Possibilities of Overcoming Resistance to Osimertinib in NSCLC Patients with Mutations in the EGFR Gene
Simple Summary
Abstract
1. Introduction
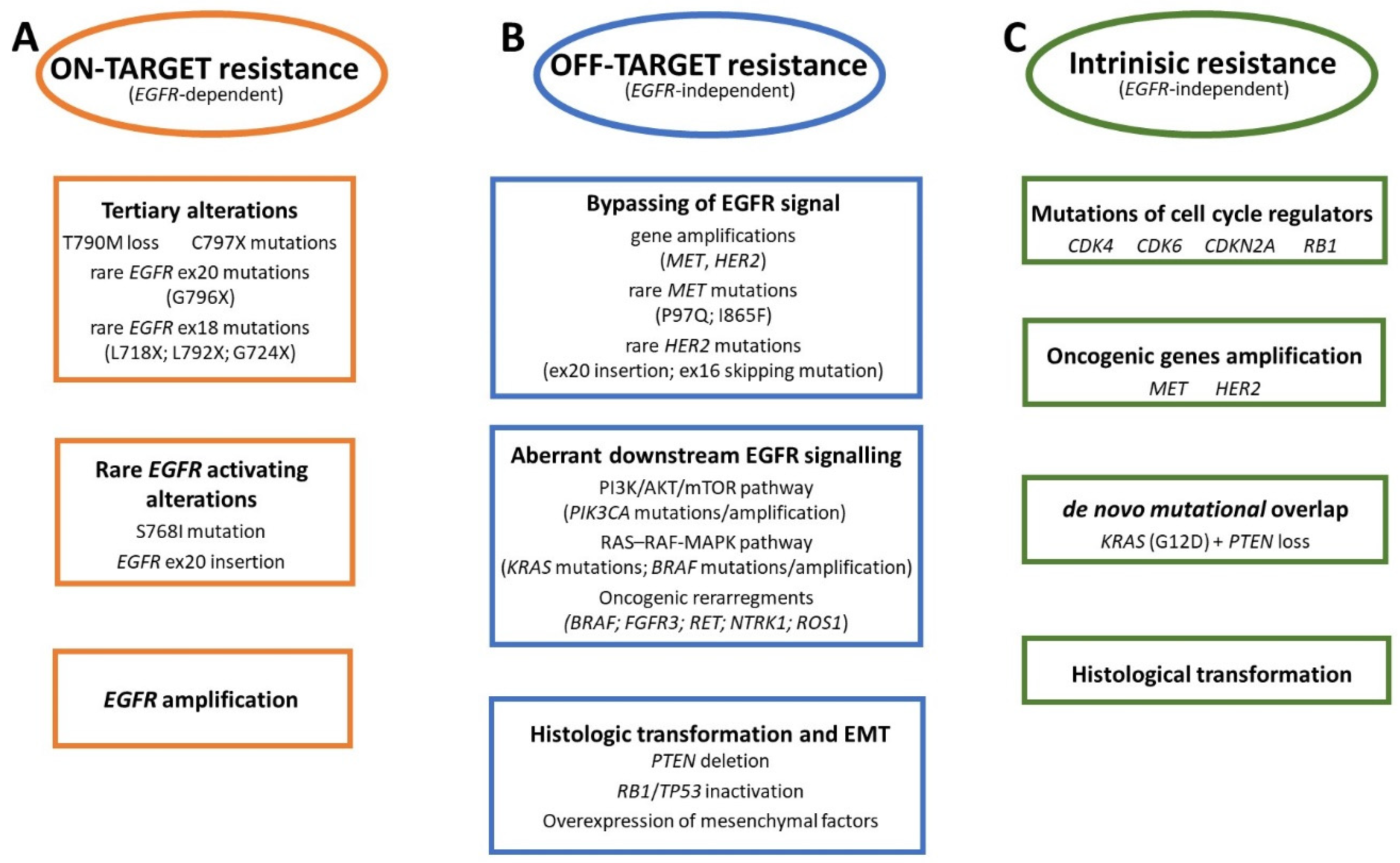
2. Mechanism of Resistance to Osimertinib
2.1. On-Target Resistance Mechanisms
2.2. Off-Target Resistance Mechanisms
3. Diagnosis of Osimertinib Resistance
4. Amivantamab with Chemotherapy as an Approved Treatment Option for Osimertinib-Resistant Patients with EGFR Mutations
5. Clinical Trials with New Drugs Overcoming the Resistance to Osimertinib (Efficacy and Safety)
5.1. Treatment Targeting the Mechanism of Resistance to Osimertinib Driven by MET Amplification and Overexpression or C797S Mutation
5.2. Treatment Independent of the Mechanism of Resistance
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuler, M.; Bölükbas, S.; Darwiche, K.; Theegarten, D.; Herrmann, K.; Stuschke, M. Personalized Treatment for Patients with Lung Cancer. Dtsch. Ärzteblatt Int. 2023, 120, 300. [Google Scholar] [CrossRef] [PubMed]
- Red Brewer, M.; Yun, C.-H.; Lai, D.; Lemmon, M.A.; Eck, M.J.; Pao, W. Mechanism for Activation of Mutated Epidermal Growth Factor Receptors in Lung Cancer. Proc. Natl. Acad. Sci. USA 2013, 110, E3595–E3604. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal Growth Factor Receptor (EGFR) in Lung Cancer: An Overview and Update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Melosky, B.; Kambartel, K.; Häntschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2022, 26, 7–18. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Cai, X.; He, M.; Li, C.; Liang, H.; Cheng, B.; Xia, X.; Guo, M.; Liang, P.; et al. Comparison of First-Generation EGFR TKIs (Gefitinib, Erlotinib, and Icotinib) as Adjuvant Therapy in Resected NSCLC Patients with Sensitive EGFR Mutations. Transl. Lung Cancer Res. 2021, 10, 4120–4129. [Google Scholar] [CrossRef]
- Giordano, P.; Manzo, A.; Montanino, A.; Costanzo, R.; Sandomenico, C.; Piccirillo, M.C.; Daniele, G.; Normanno, N.; Carillio, G.; Rocco, G.; et al. Afatinib: An Overview of Its Clinical Development in Non-Small-Cell Lung Cancer and Other Tumors. Crit. Rev. Oncol. Hematol. 2016, 97, 143–151. [Google Scholar] [CrossRef]
- Andrews Wright, N.M.; Goss, G.D. Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for the Treatment of Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2019, 8, S247–S264. [Google Scholar] [CrossRef]
- Karachaliou, N.; Fernandez-Bruno, M.; Bracht, J.W.P.; Rosell, R. EGFR First- and Second-Generation TKIs—There Is Still Place for Them in EGFR-Mutant NSCLC Patients. Transl. Cancer Res. 2019, 8, S23. [Google Scholar] [CrossRef]
- Floc’h, N.; Lim, S.; Bickerton, S.; Ahmed, A.; Orme, J.; Urosevic, J.; Martin, M.J.; Cross, D.A.E.; Cho, B.C.; Smith, P.D. Osimertinib, an Irreversible Next-Generation EGFR Tyrosine Kinase Inhibitor, Exerts Antitumor Activity in Various Preclinical NSCLC Models Harboring the Uncommon EGFR Mutations G719X or L861Q or S768I. Mol. Cancer Ther. 2020, 19, 2298–2307. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V.A.; Han, J.-Y.; Ahn, M.-J.; Ramalingam, S.S.; Delmonte, A.; Hsia, T.-C.; Laskin, J.; Kim, S.-W.; He, Y.; Tsai, C.-M.; et al. Epidermal Growth Factor Receptor Mutation Analysis in Tissue and Plasma from the AURA3 Trial: Osimertinib versus Platinum-Pemetrexed for T790M Mutation-Positive Advanced Non-Small Cell Lung Cancer. Cancer 2020, 126, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Eide, I.J.Z.; Stensgaard, S.; Helland, Å.; Ekman, S.; Mellemgaard, A.; Hansen, K.H.; Cicenas, S.; Koivunen, J.; Grønberg, B.H.; Sørensen, B.S.; et al. Osimertinib in Non-Small Cell Lung Cancer with Uncommon EGFR-Mutations: A Post-Hoc Subgroup Analysis with Pooled Data from Two Phase II Clinical Trials. Transl. Lung Cancer Res. 2022, 11, 953–963. [Google Scholar] [CrossRef]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Kato, T.; Dong, X.; Ahn, M.-J.; Quang, L.-V.; Soparattanapaisarn, N.; Inoue, T.; Wang, C.-L.; Huang, M.; Yang, J.C.-H.; et al. Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N. Engl. J. Med. 2024, 391, 585–597. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance Mechanisms to Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Gomatou, G.; Syrigos, N.; Kotteas, E. Osimertinib Resistance: Molecular Mechanisms and Emerging Treatment Options. Cancers 2023, 15, 841. [Google Scholar] [CrossRef]
- Volta, F.; La Monica, S.; Leonetti, A.; Gnetti, L.; Bonelli, M.; Cavazzoni, A.; Fumarola, C.; Galetti, M.; Eltayeb, K.; Minari, R.; et al. Intrinsic Resistance to Osimertinib in EGFR Mutated NSCLC Cell Lines Induced by Alteration in Cell-Cycle Regulators. Target. Oncol. 2023, 18, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, E.; De Carlo, E.; Del Conte, A.; Stanzione, B.; Revelant, A.; Fassetta, K.; Spina, M.; Bearz, A. Acquired Resistance to Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer: How Do We Overcome It? Int. J. Mol. Sci. 2022, 23, 6936. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Yu, H.A. The Evolving Landscape of Resistance to Osimertinib. J. Thorac. Oncol. 2020, 15, 18–21. [Google Scholar] [CrossRef]
- Le, X.; Puri, S.; Negrao, M.V.; Nilsson, M.B.; Robichaux, J.; Boyle, T.; Hicks, J.K.; Lovinger, K.L.; Roarty, E.; Rinsurongkawong, W.; et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin. Cancer Res. 2018, 24, 6195–6203. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Passaro, A.; Jänne, P.A.; Mok, T.; Peters, S. Overcoming Therapy Resistance in EGFR-Mutant Lung Cancer. Nat. Cancer 2021, 2, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Hu, Y.; Mileham, K.F.; Husain, H.; Costa, D.B.; Tracy, P.; Feeney, N.; Sholl, L.M.; Dahlberg, S.E.; Redig, A.J.; et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients with EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018, 4, 1527–1534. [Google Scholar] [CrossRef]
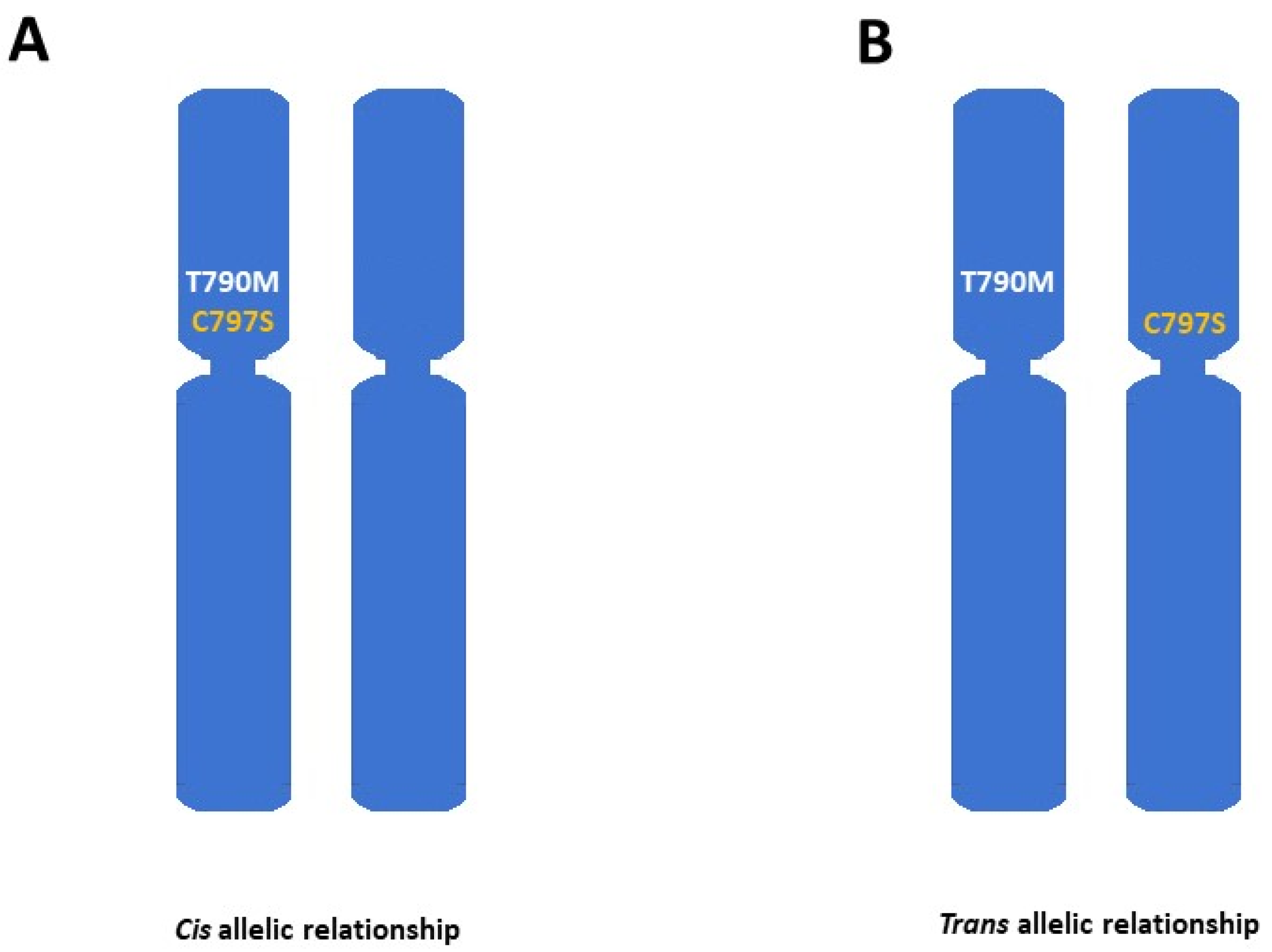
- Niederst, M.J.; Hu, H.; Mulvey, H.E.; Lockerman, E.L.; Garcia, A.R.; Piotrowska, Z.; Sequist, L.V.; Engelman, J.A. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin. Cancer Res. 2015, 21, 3924–3933. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.-J.; Huang, J.; Ye, J.-Y.; Zhang, X.-C.; Tu, H.-Y.; Han-Zhang, H.; Wu, Y.-L. Lung Adenocarcinoma Harboring EGFR T790M and In Trans C797S Responds to Combination Therapy of First- and Third-Generation EGFR TKIs and Shifts Allelic Configuration at Resistance. J. Thorac. Oncol. 2017, 12, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, N.; Ou, Q.; Xiang, Y.; Jiang, T.; Wu, X.; Bao, H.; Tong, X.; Wang, X.; Shao, Y.W.; et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, X.-C.; Yang, J.-J.; Yang, Z.-F.; Bai, Y.; Su, J.; Wang, Z.; Zhang, Z.; Shao, Y.; Zhou, Q.; et al. EGFR L792H and G796R: Two Novel Mutations Mediating Resistance to the Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib. J. Thorac. Oncol. 2018, 13, 1415–1421. [Google Scholar] [CrossRef]
- Ou, S.-H.I.; Cui, J.; Schrock, A.B.; Goldberg, M.E.; Zhu, V.W.; Albacker, L.; Stephens, P.J.; Miller, V.A.; Ali, S.M. Emergence of Novel and Dominant Acquired EGFR Solvent-Front Mutations at Gly796 (G796S/R) Together with C797S/R and L792F/H Mutations in One EGFR (L858R/T790M) NSCLC Patient Who Progressed on Osimertinib. Lung Cancer 2017, 108, 228–231. [Google Scholar] [CrossRef]
- Ercan, D.; Choi, H.G.; Yun, C.-H.; Capelletti, M.; Xie, T.; Eck, M.J.; Gray, N.S.; Jänne, P.A. EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin. Cancer Res. 2015, 21, 3913–3923. [Google Scholar] [CrossRef]
- Bersanelli, M.; Minari, R.; Bordi, P.; Gnetti, L.; Bozzetti, C.; Squadrilli, A.; Lagrasta, C.A.M.; Bottarelli, L.; Osipova, G.; Capelletto, E.; et al. L718Q Mutation as New Mechanism of Acquired Resistance to AZD9291 in EGFR-Mutated NSCLC. J. Thorac. Oncol. 2016, 11, e121–e123. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.V.; Uhrin, P.; Sixt, M.; et al. Lymph Node Blood Vessels Provide Exit Routes for Metastatic Tumor Cell Dissemination in Mice. Science 2018, 359, 1408–1411. [Google Scholar] [CrossRef]
- Li, Y.; Mao, T.; Wang, J.; Zheng, H.; Hu, Z.; Cao, P.; Yang, S.; Zhu, L.; Guo, S.; Zhao, X.; et al. Toward the next Generation EGFR Inhibitors: An Overview of Osimertinib Resistance Mediated by EGFR Mutations in Non-Small Cell Lung Cancer. Cell Commun. Signal 2023, 21, 71. [Google Scholar] [CrossRef]
- Knebel, F.H.; Bettoni, F.; Shimada, A.K.; Cruz, M.; Alessi, J.V.; Negrão, M.V.; Reis, L.F.L.; Katz, A.; Camargo, A.A. Sequential Liquid Biopsies Reveal Dynamic Alterations of EGFR Driver Mutations and Indicate EGFR Amplification as a New Mechanism of Resistance to Osimertinib in NSCLC. Lung Cancer 2017, 108, 238–241. [Google Scholar] [CrossRef]
- Nukaga, S.; Yasuda, H.; Tsuchihara, K.; Hamamoto, J.; Masuzawa, K.; Kawada, I.; Naoki, K.; Matsumoto, S.; Mimaki, S.; Ikemura, S.; et al. Amplification of EGFR Wild-Type Alleles in Non-Small Cell Lung Cancer Cells Confers Acquired Resistance to Mutation-Selective EGFR Tyrosine Kinase Inhibitors. Cancer Res. 2017, 77, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Roper, N.; Brown, A.-L.; Wei, J.S.; Pack, S.; Trindade, C.; Kim, C.; Restifo, O.; Gao, S.; Sindiri, S.; Mehrabadi, F.; et al. Clonal Evolution and Heterogeneity of Osimertinib Acquired Resistance Mechanisms in EGFR Mutant Lung Cancer. Cell Rep. Med. 2020, 1, 100007. [Google Scholar] [CrossRef]
- Chabon, J.J.; Simmons, A.D.; Lovejoy, A.F.; Esfahani, M.S.; Newman, A.M.; Haringsma, H.J.; Kurtz, D.M.; Stehr, H.; Scherer, F.; Karlovich, C.A.; et al. Circulating Tumour DNA Profiling Reveals Heterogeneity of EGFR Inhibitor Resistance Mechanisms in Lung Cancer Patients. Nat. Commun. 2016, 7, 11815. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Oh, Y.-T.; Zhang, G.; Yao, W.; Yue, P.; Li, Y.; Kanteti, R.; Riehm, J.; Salgia, R.; Owonikoko, T.K.; et al. Met Gene Amplification and Protein Hyperactivation Is a Mechanism of Resistance to Both First and Third Generation EGFR Inhibitors in Lung Cancer Treatment. Cancer Lett. 2016, 380, 494–504. [Google Scholar] [CrossRef]
- Suzawa, K.; Offin, M.; Schoenfeld, A.J.; Plodkowski, A.J.; Odintsov, I.; Lu, D.; Lockwood, W.W.; Arcila, M.E.; Rudin, C.M.; Drilon, A.; et al. Acquired MET Exon 14 Alteration Drives Secondary Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in EGFR-Mutated Lung Cancer. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, K.; Pirazzoli, V.; Arcila, M.E.; Nebhan, C.A.; Song, X.; de Stanchina, E.; Ohashi, K.; Janjigian, Y.Y.; Spitzler, P.J.; Melnick, M.A.; et al. HER2 Amplification: A Potential Mechanism of Acquired Resistance to EGFR Inhibition in EGFR-Mutant Lung Cancers That Lack the Second-Site EGFRT790M Mutation. Cancer Discov. 2012, 2, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Liao, B.-C.; Liao, W.-Y.; Markovets, A.; Stetson, D.; Thress, K.; Yang, J.C.-H. Exon 16-Skipping HER2 as a Novel Mechanism of Osimertinib Resistance in EGFR L858R/T790M-Positive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2020, 15, 50–61. [Google Scholar] [CrossRef]
- Hong, M.H.; Kim, M.H.; Kim, S.-Y.; Heo, S.G.; Kang, H.-N.; Park, C.-W.; Barrett, J.C.; Stetson, D.; Chmielecki, J.; Markovets, A.; et al. Molecular Landscape of Osimertinib Resistance Revealed by Targeted Panel Sequencing and Patient-Derived Cancer Models in Non-Small Cell Lung Cancer Patients. Ann. Oncol. 2018, 29, viii516. [Google Scholar] [CrossRef]
- Xu, C.; Wang, W.; Zhu, Y.; Yu, Z.; Zhang, H.; Wang, H.; Zhang, J.; Zhuang, W.; Lv, T.; Song, Y. Potential Resistance Mechanisms Using next Generation Sequencing from Chinese EGFR T790M+ Non-Small Cell Lung Cancer Patients with Primary Resistance to Osimertinib: A Multicenter Study. Ann. Oncol. 2019, 30, ii48. [Google Scholar] [CrossRef]
- Eng, J.; Woo, K.M.; Sima, C.S.; Plodkowski, A.; Hellmann, M.D.; Chaft, J.E.; Kris, M.G.; Arcila, M.E.; Ladanyi, M.; Drilon, A. Impact of Concurrent PIK3CA Mutations on Response to EGFR Tyrosine Kinase Inhibition in EGFR-Mutant Lung Cancers and on Prognosis in Oncogene-Driven Lung Adenocarcinomas. J. Thorac. Oncol. 2015, 10, 1713–1719. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.S.; Lee, B.; Kim, H.K.; Sun, J.-M.; Ahn, J.S.; Ahn, M.-J.; Park, K.; Lee, S.-H. Genomic Landscape of Acquired Resistance to Third-Generation EGFR Tyrosine Kinase Inhibitors in EGFR T790M-Mutant Non-Small Cell Lung Cancer. Cancer 2020, 126, 2704–2712. [Google Scholar] [CrossRef]
- Park, S.; Shim, J.H.; Lee, B.; Cho, I.; Park, W.-Y.; Kim, Y.; Lee, S.-H.; Choi, Y.L.; Han, J.; Ahn, J.S.; et al. Paired Genomic Analysis of Squamous Cell Carcinoma Transformed from EGFR-Mutated Lung Adenocarcinoma. Lung Cancer 2019, 134, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Minari, R.; Bordi, P.; La Monica, S.; Squadrilli, A.; Leonetti, A.; Bottarelli, L.; Azzoni, C.; Lagrasta, C.A.M.; Gnetti, L.; Campanini, N.; et al. Concurrent Acquired BRAF V600E Mutation and MET Amplification as Resistance Mechanism of First-Line Osimertinib Treatment in a Patient with EGFR-Mutated NSCLC. J. Thorac. Oncol. 2018, 13, e89–e91. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-C.; Liao, W.-Y.; Lin, C.-A.; Shih, J.-Y.; Yu, C.-J.; Yang, J.C.-H. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Osimertinib. J. Thorac. Oncol. 2017, 12, 567–572. [Google Scholar] [CrossRef]
- Offin, M.; Somwar, R.; Rekhtman, N.; Benayed, R.; Chang, J.C.; Plodkowski, A.; Lui, A.J.W.; Eng, J.; Rosenblum, M.; Li, B.T.; et al. Acquired ALK and RET Gene Fusions as Mechanisms of Resistance to Osimertinib in EGFR-Mutant Lung Cancers. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Chan, J.M.; Kubota, D.; Sato, H.; Rizvi, H.; Daneshbod, Y.; Chang, J.C.; Paik, P.K.; Offin, M.; Arcila, M.E.; et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-Line Osimertinib in EGFR-Mutant Lung Cancer. Clin. Cancer Res. 2020, 26, 2654–2663. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Li, C.; Wang, Z.; Zhang, P.; Yan, X. Transformation to Small-Cell Carcinoma as an Acquired Resistance Mechanism to AZD9291: A Case Report. Oncotarget 2017, 8, 18609–18614. [Google Scholar] [CrossRef]
- Lee, J.-K.; Lee, J.; Kim, S.; Kim, S.; Youk, J.; Park, S.; An, Y.; Keam, B.; Kim, D.-W.; Heo, D.S.; et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J. Clin. Oncol. 2017, 35, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Niederst, M.J.; Sequist, L.V.; Poirier, J.T.; Mermel, C.H.; Lockerman, E.L.; Garcia, A.R.; Katayama, R.; Costa, C.; Ross, K.N.; Moran, T.; et al. RB Loss in Resistant EGFR Mutant Lung Adenocarcinomas That Transform to Small-Cell Lung Cancer. Nat. Commun. 2015, 6, 6377. [Google Scholar] [CrossRef]
- Offin, M.; Chan, J.M.; Tenet, M.; Rizvi, H.A.; Shen, R.; Riely, G.J.; Rekhtman, N.; Daneshbod, Y.; Quintanal-Villalonga, A.; Penson, A.; et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at Risk for Histologic Transformation and Inferior Clinical Outcomes. J. Thorac. Oncol. 2019, 14, 1784–1793. [Google Scholar] [CrossRef]
- Hakozaki, T.; Kitazono, M.; Takamori, M.; Kiriu, T. Combined Small and Squamous Transformation in EGFR-Mutated Lung Adenocarcinoma. Intern. Med. 2020, 59, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-H.; Chen, L.-Y.; Lin, Y.-C.; Shih, J.-Y.; Lin, Y.-C.; Tseng, R.-Y.; Chiu, A.-C.; Yeh, Y.-H.; Liu, C.; Lin, Y.-T.; et al. Epithelial-Mesenchymal Transition (EMT) beyond EGFR Mutations per Se Is a Common Mechanism for Acquired Resistance to EGFR TKI. Oncogene 2019, 38, 455–468. [Google Scholar] [CrossRef]
- Yochum, Z.A.; Cades, J.; Wang, H.; Chatterjee, S.; Simons, B.W.; O’Brien, J.P.; Khetarpal, S.K.; Lemtiri-Chlieh, G.; Myers, K.V.; Huang, E.H.-B.; et al. Targeting the EMT Transcription Factor TWIST1 Overcomes Resistance to EGFR Inhibitors in EGFR-Mutant Non-Small-Cell Lung Cancer. Oncogene 2019, 38, 656–670. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S.; Goldberg, R.M.; Lenz, H.-J.; Shields, A.F.; Gibney, G.T.; Tan, A.R.; Brown, J.; Eisenberg, B.; Heath, E.I.; Phuphanich, S.; et al. The Current State of Molecular Testing in the Treatment of Patients with Solid Tumors, 2019. CA Cancer J. Clin. 2019, 69, 305–343. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.N.; Peneva, D.; Cuyun Carter, G.; Palomares, M.R.; Thakkar, S.; Hall, D.W.; Dalglish, H.; Campos, C.; Yermilov, I. Comprehensive Review on the Clinical Impact of Next-Generation Sequencing Tests for the Management of Advanced Cancer. JCO Precis. Oncol. 2023, 7, e2200715. [Google Scholar] [CrossRef] [PubMed]
- Palacín-Aliana, I.; García-Romero, N.; Asensi-Puig, A.; Carrión-Navarro, J.; González-Rumayor, V.; Ayuso-Sacido, Á. Clinical Utility of Liquid Biopsy-Based Actionable Mutations Detected via ddPCR. Biomedicines 2021, 9, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Bożyk, A.; Nicoś, M. The Overview of Perspectives of Clinical Application of Liquid Biopsy in Non-Small-Cell Lung Cancer. Life 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, L.; Dal Maso, A.; Pavan, A.; Zulato, E.; Calvetti, L.; Pasello, G.; Guarneri, V.; Conte, P.; Indraccolo, S. Liquid Biopsy and Non-Small Cell Lung Cancer: Are We Looking at the Tip of the Iceberg? Br. J. Cancer 2022, 127, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Tuzi, A.; Bolzacchini, E.; Suter, M.B.; Giaquinto, A.; Passaro, A.; Gobba, S.; Vallini, I.; Pinotti, G. Biopsy and Re-Biopsy in Lung Cancer: The Oncologist Requests and the Role of Endobronchial Ultrasounds Transbronchial Needle Aspiration. J. Thorac. Dis. 2017, 9, S405–S409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ariyasu, R.; Uchibori, K.; Sasaki, T.; Tsukahara, M.; Kiyotani, K.; Yoshida, R.; Ono, Y.; Kitazono, S.; Ninomiya, H.; Ishikawa, Y.; et al. Monitoring Epidermal Growth Factor Receptor C797S Mutation in Japanese Non-Small Cell Lung Cancer Patients with Serial Cell-Free DNA Evaluation Using Digital Droplet PCR. Cancer Sci. 2021, 112, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.; Sousa, A.C.; Janeiro, A.; Malveiro, S.; Teixeira, E.; Brysch, E.; Pantarotto, M.; Felizardo, M.; Madureira, R.; Nogueira, F.; et al. Detection and Quantification of EGFR T790M Mutation in Liquid Biopsies by Droplet Digital PCR. Transl. Lung Cancer Res. 2021, 10, 1200–1208. [Google Scholar] [CrossRef]
- Watanabe, K.; Saito, R.; Miyauchi, E.; Nagashima, H.; Nakamura, A.; Sugawara, S.; Tanaka, N.; Terasaki, H.; Fukuhara, T.; Maemondo, M. Monitoring of Plasma EGFR Mutations during Osimertinib Treatment for NSCLC Patients with Acquired T790M Mutation. Cancers 2023, 15, 4231. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhan, C.; Tong, Z.; Yin, H.; Hui, J.; Qiu, S.; Li, Q.; Xu, X.; Ma, H.; Wu, Z.; et al. Detecting EGFR Gene Amplification Using a Fluorescence in Situ Hybridization Platform Based on Digital Microfluidics. Talanta 2024, 269, 125444. [Google Scholar] [CrossRef] [PubMed]
- Press, M.F.; Sauter, G.; Buyse, M.; Fourmanoir, H.; Quinaux, E.; Tsao-Wei, D.D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Crown, J.; et al. HER2 Gene Amplification Testing by Fluorescent In Situ Hybridization (FISH): Comparison of the ASCO-College of American Pathologists Guidelines With FISH Scores Used for Enrollment in Breast Cancer International Research Group Clinical Trials. J. Clin. Oncol. 2016, 34, 3518–3528. [Google Scholar] [CrossRef]
- Guo, R.; Luo, J.; Chang, J.; Rekhtman, N.; Arcila, M.; Drilon, A. MET-Dependent Solid Tumours—Molecular Diagnosis and Targeted Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Noonan, S.A.; Berry, L.; Lu, X.; Gao, D.; Barón, A.E.; Chesnut, P.; Sheren, J.; Aisner, D.L.; Merrick, D.; Doebele, R.C.; et al. Identifying the Appropriate FISH Criteria for Defining MET Copy Number-Driven Lung Adenocarcinoma through Oncogene Overlap Analysis. J. Thorac. Oncol. 2016, 11, 1293–1304. [Google Scholar] [CrossRef]
- Yu, H.A.; Kerr, K.; Rolfo, C.; Fang, J.; Finocchiaro, G.; Wong, K.H.; Veillon, R.; Kato, T.; Yang, C.-H.; Nadal, E.; et al. Detection of MET Amplification (METamp) in Patients with EGFR Mutant (m) NSCLC after First-Line (1L) Osimertinib. J. Clin. Oncol. 2023, 41, 9074. [Google Scholar] [CrossRef]
- Remon, J.; Hendriks, L.E.L.; Mountzios, G.; García-Campelo, R.; Saw, S.P.L.; Uprety, D.; Recondo, G.; Villacampa, G.; Reck, M. MET Alterations in NSCLC-Current Perspectives and Future Challenges. J. Thorac. Oncol. 2023, 18, 419–435. [Google Scholar] [CrossRef]
- Hartmaier, R.J.; Markovets, A.A.; Ahn, M.J.; Sequist, L.V.; Han, J.-Y.; Cho, B.C.; Yu, H.A.; Kim, S.-W.; Yang, J.C.-H.; Lee, J.-S.; et al. Osimertinib + Savolitinib to Overcome Acquired MET-Mediated Resistance in Epidermal Growth Factor Receptor-Mutated, MET-Amplified Non-Small Cell Lung Cancer: TATTON. Cancer Discov. 2023, 13, 98–113. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Marinis, F.D.; Bonanno, L.; Cho, B.C.; Kim, T.-M.; Cheng, S.; Novello, S.; Proto, C.; Kim, S.-W.; Lee, J.S.; et al. EP08.02-140 MET Biomarker-Based Preliminary Efficacy Analysis in SAVANNAH: Savolitinib + osimertinib in EGFRm NSCLC Post-Osimertinib. J. Thorac. Oncol. 2022, 17, S469–S470. [Google Scholar] [CrossRef]
- Spagnolo, C.C.; Ciappina, G.; Giovannetti, E.; Squeri, A.; Granata, B.; Lazzari, C.; Pretelli, G.; Pasello, G.; Santarpia, M. Targeting MET in Non-Small Cell Lung Cancer (NSCLC): A New Old Story? Int. J. Mol. Sci. 2023, 24, 10119. [Google Scholar] [CrossRef]
- Chmielecki, J.; Mok, T.; Wu, Y.-L.; Han, J.-Y.; Ahn, M.-J.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.-H.; Shepherd, F.A.; et al. Analysis of Acquired Resistance Mechanisms to Osimertinib in Patients with EGFR-Mutated Advanced Non-Small Cell Lung Cancer from the AURA3 Trial. Nat. Commun. 2023, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Chmielecki, J.; Gray, J.E.; Cheng, Y.; Ohe, Y.; Imamura, F.; Cho, B.C.; Lin, M.-C.; Majem, M.; Shah, R.; Rukazenkov, Y.; et al. Candidate Mechanisms of Acquired Resistance to First-Line Osimertinib in EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Nat. Commun. 2023, 14, 1070. [Google Scholar] [CrossRef] [PubMed]
- Lanman, R.B.; Mortimer, S.A.; Zill, O.A.; Sebisanovic, D.; Lopez, R.; Blau, S.; Collisson, E.A.; Divers, S.G.; Hoon, D.S.B.; Kopetz, E.S.; et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PLoS ONE 2015, 10, e0140712. [Google Scholar] [CrossRef]
- Ferro, A.; Marinato, G.M.; Mulargiu, C.; Marino, M.; Pasello, G.; Guarneri, V.; Bonanno, L. The Study of Primary and Acquired Resistance to First-Line Osimertinib to Improve the Outcome of EGFR-Mutated Advanced Non-Small Cell Lung Cancer Patients: The Challenge Is Open for New Therapeutic Strategies. Crit. Rev. Oncol. Hematol. 2024, 196, 104295. [Google Scholar] [CrossRef]
- Dietel, M.; Bubendorf, L.; Dingemans, A.-M.C.; Dooms, C.; Elmberger, G.; García, R.C.; Kerr, K.M.; Lim, E.; López-Ríos, F.; Thunnissen, E.; et al. Diagnostic Procedures for Non-Small-Cell Lung Cancer (NSCLC): Recommendations of the European Expert Group. Thorax 2016, 71, 177–184. [Google Scholar] [CrossRef]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.-H.; Melosky, B.; Shih, J.-Y.; Wang, J.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; et al. Amivantamab plus Chemotherapy with and without Lazertinib in EGFR-Mutant Advanced NSCLC after Disease Progression on Osimertinib: Primary Results from the Phase III MARIPOSA-2 Study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Cho, B.C.; Wang, Y.; Melosky, B.; Califano, R.; Lee, S.-H.; Girard, N.; Reckamp, K.L.; Takahashi, T.; Felip, E.; et al. LBA15 Amivantamab plus Chemotherapy (with or without Lazertinib) vs Chemotherapy in EGFR-Mutated Advanced NSCLC after Progression on Osimertinib: MARIPOSA-2, a Phase III, Global, Randomized, Controlled Trial. Ann. Oncol. 2023, 34, S1307. [Google Scholar] [CrossRef]
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; AlShahrani, S.; Bashir, S.; Bukhari, N. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep. Oncol. 2019, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Reckamp, K.L.; Califano, R.; Lee, S.-H.; Melosky, B.; Wang, J.; Wang, Y.; Campelo, M.R.G.; Felip, E.; Girard, N.; et al. LBA54 Amivantamab plus Chemotherapy vs Chemotherapy in EGFR-Mutated, Advanced Non-Small Cell Lung Cancer after Disease Progression on Osimertinib: Second Interim Overall Survival from MARIPOSA-2. Ann. Oncol. 2024, 35, S1244–S1245. [Google Scholar] [CrossRef]
- Gentzler, R.D.; Spira, A.; Melosky, B.; Owen, S.; Burns, T.; Massarelli, E.; Nagasaka, M.; Fang, B.; Sanborn, R.E.; Arrieta Rodriguez, O.G.; et al. Amivantamab plus Chemotherapy vs Chemotherapy in EGFR-Mutant Advanced NSCLC after Progression on Osimertinib: A Post-Progression Analysis of MARIPOSA-2. ESMO Open 2024, 9, 102582. [Google Scholar] [CrossRef]
- Lopes, G.; Spira, A.I.; Han, J.-Y.; Shih, J.-Y.; Mascaux, C.; Roy, U.B.; Zugazagoitia, J.; Kim, Y.J.; Chiu, C.-H.; Kim, S.-W.; et al. MA12.08 Preventing Infusion-Related Reactions with Intravenous Amivantamab: Primary Results from SKIPPirr, a Phase 2 Study. J. Thorac. Oncol. 2024, 19, S104–S105. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Spira, A.I.; Han, J.-Y.; Shih, J.-Y.; Mascaux, C.; Roy, U.B.; Zugazagoitia, J.; Kim, Y.J.; Chiu, C.H.; Kim, S.-W.; et al. 1269P Preventing Infusion-Related Reactions with Intravenous Amivantamab: Updated Results from SKIPPirr, a Phase II Study. Ann. Oncol. 2024, 35, S812. [Google Scholar] [CrossRef]
- Leighl, N.B.; Akamatsu, H.; Lim, S.M.; Cheng, Y.; Minchom, A.R.; Marmarelis, M.E.; Sanborn, R.E.; Chih-Hsin Yang, J.; Liu, B.; John, T.; et al. Subcutaneous Versus Intravenous Amivantamab, Both in Combination with Lazertinib, in Refractory Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer: Primary Results From the Phase III PALOMA-3 Study. J. Clin. Oncol. 2024, 42, 3593–3605. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Ramalingam, S.S.; Ahn, M.-J.; Kim, S.-W.; Yu, H.A.; Saka, H.; Horn, L.; Goto, K.; Ohe, Y.; Cantarini, M.; et al. Preliminary Results of TATTON, a Multi-Arm Phase Ib Trial of AZD9291 Combined with MEDI4736, AZD6094 or Selumetinib in EGFR-Mutant Lung Cancer. J. Clin. Oncol. 2015, 33, 2509. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Cantarini, M.; Frewer, P.; Hawkins, G.; Peters, J.; Howarth, P.; Ahmed, G.F.; Sahota, T.; Hartmaier, R.; Li-Sucholeiki, X.; et al. SAVANNAH: A Phase II Trial of Osimertinib plus Savolitinib for Patients (Pts) with EGFR-Mutant, MET-Driven (MET+), Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC), Following Disease Progression on Osimertinib. J. Clin. Oncol. 2019, 37, TPS9119. [Google Scholar] [CrossRef]
- Kowalski, D.M.; Zaborowska-Szmit, M.; Szmit, S.; Jaśkiewicz, P.; Krzakowski, M. The Combination of Osimertinib and Savolitinib as Molecular Inhibition of EGFR and MET Receptors May Be Selected to Provide Maximum Effectiveness and Acceptable Toxicity. Transl. Lung Cancer Res. 2024, 13, 1426–1431. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 13 February 2022).
- Zhou, Q.; Li, J.; Wang, J.; Yang, L.; Fang, J.; Dong, X.; Yi, T.; Min, X.; Xu, F.; Chen, J.; et al. EP08.02-063 SANOVO: A Phase 3 Study of Savolitinib or Placebo in Combination with Osimertinib in Patients with EGFR-Mutant and MET Overexpressed NSCLC. J. Thorac. Oncol. 2022, 17, S429. [Google Scholar] [CrossRef]
- Liam, C.K.; Ahmad, A.R.; Hsia, T.-C.; Zhou, J.; Kim, D.-W.; Soo, R.A.; Cheng, Y.; Lu, S.; Shin, S.W.; Yang, J.C.-H.; et al. Randomized Trial of Tepotinib Plus Gefitinib versus Chemotherapy in EGFR-Mutant NSCLC with EGFR Inhibitor Resistance Due to MET Amplification: INSIGHT Final Analysis. Clin. Cancer Res. 2023, 29, 1879–1886. [Google Scholar] [CrossRef]
- Smit, E.F.; Dooms, C.; Raskin, J.; Nadal, E.; Tho, L.M.; Le, X.; Mazieres, J.; Hin, H.S.; Morise, M.; Zhu, V.W.; et al. INSIGHT 2: A Phase II Study of Tepotinib plus Osimertinib in MET-Amplified NSCLC and First-Line Osimertinib Resistance. Future Oncol. 2022, 18, 1039–1054. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Guarneri, V.; Voon, P.J.; Lim, B.K.; Yang, J.-J.; Wislez, M.; Huang, C.; Liam, C.K.; Mazieres, J.; Tho, L.M.; et al. Tepotinib plus Osimertinib in Patients with EGFR-Mutated Non-Small-Cell Lung Cancer with MET Amplification Following Progression on First-Line Osimertinib (INSIGHT 2): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2024, 25, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Elamin, Y.Y.; Nagasaka, M.; Shum, E.; Bazhenova, L.; Camidge, D.R.; Cho, B.C.; Felip, E.; Goto, K.; Lin, C.-C.; Piotrowska, Z.; et al. BLU-945 Monotherapy and in Combination with Osimertinib (OSI) in Previously Treated Patients with Advanced EGFR-Mutant (EGFRm) NSCLC in the Phase 1/2 SYMPHONY Study. J. Clin. Oncol. 2023, 41, 9011. [Google Scholar] [CrossRef]
- Mok, T.; Nakagawa, K.; Park, K.; Ohe, Y.; Girard, N.; Kim, H.R.; Wu, Y.-L.; Gainor, J.; Lee, S.-H.; Chiu, C.-H.; et al. Nivolumab Plus Chemotherapy in Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small-Cell Lung Cancer After Disease Progression on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: Final Results of CheckMate 722. J. Clin. Oncol. 2024, 42, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Lee, D.H.; Lee, J.-S.; Fan, Y.; de Marinis, F.; Iwama, E.; Inoue, T.; Rodríguez-Cid, J.; Zhang, L.; Yang, C.-T.; et al. Phase III KEYNOTE-789 Study of Pemetrexed and Platinum with or Without Pembrolizumab for Tyrosine Kinase Inhibitor–Resistant, EGFR–Mutant, Metastatic Nonsquamous Non–Small Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 4029–4039. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, L.; Jian, H.; Cheng, Y.; Wang, Q.; Fang, J.; Wang, Z.; Hu, Y.; Han, L.; Sun, M.; et al. Sintilimab plus Chemotherapy for Patients with EGFR-Mutated Non-Squamous Non-Small-Cell Lung Cancer with Disease Progression after EGFR Tyrosine-Kinase Inhibitor Therapy (ORIENT-31): Second Interim Analysis from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2023, 11, 624–636. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ahn, M. -j; Lisberg, A.E.; Kitazono, S.; Cho, B.C.; Blumenschein, G.R.; Shum, E.; Pons-Tostivint, E.; Goto, Y.; Yoh, K.; et al. 1314MO TROPION-Lung05: Datopotamab Deruxtecan (Dato-DXd) in Previously Treated Non-Small Cell Lung Cancer (NSCLC) with Actionable Genomic Alterations (AGAs). Ann. Oncol. 2023, 34, S755–S851. [Google Scholar]
- Yu, H.A.; Goto, Y.; Hayashi, H.; Felip, E.; Chih-Hsin Yang, J.; Reck, M.; Yoh, K.; Lee, S.-H.; Paz-Ares, L.; Besse, B.; et al. HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor-Mutated Non-Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J. Clin. Oncol. 2023, 41, 5363–5375. [Google Scholar] [CrossRef]
- Mok, T.; Jänne, P.A.; Nishio, M.; Novello, S.; Reck, M.; Steuer, C.; Wu, Y.-L.; Fougeray, R.; Fan, P.-D.; Meng, J.; et al. HERTHENA-Lung02: Phase III Study of Patritumab Deruxtecan in Advanced EGFR-Mutated NSCLC after a Third-Generation EGFR TKI. Future Oncol. 2024, 20, 969–980. [Google Scholar] [CrossRef] [PubMed]
| Amivanatamab + Chemotherapy | Amivanatamb + Lazertinib + Chemotherapy | Chemotherapy | |
|---|---|---|---|
| mPFS | 6.3 months | 8.3 months | 4.2 months |
| 1-year PFS | 22% | 37% | 13% |
| ORR | 64% | 63% | 36% |
| DCR | 87% | 87% | 68.4% |
| mDoR | 6.9 months | 9.4 months | 5.6 months |
| IC mPFS | 12.5 months | 12.8 months | 8.3 months |
| 1-year IC PFS | 50% | 54% | 34% |
| mTTSP | 16.0 months | No data available | 11.8 months |
| mTTTD | 10.4 months | No data available | 4.5 months |
| mTTST | 12.2 months | No data available | 6.6 months |
| mPFS2 | 16.0 months | No data available | 11.6 months |
| mOS | 17.7 months | No data available | 15.3 months |
| 18-months OS | 50% | No data available | 14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoś, M.; Sroka-Bartnicka, A.; Kalinka, E.; Krawczyk, P. Possibilities of Overcoming Resistance to Osimertinib in NSCLC Patients with Mutations in the EGFR Gene. Cancers 2025, 17, 563. https://doi.org/10.3390/cancers17040563
Nicoś M, Sroka-Bartnicka A, Kalinka E, Krawczyk P. Possibilities of Overcoming Resistance to Osimertinib in NSCLC Patients with Mutations in the EGFR Gene. Cancers. 2025; 17(4):563. https://doi.org/10.3390/cancers17040563
Chicago/Turabian StyleNicoś, Marcin, Anna Sroka-Bartnicka, Ewa Kalinka, and Paweł Krawczyk. 2025. "Possibilities of Overcoming Resistance to Osimertinib in NSCLC Patients with Mutations in the EGFR Gene" Cancers 17, no. 4: 563. https://doi.org/10.3390/cancers17040563
APA StyleNicoś, M., Sroka-Bartnicka, A., Kalinka, E., & Krawczyk, P. (2025). Possibilities of Overcoming Resistance to Osimertinib in NSCLC Patients with Mutations in the EGFR Gene. Cancers, 17(4), 563. https://doi.org/10.3390/cancers17040563






