The Challenge of Managing Neuropathic Pain in Children and Adolescents with Cancer
Simple Summary
Abstract
1. Introduction
2. Causes of NP in Children and Adolescents with Cancer
2.1. Cancer-Related NP in Children and Adolescents
2.2. Chemotherapy-Induced NP in Children and Adolescents
2.3. Radiotherapy-Induced NP in Children and Adolescents
2.4. Chronic Post-Surgical Pain in Children and Adolescents
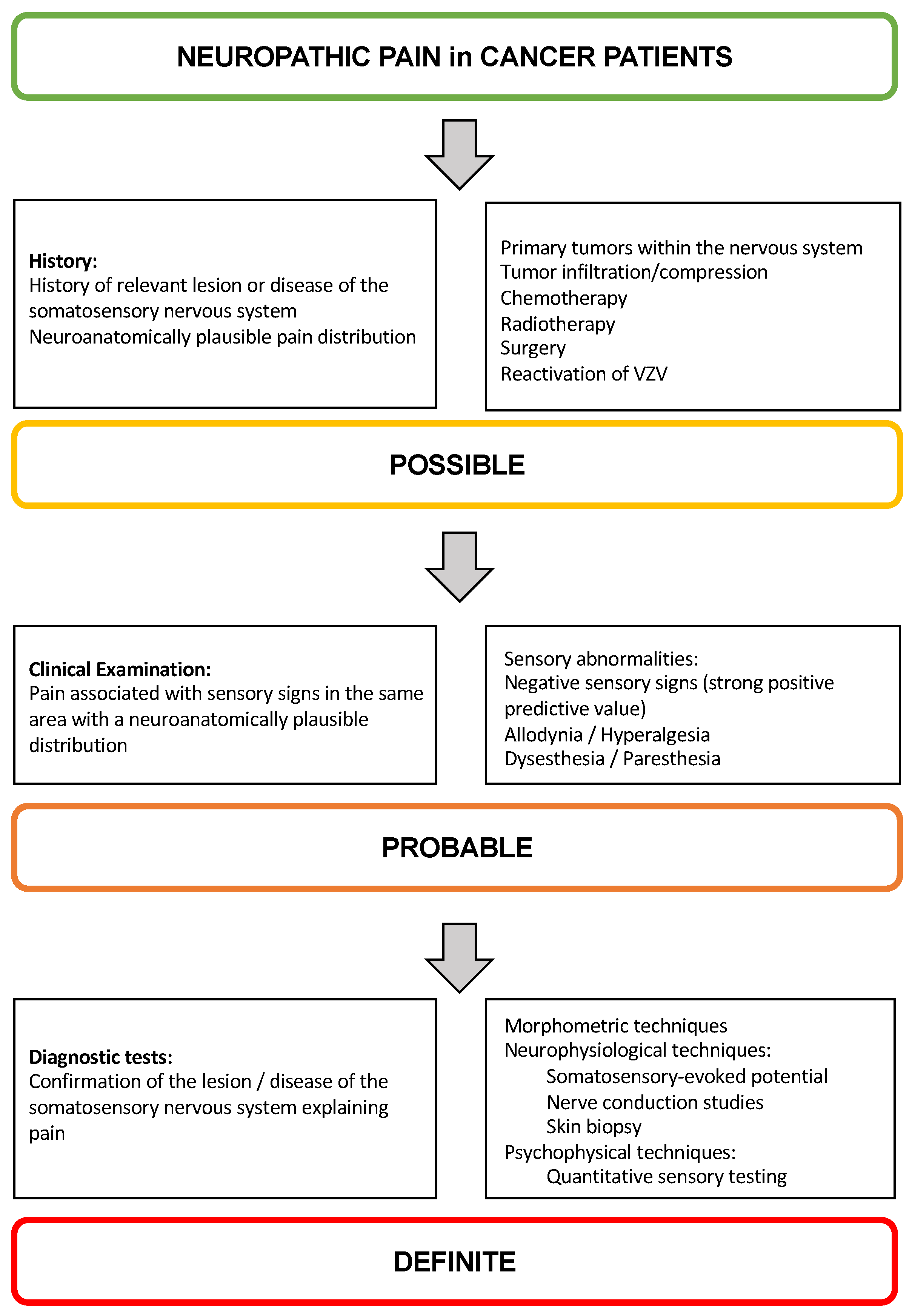
3. Diagnostic Approach to NP in Children and Adolescents
4. Managing NP in Children and Adolescents with Cancer
5. Managing Anxiety and Emotions in Children and Adolescents with Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huguet, A.; Miró, J. The severity of chronic pediatric pain: An epidemiological study. J. Pain 2008, 9, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.F.; Wiener, S.; Walker, S.M. Neuropathic pain in children. Arch. Dis. Child. 2014, 99, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Walco, G.A.; Dworkin, R.H.; Krane, E.J.; LeBel, A.A.; Treede, R.D. Neuropathic pain in children: Special considerations. Mayo Clin. Proc. 2010, 85 (Suppl. 3), S33–S41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, S.M. Neuropathic pain in children: Steps towards improved recognition and management. EBioMedicine 2020, 62, 103124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller-Schwefe, G.; Ahlbeck, K.; Aldington, D.; Alon, E.; Coaccioli, S.; Coluzzi, F.; Huygen, F.; Jaksch, W.; Kalso, E.; Kocot-Kępska, M.; et al. Pain in the cancer patient: Different pain characteristics CHANGE pharmacological treatment requirements. Curr. Med. Res. Opin. 2014, 30, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, C.; Albanese, A.A.; Contreras, N.E.; Gobetto, M.N.; Castellanos, L.C.S.; Uchitel, O.D. Ion channels and pain in Fabry disease. Mol. Pain 2021, 17, 17448069211033172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, Z.; Chen, Z.; Tang, B.; Jiang, H. Primary erythromelalgia: A review. Orphanet J. Rare Dis. 2015, 10, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langille, M.M. Guillain-Barre Syndrome in Children and Adolescents. Adv. Pediatr. 2023, 70, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; Squintani, G.; Taioli, F.; Tagliapietra, M.; Cavallaro, T.; Fabrizi, G.M. Neuropathic pain in Charcot-Marie-Tooth disease: A clinical and laser-evoked potential study. Eur. J. Pain 2022, 26, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- NCI National Cancer Institute 2024. Available online: https://www.cancer.gov/types/childhood-cancers/ccss (accessed on 23 July 2024).
- Pro, S.; Vinti, L.; Boni, A.; Mastronuzzi, A.; Scilipoti, M.; Velardi, M.; Caroleo, A.M.; Farina, E.; Badolato, F.; Alessi, I.; et al. Peripheral Nervous System Involvement in Non-Primary Pediatric Cancer: From Neurotoxicity to Possible Etiologies. J. Clin. Med. 2021, 10, 3016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Snaman, J.M.; Baker, J.N.; Ehrentraut, J.H.; Anghelescu, D.L. Pediatric Oncology: Managing Pain at the End of Life. Paediatr. Drugs 2016, 18, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Su, Y.; Zhao, W.; Duan, C.; Li, Y.; Zhou, Y.; Wang, L.; Cai, S.; Zhou, X.; Ni, X.; et al. Pain management of newly diagnosed sarcoma patients at a single center. Medicine 2022, 101, e31422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, D.D.; Luu, H.H. Osteosarcoma. Cancer Treat. Res. 2014, 162, 65–92. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, E.; Woodgate, R.L.; Sawatzky, J.A. Pain in children with central nervous system cancer: A review of the literature. Oncol. Nurs. Forum 2010, 37, E318–E330. [Google Scholar] [CrossRef] [PubMed]
- Tay, N.; Laakso, E.L.; Schweitzer, D.; Endersby, R.; Vetter, I.; Starobova, H. Chemotherapy-induced peripheral neuropathy in children and adolescent cancer patients. Front. Mol. Biosci. 2022, 9, 1015746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coluzzi, F.; Rocco, M.; Green Gladden, R.; Persiani, P.; Thur, L.A.; Milano, F. Pain Management in Childhood Leukemia: Diagnosis and Available Analgesic Treatments. Cancers 2020, 12, 3671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomez-Deza, J.; Slavutsky, A.L.; Nebiyou, M.; Le Pichon, C.E. Local production of reactive oxygen species drives vincristine-induced axon degeneration. Cell Death Dis. 2023, 14, 807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Postma, T.J.; Heimans, J.J. Grading of chemotherapy-induced peripheral neuropathy. Ann. Oncol. 2000, 11, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Rosca, L.; Robert-Boire, V.; Delisle, J.F.; Samson, Y.; Perreault, S. Carboplatin and vincristine neurotoxicity in the treatment of pediatric low-grade gliomas. Pediatr. Blood Cancer 2018, 65, e27351. [Google Scholar] [CrossRef] [PubMed]
- Śliwa-Tytko, P.; Kaczmarska, A.; Lejman, M.; Zawitkowska, J. Neurotoxicity Associated with Treatment of Acute Lymphoblastic Leukemia Chemotherapy and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 5515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loss, J.F.; Santos, P.P.; Leone, L.D.; Brunetto, A.L. Outcome of pediatric recurrent and refractory malignant solid tumors following ifosfamide/carboplatin/etoposide (ICE): A phase II study in a pediatric oncology centre in Brazil. Pediatr. Blood Cancer 2004, 42, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Domingo, I.K.; Latif, A.; Bhavsar, A.P. Pro-Inflammatory Signalling PRRopels Cisplatin-Induced Toxicity. Int. J. Mol. Sci. 2022, 23, 7227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, J.; Gao, X.; Wang, H. An Update on Clinical Trials and Potential Therapeutic Strategies in T-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2023, 24, 7201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, J.; Tanner, L.; Gilchrist, L.; Bostrom, B. Switching to Bortezomib may Improve Recovery From Severe Vincristine Neuropathy in Pediatric Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Rivetti, S.; Triarico, S.; Romano, A.; Attinà, G.; Maurizi, P.; Ruggiero, A. Mechanisms, Characteristics, and Treatment of Neuropathic Pain and Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients. Int. J. Mol. Sci. 2021, 22, 12648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorkin, L.S.; Yu, A.L.; Junger, H.; Doom, C.M. Antibody directed against GD(2) produces mechanical allodynia, but not thermal hyperalgesia when administered systemically or intrathecally despite its dependence on capsaicin sensitive afferents. Brain Res. 2002, 930, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Tecchio, C.; Sorio, M.; Bertolasi, L.; Turatti, M.; Tozzi, M.C.; Benedetti, F.; Cavaletti, G.; Monaco, S.; Ferrari, S. Clinical and neurophysiological serial assessments of brentuximab vedotin-associated peripheral neuropathy. Leuk. Lymphoma 2019, 60, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.; Widder, J.; Pötter, R. Long-term side effects of radiotherapy in survivors of childhood cancer. Front. Radiat. Ther. Oncol. 2002, 37, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Hua, C.H.; Olch, A.; Yorke, E.D.; Rancati, T.; Milano, M.T.; Constine, L.S.; Marks, L.B.; Bentzen, S.M. Reporting Standards for Complication Studies of Radiation Therapy for Pediatric Cancer: Lessons From PENTEC. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Chua, G.W.Y.; Vig, P.S. Overview of radiotherapy-induced chronic pain in childhood cancer survivors: A narrative review. Paediatr. Neonatal Pain 2023, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delanian, S.; Lefaix, J.L.; Pradat, P.F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Anghelescu, D.L.; Steen, B.D.; Wu, H.; Wu, J.; Daw, N.C.; Rao, B.N.; Neel, M.D.; Navid, F. Prospective study of neuropathic pain after definitive surgery for extremity osteosarcoma in a pediatric population. Pediatr. Blood Cancer 2017, 64, e26162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burgoyne, L.L.; Billups, C.A.; Jirón JLJr Kaddoum, R.N.; Wright, B.B.; Bikhazi, G.B.; Parish, M.E.; Pereiras, L.A. Phantom limb pain in young cancer-related amputees: Recent experience at St Jude children’s research hospital. Clin. J. Pain 2012, 28, 222–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeMoss, P.; Ramsey, L.H.; Karlson, C.W. Phantom Limb Pain in Pediatric Oncology. Front. Neurol. 2018, 9, 219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, J.; Thompson, J.M. Phantom limb pain and chemotherapy in pediatric amputees. Mayo Clin. Proc. 1995, 70, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Manworren, R.C.B.; Stinson, J. Pediatric Pain Measurement, Assessment, and Evaluation. Semin. Pediatr. Neurol. 2016, 23, 189–200. [Google Scholar] [CrossRef]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting Symptoms of Cancer and Stage at Diagnosis: Evidence from a Cross-Sectional, Population-Based Study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef]
- Crellin, D.J.; Harrison, D.; Santamaria, N.; Babl, F.E. Systematic Review of the Face, Legs, Activity, Cry and Consolability Scale for Assessing Pain in Infants and Children: Is It Reliable, Valid, and Feasible for Use? Pain 2015, 156, 2132–2151. [Google Scholar] [CrossRef]
- Marchetti, G.; Vittori, A.; Cascella, M.; Mascilini, I.; Piga, S.; Petrucci, E.; Castellano, A.; Caruso, R.; Francia, E.; Stocchi, F.; et al. Pain Prevalence and Pain Management in Children and Adolescents in an Italian Third Level Pediatric Hospital: A Cross-Sectional Study. Ital. J. Pediatr. 2023, 49, 41. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Bimonte, S.; Saettini, F.; Muzio, M.R. The Challenge of Pain Assessment in Children with Cognitive Disabilities: Features and Clinical Applicability of Different Observational Tools. J. Paediatr. Child. Health 2019, 55, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bennett, M.I.; Rayment, C.; Hjermstad, M.; Aass, N. Prevalence and aetiology of neuropathic pain in cancer patients: A systematic review. Pain 2012, 153, 359–365. [Google Scholar] [CrossRef]
- Jacob, E.; Mack, A.K.; Savedra, M.; Van Cleve, L.; Wilkie, D.J. Adolescent pediatric pain tool for multidimensional measurement of pain in children and adolescents. Pain Manag. Nurs. 2014, 15, 694–706. [Google Scholar] [CrossRef]
- Friedrichsdorf, S.J.; Nugent, A.P. Management of neuropathic pain in children with cancer. Curr. Opin. Support. Palliat. Care 2013, 7, 131–138. [Google Scholar] [CrossRef]
- Gilchrist, L.S.; Tanner, L. The pediatric-modified total neuropathy score: A reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support. Care Cancer 2013, 21, 847–856. [Google Scholar] [CrossRef]
- Anghelescu, D.L.; Tesney, J.M. Neuropathic Pain in Pediatric Oncology: A Clinical Decision Algorithm. Paediatr. Drugs 2019, 21, 59–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavoie Smith, E.M.; Li, L.; Hutchinson, R.J.; Ho, R.; Burnette, W.B.; Wells, E.; Bridges, C.; Renbarger, J. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs. 2013, 36, E49–E60. [Google Scholar] [CrossRef]
- O’Donnell, F.T.; Rosen, K.R. Pediatric Pain Management: A Review. Mo. Med. 2014, 111, 231–237. [Google Scholar]
- Hess, C.W.; Van Orden, A.R.; Mesaroli, G.; Stinson, J.N.; Borsook, D.; Simons, L.E. Application of PainDETECT in pediatric chronic pain: How well does it identify neuropathic pain and its characteristics? Pain Rep. 2023, 8, e1109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Truini, A.; Aleksovska, K.; Anderson, C.C.; Attal, N.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Cruccu, G.; Eisenberg, E.; Enax-Krumova, E.; et al. Joint European Academy of Neurology-European Pain Federation-Neuropathic Pain Special Interest Group of the International Association for the Study of Pain guidelines on neuropathic pain assessment. Eur. J. Neurol. 2023, 30, 2177–2196. [Google Scholar] [CrossRef] [PubMed]
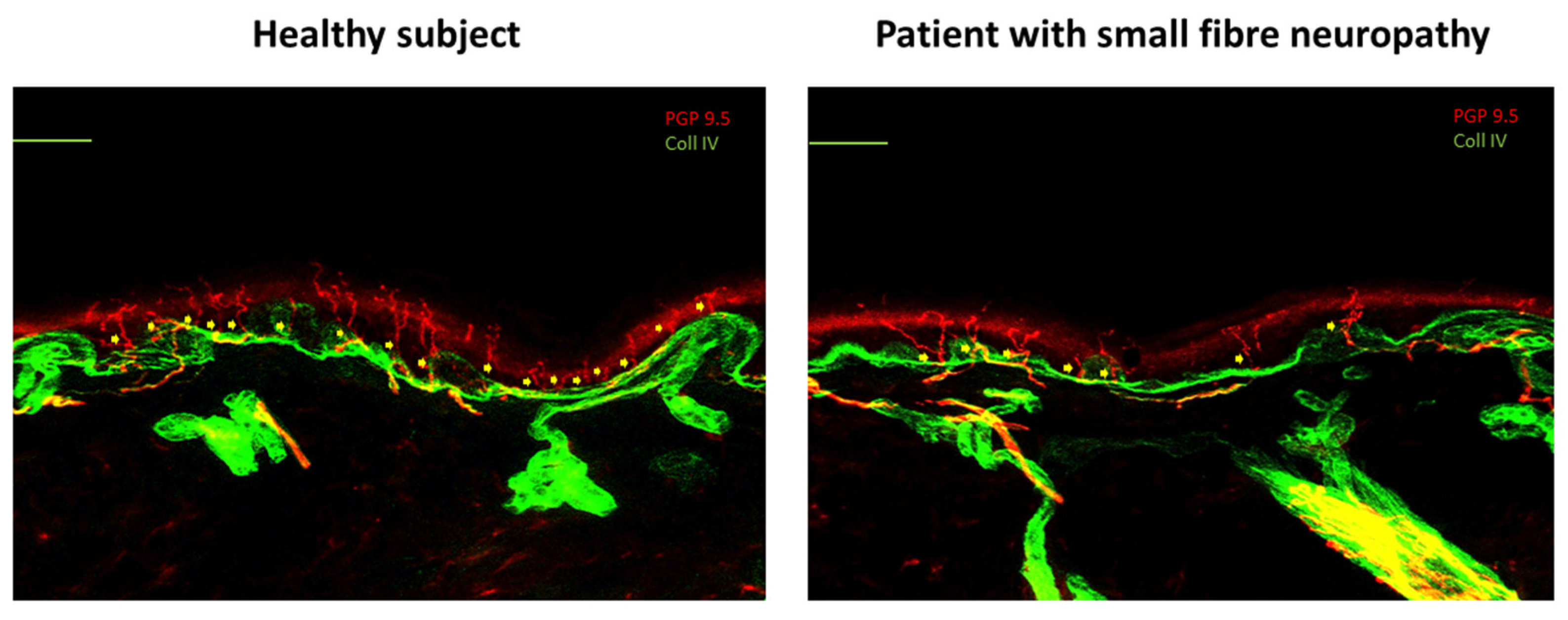
- Görlach, J.; Amsel, D.; Kölbel, H.; Grzybowsky, M.; Rutsch, F.; Schlierbach, H.; Vanlander, A.; Pogatzki-Zahn, E.; Habig, K.; Garkisch, S.; et al. Diagnostic utility of small fiber analysis in skin biopsies from children with chronic pain. Muscle Nerve 2020, 61, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Devigili, G.; Tugnoli, V.; Penza, P.; Camozzi, F.; Lombardi, R.; Melli, G.; Broglio, L.; Granieri, E.; Lauria, G. The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain 2008, 131 Pt 7, 1912–1925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lauria, G.; Cornblath, D.R.; Johansson, O.; McArthur, J.C.; Mellgren, S.I.; Nolano, M.; Rosenberg, N.; Sommer, C.; European Federation of Neurological Societies. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur. J. Neurol. 2005, 12, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, C.J.; Rydh, M.; Haegerstrand, A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry 1989, 92, 385–390. [Google Scholar] [CrossRef]
- Cruccu, G.; Sommer, C.; Anand, P.; Attal, N.; Baron, R.; Garcia-Larrea, L.; Haanpaa, M.; Jensen, T.S.; Serra, J.; Treede, R.D. EFNS guidelines on neuropathic pain assessment: Revised 2009. Eur. J. Neurol. 2010, 17, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, G.; La Cesa, S.; Leone, C.; Pepe, A.; Galosi, E.; Fiorelli, M.; Valeriani, M.; Lacerenza, M.; Pergolini, M.; Biasiotta, A.; et al. Diagnostic accuracy of laser-evoked potentials in diabetic neuropathy. Pain 2017, 158, 1100–1107. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, D.R.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Blankenburg, M.; Boekens, H.; Hechler, T.; Maier, C.; Krumova, E.; Scherens, A.; Magerl, W.; Aksu, F.; Zernikow, B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain 2010, 149, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Landry, B.W.; Fischer, P.R.; Driscoll, S.W.; Koch, K.M.; Harbeck-Weber, C.; Mack, K.J.; Wilder, R.T.; Bauer, B.A.; Brandenburg, J.E. Managing Chronic Pain in Children and Adolescents: A Clinical Review. PM&R 2015, 7, S295–S315. [Google Scholar] [CrossRef]
- Eccleston, C.; Fisher, E.; Cooper, T.E.; Grégoire, M.-C.; Heathcote, L.C.; Krane, E.; Lord, S.M.; Sethna, N.F.; Anderson, A.-K.; Anderson, B.; et al. Pharmacological Interventions for Chronic Pain in Children: An Overview of Systematic Reviews. Pain 2019, 160, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Guidelines on the Management of Chronic Pain in Children. Available online: https://www.who.int/publications/i/item/9789240017870 (accessed on 27 December 2023).
- Mao, J.J.; Ismaila, N.; Bao, T.; Barton, D.; Ben-Arye, E.; Garland, E.L.; Greenlee, H.; Leblanc, T.; Lee, R.T.; Lopez, A.M.; et al. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline. J. Clin. Oncol. 2022, 40, 3998–4024. [Google Scholar] [CrossRef]
- Deng, G. Integrative Medicine Therapies for Pain Management in Cancer Patients. Cancer J. 2019, 25, 343–348. [Google Scholar] [CrossRef]
- Coluzzi, F.; Scerpa, M.S.; Rocco, M.; Fornasari, D. The Impact of P-Glycoprotein on Opioid Analgesics: What’s the Real. Meaning in Pain. Management and Palliative Care? Int. J. Mol. Sci. 2022, 23, 14125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, S.Y.; Oh, J. Neuropathic cancer pain: Prevalence, pathophysiology, and management. Korean J. Intern. Med. 2018, 33, 1058–1069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cascella, M.; Vittori, A.; Petrucci, E.; Marinangeli, F.; Giarratano, A.; Cacciagrano, C.; Tizi, E.S.; Miceli, L.; Natoli, S.; Cuomo, A. Strengths and Weaknesses of Cancer Pain Management in Italy: Findings from a Nationwide SIAARTI Survey. Healthcare 2022, 10, 441. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar] [PubMed]
- Moore, R.A.; Chi, C.-C.; Wiffen, P.J.; Derry, S.; Rice, A.S.C. Oral Nonsteroidal Anti-Inflammatory Drugs for Neuropathic Pain. Cochrane Database Syst. Rev. 2015, 2015, CD010902. [Google Scholar] [CrossRef]
- Ziesenitz, V.C.; Welzel, T.; van Dyk, M.; Saur, P.; Gorenflo, M.; van den Anker, J.N. Efficacy and Safety of NSAIDs in Infants: A Comprehensive Review of the Literature of the Past 20 Years. Paediatr. Drugs 2022, 24, 603–655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coluzzi, F. Assessing and Treating Chronic Pain in Patients with End-Stage Renal Disease. Drugs 2018, 78, 1459–1479. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, A.N.; Kim, P.Y. Ketorolac. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Star, K.; Caster, O.; Bate, A.; Edwards, I.R. Dose Variations Associated with Formulations of NSAID Prescriptions for Children: A Descriptive Analysis of Electronic Health Records in the UK. Drug Saf. 2011, 34, 307–317. [Google Scholar] [CrossRef]
- Cooney, M.F. Pain Management in Children: NSAID Use in the Perioperative and Emergency Department Settings. Paediatr. Drugs 2021, 23, 361–372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooper, T.E.; Heathcote, L.C.; Anderson, B.; Grégoire, M.C.; Ljungman, G.; Eccleston, C. Non-steroidal anti-inflammatory drugs (NSAIDs) for cancer-related pain in children and adolescents. Cochrane Database Syst. Rev. 2017, 7, CD012563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eccleston, C.; Cooper, T.E.; Fisher, E.; Anderson, B.; Wilkinson, N.M. Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst. Rev. 2017, 8, CD012537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Motov, S.; Butt, M.; Masoudi, A.; Palacios, W.; Fassassi, C.; Drapkin, J.; Likourezos, A.; Hossain, R.; Brady, J.; Rothberger, N.; et al. Comparison of Oral Ibuprofen and Acetaminophen with Either Analgesic Alone for Pediatric Emergency Department Patients with Acute Pain. J. Emerg. Med. 2020, 58, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Archer, V.; Cloutier, Z.; Park, L.; Briatico, D.; Walton, J.M. Intravenous acetaminophen for postoperative pain control after open abdominal and thoracic surgery in pediatric patients: A systematic review and meta-analysis. Pediatr. Surg. Int. 2022, 39, 7. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Krumbach, B.; Gregory, J.A.; Biput, S.J.; Shay, A.; Gorantla, V.R. Pain Management Throughout Pediatric Laparoscopic Appendectomy: A Systematic Review. Cureus 2023, 15, e49581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radman, M.; Babic, A.; Runjic, E.; Jelicic Kadic, A.; Jeric, M.; Moja, L.; Puljak, L. Revisiting established medicines: An overview of systematic reviews about ibuprofen and paracetamol for treating pain in children. Eur. J. Pain 2019, 23, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Braithwaite, I.; McKinlay, C.J.D.; Dalziel, S.R. Comparison of Acetaminophen (Paracetamol) with Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2022398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parri, N.; Silvagni, D.; Chiarugi, A.; Cortis, E.; D’Avino, A.; Lanari, M.; Marchisio, P.G.; Vezzoli, C.; Zampogna, S.; Staiano, A. Paracetamol and ibuprofen combination for the management of acute mild-to-moderate pain in children: Expert consensus using the Nominal Group Technique (NGT). Ital. J. Pediatr. 2023, 49, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooper, T.E.; Fisher, E.; Anderson, B.; Wilkinson, N.M.; Williams, D.G.; Eccleston, C. Paracetamol (acetaminophen) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst. Rev. 2017, 8, CD012539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parker, W.; Anderson, L.G.; Jones, J.P.; Anderson, R.; Williamson, L.; Bono-Lunn, D.; Konsoula, Z. The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits. Children 2023, 11, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coluzzi, F.; Valensise, H.; Sacco, M.; Allegri, M. Chronic pain management in pregnancy and lactation. Minerva Anestesiol. 2014, 80, 211–224. [Google Scholar] [PubMed]
- Baenziger, P.H.; Moody, K. Palliative Care for Children with Central Nervous System Malignancies. Bioengineering 2018, 5, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meaadi, J.; Obara, I.; Eldabe, S.; Nazar, H. The safety and efficacy of gabapentinoids in the management of neuropathic pain: A systematic review with meta-analysis of randomised controlled trials. Int. J. Clin. Pharm. 2023, 45, 556–565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Connell, N. Clinical Management in an Evidence Vacuum: Pharmacological Management of Children with Persistent Pain. Cochrane Database Syst. Rev. 2019, 6, ED000135. [Google Scholar] [CrossRef]
- Bakır, M.; Rumeli, Ş.; Pire, A. Multimodal Analgesia in Pediatric Cancer Pain Management: A Retrospective Single-Center Study. Cureus 2023, 15, e45223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Healy, P.; Verrest, L.; Felisi, M.; Ceci, A.; Della Pasqua, O.; GAPP Consortium. Dose rationale for gabapentin and tramadol in pediatric patients with chronic pain. Pharmacol. Res. Perspect. 2023, 11, e01138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banerjee, S.; Butcher, R. Pharmacological Interventions for Chronic Pain in Pediatric Patients: A Review of Guidelines [Internet]; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar] [PubMed]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20 (Suppl. 1), S2–S12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin. Oncol. 2021, 48, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.E.; Heathcote, L.C.; Clinch, J.; Gold, J.I.; Howard, R.; Lord, S.M.; Schechter, N.; Wood, C.; Wiffen, P.J. Antidepressants for chronic non-cancer pain in children and adolescents. Cochrane Database Syst. Rev. 2017, 8, CD012535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2015, 2015, CD008242. [Google Scholar] [CrossRef]
- Wren, A.A.; Ross, A.C.; D’Souza, G.; Almgren, C.; Feinstein, A.; Marshall, A.; Golianu, B. Multidisciplinary Pain Management for Pediatric Patients with Acute and Chronic Pain: A Foundational Treatment Approach When Prescribing Opioids. Children 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.S.; Merchant, S.; Chidambaran, V. Postoperative Pain Management in Pediatric Spinal Fusion Surgery for Idiopathic Scoliosis. Paediatr. Drugs 2020, 22, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Sieberg, C.B.; Karunakaran, K.D.; Kussman, B.; Borsook, D. Preventing pediatric chronic postsurgical pain: Time for increased rigor. Can. J. Pain 2022, 6, 73–84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.H.; Sadhasivam, S.; DeMedal, S.; Visoiu, M. Short-acting versus long-acting opioids for pediatric postoperative pain management. Expert. Rev. Clin. Pharmacol. 2023, 16, 813–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiffen, P.J.; Cooper, T.E.; Anderson, A.K.; Gray, A.L.; Grégoire, M.C.; Ljungman, G.; Zernikow, B. Opioids for cancer-related pain in children and adolescents. Cochrane Database Syst. Rev. 2017, 7, CD012564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isaac, L.; Rosenbloom, B.N.; Tyrrell, J.; Ruskin, D.A.; Birnie, K.A. Development and expansion of a pediatric transitional pain service to prevent complex chronic pain. Front. Pain Res. 2023, 4, 1173675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandow, A.M.; Carroll, C.P.; Creary, S.; Edwards-Elliott, R.; Glassberg, J.; Hurley, R.W.; Kutlar, A.; Seisa, M.; Stinson, J.; Strouse, J.J.; et al. American Society of Hematology 2020 guidelines for sickle cell disease: Management of acute and chronic pain. Blood Adv. 2020, 4, 2656–2701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grond, S.; Sablotzki, A. Clinical Pharmacology of Tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Risks of Codeine and Tramadol in Children. JAMA 2017, 318, 1514. [Google Scholar] [CrossRef] [PubMed]
- Rodieux, F.; Vutskits, L.; Posfay-Barbe, K.M.; Habre, W.; Piguet, V.; Desmeules, J.A.; Samer, C.F. When the Safe Alternative Is Not That Safe: Tramadol Prescribing in Children. Front. Pharmacol. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hedenmalm, K.; Blake, K.; Donegan, K.; Macia, M.A.; Gil, M.; Williams, J.; Montero, D.; Candore, G.; Morales, D.; Kurz, X.; et al. European multicentre drug utilisation study of the impact of regulatory measures on prescribing of codeine for pain in children. Pharmacoepidemiol. Drug Saf. 2019, 28, 1086–1096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedrichsdorf, S.J.; Goubert, L. Pediatric pain treatment and prevention for hospitalized children. Pain Rep. 2019, 5, e804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Windsor, R.B.; Tham, S.W.; Adams, T.L.; Anderson, A. The Use of Opioids for Treatment of Pediatric Neuropathic Pain: A Literature Review. Clin. J. Pain 2019, 35, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017, 6 (Suppl. 1), 25–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Partanen, M.; Alberts, N.M.; Conklin, H.M.; Krull, K.R.; Pui, C.H.; Anghelescu, D.A.; Jacola, L.M. Neuropathic pain and neurocognitive functioning in children treated for acute lymphoblastic leukemia. Pain 2022, 163, 1070–1077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramkiran, S.; Thota, R.S. Methadone in Cancer Pain. Indian. J. Palliat. Care 2020, 26, 215–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pușcașu, C.; Chiriță, C.; Negreș, S.; Blebea, N.M. Exploring the Therapeutic Potential of N-Methyl-D-Aspartate Receptor Antagonists in Neuropathic Pain Management. Int. J. Mol. Sci. 2024, 25, 11111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Billeci, D.; Coluzzi, F. Tapentadol extended release for the management of chronic neck pain. J. Pain Res. 2017, 10, 495–505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coluzzi, F.; Polati, E.; Freo, U.; Grilli, M. Tapentadol: An effective option for the treatment of back pain. J. Pain Res. 2019, 12, 1521–1528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kress, H.G.; Coluzzi, F. Tapentadol in the management of cancer pain: Current evidence and future perspectives. J. Pain Res. 2019, 12, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eerdekens, M.; Radic, T.; Sohns, M.; Khalil, F.; Bulawa, B.; Elling, C. Outcomes of the Pediatric Development Plan of Tapentadol. J. Pain Res. 2021, 14, 249–261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finkel, J.C.; Goldberg, J.; Rosenburg, R.; Ariyawansa, J.; Sun, T.; Ochs-Ross, R.; Zannikos, P.; Zhang, L.; Etropolski, M. First evaluation of tapentadol oral solution for the treatment of moderate to severe acute pain in children aged 6 to <18. J. Pain Res. 2019, 12, 1925–1936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muse, D.; Tarau, E.; Lefeber, C.; Sohns, M.; Brett, M.; Goldberg, J.; Rosenburg, R. Pharmacokinetics, safety, and efficacy of tapentadol oral solution for treating moderate to severe pain in pediatric patients. J. Pain Res. 2019, 12, 1777–1790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beuter, C.; Volkers, G.; Radic, T.; Goldberg, J.; van den Anker, J. Efficacy and safety of multiple doses of tapentadol oral solution in the treatment of moderate to severe acute pain in children aged 2 to <18 years—A randomized, double-blind, placebo-controlled trial. J. Pain Res. 2019, 12, 3099–3112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eissa, A.; Tarau, E.; Beuter, C.; Radic, T.; Watson, E.; Sohns, M.; Lefeber, C.; Hammer, G.B. Tapentadol for the Treatment of Moderate-to-Severe Acute Pain in Children Under the Age of Two Years. J. Pain Res. 2021, 14, 229–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howard, R.F.; Radic, T.; Sohns, M.; Eerdekens, M.; Waßmuth, A. Tapentadol Prolonged Release for Long-Term Treatment of Pain in Children. J. Pain Res. 2020, 13, 3157–3170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coluzzi, F.; Bifulco, F.; Cuomo, A.; Dauri, M.; Leonardi, C.; Melotti, R.M.; Natoli, S.; Romualdi, P.; Savoia, G.; Corcione, A. The challenge of perioperative pain management in opioid-tolerant patients. Ther. Clin. Risk Manag. 2017, 13, 1163–1173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webster, L.; Gudin, J.; Raffa, R.B.; Kuchera, J.; Rauck, R.; Fudin, J.; Adler, J.; Mallick-Searle, T. Understanding Buprenorphine for Use in Chronic Pain: Expert Opinion. Pain Med. 2020, 21, 714–723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Degnan, M.; Mousa, S.A. A narrative review of buprenorphine in adult cancer pain. Expert. Rev. Clin. Pharmacol. 2020, 13, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Attinà, G.; Romano, A.; Triarico, S.; Mastrangelo, S.; Maurizi, P.; Ruggiero, A. Transdermal buprenorphine for pain management in children. Drugs Context 2021, 10, 2021-6-1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruggiero, A.; Coccia, P.; Arena, R.; Maurizi, P.; Battista, A.; Ridola, V.; Attinà, G.; Riccardi, R. Efficacy and safety of transdermal buprenorphine in the management of children with cancer-related pain. Pediatr. Blood Cancer 2013, 60, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Haupt, T.S.; Smyth, M.; Gregoire, M.C. A Scoping Review of Transdermal Buprenorphine Use for Non-surgical Pain in the Pediatric Population. Cureus 2019, 11, e5954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quirk, K.; Wright, J.; Marks, A.; Smith, M.A. Sublingual Buprenorphine for Pediatric Cancer Pain: A Case Report and Review of the Literature. J. Pain Symptom Manag. 2020, 60, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.; Malla, U.; Vlok, R.; Scott, A.; Chua, O.; Melhuish, T.; White, L. Buprenorphine versus Morphine in Paediatric Acute Pain: A Systematic Review and Meta-Analysis. Crit. Care Res. Pract. 2018, 2018, 3792043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irwin, M.; Gunther, W.; Keefer, P.; Saul, D.; Singh, S.A.; Wright, J.; Smith, M.A. Buprenorphine for Chronic Pain in a Pediatric Patient with Sickle-Cell Disease. J. Pain Symptom Manag. 2021, 62, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Rullo, L.; Scerpa, M.S.; Losapio, L.M.; Rocco, M.; Billeci, D.; Candeletti, S.; Romualdi, P. Current and Future Therapeutic Options in Pain Management: Multi-mechanistic Opioids Involving Both MOR and NOP Receptor Activation. CNS Drugs 2022, 36, 617–632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mastrangelo, S.; Capozza, M.A.; Triarico, S.; Attinà, G.; Maurizi, P.; Romano, A.; Ruggiero, A. Opioid transdermal delivery system: A useful method for pain management in children. Ann. Transl. Med. 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewitt, M.; Goldman, A.; Collins, G.S.; Childs, M.; Hain, R. Opioid use in palliative care of children and young people with cancer. J. Pediatr. 2008, 152, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Culp, C.; Kim, H.K.; Abdi, S. Ketamine Use for Cancer and Chronic Pain Management. Front. Pharmacol. 2020, 11, 599721. [Google Scholar] [CrossRef] [PubMed]
- Natoli, S. The Multiple Faces of Ketamine in Anaesthesia and Analgesia. Drugs Context 2021, 10, 2020-12-8. [Google Scholar] [CrossRef] [PubMed]
- Simonini, A.; Brogi, E.; Cascella, M.; Vittori, A. Advantages of Ketamine in Pediatric Anesthesia. Open Med. (Wars.) 2022, 17, 1134–1147. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Jakacki, R.; May, C.; Howrie, D.; Maurer, S. Ketamine PCA for treatment of end-of-life neuropathic pain in pediatrics. Am. J. Hosp. Palliat. Care 2015, 32, 841–848. [Google Scholar] [CrossRef] [PubMed]
- AlGhamdi, K.; Sadler, K. The Use of Ketamine for Malignant and Nonmalignant Chronic Pain in Children: A Review of Current Evidence. J. Pain Palliat. Care Pharmacother. 2024, 38, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Pansini, V.; Curatola, A.; Gatto, A.; Lazzareschi, I.; Ruggiero, A.; Chiaretti, A. Intranasal drugs for analgesia and sedation in children admitted to pediatric emergency department: A narrative review. Ann. Transl. Med. 2021, 9, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keating, G.M. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs 2015, 75, 1119–1130. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Yu, N.; Jia, C.; Wang, S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Portelli, K.; Kandraju, H.; Ryu, M.; Shah, P.S. Efficacy and safety of dexmedetomidine for analgesia and sedation in neonates: A systematic review. J. Perinatol. 2024, 44, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yasaei, R.; Saadabadi, A. Clonidine. 2023 Jul 17. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Su, F.; Hammer, G.B. Dexmedetomidine: Pediatric Pharmacology, Clinical Uses and Safety. Expert. Opin. Drug Saf. 2011, 10, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Petzke, F.; Tölle, T.; Fitzcharles, M.A.; Häuser, W. Cannabis-Based Medicines and Medical Cannabis for Chronic Neuropathic Pain. CNS Drugs 2022, 36, 31–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. 4), iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.H.; Caspi, A.; RKnodt, A.; Hall, W.; Ambler, A.; Harrington, H.; Hogan, S.; MHouts, R.; Poulton, R.; Ramrakha, S.; et al. Long-Term Cannabis Use and Cognitive Reserves and Hippocampal Volume in Midlife. Am. J. Psychiatry 2022, 179, 362–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Figueiredo, P.R.; Tolomeo, S.; Steele, J.D.; Baldacchino, A. Neurocognitive consequences of chronic cannabis use: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 108, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J. Physicians disagree on legal age for cannabis. CMAJ 2017, 189, E174–E175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Woo, J.J.; van Reekum, E.A.; Rosic, T.; Samaan, Z. Children and Youth Who Use Cannabis for Pain Relief: Benefits, Risks, and Perceptions. Adolesc. Health Med. Ther. 2020, 11, 53–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lang-Illievich, K.; Klivinyi, C.; Lasser, C.; Brenna, C.T.A.; Szilagyi, I.S.; Bornemann-Cimenti, H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2023, 15, 1350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papetti, L.; Sforza, G.; Tullo, G.; Alaimo di Loro, P.; Moavero, R.; Ursitti, F.; Ferilli, M.A.N.; Tarantino, S.; Vigevano, F.; Valeriani, M. Tolerability of Palmitoylethanolamide in a Pediatric Population Suffering from Migraine: A Pilot Study. Pain Res. Manag. 2020, 2020, 3938640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Nardo, G.; Bernardo, L.; Cremon, C.; Barbara, G.; Felici, E.; Evangelisti, M.; Ferretti, A.; Furio, S.; Piccirillo, M.; Coluzzi, F.; et al. Palmitoylethanolamide and polydatin in pediatric irritable bowel syndrome: A multicentric randomized controlled trial. Nutrition 2024, 122, 112397. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P. Novel drug treatments for pain in advanced cancer and serious illness: A focus on neuropathic pain and chemotherapy-induced peripheral neuropathy. Palliat. Care Soc. Pract. 2024, 18, 26323524241266603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, E.A.; Sauer, H.E.; Davis, M.S.; Anghelescu, D.L. Lidocaine Infusions for Pain Management in Pediatrics. Paediatr. Drugs 2021, 23, 349–359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goddard, J.M.; Reaney, R.L. Lidocaine 5%-medicated plaster (Versatis) for localised neuropathic pain: Results of a multicentre evaluation of use in children and adolescents. Br. J. Pain 2018, 12, 189–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glaros, A.K.; Callaghan, M.U.; Smith, W.R.; Zaidi, A.U. Targeting TRPV1 activity via high-dose capsaicin in patients with sickle cell disease. EJHaem 2022, 3, 653–659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, D.; Ashkan, K. Deep brain stimulation for phantom limb pain. Eur. J. Paediatr. Neurol. 2022, 39, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; Godai, K.; Daitoku, Y.; Sato, T.; Enohata, K.; Kiyonaga, N.; Maekawa, K.; Kanmura, Y. Successful intrathecal neurolytic block for the management of cancer pain in a 10-year-old child: A case report. JA Clin. Rep. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, H.S.; Kim, W.J.; Kim, H.G.; Yoo, S.H. Scrambler therapy for the treatment of neuropathic pain related to leukemia in a pediatric patient: A case report. Medicine 2017, 96, e8629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacobs, B.M.; Kerr, M.S.; Broadnax, J.P.; Anderson, E. Spinal Cord Stimulation (SCS) reduces Morphine Milligram Equivalents (MME) in patients using Opioid analgesics for Chronic Non-Cancer Pain. Interv. Pain Med. 2023, 2, 100275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheldon, B.L.; Bao, J.; Khazen, O.; Pilitsis, J.G. Spinal Cord Stimulation as Treatment for Cancer and Chemotherapy-Induced Pain. Front. Pain Res. 2021, 2, 699993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karri, J.; Palmer, J.S.; Charnay, A.; Garcia, C.; Orhurhu, V.; Shah, S.; Abd-Elsayed, A. Utility of Electrical Neuromodulation for Treating Chronic Pain Syndromes in the Pediatric Setting: A Systematic Review. Neuromodulation 2021, 25, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Bakr, S.M.; Knight, J.A.; Shlobin, N.A.; Budnick, H.; Desai, V.; Hill, H.; Johnson, S.K.; Williams, A.E.; Tolley, J.A.; Raskin, J.S. Spinal cord stimulation for treatment of chronic neuropathic pain in adolescent patients: A single-institution series, systematic review, and individual participant data meta-analysis. Neurosurg. Focus. 2022, 53, E13. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.; Wand, B.M.; O’Connell, N.E. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 9, CD011976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomanovic Vujadinovic, S.; Ilic, N.; Selakovic, I.; Nedeljkovic, U.; Krstic, N.; Mujovic, N.; Dubljanin Raspopovic, E.; Jovanovic, D. TENS Improves Cisplatin-Induced Neuropathy in Lung Cancer Patients. Medicina 2022, 58, 1405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cebalo, N.; Bašić Kes, V.; Urlić, I.; Karlović, Z.; Negovetić Vranić, D. The Effect of Transcoutaneous Electrical Nerve Stimulation on Pain Control during Dental Procedure in Children 9–14 Years Old. Psychiatr. Danub. 2021, 33 (Suppl. 4), 1316–1319. [Google Scholar] [PubMed]
- Li, Y.; Ma, Y.; Guo, W.; Ge, W.; Cheng, Y.; Jin, C.; Guo, H. Effect of transcutaneous electrical acupoint stimulation on postoperative pain in pediatric orthopedic surgery with the enhanced recovery after surgery protocol: A prospective, randomized controlled trial. Anaesth. Crit. Care Pain Med. 2023, 42, 101273. [Google Scholar] [CrossRef] [PubMed]
- Anghelescu, D.L.; Johns, E.; Bhatia, S.; Frett, M.J.; Lu, Z. Chronic postsurgical pain in children and young adults with cancer and choice of regional anesthesia for amputation and limb-sparing surgery. Cancer Rep. 2023, 6, e1719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Askins, M.A.; Moore, B.D. Psychosocial support of the pediatric cancer patient: Lessons learned over the past 50 years. Curr. Oncol. Rep. 2008, 10, 469–476. [Google Scholar] [CrossRef]
- Melesse, T.G.; Chau, J.P.C.; Nan, M.A. Effectiveness of psychosocial interventions on health outcomes of children with cancer: A systematic review of randomised controlled trials. Eur. J. Cancer Care 2022, 31, e13695. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, F.; Feng, S.; Wang, Y.; Gu, Y.; Kang, Q. Symptom Experience of Children with Cancer Younger Than Eight Years of Age: An Integrative Review. J. Pain Symptom Manag. 2019, 58, 157–166. [Google Scholar] [CrossRef]
- Gupta, S.; Li, Q.; Nathan, P.C.; D’Agostino, N.; Baxter, N.N.; Fox, C.; Chalifour, K.; Coburn, N.; Sutradhar, R. Prevalence, severity, and predictors of symptom burden among adolescents and young adults with cancer. Cancer Med. 2023, 12, 11773–11785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunin-Batson, A.S.; Lu, X.; Balsamo, L.; Graber, K.; Devidas, M.; Hunger, S.P.; Carroll, W.L.; Winick, N.J.; Mattano, L.A., Jr.; Maloney, K.W.; et al. Prevalence and predictors of anxiety and depression after completion of chemotherapy for childhood acute lymphoblastic leukemia: A prospective longitudinal study. Cancer 2016, 122, 1608–1617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yardeni, M.; Abebe Campino, G.; Bursztyn, S.; Shamir, A.; Mekori-Domachevsky, E.; Toren, A.; Gothelf, D. A three-tier process for screening depression and anxiety among children and adolescents with cancer. Psychooncology 2020, 29, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Karlson, C.W.; Alberts, N.M.; Liu, W.; Brinkman, T.M.; Annett, R.D.; Mulrooney, D.A.; Schulte, F.; Leisenring, W.M.; Gibson, T.M.; Howell, R.M.; et al. Longitudinal pain and pain interference in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2020, 126, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.-C.; Brinkman, T.M.; Armstrong, G.T.; Leisenring, W.; Robison, L.L.; Krull, K.R. Emotional distress impacts quality of life evaluation: A report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2017, 11, 309–319. [Google Scholar] [CrossRef]
- Simons, L.E.; Kaczynski, K.J.; Conroy, C.; Logan, D.E. Fear of Pain in the Context of Intensive Pain Rehabilitation Among Children and Adolescents with Neuropathic Pain: Associations with Treatment Response. J. Pain 2012, 13, 1151–1161. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Tétreault, P.; Petre, B.; Huang, L.; Berger, S.E.; Torbey, S.; Baria, A.T.; Mansour, A.R.; Hashmi, J.A.; Griffith, J.W.; et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016, 139, 1958–1970. [Google Scholar] [CrossRef]
- Coughtrey, A.; Millington, A.; Bennett, S.; Christie, D.; Hough, R.; Su, M.T.; Constantinou, M.P.; Shafran, R. The Effectiveness of Psychosocial Interventions for Psychological Outcomes in Pediatric Oncology: A Systematic Review. J. Pain Symptom Manag. 2018, 55, 1004–1017. [Google Scholar] [CrossRef]
- Moryl, N.; Coyle, N.; Essandoh, S.; Glare, P. Chronic Pain Management in Cancer Survivors. J. Natl. Compr. Cancer Netw. 2010, 8, 1104–1110. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Hou, Y. Effects of mindfulness-based stress reduction combined with music therapy on pain, anxiety, and sleep quality in patients with osteosarcoma. Braz. J. Psychiatry 2019, 41, 540–545. [Google Scholar] [CrossRef]
- Melesse, T.G.; Chau, J.P.C.; Nan, M. Effects of cognitive-behavioural therapy on psychological, physical and social outcomes of children with cancer: A systematic review and meta-analysis. J. Psychosom. Res. 2022, 157, 110805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Mo, L.; Torres, J.; Huang, X. Effects of cognitive behavioral therapy on psychological adjustment in Chinese pediatric cancer patients receiving chemotherapy. Medicine 2019, 98, e16319. [Google Scholar] [CrossRef] [PubMed]
- Balck, F.; Zschieschang, A.; Zimmermann, A.; Ordemann, R. A randomized controlled trial of problem-solving training (PST) for hematopoietic stem cell transplant (HSCT) patients: Effects on anxiety, depression, distress, coping and pain. J. Psychosoc. Oncol. 2019, 37, 541–556. [Google Scholar] [CrossRef]
- Abdulah, D.M.; Abdulla, B.M.O. Effectiveness of group art therapy on quality of life in paediatric patients with cancer: A randomized controlled trial. Complement. Ther. Med. 2018, 41, 180–185. [Google Scholar] [CrossRef]
- Da Silva Santa, I.N.; Schveitzer, M.C.; dos Santos, M.L.B.M.; Ghelman, R.; Filho, V.O. Music interventions in pediatric oncology: Systematic review and meta-analysis. Complement. Ther. Med. 2021, 59, 102725. [Google Scholar] [CrossRef]
- Thanh Nhan Nguyen Nilsson, S.; Hellström, A.-L.; Bengtson, A. Music Therapy to Reduce Pain and Anxiety in Children with Cancer Undergoing Lumbar Puncture: A Randomized Clinical Trial. J. Pediatr. Oncol. Nurs. 2010, 27, 146–155. [Google Scholar] [CrossRef]
- Uggla, L.; Bonde, L.; Hammar, U.; Wrangsjö, B.; Gustafsson, B. Music therapy supported the health-related quality of life for children undergoing haematopoietic stem cell transplants. Acta Paediatr. 2018, 107, 1986–1994. [Google Scholar] [CrossRef]
- Cheung, A.T.; Li, W.H.C.; Ho, K.Y.; Lam, K.K.W.; Ho, L.L.K.; Chiu, S.Y.; Chan, G.C.F.; Chung, J.O.K. Efficacy of musical training on psychological outcomes and quality of life in Chinese pediatric brain tumor survivors. Psychooncology 2019, 28, 174–180. [Google Scholar] [CrossRef]
- Gerçeker, G.Ö.; Bektaş, M.; Aydınok, Y.; Ören, H.; Ellidokuz, H.; Olgun, N. The effect of virtual reality on pain, fear, and anxiety during access of a port with huber needle in pediatric hematology-oncology patients: Randomized controlled trial. Eur. J. Oncol. Nurs. 2021, 50, 101886. [Google Scholar] [CrossRef]
- Czech, O.; Rutkowski, S.; Kowaluk, A.; Kiper, P.; Malicka, I. Virtual reality in chemotherapy support for the treatment of physical functions, fear, and quality of life in pediatric cancer patients: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1039720. [Google Scholar] [CrossRef]
- Erdős, S.; Horváth, K. The Impact of Virtual Reality (VR) on Psychological and Physiological Variables in Children Receiving Chemotherapy: A Pilot Cross-Over Study. Integr. Cancer Ther. 2023, 22, 153473542311689. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, M.F.; Kelada, L.; Nur, A.B.Y.; Wakefield, C.E.; Wewege, M.A.; Karpelowsky, J.; Akimana, B.; Darlington, A.-S.; Signorelli, C. Interactive video games to reduce paediatric procedural pain and anxiety: A systematic review and meta-analysis. Br. J. Anaesth. 2021, 127, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Spector, D. Yoga in the Pediatric Oncology Population: A Review of the Literature. J. Pediatr. Oncol. Nurs. 2021, 38, 410–419. [Google Scholar] [CrossRef]
- Fukuhara, J.S.; O’Haver, J.; Proudfoot, J.A.; Spies, J.M.; Kuo, D.J. Yoga as a Complementary and Alternative Therapy in Children with Hematologic and Oncologic Disease. J. Pediatr. Oncol. Nurs. 2020, 37, 278–283. [Google Scholar] [CrossRef]
- Spikestein, A.; Musante, J.; Huang, H.H.; Stojanowski, M.; Rode, D.; Pillai, P.; Crouch, G.D. Impact of Facility Dog and Certified Child Life Specialist Dyad on Children’s Pain and Anxiety During Needlestick Procedures in a Pediatric Hematology Oncology Clinic Setting. J. Pediatr. Hematol. Oncol. 2024, 46, 51–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Y.; Lin, Y.; Zhang, N.; Jiang, X.; Zhang, L. Effects of Animal-Assisted Therapy on Hospitalized Children and Teenagers: A Systematic Review and Meta-Analysis. J. Pediatr. Nurs. 2021, 60, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Goren, K.; Cen, Y.; Montemurri, V.; Moodley, D.; Sutton, A.; Ahmed, A.; Alphonsus, L.; Denezis, P.; Fleming, C.; Guertin, H.; et al. The impact of music, play, and pet therapies in managing pain and anxiety in paediatric patients in hospital: A rapid systematic review. Paediatr. Child. Health. 2023, 28, 218–224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singhal, N.R. Chronic pain in pediatrics. Semin. Pediatr. Surg. 2024, 33, 151458. [Google Scholar] [CrossRef] [PubMed]
| P.O. Dose/Starting Dose | EMA (Europe) Approved | FDA (US) Approved | |
|---|---|---|---|
| NSAIDs/APAP | |||
| Ibuprofen | 5–10 mg/kg q 6–8 h | 3 mo | 6 mo |
| Naproxen | 5 mg/kg q 8–12 h | 1 year and > 10 kg | 2 years (rheumatoid arthritis) |
| Acetaminophen | 10–15 mg/kg q 6 h | Neonates | Neonates |
| Corticosteroids | |||
| Dexamethasone | 0.25 mg/kg q 6 h | Neonates | Neonates |
| Opioids | |||
| Codeine/acetaminophen | 30/500 mg q 6 h | 12 years | 12 years |
| Tramadol | 1–2 mg/kg q 6 h (1–12 years) 50 mg q 4–6 h (≥12 years) | 1 year and >10 kg | 12 years |
| Morphine | 0.3 mg/kg q 3–4-h | Neonates | Not authorized |
| Oxycodone | 0.1–0.15 mg/kg q 4–6 h | 12 years | 11 years (extended release) |
| Tapentadol | 1.25 mg/kg q 4 h | 2 years | Not authorized |
| TTS Fentanyl | 12 mcg/h every q 72 h | 2 years | 2 years |
| TTS Buprenorphine | 5 mcg/h q 7 d | 18 years | 16 years (subdermal implant for opioid dependence) |
| Anticonvulsants | |||
| Gabapentin | 5–40 mg/kg/day in 3 divided doses | 6 years | 3 years (partial seizures) |
| Pregabalin | 2.5–3.5 mg/kg/day in 3 divided doses | Not authorized | 1 mo (partial seizures) |
| Antidepressants | |||
| Duloxetine | 30 mg/day, 60 mg/day after 2 weeks | Not authorized | 7 years (generalized anxiety disorder), 13 years (fibromyalgia) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluzzi, F.; Di Stefano, G.; Scerpa, M.S.; Rocco, M.; Di Nardo, G.; Innocenti, A.; Vittori, A.; Ferretti, A.; Truini, A. The Challenge of Managing Neuropathic Pain in Children and Adolescents with Cancer. Cancers 2025, 17, 460. https://doi.org/10.3390/cancers17030460
Coluzzi F, Di Stefano G, Scerpa MS, Rocco M, Di Nardo G, Innocenti A, Vittori A, Ferretti A, Truini A. The Challenge of Managing Neuropathic Pain in Children and Adolescents with Cancer. Cancers. 2025; 17(3):460. https://doi.org/10.3390/cancers17030460
Chicago/Turabian StyleColuzzi, Flaminia, Giulia Di Stefano, Maria Sole Scerpa, Monica Rocco, Giovanni Di Nardo, Alice Innocenti, Alessandro Vittori, Alessandro Ferretti, and Andrea Truini. 2025. "The Challenge of Managing Neuropathic Pain in Children and Adolescents with Cancer" Cancers 17, no. 3: 460. https://doi.org/10.3390/cancers17030460
APA StyleColuzzi, F., Di Stefano, G., Scerpa, M. S., Rocco, M., Di Nardo, G., Innocenti, A., Vittori, A., Ferretti, A., & Truini, A. (2025). The Challenge of Managing Neuropathic Pain in Children and Adolescents with Cancer. Cancers, 17(3), 460. https://doi.org/10.3390/cancers17030460










