Genomic Characterization of Chordoma: Insights from the AACR Project GENIE Database
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
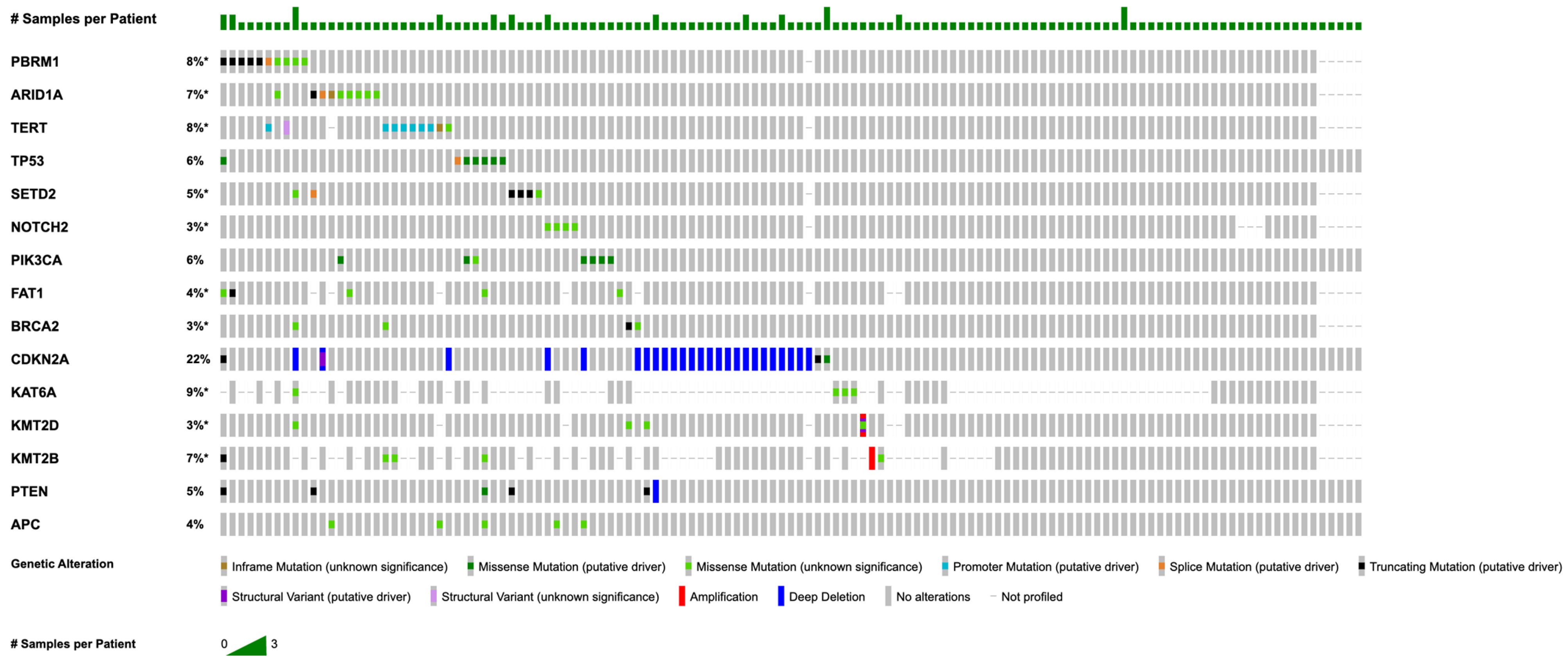
3. Results
3.1. Chordoma Patient Demographics
3.2. Somatic Mutations and Copy Number Alterations
3.3. Chordoma SWI/SNF Complex Mutations
3.4. Chordoma NOS, Conventional Type Chordoma, and Dedifferentiated Chordoma Subtypes Mutational Landscape
3.5. Adult vs. Pediatric Mutational Landscape
3.6. Primary vs. Metastatic Disease Mutational Landscape
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kremenevski, N.; Schlaffer, S.-M.; Coras, R.; Kinfe, T.M.; Graillon, T.; Buchfelder, M. Skull Base Chordomas and Chondrosarcomas. Neuroendocrinology 2020, 110, 836–847. [Google Scholar] [CrossRef] [PubMed]
- McMaster, M.L.; Goldstein, A.M.; Bromley, C.M.; Ishibe, N.; Parry, D.M. Chordoma: Incidence and Survival Patterns in the United States, 1973–1995. Cancer Causes Control 2001, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Soni, P.; Jones, J.; Habboub, G.; Barnholtz-Sloan, J.S.; Recinos, P.F.; Kshettry, V.R. Descriptive Epidemiology of Chordomas in the United States. J. Neurooncol. 2020, 148, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Tsitouras, V.; Wang, S.; Dirks, P.; Drake, J.; Bouffet, E.; Hawkins, C.; Laughlin, S.; Rutka, J.T. Management and Outcome of Chordomas in the Pediatric Population: The Hospital for Sick Children Experience and Review of the Literature. J. Clin. Neurosci. 2016, 34, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Song, P.H.; Beyhaghi, H.; Sommer, J.; Bennett, A.V. Symptom Burden and Life Challenges Reported by Adult Chordoma Patients and Their Caregivers. Qual. Life Res. 2017, 26, 2237–2244. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Ulloa, R.; Soffer, J.; Alcazar-Felix, R.J.; Snyderman, C.H.; Gardner, P.A.; Patel, V.A.; Polster, S.P. Chordoma: A Comprehensive Systematic Review of Clinical Trials. Cancers 2023, 15, 5800. [Google Scholar] [CrossRef]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma—Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef]
- Ailon, T.; Torabi, R.; Fisher, C.G.; Rhines, L.D.; Clarke, M.J.; Bettegowda, C.; Boriani, S.; Yamada, Y.J.; Kawahara, N.; Varga, P.P.; et al. Management of Locally Recurrent Chordoma of the Mobile Spine and Sacrum: A Systematic Review. Spine 2016, 41, S193. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Gerszten, P.; Laufer, I.; Yamada, Y. Radiation for Primary Spine Tumors. Neurosurg. Clin. N. Am. 2008, 19, 119–123. [Google Scholar] [CrossRef]
- Colia, V.; Stacchiotti, S. Medical Treatment of Advanced Chordomas. Eur. J. Cancer 2017, 83, 220–228. [Google Scholar] [CrossRef]
- Kesari, S.; Wagle, N.; Carrillo, J.A.; Sharma, A.; Nguyen, M.; Truong, J.; Gill, J.M.; Nersesian, R.; Nomura, N.; Rahbarlayegh, E.; et al. Pilot Study of High-Dose Pemetrexed in Patients with Progressive Chordoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Passeri, T.; Dahmani, A.; Masliah-Planchon, J.; Naguez, A.; Michou, M.; El Botty, R.; Vacher, S.; Bouarich, R.; Nicolas, A.; Polivka, M.; et al. Dramatic In Vivo Efficacy of the EZH2-Inhibitor Tazemetostat in PBRM1-Mutated Human Chordoma Xenograft. Cancers 2022, 14, 1486. [Google Scholar] [CrossRef] [PubMed]
- Zenonos, G.A.; Fernandez-Miranda, J.C.; Mukherjee, D.; Chang, Y.F.; Panayidou, K.; Snyderman, C.H.; Wang, E.W.; Seethala, R.R.; Gardner, P.A. Prospective Validation of a Molecular Prognostication Panel for Clival Chordoma. J. Neurosurg. 2018, 130, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Lehrich, B.M.; Abiri, A.; Nguyen, T.V.; Bitner, B.F.; Tong, C.C.L.; Kuan, E.C. Mutational Landscape and Predictors of Survival in Head and Neck Mucosal Melanoma. Int. Forum Allergy Rhinol. 2024, 14, 858–861. [Google Scholar] [CrossRef]
- Passeri, T.; Gutman, T.; Hamza, A.; Adle-Biassette, H.; Girard, E.; Beaurepere, R.; Tariq, Z.; Mariani, O.; Dahmani, A.; Bourneix, C.; et al. The Mutational Landscape of Skull Base and Spinal Chordomas and the Identification of Potential Prognostic and Theranostic Biomarkers. J. Neurosurg. 2023, 139, 1270–1280. [Google Scholar] [CrossRef]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different Roles in a Common Pathway of Genome Protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef]
- Dhar, S.S.; Lee, M.G. Cancer-Epigenetic Function of the Histone Methyltransferase KMT2D and Therapeutic Opportunities for the Treatment of KMT2D-Deficient Tumors. Oncotarget 2021, 12, 1296–1308. [Google Scholar] [CrossRef]
- Jambhekar, N.A.; Rekhi, B.; Thorat, K.; Dikshit, R.; Agrawal, M.; Puri, A. Revisiting Chordoma with Brachyury, a “New Age” Marker: Analysis of a Validation Study on 51 Cases. Arch. Pathol. Lab. Med. 2010, 134, 1181–1187. [Google Scholar] [CrossRef]
- Beccaria, K.; Sainte-Rose, C.; Zerah, M.; Puget, S. Paediatric Chordomas. Orphanet J. Rare Dis. 2015, 10, 116. [Google Scholar] [CrossRef]
- O’Halloran, K.; Hakimjavadi, H.; Bootwalla, M.; Ostrow, D.; Kerawala, R.; Cotter, J.A.; Yellapantula, V.; Kaneva, K.; Wadhwani, N.R.; Treece, A.; et al. Pediatric Chordoma: A Tale of Two Genomes. Mol. Cancer Res. MCR 2024, 22, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Meyers, A.D. Malignant Tumors of the Sinuses. Medscape, 9 May 2024. Available online: https://emedicine.medscape.com/article/847189-overview (accessed on 2 January 2025).
- Bai, J.; Shi, J.; Li, C.; Wang, S.; Zhang, T.; Hua, X.; Zhu, B.; Koka, H.; Wu, H.-H.; Song, L.; et al. Whole Genome Sequencing of Skull-Base Chordoma Reveals Genomic Alterations Associated with Recurrence and Chordoma-Specific Survival. Nat. Commun. 2021, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Hallor, K.H.; Staaf, J.; Jönsson, G.; Heidenblad, M.; Vult von Steyern, F.; Bauer, H.C.F.; IJszenga, M.; Hogendoorn, P.C.W.; Mandahl, N.; Szuhai, K.; et al. Frequent Deletion of the CDKN2A Locus in Chordoma: Analysis of Chromosomal Imbalances Using Array Comparative Genomic Hybridisation. Br. J. Cancer 2008, 98, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Dreier, M.R.; Walia, J.; de la Serna, I.L. Targeting SWI/SNF Complexes in Cancer: Pharmacological Approaches and Implications. Epigenomes 2024, 8, 7. [Google Scholar] [CrossRef]
- Shain, A.H.; Pollack, J.R. The Spectrum of SWI/SNF Mutations, Ubiquitous in Human Cancers. PLoS ONE 2013, 8, e55119. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhu, W.; Xu, Y.; Ma, J.; He, C.; Wang, F. SWI/SNF Complex Gene Variations Are Associated with a Higher Tumor Mutational Burden and a Better Response to Immune Checkpoint Inhibitor Treatment: A Pan-Cancer Analysis of next-Generation Sequencing Data Corresponding to 4591 Cases. Cancer Cell Int. 2022, 22, 347. [Google Scholar] [CrossRef]
- Mittal, P.; Roberts, C.W.M. The SWI/SNF Complex in Cancer—Biology, Biomarkers and Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 435–448. [Google Scholar] [CrossRef]
- Zhou, M.; Yuan, J.; Deng, Y.; Fan, X.; Shen, J. Emerging Role of SWI/SNF Complex Deficiency as a Target of Immune Checkpoint Blockade in Human Cancers. Oncogenesis 2021, 10, 3. [Google Scholar] [CrossRef]
- Saqcena, M.; Leandro-Garcia, L.J.; Maag, J.L.V.; Tchekmedyian, V.; Krishnamoorthy, G.P.; Tamarapu, P.P.; Tiedje, V.; Reuter, V.; Knauf, J.A.; de Stanchina, E.; et al. SWI/SNF Complex Mutations Promote Thyroid Tumor Progression and Insensitivity to Redifferentiation Therapies. Cancer Discov. 2021, 11, 1158–1175. [Google Scholar] [CrossRef]
- Jin, M.C.; Connolly, I.D.; Ravi, K.; Tobert, D.G.; MacDonald, S.M.; Shin, J.H. Unraveling Molecular Advancements in Chordoma Tumorigenesis and Treatment Response: A Review of Scientific Discoveries and Clinical Implications. Neurosurg. Focus 2024, 56, E18. [Google Scholar] [CrossRef]
| Demographics | Category | N (%) |
|---|---|---|
| Conventional Chordoma | 14 (10.5) | |
| Histology | Dedifferentiated Chordoma | 3 (2.3) |
| Not Otherwise Specified | 116 (87.2) | |
| Age category | Adult | 108 (91.5) |
| Pediatric | 10 (8.5) | |
| Cancer Center 1 | MSK | 70 (59.3) |
| UCSF | 18 (15.3) | |
| DFCI | 10 (8.5) | |
| MDA | 5 (4.2) | |
| CHOP | 4 (3.4) | |
| PROV | 4 (3.4) | |
| VICC | 2 (1.7) | |
| COLU | 2 (1.7) | |
| JHU | 1 (0.8) | |
| VHIO | 1 (0.8) | |
| UHN | 1 (0.8) | |
| Ethnicity | Non-Hispanic | 96 (81.4) |
| Unknown | 11 (9.3) | |
| Hispanic | 10 (8.5) | |
| Race | White | 85 (72.0) |
| Unknown | 13 (11.0) | |
| Asian | 8 (6.8) | |
| Black | 7 (5.9) | |
| Other | 5 (4.2) |
| PBRM1 | ARID1A | SETD2 |
|---|---|---|
| N258Kfs*6 | D1850Gfs*4 | R400* |
| P212Afs*3 | X1709_splice | E1756* |
| X271_splice | A345_A349del | T2338Hfs*31 |
| W1417* | L2088P | T2338Hfs*31 |
| W1417* | P194L | X1485_splice |
| R1276Vfs*6 | P1627A | A1617V |
| D1055Y | A247V | D1616N |
| D1055Y | M50V | |
| R1088W | W1073L | |
| H770P | G1770V |
| Demographics (Chi-Squared Test) | Category | Not Otherwise Specified N (%) | Conventional Type N (%) | Dedifferentiated N (%) | p-Value |
|---|---|---|---|---|---|
| Age category | Pediatric | 8 (6.9) | 0 (0.0) | 0 (0.0) | p = 0.028 |
| Adult | 108 (93.1) | 14 (100.0) | 3 (100.0) | ||
| Sex | Male | 64 (55.2) | 5 (35.7) | 3 (100.0) | p = 0.145 |
| Female | 52 (44.8) | 9 (64.3) | 0 (0.0) | ||
| Ethnicity | Non-Hispanic | 97 (83.6) | 9 (64.3) | 2 (66.7) | p = 0.120 |
| Hispanic | 9 (7.8) | 2 (14.3) | 1 (33.3) | ||
| Unknown | 9 (7.8) | 3 (21.4) | 0 (0.0) | ||
| Race | White | 83 (71.6) | 10 (71.4) | 2 (66.7) | p = 0.755 |
| Black | 6 (5.2) | 2 (14.3) | 0 (0.0) | ||
| Asian | 8 (6.9) | 0 (0.0) | 0 (0.0) | ||
| Other | 6 (5.2) | 1 (7.1) | 0 (0.0) | ||
| Unknown | 13 (11.2) | 1 (7.1) | 1 (33.3) | ||
| Sample Type | Primary | 58 (50.0) | 7 (50.0) | 2 (66.7) | p = 0.678 |
| Metastasis | 47 (40.5) | 6 (42.9) | 1 (33.3) | ||
| Other/Unknown | 11 (9.5) | 1 (7.0) | 0 (0.0) |
| Demographics (Chi-Squared Test) | Category | Adult, N (%) | Pediatric, N (%) | p Value |
|---|---|---|---|---|
| Sex | Male | 66 (54.5) | 6 (50.0) | p = 0.503 |
| Female | 55 (45.5) | 6 (50.0) | ||
| Ethnicity | Non-Hispanic | 99 (81.8) | 9 (75.0) | p = 0.369 |
| Hispanic | 11 (9.1) | 2 (16.7) | ||
| Unknown | 10 (8.3) | 1 (8.3) | ||
| Race | White | 91 (75.2) | 4 (33.3) | p = 0.562 |
| Black | 7 (5.8) | 1 (8.3) | ||
| Asian | 8 (6.6) | 0 (0.0) | ||
| Other | 7 (5.8) | 0 (0.0) | ||
| Unknown | 8 (6.6) | 7 (58.3) | ||
| Sample Type | Primary | 61 (50.4) | 6 (50.0) | p = 0.0017 |
| Metastasis | 51 (42.1) | 3 (25.0) | ||
| Other/Unknown | 45 (37.2) | 3 (25.0) |
| Sample Type | Gene | Mutations, N (%) | p Value |
|---|---|---|---|
| Primary | ARID1A | 5 (7.5) | p = 1.00 |
| PIK3CA | 5 (7.5) | p = 0.137 | |
| TP53 | 4 (6.0) | p = 1.00 | |
| PBRM1 | 4 (6.0) | p = 0.759 | |
| Metastatic | SETD2 | 6 (11.1) | p = 0.0411 (enriched) |
| BRCA2 | 6 (11.1) | p = 0.0138 (enriched) | |
| PBRM1 | 5 (9.3) | p = 0.759 | |
| ARID1A | 5 (9.3) | p = 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsia, B.; Bitar, G.; Alshaka, S.A.; Kim, J.D.; Valencia-Sanchez, B.A.; Faraji, F.; Brandel, M.G.; Sato, M.; Crawford, J.R.; Levy, M.L.; et al. Genomic Characterization of Chordoma: Insights from the AACR Project GENIE Database. Cancers 2025, 17, 536. https://doi.org/10.3390/cancers17030536
Hsia B, Bitar G, Alshaka SA, Kim JD, Valencia-Sanchez BA, Faraji F, Brandel MG, Sato M, Crawford JR, Levy ML, et al. Genomic Characterization of Chordoma: Insights from the AACR Project GENIE Database. Cancers. 2025; 17(3):536. https://doi.org/10.3390/cancers17030536
Chicago/Turabian StyleHsia, Beau, Gabriel Bitar, Saif A. Alshaka, Jeeho D. Kim, Bastien A. Valencia-Sanchez, Farhoud Faraji, Michael G. Brandel, Mariko Sato, John Ross Crawford, Michael L. Levy, and et al. 2025. "Genomic Characterization of Chordoma: Insights from the AACR Project GENIE Database" Cancers 17, no. 3: 536. https://doi.org/10.3390/cancers17030536
APA StyleHsia, B., Bitar, G., Alshaka, S. A., Kim, J. D., Valencia-Sanchez, B. A., Faraji, F., Brandel, M. G., Sato, M., Crawford, J. R., Levy, M. L., Patel, V. A., & Polster, S. P. (2025). Genomic Characterization of Chordoma: Insights from the AACR Project GENIE Database. Cancers, 17(3), 536. https://doi.org/10.3390/cancers17030536







