Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies
Simple Summary
Abstract
1. Introduction
2. Characteristics of Cancer Stem Cells
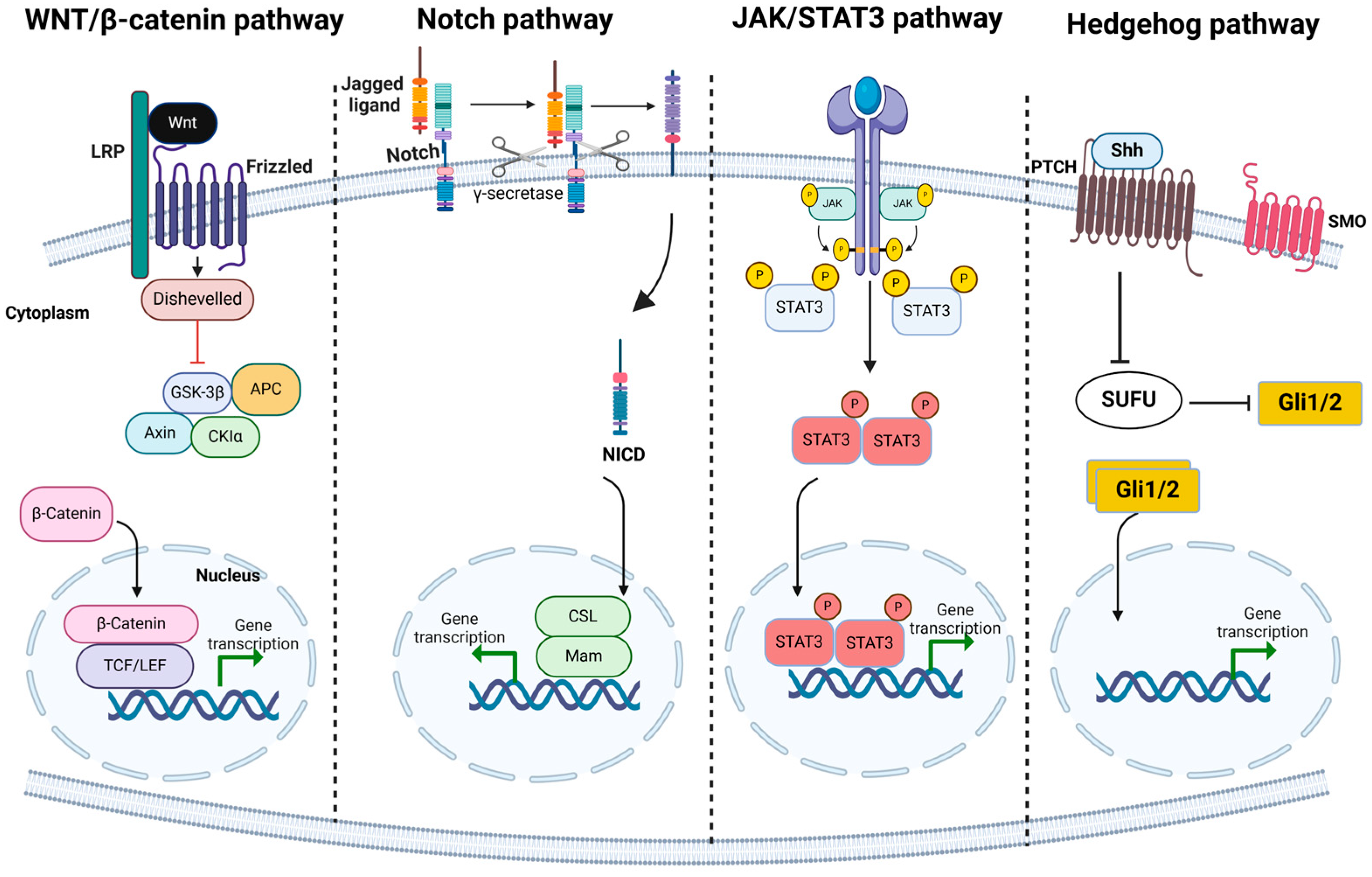
3. Molecular Markers and Signaling Pathways That Define CSCs Across Different Cancer Types
4. Techniques for Isolating and Studying CSCs in the Laboratory and Clinical Settings
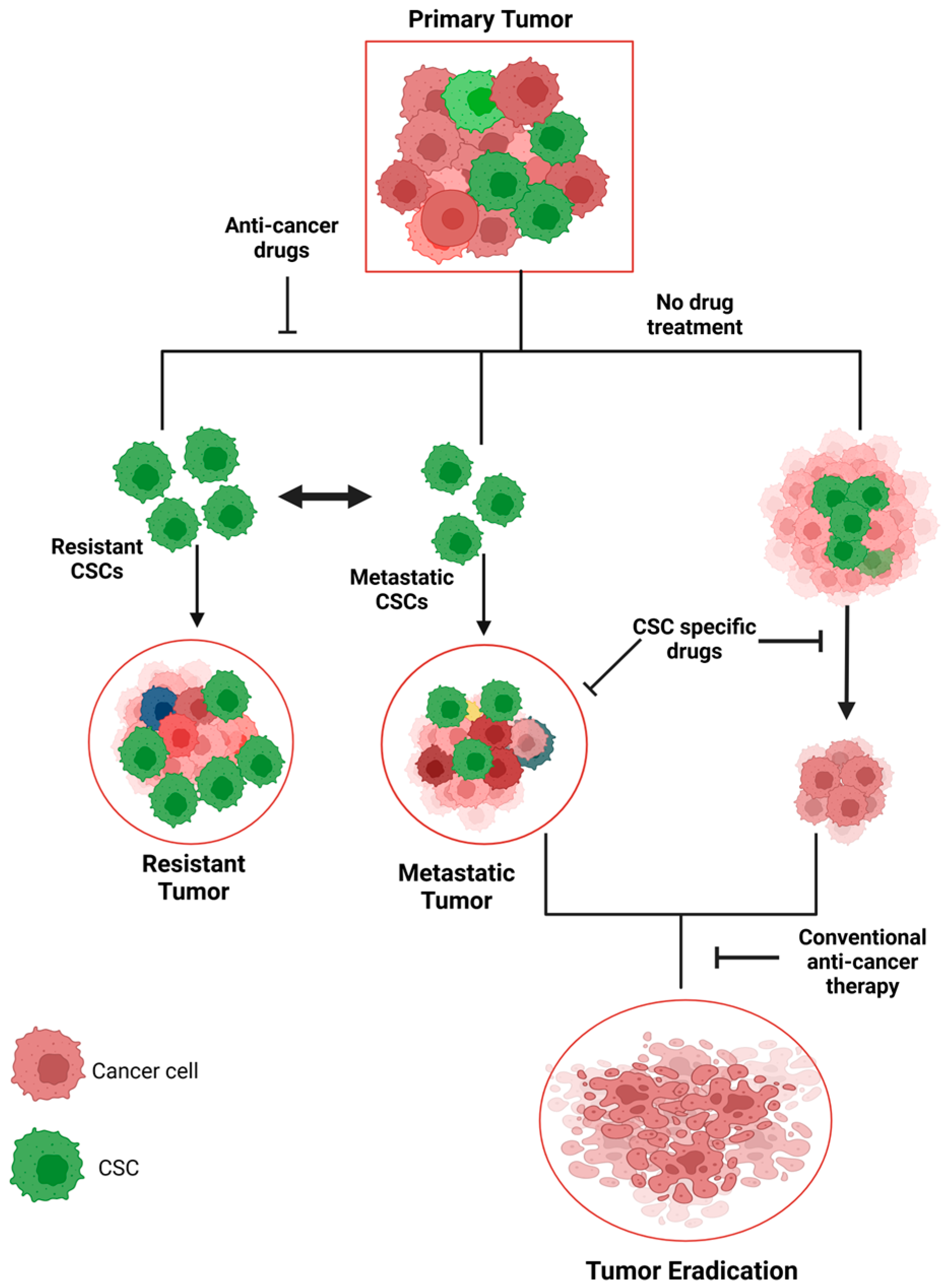
5. CSCs and Tumor Heterogeneity
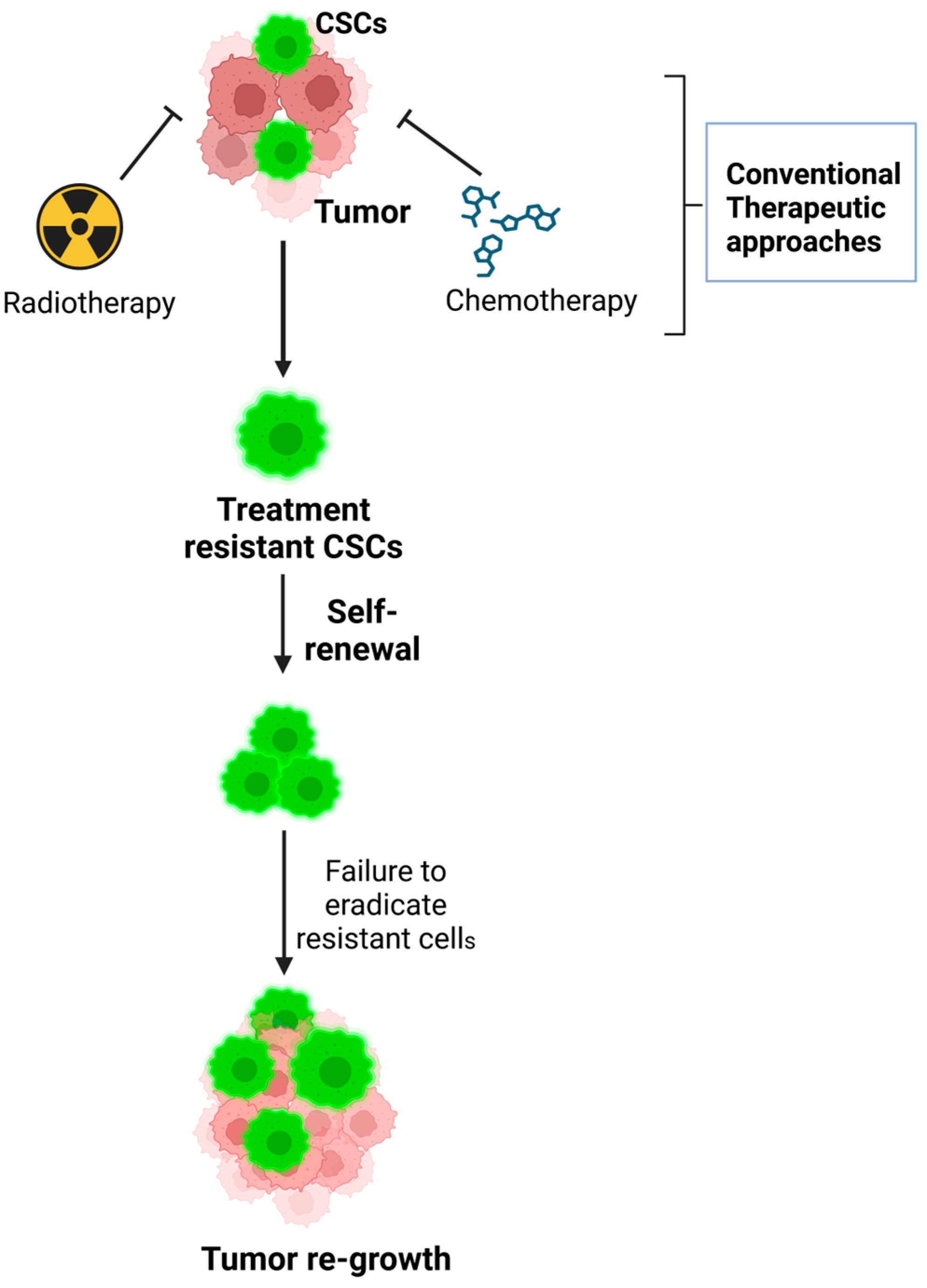
5.1. Role of Tumor Heterogeneity in Treatment Resistance
- Mechanisms of treatment resistance: CSCs play a significant role in promoting tumor treatment resistance. This resistance is attributed to several features inherent to CSCs, including the following:
- Quiescence: Many CSCs exist in a dormant or slow-dividing state, making them less susceptible to treatments like chemotherapy or radiation, which primarily target rapidly dividing cells.
- Enhanced DNA repair mechanisms: CSCs have efficient DNA repair pathways that enable them to survive the DNA damage caused by therapies.
- Drug efflux pumps: CSCs often express high levels of drug efflux pumps, such as P-glycoprotein, which actively pump out therapeutic agents, reducing their effectiveness.
5.2. Role of Tumor Heterogeneity in Disease Progression
5.3. Challenges Posed by Tumor Heterogeneity in Identifying Molecular Profiles
5.4. Strategies to Overcome Tumor Heterogeneity and Enhance Treatment Efficacy
- Targeting CSC populations: Directly targeting CSCs and their niche within the tumor could help eliminate the cells responsible for tumor initiation and relapse.
- Combination therapies: Multi-drug approaches that target different subpopulations of cancer cells within the tumor could reduce the likelihood of resistance and improve overall treatment efficacy.
- Adaptive treatment strategies: Treatment plans that can be adjusted dynamically in response to the changing tumor landscape may help counter the evolving nature of tumor heterogeneity.
6. The Dynamic Interplay Between CSCs and the Tumor Microenvironment
7. CSCs in Metastasis and Recurrence
8. Role of CSCs in Tumor Relapse and Resistance to Therapy
9. Exploration of the Concept of “Dormant” CSCs and Their Impact on Disease Recurrence
10. Strategies for Specifically Targeting CSCs, Including Novel Therapeutic Agents and Approaches
Challenges in Targeting CSCs Without Harming Normal Stem Cells
11. Review of Clinical Trials Focusing on CSC-Targeted Therapies and Their Outcomes
12. Overcoming Therapeutic Resistance Mediated by CSCs
12.1. Mechanisms of Resistance in CSCs to Conventional Chemotherapy and Radiation Therapy
12.2. Approaches to Sensitize CSCs to Current Treatments and Prevent Resistance
12.3. Potential of Combination Therapies in Effectively Targeting CSCs and Non-CSC Cancer Cells
13. Advances in Single-Cell Sequencing and Other Technologies for Dissecting CSC Heterogeneity
13.1. The Potential of CRISPR/Cas9 and Other Gene-Editing Tools in CSC Research
13.2. Role of Artificial Intelligence and Computational Models in Understanding CSC Behavior and Designing Therapies
14. Ethical Challenges in CSC Research and Therapy Development
15. The Future Landscape of CSC-Targeted Therapies and Personalized Medicine
16. Key Research Areas and Collaborations Needed to Advance the Understanding and Treatment of CSCs
17. Polar View
18. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Brien, C.A.; Kreso, A.; Jamieson, C.H. Cancer stem cells and self-renewal. Clin. Cancer Res. 2010, 16, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Maitland, N.; Bryce, S.; Stower, M.; Collins, A. Prostate cancer stem cells: A target for new therapies. In Cancer Stem Cells: Novel Concepts and Prospects for Tumor Therapy; Springer: Berlin/Heidelberg, Germany, 2007; pp. 155–179. [Google Scholar]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.-C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef]
- Burkert, J.; Wright, N.; Alison, M. Stem cells and cancer: An intimate relationship. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2006, 209, 287–297. [Google Scholar] [CrossRef]
- Lim, J.R.; Mouawad, J.; Gorton, O.K.; Bubb, W.A.; Kwan, A.H. Cancer stem cell characteristics and their potential as therapeutic targets. Med. Oncol. 2021, 38, 76. [Google Scholar] [CrossRef]
- Moitra, K.; Lou, H.; Dean, M. Multidrug efflux pumps and cancer stem cells: Insights into multidrug resistance and therapeutic development. Clin. Pharmacol. Ther. 2011, 89, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.-Z.; Ren, N. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Nie, B.; Pienta, K.J.; Morgan, T.M.; Taichman, R.S. Cancer stem cells and their role in metastasis. Pharmacol. Ther. 2013, 138, 285–293. [Google Scholar] [CrossRef]
- Alison, M.R.; Lim, S.M.; Nicholson, L.J. Cancer stem cells: Problems for therapy? J. Pathol. 2011, 223, 148–162. [Google Scholar] [CrossRef]
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer stem cells—Key players in tumor relapse. Cancers 2021, 13, 376. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef]
- Aramini, B.; Masciale, V.; Grisendi, G.; Bertolini, F.; Maur, M.; Guaitoli, G.; Chrystel, I.; Morandi, U.; Stella, F.; Dominici, M. Dissecting tumor growth: The role of cancer stem cells in drug resistance and recurrence. Cancers 2022, 14, 976. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, H.-Y. Targeting cancer stem cell markers or pathways: A potential therapeutic strategy for oral cancer treatment. Int. J. Stem Cells 2021, 14, 386–399. [Google Scholar] [CrossRef]
- Yoo, Y.D.; Kwon, Y.T. Molecular mechanisms controlling asymmetric and symmetric self-renewal of cancer stem cells. J. Anal. Sci. Technol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Matsui, W.H. Cancer stem cell signaling pathways. Medicine 2016, 95, S8–S19. [Google Scholar] [CrossRef]
- Hill, R.P. Identifying cancer stem cells in solid tumors: Case not proven. Cancer Res. 2006, 66, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Huntly, B.J.; Gilliland, D.G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat. Rev. Cancer 2005, 5, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Yu, Y.; Nautiyal, J.; Patel, B.B.; Majumdar, A.P. The Wnt/β-catenin pathway regulates growth and maintenance of colonospheres. Mol. Cancer 2010, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wakeman, T.P.; Lathia, J.D.; Hjelmeland, A.B.; Wang, X.-F.; White, R.R.; Rich, J.N.; Sullenger, B.A. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010, 28, 17–28. [Google Scholar] [CrossRef]
- Cochrane, C.R.; Szczepny, A.; Watkins, D.N.; Cain, J.E. Hedgehog signaling in the maintenance of cancer stem cells. Cancers 2015, 7, 1554–1585. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, T.; Liu, A.Y.; Ouyang, G. Differentiation and transdifferentiation potentials of cancer stem cells. Oncotarget 2015, 6, 39550. [Google Scholar] [CrossRef]
- Bussolati, B.; Bruno, S.; Grange, C.; Ferrando, U.; Camussi, G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008, 22, 3696–3705. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Sun, H.-C.; Zhang, W.; Zhu, X.-D.; Zhuang, P.-Y.; Zhang, J.-B.; Wang, L.; Wu, W.-Z.; Qin, L.-X.; Tang, Z.-Y. Human hepatocellular carcinoma tumor–derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin. Cancer Res. 2009, 15, 4838–4846. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; LLeonart, M. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019, 234, 116781. [Google Scholar] [CrossRef]
- Sulaiman, A.; McGarry, S.; Han, X.; Liu, S.; Wang, L. CSCs in breast cancer—One size does not fit all: Therapeutic advances in targeting heterogeneous epithelial and mesenchymal CSCs. Cancers 2019, 11, 1128. [Google Scholar] [CrossRef]
- Hardt, O.; Bissels, U.; Bosio, A.; Knöbel, S. Stem Cell Markers. In Stem Cells: From Basic Research to; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Bhosale, R.; Gangadharappa, H.V.; Gowda, D.V.; Osmani, R.A.M.A.; Vaghela, R.; Kulkarni, P.K.; Sairam, K.V.; Gurupadayya, B. Current perspectives on novel drug carrier systems and therapies for management of pancreatic cancer: An updated inclusive review. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 195–292. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; Graveel, C.R.; Zylstra-Diegel, C.R.; Zhong, Z.; Williams, B.O. Wnt/β-catenin signaling in normal and cancer stem cells. Cancers 2011, 3, 2050–2079. [Google Scholar] [CrossRef]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Turner, S. The role of autophagy and lncRNAs in the maintenance of cancer stem cells. Cancers 2021, 13, 6. [Google Scholar] [CrossRef]
- Liu, A.; Yu, X.; Liu, S. Pluripotency transcription factors and cancer stem cells: Small genes make a big difference. Chin. J. Cancer 2013, 32, 483. [Google Scholar] [CrossRef]
- De Wynter, E.A.; Coutinho, L.H.; Pei, X.; Marsh, J.; Hows, J.; Luft, T.; Testa, N.G. Comparison of purity and enrichment of CD34+ cells from bone marrow, umbilical cord and peripheral blood (primed for apheresis) using five separation systems. Stem Cells 1995, 13, 524–532. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Ingram, P.N.; Fouladdel, S.; McDermott, S.P.; Azizi, E.; Wicha, M.S.; Yoon, E. High-throughput single-cell derived sphere formation for cancer stem-like cell identification and analysis. Sci. Rep. 2016, 6, 27301. [Google Scholar] [CrossRef]
- Taylor, M.D.; Poppleton, H.; Fuller, C.; Su, X.; Liu, Y.; Jensen, P.; Magdaleno, S.; Dalton, J.; Calabrese, C.; Board, J. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 2005, 8, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xu, W.; Tan, J.; Liu, Z.; Huang, G.; Wang, S.; He, Z. Fluorescence detection of cancer stem cell markers using a sensitive nano-aptamer sensor. Front. Chem. 2022, 10, 920123. [Google Scholar] [CrossRef]
- Intartaglia, M.; Sabetta, R.; Gargiulo, M.; Roncador, G.; Marino, F.Z.; Franco, R. Immunohistochemistry for cancer stem cells detection: Principles and methods. In Cancer Stem Cells: Methods and Protocols; Humana Press: New York, NY, USA, 2018; pp. 195–211. [Google Scholar]
- Aiken, C.; Werbowetski-Ogilvie, T. Animal models of cancer stem cells: What are they really telling us? Curr. Pathobiol. Rep. 2013, 1, 91–99. [Google Scholar] [CrossRef][Green Version]
- Ho, D.W.; Yang, Z.F.; Yi, K.; Lam, C.T.; Ng, M.N.; Yu, W.C.; Lau, J.; Wan, T.; Wang, X.; Yan, Z. Gene expression profiling of liver cancer stem cells by RNA-sequencing. PLoS ONE 2012, 7, e37159. [Google Scholar] [CrossRef]
- Jafari, A.; Farahani, M.; Abdollahpour-Alitappeh, M.; Manzari-Tavakoli, A.; Yazdani, M.; Rezaei-Tavirani, M. Unveiling diagnostic and therapeutic strategies for cervical cancer: Biomarker discovery through proteomics approaches and exploring the role of cervical cancer stem cells. Front. Oncol. 2024, 13, 1277772. [Google Scholar] [CrossRef]
- D’Antonio, L.; Fieni, C.; Ciummo, S.L.; Vespa, S.; Lotti, L.; Sorrentino, C.; Di Carlo, E. Inactivation of interleukin-30 in colon cancer stem cells via CRISPR/Cas9 genome editing inhibits their oncogenicity and improves host survival. J. Immunother. Cancer 2023, 11, e006056. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Yang, Y.; Zhong, C.; Ji, T.; Duan, J.; Wang, Y. RNAi mediated silencing of Nanog expression suppresses the growth of human colorectal cancer stem cells. Biochem. Biophys. Res. Commun. 2021, 534, 254–260. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, C.; An, C.; Zheng, X.; Wen, S.; Chen, W.; Liu, X.; Lv, Z.; Yang, P.; Xu, W.; et al. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Mol. Cancer 2021, 20, 126. [Google Scholar] [CrossRef]
- Greve, B.; Kelsch, R.; Spaniol, K.; Eich, H.T.; Götte, M. Flow cytometry in cancer stem cell analysis and separation. Cytom. Part A 2012, 81, 284–293. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Cheaito, K.; Chalhoub, R.M.; Hadadeh, O.; Monzer, A.; Ballout, F.; El-Hajj, A.; Mukherji, D.; Liu, Y.-N.; Daoud, G. Sphere-formation assay: Three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front. Oncol. 2018, 8, 347. [Google Scholar] [CrossRef]
- Balic, M.; Rapp, N.; Stanzer, S.; Lin, H.; Strutz, J.; Szkandera, J.; Daidone, M.G.; Samonigg, H.; Cote, R.J.; Dandachi, N. Novel immunofluorescence protocol for multimarker assessment of putative disseminating breast cancer stem cells. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Margaritescu, C.; Pirici, D.; Cherciu, I.; Barbalan, A.; Cârtân, T.; Saftoiu, A. CD133/CD166/Ki-67 triple immunofluorescence assessment for putative cancer stem cells in colon carcinoma. J. Gastrointest. Liver Dis. 2014, 23, 161–170. [Google Scholar] [CrossRef]
- Bao, B.; Azmi, A.S.; Li, Y.; Ahmad, A.; Ali, S.; Banerjee, S.; Kong, D.; Sarkar, F.H. Targeting CSCs in tumor microenvironment: The potential role of ROS-associated miRNAs in tumor aggressiveness. Curr. Stem Cell Res. Ther. 2014, 9, 22–35. [Google Scholar] [CrossRef]
- Yang, X.-G.; Zhu, L.-C.; Wang, Y.-J.; Li, Y.-Y.; Wang, D. Current advance of therapeutic agents in clinical trials potentially targeting tumor plasticity. Front. Oncol. 2019, 9, 887. [Google Scholar] [CrossRef]
- Shiino, S.; Tokura, M.; Nakayama, J.; Yoshida, M.; Suto, A.; Yamamoto, Y. Investigation of Tumor Heterogeneity Using Integrated Single-Cell RNA Sequence Analysis to Focus on Genes Related to Breast Cancer-, EMT-, CSC-, and Metastasis-Related Markers in Patients with HER2-Positive Breast Cancer. Cells 2023, 12, 2286. [Google Scholar] [CrossRef]
- Jung, H.J. Chemical proteomic approaches targeting cancer stem cells: A review of current literature. Cancer Genom. Proteom. 2017, 14, 315–327. [Google Scholar]
- Zhao, R.; Kaakati, R.; Liu, X.; Xu, L.; Lee, A.K.; Bachelder, R.; Li, C.-Y.; Hollenbeck, S.T. CRISPR/Cas9-mediated BRCA1 knockdown adipose stem cells promote breast cancer progression. Plast. Reconstr. Surg. 2019, 143, 747–756. [Google Scholar] [CrossRef]
- Chen, J.; Pan, Y.; He, B.; Ying, H.; Wang, F.; Sun, H.; Deng, Q.; Liu, X.; Lin, K.; Peng, H. Inhibition of CD147 expression by RNA interference reduces proliferation, invasion and increases chemosensitivity in cancer stem cell-like HT-29 cells. Int. J. Oncol. 2015, 47, 1476–1484. [Google Scholar] [CrossRef][Green Version]
- Liang, D.; Fang, Z.; Dong, M.; Liang, C.; Xing, C.; Zhao, J.; Yang, Y. Effect of RNA interference-related HiWi gene expression on the proliferation and apoptosis of lung cancer stem cells. Oncol. Lett. 2012, 4, 146–150. [Google Scholar] [CrossRef]
- Yadav, A.K.; Desai, N.S. Cancer stem cells: Acquisition, characteristics, therapeutic implications, targeting strategies and future prospects. Stem Cell Rev. Rep. 2019, 15, 331–355. [Google Scholar] [CrossRef]
- Kapoor-Narula, U.; Lenka, N. Cancer stem cells and tumor heterogeneity: Deciphering the role in tumor progression and metastasis. Cytokine 2022, 157, 155968. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Raza, S.S.; Haque, R. Transcriptional factors targeting in cancer stem cells for tumor modulation. Semin. Cancer Biol. 2023, 88, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Vasefifar, P.; Motafakkerazad, R.; Maleki, L.A.; Najafi, S.; Ghrobaninezhad, F.; Najafzadeh, B.; Alemohammad, H.; Amini, M.; Baghbanzadeh, A.; Baradaran, B. Nanog, as a key cancer stem cell marker in tumor progression. Gene 2022, 827, 146448. [Google Scholar] [CrossRef]
- Gong, S.; Li, Q.; Jeter, C.R.; Fan, Q.; Tang, D.G.; Liu, B. Regulation of NANOG in cancer cells. Mol. Carcinog. 2015, 54, 679–687. [Google Scholar] [CrossRef]
- Lau, E.Y.-T.; Ho, N.P.-Y.; Lee, T.K.-W. Cancer stem cells and their microenvironment: Biology and therapeutic implications. Stem Cells Int. 2017, 2017, 3714190. [Google Scholar] [CrossRef]
- Kise, K.; Kinugasa-Katayama, Y.; Takakura, N. Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev. 2016, 99, 197–205. [Google Scholar] [CrossRef]
- Li, Y.-R.; Fang, Y.; Lyu, Z.; Zhu, Y.; Yang, L. Exploring the dynamic interplay between cancer stem cells and the tumor microenvironment: Implications for novel therapeutic strategies. J. Transl. Med. 2023, 21, 686. [Google Scholar] [CrossRef]
- Wu, B.; Shi, X.; Jiang, M.; Liu, H. Cross-talk between cancer stem cells and immune cells: Potential therapeutic targets in the tumor immune microenvironment. Mol. Cancer 2023, 22, 38. [Google Scholar] [CrossRef]
- Li, Z.; Bao, S.; Wu, Q.; Wang, H.; Eyler, C.; Sathornsumetee, S.; Shi, Q.; Cao, Y.; Lathia, J.; McLendon, R.E. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 2009, 15, 501–513. [Google Scholar] [CrossRef]
- Seidel, S.; Garvalov, B.K.; Wirta, V.; Von Stechow, L.; Schänzer, A.; Meletis, K.; Wolter, M.; Sommerlad, D.; Henze, A.-T.; Nister, M. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2α. Brain 2010, 133, 983–995. [Google Scholar] [CrossRef]
- Bhat, K.M. Notch signaling acts before cell division to promote asymmetric cleavage and cell fate of neural precursor cells. Sci. Signal. 2014, 7, ra101. [Google Scholar] [CrossRef] [PubMed]
- Heddleston, J.M.; Li, Z.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 2009, 8, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- Galassi, C.; Musella, M.; Manduca, N.; Maccafeo, E.; Sistigu, A. The immune privilege of cancer stem cells: A key to understanding tumor immune escape and therapy failure. Cells 2021, 10, 2361. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Coyle, K.M.; Vidovic, D.; Thomas, M.L.; Gujar, S.; Marcato, P. Hide-and-seek: The interplay between cancer stem cells and the immune system. Carcinogenesis 2017, 38, 107–118. [Google Scholar] [CrossRef]
- Patel, P.; Chen, E.I. Cancer stem cells, tumor dormancy, and metastasis. Front. Endocrinol. 2012, 3, 125. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, W.-J.; Wang, Z.-Q. Cancer stem cells and the tumor microenvironment in gastric cancer. Front. Oncol. 2022, 11, 803974. [Google Scholar] [CrossRef]
- Liang, L.; Kaufmann, A.M. The significance of cancer stem cells and epithelial–mesenchymal transition in metastasis and anti-cancer therapy. Int. J. Mol. Sci. 2023, 24, 2555. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The role of tumor associated macrophages (TAMs) in cancer progression, chemoresistance, angiogenesis and metastasis-current status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef]
- Muppala, S. Significance of the tumor microenvironment in liver cancer progression. Crit. Rev.™ Oncog. 2020, 25, 1–9. [Google Scholar] [CrossRef]
- Nengroo, M.A.; Verma, A.; Datta, D. Cytokine chemokine network in tumor microenvironment: Impact on CSC properties and therapeutic applications. Cytokine 2022, 156, 155916. [Google Scholar] [CrossRef]
- Su, Y.-C.; Li, S.-C.; Wu, Y.-C.; Wang, L.-M.; Chao, K.C.; Liao, H.-F. Research Article Resveratrol Downregulates Interleukin-6-Stimulated Sonic Hedgehog Signaling in Human Acute Myeloid Leukemia. Evid.-Based Complement. Altern. Med. 2013, 2013, 547430. [Google Scholar] [CrossRef] [PubMed]
- Sipos, F.; Műzes, G. Cancer stem cell relationship with pro-tumoral inflammatory microenvironment. Biomedicines 2023, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Z.; Zhu, Y.; Chen, J.; Li, W. Role of hypoxia inducible factor-1 in cancer stem cells. Mol. Med. Rep. 2021, 23, 17. [Google Scholar] [CrossRef]
- Wang, D. Towards a More Personalized Approach in the Treatment of Esophageal Cancer Focusing on Predictive Factors in Response to Chemoradiation. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2017. [Google Scholar]
- El Marsafy, S.; Larghero, J. Cancer Cell De-Differentiation: Plasticity-Driven Stratagem For Tumor Metastasis and Recurrence. Curr. Stem Cell Res. Ther. 2023, 18, 54–61. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.-J.; Wu, J. The Tumor Immune Microenvironment plays a key role in driving the progression of cholangiocarcinoma. Curr. Cancer Drug Targets 2024, 24, 681–700. [Google Scholar] [CrossRef]
- Li, S.; Li, Q. Cancer stem cells and tumor metastasis. Int. J. Oncol. 2014, 44, 1806–1812. [Google Scholar] [CrossRef]
- Lei, Z.-N.; Teng, Q.-X.; Koya, J.; Liu, Y.; Chen, Z.; Zeng, L.; Chen, Z.-S.; Fang, S.; Wang, J.; Liu, Y. The correlation between cancer stem cells and epithelial-mesenchymal transition: Molecular mechanisms and significance in cancer theragnosis. Front. Immunol. 2024, 15, 1417201. [Google Scholar] [CrossRef]
- Tanabe, S. Epithelial–Mesenchymal Transition and Cancer Stem Cells. In Cancer Stem Cell Markers and Related Network Pathways; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–49. [Google Scholar]
- Li, F.; Tiede, B.; Massagué, J.; Kang, Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res. 2007, 17, 3–14. [Google Scholar] [CrossRef]
- Liu, X.; Fan, D. The epithelial-mesenchymal transition and cancer stem cells: Functional and mechanistic links. Curr. Pharm. Des. 2015, 21, 1279–1291. [Google Scholar] [CrossRef]
- Jing, Y.; Han, Z.; Zhang, S.; Liu, Y.; Wei, L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci. 2011, 1, 29. [Google Scholar] [CrossRef]
- Kim, B.N.; Ahn, D.H.; Kang, N.; Yeo, C.D.; Kim, Y.K.; Lee, K.Y.; Kim, T.-J.; Lee, S.H.; Park, M.S.; Yim, H.W. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci. Rep. 2020, 10, 10597. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-l.; Zhang, M.; Tang, Y.-l.; Liang, X.-h. Cancer cell dormancy: Mechanisms and implications of cancer recurrence and metastasis. OncoTargets Ther. 2017, 10, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Lima, L.G.; Möller, A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Strippoli, R.; Niayesh-Mehr, R.; Adelipour, M.; Khosravi, A.; Cordani, M.; Zarrabi, A.; Allameh, A. Contribution of Autophagy to Epithelial Mesenchymal Transition Induction during Cancer Progression. Cancers 2024, 16, 807. [Google Scholar] [CrossRef]
- Salehloo, E.B.; Mozdoori, N.; Esmatabadi, M.J.D.; Hajigholami, S.; Bozorgmehr, A. Cancer Stem Cell Biomarkers: Critical Roles, Challenges, Clinical Application, and Perspectives in Cancer Therapy. Basic Clin. Cancer Res. 2022, 13, 156–174. [Google Scholar]
- Sravani, A.; Chandrasekaran, N. Overview: Cancer Stem Cells. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Springer: Cham, Switzerland, 2024; pp. 1–18. [Google Scholar]
- Pourmahdi, M.; Saber, A.; Rajabi, A.; Abdolahi, S.; Ebrahimi, P.; Safaralizadeh, R. Key epigenetic events involved in the maintenance of breast cancer stem cells. Curr. Stem Cell Res. Ther 2021, 16, 877–887. [Google Scholar] [CrossRef]
- Li, S.C.; Vu, L.T.; Luo, J.J.; Zhong, J.F.; Li, Z.; Dethlefs, B.A.; Loudon, W.G.; Kabeer, M.H. Tissue elasticity bridges cancer stem cells to the tumor microenvironment through microRNAs: Implications for a “watch-and-wait” approach to cancer. Curr. Stem Cell Res. Ther. 2017, 12, 455–470. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Jolly, M.K. Cancer stem cells and epithelial-to-mesenchymal transition in cancer metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a036905. [Google Scholar] [CrossRef]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef]
- Amissah, H.A.; Combs, S.E.; Shevtsov, M. Tumor dormancy and reactivation: The role of heat shock proteins. Cells 2024, 13, 1087. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Devo, P.; Goodall, I.C.; Sirlantzis, K.; Ghose, A.; Shinde, S.D.; Papadopoulos, V.; Sanchez, E.; Rassy, E.; Ovsepian, S.V. Exosomes in the diagnosis and treatment of renal cell cancer. Int. J. Mol. Sci. 2023, 24, 14356. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Masciale, V.; Banchelli, F.; Grisendi, G.; D’Amico, R.; Maiorana, A.; Stefani, A.; Morandi, U.; Stella, F.; Dominici, M.; Aramini, B. The influence of cancer stem cells on the risk of relapse in adenocarcinoma and squamous cell carcinoma of the lung: A prospective cohort study. Stem Cells Transl. Med. 2022, 11, 239–247. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug resistance and Cancer stem cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef]
- Robinson, N.J.; Parker, K.A.; Schiemann, W.P. Epigenetic plasticity in metastatic dormancy: Mechanisms and therapeutic implications. Ann. Transl. Med. 2020, 8, 903. [Google Scholar] [CrossRef]
- Chen, W.; Dong, J.; Haiech, J.; Kilhoffer, M.-C.; Zeniou, M. Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem Cells Int. 2016, 2016, 1740936. [Google Scholar] [CrossRef]
- Lv, S.; Liu, Y.; Xie, C.; Xue, C.; Du, S.; Yao, J. Emerging role of interactions between tumor angiogenesis and cancer stem cells. J. Control. Release 2023, 360, 468–481. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar]
- Talukdar, S.; Bhoopathi, P.; Emdad, L.; Das, S.; Sarkar, D.; Fisher, P.B. Dormancy and cancer stem cells: An enigma for cancer therapeutic targeting. Adv. Cancer Res. 2019, 141, 43–84. [Google Scholar]
- Francescangeli, F.; De Angelis, M.L.; Rossi, R.; Cuccu, A.; Giuliani, A.; De Maria, R.; Zeuner, A. Dormancy, stemness, and therapy resistance: Interconnected players in cancer evolution. Cancer Metastasis Rev. 2023, 42, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, J.; Sun, J.; Hou, K.; Yang, C.; Guo, Y.; Liu, X.; Kalvakolanu, D.V.; Zhang, L.; Guo, B. Epigenetic regulation of cancer stem cells: Shedding light on the refractory/relapsed cancers. Biochem. Pharmacol. 2022, 202, 115110. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Musella, M.; Galassi, C.; Vitale, I.; De Maria, R. Tuning cancer fate: Tumor microenvironment’s role in cancer stem cell quiescence and reawakening. Front. Immunol. 2020, 11, 2166. [Google Scholar] [CrossRef]
- Ismaeel, G.L.; Abdul-Hussein, A.H.; Qasim, H.M.; Abed, N.K.; Jalil, A.T.; Suleiman, A.A.; Dilfy, S.H. Therapeutic targeting of dormant cancer stem cells in solid tumors. Gene Rep. 2023, 30, 101717. [Google Scholar] [CrossRef]
- Lee, S.H.; Reed-Newman, T.; Anant, S.; Ramasamy, T.S. Regulatory role of quiescence in the biological function of cancer stem cells. Stem Cell Rev. Rep. 2020, 16, 1185–1207. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, R. Stem cell quiescence. Clin. Cancer Res. 2011, 17, 4936–4941. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC radioresistance: A therapeutic challenge to improve radiotherapy effectiveness in cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef]
- Ferrer, A.I.; Trinidad, J.R.; Sandiford, O.; Etchegaray, J.-P.; Rameshwar, P. Epigenetic dynamics in cancer stem cell dormancy. Cancer Metastasis Rev. 2020, 39, 721–738. [Google Scholar] [CrossRef]
- Jiang, X.; Liang, L.; Chen, G.; Liu, C. Modulation of immune components on stem cell and dormancy in cancer. Cells 2021, 10, 2826. [Google Scholar] [CrossRef]
- Jin, L.; Hope, K.J.; Zhai, Q.; Smadja-Joffe, F.; Dick, J.E. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006, 12, 1167–1174. [Google Scholar] [CrossRef]
- Bar, E.E.; Chaudhry, A.; Lin, A.; Fan, X.; Schreck, K.; Matsui, W.; Piccirillo, S.; Vescovi, A.L.; DiMeco, F.; Olivi, A. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 2007, 25, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Nobusue, H.; Saya, H. Targeting of cancer stem cells by differentiation therapy. Cancer Sci. 2020, 111, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Guo, Y.; Hu, Y.; Bao, X.; Zhu, X.; Fu, Q.; Zhang, H.; Tong, Z.; Liu, L.; Zheng, Y. Immunotherapy for targeting cancer stem cells in hepatocellular carcinoma. Theranostics 2021, 11, 3489. [Google Scholar] [CrossRef] [PubMed]
- Badrinath, N.; Yoo, S.Y. Recent advances in cancer stem cell-targeted immunotherapy. Cancers 2019, 11, 310. [Google Scholar] [CrossRef]
- Wang, T.; Narayanaswamy, R.; Ren, H.; Torchilin, V.P. Combination therapy targeting both cancer stem-like cells and bulk tumor cells for improved efficacy of breast cancer treatment. Cancer Biol. Ther. 2016, 17, 698–707. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Cojoc, M.; Mäbert, K.; Muders, M.H.; Dubrovska, A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin. Cancer Biol. 2015, 31, 16–27. [Google Scholar] [CrossRef]
- Dragu, D.L.; Necula, L.G.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Therapies targeting cancer stem cells: Current trends and future challenges. World J. Stem Cells 2015, 7, 1185. [Google Scholar] [CrossRef]
- Hazafa, A.; Mumtaz, M.; Farooq, M.F.; Bilal, S.; Chaudhry, S.N.; Firdous, M.; Naeem, H.; Ullah, M.O.; Yameen, M.; Mukhtiar, M.S. CRISPR/Cas9: A powerful genome editing technique for the treatment of cancer cells with present challenges and future directions. Life Sci. 2020, 263, 118525. [Google Scholar] [CrossRef]
- Ni, M.; Xiong, M.; Zhang, X.; Cai, G.; Chen, H.; Zeng, Q.; Yu, Z. Poly (lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. Int. J. Nanomed. 2015, 10, 2537–2554. [Google Scholar]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S.V. Hyaluronic acid-based nanogel–drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjugate Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, W.-L.; Guo, J.; Du, J.; Li, T.; Wu, J.-W.; Wang, G.-L.; Wang, J.-C.; Zhang, X.; Zhang, Q. A potential target associated with both cancer and cancer stem cells: A combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J. Control. Release 2008, 129, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fiori, M.E.; Villanova, L.; De Maria, R. Cancer stem cells: At the forefront of personalized medicine and immunotherapy. Curr. Opin. Pharmacol. 2017, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M. Normal stem cells and cancer stem cells: Similar and different. Semin. Cancer Biol. 2010, 20, 85–92. [Google Scholar] [CrossRef]
- Kim, W.-T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285. [Google Scholar] [CrossRef]
- Thankamony, A.P.; Saxena, K.; Murali, R.; Jolly, M.K.; Nair, R. Cancer stem cell plasticity–a deadly deal. Front. Mol. Biosci. 2020, 7, 79. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in cancer: Stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Lan, L.; Behrens, A. Are there specific cancer stem cell markers? Cancer Res. 2023, 83, 170–172. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, Q.; Li, J.; Zhang, K.; Qin, J.; Zhao, J.; Sun, Q.; Wang, Z.; Wartmann, T.; Jauch, K.W. Targeting cancer stem cells and their niche: Perspectives for future therapeutic targets and strategies. Semin. Cancer Biol. 2018, 53, 139–155. [Google Scholar] [CrossRef]
- Marquardt, S.; Solanki, M.; Spitschak, A.; Vera, J.; Puetzer, B.M. Emerging functional markers for cancer stem cell-based therapies: Understanding signaling networks for targeting metastasis. Semin. Cancer Biol. 2018, 53, 90–109. [Google Scholar] [CrossRef]
- Desai, A.; Yan, Y.; Gerson, S.L. Concise reviews: Cancer stem cell targeted therapies: Toward clinical success. Stem Cells Transl. Med. 2019, 8, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Yoshino, T.; Tebbutt, N.C.; Grothey, A.; Tabernero, J.; Xu, R.-H.; Cervantes, A.; Oh, S.C.; Yamaguchi, K.; Fakih, M. Napabucasin plus FOLFIRI in patients with previously treated metastatic colorectal cancer: Results from the open-label, randomized phase III canStem303C study. Clin. Color. Cancer 2023, 22, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.; Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2019, 33, 379–389. [Google Scholar] [CrossRef]
- Siu, L.; Papadopoulos, K.; Alberts, S.; Kirchoff-Ross, R.; Vakkalagadda, B.; Lang, L.; Ahlers, C.; Bennett, K.; Van Tornout, J. A first-in-human, phase I study of an oral hedgehog (HH) pathway antagonist, BMS-833923 (XL139), in subjects with advanced or metastatic solid tumors. J. Clin. Oncol. 2010, 28 (Suppl. S15), 2501. [Google Scholar] [CrossRef]
- Gracian, A.C.; Jameson, M.B.; Grande, E.; Cooray, P.; Parnis, F.; Grimison, P.; Jeffery, M.; Stagg, R.J.; Dupont, J.; Tebbutt, N.C. A Phase 1b Study of the Anticancer Stem Cell Agent Demcizumab (DEM) and Gemcitabine (GEM) with or Without Paclitaxel Protein Bound Particles (Nab-Paclitaxel) in Patients with Pancreatic Cancer; American Society of Clinical Oncology: Alexandria, VA, USA, 2014. [Google Scholar]
- Locatelli, M.A.; Aftimos, P.; Dees, E.C.; LoRusso, P.M.; Pegram, M.D.; Awada, A.; Huang, B.; Cesari, R.; Jiang, Y.; Shaik, M.N. Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer. Oncotarget 2016, 8, 2320. [Google Scholar] [CrossRef]
- Brenner, A.J.; Peters, K.B.; Vredenburgh, J.; Bokstein, F.; Blumenthal, D.T.; Yust-Katz, S.; Peretz, I.; Oberman, B.; Freedman, L.S.; Ellingson, B.M. Safety and efficacy of VB-111, an anticancer gene therapy, in patients with recurrent glioblastoma: Results of a phase I/II study. Neuro-Oncol. 2020, 22, 694–704. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Takahashi, R.-u.; Usuba, W.; Kohama, I.; Ochiya, T. Drug resistance driven by cancer stem cells and their niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Nathansen, J.; Meyer, F.; Müller, L.; Schmitz, M.; Borgmann, K.; Dubrovska, A. Beyond the double-strand breaks: The role of DNA repair proteins in cancer stem-cell regulation. Cancers 2021, 13, 4818. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed]
- Chenna, V.; Hu, C.; Pramanik, D.; Aftab, B.T.; Karikari, C.; Campbell, N.R.; Hong, S.-M.; Zhao, M.; Rudek, M.A.; Khan, S.R. A polymeric nanoparticle encapsulated small-molecule inhibitor of Hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to Smoothened antagonists. Mol. Cancer Ther. 2012, 11, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-X.; Gao, D.; Shao, Z.-Z.; Chen, L.; Ding, W.-J.; Yu, Q.-F. Wnt/β-catenin signaling: Causes and treatment targets of drug resistance in colorectal cancer. Mol. Med. Rep. 2021, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- BeLow, M.; Osipo, C. Notch signaling in breast cancer: A role in drug resistance. Cells 2020, 9, 2204. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Das, T.; Damodaran, C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br. J. Cancer 2013, 109, 2587–2596. [Google Scholar] [CrossRef]
- Caruana, B.T.; Byrne, F.L. The NF-κB signalling pathway regulates GLUT6 expression in endometrial cancer. Cell. Signal. 2020, 73, 109688. [Google Scholar] [CrossRef]
- Garza-Treviño, E.N.; Martínez-Rodríguez, H.G.; Delgado-González, P.; Solís-Coronado, O.; Ortíz-Lopez, R.; Soto-Domínguez, A.; Treviño, V.M.; Padilla-Rivas, G.R.; Islas-Cisneros, J.F.; Quiroz-Reyes, A.G. Chemosensitivity analysis and study of gene resistance on tumors and cancer stem cell isolates from patients with colorectal cancer. Mol. Med. Rep. 2021, 24, 721. [Google Scholar] [CrossRef]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef]
- Dai, L.; Li, C.; Shedden, K.A.; Lee, C.J.; Li, C.; Quoc, H.; Simeone, D.M.; Lubman, D.M. Quantitative proteomic profiling studies of pancreatic cancer stem cells. J. Proteome Res. 2010, 9, 3394–3402. [Google Scholar] [CrossRef]
- Mahgoub, E.O.; Cho, W.C.; Sharifi, M.; Falahati, M.; Zeinabad, H.A.; Mare, H.E.; Hasan, A. Role of functional genomics in identifying cancer drug resistance and overcoming cancer relapse. Heliyon 2024, 10, e22095. [Google Scholar] [CrossRef]
- Asghari, F.; Khademi, R.; Ranjbar, F.E.; Malekshahi, Z.V.; Majidi, R.F. Application of nanotechnology in targeting of cancer stem cells: A review. Int. J. Stem Cells 2019, 12, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination therapy with vitamin C could eradicate cancer stem cells. Biomolecules 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Shen, Y.; Chen, X.; He, J.; Liu, J.; Zu, X. Self-renewal signalling pathway inhibitors: Perspectives on therapeutic approaches for cancer stem cells. OncoTargets Ther. 2020, 13, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cang, W.; Li, Q.; Liao, X.; Zhan, M.; Deng, H.; Li, S.; Jin, W.; Pang, Z.; Qiu, X. Erlotinib overcomes paclitaxel-resistant cancer stem cells by blocking the EGFR-CREB/GRβ-IL-6 axis in MUC1-positive cervical cancer. Oncogenesis 2019, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Tsujii, M.; Kondo, J.; Hayashi, Y.; Kato, M.; Akasaka, T.; Inoue, T.; Shiraishi, E.; Inoue, T.; Hiyama, S. The effectiveness of an anti-human IL-6 receptor monoclonal antibody combined with chemotherapy to target colon cancer stem-like cells. Int. J. Oncol. 2015, 46, 1551–1559. [Google Scholar] [CrossRef]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 205. [Google Scholar] [CrossRef]
- Zheng, H.; Pomyen, Y.; Hernandez, M.O.; Li, C.; Livak, F.; Tang, W.; Dang, H.; Greten, T.F.; Davis, J.L.; Zhao, Y. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology 2018, 68, 127–140. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, F.; Yang, X.; Han, T.; Long, Z.; Wen, J.; Huang, J.; Shen, J.; Guo, Q. Single-cell sequencing technology applied to epigenetics for the study of tumor heterogeneity. Clin. Epigenetics 2023, 15, 161. [Google Scholar] [CrossRef]
- Jia, X.; Shen, G.; Jia, J.; Zhang, Y.; Zhang, D.; Li, W.; Zhang, J.; Huang, X.; Tian, J. Lineage Tracing and Molecular Real-Time Imaging of Cancer Stem Cells. Biosensors 2022, 12, 703. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Zaman, T.; Chowdhury, F.; Mraiche, F.; Tariq, M.; Ahmad, I.S.; Hasan, A. Single-cell RNA sequencing with spatial transcriptomics of cancer tissues. Int. J. Mol. Sci. 2022, 23, 3042. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wong, A.S. CRISPR screening in cancer stem cells. Essays Biochem. 2022, 66, 305–318. [Google Scholar] [PubMed]
- Wang, Y.; Wu, J.; Chen, H.; Yang, Y.; Xiao, C.; Yi, X.; Shi, C.; Zhong, K.; He, H.; Li, Y. Genome-wide CRISPR-Cas9 screen identified KLF11 as a druggable suppressor for sarcoma cancer stem cells. Sci. Adv. 2021, 7, eabe3445. [Google Scholar] [CrossRef]
- Czerwińska, P.; Mazurek, S.; Kołodziejczak, I.; Wiznerowicz, M. Gene delivery methods and genome editing of human pluripotent stem cells. Rep. Pract. Oncol. Radiother. 2019, 24, 180–187. [Google Scholar] [CrossRef]
- Davis, D.; Stokoe, D. Zinc finger nucleases as tools to understand and treat human diseases. BMC Med. 2010, 8, 42. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Jia, Y.; Wang, J.; Sang, R.; Zhang, Y.; Rong, R. Single-cell sequencing in the field of stem cells. Curr. Genom. 2020, 21, 576–584. [Google Scholar] [CrossRef]
- Saber, A.; Liu, B.; Ebrahimi, P.; Haisma, H.J. CRISPR/Cas9 for overcoming drug resistance in solid tumors. DARU J. Pharm. Sci. 2020, 28, 295–304. [Google Scholar] [CrossRef]
- Khatami, F.; Aghamir, Z.S.; Jahanshahi, F.; Feiz-Abadi, S.A.; Birang, F.; Khoshchehreh, M.; Shabestari, A.N.; Aghamir, S.M.K. The gene manipulation and cellular immunotherapy combination in the treatment of cancer. Iran. J. Biotechnol. 2022, 20, e3094. [Google Scholar]
- Liang, G.; Fan, W.; Luo, H.; Zhu, X. The emerging roles of artificial intelligence in cancer drug development and precision therapy. Biomed. Pharmacother. 2020, 128, 110255. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Li, X.; Park, S. Deep learning models for cancer stem cell detection: A brief review. Front. Immunol. 2023, 14, 1214425. [Google Scholar] [CrossRef] [PubMed]
- Lobanova, O.A.; Kolesnikova, A.O.; Ponomareva, V.A.; Vekhova, K.A.; Shaginyan, A.L.; Semenova, A.B.; Nekhoroshkov, D.P.; Kochetkova, S.E.; Kretova, N.V.; Zanozin, A.S. Artificial intelligence (AI) for tumor microenvironment (TME) and tumor budding (TB) identification in colorectal cancer (CRC) patients: A systematic review. J. Pathol. Inform. 2024, 15, 100353. [Google Scholar] [CrossRef] [PubMed]
- Sigal, D.; Przedborski, M.; Sivaloganathan, D.; Kohandel, M. Mathematical modelling of cancer stem cell-targeted immunotherapy. Math. Biosci. 2019, 318, 108269. [Google Scholar] [CrossRef]
- Marcu, L.G.; Marcu, D. In silico modelling of a cancer stem cell-targeting agent and its effects on tumour control during radiotherapy. Sci. Rep. 2016, 6, 32332. [Google Scholar] [CrossRef]
- Khatami, F.; Tavangar, S.M.; Pour, N.K. Genomics, Proteomics, and Metabolomics of Cancer Stem Cells (CSCs). In Genomics, Proteomics, and Metabolomics. Stem Cell Biology and Regenerative Medicine; Humana: Cham, Switzerland, 2019; pp. 159–179. [Google Scholar]
- Ye, Z.; Zheng, M.; Zeng, Y.; Wei, S.; Wang, Y.; Lin, Z.; Shu, C.; Xie, Y.; Zheng, Q.; Chen, L. Bioinformatics analysis reveals an association between cancer cell stemness, gene mutations, and the immune microenvironment in stomach adenocarcinoma. Front. Genet. 2020, 11, 595477. [Google Scholar] [CrossRef]
- Dogan, E.; Kisim, A.; Bati-Ayaz, G.; Kubicek, G.J.; Pesen-Okvur, D.; Miri, A.K. Cancer stem cells in tumor modeling: Challenges and future directions. Adv. Nanobiomed Res. 2021, 1, 2100017. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.-K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer stem cells—Origins and biomarkers: Perspectives for targeted personalized therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Corti, C.; Cobanaj, M.; Dee, E.C.; Criscitiello, C.; Tolaney, S.M.; Celi, L.A.; Curigliano, G. Artificial intelligence in cancer research and precision medicine: Applications, limitations and priorities to drive transformation in the delivery of equitable and unbiased care. Cancer Treat. Rev. 2023, 112, 102498. [Google Scholar] [CrossRef]
- Hanai, Y.; Ishihata, H.; Zhang, Z.; Maruyama, R.; Kasai, T.; Kameda, H.; Sugiyama, T. Temporal and locational values of images affecting the deep learning of cancer stem cell morphology. Biomedicines 2022, 10, 941. [Google Scholar] [CrossRef]
- Cianciosi, D.; Ansary, J.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Gracia Villar, S.; Garcia Villena, E.; Tutusaus Pifarre, K.; Alvarez-Suarez, J.M.; Battino, M. The molecular basis of different approaches for the study of cancer stem cells and the advantages and disadvantages of a three-dimensional culture. Molecules 2021, 26, 2615. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Guo, T.; Nie, D.-Y.; Zhu, Y.-X.; Lin, M. Advances of nanotechnology applied to cancer stem cells. World J. Stem Cells 2023, 15, 514. [Google Scholar] [CrossRef] [PubMed]
- Cortina, C.; Turon, G.; Stork, D.; Hernando-Momblona, X.; Sevillano, M.; Aguilera, M.; Tosi, S.; Merlos-Suárez, A.; Stephan-Otto Attolini, C.; Sancho, E. A genome editing approach to study cancer stem cells in human tumors. EMBO Mol. Med. 2017, 9, 869–879. [Google Scholar] [CrossRef]
- Dianat-Moghadam, H.; Mahari, A.; Salahlou, R.; Khalili, M.; Azizi, M.; Sadeghzadeh, H. Immune evader cancer stem cells direct the perspective approaches to cancer immunotherapy. Stem Cell Res. Ther. 2022, 13, 150. [Google Scholar] [CrossRef]


| Characteristic | Cancer Stem Cells (CSCs) | Regular Cancer Cells |
|---|---|---|
| Self-renewal | CSCs can self-renew, maintaining their population over time. They divide symmetrically to produce more CSCs or a CSC and a non-CSC. | Regular cancer cells do not exhibit the same self-renewal ability and have limited proliferative potential. |
| Differentiation | CSCs can differentiate into multiple cell types within the tumor, contributing to cellular diversity. | Regular cancer cells have limited differentiation potential and often form a homogeneous population. |
| Transdifferentiation | CSCs can undergo transdifferentiation, converting into different cell types (e.g., endothelial cells for angiogenesis), promoting tumor growth. | Regular cancer cells typically do not exhibit transdifferentiation or the ability to form specialized cell types. |
| Resistance to Chemotherapy | CSCs are highly resistant to chemotherapy, employing mechanisms like DNA repair, drug efflux pumps, and cell cycle arrest. | Regular cancer cells are more susceptible to chemotherapy, particularly during the proliferative phases of the cell cycle. |
| Role in Tumor Formation | CSCs are responsible for initiating and sustaining tumor growth, driving tumor progression and metastasis. | Regular cancer cells contribute to tumor mass but are less involved in tumor initiation and persistence. |
| Involvement in Tumor Progression | CSCs drive tumor progression and recurrence, even after chemotherapy, due to their ability to survive treatments. | Regular cancer cells are more likely to be eliminated by standard therapies and do not contribute to tumor recurrence as effectively as CSCs. |
| Cancer Type | Molecular Markers | Key Signaling Pathways Involved | Role of Pathways in CSC Biology |
|---|---|---|---|
| Breast Cancer | CD44+/CD24−/low, ALDH+ | Wnt/β-catenin, Notch, Hedgehog | Self-renewal, differentiation, and resistance to therapies |
| Melanoma | CD34+CD38− | Notch, Wnt/β-catenin | Tumor initiation, progression, and metastasis |
| Glioblastoma | CD133 | Notch, Hedgehog, Wnt/β-catenin | Self-renewal, differentiation into endothelial cells, tumor relapse |
| Colorectal Cancer | CD44+ | Wnt/β-catenin | Self-renewal and expansion of CSCs |
| Pancreatic Cancer | CD133, CD44+ | Notch, Hedgehog | Tumorigenesis and differentiation potential |
| Leukemia | CD34+ | Hedgehog | Maintenance of CSC properties and chemoresistance |
| Technique | Short Principle | Advantages | Limitations | Application | References |
|---|---|---|---|---|---|
| Flow Cytometry and Cell Sorting | Uses fluororeacted antibodies to bind to specific CSC surface markers, enabling the isolation of CSCs from the bulk tumor population. | Highly specific and quantitative; allows for precise identification and separation of CSCs. | Limited to known CSC markers; requires expensive equipment and specialized expertise. | Isolation and analysis of CSCs for further studies. | [43,52] |
| Sphere Formation Assay | Measures CSCs’ ability to grow in non-adherent, serum-free conditions, forming spheroids. This assesses self-renewal and enriches CSC populations for further analysis. | Reflects CSC self-renewal ability; easy to perform and provides a functional readout of CSC activity. | Does not perfectly mimic the in vivo environment; can be influenced by culture conditions. | Studying CSC proliferation, self-renewal, and enrichment for further analysis. | [5,53] |
| Immunohistochemistry (IHC) | Uses antibodies to detect CSC markers in tissue samples, providing location and quantity of CSCs within the tumor. | Visualizes CSCs in tissue context, offering spatial information on their distribution. | Limited by the availability of specific antibodies; requires proper tissue preservation and preparation. | Localization and quantification of CSCs in tumor tissues. | [45] |
| Immunofluorescence (IF) | Fluorescently labeled antibodies bind to CSC-specific markers to visualize their presence and distribution in tissue or cultured cells. | Provides high-resolution images and helps in co-localization studies of multiple markers. | Requires specialized equipment (fluorescent microscopes); potential for signal overlap in multiplex assays. | Study of CSC marker expression and distribution in cells and tissues. | [54,55] |
| CSC Transplantation in Immunocompromised Mice | Involves transplanting CSCs into immunocompromised mice to observe tumorigenic potential by their ability to generate tumors. | Directly tests the tumorigenic potential of CSCs, considered the “gold standard” for evaluating their cancer-initiating capacity. | Ethical concerns; long duration; requires appropriate animal models and conditions. | Evaluating the tumorigenic potential of CSCs. | [56,57] |
| RNA Sequencing (RNA-seq) | Uses next-generation sequencing to analyze the transcriptome of CSCs, identifying unique gene expression profiles. | High throughput; provides comprehensive gene expression data; helps in understanding CSC molecular characteristics. | Expensive; requires bioinformatics expertise for data analysis; large amount of tissue may be required. | Identifying gene expression profiles and pathways specific to CSCs. | [58] |
| Proteomics | Analyzes protein expression profiles of CSCs to understand their functional roles and molecular pathways. | Provides a detailed view of the proteome; helps identify biomarkers and therapeutic targets. | Complex data interpretation; requires extensive data analysis; high cost. | Identifying proteins and pathways associated with CSC functions. | [59] |
| CRISPR-Cas9 | Gene-editing tool that enables targeted mutations in CSCs to study the role of specific genes in CSC proliferation and differentiation. | Allows precise modification of genes in CSCs; can be used to identify essential genes and pathways for CSC functions. | Requires optimization for CSC culture; off-target effects; technical expertise required. | Gene functional studies to identify key genes involved in CSC self-renewal and differentiation. | [60] |
| RNA Interference (RNAi) | Uses small RNA molecules to suppress the expression of target genes in CSCs, helping identify essential pathways. | High specificity for gene silencing; enables functional analysis of specific genes. | Off-target effects; transient gene knockdown; challenges with delivery into CSCs. | Studying gene function in CSCs and identifying potential therapeutic targets. | [61,62] |
| Study/Research Article | Key Findings | Implications |
|---|---|---|
| [68,81] | The TME plays a pivotal role in the growth, progression, spread, and resistance to treatment in cancer. | The TME shields CSCs, allowing for their survival, contributing to cancer growth and metastasis. |
| [69,82] | The TME consists of various cell types, such as immune cells, fibroblasts, endothelial cells, and extracellular matrix components, that create a supportive environment for CSCs. | CSCs thrive in this environment, receiving signals that enable their survival, self-renewal, and differentiation. |
| [70,83] | CSCs communicate with the TME through signaling pathways, cytokines, and growth factors, regulating their own proliferation, differentiation, and death. | This intercommunication allows CSCs to maintain their stemness properties and resist therapeutic interventions. |
| [71,84] | CSCs release factors that recruit and differentiate stromal cells and immune cells to promote a supportive TME. | The recruitment of stromal and immune cells enhances the growth and survival of CSCs, aiding in tumor progression. |
| [72,85] | Hypoxic conditions in tumors, caused by inadequate vascularization, influence the CSC-TME relationship. | Hypoxia in tumors leads to altered metabolic and signaling pathways that support CSC survival. |
| [73,86] | Hypoxia-inducible factors (HIFs) are overexpressed in CSCs in low oxygen conditions, enhancing stemness characteristics, angiogenesis, and metabolic adaptation. | HIF overexpression in CSCs promotes their ability to survive and adapt in the harsh tumor microenvironment. |
| [74,87] | Hypoxia regulates critical CSC signaling pathways (Wnt and Notch), inducing epithelial-to-mesenchymal transition (EMT) and increasing their invasiveness and resistance to therapies. | EMT enables CSCs to become more invasive, increasing their potential for metastasis and resistance to treatment. |
| [75,88] | The interaction between CSCs and the TME enhances CSC survival and contributes to tumor progression. | This relationship is critical for tumor growth and metastasis, making it a target for therapeutic strategies. |
| [76,83] | The TME helps CSCs evade immune system detection through cytokine secretion and the expression of co-inhibitory signals. | Immune escape mechanisms in CSCs allow them to survive and evade immune surveillance, promoting disease persistence. |
| [77,89] | CSCs alter the immune microenvironment by secreting immunosuppressive cytokines or by inhibiting immune responses through co-inhibitory signals. | These immune-modulating actions enable CSCs to resist immune attacks, which complicates cancer treatment. |
| [32,78,79,90] | CSCs and the TME play a significant role in metastasis. | The interaction of CSCs with the TME facilitates metastasis, as they can colonize distant organs and form secondary tumors. |
| [80,91] | CSCs exposed to stimuli from the TME undergo epithelial-to-mesenchymal transition (EMT), enabling them to move and invade other tissues. | EMT is essential for CSCs to metastasize, facilitating the spread of cancer to other parts of the body. |
| General Understanding | CSCs and TME are interdependent; TME supports CSCs’ self-renewal, survival, and metastatic potential, while CSCs regulate and manipulate the TME. | The interrelationship between CSCs and TME is central to tumor development, progression, immune escape, and treatment resistance. Developing therapies that target both CSCs and their niche could lead to better therapeutic outcomes and tumor control. |
| Research Article | Focus | Outcome/Findings | Significance |
|---|---|---|---|
| [14,101] | CSCs in Metastasis | CSCs are essential in metastasis, facilitating the migration of cancer cells from the primary tumor. | Highlighted the crucial role of CSCs in cancer metastasis. |
| [92,102] | CSC Migration in Metastasis | CSCs help in the migration from primary tumors by mediating EMT (epithelial-to-mesenchymal transition). | Demonstrated that CSCs enhance cell migration and invasion capacities, aiding metastasis. |
| [93,94,103] | CSCs and EMT in Metastasis | CSCs undergo EMT, a process enhancing their invasive properties, aiding detachment, and spreading through blood and lymphatic systems. | Explained how EMT facilitates CSC survival in circulation and metastasis. |
| [95,96,104] | EMT Signaling Pathways | Dysregulated signaling pathways (TGF-β, Wnt/β-catenin, Notch) in CSCs mediate EMT. | Focused on how environmental and signaling factors contribute to CSC-driven metastasis. |
| [95,96,97,104,105] | CSC Survival in Circulation | CSCs withstand circulatory system stress and evade immune detection, leading to higher survival rates in harsh environments. | Emphasized the resilience of CSCs in hostile environments like the bloodstream. |
| [106,107] | CSCs and Micrometastasis | CSCs can undergo mesenchymal-to-epithelial transition in distant sites to form micrometastases. | Showed the process by which CSCs can re-establish growth in secondary sites. |
| [97,108] | Dormancy and Recurrence of CSCs | CSCs can remain dormant for extended periods and reactivate upon stimuli, leading to delayed recurrence. | Provided insight into how CSCs contribute to delayed cancer recurrence. |
| [18,98] | CSCs in Pre-Metastatic Niche Formation | CSCs help form pre-metastatic niches in target organs, modulating the tissue environment for future tumor growth. | Highlighted CSCs’ role in preparing distant tissues for metastatic growth. |
| [99,109] | Exosomes from Renal CSCs | Exosomes from CD105+ renal CSCs enhance angiogenesis and kidney cancer metastasis to lungs by increasing VEGF and MMP-2 in lung endothelial cells. | Showed how CSC-derived exosomes can influence angiogenesis and metastasis. |
| [100,110] | Exosomes in Melanoma Metastasis | Exosomes from metastatic melanoma enhance metastasis by stimulating bone marrow progenitors and forming pre-metastatic niches. | Demonstrated the metastatic role of CSC-derived exosomes in melanoma. |
| General Observation | Role of CSCs in Metastasis and Recurrence | CSCs drive metastasis via EMT, survive harsh conditions, and establish micrometastases. CSCs contribute to recurrence through prolonged dormancy and reactivation. | Emphasized the critical role of CSCs in metastasis and cancer recurrence, with a need for targeted therapies. |
| Research Article | Key Findings | Outcomes | Conclusions |
|---|---|---|---|
| Study on CSC quiescence in therapy resistance [111,113,114] | CSCs remain in a quiescent state after treatment, enabling them to evade therapeutic stress | CSCs survive therapy and are capable of reactivating, leading to tumor relapse | Tumor relapse occurs due to CSCs entering a dormant state and evading treatment; new strategies are needed to target these cells |
| CSC DNA repair and drug efflux mechanisms [31,112] | CSCs have superior DNA repair capabilities and higher levels of drug efflux pumps | CSCs are less susceptible to DNA damage from chemotherapy, leading to drug resistance | The enhanced repair capacity and drug pump activity make CSCs resistant to conventional therapies |
| Mechanisms of CSC self-renewal and tumor recurrence [16,113] | CSCs can self-renew post-treatment, leading to genetic and epigenetic alterations | Genetic and epigenetic changes enable CSCs to form a new resistant tumor | CSCs contribute to tumor recurrence through self-renewal and the evolution of resistant and aggressive clones |
| Role of CSCs in TME regulation and angiogenesis [113,115] | CSCs regulate the tumor microenvironment (TME) and promote angiogenesis | CSCs contribute to tumor recurrence by influencing the TME and stimulating angiogenesis | Targeting CSC regulation of the TME and angiogenesis may offer new treatment approaches to prevent relapse |
| Targeted therapies for CSCs [113,116] | CSC-targeted therapies, including sensitization to conventional drugs and immunotherapies | Potential therapies can target CSCs and reverse their resistance to conventional treatments | New therapies targeting CSC pathways and their microenvironment may be effective in overcoming resistance and preventing relapse |
| Research Article | Key Findings | Outcomes | Conclusions |
|---|---|---|---|
| Study on quiescence of dormant CSCs [117,122,123] | Dormant CSCs are in a quiescent state with reduced mitotic and oxidative activities | Dormant CSCs evade conventional therapies by staying in a dormant state, unaffected by treatments targeting dividing cells | Dormant CSCs contribute to disease recurrence by surviving treatment, underscoring the need for strategies targeting this quiescent state |
| CSC response to therapeutic pressure [118,124] | Dormant CSCs can survive treatment and remain latent until therapeutic pressure is relieved | When therapeutic pressure is removed, dormant CSCs reactivate and lead to tumor relapse | Disease relapse occurs due to the reactivation of dormant CSCs, which can regenerate tumors that are more invasive and resistant to prior therapies |
| Genetic and epigenetic alterations in dormant CSCs [119,125] | Dormant CSCs may undergo genetic and epigenetic changes during their latent period | These alterations contribute to more aggressive and therapy-resistant tumor recurrence | Genetic and epigenetic changes in dormant CSCs lead to more aggressive disease upon reactivation, necessitating new therapeutic approaches |
| Role of TME factors in regulating CSC dormancy [120,126] | Factors such as hypoxia, starvation, and interactions with other cells in the TME regulate CSC quiescence | Dormant CSCs are difficult to predict and target due to the influence of the TME on their state | The TME plays a crucial role in maintaining CSC dormancy, and understanding these interactions is key to preventing relapse |
| Targeting dormant CSCs for therapeutic strategies [117,121] | Strategies to target dormant CSCs include disrupting their survival pathways, altering the tumor niche, and enhancing immune responses | These approaches aim to eradicate dormant CSCs or prevent their reactivation | New therapeutic strategies targeting dormant CSCs, their survival pathways, and the tumor environment may enable permanent remission and improved outcomes |
| Research Article | Key Findings | Outcomes | Conclusions |
|---|---|---|---|
| Monoclonal antibody targeting CD44 [127,147] | Monoclonal antibody against CD44 reduced acute myeloid leukemia in vivo by over 50% | Targeting CD44, a marker for CSCs, reduces tumor burden in vivo, highlighting its potential for CSC-targeted therapy | CD44-targeted therapy shows promise in reducing CSCs and limiting tumor progression |
| Cyclopamine inhibition of Hh pathway in glioblastoma stem cells [128] | Cyclopamine blocks the Hh pathway in glioblastoma stem cells, reducing growth by 40–60% | Reduction in growth of glioma cell lines with high Gli1 expression, and no neurosphere formation | Targeting the Hh pathway in glioblastoma stem cells effectively inhibits growth, presenting a viable CSC-targeting strategy |
| Differentiation therapy for CSCs [129] | Differentiation therapy aims to induce CSC differentiation into non-tumorigenic cells | Reduces CSC pool and enhances sensitivity to conventional therapies | Differentiation therapy is a novel approach to depleting CSCs and improving tumor response to treatment |
| Immunotherapy targeting CSCs [130] | Immunotherapies, including CAR-T cell therapy and CSC vaccination, trigger immune response against CSC markers | Increased immune response against CSCs, leading to tumor reduction | Immunotherapy holds promise for overcoming tumor suppressive environments and targeting CSCs |
| Targeting the CSC niche in the tumor microenvironment [56,133] | Strategies include inhibiting angiogenesis, targeting stromal cells, and altering extracellular matrix | Prevents CSC mobility and limits nutrient supply, reducing CSC survival | Modulating the CSC niche in the tumor microenvironment presents an effective way to target CSCs while preserving normal tissue function |
| Combination therapies with CSC-targeted drugs [132,134,135] | Combining CSC-targeted drugs with chemotherapy or radiation therapy enhances efficacy | Targeting both CSCs and bulk tumor cells reduces recurrence and progression | Combination therapies that target CSCs alongside conventional treatments may prevent tumor relapse and progression |
| RNAi and CRISPR/Cas9 for gene modification [50,51,86,136] | RNAi and CRISPR/Cas9 used to knock down or modify genes essential for CSC survival | Vulnerability of CSCs to treatment is enhanced, leading to improved outcomes | Gene modification technologies such as RNAi and CRISPR/Cas9 are valuable tools for making CSCs more susceptible to therapy |
| Nanoparticles targeting CSC-specific markers [137,138] | Nanoparticles can be designed to target CSC-specific markers and enhance tumor penetration | Increased specificity and efficacy of CSC-targeted therapies | Nanotechnology holds potential for delivering drugs more effectively to CSCs, enhancing therapy precision |
| Personalized CSC-targeted therapies [140] | Personalized therapy tailored to CSC characteristics in specific patients | More effective and individualized treatment strategies based on CSC variability across tumors | Personalized therapies are crucial for addressing the heterogeneity of CSCs and ensuring treatment efficacy |
| Challenges in targeting CSCs without affecting normal stem cells [141,142] | CSCs and normal stem cells share similar properties, complicating targeted therapy | Some therapies designed for CSCs may also affect normal stem cells, causing toxicity | Identifying specific markers and pathways for CSCs, along with local delivery and temporal targeting, may minimize toxicity and enhance specificity |
| Strategies for overcoming challenges in CSC targeting [145,146] | Approaches include targeting CSC-specific markers, using local drug delivery, and temporal targeting | These strategies reduce the risk of toxicity to normal stem cells and improve treatment efficacy | Advances in precision medicine and drug delivery systems hold promise for developing safe and effective CSC-targeted therapies |
| Cancer Stem Cell (CSC)-Targeted Therapeutics/Article | Trial Phase | Cancer Type | Outcome | Limitations | Practical Implications | Summarized Abstract |
|---|---|---|---|---|---|---|
| BBI608 (Napabucasin) [149] | Phase III | Colorectal Cancer | Did not meet primary endpoint of overall survival improvement | High toxicity, lack of efficacy | Need for better biomarkers to select responsive patient populations | Napabucasin, an investigational CSC-targeting agent, combined with FOLFIRI, failed to improve overall survival in metastatic colorectal cancer patients compared to the use FOLFIRI alone. |
| GDC-0449 (Vismodegib) [150] | Phase II | Basal Cell Carcinoma | Showed significant tumor reduction in advanced cases | Development of resistance, side effects like muscle spasms | Effective in targeting Hedgehog pathway, but requires combination with other therapies to prevent resistance | Vismodegib, a Hedgehog pathway inhibitor, demonstrated significant tumor reduction in patients with advanced basal cell carcinoma, though resistance and muscle spasms were notable issues. |
| Olaparib (Lynparza) [151] | Phase II | Ovarian Cancer | Prolonged progression-free survival in patients with BRCA mutations | Limited to patients with specific genetic mutations | Highlights importance of genetic screening in CSC-targeted therapy | Olaparib maintenance therapy significantly prolonged progression-free survival in ovarian cancer patients with BRCA mutations, underscoring the necessity of genetic screening. |
| PF-04449913 (Glasdegib) [152] | Phase II | Acute Myeloid Leukemia | Improved survival in combination with low-dose cytarabine | Limited efficacy as monotherapy, high cost | Potential in combination therapy, especially in older patients | Glasdegib combined with low-dose cytarabine improved survival in elderly patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndromes. |
| BMS-833923 (XL139) [153] | Phase I | Solid Tumors | Some tumor shrinkage observed | Early trial phase, small sample size | Encouraging results warrant further investigation in larger trials | BMS-833923, targeting the Hedgehog pathway, showed preliminary signs of tumor shrinkage in patients with advanced solid tumors, meriting further research. |
| Demcizumab (OMP-21M18) [154] | Phase I/II | Pancreatic Cancer | Some evidence of delayed tumor progression | Cardiotoxicity, transient efficacy | Indicates potential but requires combination with cardioprotective agents | Demcizumab, targeting DLL4, exhibited potential in delaying tumor progression in pancreatic cancer but faced cardiotoxicity challenges. |
| PF-03084014 (Gamma-secretase inhibitor) [155] | Phase I | Breast Cancer | Partial response in some patients | Not all patients respond, gastrointestinal toxicity | Suggests need for patient selection and combination with other treatments | The gamma-secretase inhibitor PF-03084014 induced partial responses in advanced triple-negative breast cancer patients, highlighting the need for combination strategies. |
| VB-111 (Ofra-Vec) [156] | Phase I/II | Glioblastoma | Extended progression-free survival | Limited overall survival benefit, side effects | Demonstrates role of anti-angiogenic approach in CSC targeting | VB-111, an anti-angiogenic virotherapy, showed extended progression-free survival in recurrent glioblastoma patients, though overall survival benefit was limited. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Tanani, M.; Rabbani, S.A.; Satyam, S.M.; Rangraze, I.R.; Wali, A.F.; El-Tanani, Y.; Aljabali, A.A.A. Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies. Cancers 2025, 17, 382. https://doi.org/10.3390/cancers17030382
El-Tanani M, Rabbani SA, Satyam SM, Rangraze IR, Wali AF, El-Tanani Y, Aljabali AAA. Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies. Cancers. 2025; 17(3):382. https://doi.org/10.3390/cancers17030382
Chicago/Turabian StyleEl-Tanani, Mohamed, Syed Arman Rabbani, Shakta Mani Satyam, Imran Rashid Rangraze, Adil Farooq Wali, Yahia El-Tanani, and Alaa A. A. Aljabali. 2025. "Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies" Cancers 17, no. 3: 382. https://doi.org/10.3390/cancers17030382
APA StyleEl-Tanani, M., Rabbani, S. A., Satyam, S. M., Rangraze, I. R., Wali, A. F., El-Tanani, Y., & Aljabali, A. A. A. (2025). Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies. Cancers, 17(3), 382. https://doi.org/10.3390/cancers17030382








