ASPH Is a Metastatic Factor and Therapeutic Target in Chondrosarcoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
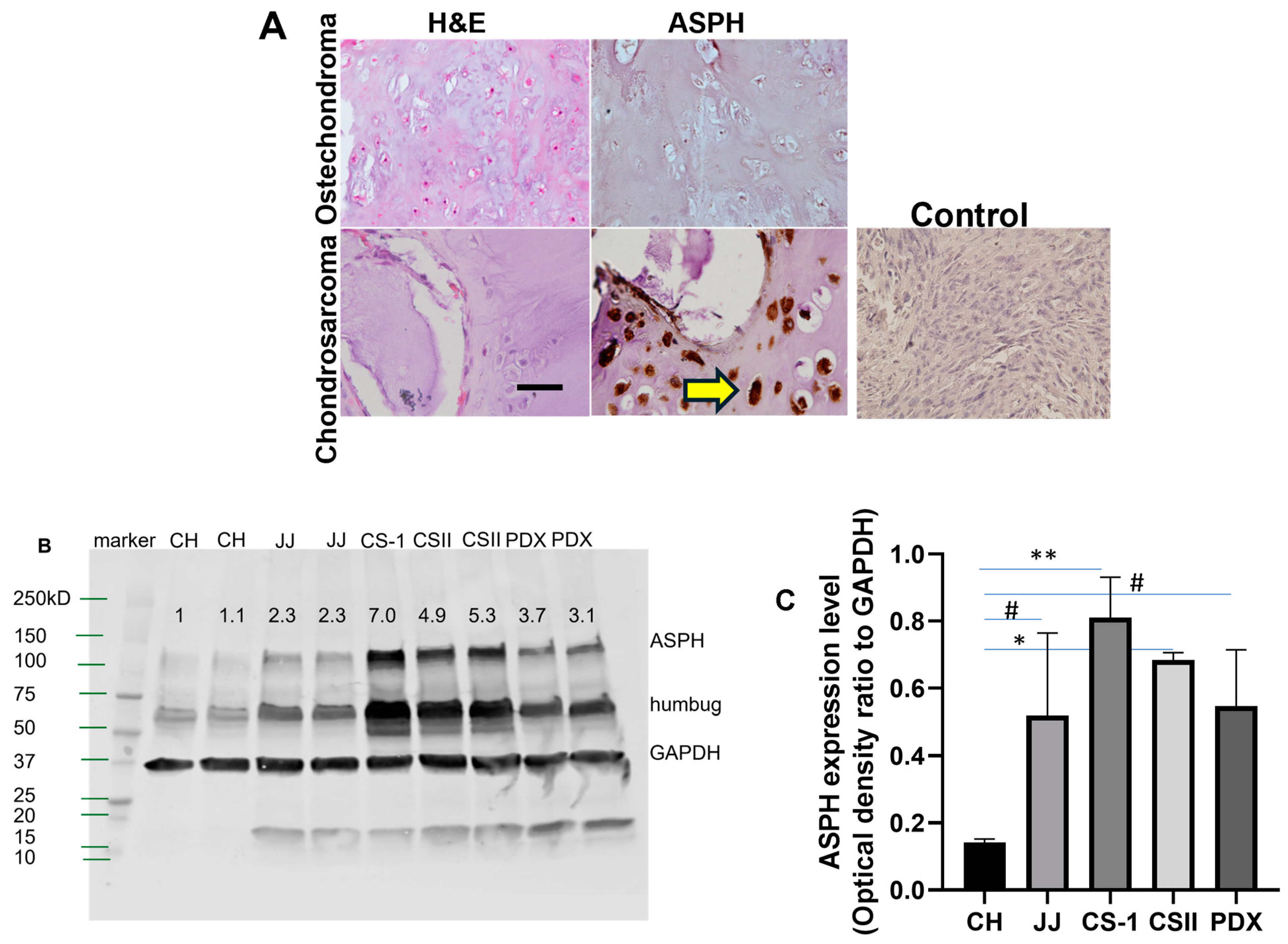
2.1. Chondrosarcoma Tissue Microarray (TMA) and Immunohistochemistry
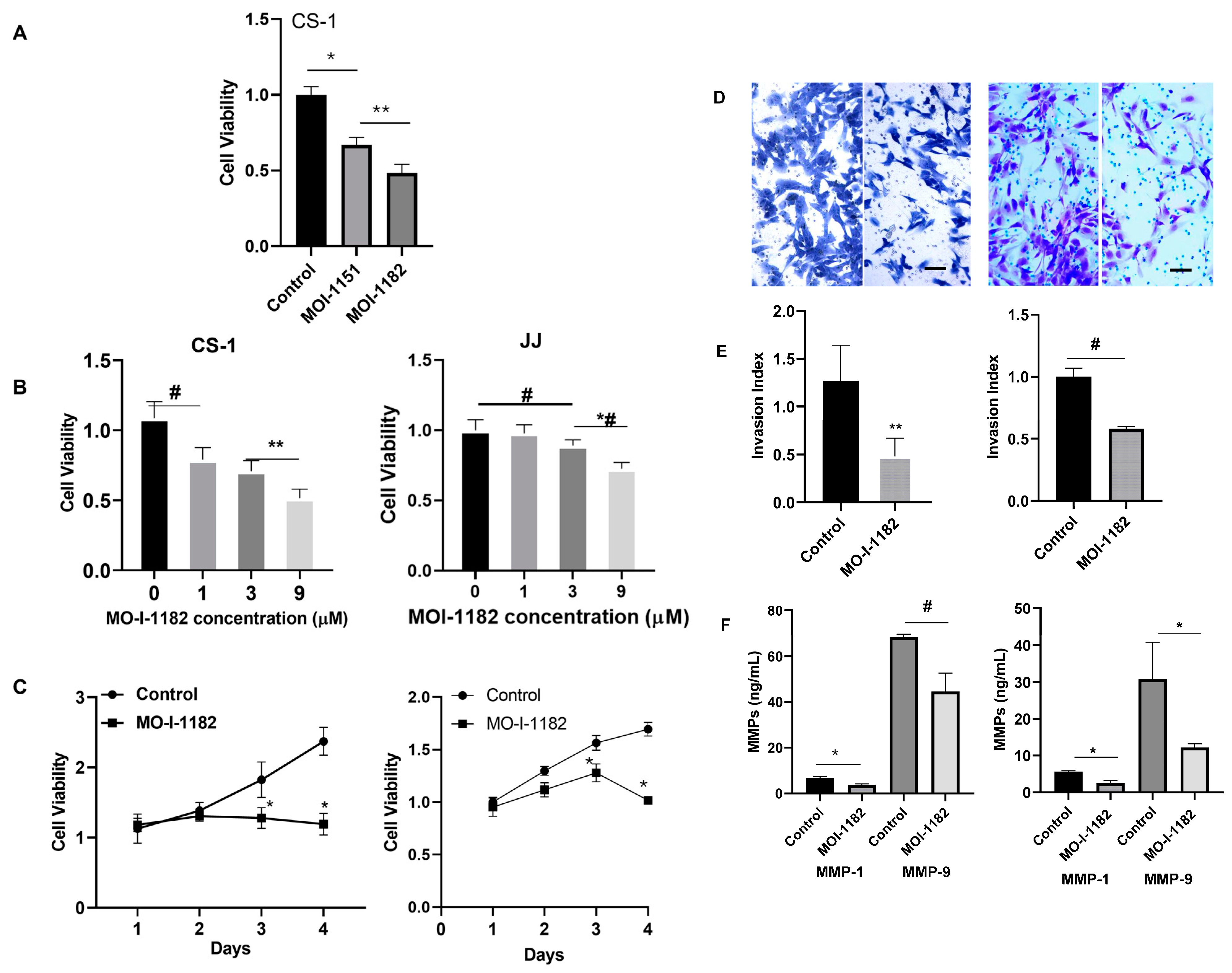
2.2. Cell Viability Assay:
2.3. Invasion Assay
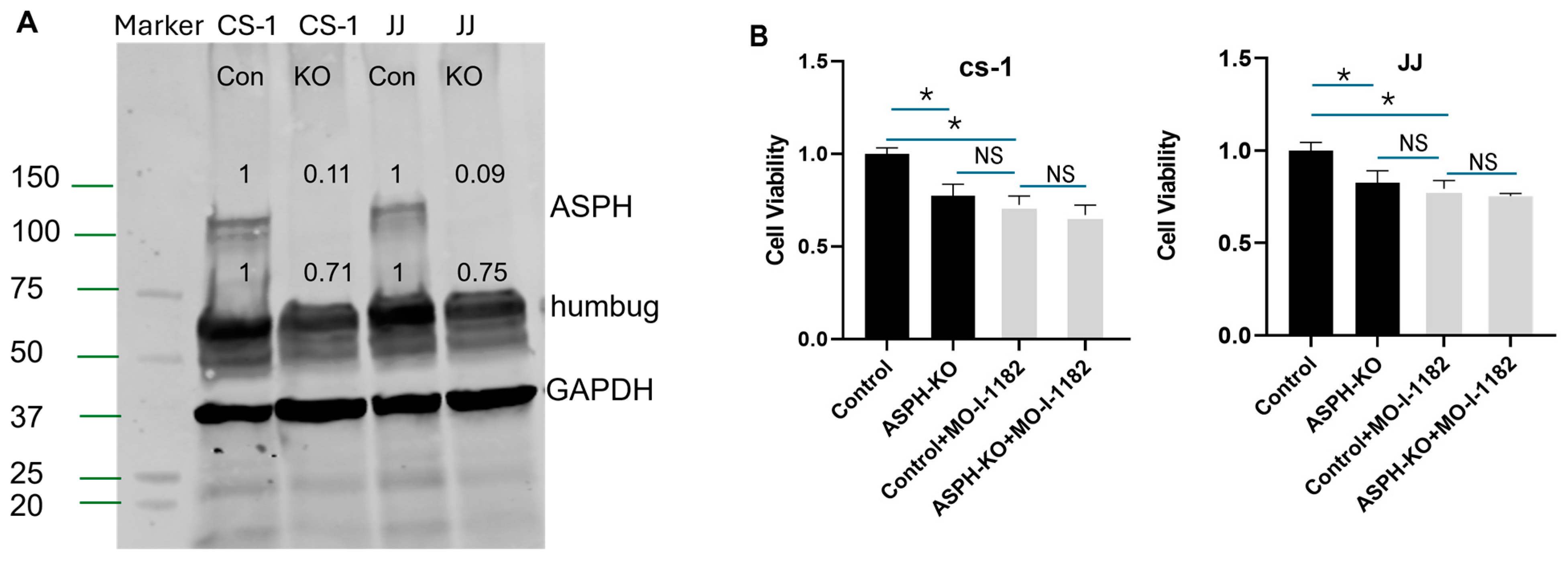
2.4. ASPH-Knockout
2.5. ELISA Assay
2.6. Western Blot Analysis
2.7. Mouse Models
2.7.1. Bioimaging
2.7.2. Primary Tumor Analysis
2.7.3. Metastasis Analysis
2.8. Statistical Analysis
2.8.1. TMA
2.8.2. Experimental
3. Results
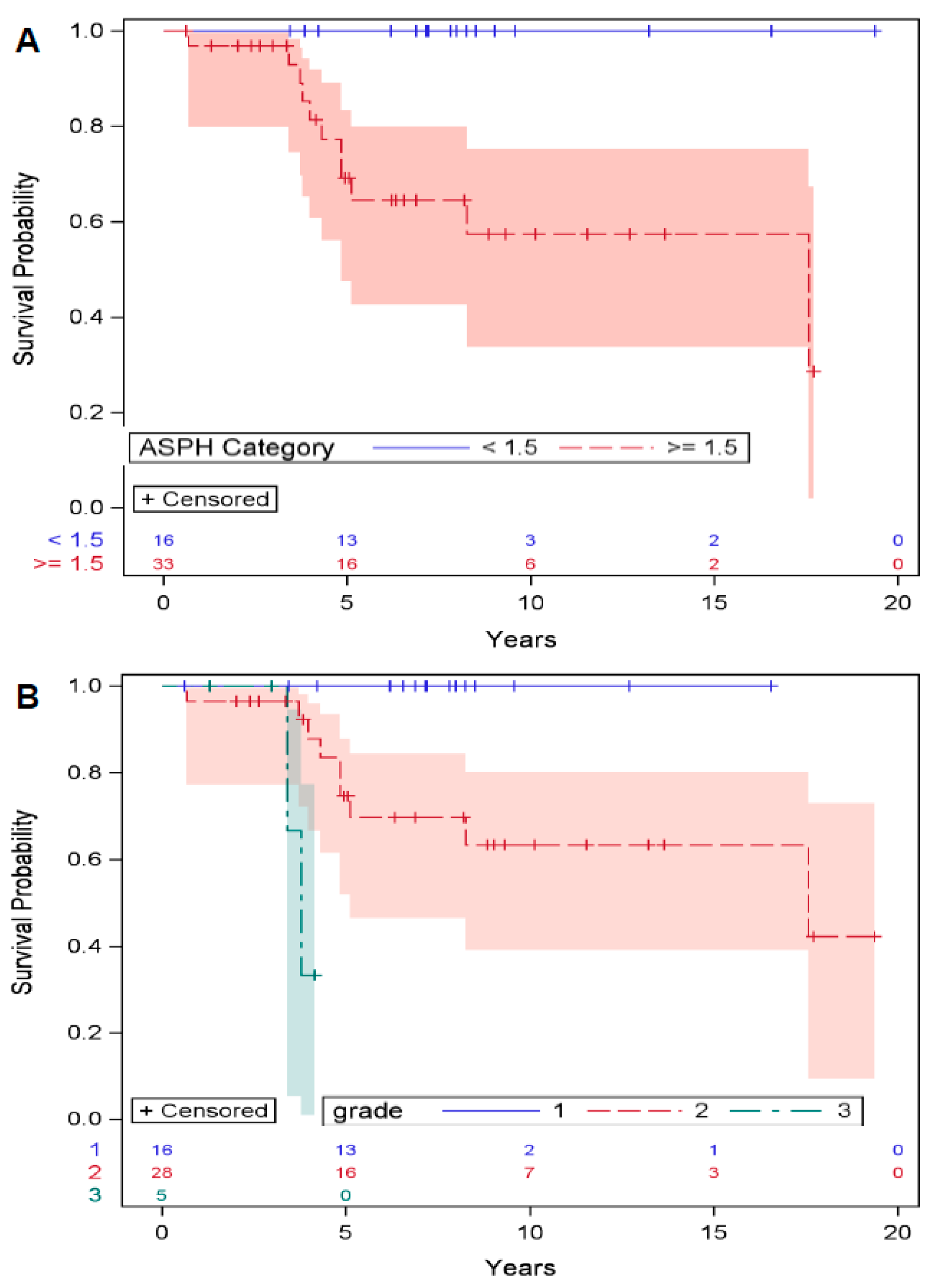
3.1. ASPH Expression in Chondrosarcoma Predicts Survival
3.2. ASPH Inhibition Decreases CS Proliferation and Invasion In Vitro
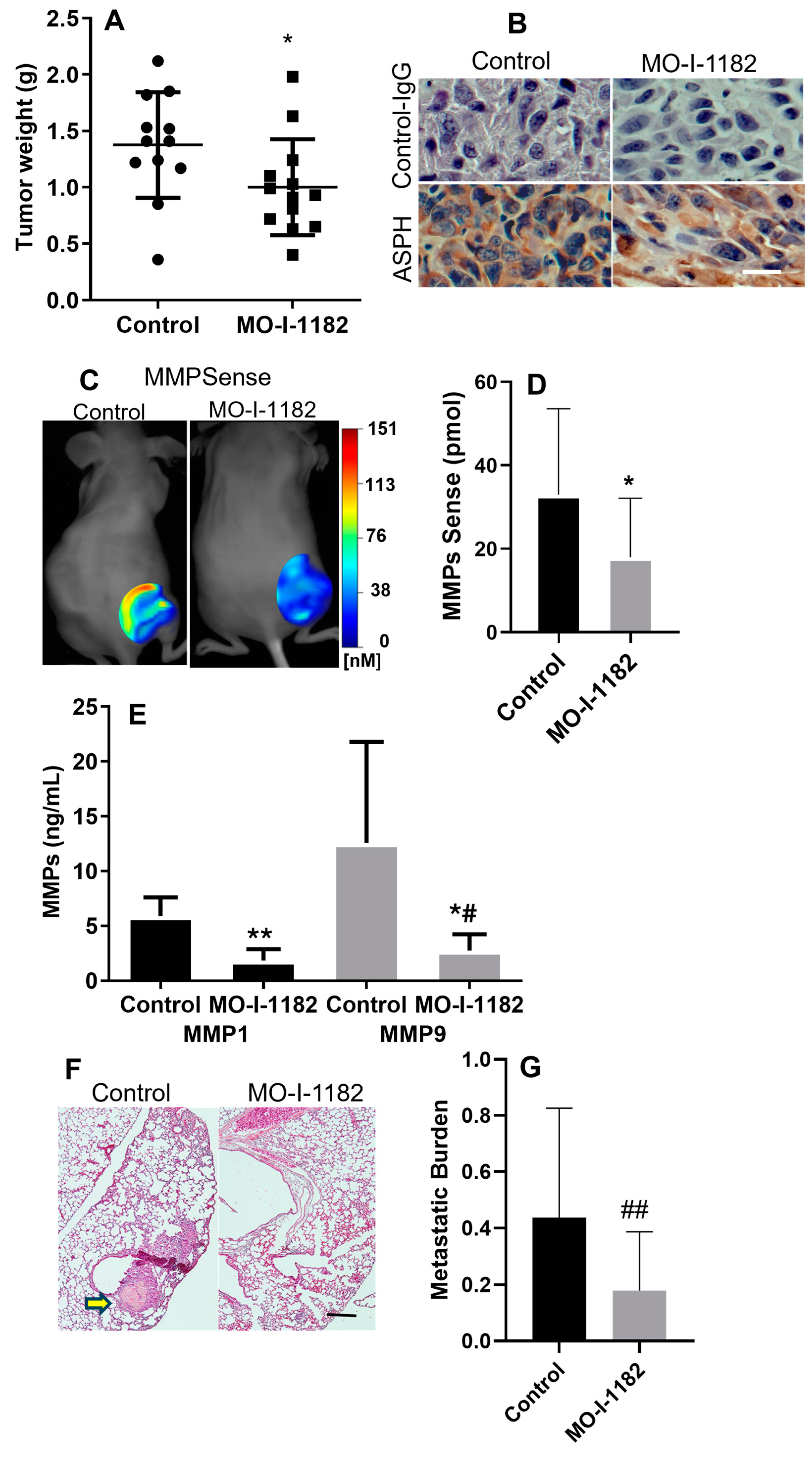
3.3. SMI Inhibits Tumor Growth and Metastasis in Xenograft Chondrosarcoma Model
3.4. SMI Inhibits MMP Expression in PDX Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damron, T.A.; Ward, W.G.; Stewart, A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin. Orthop. Relat. Res. 2007, 459, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Mankin, H.J.; Fondren, G.; Gebhardt, M.C.; Springfield, D.S.; Rosenberg, A.E.; Jennings, L.C. Chondrosarcoma of bone: An assessment of outcome. J. Bone Jt. Surg. Am. 1999, 81, 326–338. [Google Scholar] [CrossRef]
- van Maldegem, A.M.; Bovee, J.V.; Gelderblom, H. Comprehensive analysis of published studies involving systemic treatment for chondrosarcoma of bone between 2000 and 2013. Clin. Sarcoma Res. 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, A.Y.; Burgueno, J.E.; Koniaris, L.G.; Gutierrez, J.C.; Duncan, R.; Scully, S.P. Chondrosarcoma in the United States (1973 to 2003): An analysis of 2890 cases from the SEER database. J. Bone Jt. Surg. Am. 2009, 91, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, M.; Ekfors, T.O.; Bohling, T.O.; Teppo, L.H.; Vuorio, E.I.; Aro, H.T. No improvement in the overall survival of 194 patients with chondrosarcoma in Finland in 1971–1990. Acta Orthop. Scand. 2003, 74, 344–350. [Google Scholar] [CrossRef]
- Landuzzi, L.; Ruzzi, F.; Lollini, P.-L.; Scotlandi, K. Chondrosarcoma: New Molecular Insights, Challenges in Near-Patient Preclinical Modeling, and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1542. [Google Scholar] [CrossRef]
- Korioth, F.; Gieffers, C.; Frey, J. Cloning and characterization of the human gene encoding aspartyl beta-hydroxylase. Gene 1994, 150, 395–399. [Google Scholar] [CrossRef]
- Lavaissiere, L.; Jia, S.; Nishiyama, M.; de la Monte, S.; Stern, A.M.; Wands, J.R.; Friedman, P.A. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J. Clin. Investig. 1996, 98, 1313–1323. [Google Scholar] [CrossRef]
- Jia, S.; VanDusen, W.J.; Diehl, R.E.; Kohl, N.E.; Dixon, R.A.; Elliston, K.O.; Stern, A.M.; Friedman, P.A. cDNA cloning and expression of bovine aspartyl (asparaginyl) beta-hydroxylase. J. Biol. Chem. 1992, 267, 14322–14327. [Google Scholar] [CrossRef]
- Patel, N.; Khan, A.O.; Mansour, A.; Mohamed, J.Y.; Al-Assiri, A.; Haddad, R.; Jia, X.; Xiong, Y.; Megarbane, A.; Traboulsi, E.I.; et al. Mutations in ASPH cause facial dysmorphism, lens dislocation, anterior-segment abnormalities, and spontaneous filtering blebs, or Traboulsi syndrome. Am. J. Hum. Genet. 2014, 94, 755–759. [Google Scholar] [CrossRef]
- Zou, Q.; Hou, Y.; Wang, H.; Wang, K.; Xing, X.; Xia, Y.; Wan, X.; Li, J.; Jiao, B.; Liu, J.; et al. Hydroxylase Activity of ASPH Promotes Hepatocellular Carcinoma Metastasis Through Epithelial-to-Mesenchymal Transition Pathway. EBioMedicine 2018, 31, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Lin, Q.; Li, L.; Bai, X.; Chen, X.; Chen, H.; Kong, R.; Wang, Y.; Zhu, H.; He, F.; et al. Aspartate beta-hydroxylase promotes pancreatic ductal adenocarcinoma metastasis through activation of SRC signaling pathway. J. Hematol. Oncol. 2019, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Charbonneau, C.; Wei, L.; Chen, Q.; Terek, R.M. miR-181a Targets RGS16 to Promote Chondrosarcoma Growth, Angiogenesis, and Metastasis. Mol. Cancer Res. 2015, 13, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, M.; Polakova, I.; Olsen, M.; Kasi, M.K.; Tachezy, R.; Smahel, M. Heterogeneous Response of Tumor Cell Lines to Inhibition of Aspartate β-hydroxylase. J. Cancer 2024, 15, 3466–3480. [Google Scholar] [CrossRef]
- Nota, S.; Al-Sukaini, A.; Patel, S.S.; Sabbatino, F.; Nielsen, G.P.; Deshpande, V.; Yearley, J.H.; Ferrone, S.; Wang, X.; Schwab, J.H. High TIL, HLA, and Immune Checkpoint Expression in Conventional High-Grade and Dedifferentiated Chondrosarcoma and Poor Clinical Course of the Disease. Front. Oncol. 2021, 11, 598001. [Google Scholar] [CrossRef]
- Dinchuk, J.E.; Henderson, N.L.; Burn, T.C.; Huber, R.; Ho, S.P.; Link, J.; O’Neil, K.T.; Focht, R.J.; Scully, M.S.; Hollis, J.M.; et al. Aspartyl beta -hydroxylase (Asph) and an evolutionarily conserved isoform of Asph missing the catalytic domain share exons with junctin. J. Biol. Chem. 2000, 275, 39543–39554. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Li, Y.; Zhang, X.; Xie, H. Characterization of the Relationship Between the Expression of Aspartate beta-Hydroxylase and the Pathological Characteristics of Breast Cancer. Med. Sci. Monit. 2020, 26, e926752. [Google Scholar]
- Gan, X.; Li, S.; Wang, Y.; Du, H.; Hu, Y.; Xing, X.; Cheng, X.; Yan, Y.; Li, Z. Aspartate beta-Hydroxylase Serves as a Prognostic Biomarker for Neoadjuvant Chemotherapy in Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 5482. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Hu, J.; Bai, B.; Zhu, H. Diverse molecular functions of aspartate beta-hydroxylase in cancer (Review). Oncol. Rep. 2020, 44, 2364–2372. [Google Scholar] [CrossRef]
- Engin, F.; Bertin, T.; Ma, O.; Jiang, M.M.; Wang, L.; Sutton, R.E.; Donehower, L.A.; Lee, B. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum. Mol. Genet. 2009, 18, 1464–1470. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Z.Q.; Fang, Y.C.; Li, X.L.; Sun, Y.; Xiong, C.Z.; Yan, L.Q.; Wang, Q. Metastasis-associated lung adenocarcinoma transcript 1 promotes the proliferation of chondrosarcoma cell via activating Notch-1 signaling pathway. Oncol. Targets Ther. 2016, 9, 2143–2151. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Berend, K.R.; Toth, A.P.; Harrelson, J.M.; Layfield, L.J.; Hey, L.A.; Scully, S.P. Association between ratio of matrix metalloproteinase-1 to tissue inhibitor of metalloproteinase-1 and local recurrence, metastasis, and survival in human chondrosarcoma. J. Bone Jt. Surg. Am. 1998, 80, 11–17. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, T.; Wang, W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine 2018, 97, e13051. [Google Scholar] [CrossRef] [PubMed]
- Zhdanovskaya, N.; Firrincieli, M.; Lazzari, S.; Pace, E.; Scribani Rossi, P.; Felli, M.P.; Talora, C.; Screpanti, I.; Palermo, R. Targeting Notch to Maximize Chemotherapeutic Benefits: Rationale, Advanced Strategies, and Future Perspectives. Cancers 2021, 13, 5106. [Google Scholar] [CrossRef]
- Lee, J.H. Overexpression of humbug promotes malignant progression in human gastric cancer cells. Oncol. Rep. 2008, 19, 795–800. [Google Scholar] [CrossRef]
- Jeys, L.M.; Thorkildsen, J.; Kurisunkal, V.; Puri, A.; Ruggieri, P.; Houdek, M.T.; Boyle, R.A.; Ebeid, W.; Botello, E.; Morris, G.V.; et al. Controversies in orthopaedic oncology. Bone Joint J. 2024, 106, 425–429. [Google Scholar] [CrossRef]
- Walter, S.G.; Knoll, P.; Eysel, P.; Quaas, A.; Gaisendrees, C.; Nissler, R.; Hieggelke, L. Molecular In-Depth Characterization of Chondrosarcoma for Current and Future Targeted Therapies. Cancers 2023, 15, 2556. [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, M.S.; Jiang, S.K.; Eaw, C.Y.; Han, Y.J.; Tang, C.H. Transcriptomic data integration and analysis revealing potential mechanisms of doxorubicin resistance in chondrosarcoma cells. Biochem. Pharmacol. 2025, 232, 116733. [Google Scholar] [CrossRef]
- van Oosterwijk, J.G.; Herpers, B.; Meijer, D.; Briaire-de Bruijn, I.H.; Cleton-Jansen, A.M.; Gelderblom, H.; van de Water, B.; Bovee, J.V. Restoration of chemosensitivity for doxorubicin and cisplatin in chondrosarcoma in vitro: BCL-2 family members cause chemoresistance. Ann. Oncol. 2012, 23, 1617–1626. [Google Scholar] [CrossRef]
| Patient Demographics | |
| Male, n (%) | 23 (46%) |
| Age at Presentation, mean (SD) | 53.4 (14.7) |
| Disease Characteristics | |
| Average ASPH Score, n (%) | |
| 0 | 0 (0%) |
| 1 | 16 (32%) |
| 1.5 | 8 (16%) |
| 2 | 17 (34%) |
| 2.5 | 4 (8%) |
| 3 | 5 (10.0%) |
| Average ASPH Score, mean (SD) | 1.74 (0.65) |
| ASPH Score ≥ 1.5 | 34 (68.0%) |
| Grade, n (%) | |
| 0 | 0 (0%) |
| 1 | 16 (33%) |
| 2 | 29 (57%) |
| 3 | 5 (10%) |
| Overall Survival Outcomes | |
| Died, n (%) | 11 (22.5%) |
| Metastasis, n (%) | 8 (16.0%) |
| Local Recurrence, n (%) | 10 (20.0%) |
| Time to Occurrence | |
| Months to death, median (IQR) | 76.3 (46.7–107.7) |
| Months to metastasis, median (IQR) | 76.3 (46.7–107.7) |
| Months to local recurrence, median (IQR) | 59.1 (35.8–98.9) |
| Deceased (n = 11) | Alive (n = 48) | p-Value | |
|---|---|---|---|
| Grade | 0.02 | ||
| 0 | 0 (0%) | 0 (0%) | |
| 1 | 0 (0%) | 16 (42.1%) | |
| 2 | 9 (81.8%) | 19 (50.0%) | |
| 3 | 2 (18.2%) | 3 (7.9%) | |
| ASPH score | 0.02 | ||
| 1 | 0 (0%) | 16 (42.1%) | |
| 1.5 | 4 (36.4%) | 4 (10.5%) | |
| 2 | 5 (45.5%) | 11 (29.0%) | |
| 2.5 | 1 (9.1%) | 3 (7.9%) | |
| 3 | 1 (9.1%) | 4 (10.5%) | |
| ASPH Score | 0.009 | ||
| <1.5 | 0 (0%) | 16 (42.1%) | |
| ≥1.5 | 11 (100%) | 22 (57.9%) |
| Metastasis (n = 8) | No Metastasis (n = 42) | p-Value | |
|---|---|---|---|
| Grade | 0.053 | ||
| 0 | 0 (0%) | 0 (0%) | |
| 1 | 0 (0%) | 16 (38.1%) | |
| 2 | 6 (75.0%) | 23 (54.8%) | |
| 3 | 2 (25.0%) | 3 (7.1%) | |
| ASPH score | 0.078 | ||
| 1 | 0 (0%) | 16 (38.1%) | |
| 1.5 | 3 (37.5%) | 5 (11.9%) | |
| 2 | 4 (50.0%) | 13 (31.0%) | |
| 2.5 | 0 (0%) | 4 (9.5%) | |
| 3 | 1 (12.5%) | 4 (9.5%) | |
| ASPH Score | 0.04 | ||
| <1.5 | 0 (0%) | 16 (38.1%) | |
| ≥1.5 | 8 (100%) | 26 (61.9%) |
| Recurrence (n = 10) | No Recurrence (n = 40) | p-Value | |
|---|---|---|---|
| Grade | 0.77 | ||
| 0 | 0 (0%) | 0 (0%) | |
| 1 | 2 (20.0%) | 14 (35.0%) | |
| 2 | 7 (70.0%) | 2 (55.0%) | |
| 3 | 1 (10.0%) | 4 (10.0%) | |
| ASPH score | 0.25 | ||
| 1 | 2 (20.0%) | 14 (35.0%) | |
| 1.5 | 4 (40.0%) | 4 (10.0%) | |
| 2 | 3 (30.0%) | 14 (35.0%) | |
| 2.5 | 0 (0%) | 4 (10.0%) | |
| 3 | 1 (10.0%) | 4 (10.0%) | |
| Average ASPH Score | 0.47 | ||
| <1.5 | 2 (20%) | 14 (35.0%) | |
| ≥1.5 | 8 (80%) | 26 (65.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Hart, J.; Taliano, R.; Molino, J.; Schwab, J.H.; Nota, S.; Nagaoka, K.; Zhang, S.; Olsen, M.; Carlson, R.; et al. ASPH Is a Metastatic Factor and Therapeutic Target in Chondrosarcoma. Cancers 2025, 17, 951. https://doi.org/10.3390/cancers17060951
Sun X, Hart J, Taliano R, Molino J, Schwab JH, Nota S, Nagaoka K, Zhang S, Olsen M, Carlson R, et al. ASPH Is a Metastatic Factor and Therapeutic Target in Chondrosarcoma. Cancers. 2025; 17(6):951. https://doi.org/10.3390/cancers17060951
Chicago/Turabian StyleSun, Xiaojuan, Jesse Hart, Ross Taliano, Janine Molino, Joseph H. Schwab, Sjoerd Nota, Katsuya Nagaoka, Songhua Zhang, Mark Olsen, Rolf Carlson, and et al. 2025. "ASPH Is a Metastatic Factor and Therapeutic Target in Chondrosarcoma" Cancers 17, no. 6: 951. https://doi.org/10.3390/cancers17060951
APA StyleSun, X., Hart, J., Taliano, R., Molino, J., Schwab, J. H., Nota, S., Nagaoka, K., Zhang, S., Olsen, M., Carlson, R., Wands, J., & Terek, R. M. (2025). ASPH Is a Metastatic Factor and Therapeutic Target in Chondrosarcoma. Cancers, 17(6), 951. https://doi.org/10.3390/cancers17060951






