Simple Summary
Thyroid nodules are common, and when their diagnosis is unclear, doctors often recommend surgery to rule out cancer. However, many of these surgeries turn out to be unnecessary, leading to potential complications and increased healthcare costs. Molecular testing has emerged as a promising tool to improve decision-making by predicting whether a nodule is likely to be benign or malignant. Several molecular tests exist, but their effectiveness varies, and it is unclear which test performs best in avoiding unnecessary surgery. This study systematically reviews and analyzes data from 31 studies to compare the ability of different molecular testing platforms to reduce surgery rates. The findings suggest that all molecular tests help avoid surgery in some cases, but their performance differs. While one test (ThyGenX/ThyraMIR) showed the highest surgical avoidance rate, its results should be interpreted cautiously due to limited sample size. More research is needed to optimize the use of molecular testing in clinical practice.
Abstract
Background: The management of indeterminate thyroid nodules (Bethesda III/IV) has evolved with molecular testing, aiming to reduce unnecessary surgeries. However, the comparative effectiveness of different platforms in influencing surgical decision-making remains unclear. This systematic review and meta-analysis evaluate the impact of molecular testing on surgical avoidance rates. Methods: A systematic literature search was conducted across eight electronic databases, including Embase, PubMed, and Cochrane Library, from January 2019 to December 2024, following PRISMA guidelines to encompass most recent advancements in the last 5 years. Studies evaluating Afirma Gene Expression Classifier (GEC), Afirma Genomic Sequencing Classifier (GSC), ThyroSeq V2, ThyroSeq V3, and ThyGenX/ThyraMIR were included. The primary outcome was surgical avoidance, analyzed using a random-effects model. Results: Thirty-one studies comprising 4464 indeterminate thyroid nodules met inclusion criteria. Pooled surgical avoidance rates varied across platforms: ThyroSeq V2 (50.3%, 95% CI: 20.8–79.6%), ThyroSeq V3 (62.5%, 95% CI: 54.8–70.0%), Afirma GEC (58.8%, 95% CI: 43.6–73.1%), Afirma GSC (50.6%, 95% CI: 34.3–66.8%), and ThyGenX/ThyraMIR (68.6%, 95% CI: 63.1–73.9%). ThyGenX/ThyraMIR had the highest surgical avoidance rate and lowest heterogeneity (I2 = 51.2%), while ThyroSeq showed improvement from V2 to V3. Conclusions: Molecular testing reduces unnecessary thyroid surgeries, with avoidance rates ranging from 50.3% to 68.6%. While ThyGenX/ThyraMIR showed the highest avoidance rate, its limited representation warrants cautious interpretation. Standardized protocols are needed to optimize clinical application. Further prospective studies should compare platforms and assess long-term outcomes and cost-effectiveness.
1. Introduction
Over the last decade, the clinical management of indeterminate thyroid nodules has undergone significant transformation with the emergence of molecular testing platforms [1,2]. These nodules, classified as Bethesda III (Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance) or Bethesda IV (Follicular Neoplasm/Suspicious for Follicular Neoplasm) [3], represent a substantial diagnostic challenge, creating uncertainty in surgical decision-making [4]. Historically, this diagnostic uncertainty has led to diagnostic thyroid surgery for most patients with indeterminate cytology, resulting in numerous potentially unnecessary operations and associated healthcare costs [5].
Until recently, the standard approach for patients presenting with indeterminate thyroid nodules was diagnostic surgery, achieving definitive histological diagnosis but potentially subjecting patients to unnecessary surgical intervention and associated risks [6]. However, recognizing the need for more precise diagnostic tools, various molecular testing platforms have emerged, employing approaches ranging from gene expression analysis to next-generation sequencing. This evolution is exemplified by platforms such as Afirma GEC that uses gene expression analysis to classify nodules, while the Afirma GSC incorporates genomic sequencing for a more detailed genetic assessment. ThyroSeq V2 and V3 utilize next-generation sequencing to identify a broad spectrum of genetic alterations [7]. ThyGenX/ThyraMIR combines genetic analysis with microRNA profiling, offering a comprehensive approach to risk stratification [7].
These molecular platforms aim to improve preoperative risk stratification and reduce unnecessary surgeries through distinct methodologies to enhance diagnostic accuracy and guide surgical decision-making [8]. However, despite their growing implementation, comprehensive evidence regarding molecular testing’s effect on surgical avoidance rates remains limited [9].
Recognizing that the appropriate utilization of molecular testing remains a subject of debate, we aim to systematically review the literature evaluating the impact of molecular testing on surgical decision-making for patients with indeterminate thyroid nodules. Specifically, this review seeks to assess surgical avoidance rates following molecular testing and determine whether implementation of these platforms can effectively reduce unnecessary surgical interventions. This knowledge is crucial for institutions making informed decisions about platform selection and implementation strategies in clinical practice. Our study aligns with the 2015 ATA guidelines and ETA recommendations which advocate for molecular testing in Bethseda III/IV nodules to guide surgical decision-making [9]. Consistent with these guidelines, we prioritized platforms validated in large multicenter trials, like Thyroseq V3 and Afirma GSC, and excluded studies lacking histopathological confirmation. Previous reviews have focused primarily on individual platforms or earlier versions, leaving a critical gap in understanding the comparative effectiveness of current molecular testing platforms
2. Materials and Methods
2.1. Literature Search
The systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The protocol was registered in PROSPERO under registration number CRD42024615384. The search was conducted across eight electronic databases (Embase, PubMed, Web of Science, Crossref, CINAHL, Cochrane Library, Scopus, and ClinicalTrials.gov) from January 2019 to December 2024. The selected timeframe was chosen to reflect most recent advancements in molecular testing technologies. The search strategy utilized keywords and Medical Subject Headings (MeSH) terms related to three main categories: population terms (“thyroid nodule*”, “indeterminate thyroid*”, “Bethesda III”, “Bethesda IV”, “AUS/FLUS”, “FN/SFN”), intervention terms (“molecular test*”, “molecular marker*”, “Afirma”, “ThyroSeq”, “ThyGenX”, “ThyraMIR”), and outcome terms (“surgery”, “surgical decision*”, “surgical management”, “surgical avoid*”). The search was supplemented by manual screening of reference lists from included articles and recent reviews to ensure comprehensive coverage.
2.2. Screening and Eligibility Assessment
Studies were included if they met the following criteria: (i) published between 2019 and 2024; (ii) evaluated molecular testing platforms (Afirma GEC, Afirma GSC, ThyroSeq V2, ThyroSeq V3 or ThyGenX/ThyraMIR) in indeterminate thyroid nodules; (iii) reported surgical avoidance rates directly or provided sufficient data to calculate these rates from surgical resection data; (iv) included adult patients with Bethesda III (AUS/FLUS) or IV (FN/SFN) cytology; and (v) included a minimum follow-up period of 6 months for non-operative management.
Studies were excluded if they: (i) were case reports, reviews, editorials, or conference abstracts; (ii) included pediatric populations; (iii) evaluated molecular testing platforms other than the five specified platforms (except for two studies using custom NGS panels); (iv) lacked surgical decision-making outcomes or sufficient data to calculate surgical avoidance rates; (v) had overlapping patient populations with other included studies; or (vi) were published in languages other than English. Additionally, studies with inadequate data reporting for meta-analysis were excluded.
2.3. Quality Assessment and Risk of Bias
Risk of bias assessment was conducted independently by two reviewers using the Newcastle–Ottawa Scale [11] for observational studies and the Cochrane Risk of Bias tool for randomized controlled trials [12]. Publication bias was assessed using funnel plots and Egger’s test [13] for each molecular testing platform.
2.4. Data Extraction
Data extraction was facilitated using Covidence, a systematic review management software [14]. Two independent reviewers (R.C. and M.A.) performed initial screening of titles and abstracts, followed by full-text review of potentially eligible studies. Data were extracted into standardized forms, capturing study characteristics, patient demographics, nodule characteristics, molecular testing platform used, and surgical outcomes. The primary outcome measure was surgical avoidance rate. Authors were contacted when relevant data were unclear or missing.
2.5. Statistical Analysis
Statistical analysis employed random-effects model using JBI SUMARI (accessed February 2025) and Comprehensive Meta-Analysis software version 4.0 [15,16,17]. Effect measures were pooled using the DerSimonian and Laird method, with surgical avoidance rates calculated for each platform and presented with 95% confidence intervals. Heterogeneity was assessed using the I2 statistic, with values of 25%, 50%, and 75% considered as low, moderate, and high heterogeneity, respectively [18].
2.6. Molecular Testing Platforms
To account for methodological differences between platforms, we extracted detailed information about each test from the included studies and relevant publications. The key characteristics of each platform are summarized below.
Afirma GEC: This platform analyzes the expression levels of 167 genes using microarray technology. The test classifies nodules as either benign or suspicious based on the proprietary Gene Expression Classifier (GEC) algorithm [6,7].
Afirma GSC: This platform uses next-generation sequencing to analyze RNA from 555 genes. The test detects gene mutations, fusions, and expression alterations. Results are classified as benign, suspicious, or non-diagnostic using the Genomic Sequencing Classifier (GSC) algorithm [6,7].
Thyroseq V2 and V3: These platforms employ next-generation sequencing to detect DNA and RNA alterations in a panel of genes commonly implicated in thyroid cancer. ThyroSeq V3 has an expanded gene panel compared to V2. Both versions report the presence or absence of specific mutations, gene fusions, and gene expression alterations [5,6,7].
ThyGenX/ThyraMIR: This platform combines mutation analysis using next-generation sequencing with microRNA expression profiling. The test analyzes mutations in key thyroid cancer genes (including BRAF, RAS, TERT, TP53, and PIK3CA) and the expression levels of 10 microRNAs (including miR-29b, miR-146b, miR-221, and miR-222). Results are integrated using a proprietary algorithm to provide a risk score for malignancy [5,6,7].
3. Results
3.1. Summary of Literature Search
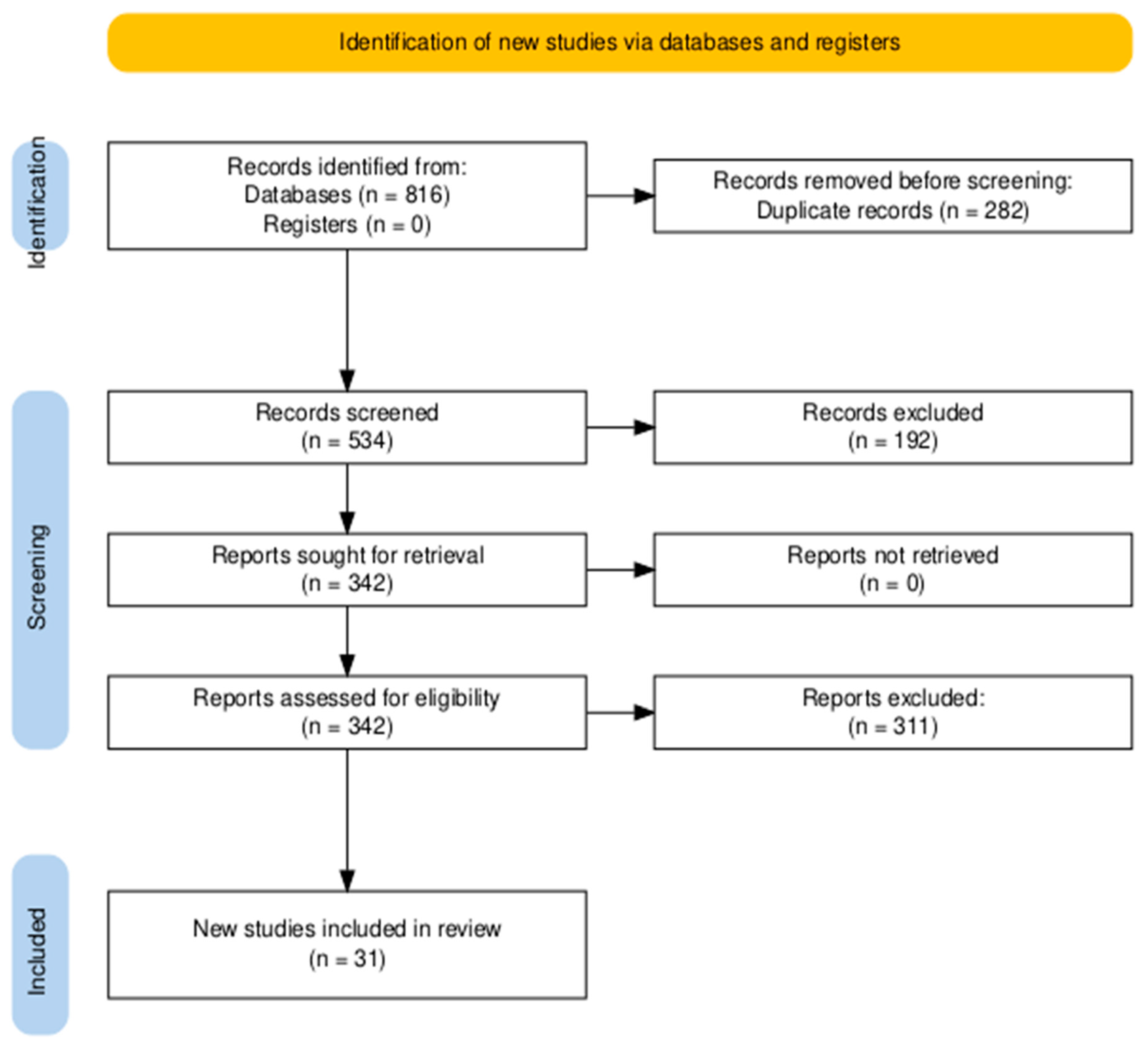
The systemic database search yielded 816 records from databases, with no additional records identified through registers. After removing 282 duplicate records, 534 articles were screened based on titles and abstracts. Of these, 342 full-text articles were assessed for eligibility. Following detailed evaluation, 311 articles were excluded based on predefined criteria. Ultimately, 31 studies [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] met the inclusion criteria and were included in the final review (Figure 1).
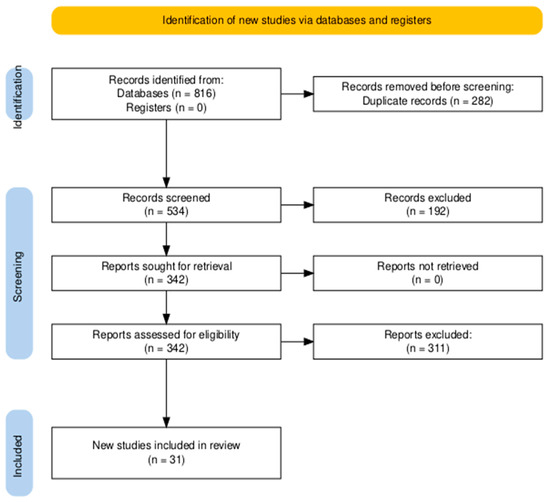
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for literature search and study selection.
3.2. Study Characteristics
The systematic review encompassed 31 studies published between 2019 and 2024, with the following distribution: 2 experimental studies (1 randomized clinical trial, 1 secondary RCT analysis) and 29 observational studies (2 prospective cohort studies, 25 retrospective cohort studies, 2 multi-institutional retrospective analyses). The study populations demonstrated median ages ranging from 47.8 to 59.3 years with 63% to 86% female predominance and nodule sizes ranging from 1.8 to 2.7 cm, with sample sizes varying from 27 to 1593 nodules. Studies were primarily conducted at North American academic medical centers and specialized endocrine surgery units, with additional studies from China, India, and Chile, spanning durations of 12 to 84 months between 2015 and 2022, with follow up periods of 6 to 72 months and majority reporting minimum 12 month follow up. The distribution of indeterminate nodules followed Bethesda classification, with Bethesda III (AUS/FLUS) comprising 46% to 85% of cases, Bethesda IV (FN/SFN) representing 15% to 42%, and Hürthle cell neoplasms accounting for 6% to 16% when specifically reported. The review evaluated five primary molecular testing platforms: Afirma Gene Expression Classifier (GEC) in 6 studies, Afirma Genomic Sequencing Classifier (GSC) in 6 studies, ThyroSeq V2 in 3 studies, ThyroSeq V3 in 10 studies, ThyGenX/ThyGeNEXT ThyraMIR in 4 studies, and other platforms (including custom NGS panels) in 2 studies (Table 1).

Table 1.
Characteristics of included studies.
3.3. Summary of Quality Assessment
The quality assessment of included studies revealed consistent patterns across different study types. For the 29 observational studies evaluated using the Newcastle–Ottawa Scale (NOS), most studies demonstrated adequate representativeness and selection of study groups, scoring 3–4 stars in the selection domain. In the comparability domain, studies generally scored 1–2 stars, with variation observed in how well they controlled for confounding factors. For outcome assessment, studies typically scored 2–3 stars, showing adequate follow-up periods but some limitations in outcome validation. The two experimental studies assessed using the Cochrane Risk of Bias tool showed low risk for random sequence generation in both studies, with allocation concealment being low risk in one study and unclear in the other. Overall, 25 out of 31 studies were of moderate to high quality, though common limitations included lack of validation in outcome measurement, incomplete test–retest reliability assessment, and variable approaches to controlling for confounding factors. The assessment revealed that while most studies maintained acceptable methodological standards, there were consistent limitations in outcome validation and reliability testing.
3.4. Surgical Avoidance Rates
3.4.1. Throseq V2
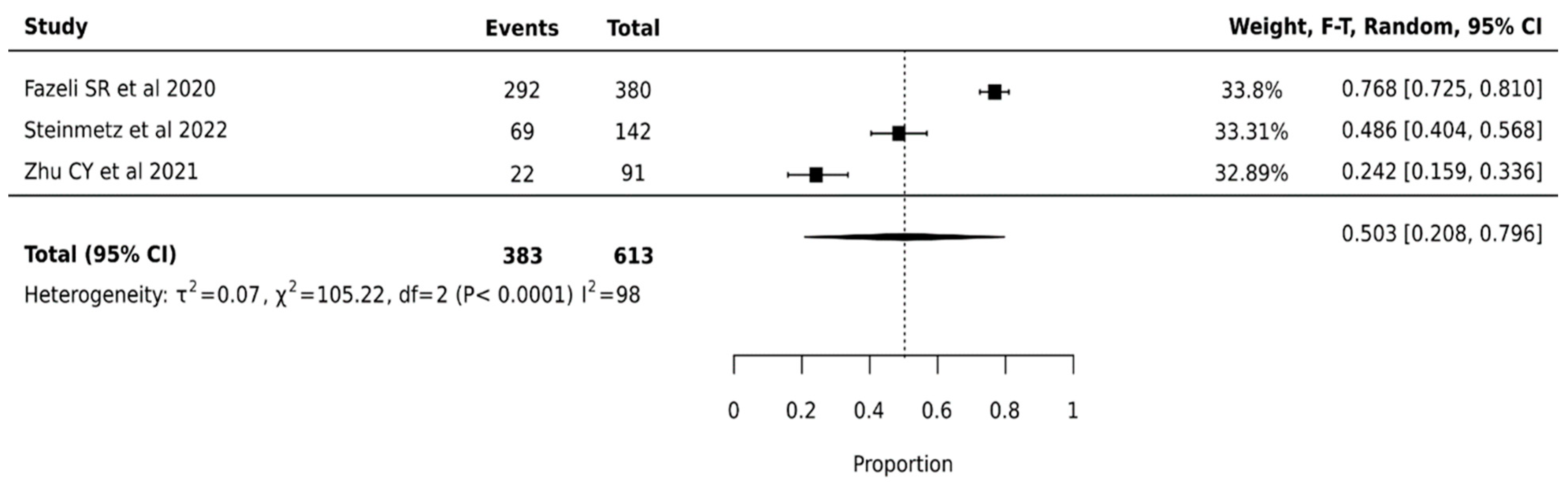
ThyroSeq V2 demonstrated a pooled surgical avoidance rate of 50.3% (95% CI: 20.8–79.6%) across 613 nodules from three studies. The wide confidence interval suggests considerable uncertainty in the true effect size. Statistical analysis revealed substantial heterogeneity (I2 = 98%, p < 0.0001), indicating significant variation in platform performance across institutions (Figure 2).
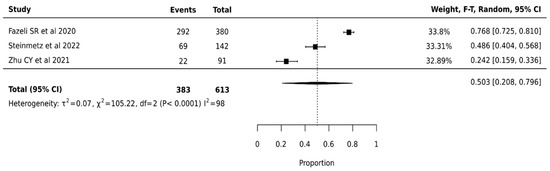
Figure 2.
Thyroseq V2—Forest Plot [26,44,49].
3.4.2. Thyroseq V3
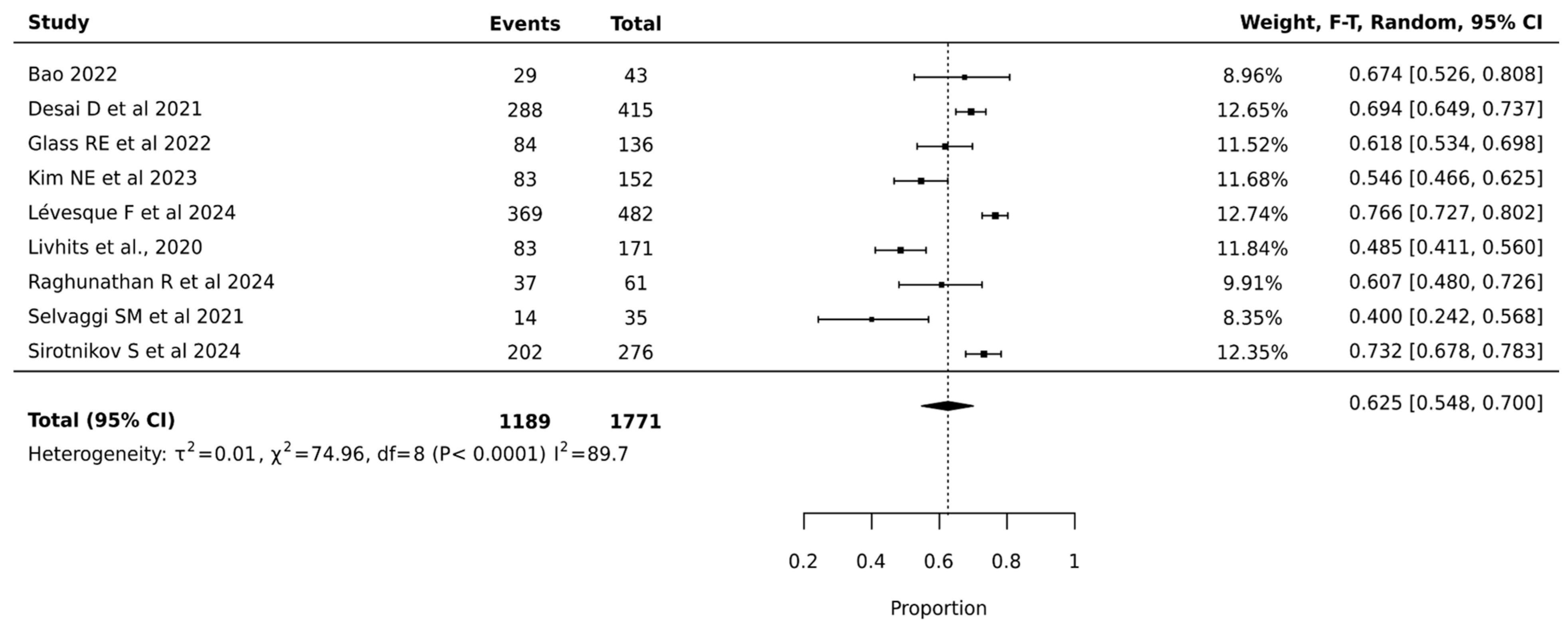
Meta-analysis of 1771 nodules across nine studies showed that ThyroSeq V3 achieved a pooled surgical avoidance rate of 62.5% (95% CI: 54.8–70.0%). The narrower confidence interval compared to V2 suggests more precise estimation. Heterogeneity remained high but improved (I2 = 89.7%, p < 0.0001, τ2 = 0.01), indicating more consistent performance across institutions. The substantial statistical heterogeneity indicated significant variation in ThyroSeq V3’s effectiveness across different clinical settings, suggesting that institutional expertise and patient selection critically influenced surgical decision-making outcomes. The relatively narrow confidence interval of the pooled estimate supports the reliability of ThyroSeq V3 in reducing unnecessary surgeries for indeterminate thyroid nodules (Figure 3).
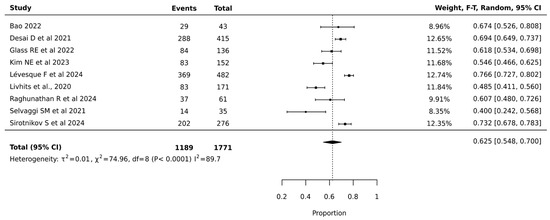
Figure 3.
Thyroseq V3—Forest Plot [21,25,27,29,30,32,39,41,42].
3.4.3. Afirma GEC
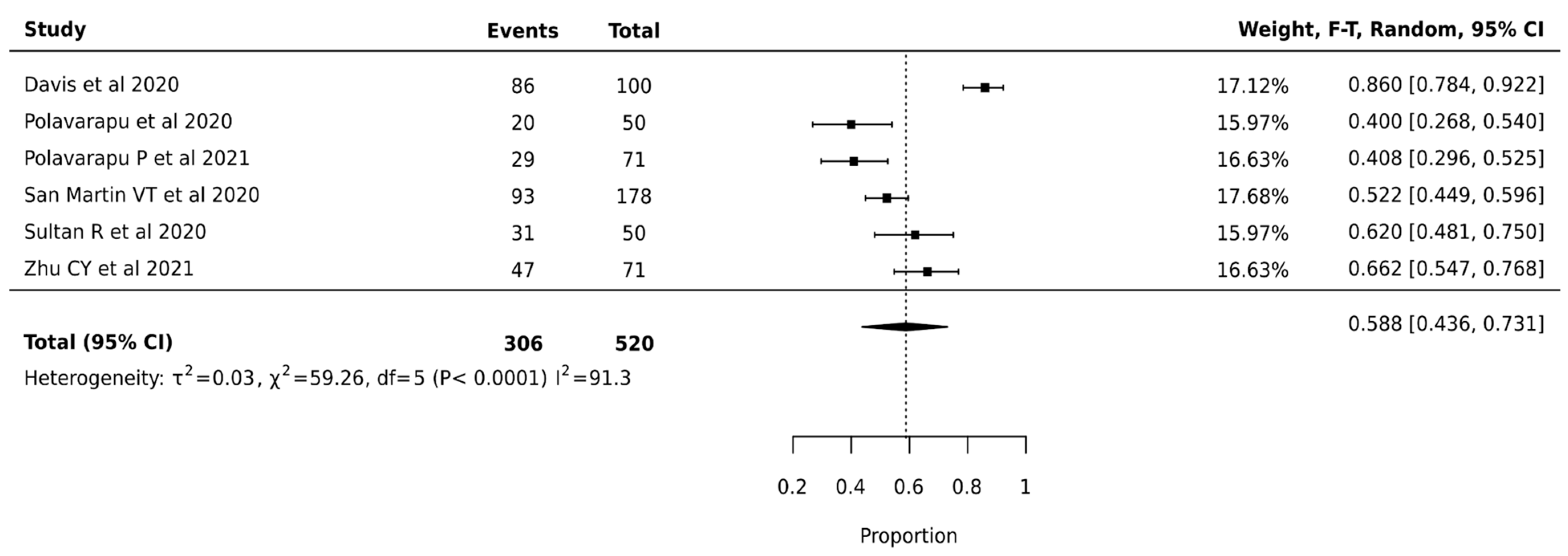
Meta-analysis of 520 nodules from 6 studies revealed a pooled surgical avoidance rate of 58.8% (95% CI: 43.6–73.1%). High heterogeneity was observed (I2 = 91.3%, p < 0.0001, τ2 = 0.03), suggesting variable effectiveness across different clinical settings (Figure 4).
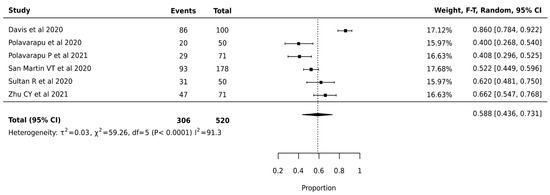
Figure 4.
Afirma GEC—Forest Plot [24,37,38,40,45,49].
3.4.4. Afirma GSC
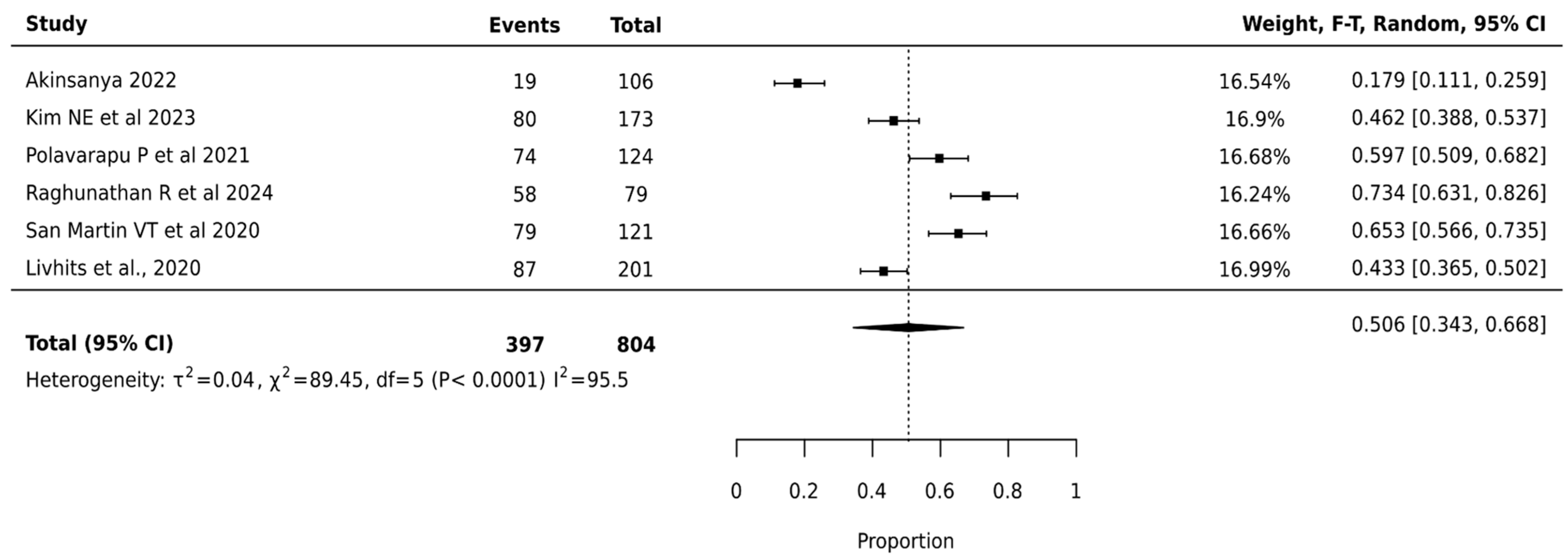
The meta-analysis of Afirma GSC involving 804 nodules across 6 studies showed a pooled surgical avoidance rate of 50.6% (95% CI: 34.3–66.8%). The platform demonstrated the highest heterogeneity among all platforms (I2 = 95.5%, p < 0.0001), indicating significant variation in institutional performance (Figure 5).
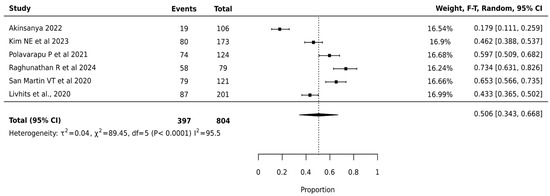
Figure 5.
Afirma GSC—Forest Plot [20,29,32,38,39,40].
3.4.5. ThyGenX/ThyraMIR
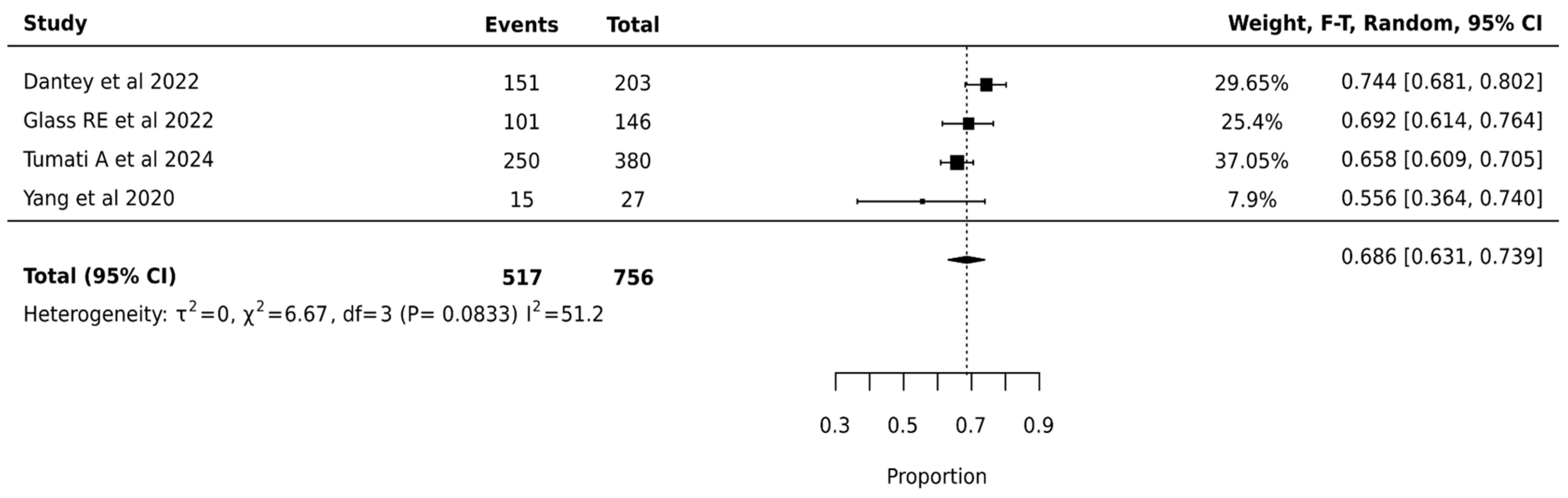
ThyGenX/ThyraMIR showed the highest pooled surgical avoidance rates of 68.6% (95% CI: 63.1–73.9%) after analysis of 756 nodules from 4 studies. The moderate statistical heterogeneity (I2 = 51.2%, p = 0.0833) suggested some variation in ThyGenX/ThyraMIR’s effectiveness across different clinical settings, though less pronounced than other molecular testing platforms. The relatively narrow confidence interval of the pooled estimate (63.1–73.9%) and lower heterogeneity compared to other platforms indicate more consistent performance of ThyGenX/ThyraMIR in surgical decision-making for indeterminate thyroid nodules (Figure 6).
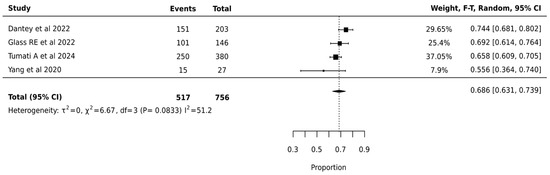
Figure 6.
ThyGenX/ThyraMIR—Forest Plot [23,27,47,48].
3.5. Mutation Frequencies Across Platforms and Coherence with Bethseda Classifications
The pooled analysis of BRAF and RAS mutations across different platforms revealed significant variability in mutation frequencies, highlighting the influence of platform-specific detection capabilities and study designs. For BRAF mutations, Thyroseq demonstrated a pooled mutation proportion of 2.7% (95% CI: 0.3%, 6.7%), with high heterogeneity (I2 = 85.5%), while NGS showed a higher pooled mutation proportion of 6.7% (95% CI: 3.4%, 10.7%), with no heterogeneity (I2 = 0%). For RAS mutations, ThyroSeq showed a pooled mutation proportion of 18.8% (95% CI: 9.3%, 30.6%), with significant heterogeneity (I2 = 95.7%), whereas NGS demonstrated a proportion of 15.7% (95% CI: 3.8%, 32.9%), with moderate heterogeneity (I2 = 67.1%). However, due to the lack of detailed data on mutation frequencies stratified by Bethesda categories in the included studies, the coherence of specific gene mutations with Bethesda classifications could not be fully addressed, limiting the ability to draw definitive conclusions about their diagnostic or prognostic implications (Table 2).

Table 2.
Summary of pooled mutation proportions by gene and platform.
4. Discussion
This meta-analysis evaluated the impact of molecular testing on surgical decision-making in indeterminate thyroid nodules. The analysis included 31 studies and 4464 indeterminate thyroid nodules, assessing the impact of molecular testing on surgical decision-making. The analysis revealed that ThyGenX/ThyraMIR had the highest surgical avoidance rate at 68.6% (95% CI: 63.1–73.9%), with the lowest heterogeneity (I2 = 51.2%). This superior performance likely stems from its combined approach, utilizing both mutation analysis and microRNA expression [50]. However, the sample size for ThyGenX/ThyraMIR was smaller compared to ThyroSeq and Afirma, which may limit the generalizability of its results.
ThyroSeq showed improvement from V2 to V3, with rates increasing from 50.3% (95% CI: 20.8–79.6%) to 62.5% (95% CI: 54.8–70.0%). This advancement reflects enhanced genomic sequencing capabilities and refined algorithmic interpretation.
Afirma platforms showed comparable performance between iterations, with GEC achieving 58.8% (95% CI: 43.6–73.1%) and GSC showing 50.6% (95% CI: 34.3–66.8%). Significant heterogeneity [18] observed across most platforms (I2 ranging from 51.2% to 98%) indicates substantial variation in real-world effectiveness. This variability likely stems from differences in institutional expertise, patient selection criteria, and implementation protocols. The notably lower heterogeneity in ThyGenX/ThyraMIR suggests more consistent performance across different clinical settings, suggesting variation in implementation effectiveness. The risk of bias was assessed using the NOS and Cochrane Risk of Bias tool, with most studies being observational and of moderate to high quality. A few studies reported complication rates, but these did not show significant differences.
Our findings demonstrating the surgical avoidance rate observed for ThyroSeq V3 (62.5%) aligned with a similar study by Chen et al. (2020) [51], which showed ThroSeq V3’s contribution in preventing unnecessary diagnostic surgeries by classifying indeterminate thyroid nodules as either negative or positive. In addition, our study aligned with a meta-analysis by Vardarli et al. (2024) [52] that showed that molecular testing in patients with indeterminate thyroid nodules helps avoid unnecessary thyroid surgery, with Thyroseq V3 having the best diagnostic performance. The findings that ThyGen/ThyraMIR had the highest surgical avoidance rate in comparison with other molecular tests, corresponded with findings by Glass et al. (2021) [27] who reported that ThyGenX/ThyraMIR had a surgical avoidance rate of 38.9% compared with Thyroseq at 24.2%.
This analysis underscores the potential of molecular testing to substantially reduce unnecessary thyroid surgeries [52], with observed surgical avoidance rates ranging from 50.3% to 68.6% across different platforms. The consistent performance of ThyGenX/ThyraMIR, with the highest surgical avoidance rate (68.6%), suggests that this platform can offer more reliable clinical decision-making support, aiding in surgical planning and patient counseling. However, given the relatively limited number of studies assessing this platform, further validation in larger, multi-center cohorts is necessary before drawing definitive conclusions. The demonstrated improvement from ThyroSeq V2 to V3 further highlights the clinical value of platform evolution in enhancing diagnostic precision. While these findings support the adoption of molecular testing, the observed variation in surgical avoidance rates across institutions emphasizes the need for standardized implementation protocols and integration of test results within a multidisciplinary team, ultimately ensuring optimal patient selection and surgical outcomes [53]. Moreover, providing patients with clear and comprehensive information about their treatment options and the role of molecular testing is crucial for facilitating informed decision-making and promoting active coping strategies, which can reduce anxiety and improve patient satisfaction [54,55].
Our meta-analysis is strengthened by its comprehensive search strategy across multiple databases and rigorous data extraction and quality assessment. However, the predominance of observational studies (29 out of 31) limits causal inference and introduces potential selection bias. Sample sizes varied significantly across studies and platforms, with limited representation for ThyroSeq V2 and ThyGenX/ThyraMIR, making direct comparisons less robust. The substantial variation in follow-up periods (6 to 84 months) also restricts the assessment of long-term outcomes. While publication bias was minimal, the substantial heterogeneity observed in most platforms, coupled with the geographic concentration of studies in North America, suggests that unmeasured factors and limited generalizability may influence the findings [56]. The lack of standardized reporting further complicates the interpretation and application of results across different clinical settings. While we attempted to analyze the impact of specific mutations on surgical avoidance rates, the available data were limited. Our analysis of BRAF and RAS mutation frequencies revealed variations across platforms. Due to inconsistencies in reporting and a lack of stratification by Bethesda category, we were unable to draw definitive conclusions about the relationship between specific mutations, platform performance, and surgical outcomes. With molecular testing significantly reducing unnecessary surgeries, there remains a risk of false-negative results, where a malignant nodule is incorrectly classified as benign. The accuracy of each molecular testing platform, including its ability to correctly identify benign nodules and avoid unnecessary surgeries, is crucial. ThyGenX/ThyraMIR demonstrated a high surgical avoidance rate; however, to mitigate the risk of missing malignant nodules, clinical practice should integrate molecular testing with thorough clinical and radiological evaluations. Regular follow-up is also essential for nodules.
Future research should focus on standardizing implementation protocols for molecular testing and conducting long-term outcome assessments to evaluate the durability of surgical avoidance. Prospective studies comparing different molecular testing platforms head-to-head are needed to provide more definitive evidence on their relative effectiveness. Furthermore, research should investigate the cost-effectiveness of molecular testing strategies and explore the impact of molecular testing on patient-reported outcomes and quality of life.
5. Conclusions
This systematic review and meta-analysis support the role of molecular testing as a valuable adjunct in the management of indeterminate thyroid nodules, contributing to improved risk stratification and reduction in unnecessary surgeries when interpreted alongside established clinical guidelines. While surgical decisions remain closely guided by the ATA and ETA frameworks, the integration of molecular testing—particularly platforms such as ThyroSeq V3 and ThyGenX/ThyraMIR—has demonstrated the potential to influence clinical management, especially in the presence of specific mutations such as BRAF. However, the broader use of multigene panels has yet to consistently impact decision-making independently.
Our findings highlight the heterogeneity in test performance and institutional practices, underscoring the need for standardized implementation protocols and clearer clinical algorithms. Looking ahead, the incorporation of additional mutations such as TERT and expanded genomic classifiers may enhance the predictive value of these platforms. Until such tools are validated through prospective studies, molecular testing should be applied as a complementary measure within multidisciplinary evaluation frameworks, ensuring informed and individualized patient care.
Author Contributions
Conceptualization, R.C., M.A. and R.J.P.; methodology, R.C., J.H. and M.A.; formal analysis, R.C.; investigation, R.C. and R.J.P.; data curation, R.C. and M.A.; writing—original draft preparation, R.C.; writing—review and editing, R.C., J.H., K.E.P., M.A., O.D., N.E., V.-I.F. and R.J.P.; supervision, R.J.P.; project administration, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roth, M.Y.; Witt, R.L.; Steward, D.L. Molecular testing for thyroid nodules: Review and current state. Cancer 2018, 124, 888–898. [Google Scholar] [PubMed]
- Patel, J.; Klopper, J.; Cottrill, E.E. Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities. Front. Endocrinol. 2023, 14, 1101410. [Google Scholar]
- Krane, J.F.; Nayar, R.; Renshaw, A.A. Atypia of undetermined significance/follicular lesion of undetermined significance. In The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes; Springer: Berlin/Heidelberg, Germany, 2018; pp. 49–70. [Google Scholar]
- AlSaedi, A.H.; Almalki, D.S.; ElKady, R.M. Approach to thyroid nodules: Diagnosis and treatment. Cureus 2024, 16, e52232. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: A prospective blinded multicenter study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar]
- de Koster, E.J.; de Geus-Oei, L.F.; Dekkers, O.M.; van Engen-van Grunsven, I.; Hamming, J.; Corssmit, E.P.; Morreau, H.; Schepers, A.; Smit, J.; Oyen, W.J.; et al. Diagnostic utility of molecular and imaging biomarkers in cytological indeterminate thyroid nodules. Endocr. Rev. 2018, 39, 154–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishino, M. Molecular Diagnostics in Thyroid Cytology. In Molecular Diagnostics in Cytopathology: A Practical Handbook for the Practicing Pathologist; Springer: Berlin/Heidelberg, Germany, 2019; pp. 249–299. [Google Scholar]
- Sipos, J.A.; Ringel, M.D. Molecular testing in thyroid cancer diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101680. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar]
- Moskalewicz, A.; Oremus, M. No clear choice between Newcastle–Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J. Clin. Epidemiol. 2020, 120, 94–103. [Google Scholar] [CrossRef]
- Jørgensen, L.; Paludan-Müller, A.S.; Laursen, D.R.; Savović, J.; Boutron, I.; Sterne, J.A.; Higgins, J.P.T.; Hróbjartsson, A. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: Overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst. Rev. 2016, 5, 1–13. [Google Scholar]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Babineau, J. Product review: Covidence (systematic review software). J. Can. Health Libr. Assoc./J. l’Association Bibliothèques Santé Can. 2014, 35, 68–71. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Aromataris, E.; Tufanaru, C.; Stern, C.; Porritt, K.; Farrow, J.; Lockwood, C.; Stephenson, M.; Moola, S.; Lizarondo, L.; et al. The development of software to support multiple systematic review types: The Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI). JBI Evid. Implement. 2019, 17, 36–43. [Google Scholar] [CrossRef]
- Borenstein, M. Comprehensive meta-analysis software. In Systematic Reviews in Health Research: Meta-Analysis in Context; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 535–548. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Abdelhakam, D.A.; Mojica, R.E.; Huenerberg, K.A.; Nassar, A. Impact of a genomic classifier on indeterminate thyroid nodules: An institutional experience. J. Am. Soc. Cytopathol. 2021, 10, 155–163. [Google Scholar] [CrossRef]
- Akinsanya, A.; Wu, H. Performance of Afirma Genomic Sequencing Classifier in Thyroid Nodules with Preoperative Cytologic Diagnosis of AUS/FLUS. J. Am. Soc. Cytopathol. 2022, 11, S74. [Google Scholar] [CrossRef]
- Bao, G.; Chang, C.; Yin, A. PSAT267 Performance of ThyroSeq V3 molecular testing in assessing indeterminate thyroid nodules for thyroid cancer at an urban endocrinology clinic. J. Endocr. Soc. 2022, 6 (Suppl. S1), A809. [Google Scholar] [CrossRef]
- Carty, S.E.; Ohori, N.P.; Hilko, D.A.; McCoy, K.L.; French, E.K.; Manroa, P.; Morariu, E.; Sridharan, S.; Seethala, R.R.; Yip, L. The clinical utility of molecular testing in the management of thyroid follicular neoplasms (Bethesda IV nodules). Ann. Surg. 2020, 272, 621–627. [Google Scholar] [CrossRef]
- Dantey, K.; Hasan, F.; Tipu, A.; Verma, T. ODP481 Frequency and outcomes of reporting Bethesda class III thyroid cytopathology-An integrated health network’s experience. J. Endocr. Soc. 2022, 6 (Suppl. S1), A766. [Google Scholar] [CrossRef]
- Davis, H.; Jug, R. Afirma “Benign” Nodules Show Stability with Long-term Follow-up. J. Am. Soc. Cytopathol. 2020, 9, S57. [Google Scholar]
- Desai, D.; Lepe, M.; Baloch, Z.W.; Mandel, S.J. ThyroSeq v3 for Bethesda III and IV: An institutional experience. Cancer Cytopathol. 2021, 129, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, S.R.; Zehr, B.; Amraei, R.; Toraldo, G.; Guan, H.; Kindelberger, D.; Lee, S.L.; Cerda, S.R. ThyroSeq v2 testing: Impact on cytologic diagnosis, management, and cost of care in patients with thyroid nodule. Thyroid 2020, 30, 1528–1534. [Google Scholar]
- Glass, R.E.; Marotti, J.D.; Kerr, D.A.; Levy, J.J.; Vaickus, L.J.; Gutmann, E.J.; Tafe, L.J.; Motanagh, S.A.; Sorensen, M.J.; Davies, L.; et al. Using molecular testing to improve the management of thyroid nodules with indeterminate cytology: An institutional experience with review of molecular alterations. J. Am. Soc. Cytopathol. 2022, 11, 79–86. [Google Scholar]
- Kannan, S.; Aggarwal, S.; Shivaprasad, K.; Sooragonda, B.; Khadilkar, K.; Gondaliya, H. Pilot results of a cost effective NGS panel for prognostication of Thyroid nodules/cancers. Indian J. Endocrinol. Metab. 2022, 26, 14. [Google Scholar] [CrossRef]
- Kim, N.E.; Raghunathan, R.S.; Hughes, E.G.; Longstaff, X.R.; Tseng, C.H.; Li, S.; Cheung, D.S.; A Gofnung, Y.; Famini, P.; Wu, J.X.; et al. Bethesda III and IV thyroid nodules managed nonoperatively after molecular testing with Afirma GSC or Thyroseq v3. J. Clin. Endocrinol. Metab. 2023, 108, e698–e703. [Google Scholar] [CrossRef]
- Lévesque, F.; Payne, R.J.; Beaudoin, D.; Boucher, A.; Fortier, P.H.; Massicotte, M.H.; Pusztaszeri, M.; Rondeau, G.; Corriveau, E.; El Malt, F.; et al. A Prospective Study of Publicly Funded Molecular Testing of Indeterminate Thyroid Nodules: Canada’s Experience. J. Clin. Endocrinol. Metab. 2025, 17, e1031–e1037. [Google Scholar] [CrossRef]
- Li, W.; Justice-Clark, T.; Cohen, M.B. The utility of ThyroSeq® in the management of indeterminate thyroid nodules by fine-needle aspiration. Cytopathology 2021, 32, 505–512. [Google Scholar]
- Livhits, M.J.; Zhu, C.Y.; Kuo, E.J.; Nguyen, D.T.; Kim, J.; Tseng, C.H.; Leung, A.M.; Rao, J.; Levin, M.; Douek, M.L.; et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: A randomized clinical trial. JAMA Oncol. 2021, 7, 70–77. [Google Scholar] [CrossRef]
- Lu, J.; Lupo, M.A. Malignancy Risk in RAS-Mutated Cytologically Indeterminate Thyroid Nodules: Real-World Clinical Experience. J. Endocr. Soc. 2021, 5 (Suppl. S1), A865. [Google Scholar]
- Munoz-Zuluaga, C.A.; Heymann, J.J.; Solomon, J.P.; Patel, A.; Siddiqui, M.T.; Scognamiglio, T.; Gokozan, H.N. Use of the Afirma Xpression Atlas for cytologically indeterminate, Afirma Genomic Sequencing Classifier suspicious thyroid nodules: Clinicopathologic analysis with postoperative molecular testing. Am. J. Clin. Pathol. 2024, 161, 463–468. [Google Scholar]
- Olmos, R.; Domínguez, J.M.; Vargas-Salas, S.; Mosso, L.; Fardella, C.E.; González, G.; Baudrand, R.; Guarda, F.; Valenzuela, F.; Arteaga, E.; et al. ThyroidPrint®: Clinical utility for indeterminate thyroid cytology. Endocr.-Relat. Cancer 2023, 30, e220409. [Google Scholar] [CrossRef] [PubMed]
- Papazian, M.R.; Dublin, J.C.; Patel, K.N.; Oweity, T.; Jacobson, A.S.; Brandler, T.C.; Givi, B. Repeat fine-needle aspiration with molecular analysis in management of indeterminate thyroid nodules. Otolaryngol. Head Neck Surg. 2023, 168, 738–744. [Google Scholar] [PubMed]
- Polavarapu, P.; Fingeret, A.; Yuil-Valdes, A.; Patel, A.; Goldner, W. MON-518 Institutional Experience with Cytologically Indeterminate Thyroid Nodules: No Molecular Testing Versus Afirma Gene Expression Classifier or Genomic Sequencing Classifier. J. Endocr. Soc. 2020, 4 (Suppl. S1), MON-518. [Google Scholar] [CrossRef]
- Polavarapu, P.; Fingeret, A.; Yuil-Valdes, A.; Olson, D.; Patel, A.; Shivaswamy, V.; Matthias, T.D.; Goldner, W. Comparison of Afirma GEC and GSC to nodules without molecular testing in cytologically indeterminate thyroid nodules. J. Endocr. Soc. 2021, 5, bvab148. [Google Scholar]
- Raghunathan, R.; Longstaff, X.R.; Hughes, E.G.; Li, S.J.; Sant, V.R.; Tseng, C.H.; Rao, J.; Wu, J.X.; Yeh, M.W.; Livhits, M.J. Diagnostic performance of molecular testing in indeterminate (Bethesda III and IV) thyroid nodules with Hürthle cell cytology. Surgery 2024, 175, 221–227. [Google Scholar]
- San Martin, V.T.; Lawrence, L.; Bena, J.; Madhun, N.Z.; Berber, E.; Elsheikh, T.M.; Nasr, C.E. Real-world comparison of Afirma GEC and GSC for the assessment of cytologically indeterminate thyroid nodules. J. Clin. Endocrinol. Metab. 2020, 105, e428–e435. [Google Scholar]
- Selvaggi, S.M. The role of ThyroSeq V3 testing in the management of patients with indeterminate thyroid nodules on fine needle aspiration. Diagn. Cytopathol. 2021, 49, 838–841. [Google Scholar]
- Sirotnikov, S.; Griffith, C.C.; Lubin, D.; Zhang, C.; Saba, N.F.; Li, D.; Kornfield, A.; Chen, A.; Shi, Q. ThyroSeq overview on indeterminate thyroid nodules: An institutional experience. Diagn. Cytopathol. 2024, 52, 353–361. [Google Scholar]
- Song, Y.; Xu, G.; Ma, T.; Zhu, Y.; Yu, H.; Yu, W.; Wei, W.; Wang, T.; Zhang, B. Utility of a multigene testing for preoperative evaluation of indeterminate thyroid nodules: A prospective blinded single center study in China. Cancer Med. 2020, 9, 8397–8405. [Google Scholar] [CrossRef]
- Steinmetz, D.; Kim, M.; Choi, J.H.; Yeager, T.; Samuel, K.; Khajoueinejad, N.; Buseck, A.; Imtiaz, S.; Fernandez-Ranvier, G.; Lee, D.; et al. How effective is the use of molecular testing in preoperative decision making for management of indeterminate thyroid nodules? World J. Surg. 2022, 46, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Sultan, R.; Levy, S.; Sulanc, E.; Honasoge, M.; Rao, S.D. Utility of Afirma gene expression classifier for evaluation of indeterminate thyroid nodules and correlation with ultrasound risk assessment: Single institutional experience. Endocr. Pract. 2020, 26, 543–551. [Google Scholar] [CrossRef]
- Torrecillas, V.; Sharma, A.; Neuberger, K.; Abraham, D. Utility of mutational analysis for risk stratification of indeterminate thyroid nodules in a real-world setting. Clin. Endocrinol. 2022, 96, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Tumati, A.; Egan, C.E.; Lee-Saxton, Y.J.; Marshall, T.E.; Lee, J.; Jain, K.; Heymann, J.J.; Gokozan, H.; Azar, S.A.; Schwarz, J.; et al. Clinical utility of a microRNA classifier in cytologically indeterminate thyroid nodules with RAS mutations: A multi-institutional study. Surgery 2024, 175, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Vazmitsel, M.; Esebua, M. Molecular Testing in Patients with Indeterminate Cytology of Thyroid Fine Needle Aspiration: A single Medical Institute Experience. J. Am. Soc. Cytopathol. 2020, 9, S48. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Donangelo, I.; Gupta, D.; Nguyen, D.T.; Ochoa, J.E.; Yeh, M.W.; Livhits, M.J. Outcomes of indeterminate thyroid nodules managed nonoperatively after molecular testing. J. Clin. Endocrinol. Metab. 2021, 106, e1240–e1247. [Google Scholar] [CrossRef]
- Rossi, E.D.; Larocca, L.M.; Pantanowitz, L. Ancillary molecular testing of indeterminate thyroid nodules. Cancer Cytopathol. 2018, 126, 654–671. [Google Scholar] [CrossRef]
- Chen, T.; Gilfix, B.M.; Rivera, J.; Sadeghi, N.; Richardson, K.; Hier, M.P.; Forest, V.; Fishman, D.; Caglar, D.; Pusztaszeri, M.; et al. The Role of the ThyroSeq v3 Molecular Test in the Surgical Management of Thyroid Nodules in the Canadian Public Health Care Setting. Thyroid 2020, 30, 1280–1287. [Google Scholar] [CrossRef]
- Vardarli, I.; Tan, S.; Görges, R.; Krämer, B.K.; Herrmann, K.; Brochhausen, C. Diagnostic accuracy of Afirma Gene Expression Classifier, Afirma Gene Sequencing Classifier, ThyroSeq v2 and ThyroSeq v3 for indeterminate (Bethesda III and IV) thyroid nodules: A meta-analysis. Endocr. Connect. 2024, 13, e240170. [Google Scholar] [CrossRef]
- Whiteman, A.R.; Dhesi, J.K.; Walker, D. The high-risk surgical patient: A role for a multi-disciplinary team approach? BJA Br. J. Anaesth. 2016, 116, 311–314. [Google Scholar] [CrossRef]
- Brouillette, K.; Chowdhury, R.; Payne, K.E.; Pusztaszeri, M.P.; Forest, V. A Scoping Review of Patient Health-Related Quality of Life Following Surgery or Molecular Testing for Individuals with Indeterminate Thyroid Nodules. Healthcare 2024, 12, 2025. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, J.G.; Wagner, G.J.; Del Bene, M. Resilience and distress among amyotrophic lateral sclerosis patients and caregivers. Psychosom. Med. 2000, 62, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Macaskill, P.; Walter, S.D.; Irwig, L. A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 2001, 20, 641–654. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).