Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer
Simple Summary
Abstract
1. Introduction
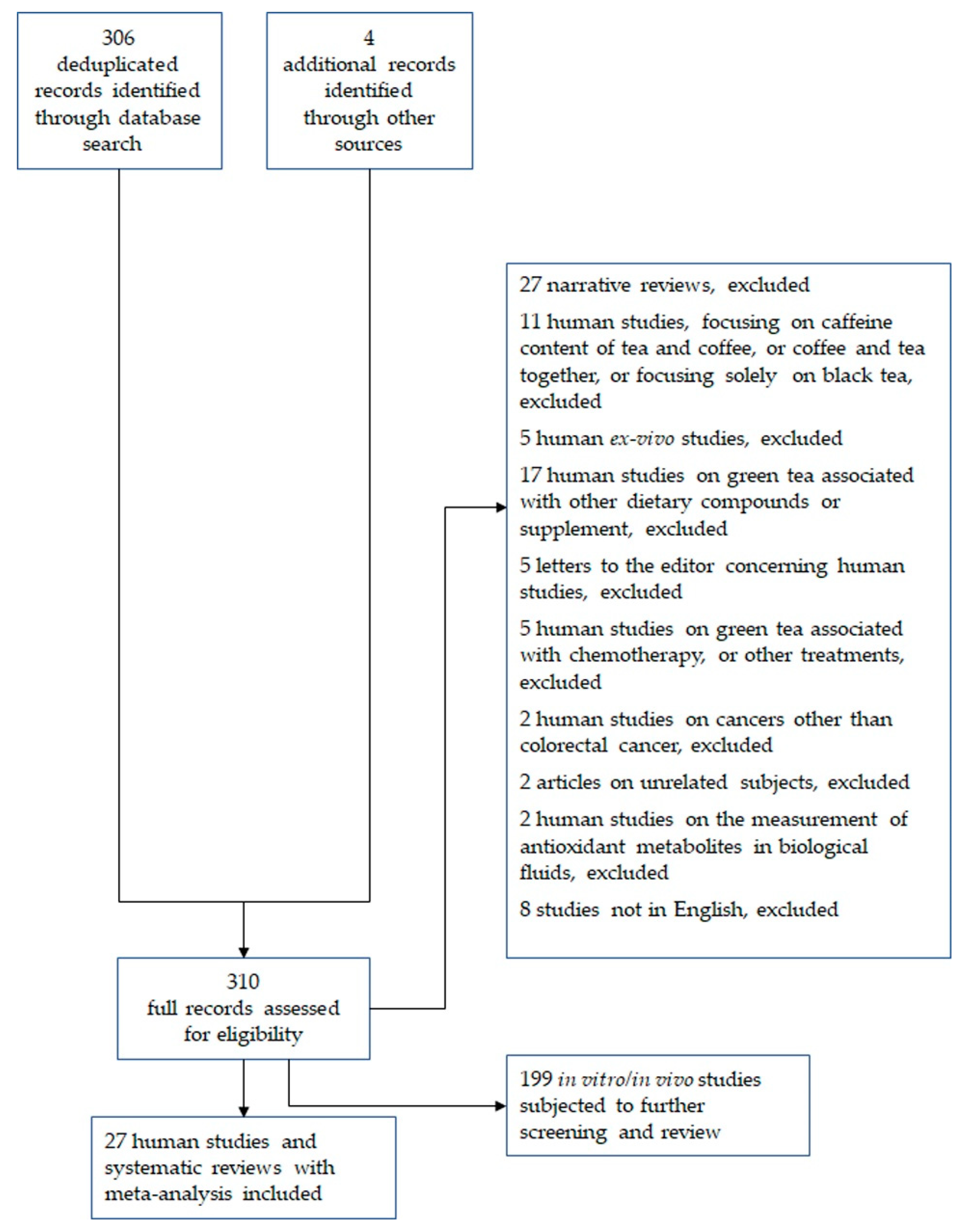
2. Methods
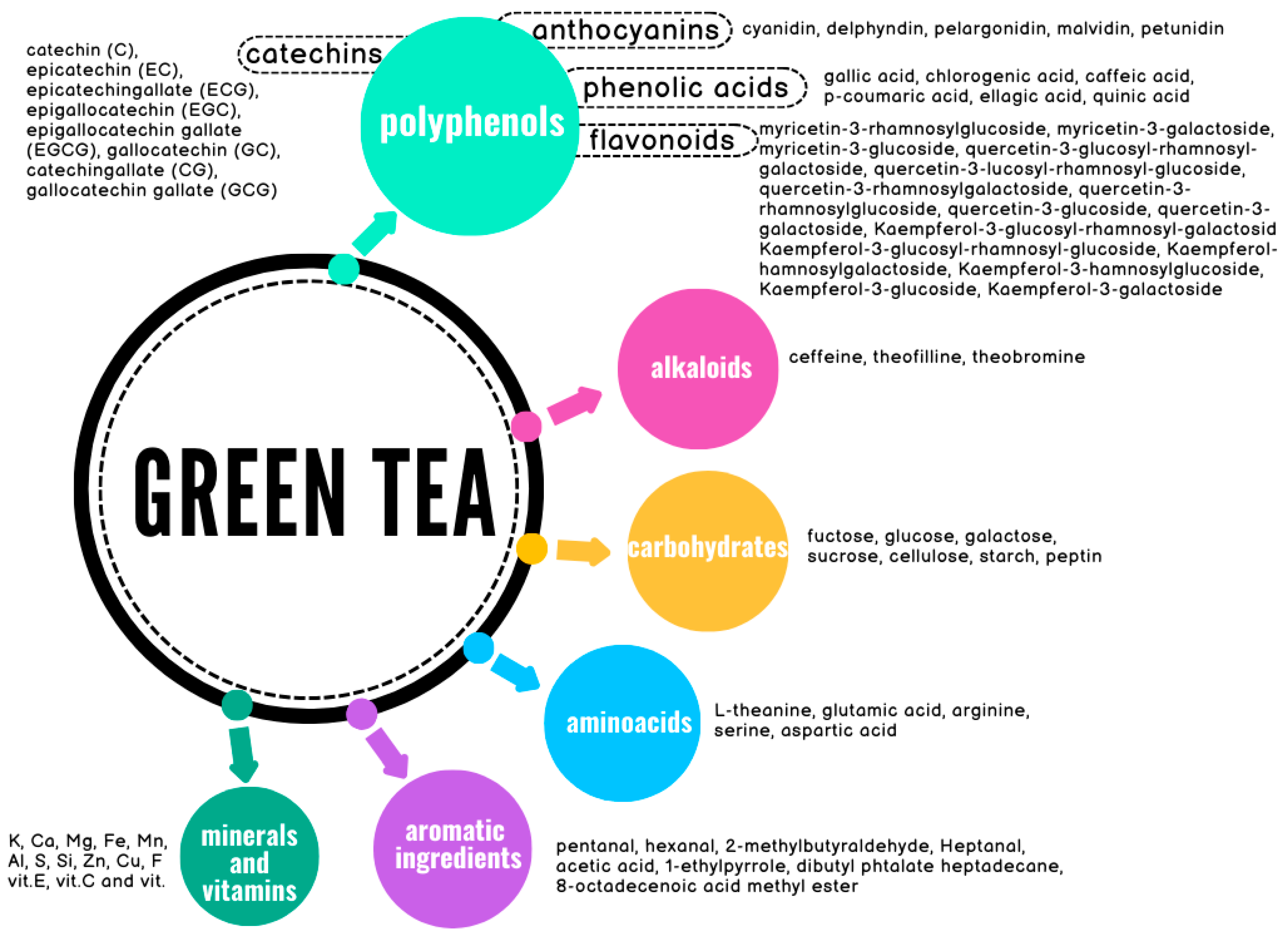
3. Green Tea Constituents
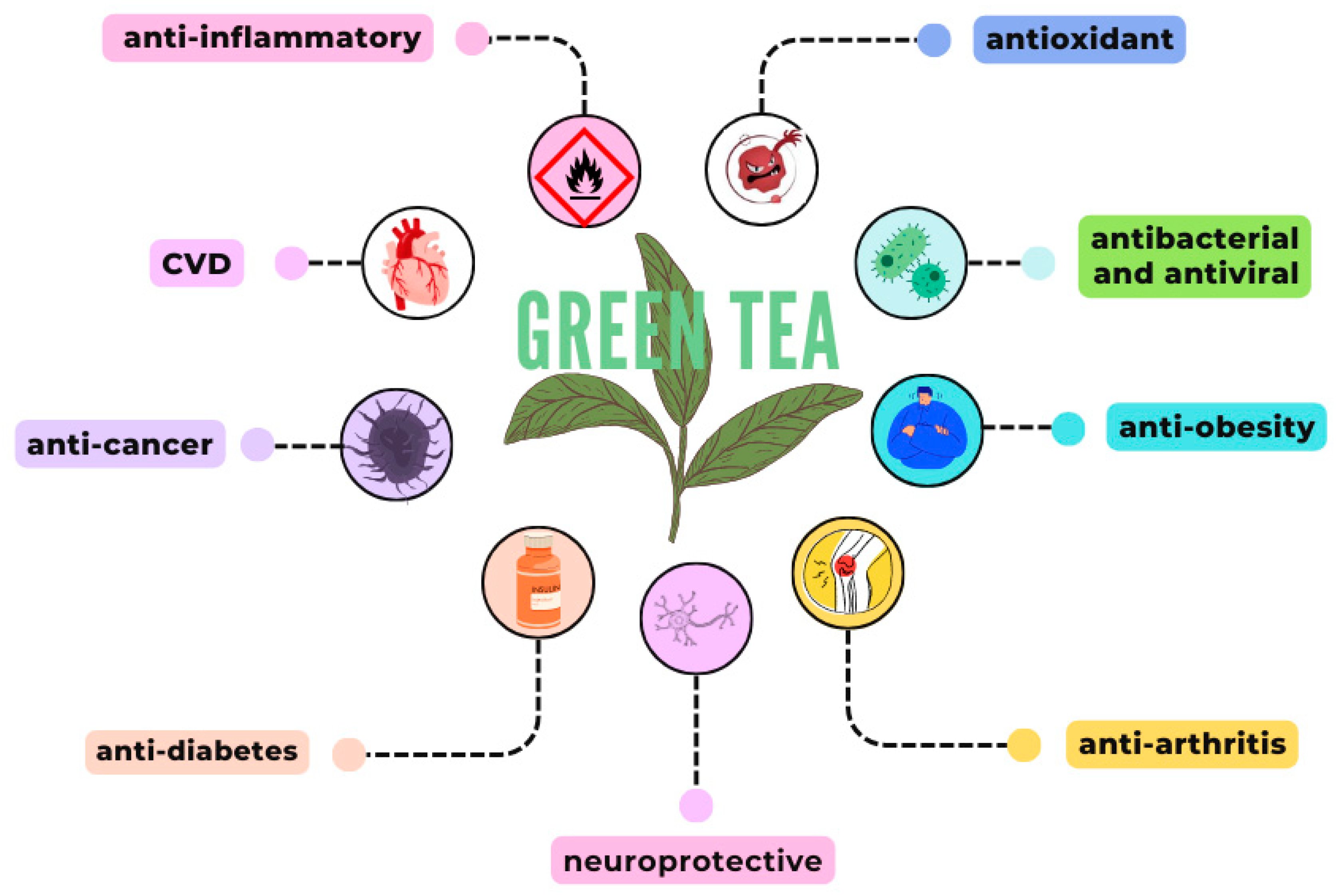
4. Green Tea Activities
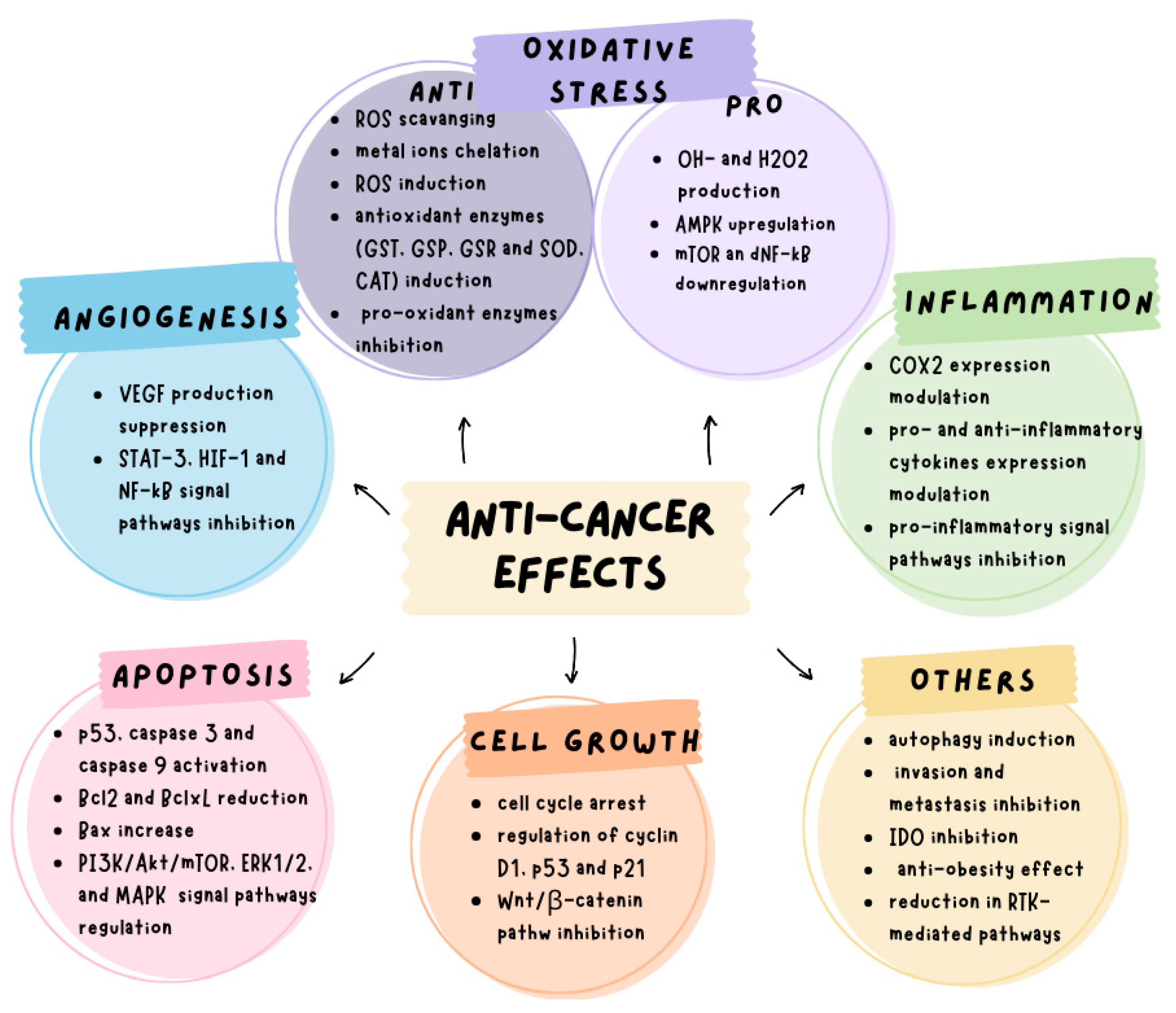
5. Anticancer Effects of Green Tea
6. Effects of Green Tea on Colorectal Cancer
6.1. In Vitro Studies
6.2. In Vivo Studies (Animal Models)
| Type of Model | Treatment | Potential Mechanisms | Effects |
|---|---|---|---|
| In vitro | |||
| SW480 and colon cancer cells from patients | 0–50 µM EGCG for 48 h | STAT3/CXCL8 signaling pathway | Significant inhibition of SW480 cells migration and invasion [80] |
| SW480, SW620 and LS411N cells | 10, 20 and 40 μg/mL EGCG | Activation of caspase-3 and PARP, downregulation of STAT3, and decrease in Bcl-2 protein levels | Significant suppression of cell proliferation and induction of apoptosis [78] |
| HT29 cells | EGCG | Inhibition of ERK1/2 signaling and VEGF expression | Significant induction of apoptosis, and angiogenesis inhibition [82] |
| LoVo, SW480, HCT8, HT29 cells lines | 0–35 µg/mL EGCG | Induction of cell cycle alterations and inhibition of Notch signaling | Significant inhibition of cell proliferation and apoptosis induction [81] |
| Caco-2 cells | 0–80 µM EGCG | Induction of G2/M cell cycle arrest and activation of laminin receptor-mediated myosin phosphatase | Significant inhibition of cell proliferation [88] |
| HCT116, HT29, SW480, IEC6 cell lines | 0–150 µM EGCG | RXRa activity restoration and RXRa promoter methylation reduction | Reversal of gene silencing in colon carcinogenesis modulation [87] |
| HCT116, HEK293, SW480 cell lines | 0–80 µM EGCG | Wnt/β-catenin signaling suppression | β-catenin phosphorylation and proteasomal degradation promotion [86] |
| HCT116, HT29, Caco-2, SW480, and SW837 cells | 0–50 µM EGCG or polyphenon E | Induction of G1 cell cycle arrest and reduction of AKT, EGFR, and activation of HER2 | Significant cell growth inhibition and apoptosis induction [85] |
| SW480 cells | 25 and 50 µM EGCG | EGFR downregulation | Significant cell growth inhibition [93] |
| HT29 cells | 0–50 µM EGCG | AKT, ERK1/2, and p38 MAPK signaling pathways modulation | Significant induction of apoptotic cell death [61] |
| HCT116 spheroids | 50 µM EGCG for 1 week | CD133 and NANOG and ABCC1 and ABCG2 gene expression decrease | Significant decrease in sphere formation, and apoptosis and cell cycle alterations induction [84] |
| DLD-1 and SW480 spheroids | 0–60 µM EGCG | Colorectal CSC properties and Wnt/β-catenin pathway inhibition | Significant reduction of cell proliferation and induction of apoptosis in colorectal CSCs [83] |
| SW480, SW620 and LS411N cell lines | 0–100 μg/mL EGCG | Activation of caspase-3 and PARP, decrease in Bcl-2, MCL-1, and vimentin levels, and increase in E-cadherin levels; downregulation of STAT3 and p-STAT3 | Significant inhibition of cell proliferation and migration [79] |
| HCT116 and HT29 cell lines | 30–60 µM GA | Activation of caspase-3 and caspase-9, decrease in STAT3, EGFR, and AKT phosphorylation | Cell proliferation inhibition, apoptosis induction [89] |
| In vivo (animal models) | |||
| SW620 xenograft in BALB/c mice | 50 and 100 mg/Kg EGCG i.p. for 4 weeks | STAT3 deregulation | Suppression of tumor volume and weight [78] |
| azoxymethane-induced premalignant lesions in mice | 0.01% and 0.1% EGCG in drinking water for 7 weeks | β-catenin, COX2, and cyclin D1 expression decrease | Significant inhibition of premalignant lesions development [90] |
| xenograft of SW837 cells in BALB/c mice | 0.01% and 0.1% EGCG in drinking water for 7 weeks | Suppression of VEGF/VEGFR signaling activation | Xenograft tumor growth inhibition [91] |
| xenograft of HT29 cells in BALB/c mice | 5, 10, and 20 mg/Kg/die EGCG intragastrically for 14 days | Inhibition of Notch signaling | Significant xenograft tumor volume reduction, and apoptosis induction [81] |
| xenograft of HT29 cells in BALB/c mice | 1.5 mg/day EGCG | VEGF decrease | Significant tumor growth and microvessels density reduction; apoptosis induction [82] |
| 1,2-dimethylhydrazine-induced colon cancer in Wistar rats | 400 mg/Kg Theanine for 2 weeks | Ki-67, AKT/mTOR, and JAK2/STAT3 reduction; Smad2 tumor suppressor increase | Precancerous and cancerous lesions, tumor volume reduction [92] |
| xenograft of HCT116 and HT29 cells in BALB/c mice | 80 mg/Kg/day GA | Activation of caspase-3 and caspase-9; decrease in STAT3, EGFR, and AKT phosphorylation | Xenograft volume reduction, apoptosis induction [89] |

7. Human Studies
7.1. Meta-Analyses
7.2. Major Studies According to Specific Geographic Regions
7.2.1. Asian Studies
Japan
Korea
Singapore
Taiwan
China
7.2.2. European, American, and Australian Studies
Europe
USA
Australia
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kaderi Kibria, K.M.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-Onset Colorectal Cancer: A Review of Current Knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-de-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A Genetic Model for Colorectal Tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Wells, K.; Wise, P.E. Hereditary Colorectal Cancer Syndromes. Surg. Clin. N. Am. 2017, 97, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Willett, C.G.; Chang, D.T.; Czito, B.G.; Meyer, J.; Wo, J.; Cancer Genome Atlas Network. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330–337. [Google Scholar]
- Karahalios, A.; English, D.R.; Simpson, J.A. Weight Change and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2015, 181, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Van Kruijsdijk, R.C.M.; Van Der Wall, E.; Visseren, F.L.J. Obesity and Cancer: The Role of Dysfunctional Adipose Tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and Beverages and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies, an Update of the Evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Bosetti, C.; Levi, F.; Polesel, J.; Zucchetto, A.; Negri, E.; La Vecchia, C. Risk Factors for Young-Onset Colorectal Cancer. Cancer Causes Control 2013, 24, 335–341. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between Vitamin D and Risk of Colorectal Cancer: A Systematic Review of Prospective Studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Yan, Y.; Colditz, G.A.; Lee, I.M. Physical Activity and Colon Cancer Prevention: A Meta-Analysis. Br. J. Cancer 2009, 100, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Dubé, C.; Lewin, G.; Tsertsvadze, A.; Barrowman, N.; Code, C.; Sampson, M.; Moher, D. Nonsteroidal Anti-Inflammatory Drugs and Cyclooxygenase-2 Inhibitors for Primary Prevention of Colorectal Cancer: A Systematic Review Prepared for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2007, 146, 376–389. [Google Scholar] [CrossRef] [PubMed]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-Analysis of 16 Studies of the Association of Alcohol with Colorectal Cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and Colorectal Cancer: A Meta-Analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and Emerging Therapeutic Approaches for Colorectal Cancer: A Comprehensive Review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef] [PubMed]
- Schuell, B.; Gruenberger, T.; Kornek, G.V.; Dworan, N.; Depisch, D.; Lang, F.; Schneeweiss, B.; Scheithauer, W. Side Effects during Chemotherapy Predict Tumour Response in Advanced Colorectal Cancer. Br. J. Cancer 2005, 93, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to Anti-EGFR Therapies in Metastatic Colorectal Cancer: Underlying Mechanisms and Reversal Strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Hu, C.; Fang, J.; Liu, G. The Protective Role of Probiotics against Colorectal Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 8884583. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, B. RNA-Based Therapeutics for Colorectal Cancer: Updates and Future Directions. Pharmacol. Res. 2020, 152, 104550. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic Virus Therapy: A New Era of Cancer Treatment at Dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, S.; Agarwal, R. Natural Products and Colon Cancer: Current Status and Future Prospects. Drug Dev. Res. 2008, 69, 460–471. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green Tea (Camellia Sinensis) for the Prevention of Cancer. Cochrane Database Syst. Rev. 2020, 2020, CD005004. [Google Scholar]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Jigisha, A.; Nishant, R.; Navin, K.; Pankaj, G. Green Tea: A Magical Herb with Miraculous Outcomes. Int. Res. J. Pharm. 2012, 3, 139–148. [Google Scholar]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Shibamoto, T. Antioxidative Activities of Volatile Extracts from Green Tea, Oolong Tea, and Black Tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-Gallate: From Green Tea to Cancer Therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef] [PubMed]
- Almatrood, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydh, F.A.; Alsahl, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia Sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y.; Kuramoto, N.; Kawada, K. The Role of Glutamine in Neurogenesis Promoted by the Green Tea Amino Acid Theanine in Neural Progenitor Cells for Brain Health. Neurochem. Int. 2019, 129, 104505. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Shimizu, M. Possible Mechanisms of Green Tea and Its Constituents against Cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The Antioxidant and Pro-Oxidant Activities of Green Tea Polyphenols: A Role in Cancer Prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, M.; Yamagishi, K.; Muraki, I.; Tamakoshi, A.; Iso, H. Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People with and Without Hypertension. J. Am. Heart Assoc. 2023, 12, e026477. [Google Scholar] [CrossRef]
- Ahmad, N.; Mukhtar, H. Green Tea Polyphenols and Cancer: Biologic Mechanisms and Practical Implications. Nutr. Rev. 1999, 57, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.N. Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Fatimawali; Niode, N.J.; Idroes, R.; Zidan, B.M.R.M.; Mitra, S.; Celik, I.; Nainu, F.; Aǧagündüz, D.; Emran, T.B.; et al. A Comprehensive Review of the Potential Use of Green Tea Polyphenols in the Management of COVID-19. Evid.-Based Complement. Altern. Med. 2021, 2021, 7170736. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Sharika, R.; Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C.; Tencomnao, T.; Prasansuklab, A. Role of Herbal Teas in Regulating Cellular Homeostasis and Autophagy and Their Implications in Regulating Overall Health. Nutrients 2021, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Qiao, X.; Fardous, R.; Lewandowski, A.; Liu, J.; Chan, T.H.; Dou, Q.P. Perspectives on the Recent Developments with Green Tea Polyphenols in Drug Discovery. Expert Opin. Drug Discov. 2018, 13, 643–660. [Google Scholar]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.J.; Brighenti, F.; Crozier, A. HPLC-MSn Analysis of Phenolic Compounds and Purine Alkaloids in Green and Black Tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia Sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Dong, E. Influence of Green Tea and Its Three Major Components upon Low-Density Lipoprotein Oxidation. Exp. Toxicol. Pathol. 1997, 49, 329–335. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T. Anti-Cariogenic Properties of Tea (Camellia Sinensis). J. Med. Microbiol. 2001, 50, 299–302. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial Effects of Green Tea: A Literature Review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Oh, J.W.; Muthu, M.; Pushparaj, S.S.C.; Gopal, J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules 2023, 28, 2151. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of Anti-Inflammatory Effects of Green Tea and Black Tea: A Comparative in Vitro Study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood–Brain Barrier Permeability of Green Tea Catechin Metabolites and Their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia Sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Wolfram, S. Effects of Green Tea and Egcg on Cardiovascular and Metabolic Health. J. Am. Coll. Nutr. 2007, 26, 373S–388S. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A Review on Anti-Cancer Effect of Green Tea Catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.F.; Zhao, C.G.; Sui, F.X.; Teng, X.; Wu, Y. Bin Therapeutic Potential of EGCG on Acute Renal Damage in a Rat Model of Obstructive Nephropathy. Mol. Med. Rep. 2013, 7, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, Y.; Li, Z.; Ma, C.; Lou, Z.; Yokoyama, W.; Wang, H. Lipase-Catalyzed Synthesis of Acetylated EGCG and Antioxidant Properties of the Acetylated Derivatives. Food Res. Int. 2014, 56, 279–286. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hasumi, K.; Woo, J.T.; Nagai, K.; Wachi, M. Generation of Hydrogen Peroxide Primarily Contributes to the Induction of Fe(II)-Dependent Apoptosis in Jurkat Cells by (−)-Epigallocatechin Gallate. Carcinogenesis 2004, 25, 1567–1574. [Google Scholar] [CrossRef]
- Shankar, S.; Suthakar, G.; Srivastava, R.K. Epigallocatechin-3-Gallate Inhibits Cell Cycle and Induces Apoptosis in Pancreatic Cancer. Front. Biosci. 2007, 12, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and Alternative P38MAPK Signalling Pathways in Human Colon Cancer Cell Apoptosis Induced by Green Tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Singh, A.; Tully, E.; Woo, J.; Le, A.; Nguyen, T.; Biswal, S.; Sharma, D.; Gabrielson, E. Nrf2 Signaling and Autophagy Are Complementary in Protecting Breast Cancer Cells during Glucose Deprivation. Free Radic. Biol. Med. 2018, 120, 407–413. [Google Scholar] [CrossRef]
- Enkhbat, T.; Nishi, M.; Yoshikawa, K.; Jun, H.; Tokunaga, T.; Takasu, C.; Kashihara, H.; Ishikawa, D.; Tominaga, M.; Shimada, M. Epigallocatechin-3-Gallate Enhances Radiation Sensitivity in Colorectal Cancer Cells through Nrf2 Activation and Autophagy. Anticancer Res. 2018, 38, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahmad, N.; Nieminen, A.L.; Mukhtar, H. Growth Inhibition, Cell-Cycle Dysregulation, and Induction of Apoptosis by Green Tea Constituent (−)-Epigallocatechin-3-Gallate in Androgen-Sensitive and Androgen-Insensitive Human Prostate Carcinoma Cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin Gallate (EGCG) Suppresses Growth and Tumorigenicity in Breast Cancer Cells by Downregulation of MiR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Yang, C.; Du, W.; Yang, D. Inhibition of Green Tea Polyphenol EGCG((−)-Epigallocatechin-3-Gallate) on the Proliferation of Gastric Cancer Cells by Suppressing Canonical Wnt/β-Catenin Signalling Pathway. Int. J. Food Sci. Nutr. 2016, 67, 818–827. [Google Scholar] [CrossRef]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated Review on Green Tea Polyphenol Epigallocatechin-3-Gallate as a Cancer Epigenetic Regulator. Semin. Cancer Biol. 2022, 83, 335–352. [Google Scholar] [CrossRef]
- Adachi, S.; Shimizu, M.; Shirakami, Y.; Yamauchi, J.; Natsume, H.; Matsushima-Nishiwaki, R.; To, S.; Weinstein, I.B.; Moriwaki, H.; Kozawa, O. (−)-Epigallocatechin Gallate Downregulates EGF Receptor via Phosphorylation at Ser1046/1047 by P38 MAPK in Colon Cancer Cells. Carcinogenesis 2009, 30, 1544–1552. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Kochi, T.; Ideta, T.; Miyazaki, T.; Moriwaki, H. Chemopreventive Potential of Green Tea Catechins in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2015, 16, 6124–6139. [Google Scholar] [CrossRef]
- Misra, S.; Ikbal, A.M.A.; Bhattacharjee, D.; Hore, M.; Mishra, S.; Karmakar, S.; Ghosh, A.; Srinivas, R.; Das, A.; Agarwal, S.; et al. Validation of Antioxidant, Antiproliferative, and in Vitro Anti-Rheumatoid Arthritis Activities of Epigallo-Catechin-Rich Bioactive Fraction from Camellia Sinensis Var. Assamica, Assam Variety White Tea, and Its Comparative Evaluation with Green Tea Fract. J. Food Biochem. 2022, 46, e14487. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Lee, J.; Lee, S.Y.; Kim, E.K.; Moon, Y.M.; Jung, Y.O.; Park, S.H.; Cho, M. La EGCG Attenuates Autoimmune Arthritis by Inhibition of STAT3 and HIF-1α with Th17/Treg Control. PLoS ONE 2014, 9, e86062. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Perletti, G.; Magri, V.; Vral, A.; Stamatiou, K.; Trinchieri, A. Green Tea Catechins for Chemoprevention of Prostate Cancer in Patients with Histologically-Proven HG-PIN or ASAP. Concise Review and Meta-Analysis. Arch. Ital. Urol. Androl. 2019, 91, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Yuan, J.M.; Koh, W.P.; Lee, H.P.; Yu, M.C. Green Tea and Black Tea Consumption in Relation to Colorectal Cancer Risk: The Singapore Chinese Health Study. Carcinogenesis 2007, 28, 2143–2148. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, W.; Xiang, Y.B.; Gao, J.; Li, H.L.; Zhang, X.; Gao, Y.T.; Shu, X.O. Green Tea Consumption and Colorectal Cancer Risk: A Report from the Shanghai Men’s Health Study. Carcinogenesis 2011, 32, 1684–1688. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Du, M.; Chu, H.; Zhu, L.; Tong, N.; Zhang, Z.; Wang, M.; Gu, D.; Chen, J. An Inverse Association between Tea Consumption and Colorectal Cancer Risk. Oncotarget 2017, 8, 37367–37376. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.T.; Chow, W.H.; Hsing, A.W.; Mclaughlin, J.K.; Dai, Q.; Gao, Y.T.; Blot, W.J.; Fraumeni, J.F. Green Tea Consumption and the Risk of Pancreatic and Colorectal Cancers. Int. J. Cancer 1997, 70, 255–258. [Google Scholar] [CrossRef]
- Luo, K.W.; Ye, W.; Li, N.; Cheng, B.H. Tea Polyphenol EGC Suppresses Colorectal Cancer Cell Proliferation Both in Vitro and in Vivo via Downregulation of STAT3. J. Funct. Foods 2024, 112, 105977. [Google Scholar] [CrossRef]
- Luo, K.W.; Xia, J.; Cheng, B.H.; Gao, H.C.; Fu, L.W.; Luo, X. Le Tea Polyphenol EGCG Inhibited Colorectal-Cancer-Cell Proliferation and Migration via Downregulation of STAT3. Gastroenterol. Rep. 2021, 9, 59–70. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Q.; Wang, S.; Shi, C. Epigallocatechin-3-Gallate Inhibits the Formation of Neutrophil Extracellular Traps and Suppresses the Migration and Invasion of Colon Cancer Cells by Regulating STAT3/CXCL8 Pathway. Mol. Cell. Biochem. 2023, 478, 887–898. [Google Scholar] [CrossRef]
- Jin, H.; Gong, W.; Zhang, C.; Wang, S. Epigallocatechin Gallate Inhibits the Proliferation of Colorectal Cancer Cells by Regulating Notch Signaling. Onco. Targets. Ther. 2013, 6, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.D.; Kim, M.S.; Shin, B.A.; Chay, K.O.; Ahn, B.W.; Liu, W.; Bucana, C.D.; Gallick, G.E.; Ellis, L.M. EGCG, a Major Component of Green Tea, Inhibits Tumour Growth by Inhibiting VEGF Induction in Human Colon Carcinoma Cells. Br. J. Cancer 2001, 84, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.T.; Wu, J.S.; Geng, S.S.; Zhong, C.Y.; et al. (−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin Gallate Hinders Human Hepatoma and Colon Cancer Sphere Formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Deguchi, A.; Lim, J.T.E.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)-Epigallocatechin Gallate and Polyphenon E Inhibit Growth and Activation of the Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor-2 Signaling Pathways in Human Colon Cancer Cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green Tea Polyphenol EGCG Suppresses Wnt/β-Catenin Signaling by Promoting GSK-3β- and PP2A-Independent β-Catenin Phosphorylation/Degradation. BioFactors 2014, 40, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Moseley, V.R.; Cabang, A.B.; Coleman, K.; Wei, W.; Garrett-Mayer, E.; Wargovich, M.J. Reduction in Promotor Methylation Utilizing EGCG (Epigallocatechin-3-Gallate) Restores RXRα Expression in Human Colon Cancer Cells. Oncotarget 2016, 7, 35313–35326. [Google Scholar] [CrossRef] [PubMed]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Involvement of 67-KDa Laminin Receptor-Mediated Myosin Phosphatase Activation in Antiproliferative Effect of Epigallocatechin-3-O-Gallate at a Physiological Concentration on Caco-2 Colon Cancer Cells. Biochem. Biophys. Res. Commun. 2008, 371, 172–176. [Google Scholar] [CrossRef]
- Lin, X.; Wang, G.; Liu, P.; Han, L.; Wang, T.; Chen, K.; Gao, Y. Gallic Acid Suppresses Colon Cancer Proliferation by Inhibiting SRC and EGFR Phosphorylation. Exp. Ther. Med. 2021, 21, 638. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Adachi, S.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (−)-Epigallocatechin Gallate Suppresses Azoxymethane-Induced Colonic Premalignant Lesions in Male C57BL/KsJ-Db/Db Mice. Cancer Prev. Res. 2008, 1, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Kubota, M.; Adachi, S.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin Gallate Inhibits Growth and Activation of the VEGF/VEGFR Axis in Human Colorectal Cancer Cells. Chem. Biol. Interact. 2010, 185, 247–252. [Google Scholar] [CrossRef]
- Shojaei-Zarghani, S.; Yari Khosroushahi, A.; Rafraf, M. Oncopreventive Effects of Theanine and Theobromine on Dimethylhydrazine-Induced Colon Cancer Model. Biomed. Pharmacother. 2021, 134, 111140. [Google Scholar] [CrossRef]
- Adachi, S.; Nagao, T.; To, S.; Joe, A.K.; Shimizu, M.; Matsushima-Nishiwaki, R.; Kozawa, O.; Moriwaki, H.; Maxfield, F.R.; Weinstein, I.B. (−)-Epigallocatechin Gallate Causes Internalization of the Epidermal Growth Factor Receptor in Human Colon Cancer Cells. Carcinogenesis 2008, 29, 1986–1993. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Liu, Y.; Tian, R.; Yin, X.; Hao, Y.; Yang, Y.; Yang, J.; Li, Z.; Yu, S.; et al. Association between Tea Consumption and Colorectal Cancer: A Systematic Review and Meta-Analysis of a Population-Based Study. BMC Gastroenterol. 2023, 23, 294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-Z.; Lu, D.-M.; Ouyang, J.; Zhou, F.; Huang, P.-F.; Gu, B.-Z.; Tang, J.-W.; Shen, F.; Li, J.-F.; Li, Y.-L.; et al. Tea Consumption and Colorectal Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2020, 59, 3603–3615. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Jin, Z.; Jiang, H.; Xiang, C.; Tang, J.; Li, T.; He, J. Tea Consumption and the Risk of Five Major Cancers: A Dose-Response Meta-Analysis of Prospective Studies. BMC Cancer 2014, 14, 197. [Google Scholar] [CrossRef]
- Wang, Z.H.; Gao, Q.Y.; Fang, J.Y. Green Tea and Incidence of Colorectal Cancer: Evidence from Prospective Cohort Studies. Nutr. Cancer 2012, 64, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Albanes, D.; Beeson, W.L.; Van Den Brandt, P.A.; Buring, J.E.; Flood, A.; Freudenheim, J.L.; Giovannucci, E.L.; Goldbohm, R.A.; Jaceldo-Siegl, K.; et al. Risk of Colon Cancer and Coffee, Tea, and Sugar-Sweetened Soft Drink Intake: Pooled Analysis of Prospective Cohort Studies. J. Natl. Cancer Inst. 2010, 102, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zeng, X.T.; Duan, X.L.; Zeng, H.C.; Shen, R.; Zhou, P. Association between Green Tea and Colorectal Cancer Risk: A Meta-Analysis of 13 Case-Control Studies. Asian Pacific J. Cancer Prev. 2012, 13, 3123–3127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Ohnaka, K.; Morita, M.; Toyomura, K.; Kono, S.; Ueki, T.; Tanaka, M.; Kakeji, Y.; Maehara, Y.; Okamura, T.; et al. Dietary Polyphenols and Colorectal Cancer Risk: The Fukuoka Colorectal Cancer Study. World J. Gastroenterol. 2013, 19, 2683–2690. [Google Scholar] [CrossRef]
- Wada, K.; Oba, S.; Tsuji, M.; Goto, Y.; Mizuta, F.; Koda, S.; Uji, T.; Hori, A.; Tanabashi, S.; Matsushita, S.; et al. Green Tea Intake and Colorectal Cancer Risk in Japan: The Takayama Study. Jpn. J. Clin. Oncol. 2019, 49, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Fukutomi, Y.; Ninomiya, M.; Nagura, K.; Kato, T.; Araki, H.; Suganuma, M.; Fujiki, H.; Moriwaki, H. Green Tea Extracts for the Prevention of Metachronous Colorectal Adenomas: A Pilot Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3020–3025. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tsubono, Y.; Nakaya, N.; Koizumi, Y.; Suzuki, Y.; Shibuya, D.; Tsuji, I. Green Tea and the Risk of Colorectal Cancer: Pooled Analysis of Two Prospective Studies in Japan. J. Epidemiol. 2005, 15, 118–124. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Protective Effect of Green Tea Consumption on Colorectal Cancer Varies by Lifestyle Factors. Nutrients 2019, 11, 2612. [Google Scholar] [CrossRef]
- Shin, C.M.; Lee, D.H.; Seo, A.Y.; Lee, H.J.; Kim, S.B.; Son, W.C.; Kim, Y.K.; Lee, S.J.; Park, S.H.; Kim, N.; et al. Green Tea Extracts for the Prevention of Metachronous Colorectal Polyps among Patients Who Underwent Endoscopic Removal of Colorectal Adenomas: A Randomized Clinical Trial. Clin. Nutr. 2018, 37, 452–458. [Google Scholar] [CrossRef]
- Chen, H.Y.; Sun, Z.J.; Li, C.H.; Chou, Y.T.; Chang, C.J.; Lu, F.H.; Yang, Y.C.; Wu, J.S. Cumulative Tea Consumption Is Inversely Associated with Colorectal Adenomas in Adults: A Cross-Sectional Study in a Taiwanese Population. Cancer Epidemiol. 2021, 73, 101945. [Google Scholar] [CrossRef]
- Yang, G.; Shu, X.O.; Li, H.; Chow, W.H.; Ji, B.T.; Zhang, X.; Gao, Y.T.; Zheng, W. Prospective Cohort Study of Green Tea Consumption and Colorectal Cancer Risk in Women. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, C.; Guo, Y.; Bian, Z.; Shen, Z.; Yang, L.; Chen, Y.; Wei, Y.; Zhang, H.; Qiu, Z.; et al. Association between Tea Consumption and Risk of Cancer: A Prospective Cohort Study of 0.5 Million Chinese Adults. Eur. J. Epidemiol. 2019, 34, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, M.; Liu, B.; Liang, X.; Yu, Y.; Li, Q.; Ma, X.; Yao, K.; Chen, K. The Association of XPC Polymorphisms and Tea Drinking with Colorectal Cancer Risk in a Chinese Population. Mol. Carcinog. 2011, 50, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.X.; Zhuo, Z.J.; Zhu, J.; Zhang, S.D.; Xue, W.Q.; Zhang, J.B.; Xu, H.M.; Li, X.Z.; Zhang, P.F.; He, J.; et al. XPG Gene Polymorphisms Contribute to Colorectal Cancer Susceptibility: A Two-Stage Case-Control Study. J. Cancer 2016, 7, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, M.; Pan, Y.; Jin, M.; Jiang, X.; Zhang, S.; Wu, Y.; Ni, Q.; Li, Q.; Chen, K. PLA2G4A Mutants Modified Protective Effect of Tea Consumption against Colorectal Cancer. Int. J. Color. Dis. 2012, 27, 1005–1013. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Cayssials, V.; Jenab, M.; Rothwell, J.A.; Fedirko, V.; Aleksandrova, K.; Tjønneland, A.; Kyrø, C.; Overvad, K.; Boutron-Ruault, M.C.; et al. Dietary Intake of Total Polyphenol and Polyphenol Classes and the Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Eur. J. Epidemiol. 2018, 33, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Dik, V.K.; Bueno-De-Mesquita, H.B.; Van Oijen, M.G.H.; Siersema, P.D.; Uiterwaal, C.S.P.M.; Van Gils, C.H.; Van Duijnhoven, F.J.B.; Cauchi, S.; Yengo, L.; Froguel, P.; et al. Coffee and Tea Consumption, Genotype-Based CYP1A2 and NAT2 Activity and Colorectal Cancer Risk—Results from the EPIC Cohort Study. Int. J. Cancer 2014, 135, 401–412. [Google Scholar] [CrossRef]
- Seufferlein, T.; Ettrich, T.J.; Menzler, S.; Messmann, H.; Kleber, G.; Zipprich, A.; Frank-Gleich, S.; Algül, H.; Metter, K.; Odemar, F.; et al. Green Tea Extract to Prevent Colorectal Adenomas, Results of a Randomized, Placebo-Controlled Clinical Trial. Am. J. Gastroenterol. 2022, 117, 884–894. [Google Scholar] [CrossRef]
- Hartman, T.J.; Tangrea, J.A.; Pietinen, P.; Malila, N.; Virtanen, M.; Taylor, P.R.; Albanes, D. Tea and Coffee Consumption and Risk of Colon and Rectal Cancer in Middle-Aged Finnish Men. Nutr. Cancer 1998, 31, 41–48. [Google Scholar] [CrossRef]
- Terry, P.; Wolk, A. Tea Consumption and the Risk of Colorectal Cancer in Sweden. Nutr. Cancer 2001, 39, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, K.; Zhong, R.; Cassidy, A.; Rimm, E.B.; Nimptsch, K.; Wu, K.; Chan, A.T.; Giovannucci, E.L.; Ogino, S.; et al. Flavonoid Intake and Survival after Diagnosis of Colorectal Cancer: A Prospective Study in 2 US Cohorts. Am. J. Clin. Nutr. 2023, 117, 1121–1129. [Google Scholar] [CrossRef]
- Su, L.J.; Arab, L. Tea Consumption and the Reduced Risk of Colon Cancer—Results from a National Prospective Cohort Study. Public Health Nutr. 2002, 5, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Putnam, S.D.; Bianchi, G.D.; Parker, A.S.; Lynch, C.F.; Cantor, K.P. Tea Consumption and Risk of Cancer of the Colon and Rectum. Nutr. Cancer 2001, 41, 33–40. [Google Scholar] [CrossRef]
- Green, C.J.; de Dauwe, P.; Boyle, T.; Tabatabaei, S.M.; Fritschi, L.; Heyworth, J.S. Tea, Coffee, and Milk Consumption and Colorectal Cancer Risk. J. Epidemiol. 2014, 24, 146–153. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic Study of Catechin Stability: Effects of Ph, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Liang, Y.F.; Ma, S.B.; Li, H.; Gao, W.Y. Stability and Stabilization of (–)-Gallocatechin Gallate under Various Experimental Conditions and Analyses of Its Epimerization, Auto-Oxidation, and Degradation by LC-MS. J. Sci. Food Agric. 2019, 99, 5984–5993. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A. Dose-Dependent Functionality and Toxicity of Green Tea Polyphenols in Experimental Rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Tessaro, L.; Lima, A.K.O.; Velloso, I.P.S.; Conte-Junior, C.A. Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients 2023, 15, 3136. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. Biomed Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Wei, X.L.; Fang, Y.P.; Gan, R.Y.; Wang, M.; Ge, Y.Y.; Zhang, D.; Cheng, L.Z.; Corke, H. Nanochemoprevention with Therapeutic Benefits: An Updated Review Focused on Epigallocatechin Gallate Delivery. Crit. Rev. Food Sci. Nutr. 2020, 60, 1243–1264. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Mondal, S.; Das, S.; Das, S.; Das Saha, K. Enhanced Anticancer Activity of (-)-Epigallocatechin-3-Gallate (EGCG) Encapsulated NPs toward Colon Cancer Cell Lines. Free Radic. Biol. Med. 2024, 58, 565–582. [Google Scholar] [CrossRef]
- Borah, G.; Bharali, M.K. Green Tea Catechins in Combination with Irinotecan Attenuates Tumorigenesis and Treatment-Associated Toxicity in an Inflammation-Associated Colon Cancer Mice Model. J. Egypt. Natl. Canc. Inst. 2021, 33, 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ren, G.; Xu, X.; Yuan, H.; Wang, Z.; Kang, L.; Yu, W.; Tian, K. Combination of Curcumin and Green Tea Catechins Prevents Dimethylhydrazine-Induced Colon Carcinogenesis. Food Chem. Toxicol. 2010, 48, 390–395. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year | Number of Pooled Studies | Comparisons | Major Findings | Reference |
|---|---|---|---|---|
| Huang et al., 2023 | 15 | Regular tea consumption vs. non-consumers | Risk of CRC not significantly decreased in the general population; significant 68% decrease in American subgroup | [94] |
| Zhu et al., 2020 | 20 | Highest vs. lowest tea consumption | Risk of CRC not significantly decreased in the general population; significant, 10% decrease in women | [95] |
| Chen et al., 2017 | 29 | Increase in tea consumption (one cup increase) | Odds of CRC not significantly decreased in the general population; significant 9% decreased odds found against rectal cancer; significant, 14% decrease in CRC odds in women stratum | [83] |
| Yu et al., 2014 | 15 | Risk estimate per 3 cups increase | No significant decrease in CRC risk | [96] |
| Wang et al., 2012 | 6 | Highest vs. lowest tea consumption | Risk of CRC not significantly decreased in the general population; significant, 30% decrease in risk in Shanghai subgroup; 36% increased CRC risk in Singapore subgroup | [97] |
| Wang et al., 2012 | 13 | No significant decrease in CRC risk | [99] | |
| Zhang et al., 2010 | 13 | Consumption increment of more than 4 cups/day of tea (type of tea not disclosed) | Significant increase in CRC risk (28%) | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randisi, F.; Perletti, G.; Marras, E.; Gariboldi, M.B. Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer. Cancers 2025, 17, 623. https://doi.org/10.3390/cancers17040623
Randisi F, Perletti G, Marras E, Gariboldi MB. Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer. Cancers. 2025; 17(4):623. https://doi.org/10.3390/cancers17040623
Chicago/Turabian StyleRandisi, Federica, Gianpaolo Perletti, Emanuela Marras, and Marzia Bruna Gariboldi. 2025. "Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer" Cancers 17, no. 4: 623. https://doi.org/10.3390/cancers17040623
APA StyleRandisi, F., Perletti, G., Marras, E., & Gariboldi, M. B. (2025). Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer. Cancers, 17(4), 623. https://doi.org/10.3390/cancers17040623







