Usefulness of Flow Cytometry Monocyte Partitioning in the Diagnosis of Chronic Myelomonocytic Leukemia in a Real-World Setting
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Flow Cytometry
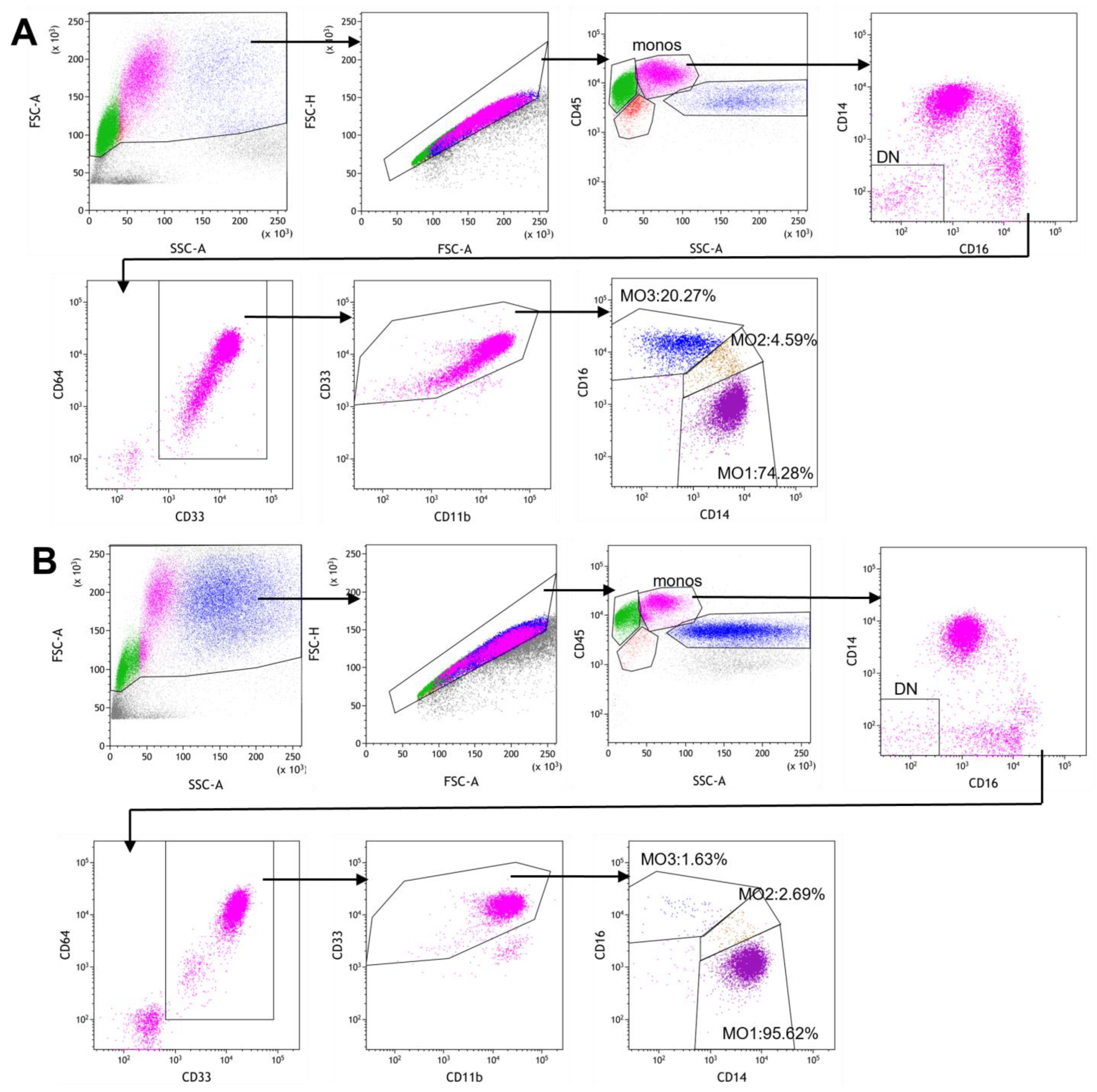
2.3. Gating Strategy for Monocyte Subset Partitioning Using a Routine 8-Color Tube
2.4. Statistical Analysis
3. Results
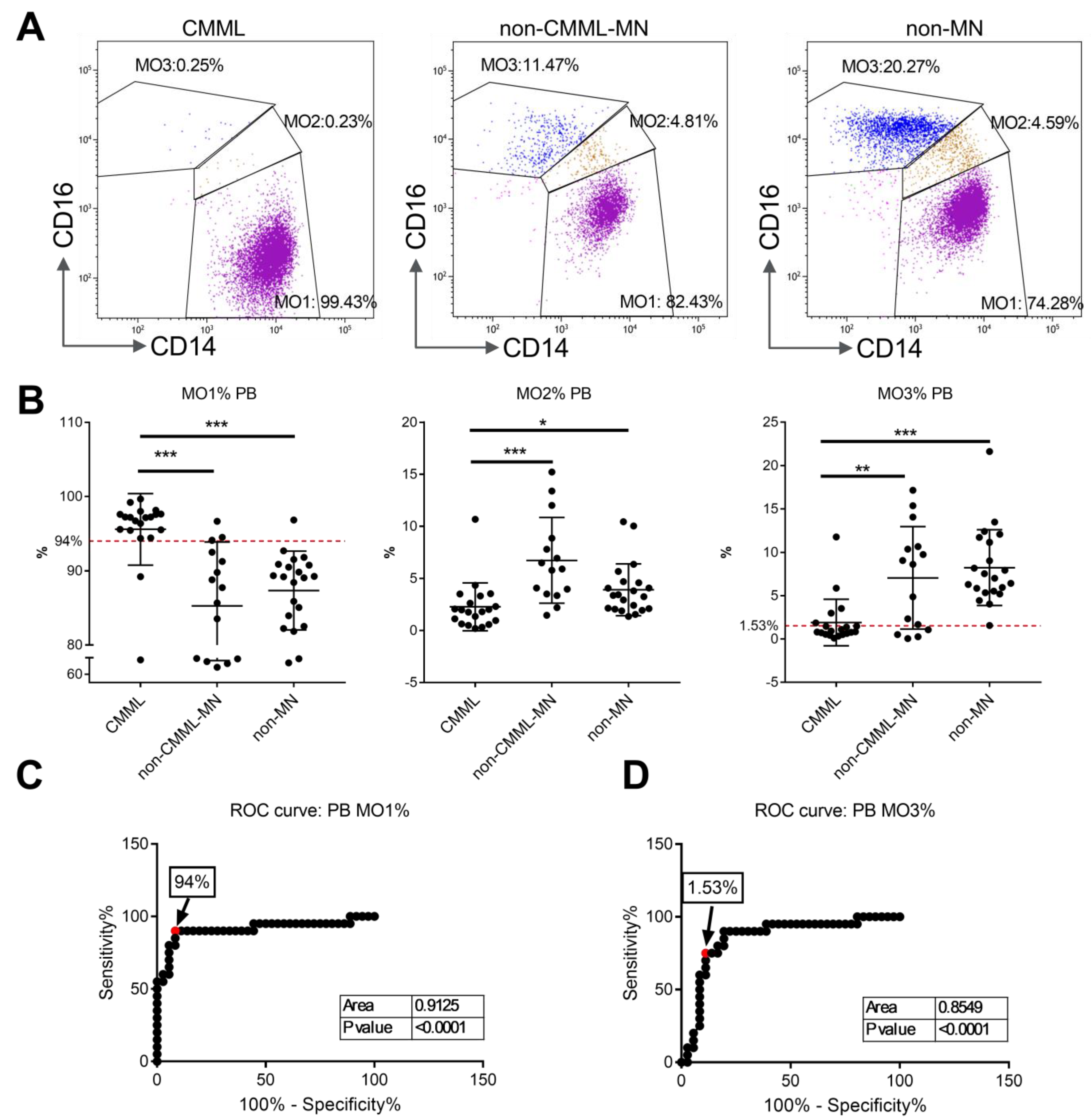
3.1. Monocyte Subset Analysis on Peripheral Blood Samples from CMML Patients and Non-CMML Controls
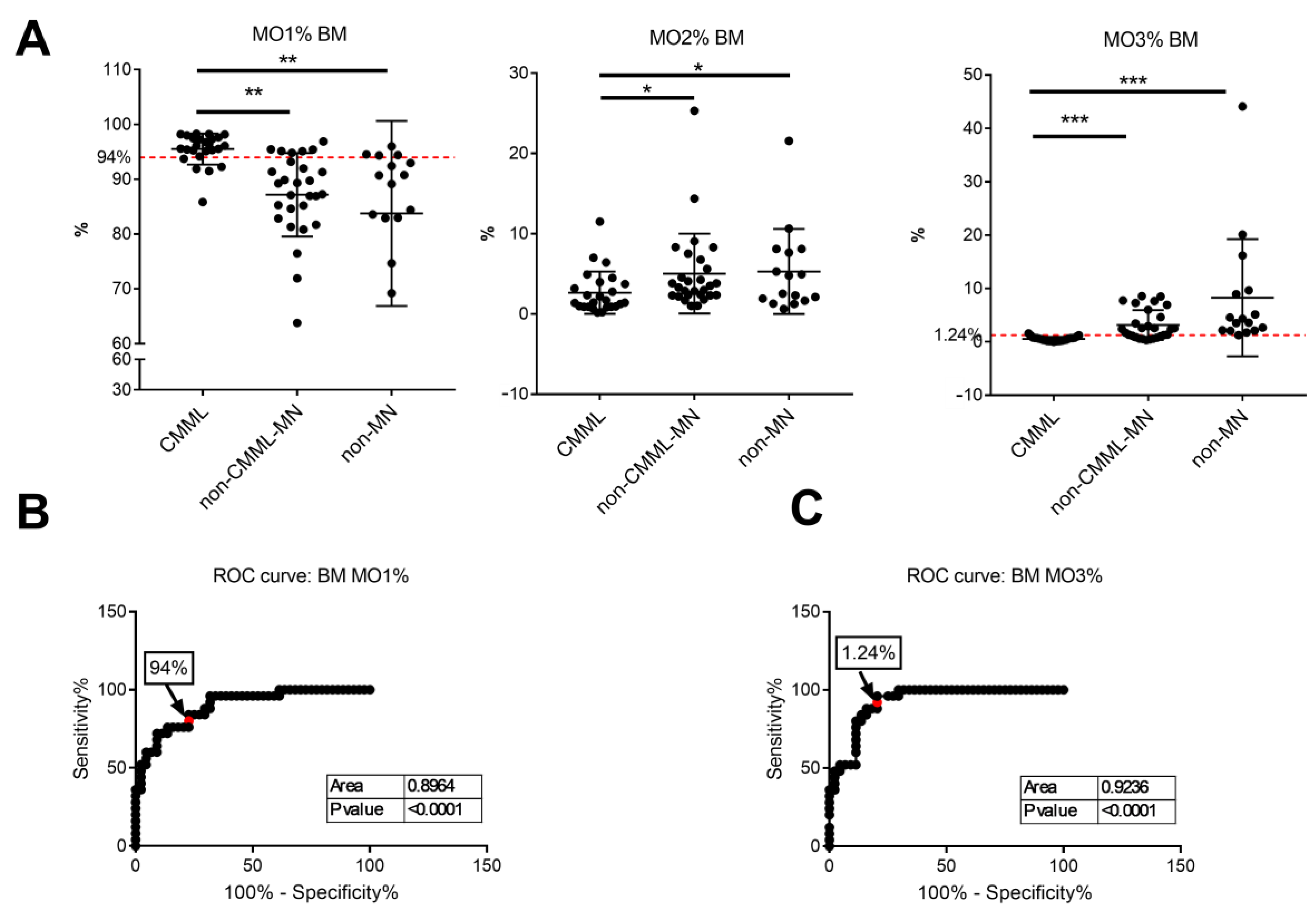
3.2. Monocyte Subset Analysis on Bone Marrow Samples
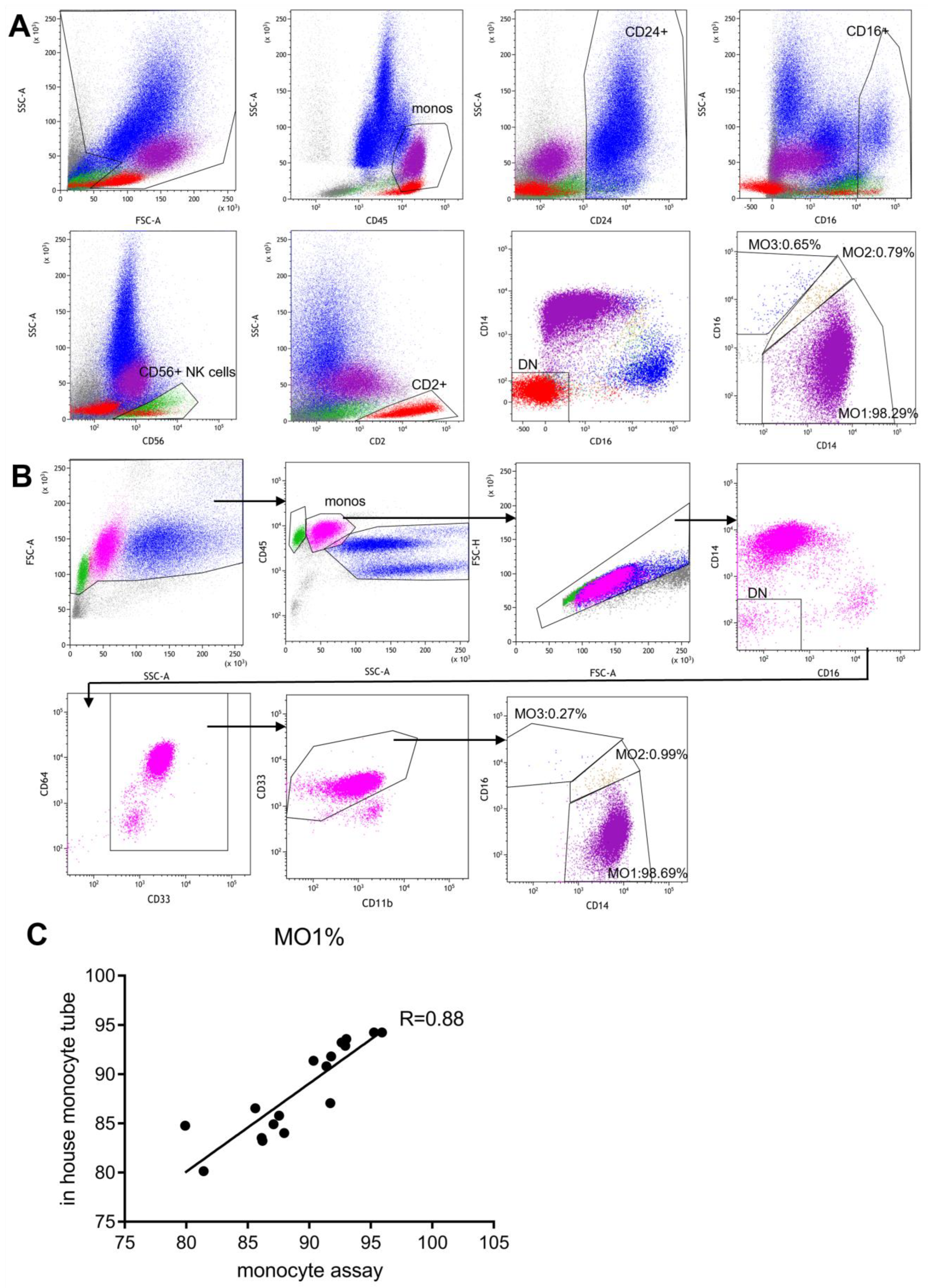
3.3. Side-by-Side Comparison with the Standard “Monocyte Assay”
3.4. Clinical Features, Chromosome Abnormalities and Gene Mutations of CMML Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.M.; Makishima, H.; Tiu, R.V.; Szpurka, H.; Huang, Y.; Traina, F.; Visconte, V.; Sugimoto, Y.; Prince, C.; O’Keefe, C.; et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood 2011, 118, 3932–3941. [Google Scholar] [CrossRef] [PubMed]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Morabito, M.; Preudhomme, C.; Berthon, C.; Ades, L.; Fenaux, P.; Platzbecker, U.; Gagey, O.; et al. Clonal architecture of chronic myelomonocytic leukemias. Blood 2013, 121, 2186–2198. [Google Scholar] [CrossRef] [PubMed]
- Merlevede, J.; Droin, N.; Qin, T.; Meldi, K.; Yoshida, K.; Morabito, M.; Chautard, E.; Auboeuf, D.; Fenaux, P.; Braun, T.; et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat. Commun. 2016, 7, 10767. [Google Scholar] [CrossRef]
- Xu, Y.; McKenna, R.W.; Karandikar, N.J.; Pildain, A.J.; Kroft, S.H. Flow cytometric analysis of monocytes as a tool for distinguishing chronic myelomonocytic leukemia from reactive monocytosis. Am. J. Clin. Pathol. 2005, 124, 799–806. [Google Scholar] [CrossRef]
- Harrington, A.M.; Schelling, L.A.; Ordobazari, A.; Olteanu, H.; Hosking, P.R.; Kroft, S.H. Immunophenotypes of Chronic Myelomonocytic Leukemia (CMML) Subtypes by Flow Cytometry: A Comparison of CMML-1 vs CMML-2, Myeloproliferative vs Dysplastic, De Novo vs Therapy-Related, and CMML-Specific Cytogenetic Risk Subtypes. Am. J. Clin. Pathol. 2016, 146, 170–181. [Google Scholar] [CrossRef]
- Sojitra, P.; Gandhi, P.; Fitting, P.; Kini, A.R.; Alkan, S.; Velankar, M.M.; Venkataraman, G. Chronic myelomonocytic leukemia monocytes uniformly display a population of monocytes with CD11c underexpression. Am. J. Clin. Pathol. 2013, 140, 686–692. [Google Scholar] [CrossRef]
- Lacronique-Gazaille, C.; Chaury, M.P.; Le Guyader, A.; Faucher, J.L.; Bordessoule, D.; Feuillard, J. A simple method for detection of major phenotypic abnormalities in myelodysplastic syndromes: Expression of CD56 in CMML. Haematologica 2007, 92, 859–860. [Google Scholar] [CrossRef]
- Feng, R.; Bhatt, V.R.; Fu, K.; Pirruccello, S.; Yuan, J. Application of immunophenotypic analysis in distinguishing chronic myelomonocytic leukemia from reactive monocytosis. Cytom. B Clin. Cytom. 2018, 94, 901–909. [Google Scholar] [CrossRef]
- Hadjadj, J.; Michel, M.; Chauveheid, M.P.; Godeau, B.; Papo, T.; Sacre, K. Immune thrombocytopenia in chronic myelomonocytic leukemia. Eur. J. Haematol. 2014, 93, 521–526. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef]
- Selimoglu-Buet, D.; Wagner-Ballon, O.; Saada, V.; Bardet, V.; Itzykson, R.; Bencheikh, L.; Morabito, M.; Met, E.; Debord, C.; Benayoun, E.; et al. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood 2015, 125, 3618–3626. [Google Scholar] [CrossRef]
- Selimoglu-Buet, D.; Badaoui, B.; Benayoun, E.; Toma, A.; Fenaux, P.; Quesnel, B.; Etienne, G.; Braun, T.; Abermil, N.; Morabito, M.; et al. Accumulation of classical monocytes defines a subgroup of MDS that frequently evolves into CMML. Blood 2017, 130, 832–835. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Timm, M.M.; Vallapureddy, R.; Lasho, T.L.; Ketterling, R.P.; Gangat, N.; Shi, M.; Tefferi, A.; Solary, E.; Reichard, K.K.; et al. Flow cytometry based monocyte subset analysis accurately distinguishes chronic myelomonocytic leukemia from myeloproliferative neoplasms with associated monocytosis. Blood Cancer J. 2017, 7, e584. [Google Scholar] [CrossRef]
- Tarfi, S.; Harrivel, V.; Dumezy, F.; Guy, J.; Roussel, M.; Mimoun, A.; Fenaux, P.; Chapuis, N.; Solary, E.; Selimoglu-Buet, D.; et al. Multicenter validation of the flow measurement of classical monocyte fraction for chronic myelomonocytic leukemia diagnosis. Blood Cancer J. 2018, 8, 114. [Google Scholar] [CrossRef]
- Hudson, C.A.; Burack, W.R.; Leary, P.C.; Bennett, J.M. Clinical Utility of Classical and Nonclassical Monocyte Percentage in the Diagnosis of Chronic Myelomonocytic Leukemia. Am. J. Clin. Pathol. 2018, 150, 293–302. [Google Scholar] [CrossRef]
- Talati, C.; Zhang, L.; Shaheen, G.; Kuykendall, A.; Ball, M.; Zhang, Q.; Lancet, J.E.; Zuckerman, K.S.; List, A.F.; Komrokji, R.; et al. Monocyte subset analysis accurately distinguishes CMML from MDS and is associated with a favorable MDS prognosis. Blood 2017, 129, 1881–1883. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Pophali, P.A.; Timm, M.M.; Mangaonkar, A.A.; Shi, M.; Reichard, K.; Tefferi, A.; Pavelko, K.; Villasboas, J.C.; Jevremovic, D.; Patnaik, M.M. Practical limitations of monocyte subset repartitioning by multiparametric flow cytometry in chronic myelomonocytic leukemia. Blood Cancer J. 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Barge, L.; Gooch, M.; Hendle, M.; Simleit, E. Real world implementation of flow cytometric monocyte subset partitioning for distinguishing chronic myelomonocytic leukaemia from other causes of monocytosis. Pathology 2023, 55, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Cross, D.; Mollee, P. The use of monocyte subset repartitioning by flow cytometry for diagnosis of chronic myelomonocytic leukaemia. Blood Cancer J. 2021, 11, 6. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Wagner-Ballon, O.; Bettelheim, P.; Lauf, J.; Bellos, F.; Della Porta, M.; Travaglino, E.; Subira, D.; Lopez, I.N.; Tarfi, S.; Westers, T.M.; et al. ELN iMDS flow working group validation of the monocyte assay for chronic myelomonocytic leukemia diagnosis by flow cytometry. Cytom. B Clin. Cytom. 2023, 104, 66–76. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. Chronic Myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 97–115. [Google Scholar] [CrossRef]
- Jurado, R.; Huguet, M.; Xicoy, B.; Cabezon, M.; Jimenez-Ponce, A.; Quintela, D.; De La Fuente, C.; Raya, M.; Vinets, E.; Junca, J.; et al. Optimization of monocyte gating to quantify monocyte subsets for the diagnosis of chronic myelomonocytic leukemia. Cytom. B Clin. Cytom. 2023, 104, 319–330. [Google Scholar] [CrossRef]
- Frisanco Oliveira, A.; Tansini, A.; Toledo, T.R.; Balceiro, R.; Onofre Vidal, D.; de Martino Lee, M.L.; Lorand-Metze, I.; Lopes, L.F. Immunophenotypic characteristics of juvenile myelomonocytic leukaemia and their relation with the molecular subgroups of the disease. Br. J. Haematol. 2021, 192, 129–136. [Google Scholar] [CrossRef]
- Gadgeel, M.; Bagla, S.; Buck, S.; Shamoun, M.; Ravindranath, Y. CD14/16 monocyte profiling in juvenile myelomonocytic leukemia. Pediatr. Blood Cancer 2020, 67, e28555. [Google Scholar] [CrossRef]
- Allen, S.A.; Ng, E.; Hahn, U.H.; Banovic, T.; Ross, D.M. Flow cytometric monocyte repartition demonstrates overlap between chronic myelomonocytic leukaemia and myeloid neoplasms with monocytosis. Pathology 2022, 54, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Quang, V.T.; Podvin, B.; Desterke, C.; Tarfi, S.; Barathon, Q.; Badaoui, B.; Freynet, N.; Parinet, V.; Leclerc, M.; Maury, S.; et al. TET2 mutational status affects myelodysplastic syndrome evolution to chronic myelomonocytic leukemia. Haematologica 2023, 108, 3135–3141. [Google Scholar] [CrossRef] [PubMed]
- Calvo, X. Should we give oligomonocytic chronic myelomonocytic leukemia a higher prominence in the next WHO Classification of Haematolymphoid Tumors? Leukemia 2023, 37, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Calvo, X.; Roman-Bravo, D.; Garcia-Gisbert, N.; Rodriguez-Sevilla, J.J.; Garcia-Avila, S.; Florensa, L.; Gibert, J.; Fernandez-Rodriguez, C.; Salido, M.; Puiggros, A.; et al. Outcomes and molecular profile of oligomonocytic CMML support its consideration as the first stage in the CMML continuum. Blood Adv. 2022, 6, 3921–3931. [Google Scholar] [CrossRef]
| Age Median (Range) | 72 (23–93) | |
|---|---|---|
| Sex | Male | 25 |
| Female | 13 | |
| WBC (109/L), median (range) | 11.3 (2.7–84.7) | |
| AMC (109/L), median (range) | 2.5 (1.0–24.6) | |
| Monocyte %, median (range) | 23% (9–44%) | |
| Hemoglobin (g/dL), median (range) | 10.4 (6.4–14.9) | |
| Platelets (109/L), median (range) | 100 (11–313) | |
| Cytogenetics (n = 36) | Normal karyotype | 22 |
| Abnormal (non-complex) | 13 | |
| Abnormal (complex) | 1 | |
| Next-generation sequencing (n = 31) | ||
| Epigenetic regulator | TET2 | 16 |
| ASXL1 | 7 | |
| E2H2 | 4 | |
| BCOR | 4 | |
| Spliceosome components | SRSF2 | 13 |
| SF3B1 | 2 | |
| U2AF1 | 4 | |
| Transcription factor | RUNX1 | 6 |
| Signal genes | KRAS/NRAS | 10 |
| CBL | 3 | |
| JAK2 | 0 | |
| SH2B3 | 4 | |
| PTPN11 | 2 | |
| Others | TP53 | 1 |
| SETBP1 | 2 | |
| PHF6 | 2 | |
| CMML subtypes | ||
| CMML-1 | 28 | |
| CMML-2 | 10 | |
| CMML-P | 21 | |
| CMML-D | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tariq, H.; Fu, L.; Gao, J.; Jagtiani, T.; Wolniak, K.; Aqil, B.; Ji, P.; Chen, Y.-H.; Chen, Q.C. Usefulness of Flow Cytometry Monocyte Partitioning in the Diagnosis of Chronic Myelomonocytic Leukemia in a Real-World Setting. Cancers 2025, 17, 1229. https://doi.org/10.3390/cancers17071229
Liu Y, Tariq H, Fu L, Gao J, Jagtiani T, Wolniak K, Aqil B, Ji P, Chen Y-H, Chen QC. Usefulness of Flow Cytometry Monocyte Partitioning in the Diagnosis of Chronic Myelomonocytic Leukemia in a Real-World Setting. Cancers. 2025; 17(7):1229. https://doi.org/10.3390/cancers17071229
Chicago/Turabian StyleLiu, Yijie, Hamza Tariq, Lucy Fu, Juehua Gao, Taruna Jagtiani, Kristy Wolniak, Barina Aqil, Peng Ji, Yi-Hua Chen, and Qing Ching Chen. 2025. "Usefulness of Flow Cytometry Monocyte Partitioning in the Diagnosis of Chronic Myelomonocytic Leukemia in a Real-World Setting" Cancers 17, no. 7: 1229. https://doi.org/10.3390/cancers17071229
APA StyleLiu, Y., Tariq, H., Fu, L., Gao, J., Jagtiani, T., Wolniak, K., Aqil, B., Ji, P., Chen, Y.-H., & Chen, Q. C. (2025). Usefulness of Flow Cytometry Monocyte Partitioning in the Diagnosis of Chronic Myelomonocytic Leukemia in a Real-World Setting. Cancers, 17(7), 1229. https://doi.org/10.3390/cancers17071229





