resistance and Exploring Combination Therapeutics. Cancers, 17(3), 459. https://doi.org/10.3390/cancers17030459





