Unveiling Another Dimension: Advanced Visualization of Cancer Invasion and Metastasis via Micro-CT Imaging
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Animal Care
2.2. Patient-Derived Tumor Collection
2.3. Establishment of the Orthotopic Patient-Derived Xenograft Model
2.4. Sample Preparation
2.5. Micro-CT Imaging
2.6. Micro-CT Data Analysis
2.7. Histopathological Analysis
3. Results
3.1. Method Validation
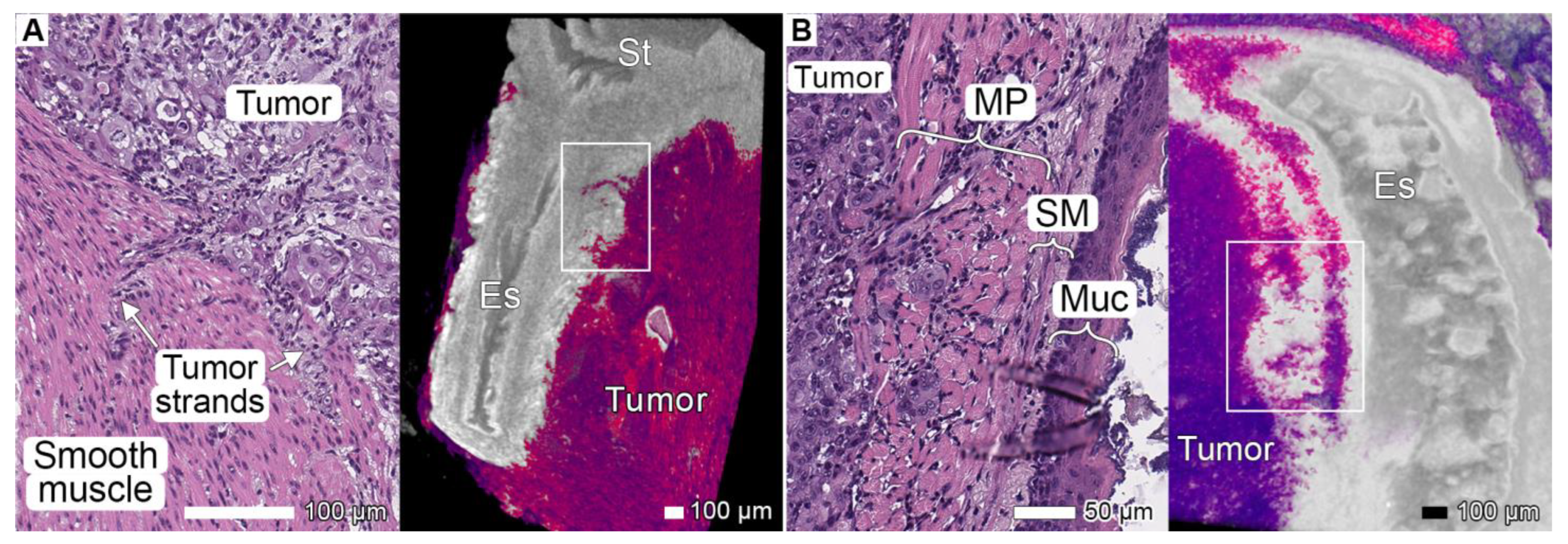
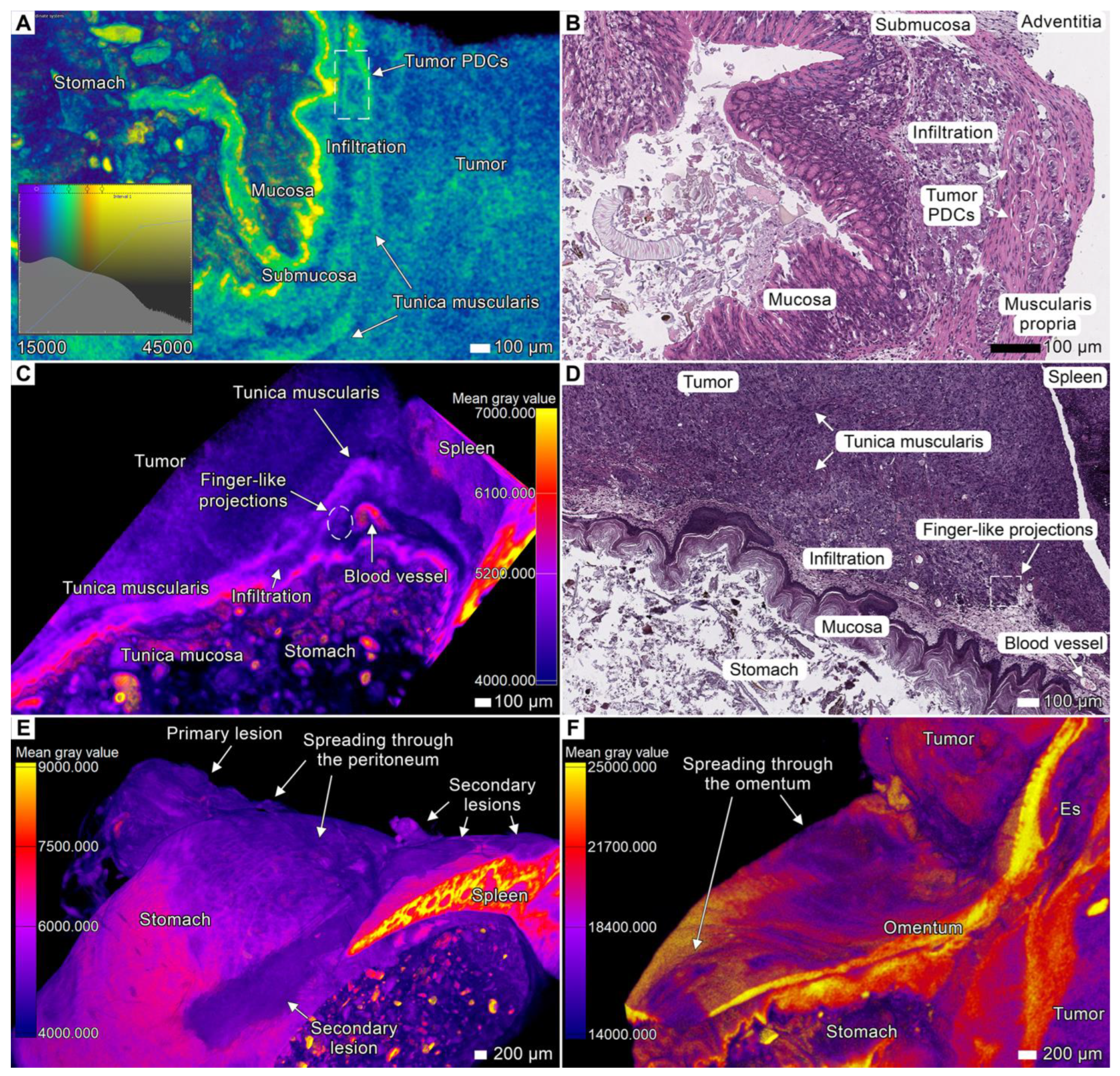
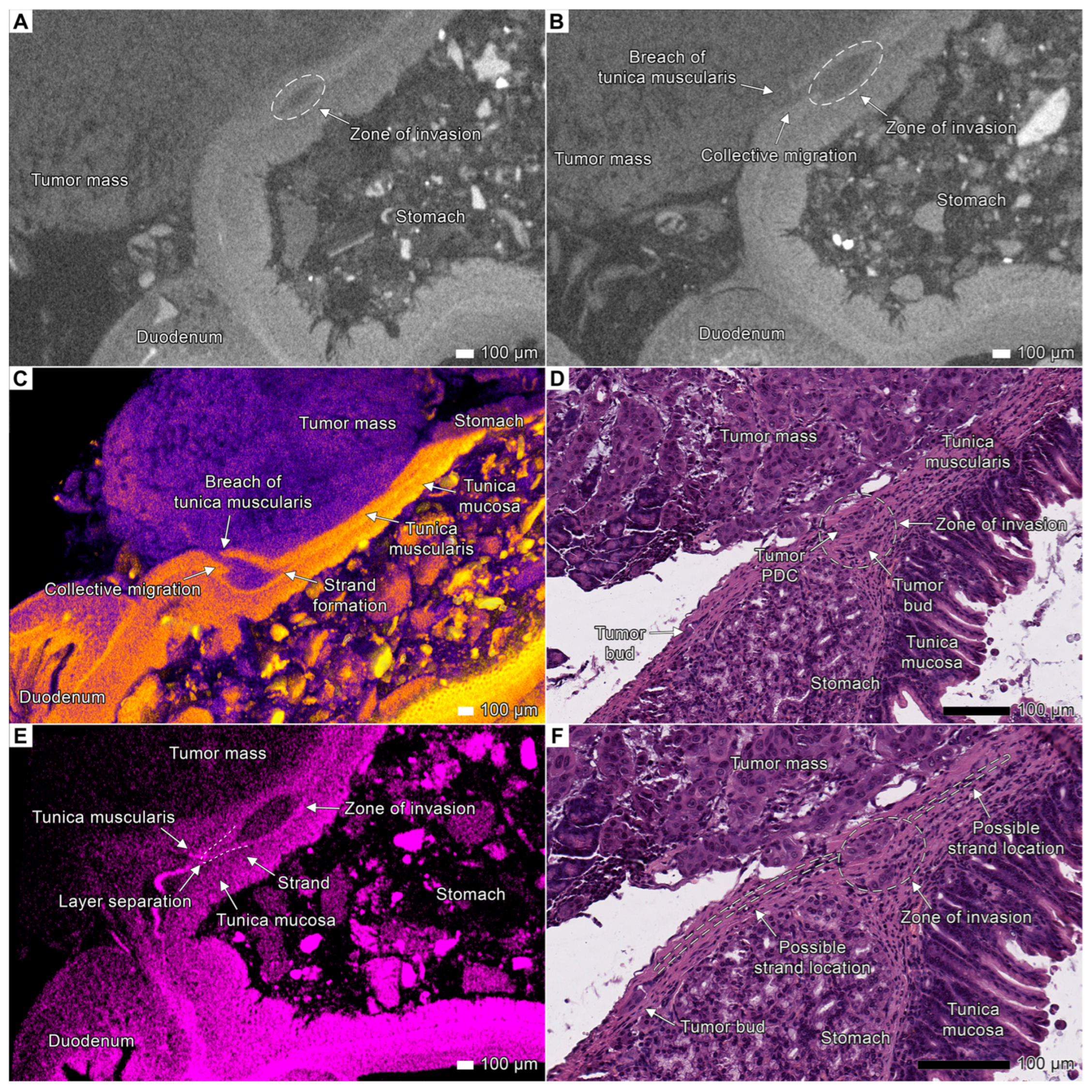
3.2. Tumor Invasion into the Esophagus and Stomach
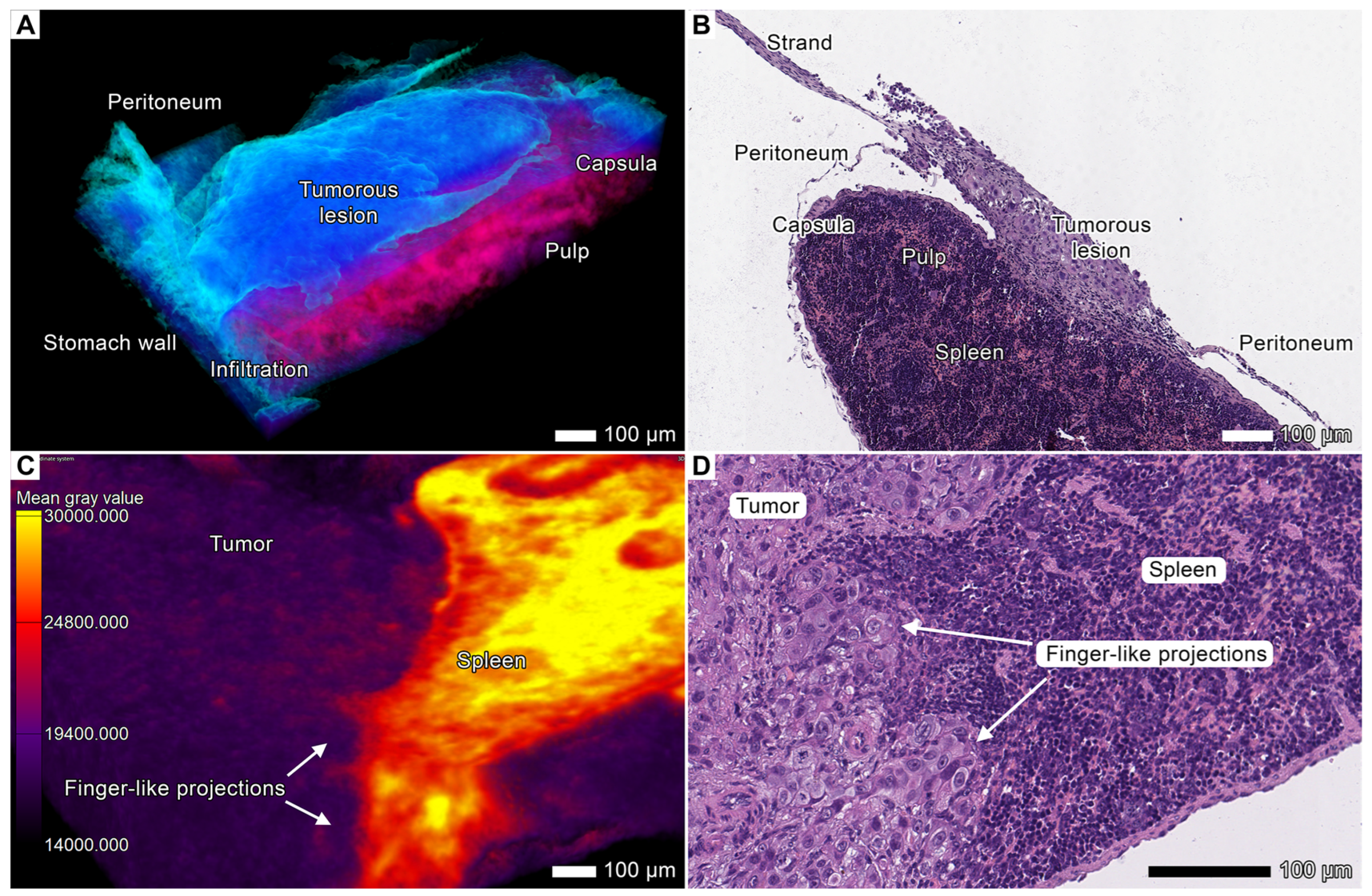
3.3. Tumor Invasion into the Spleen
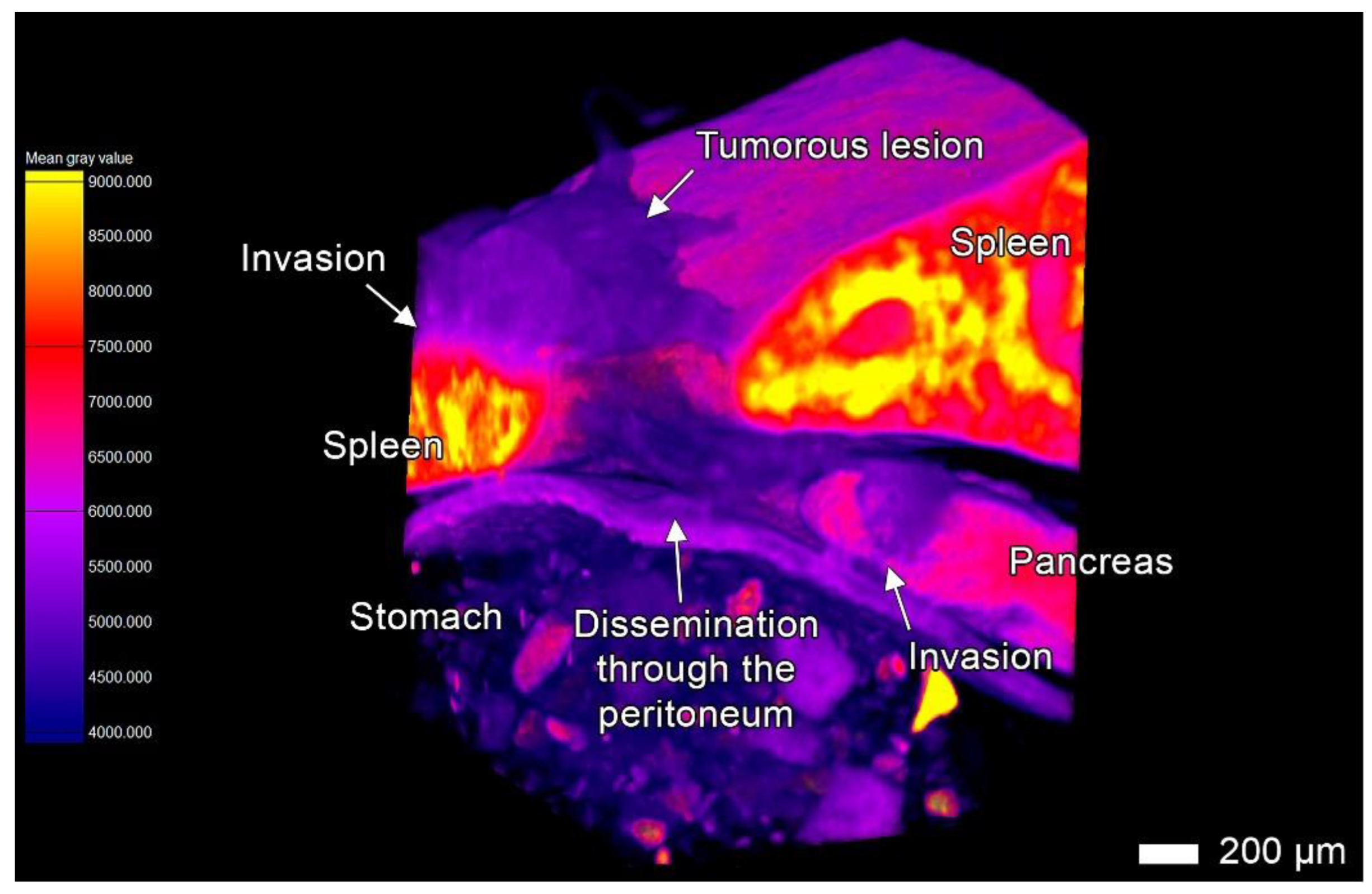
3.4. Tumor Invasion into the Pancreas

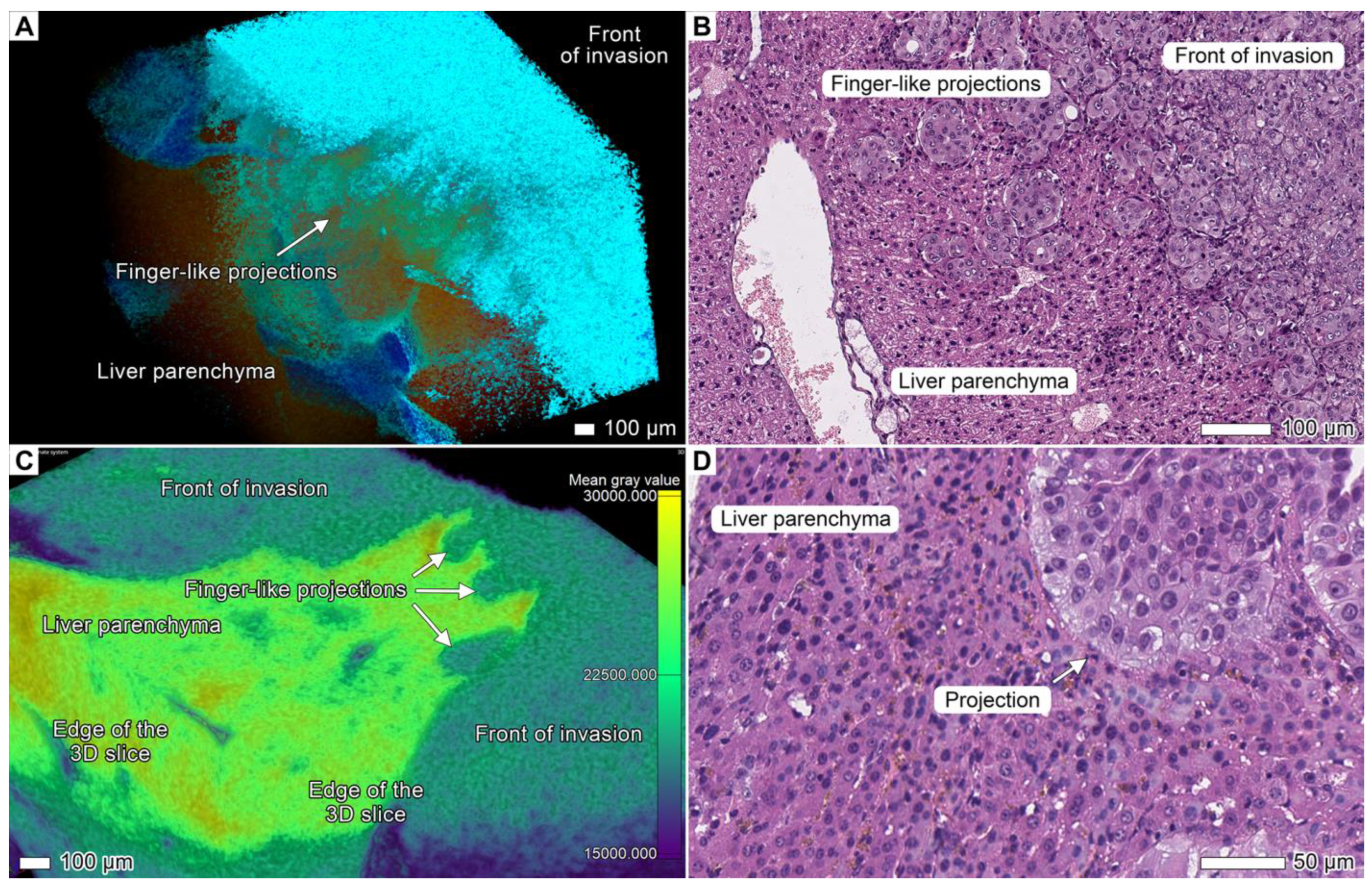
3.5. Tumor Invasion into the Liver
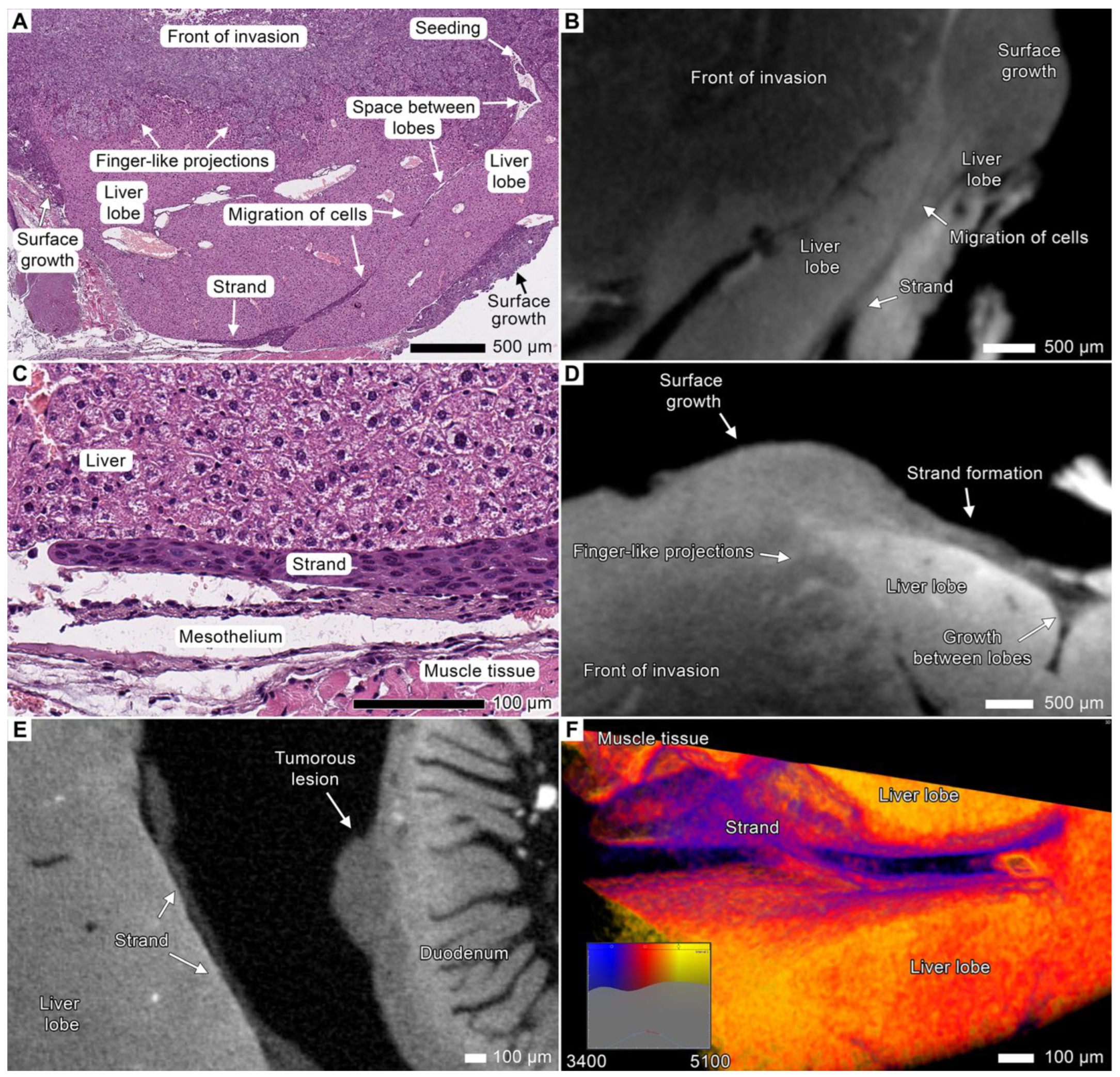
3.6. Peritoneal Dissemination
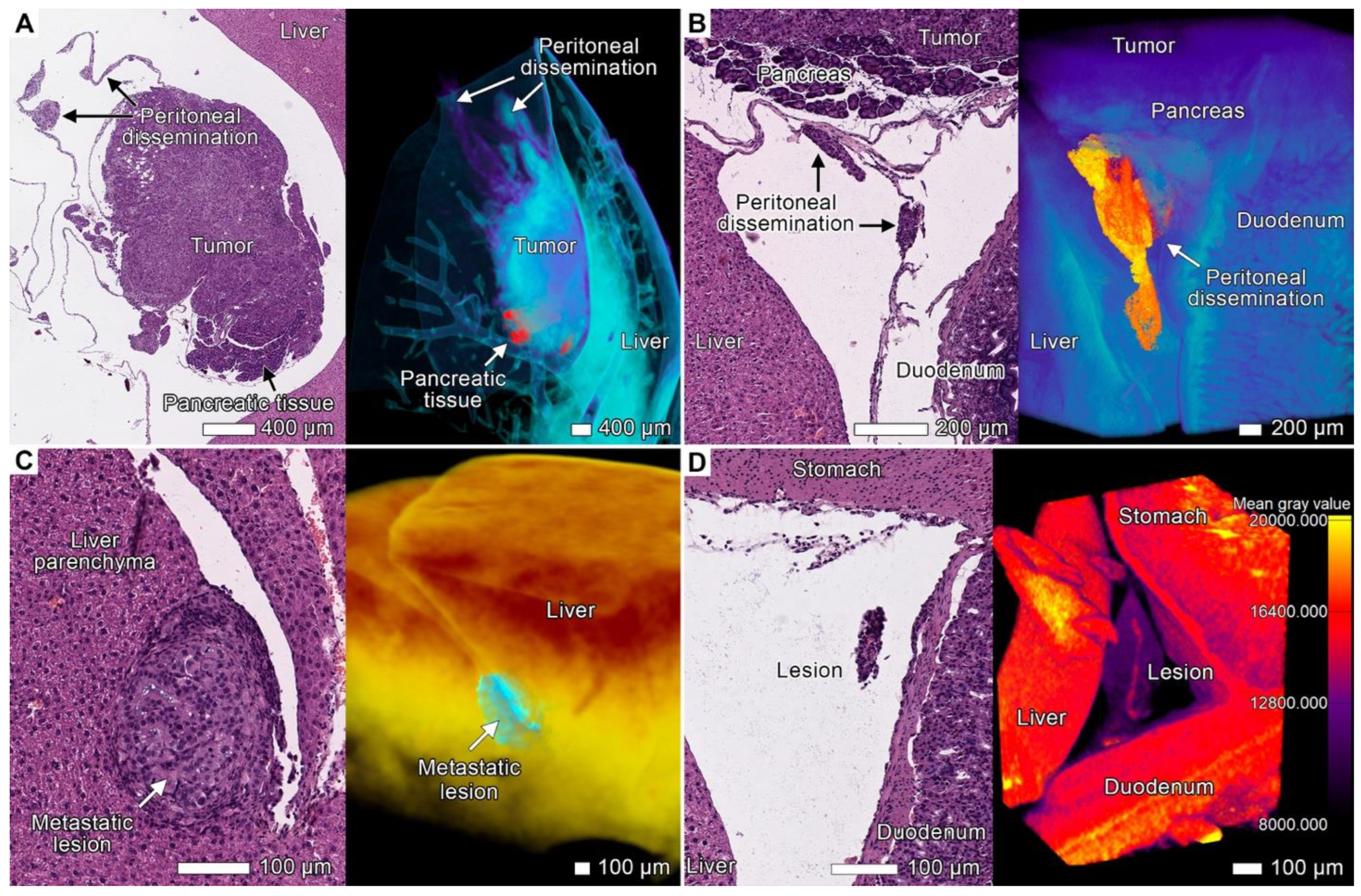
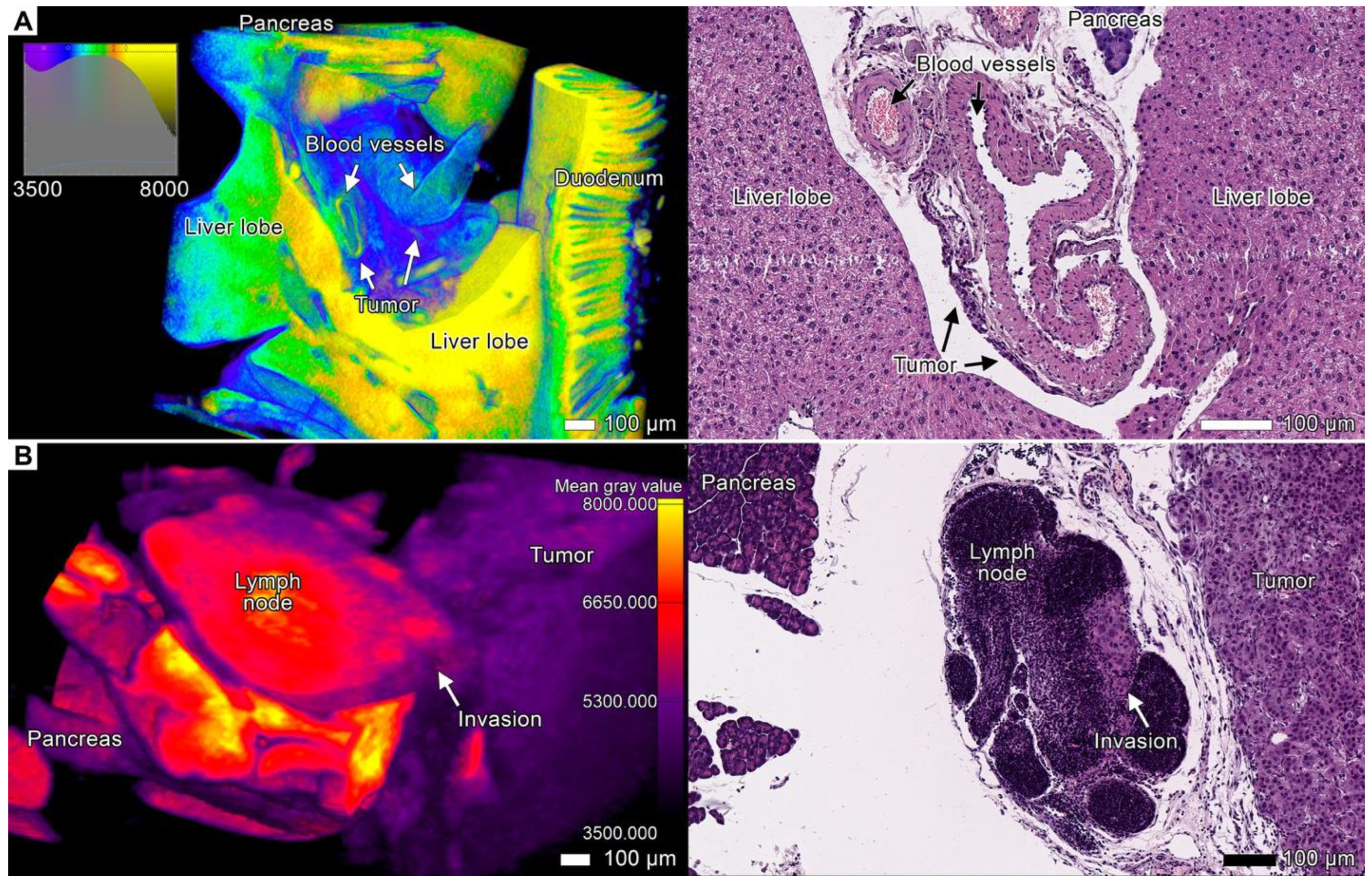
3.7. Stromal Connective Tissues Define Patterns of Invasion
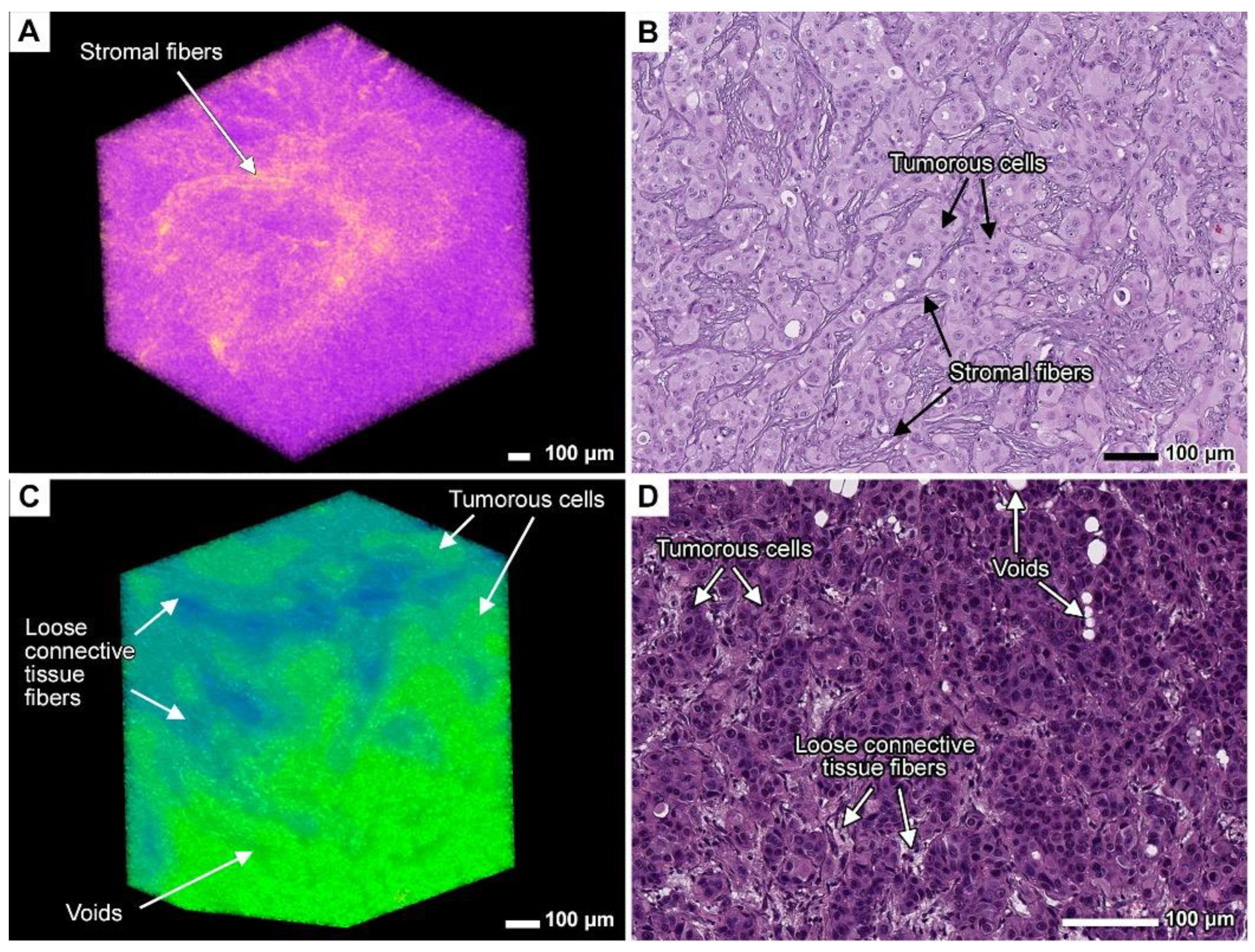
3.8. Expanding the Concept of Tumor Clusters
4. Discussion
4.1. Visualization of Tumor Invasion
4.2. Considerations for the Model Selection
4.3. Invasion Patterns
4.4. Tumor Budding Revisited
4.5. Tumor Invasion as a Process of Coordinated Tissue Remodeling
4.6. The Cyclical Nature of Tumor Dissemination
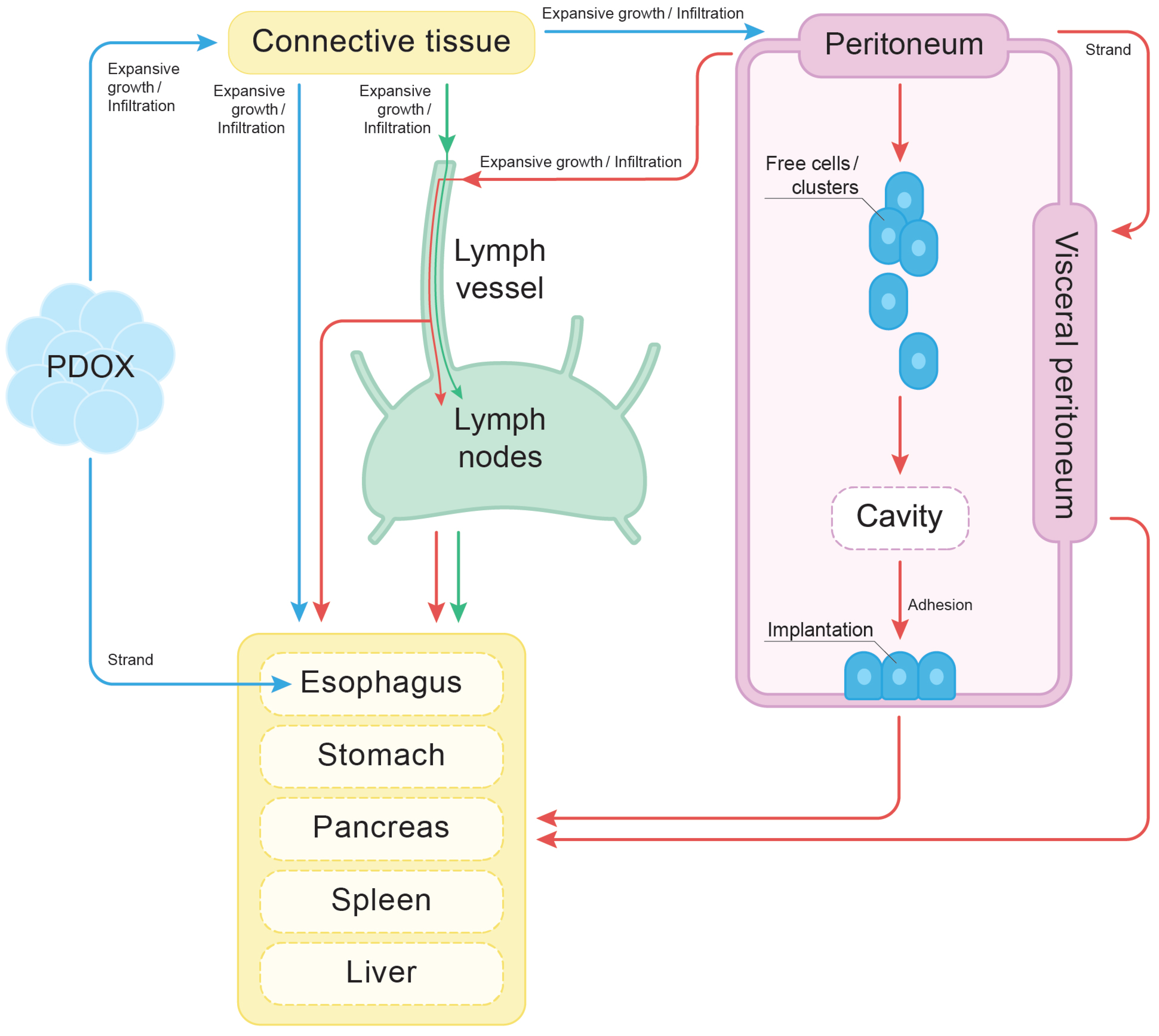
4.7. The Finger-like Projection Type of Invasion in the Connective Tissue
4.8. Peritoneal Dissemination
4.9. Cellular Heterogeneity and Adaptive Phenotypic Plasticity
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of Deaths from Cancer Caused by Metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, M.; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of Cancer Metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the Tumor Stroma for Cancer Therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of Cancer Cell Invasion: Patterns and Mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef]
- Micalet, A.; Pape, J.; Bakkalci, D.; Javanmardi, Y.; Hall, C.; Cheema, U.; Moeendarbary, E. Evaluating the Impact of a Biomimetic Mechanical Environment on Cancer Invasion and Matrix Remodeling. Adv. Healthc. Mater. 2023, 12, 2201749. [Google Scholar] [CrossRef]
- Rice, T.W.; Zuccaro, G.; Adelstein, D.J.; Rybicki, L.A.; Blackstone, E.H.; Goldblum, J.R. Esophageal Carcinoma: Depth of Tumor Invasion Is Predictive of Regional Lymph Node Status. Ann. Thorac. Surg. 1998, 65, 787–792. [Google Scholar] [CrossRef]
- Shaheen, O.; Ghibour, A.; Alsaid, B. Esophageal Cancer Metastases to Unexpected Sites: A Systematic Review. Gastroenterol. Res. Pract. 2017, 2017, 1657310. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal Cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, H.; Fang, K.; Zheng, Y.; Wu, J. International Trends in Esophageal Cancer Incidence Rates by Histological Subtype (1990–2012) and Prediction of the Rates to 2030. Esophagus 2022, 19, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Roshandel, G.; McCormack, V.; Malekzadeh, R. Current Status and Future Prospects for Esophageal Cancer. Cancers 2023, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-X.; Zhao, H.-P.; Zhao, M.-Y.; Yu, Y.; Qi, X.; Wang, J.-H.; Lv, J. Latest Insights into the Global Epidemiological Features, Screening, Early Diagnosis and Prognosis Prediction of Esophageal Squamous Cell Carcinoma. World J. Gastroenterol. 2024, 30, 2638–2656. [Google Scholar] [CrossRef]
- Ito, E.; Ozawa, S.; Kijima, H.; Kazuno, A.; Nishi, T.; Chino, O.; Shimada, H.; Tanaka, M.; Inoue, S.; Inokuchi, S.; et al. New Invasive Patterns as a Prognostic Factor for Superficial Esophageal Cancer. J. Gastroenterol. 2012, 47, 1279–1289. [Google Scholar] [CrossRef]
- Jakab, Á.; Zarándy, L.; Kocsmár, I.; Várkonyi, T.; Kenessey, I.; Szijártó, A.; Kiss, A.; Vass, T.; Lotz, G.; Kocsmár, É. Local Invasion Patterns Characterized by SARIFA and Tumor Budding Differ and Have Distinct Prognostic Significance in Esophageal Adenocarcinoma and Squamous Cell Carcinoma. Cancers 2024, 16, 3144. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, J.; Yao, X.; Li, Z.; Li, W.; Wang, H.; Zhu, J. Patterns and Prognostic Predictive Value of Perineural Invasion in Esophageal Squamous Cell Carcinoma. BMC Cancer 2022, 22, 1287. [Google Scholar] [CrossRef]
- Liu, C.; Ye, L.; Peng, T.; Chen, L.; Zhang, Y.; Zhou, Y.; Du, J.; Bai, S.; Luo, Y.; Zhang, W.; et al. Correlation between Tumor Budding and the Long-Term Follow-up Outcomes after Endoscopic Submucosal Dissection for Superficial Esophageal Squamous Cell Carcinoma. Surg. Endosc. 2024, 38, 5575–5583. [Google Scholar] [CrossRef]
- Kato, Y.; Ito, T.; Yamamoto, K.; Furukawa, A.; Shintaku, H.; Yamamoto, K.; Sakai, M.; Tomita, Y.; Chino, Y.; Uchida, K.; et al. Invasive Features of Superficial Oesophageal Squamous Cell Carcinoma—Analysis of Risk Factors for Lymph Node Metastasis. Virchows Archiv 2023, 483, 645–653. [Google Scholar] [CrossRef]
- Regmi, S.M.; Regmi, P.; Paudyal, A.; Upadhyay, D.; Lamichhane, S.; Dahal, A.; Thatal, S.; Tiwari, A. Tumor Budding Is Associated with Poor Prognosis and Clinicopathologic Factors in Esophageal Carcinoma: A Meta-Analysis. Eur. J. Surg. Oncol. 2024, 50, 108668. [Google Scholar] [CrossRef]
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying Collective Cancer Cell Invasion. Nat. Cell Biol. 2012, 14, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Taqi, S.A.; Sami, S.A.; Sami, L.B.; Zaki, S.A. A Review of Artifacts in Histopathology. J. Oral Maxillofac. Pathol. 2018, 22, 279. [Google Scholar] [CrossRef]
- Liu, G.; Xu, J.; Chen, W.; Zhuang, L.; Chen, J.; Zhang, C.; He, Y. Narrative Review: Research Progress of Tumor Budding in Gastrointestinal Tumor. Dig. Med. Res. 2020, 3. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.-F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for Reporting Tumor Budding in Colorectal Cancer Based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Lugli, A.; Zlobec, I.; Berger, M.D.; Kirsch, R.; Nagtegaal, I.D. Tumour Budding in Solid Cancers. Nat. Rev. Clin. Oncol. 2021, 18, 101–115. [Google Scholar] [CrossRef]
- Bronsert, P.; Enderle-Ammour, K.; Bader, M.; Timme, S.; Kuehs, M.; Csanadi, A.; Kayser, G.; Kohler, I.; Bausch, D.; Hoeppner, J.; et al. Cancer Cell Invasion and EMT Marker Expression: A Three-Dimensional Study of the Human Cancer-Host Interface: 3D Cancer-Host Interface. J. Pathol. 2014, 234, 410–422. [Google Scholar] [CrossRef]
- Frieboes, H.B.; Jin, F.; Chuang, Y.-L.; Wise, S.M.; Lowengrub, J.S.; Cristini, V. Three-Dimensional Multispecies Nonlinear Tumor Growth—II: Tumor Invasion and Angiogenesis. J. Theor. Biol. 2010, 264, 1254–1278. [Google Scholar] [CrossRef]
- Ruusuvuori, P.; Valkonen, M.; Kartasalo, K.; Valkonen, M.; Visakorpi, T.; Nykter, M.; Latonen, L. Spatial Analysis of Histology in 3D: Quantification and Visualization of Organ and Tumor Level Tissue Environment. Heliyon 2022, 8, e08762. [Google Scholar] [CrossRef]
- Albers, J.; Pacilé, S.; Markus, M.A.; Wiart, M.; Vande Velde, G.; Tromba, G.; Dullin, C. X-Ray-Based 3D Virtual Histology-Adding the Next Dimension to Histological Analysis. Mol. Imaging Biol. 2018, 20, 732–741. [Google Scholar] [CrossRef]
- Farahani, N.; Braun, A.; Jutt, D.; Huffman, T.; Reder, N.; Liu, Z.; Yagi, Y.; Pantanowitz, L. Three-Dimensional Imaging and Scanning: Current and Future Applications for Pathology. J. Pathol. Inform. 2017, 8, 36. [Google Scholar] [CrossRef]
- Oliveira, L.M.C.; Tuchin, V.V. (Eds.) Optical Clearing and Tissue Imaging. In The Optical Clearing Method: A New Tool for Clinical Practice and Biomedical Engineering; Springer International Publishing: Cham, Switzerland, 2019; pp. 107–138. ISBN 978-3-030-33055-2. [Google Scholar]
- Orhan, K. (Ed.) Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-16640-3. [Google Scholar]
- Bidola, P.; Martins de Souza E Silva, J.; Achterhold, K.; Munkhbaatar, E.; Jost, P.J.; Meinhardt, A.-L.; Taphorn, K.; Zdora, M.-C.; Pfeiffer, F.; Herzen, J. A Step towards Valid Detection and Quantification of Lung Cancer Volume in Experimental Mice with Contrast Agent-Based X-Ray Microtomography. Sci. Rep. 2019, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Zanette, I.; Noël, P.B.; Cardoso, M.B.; Kimm, M.A.; Pfeiffer, F. Three-Dimensional Non-Destructive Soft-Tissue Visualization with X-Ray Staining Micro-Tomography. Sci. Rep. 2015, 5, 14088. [Google Scholar] [CrossRef]
- Gersing, A.S.; Kimm, M.A.; Bollwein, C.; Ilg, P.; Mogler, C.; Gassert, F.G.; Feuerriegel, G.C.; Knebel, C.; Woertler, K.; Pfeiffer, D.; et al. Chondrosarcoma Evaluation Using Hematein-Based X-Ray Staining and High-Resolution 3D Micro-CT: A Feasibility Study. Eur. Radiol. Exp. 2024, 8, 58. [Google Scholar] [CrossRef]
- Tkachev, S.Y.; Mitrin, B.I.; Karnaukhov, N.S.; Sadyrin, E.V.; Voloshin, M.V.; Maksimov, A.Y.; Goncharova, A.S.; Lukbanova, E.A.; Zaikina, E.V.; Volkova, A.V.; et al. Visualization of Different Anatomical Parts of the Enucleated Human Eye Using X-Ray Micro-CT Imaging. Exp. Eye Res. 2021, 203, 108394. [Google Scholar] [CrossRef]
- Kogan, M.I.; Popov, I.V.; Kirichenko, E.Y.; Mitrin, B.I.; Sadyrin, E.V.; Kulaeva, E.D.; Popov, I.V.; Kulba, S.N.; Logvinov, A.K.; Akimenko, M.A.; et al. X-Ray Micro-Computed Tomography in the Assessment of Penile Cavernous Fibrosis in a Rabbit Castration Model. Andrology 2021, 9, 1467–1480. [Google Scholar] [CrossRef]
- Fayzullin, A.; Vladimirov, G.; Kuryanova, A.; Gafarova, E.; Tkachev, S.; Kosheleva, N.; Istranova, E.; Istranov, L.; Efremov, Y.; Novikov, I.; et al. A Defined Road to Tracheal Reconstruction: Laser Structuring and Cell Support for Rapid Clinic Translation. Stem Cell Res. Ther. 2022, 13, 317. [Google Scholar] [CrossRef]
- Tkachev, S.; Chepelova, N.; Galechyan, G.; Ershov, B.; Golub, D.; Popova, E.; Antoshin, A.; Giliazova, A.; Voloshin, S.; Efremov, Y.; et al. Three-Dimensional Cell Culture Micro-CT Visualization within Collagen Scaffolds in an Aqueous Environment. Cells 2024, 13, 1234. [Google Scholar] [CrossRef]
- Apps, J.R.; Hutchinson, J.C.; Arthurs, O.J.; Virasami, A.; Joshi, A.; Zeller-Plumhoff, B.; Moulding, D.; Jacques, T.S.; Sebire, N.J.; Martinez-Barbera, J.P. Imaging Invasion: Micro-CT Imaging of Adamantinomatous Craniopharyngioma Highlights Cell Type Specific Spatial Relationships of Tissue Invasion. Acta Neuropathol. Commun. 2016, 4, 57. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, D.; Lin, R.; Zhou, G.; Peng, G.; Su, M. Phase-Contrast X-Ray CT Imaging of Esophagus and Esophageal Carcinoma. Sci. Rep. 2014, 4, 5332. [Google Scholar] [CrossRef]
- Lee, H.N.; Kim, S.; Park, S.; Jung, W.; Kang, J.S. Quantification and Visualization of Metastatic Lung Tumors in Mice. Toxicol. Res. 2022, 38, 503–510. [Google Scholar] [CrossRef]
- Enders, C.; Braig, E.-M.; Scherer, K.; Werner, J.U.; Lang, G.K.; Lang, G.E.; Pfeiffer, F.; Noël, P.; Rummeny, E.; Herzen, J. Advanced Non-Destructive Ocular Visualization Methods by Improved X-Ray Imaging Techniques. PLoS ONE 2017, 12, e0170633. [Google Scholar] [CrossRef] [PubMed]
- Kayı Cangır, A.; Dizbay Sak, S.; Güneş, G.; Orhan, K. Differentiation of Benign and Malignant Regions in Paraffin Embedded Tissue Blocks of Pulmonary Adenocarcinoma Using Micro CT Scanning of Paraffin Tissue Blocks: A Pilot Study for Method Validation. Surg. Today 2021, 51, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Kayı Cangır, A.; Güneş, S.G.; Orhan, K.; Özakıncı, H.; Kahya, Y.; Karasoy, D.; Dizbay Sak, S. Microcomputed Tomography as a Diagnostic Tool for Detection of Lymph Node Metastasis in Non-Small Cell Lung Cancer: A Decision-Support Approach for Pathological Examination “A Pilot Study for Method Validation”. J. Pathol. Inform. 2024, 15, 100373. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Nishimura, M.; Teplov, A.; Leung, G.; Ntiamoah, P.; Cesmecioglu, E.; Kawata, N.; Ohnishi, T.; Kareem, I.; Shia, J.; et al. A Pilot Study of Micro-CT-Based Whole Tissue Imaging (WTI) on Endoscopic Submucosal Dissection (ESD) Specimens. Sci. Rep. 2022, 12, 9889. [Google Scholar] [CrossRef]
- Ohnishi, T.; Teplov, A.; Kawata, N.; Ibrahim, K.; Ntiamoah, P.; Firat, C.; Haneishi, H.; Hameed, M.; Shia, J.; Yagi, Y. Three-Dimensional Vessel Segmentation in Whole-Tissue and Whole-Block Imaging Using a Deep Neural Network: Proof-of-Concept Study. Am. J. Pathol. 2021, 191, 463–474. [Google Scholar] [CrossRef]
- Donato, S.; Agostino, R.G.; Arana Peña, L.M.; Arfelli, F.; Brombal, L.; Longo, R.; Martellani, F.; Romano, A.; Rosano, I.; Saccomano, G.; et al. Unveiling Tumor Invasiveness: Enhancing Cancer Diagnosis with Phase-Contrast Microtomography for 3D Virtual Histology. Eur. Phys. J. Plus 2024, 139, 413. [Google Scholar] [CrossRef]
- Tajbakhsh, K.; Stanowska, O.; Neels, A.; Perren, A.; Zboray, R. 3D Virtual Histopathology by Phase-Contrast X-Ray Micro-CT for Follicular Thyroid Neoplasms. IEEE Trans. Med. Imaging 2024, 43, 2670–2678. [Google Scholar] [CrossRef]
- Albus, U. Guide for the Care and Use of Laboratory Animals (8th Edn). Lab. Anim. 2012, 46, 267–268. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Grigore, A.D.; Jolly, M.K.; Jia, D.; Farach-Carson, M.C.; Levine, H. Tumor Budding: The Name Is EMT. Partial EMT. J. Clin. Med. 2016, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Liu, B.; Guan, X. The Role of Tumor Microenvironment in Invasion and Metastasis of Esophageal Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 911285. [Google Scholar]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Modes of Invasion during Tumour Dissemination. Mol. Oncol. 2017, 11, 5–27. [Google Scholar] [CrossRef]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D Cell Migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef]
- Mine, S.; Tanaka, K.; Kawachi, H.; Shirakawa, Y.; Kitagawa, Y.; Toh, Y.; Yasuda, T.; Watanabe, M.; Kamei, T.; Oyama, T.; et al. Japanese Classification of Esophageal Cancer, 12th Edition: Part I. Esophagus 2024, 21, 179–215. [Google Scholar] [CrossRef]
- Shimazu, Y.; Kudo, T.; Yagishita, H.; Aoba, T. Three-Dimensional Visualization and Quantification for the Growth and Invasion of Oral Squamous Cell Carcinoma. Jpn. Dent. Sci. Rev. 2010, 46, 17–25. [Google Scholar] [CrossRef]
- Kudo, T.; Shimazu, Y.; Yagishita, H.; Izumo, T.; Soeno, Y.; Sato, K.; Taya, Y.; Aoba, T. Three-Dimensional Reconstruction of Oral Tongue Squamous Cell Carcinoma at Invasion Front. Int. J. Dent. 2013, 2013, 482765. [Google Scholar] [CrossRef]
- Li, M.; Jirapatnakul, A.; Biancardi, A.; Riccio, M.L.; Weiss, R.S.; Reeves, A.P. Growth Pattern Analysis of Murine Lung Neoplasms by Advanced Semi-Automated Quantification of Micro-CT Images. PLoS ONE 2013, 8, e83806. [Google Scholar] [CrossRef]
- Xu, B.; Teplov, A.; Ibrahim, K.; Inoue, T.; Stueben, B.; Katabi, N.; Hameed, M.; Yagi, Y.; Ghossein, R. Detection and Assessment of Capsular Invasion, Vascular Invasion and Lymph Node Metastasis Volume in Thyroid Carcinoma Using microCT Scanning of Paraffin Tissue Blocks (3D Whole Block Imaging): A Proof of Concept. Mod. Pathol. 2020, 33, 2449–2457. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T. Advances in Micro-CT Imaging of Small Animals. Phys. Med. Eur. J. Med. Phys. 2021, 88, 175–192. [Google Scholar] [CrossRef]
- Jensen, M.; Clemmensen, A.; Hansen, J.G.; van Krimpen Mortensen, J.; Christensen, E.N.; Kjaer, A.; Ripa, R.S. 3D Whole Body Preclinical Micro-CT Database of Subcutaneous Tumors in Mice with Annotations from 3 Annotators. Sci. Data 2024, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Stribbling, S.M.; Beach, C.; Ryan, A.J. Orthotopic and Metastatic Tumour Models in Preclinical Cancer Research. Pharmacol. Ther. 2024, 257, 108631. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.E.; Junttila, M.R.; De Sauvage, F.J. Translational Value of Mouse Models in Oncology Drug Development. Nat. Med. 2015, 21, 431–439. [Google Scholar] [PubMed]
- Bhimani, J.; Ball, K.; Stebbing, J. Patient-Derived Xenograft Models—The Future of Personalised Cancer Treatment. Br. J. Cancer 2020, 122, 601–602. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Cogels, M.M.; Rouas, R.; Ghanem, G.E.; Martinive, P.; Awada, A.; Van Gestel, D.; Krayem, M. Humanized Mice as a Valuable Pre-Clinical Model for Cancer Immunotherapy Research. Front. Oncol. 2021, 11, 784947. [Google Scholar] [CrossRef]
- Guil-Luna, S.; Sedlik, C.; Piaggio, E. Humanized Mouse Models to Evaluate Cancer Immunotherapeutics. Annu. Rev. Cancer Biol. 2021, 5, 119–136. [Google Scholar]
- Lang, J.; Capasso, A.; Jordan, K.R.; French, J.D.; Kar, A.; Bagby, S.M.; Barbee, J.; Yacob, B.W.; Head, L.S.; Tompkins, K.D.; et al. Development of an Adrenocortical Cancer Humanized Mouse Model to Characterize Anti-PD1 Effects on Tumor Microenvironment. J. Clin. Endocrinol. Metab. 2020, 105, 26–42. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Wang, B.; Ying, J.; He, J.; Xue, L. Tumor Budding and Tumor-Infiltrating Lymphocytes Can Predict Prognosis in pT1b Esophageal Squamous Cell Carcinoma. Thorac. Cancer 2023, 14, 2608–2617. [Google Scholar] [CrossRef]
- Lee, N.P.; Chan, C.M.; Tung, L.N.; Wang, H.K.; Law, S. Tumor Xenograft Animal Models for Esophageal Squamous Cell Carcinoma. J. Biomed. Sci. 2018, 25, 66. [Google Scholar] [CrossRef]
- Tung, L.N.; Song, S.; Chan, K.T.; Choi, M.Y.; Lam, H.Y.; Chan, C.M.; Chen, Z.; Wang, H.K.; Leung, H.T.; Law, S.; et al. Preclinical Study of Novel Curcumin Analogue SSC-5 Using Orthotopic Tumor Xenograft Model for Esophageal Squamous Cell Carcinoma. Cancer Res. Treat. 2018, 50, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Hong, S.-M.; Jung, D.; Noë, M.; Kiemen, A.; Wu, P.-H.; Wirtz, D.; Hruban, R.H.; Wood, L.D.; Oshima, K. Three-Dimensional Analysis of Extrahepatic Cholangiocarcinoma and Tumor Budding. J. Pathol. 2020, 251, e5474. [Google Scholar] [CrossRef]
- Parlani, M.; Jorgez, C.; Friedl, P. Plasticity of Cancer Invasion and Energy Metabolism. Trends Cell Biol. 2023, 33, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Toret, C.; Picco, A.; Boiero-Sanders, M.; Michelot, A.; Kaksonen, M. The Cellular Slime Mold Fonticula Alba Forms a Dynamic, Multicellular Collective While Feeding on Bacteria. Curr. Biol. 2022, 32, 1961–1973.e4. [Google Scholar] [CrossRef] [PubMed]
- Hashimura, H.; Morimoto, Y.V.; Yasui, M.; Ueda, M. Collective Cell Migration of Dictyostelium without cAMP Oscillations at Multicellular Stages. Commun. Biol. 2019, 2, 34. [Google Scholar] [CrossRef]
- Capp, J.-P.; Thomas, F.; Marusyk, A.; Dujon, A.M.; Tissot, S.; Gatenby, R.; Roche, B.; Ujvari, B.; DeGregori, J.; Brown, J.S.; et al. The Paradox of Cooperation among Selfish Cancer Cells. Evol. Appl. 2023, 16, 1239–1256. [Google Scholar] [CrossRef]
- Bischoff, M.C.; Bogdan, S. Collective Cell Migration Driven by Filopodia—New Insights from the Social Behavior of Myotubes. BioEssays 2021, 43, 2100124. [Google Scholar] [CrossRef]
- Plou, J.; Juste-Lanas, Y.; Olivares, V.; del Amo, C.; Borau, C.; García-Aznar, J.M. From Individual to Collective 3D Cancer Dissemination: Roles of Collagen Concentration and TGF-β. Sci. Rep. 2018, 8, 12723. [Google Scholar] [CrossRef]
- Tétreault, M.-P. Esophageal Cancer: Insights from Mouse Models. Cancer Growth Metastasis 2015, 8s1, CGM.S21218. [Google Scholar] [CrossRef]
- Yonemura, Y.; Ishibashi, H.; Mizumoto, A.; Nishihara, K.; Liu, Y.; Wakama, S.; Sako, S.; Takao, N.; Ichinose, M.; Motoi, S.; et al. Mechanisms of Peritoneal Metastasis Formation. In Pathology of Peritoneal Metastases: The Unchartered Fields; Glehen, O., Bhatt, A., Eds.; Springer: Singapore, 2020; pp. 1–26. ISBN 978-981-15-3773-8. [Google Scholar]
- Al Habyan, S.; Kalos, C.; Szymborski, J.; McCaffrey, L. Multicellular Detachment Generates Metastatic Spheroids during Intra-Abdominal Dissemination in Epithelial Ovarian Cancer. Oncogene 2018, 37, 5127–5135. [Google Scholar] [CrossRef]
- Sodek, K.L.; Murphy, K.J.; Brown, T.J.; Ringuette, M.J. Cell–Cell and Cell–Matrix Dynamics in Intraperitoneal Cancer Metastasis. Cancer Metastasis Rev. 2012, 31, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Friedl, P. Determinants of Leader Cells in Collective Cell Migration. Integr. Biol. 2010, 2, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Barbazán, J.; Matic Vignjevic, D. Cancer Associated Fibroblasts: Is the Force the Path to the Dark Side? Curr. Opin. Cell Biol. 2019, 56, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, K.J.; Wong, K.K.; Rustgi, A.K. Cancer-Associated Fibroblasts in Esophageal Cancer. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 687–695. [Google Scholar] [CrossRef]
- Laplane, L.; Maley, C.C. The Evolutionary Theory of Cancer: Challenges and Potential Solutions. Nat. Rev. Cancer 2024, 24, 718–733. [Google Scholar] [CrossRef]
- Arozarena, I.; Wellbrock, C. Phenotype Plasticity as Enabler of Melanoma Progression and Therapy Resistance. Nat. Rev. Cancer 2019, 19, 377–391. [Google Scholar] [CrossRef]
- de Bournonville, S.; Vangrunderbeeck, S.; Kerckhofs, G. Contrast-Enhanced MicroCT for Virtual 3D Anatomical Pathology of Biological Tissues: A Literature Review. Contrast Media Mol. Imaging 2019, 2019, 8617406. [Google Scholar] [CrossRef]
- Chomet, M.; Schreurs, M.; Vos, R.; Verlaan, M.; Kooijman, E.J.; Poot, A.J.; Boellaard, R.; Windhorst, A.D.; van Dongen, G.A.; Vugts, D.J.; et al. Performance of nanoScan PET/CT and PET/MR for Quantitative Imaging of 18F and 89Zr as Compared with Ex Vivo Biodistribution in Tumor-Bearing Mice. EJNMMI Res. 2021, 11, 57. [Google Scholar] [CrossRef]
- Desmonts, C.; Lasnon, C.; Jaudet, C.; Aide, N. PET Imaging and Quantification of Small Animals Using a Clinical SiPM-Based Camera. EJNMMI Phys. 2023, 10, 61. [Google Scholar] [CrossRef]
- Clément, A.; Zaragori, T.; Filosa, R.; Ovdiichuk, O.; Beaumont, M.; Collet, C.; Roeder, E.; Martin, B.; Maskali, F.; Barberi-Heyob, M.; et al. Multi-Tracer and Multiparametric PET Imaging to Detect the IDH Mutation in Glioma: A Preclinical Translational In Vitro, In Vivo, and Ex Vivo Study. Cancer Imaging 2022, 22, 16. [Google Scholar] [CrossRef]
- Benfante, V.; Stefano, A.; Comelli, A.; Giaccone, P.; Cammarata, F.P.; Richiusa, S.; Scopelliti, F.; Pometti, M.; Ficarra, M.; Cosentino, S.; et al. A New Preclinical Decision Support System Based on PET Radiomics: A Preliminary Study on the Evaluation of an Innovative 64Cu-Labeled Chelator in Mouse Models. J. Imaging 2022, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Lecker, D.N.; Kumari, S.; Khan, A. Iodine Binding Capacity and Iodine Binding Energy of Glycogen. J. Polym.Sci. Part A: Polym. Chem. 1997, 38, 1409–1412. [Google Scholar] [CrossRef]
- Busse, M.; Müller, M.; Kimm, M.A.; Ferstl, S.; Allner, S.; Achterhold, K.; Herzen, J.; Pfeiffer, F. Three-Dimensional Virtual Histology Enabled through Cytoplasm-Specific X-Ray Stain for Microscopic and Nanoscopic Computed Tomography. Proc. Natl. Acad. Sci. USA 2018, 115, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Kimm, M.A.; Ferstl, S.; Allner, S.; Achterhold, K.; Herzen, J.; Pfeiffer, F.; Busse, M. Nucleus-Specific X-Ray Stain for 3D Virtual Histology. Sci. Rep. 2018, 8, 17855. [Google Scholar] [CrossRef]
- Sanna, A.; Quarta, A.; Pieroni, N.; Parodi, B.; Palermo, F.; Bukreeva, I.; Fratini, M.; Massimi, L.; Simeone, D.; Le Guével, X.; et al. Combining Metal Nanoparticles and Nanobodies to Boost the Biomedical Imaging in Neurodegenerative Diseases. Front. Nanotechnol. 2023, 5, 1236810. [Google Scholar] [CrossRef]
- Metscher, B. A Simple Nuclear Contrast Staining Method for microCT-Based 3D Histology Using Lead(II) Acetate. J. Anat. 2021, 238, 1036–1041. [Google Scholar] [CrossRef]
- Kwon, K.A.; Bax, D.V.; Shepherd, J.H.; Cameron, R.E.; Best, S.M. Avoiding Artefacts in MicroCT Imaging of Collagen Scaffolds: Effect of Phosphotungstic Acid (PTA)-Staining and Crosslink Density. Bioact. Mater. 2022, 8, 210–219. [Google Scholar] [CrossRef]
- Lesciotto, K.M.; Motch Perrine, S.M.; Kawasaki, M.; Stecko, T.; Ryan, T.M.; Kawasaki, K.; Richtsmeier, J.T. Phosphotungstic Acid-Enhanced microCT: Optimized Protocols for Embryonic and Early Postnatal Mice. Dev. Dyn. 2020, 249, 573–585. [Google Scholar] [CrossRef]
- Schöberl, E.; Breite, C.; Melnikov, A.; Swolfs, Y.; Mavrogordato, M.N.; Sinclair, I.; Spearing, S.M. Fibre-Direction Strain Measurement in a Composite Ply under Quasi-Static Tensile Loading Using Digital Volume Correlation and in Situ Synchrotron Radiation Computed Tomography. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105935. [Google Scholar] [CrossRef]
- Ivanishko, Y.; Bravin, A.; Kovalev, S.; Lisutina, P.; Lotoshnikov, M.; Mittone, A.; Tkachev, S.; Tkacheva, M. Feasibility Study of the 3D Visualization at High Resolution of Intra-Cranial Rabbit Eyes with X-Ray CT Phase-Contrast Imaging. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5941–5948. [Google Scholar] [CrossRef]
- Saccomano, G.; Pinamonti, M.; Longo, E.; Marcuzzo, T.; Tromba, G.; Dreossi, D.; Brun, F. The Potential of X-Ray Virtual Histology in the Diagnosis of Skin Tumors. Skin Res. Technol. 2024, 30, e13801. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.D.; Miller, M.A.; Dusold, D.; Ramos-Vara, J. Effects of Prolonged Formalin Fixation on Diagnostic Immunohistochemistry in Domestic Animals. J. Histochem. Cytochem. 2009, 57, 753–761. [Google Scholar] [CrossRef] [PubMed]
- van Seijen, M.; Brcic, L.; Gonzales, A.N.; Sansano, I.; Bendek, M.; Brcic, I.; Lissenberg-Witte, B.; Korkmaz, H.I.; Geiger, T.; Kammler, R.; et al. Impact of Delayed and Prolonged Fixation on the Evaluation of Immunohistochemical Staining on Lung Carcinoma Resection Specimen. Virchows Archiv 2019, 475, 191–199. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Fan, M.; Zhou, Q.; Deng, H.; Aisharif, M.J.; Chen, X. The Expression of E-Cadherin at the Invasive Tumor Front of Oral Squamous Cell Carcinoma: Immunohistochemical and RT-PCR Analysis with Clinicopathological Correlation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 547–554. [Google Scholar] [CrossRef]
- Guadagno, E.; Campione, S.; Pignatiello, S.; Borrelli, G.; De Dominicis, G.; De Rosa, N.; De Caro, M.D.B. Epithelial-Mesenchymal Transition Proteins in Neuroendocrine Neoplasms: Differential Immunohistochemical Expression in Different Sites and Correlation with Clinico-Pathological Features. Diagnostics 2020, 10, 351. [Google Scholar] [CrossRef]
- Kit, O.I.; Vodolazhskiy, D.I.; Kolesnikov, E.N.; Timoshkina, N.N. Epigenetic Markers of Esophageal Cancer: DNA Methylation. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2017, 11, 55–61. [Google Scholar] [CrossRef]
- Zhou, Y.; Mo, S.; Cui, H.; Sun, R.; Zhang, W.; Zhuang, X.; Xu, E.; Li, H.; Cheng, Y.; Meng, Y.; et al. Immune-Tumor Interaction Dictates Spatially Directed Evolution of Esophageal Squamous Cell Carcinoma. Natl. Sci. Rev. 2024, 11, nwae150. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Ahmed, M.; Urbina, J.; Huang, T.-Y.; Wong, P.K. Three-Dimensional Computer Vision for Exploring Heterogeneity in Collective Cancer Invasion. Sci. Rep. 2024, 14, 23560. [Google Scholar] [CrossRef]
- Buccardi, M.; Ferrini, E.; Pennati, F.; Vincenzi, E.; Ledda, R.E.; Grandi, A.; Buseghin, D.; Villetti, G.; Sverzellati, N.; Aliverti, A.; et al. A Fully Automated micro-CT Deep Learning Approach for Precision Preclinical Investigation of Lung Fibrosis Progression and Response to Therapy. Respir. Res. 2023, 24, 126. [Google Scholar] [CrossRef]
- Schoppe, O.; Pan, C.; Coronel, J.; Mai, H.; Rong, Z.; Todorov, M.I.; Müskes, A.; Navarro, F.; Li, H.; Ertürk, A.; et al. Deep Learning-Enabled Multi-Organ Segmentation in Whole-Body Mouse Scans. Nat. Commun. 2020, 11, 5626. [Google Scholar] [CrossRef]
- Ferl, G.Z.; Barck, K.H.; Patil, J.; Jemaa, S.; Malamut, E.J.; Lima, A.; Long, J.E.; Cheng, J.H.; Junttila, M.R.; Carano, R.A.D. Automated Segmentation of Lungs and Lung Tumors in Mouse Micro-CT Scans. iScience 2022, 25, 105712. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Duan, C.; Manzuk, L.; Chang, S.; Cubias, A.; Brun, S.; Giddabasappa, A.; Jiang, Z.K. Applying Deep Learning to Segmentation of Murine Lung Tumors in Pre-Clinical Micro-Computed Tomography. Transl. Oncol. 2024, 40, 101833. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkachev, S.; Brosalov, V.; Kit, O.; Maksimov, A.; Goncharova, A.; Sadyrin, E.; Dalina, A.; Popova, E.; Osipenko, A.; Voloshin, M.; et al. Unveiling Another Dimension: Advanced Visualization of Cancer Invasion and Metastasis via Micro-CT Imaging. Cancers 2025, 17, 1139. https://doi.org/10.3390/cancers17071139
Tkachev S, Brosalov V, Kit O, Maksimov A, Goncharova A, Sadyrin E, Dalina A, Popova E, Osipenko A, Voloshin M, et al. Unveiling Another Dimension: Advanced Visualization of Cancer Invasion and Metastasis via Micro-CT Imaging. Cancers. 2025; 17(7):1139. https://doi.org/10.3390/cancers17071139
Chicago/Turabian StyleTkachev, Sergey, Vladimir Brosalov, Oleg Kit, Alexey Maksimov, Anna Goncharova, Evgeniy Sadyrin, Alexandra Dalina, Elena Popova, Anton Osipenko, Mark Voloshin, and et al. 2025. "Unveiling Another Dimension: Advanced Visualization of Cancer Invasion and Metastasis via Micro-CT Imaging" Cancers 17, no. 7: 1139. https://doi.org/10.3390/cancers17071139
APA StyleTkachev, S., Brosalov, V., Kit, O., Maksimov, A., Goncharova, A., Sadyrin, E., Dalina, A., Popova, E., Osipenko, A., Voloshin, M., Karnaukhov, N., & Timashev, P. (2025). Unveiling Another Dimension: Advanced Visualization of Cancer Invasion and Metastasis via Micro-CT Imaging. Cancers, 17(7), 1139. https://doi.org/10.3390/cancers17071139





