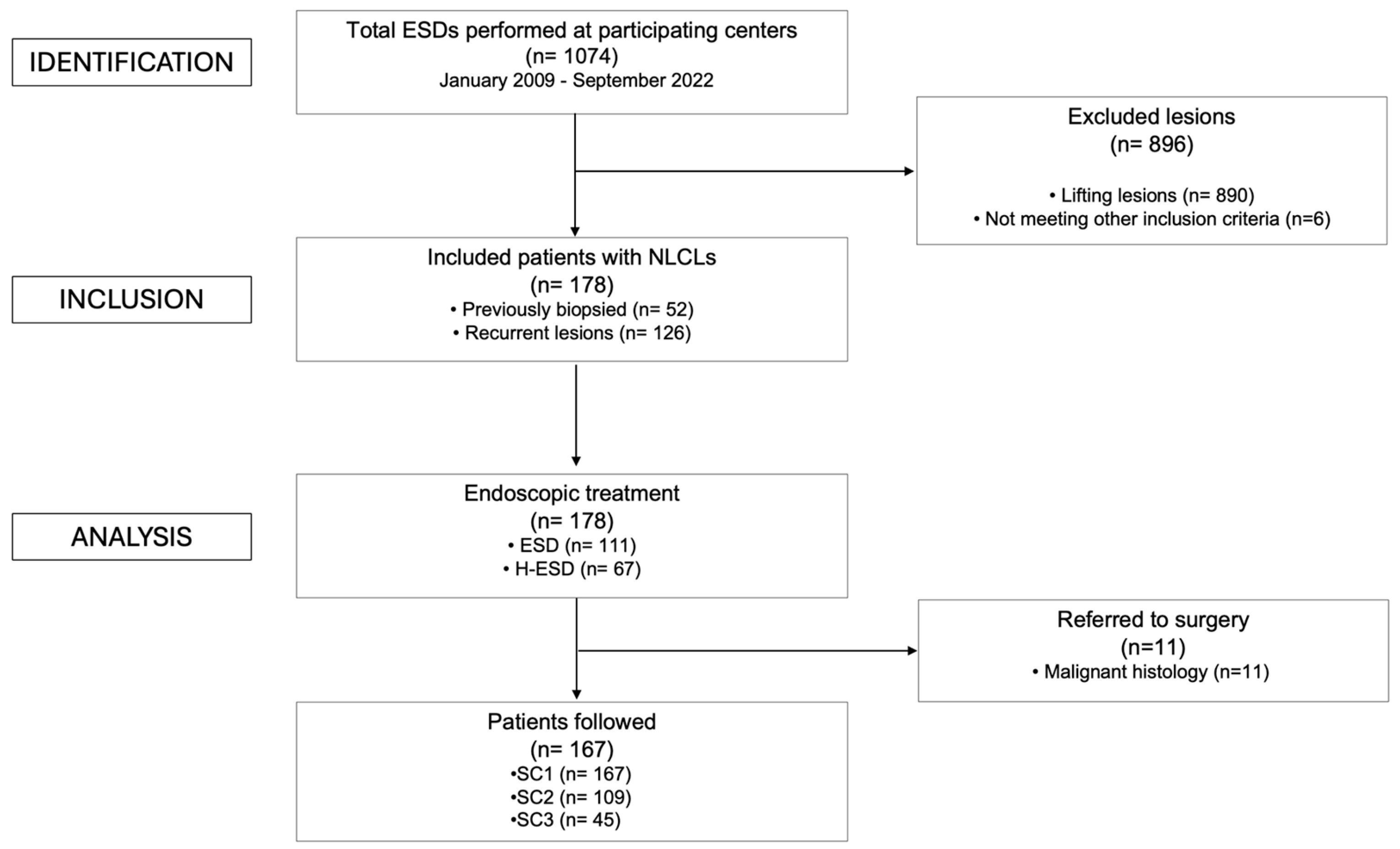
Endoscopic Submucosal Dissection (ESD) for the Management of Fibrotic Non-Lifting Colorectal Lesions (NLCLs): Results from a Large Multicenter Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Endpoint
2.3. Endoscopic Procedure
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Endpoint
3.3. Secondary Endpoints
3.4. Safety
3.5. Univariate and Multivariate Analysis
4. Discussion
| Author | Year | Country | Study Design | Endoscopic Techniques | Patients (n) | Age (Mean, years) | Sex (Male, %) | Lesion Size Median (Range, mm) | Procedural Time (Median, min) | En Bloc Resection (%) | Adverse Events (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hurlstone et al. [55] | 2008 | United Kingdom | Prospective monocentric | ESD | 30 | 65 | 72 | NA (5–45) | 62 | 93% | 16 |
| Spychalski et al. [56] | 2019 | Poland | Retrospective monocentric | ESD | 70 | 65 | 55.7 | 34.8 (23–46) | 77 | NA | 12.8 |
| Faller et al. [57] | 2020 | France | Retrospective multicentric | ESD | 53 | 70 | 50.9 | 40 (20–65) | 43 | 92.5 | 9.4 |
| Yzet et al. [29] * | 2023 | France | Retrospective multicentric | ESD/eFTR | 177 | 69 | 76 | 35 (5–140) | 45 | 94.3 | 16.3 |
| Tanaka et al. [35] | 2021 | Japan | Retrospective multicentric | ESD | 102 | 69 | 55 | 20 (4–50) | 84 | 95 | 8.8 |
| Tanaka et al. [58] | 2024 | Japan | Prospective multicentric | ESD | 54 | 70 | 63 | 31 (24.5–40) | 65 | 96.3 | 9.5 |
| Dell’Anna et al. | 2025 | Italy | Retrospective multicentric | ESD/H-ESD | 178 | 68 | 56.2 | 30 (10–120) | 80 | 71.9 | 13.4 |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishiguro, A.; Uno, Y.; Ishiguro, Y.; Munakata, A.; Morita, T. Correlation of Lifting versus Non-Lifting and Microscopic Depth of Invasion in Early Colorectal Cancer. Gastrointest. Endosc. 1999, 50, 329–333. [Google Scholar] [CrossRef]
- Kobayashi, N.; Saito, Y.; Sano, Y.; Uragami, N.; Michita, T.; Nasu, J.; Matsuda, T.; Fu, K.; Fujii, T.; Fujimori, T.; et al. Determining the Treatment Strategy for Colorectal Neoplastic Lesions: Endoscopic Assessment or the Non-Lifting Sign for Diagnosing Invasion Depth? Endoscopy 2007, 39, 701–705. [Google Scholar] [CrossRef]
- Chiba, H.; Tachikawa, J.; Arimoto, J.; Ashikari, K.; Kuwabara, H.; Nakaoka, M.; Goto, T.; Ohata, K.; Nakajima, A. Predictive Factors of Mild and Severe Fibrosis in Colorectal Endoscopic Submucosal Dissection. Dig. Dis. Sci. 2020, 65, 232–242. [Google Scholar] [CrossRef]
- Cecinato, P.; Lisotti, A.; Azzolini, F.; Lucarini, M.; Bassi, F.; Fusaroli, P.; Sassatelli, R. Left Colonic Localization, Non-Granular Morphology, and Pit Pattern Independently Predict Submucosal Fibrosis of Naïve Colorectal Neoplasms before Endoscopic Submucosal Dissection. Surg. Endosc. 2023, 37, 3037–3045. [Google Scholar] [CrossRef]
- Sferrazza, S.; Maida, M.; Calabrese, G.; Facciorusso, A.; Fuccio, L.; Frazzoni, L.; Maselli, R.; Repici, A.; Di Mitri, R.; Santos-Antunes, J. The Derivation and External Validation of a Fibrosis Risk Model for Colorectal Tumours Undergoing Endoscopic Submucosal Dissection. J. Clin. Med. 2024, 13, 4517. [Google Scholar] [CrossRef]
- Azzolini, F.; Biolchini, F.; Sassatelli, R.; Camellini, L.; Decembrino, F.; Iori, V.; Tioli, C.; Sereni, G.; Bedogni, G. Endoscopic Submucosal Dissection for Residual Rectal Polyps Embedded in Tissue Scar: A “Rescue Therapy” to Prevent Surgical Intervention? Endoscopy 2008, 40, E222–E223. [Google Scholar] [CrossRef]
- Azzolini, F.; Camellini, L.; Sassatelli, R.; Sereni, G.; Biolchini, F.; Decembrino, F.; De Marco, L.; Iori, V.; Tioli, C.; Cavina, M.; et al. Endoscopic Submucosal Dissection of Scar-Embedded Rectal Polyps: A Prospective Study (Esd in Scar-Embedded Rectal Polyps). Clin. Res. Hepatol. Gastroenterol. 2011, 35, 572–579. [Google Scholar] [CrossRef]
- Maselli, R.; Iacopini, F.; Azzolini, F.; Petruzziello, L.; Manno, M.; De Luca, L.; Cecinato, P.; Fiori, G.; Staiano, T.; Rosa Rizzotto, E.; et al. Endoscopic Submucosal Dissection: Italian National Survey on Current Practices, Training and Outcomes. Dig. Liver Dis. 2020, 52, 64–71. [Google Scholar] [CrossRef]
- Gupta, N.; Rodríguez-Ruiz, G.; Siddiqui, U.D.; Chapman, C.G.; Donboli, K.; Hart, J.; Xiao, S.-Y.; Waxman, I. Endoscopic Submucosal Dissection for Colorectal Lesions: Outcomes from a United States Experience. Surg. Endosc. 2022, 36, 236–243. [Google Scholar] [CrossRef]
- Steinbrück, I.; Faiss, S.; Dumoulin, F.L.; Oyama, T.; Pohl, J.; von Hahn, T.; Schmidt, A.; Allgaier, H.-P. Learning Curve of Endoscopic Submucosal Dissection (ESD) with Prevalence-Based Indication in Unsupervised Western Settings: A Retrospective Multicenter Analysis. Surg. Endosc. 2023, 37, 2574–2586. [Google Scholar] [CrossRef]
- Fujiya, M.; Tanaka, K.; Dokoshi, T.; Tominaga, M.; Ueno, N.; Inaba, Y.; Ito, T.; Moriichi, K.; Kohgo, Y. Efficacy and Adverse Events of EMR and Endoscopic Submucosal Dissection for the Treatment of Colon Neoplasms: A Meta-Analysis of Studies Comparing EMR and Endoscopic Submucosal Dissection. Gastrointest. Endosc. 2015, 81, 583–595. [Google Scholar] [CrossRef]
- McCarty, T.R.; Bazarbashi, A.N.; Thompson, C.C.; Aihara, H. Hybrid Endoscopic Submucosal Dissection (ESD) Compared with Conventional ESD for Colorectal Lesions: A Systematic Review and Meta-Analysis. Endoscopy 2021, 53, 1048–1058. [Google Scholar] [CrossRef]
- Morimoto, S.; Tanaka, H.; Takehara, Y.; Yamamoto, N.; Tanino, F.; Kamigaichi, Y.; Yamashita, K.; Takigawa, H.; Yuge, R.; Urabe, Y.; et al. Hybrid Endoscopic Submucosal Dissection as a Salvage Option for Difficult Colorectal Conventional Endoscopic Submucosal Dissection. Surg. Endosc. 2024, 38, 222–228. [Google Scholar] [CrossRef]
- Okamoto, K.; Muguruma, N.; Kagemoto, K.; Mitsui, Y.; Fujimoto, D.; Kitamura, S.; Kimura, T.; Sogabe, M.; Miyamoto, H.; Takayama, T. Efficacy of Hybrid Endoscopic Submucosal Dissection (ESD) as a Rescue Treatment in Difficult Colorectal ESD Cases. Dig. Endosc. 2017, 29, 45–52. [Google Scholar] [CrossRef]
- Libânio, D.; Pimentel-Nunes, P.; Bastiaansen, B.; Bisschops, R.; Bourke, M.J.; Deprez, P.H.; Esposito, G.; Lemmers, A.; Leclercq, P.; Maselli, R.; et al. Endoscopic Submucosal Dissection Techniques and Technology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2023, 55, 361–389. [Google Scholar] [CrossRef]
- Chedgy, F.J.Q.; Bhattacharyya, R.; Kandiah, K.; Longcroft-Wheaton, G.; Bhandari, P. Knife-Assisted Snare Resection: A Novel Technique for Resection of Scarred Polyps in the Colon. Endoscopy 2016, 48, 277–280. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Chedgy, F.J.; Kandiah, K.; Longcroft-Wheaton, G.; Bhandari, P. Knife-Assisted Snare Resection (KAR) of Large and Refractory Colonic Polyps at a Western Centre: Feasibility, Safety and Efficacy Study to Guide Future Practice. United Eur. Gastroenterol. J. 2016, 4, 466–473. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic Submucosal Dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Bastiaansen, B.A.J.; Bhandari, P.; Bisschops, R.; Bourke, M.J.; Esposito, G.; Lemmers, A.; Maselli, R.; Messmann, H.; et al. Endoscopic Submucosal Dissection for Superficial Gastrointestinal Lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2022. Endoscopy 2022, 54, 591–622. [Google Scholar] [CrossRef]
- Matsumoto, A.; Tanaka, S.; Oba, S.; Kanao, H.; Oka, S.; Yoshihara, M.; Chayama, K. Outcome of Endoscopic Submucosal Dissection for Colorectal Tumors Accompanied by Fibrosis. Scand. J. Gastroenterol. 2010, 45, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Nass, K.J.; Zwager, L.W.; van der Vlugt, M.; Dekker, E.; Bossuyt, P.M.M.; Ravindran, S.; Thomas-Gibson, S.; Fockens, P. Novel Classification for Adverse Events in GI Endoscopy: The AGREE Classification. Gastrointest. Endosc. 2022, 95, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Pioche, M.; Albéniz, E.; Berr, F.; Deprez, P.; Ebigbo, A.; Dewint, P.; Haji, A.; Panarese, A.; Weusten, B.L.; et al. Curriculum for Endoscopic Submucosal Dissection Training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2019, 51, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 2021, 135, 904–919. [Google Scholar] [CrossRef]
- Endoscopic Classification Review Group. Update on the Paris Classification of Superficial Neoplastic Lesions in the Digestive Tract. Endoscopy 2005, 37, 570–578. [Google Scholar] [CrossRef]
- Kudo, S.; Tamura, S.; Nakajima, T.; Yamano, H.; Kusaka, H.; Watanabe, H. Diagnosis of Colorectal Tumorous Lesions by Magnifying Endoscopy. Gastrointest. Endosc. 1996, 44, 8–14. [Google Scholar] [CrossRef]
- Gupta, S.; Miskovic, D.; Bhandari, P.; Dolwani, S.; McKaig, B.; Pullan, R.; Rembacken, B.; Rutter, M.D.; Riley, S.; Valori, R.; et al. The “SMSA” Scoring System for Determining the Complexity of a Polyp. Gut 2011, 60, A129. [Google Scholar] [CrossRef]
- Kumar, V.; Broadley, H.; Rex, D.K. Safety and Efficacy of Hot Avulsion as an Adjunct to EMR (with Videos). Gastrointest. Endosc. 2019, 89, 999–1004. [Google Scholar] [CrossRef]
- Yzet, C.; Le Baleur, Y.; Albouys, J.; Jacques, J.; Doumbe-Mandengue, P.; Barret, M.; Abou Ali, E.; Schaefer, M.; Chevaux, J.-B.; Leblanc, S.; et al. Use of Endoscopic Submucosal Dissection or Full-Thickness Resection Device to Treat Residual Colorectal Neoplasia after Endoscopic Resection: A Multicenter Historical Cohort Study. Endoscopy 2023, 55, 1002–1009. [Google Scholar] [CrossRef]
- Tanaka, H.; Uraoka, T.; Kobayashi, N.; Ohata, K.; Takeuchi, Y.; Chino, A.; Yamada, M.; Tsuji, Y.; Hotta, K.; Harada, K.; et al. Short-term and Long-term Outcomes of Submucosal Dissection for Residual or Recurrent Colorectal Tumors after Endoscopic Resection: Analysis of a Multicenter Prospective Study. Dig. Endosc. 2024, 36, 1003–1011. [Google Scholar] [CrossRef]
- Despott, E.J.; Murino, A. Saline-Immersion Therapeutic Endoscopy (SITE): An Evolution of Underwater Endoscopic Lesion Resection. Dig. Liver Dis. 2017, 49, 1376. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Sunada, K.; Takahashi, H.; Shinhata, H.; Lefor, A.T.; Tanaka, A.; Yamamoto, H. Pocket-Creation Method of Endoscopic Submucosal Dissection to Achieve En Bloc Resection of Giant Colorectal Subpedunculated Neoplastic Lesions. Endoscopy 2014, 46, E421–E422. [Google Scholar] [CrossRef] [PubMed]
- Masgnaux, L.-J.; Grimaldi, J.; Rivory, J.; Ponchon, T.; Yzet, C.; Wallenhorst, T.; Lupu, A.; Lafeuille, P.; Legros, R.; Rostain, F.; et al. Endoscopic Submucosal Dissection Assisted by Adaptive Traction: Results of the First 54 Procedures. Endoscopy 2024, 56, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Straus Takahashi, M.; Eyileten Postula, C.; Buccino, V.R.; Muscatiello, N. Efficacy of Hemostatic Powders in Upper Gastrointestinal Bleeding: A Systematic Review and Meta-Analysis. Dig. Liver Dis. 2019, 51, 1633–1640. [Google Scholar] [CrossRef]
- Tanaka, H.; Oka, S.; Tanaka, S.; Nagata, S.; Kunihiro, M.; Kuwai, T.; Hiraga, Y.; Mizumoto, T.; Okanobu, H.; Chayama, K. Salvage Endoscopic Submucosal Dissection for Local Residual/Recurrent Colorectal Tumor after Endoscopic Resection: Large Multicenter 10-year Study. Dig. Endosc. 2021, 33, 608–615. [Google Scholar] [CrossRef]
- Holmes, I.; Kim, H.G.; Yang, D.-H.; Friedland, S. Avulsion Is Superior to Argon Plasma Coagulation for Treatment of Visible Residual Neoplasia during EMR of Colorectal Polyps (with Videos). Gastrointest. Endosc. 2016, 84, 822–829. [Google Scholar] [CrossRef]
- Tsiamoulos, Z.P.; Bourikas, L.A.; Saunders, B.P. Endoscopic Mucosal Ablation: A New Argon Plasma Coagulation/Injection Technique to Assist Complete Resection of Recurrent, Fibrotic Colon Polyps (with Video). Gastrointest. Endosc. 2012, 75, 400–404. [Google Scholar] [CrossRef]
- Tate, D.J.; Desomer, L.; Argenziano, M.E.; Mahajan, N.; Sidhu, M.; Vosko, S.; Shahidi, N.; Lee, E.; Williams, S.J.; Burgess, N.G.; et al. Treatment of Adenoma Recurrence after Endoscopic Mucosal Resection. Gut 2023, 72, 1875–1886. [Google Scholar] [CrossRef]
- Rashid, M.U.; Khetpal, N.; Zafar, H.; Ali, S.; Idrisov, E.; Du, Y.; Stein, A.; Jain, D.; Hasan, M.K. Colon Mucosal Neoplasia Referred for Endoscopic Mucosal Resection: Recurrence of Adenomas and Prediction of Submucosal Invasion. World J. Gastrointest. Endosc. 2020, 12, 198–211. [Google Scholar] [CrossRef]
- Van der Voort, V.R.H.; Moons, L.M.G.; de Graaf, W.; Schrauwen, R.W.M.; Hazen, W.L.; Seerden, T.C.J.; Vleggaar, F.P.; Didden, P. Efficacy and Safety of Cap-Assisted Endoscopic Mucosal Resection for Treatment of Nonlifting Colorectal Polyps. Endoscopy 2022, 54, 509–514. [Google Scholar] [CrossRef]
- Shahidi, N.; Vosko, S.; Gupta, S.; van Hattem, W.A.; Sidhu, M.; Tate, D.J.; Williams, S.J.; Lee, E.Y.T.; Burgess, N.; Bourke, M.J. Previously Attempted Large Nonpedunculated Colorectal Polyps Are Effectively Managed by Endoscopic Mucosal Resection. Am. J. Gastroenterol. 2021, 116, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Belderbos, T.D.G.; Leenders, M.; Moons, L.M.G.; Siersema, P.D. Local Recurrence after Endoscopic Mucosal Resection of Nonpedunculated Colorectal Lesions: Systematic Review and Meta-Analysis. Endoscopy 2014, 46, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Repici, A.; Sharma, P.; Correale, L.; Zullo, A.; Bretthauer, M.; Senore, C.; Spada, C.; Bellisario, C.; Bhandari, P.; et al. Efficacy and Safety of Endoscopic Resection of Large Colorectal Polyps: A Systematic Review and Meta-Analysis. Gut 2016, 65, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Schaefer, M.; Wallenhorst, T.; Rösch, T.; Lépilliez, V.; Chaussade, S.; Rivory, J.; Legros, R.; Chevaux, J.-B.; Leblanc, S.; et al. Endoscopic En Bloc Versus Piecemeal Resection of Large Nonpedunculated Colonic Adenomas: A Randomized Comparative Trial. Ann. Intern. Med. 2024, 177, 29–38. [Google Scholar] [CrossRef]
- Burgess, N.G.; Williams, S.J.; Hourigan, L.F.; Brown, G.J.; Zanati, S.A.; Singh, R.; Tam, W.; Butt, J.; Byth, K.; Bourke, M.J. A Management Algorithm Based on Delayed Bleeding After Wide-Field Endoscopic Mucosal Resection of Large Colonic Lesions. Clin. Gastroenterol. Hepatol. 2014, 12, 1525–1533. [Google Scholar] [CrossRef]
- Binmoeller, K.F.; Weilert, F.; Shah, J.; Bhat, Y.; Kane, S. “Underwater” EMR without Submucosal Injection for Large Sessile Colorectal Polyps (with Video). Gastrointest. Endosc. 2012, 75, 1086–1091. [Google Scholar] [CrossRef]
- Kim, H.G.; Thosani, N.; Banerjee, S.; Chen, A.; Friedland, S. Underwater Endoscopic Mucosal Resection for Recurrences after Previous Piecemeal Resection of Colorectal Polyps (with Video). Gastrointest. Endosc. 2014, 80, 1094–1102. [Google Scholar] [CrossRef]
- Ohmori, M.; Yamasaki, Y.; Iwagami, H.; Nakahira, H.; Matsuura, N.; Shichijo, S.; Maekawa, A.; Kanesaka, T.; Yamamoto, S.; Higashino, K.; et al. Propensity Score-matched Analysis of Endoscopic Resection for Recurrent Colorectal Neoplasms: A Pilot Study. J. Gastro Hepatol. 2021, 36, 2568–2574. [Google Scholar] [CrossRef]
- Tate, D.J.; Bahin, F.F.; Desomer, L.; Sidhu, M.; Gupta, V.; Bourke, M.J. Cold-Forceps Avulsion with Adjuvant Snare-Tip Soft Coagulation (CAST) Is an Effective and Safe Strategy for the Management of Non-Lifting Large Laterally Spreading Colonic Lesions. Endoscopy 2018, 50, 52–62. [Google Scholar] [CrossRef]
- Mandarino, F.V.; O’Sullivan, T.; Gauci, J.L.; Kerrison, C.; Whitfield, A.; Lam, B.; Perananthan, V.; Gupta, S.; Cronin, O.; Medas, R.; et al. Impact of Margin Thermal Ablation after Cold-Forceps Avulsion with Snare-Tip Soft Coagulation for Non-Lifting Large Non-Pedunculated Colorectal Polyps. Endoscopy 2025. [Google Scholar] [CrossRef]
- Von Helden, A.; Hildenbrand, R.; Sido, B.; Dumoulin, F.L. Endoscopic Full-Thickness Resection Using an over-the-Scope Device for Treatment of Recurrent/Residual Colorectal Neoplasia: A Single-Center Case Series. BMC Gastroenterol. 2019, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, B.; Gong, S.; Zhang, X.; Li, W. Efficacy and Safety of Endoscopic Full-Thickness Resection in the Colon and Rectum Using an over-the-Scope Device: A Meta-Analysis. Surg. Endosc. 2021, 35, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zwager, L.W.; Bastiaansen, B.A.J.; Bronzwaer, M.E.S.; van der Spek, B.W.; Heine, G.D.N.; Haasnoot, K.J.C.; van der Sluis, H.; Perk, L.E.; Boonstra, J.J.; Rietdijk, S.T.; et al. Endoscopic Full-Thickness Resection (eFTR) of Colorectal Lesions: Results from the Dutch Colorectal eFTR Registry. Endoscopy 2020, 52, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, G.; Soriani, P.; Manno, M.; Pizzicannella, M.; Pugliese, F.; Mutignani, M.; Naspetti, R.; Petruzziello, L.; Iacopini, F.; Grossi, C.; et al. Colo-Rectal Endoscopic Full-Thickness Resection (EFTR) with the over-the-Scope Device (FTRD®): A Multicenter Italian Experience. Dig. Liver Dis. 2019, 51, 375–381. [Google Scholar] [CrossRef]
- Barbaro, F.; Papparella, L.G.; Chiappetta, M.F.; Ciuffini, C.; Fukuchi, T.; Hamanaka, J.; Quero, G.; Pecere, S.; Gibiino, G.; Petruzziello, L.; et al. Endoscopic Full-Thickness Resection vs. Endoscopic Submucosal Dissection of Residual/Recurrent Colonic Lesions on Scars: A Retrospective Italian and Japanese Comparative Study. Eur. J. Gastroenterol. Hepatol. 2024, 36, 162–167. [Google Scholar] [CrossRef]
- Hurlstone, D.P.; Shorthouse, A.J.; Brown, S.R.; Tiffin, N.; Cross, S.S. Salvage Endoscopic Submucosal Dissection for Residual or Local Recurrent Intraepithelial Neoplasia in the Colorectum: A Prospective Analysis. Color. Dis. 2008, 10, 891–897. [Google Scholar] [CrossRef]
- Spychalski, M.; Skulimowski, A.; Nishimura, M.; Dziki, A. Comparison of Endoscopic Submucosal Dissection for Primary and Recurrent Colorectal Lesions: A Single-Center European Study. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 366–373. [Google Scholar] [CrossRef]
- Faller, J.; Jacques, J.; Oung, B.; Legros, R.; Rivory, J.; Subtil, F.; Saurin, J.-C.; Robinson, P.; Ponchon, T.; Pioche, M. Endoscopic Submucosal Dissection with Double Clip and Rubber Band Traction for Residual or Locally Recurrent Colonic Lesions after Previous Endoscopic Mucosal Resection. Endoscopy 2020, 52, 383–388. [Google Scholar] [CrossRef]
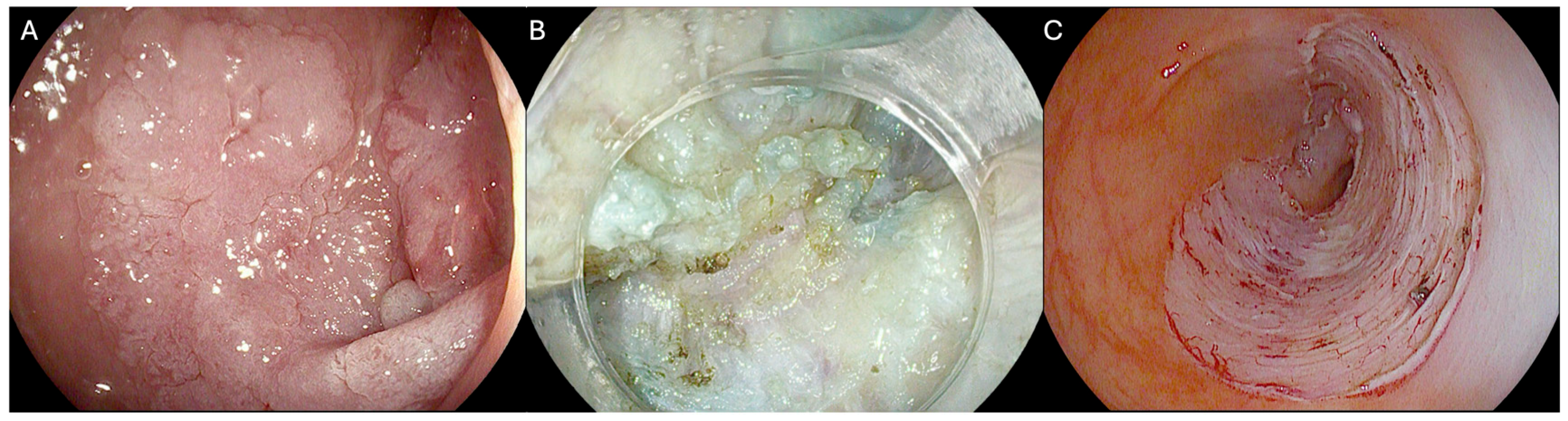
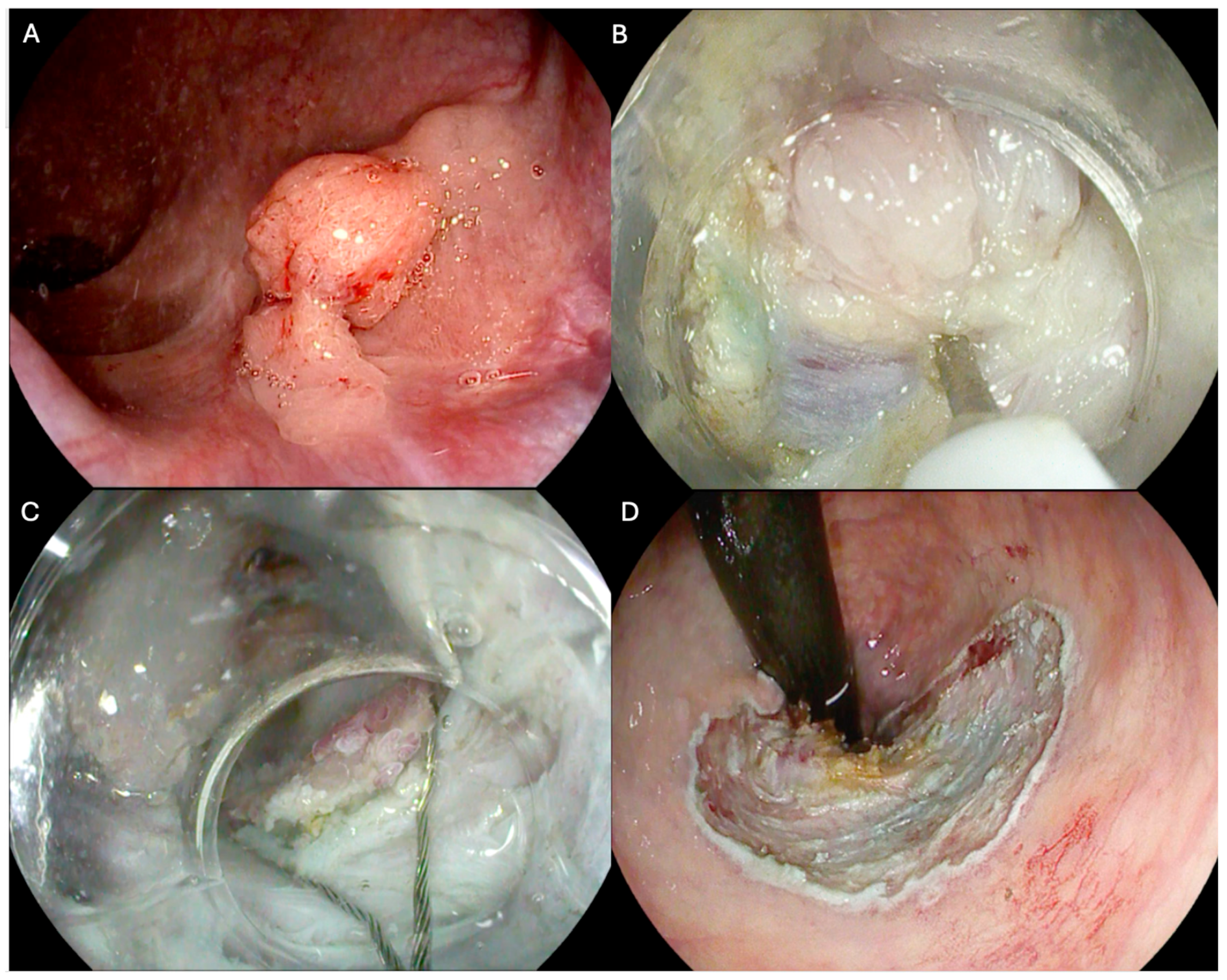

| Overall (n = 178) | Previously Biopsied (n = 52) | Recurrencies (n = 126) | p Value | ESD (111) | H-ESD (67) | p Value | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD) (years) * | 68.4 (11.3) | 68.6 (11.8) | 68.3 (11.1) | 0.871 | 67.5 (10.6) | 69.9 (12.1) | 0.161 |
| Sex, (n, %) | 0.794 | 0.842 | |||||
| Female | 78 (43.8) | 22 (42.3) | 56 (44.4) | 48 (43.2) | 30 (44.7) | ||
| Male | 100 (56.2) | 30 (57.7) | 70 (55.6) | 63 (56.7) | 37 (55.2) | ||
| ASA (n, %) ** | 0.683 | 0.322 | |||||
| I-II | 153 (87.4) | 43 (82.7) | 110 (87.3) | 93 (83.8) | 60 (89.6) | ||
| III | 25 (12.6) | 9 (17.3) | 16 (12.7) | 18 (16.2) | 7 (10.4) | ||
| Localization (n, %) * | 0.804 | <0.001 | |||||
| Rectum | 90 (50.6) | 24 (46.2) | 66 (52.4) | 69 (62.1) | 21 (31.3) | ||
| Left colon | 25 (14.0) | 7 (13.5) | 18 (14.3) | 14 (12.6) | 11 (16.4) | ||
| Transverse colon | 17 (9.6) | 5 (9.6) | 12 (9.5) | 9 (8.1) | 8 (11.9) | ||
| Right colon | 46 (25.8) | 16 (30.8) | 30 (23.8) | 19 (17.1) | 27 (40.3) | ||
| Fibrosis degree (n, %) ** | 15 (11.9) | <0.01 | <0.001 | ||||
| F1 | 34 (19.1) | 19 (36.5) | 111 (88.1) | 31 (27.9) | 3 (4.5) | ||
| F2 | 144 (66.9) | 33 (63.5) | 80 (72.1) | 64 (95.5) | |||
| Paris morphology (n, %) ** | 0.696 | 0.925 | |||||
| Is/IIa + Is | 92 (51.7) | 25 (48.1) | 67 (47.6) | 58 (52.3) | 34 (50.7) | ||
| IIa | 84 (47.2) | 26 (50.0) | 58 (46.0) | 52 (46.8) | 32 (47.8) | ||
| IIb/IIc | 2 (1.1) | 1 (1.9) | 1 (0.8) | 1 (0.9) | 1 (1.5) | ||
| LST morphology (n, %) ** | 0.535 | 0.508 | |||||
| Granular | 44 (31.4) | 15 (37.5) | 29 (29.0) | 30 (27.0) | 14 (20.9) | ||
| Non granular | 41 (29.2) | 13 (32.5) | 28 (28.0) | 23 (20.7) | 18 (26.9) | ||
| Mixed | 55 (39.2) | 12 (30.0) | 43 (43.0) | 32 (28.8) | 23 (34.3) | ||
| Kudo classification (n, %) | |||||||
| III/IV | 151 (84.8) | 35 (67.3) | 116 (92.1) | <0.01 | 89 (80.2) | 62 (92.5) | 0.026 |
| Vi | 27 (15.2) | 17 (32.7) | 10 (7.9) | 22 (19.8) | 5 (7.5) | ||
| Dimension long axis, mm (median, IQR) *** | 30 (20) | 35 (20) | 30 (20) | 0.006 | 30 (21) | 30 (20) | 0.161 |
| Dimension short axis, mm (median, IQR) *** | 20 (15) | 25 (20) | 20 (15) | 0.035 | 25 (20) | 20 (15) | 0.037 |
| Area, cm2 × 0.25π (median, IQR) *** | 4.9 (7.1) | 7.1 (9.1) | 4.7 (6.3) | 0.011 | 6.8 (10.1) | 4.7 (7.1) | 0.021 |
| Procedure time, min (median, IQR) ** | 80 (60) | 85 (69) | 79.5 (65) | 0.044 | 30 (60) | 82 (60) | 0.647 |
| Knife type (n, %) *** | 0.271 | ||||||
| Hybrid type | 125 (70.2) | 29 (55.7) | 96 (76.1) | 79 (71.2) | 46 (68.7) | ||
| Dual type | 39 (21.9) | 22 (42.3) | 17 (13.5) | <0.001 | 26 (23.4) | 13 (19.4) | |
| Hook type | 14 (7.9) | 1 (1.9) | 13 (10.3) | 6 (5.4) | 8 (11.9) | ||
| ESD technique (n, %) *** | 0.459 | 0.018 | |||||
| Standard | 167 (93.8) | 47 (90.4) | 120 (95.2) | 100 (90.1) | 67(100) | ||
| Tunnel/Pocket | 11 (6.2) | 5 (9.6) | 6 (4.8) | 11 (9.9) | 0 (0) | ||
| Traction (n, %) | 0.137 | ||||||
| Yes | 10 (5.6) | 5 (9.6) | 5 (4.0) | 10 (90) | 0 (0) | 0.007 | |
| No | 168 (94.4) | 47 (90.4) | 121 (96.0) | 101 (10) | 67 (100) | ||
| Dissection speed, mm2/min (median, IQR) * | 6.1 (8.1) | 7.0 (10.8) | 6.1 (7.3) | 0.057 | 7.5 (8.8) | 5.2 (5.2) | 0.018 |
| Early Recurrence | Late Recurrence | |||||
|---|---|---|---|---|---|---|
| SC1 | SC2 | |||||
| Recurrence | Treatment | Histology | Recurrence | Treatment | Histology | |
| Fibrosis cause (n, %) | ||||||
| Previously biopsied | 1 (16.7) | EMR 0 | LGD 0 | 1 (50) | EMR 1 (50) | LGD 1 (50) |
| HBA 0 | HGD 1 (16.7) | HBA 0 | HGD 0 | |||
| ESD 0 | Other 0 | ESD 0 | Other 0 | |||
| H-ESD 1 (16.7) | H-ESD | |||||
| FTR 0 | FTR 0 | |||||
| Recurrent | 5 (83.3) | EMR 2 (33.3) | LGD 5 (83.3) | 1 (50) | EMR 0 | LGD 1 (50) |
| HBA 2 (33.3) | HGD 0 | HBA 0 | HGD 0 | |||
| ESD 1 (16.7) | Other 0 | ESD 1 (50) | Other 0 | |||
| H-ESD 0 | H-ESD 0 (50) | |||||
| FTR 0 | FTR 0 | |||||
| Procedure (n, %) | ||||||
| ESD | 2 (33.3) | EMR 1 (16.7) | LGD 2 (33.3) | 1 (50) | EMR 1 (50) | LGD 1 (50) |
| HBA 1 (16.7) | HGD 2 (33.3) | HBA 0 | HGD 0 | |||
| ESD 1 (16.7) | Other 0 | ESD 0 | Other 0 | |||
| H-ESD 1 (16.7) | H-ESD 0 | |||||
| FTR 0 | FTR 0 | |||||
| H-ESD | 4 (66.7) | EMR 1 (16.7) | LGD 2 (33.3) | 1 (50) | EMR 0 | LGD 1 (50) |
| HBA 1 (16.7) | HGD 0 | HBA 0 | HGD 0 | |||
| ESD 0 | Other 0 | ESD 1 (50) | Other 0 | |||
| H-ESD 0 | H-ESD 0 | |||||
| FTR 0 | FTR 0 | |||||
| Overall (n, %) | 6 (100) | EMR 2 (33.3) | LGD 5 (70) | 2 (100) | EMR 1 (50) | LGD 2 (100) |
| HBA 2 (33.3) | HGD 1 (30) | HBA 0 | HGD 0 | |||
| ESD 1 (16.7) | Other 0 | ESD 1 (50) | Other 0 | |||
| H-ESD 1 (16.7) | H-ESD 0 | |||||
| FTR 0 | FTR 0 | |||||
| Overall (n = 178) | Previously Biopsied (n = 52) | Recurrencies (n = 126) | p Value | |
|---|---|---|---|---|
| Technical success/en bloc resection (n, %) | 0.004 | |||
| Yes | 128 (71.9) | 43 (82.7) | 85 (67.5) | |
| No | 60 (28.1) | 9 (17.3) | 41 (32.5) | |
| ESD technical success (n,%) | 0.04 | |||
| Yes | 111 (62.4) | 41 (78.8) | 70 (55.6) | |
| No | 67 (37.6) | 11 (21.2) | 56 (44.4) | |
| CR * rate (n, %) | 0.475 | |||
| Yes | 119 (93.0) | 39 (90.7) | 80 (94.1) | |
| No | 9 (7.0) | 4 (9.3) | 5 (5.9) | |
| cR * rate (n, %) | 0.475 | |||
| Yes | 119 (93.0) | 39 (90.7) | 80 (94.1) | |
| No | 9 (7.0) | 4 (9.3) | 5 (5.9) | |
| Histology (n, %) ** | 0.277 | |||
| LGD/HGD | 153 (86.0) | 41 (78.9) | 112 (88.9) | |
| ADK (sm1) | 11 (6.1) | 5 (9.6) | 6 (4.7) | |
| ADK (sm2) | 10 (5.6) | 5 (9.6) | 5 (4.0) | |
| Other | 4 (2.3) | 1 (1.9) | 3 (2.4) | |
| Early adverse events (n, %) ** | 0.197 | |||
| Perforation | 15 (8.4) | 6 (11.5) | 9 (7.1) | |
| Bleeding | 5 (2.8) | 0 (0) | 5 (4.0) | |
| Both | 1 (0.6) | 1 (1.9) | 0 (0) | |
| Late adverse events (n, %) ** | ||||
| Perforation | 0 (0) | 0 (0) | 0 (0) | |
| Bleeding | 3 (0.017) | 2 (0.04) | 1 (0.008) | |
| Surgery (n, %) | 0.134 | |||
| Yes | 11 (6.1) | 5 (9.6) | 6 (4.7) | |
| Histology | 11 (6.1) | 5 (9.6) | 6 (4.7) | |
| Adverse event | 0 (0) | 0 (0) | 0 (0) | |
| Follow-up time, days (median, IQR) | 373 (540) | 401 (358) | 360 (648.2) | 0.11 |
| ESD (111) | H-ESD (67) | p Value | |
|---|---|---|---|
| Technical success/en bloc resection (n, %) | |||
| En Bloc | 107 (96.4) | 21 (31.3) | <0.001 |
| Piecemeal | 4 (3.6) | 46 (68.7) | |
| Fibrosis cause (n, %) | |||
| Previously biopsied | 41 (36.9) | 11 (16.4) | <0.004 |
| Recurrent | 70 (63.1) | 56 (83.6) | |
| Histology (n, %) * | |||
| LGD/HGD | 90 (81.1) | 63 (94.0) | 0.249 |
| ADK (sm1) | 9 (8.1) | 2 (3.0) | |
| ADK (sm2) | 8 (7.2) | 2 (3.0) | |
| Other | 4 (3.6) | 0 (0.0) | |
| cR ** rate (n, %) | |||
| Yes | 98 (91.9) | 20 (95.2) | 0.656 |
| No | 9 (8.1) | 1 (4.7) | |
| CR ** rate (n, %) | |||
| Yes | 98 (91.9) | 20 (95.2) | 0.656 |
| No | 9 (8.1) | 1 (4.7) | |
| Adverse events (n, %) * | |||
| Perforation | 10 (9.9) | 5 (7.4) | 0.532 |
| Bleeding | 4 (5.4) | 1 (1.4) | |
| Both | 1 (0.9) | 0 (0) | |
| Delayed perforation | 0 (0) | 0 (0) | |
| Delayed bleeding | 3 (2.7) | 0 (0) | |
| Follow-up time, days (median, IQR) | 376.0 (438.7) | 366.0 (815.5) | 0.55 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Unadjusted Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |
| Localization (n, %) Rectum Left colon Transverse colon Right colon | 0.10 (0.03–0.28) | 0.001 | 0.10 (0.03–0.28) | <0.001 |
| Fibrosis degree (n, %) F1 F2 | 0.12 (0.03–0.44) | 0.0017 | 0.09 (0.02–0.40) | 0.0003 |
| Fibrosis cause (n, %) Previously biopsied Recurrent | 0.33 (0.58–0.86) | 0.004 | 0.33 (0.59–0.89) | 0.006 |
| Paris morphology (n, %) Is/IIa-Is IIa Other | 1.21 (0.80–1.83) | 0.351 | ||
| LST morphology (n, %) ** Granular Non granular Mixed | 0.97 (0.78–1.21) | 0.822 | ||
| Kudo classification (n, %) III/IV Vi | 0.41 (0.17–1.00) | 0.051 | ||
| Dimension long axis, mm (median, IQR) *** | 0.98 (0.96–0.99) | 0.032 | 1.01 (0.98–1.03) | 0.397 |
| Dimension short axis, mm (median, IQR) *** | 0.98 (0.96–1.01) | 0.125 | ||
| Area, cm2 × 0.25π (median, IQR) *** | 0.98 (0.95–1.01) | 0.199 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Anna, G.; Fasulo, E.; Cecinato, P.; Barbara, G.; Barchi, A.; Viale, E.; Esposito, D.; Grillo, S.; Sassatelli, R.; Malesci, A.; et al. Endoscopic Submucosal Dissection (ESD) for the Management of Fibrotic Non-Lifting Colorectal Lesions (NLCLs): Results from a Large Multicenter Retrospective Study. Cancers 2025, 17, 1242. https://doi.org/10.3390/cancers17071242
Dell’Anna G, Fasulo E, Cecinato P, Barbara G, Barchi A, Viale E, Esposito D, Grillo S, Sassatelli R, Malesci A, et al. Endoscopic Submucosal Dissection (ESD) for the Management of Fibrotic Non-Lifting Colorectal Lesions (NLCLs): Results from a Large Multicenter Retrospective Study. Cancers. 2025; 17(7):1242. https://doi.org/10.3390/cancers17071242
Chicago/Turabian StyleDell’Anna, Giuseppe, Ernesto Fasulo, Paolo Cecinato, Giovanni Barbara, Alberto Barchi, Edi Viale, Dario Esposito, Simone Grillo, Romano Sassatelli, Alberto Malesci, and et al. 2025. "Endoscopic Submucosal Dissection (ESD) for the Management of Fibrotic Non-Lifting Colorectal Lesions (NLCLs): Results from a Large Multicenter Retrospective Study" Cancers 17, no. 7: 1242. https://doi.org/10.3390/cancers17071242
APA StyleDell’Anna, G., Fasulo, E., Cecinato, P., Barbara, G., Barchi, A., Viale, E., Esposito, D., Grillo, S., Sassatelli, R., Malesci, A., Massironi, S., Annese, V., Fuccio, L., Facciorusso, A., Donatelli, G., Danese, S., & Azzolini, F. (2025). Endoscopic Submucosal Dissection (ESD) for the Management of Fibrotic Non-Lifting Colorectal Lesions (NLCLs): Results from a Large Multicenter Retrospective Study. Cancers, 17(7), 1242. https://doi.org/10.3390/cancers17071242







