Theranostics in Hematological Malignancies: Cutting-Edge Advances in Diagnosis and Targeted Therapy
Simple Summary
Abstract
1. Introduction
2. Principles of Nuclear Medicine in Hematological Malignancies
2.1. Imaging Modalities in Theranostics for Hematological Malignancies
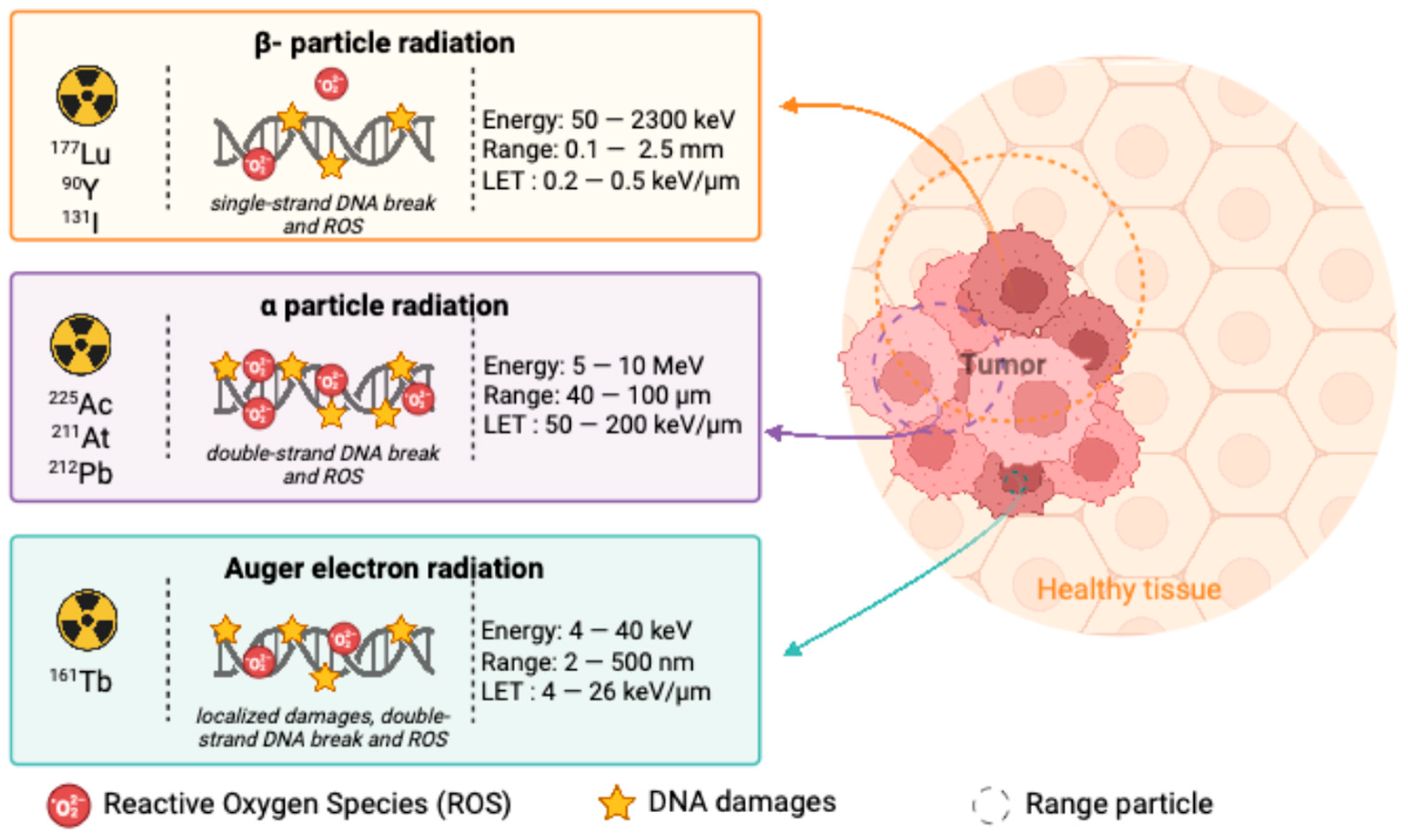
2.2. Types of Radioactive Emitters Used in the Treatment of Hematological Malignancies
2.2.1. Beta(β)-Emitters
2.2.2. Alpha(α)-Emitters
2.2.3. Auger-Emitters
2.2.4. Comparing β-Emitters and α-Emitters
2.3. TRT to Overcome Resistance Mechanisms, Improve Selectivity and Reduce Toxicity
3. Recent Advancements in Hematological Malignancies, from Preclinical to First in Human Studies
3.1. Targets in B-Cell Lymphomas
3.1.1. CD20
3.1.2. CD37
3.1.3. CD22
3.1.4. CD74
3.2. Targets in Leukemias (Myeloid and Lymphoid)
3.2.1. CD33
3.2.2. CD123
3.2.3. CD45
3.2.4. CXCR4
3.3. Targets in Multiple Myeloma
3.3.1. CD38
3.3.2. B-Cell Maturation Antigen (BCMA)
3.3.3. Signaling Lymohocytic Activation Molecule Family Member 7 (SLAMF7; CS1)
3.4. Other Emerging Targets in Hematological Malignancies
3.4.1. CD70
3.4.2. DOTA-TATE
3.4.3. Prostate-Specific Membrane Antigen (PSMA)
3.4.4. The Place of Combination Therapies in Hematological Malignancies
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| ADCs | Antibody-drug conjugates |
| AML | Acute myeloid leukemia |
| ALL | Acute lymphoblastic leukemia |
| B-ALL | B-cell acute lymphoblastic leukemia |
| BCR | B-cell receptor |
| BCMA | B-cell maturation antigen |
| BiTEs | Bispecific T-cell engagers |
| BPDCN | Blastic plasmacytoid dendritic cell neoplasm |
| CAR | Chimeric antigen receptor |
| CLL | Chronic lymphocytic leukemia |
| CML | Chronic myeloid leukemia |
| CRS | Cytokine release syndrome |
| CD | Cluster of Differentiation |
| dCR | Deep complete remission |
| HSCT | Hematopoietic stem cell transplantation |
| LET | Linear energy transfer |
| LSCs | Leukemic stem cells |
| MM | Multiple myeloma |
| mAbs | Monoclonal antibodies |
| MRD | Minimal residual disease |
| NHL | Non-Hodgkin lymphoma |
| NK | Natural killer |
| PSMA | Prostate-Specific Membrane Antigen |
| R/R | Relapsed/refractory |
| SLAMF7 | Signaling Lymphocytic Activation Molecule Family Member 7 |
| SSTR | Somatostatin receptors |
| TNF | Tumor necrosis factor |
| TRT | Targeted radiotherapy |
References
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and Classification of Hematologic Malignancies on the Basis of Genetics. Blood 2017, 130, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global Burden of Hematologic Malignancies and Evolution Patterns over the Past 30 Years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef]
- Brown, G. Lessons to Cancer from Studies of Leukemia and Hematopoiesis. Front. Cell Dev. Biol. 2022, 10, 993915. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Abdal, M.N.; Akram, T.; Alasiry, A.; Marzougui, M.; Masood, A. An Efficient Decision Support System for Leukemia Identification Utilizing Nature-Inspired Deep Feature Optimization. Front. Oncol. 2024, 14, 1328200. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple Myeloma: 2024 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Sochacka-Ćwikła, A.; Mączyński, M.; Regiec, A. FDA-Approved Drugs for Hematological Malignancies—The Last Decade Review. Cancers 2021, 14, 87. [Google Scholar] [CrossRef]
- Weber, W.A.; Barthel, H.; Bengel, F.; Eiber, M.; Herrmann, K.; Schäfers, M. What Is Theranostics? J. Nucl. Med. 2023, 64, 669–670. [Google Scholar] [CrossRef]
- Di Franco, M.; Zanoni, L.; Fortunati, E.; Fanti, S.; Ambrosini, V. Radionuclide Theranostics in Neuroendocrine Neoplasms: An Update. Curr. Oncol. Rep. 2024, 26, 538–550. [Google Scholar] [CrossRef]
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.A.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in Nuclear Medicine Practice. Onco Targets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef]
- Echavidre, W.; Fagret, D.; Faraggi, M.; Picco, V.; Montemagno, C. Recent Pre-Clinical Advancements in Nuclear Medicine: Pioneering the Path to a Limitless Future. Cancers 2023, 15, 4839. [Google Scholar] [CrossRef] [PubMed]
- Grillo-López, A.J. Zevalin: The First Radioimmunotherapy Approved for the Treatment of Lymphoma. Expert Rev. Anticancer Ther. 2002, 2, 485–493. [Google Scholar] [CrossRef]
- Jacene, H.A.; Filice, R.; Kasecamp, W.; Wahl, R.L. Comparison of 90Y-Ibritumomab Tiuxetan and 131I-Tositumomab in Clinical Practice. J. Nucl. Med. 2007, 48, 1767–1776. [Google Scholar] [CrossRef]
- Cheung, M.C.; MacEachern, J.A.; Haynes, A.E.; Meyer, R.M.; Imrie, K. 131I–Tositumomab in Lymphoma. Curr. Oncol. 2009, 16, 32–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Witzig, T.E.; Flinn, I.W.; Gordon, L.I.; Emmanouilides, C.; Czuczman, M.S.; Saleh, M.N.; Cripe, L.; Wiseman, G.; Olejnik, T.; Multani, P.S.; et al. Treatment with Ibritumomab Tiuxetan Radioimmunotherapy in Patients with Rituximab-Refractory Follicular Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 3262–3269. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; Pohlman, B.L.; Bartlett, N.L.; Wiseman, G.A.; Padre, N.; et al. Randomized Controlled Trial of Yttrium-90-Labeled Ibritumomab Tiuxetan Radioimmunotherapy versus Rituximab Immunotherapy for Patients with Relapsed or Refractory Low-Grade, Follicular, or Transformed B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 2453–2463. [Google Scholar] [CrossRef]
- Omer, M.H.; Shafqat, A.; Ahmad, O.; Alkattan, K.; Yaqinuddin, A.; Damlaj, M. Bispecific Antibodies in Hematological Malignancies: A Scoping Review. Cancers 2023, 15, 4550. [Google Scholar] [CrossRef]
- Mansoori, S.; Noei, A.; Maali, A.; Seyed-Motahari, S.S.; Sharifzadeh, Z. Recent Updates on Allogeneic CAR-T Cells in Hematological Malignancies. Cancer Cell Int. 2024, 24, 304. [Google Scholar] [CrossRef]
- Dun, Y.; Huang, G.; Liu, J.; Wei, W. ImmunoPET Imaging of Hematological Malignancies: From Preclinical Promise to Clinical Reality. Drug Discov. Today 2022, 27, 1196–1203. [Google Scholar] [CrossRef]
- Zamanian, M.; Albano, D.; Treglia, G.; Rizzo, A.; Abedi, I. The Clinical Role of CXCR4-Targeted PET on Lymphoproliferative Disorders: A Systematic Review. J. Clin. Med. 2024, 13, 2945. [Google Scholar] [CrossRef]
- Alqahtani, F.F. SPECT/CT and PET/CT, Related Radiopharmaceuticals, and Areas of Application and Comparison. Saudi Pharm. J. 2023, 31, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Banerjee, S. Theranostic Applications of Lutetium-177 in Radionuclide Therapy. Curr. Radiopharm. 2016, 9, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, C.; Rosenkrans, Z.T.; Huo, N.; Chen, Z.; Ehlerding, E.B.; Huo, Y.; Ferreira, C.A.; Barnhart, T.E.; Engle, J.W.; et al. CD38-Targeted Theranostics of Lymphoma with 89Zr/177Lu-Labeled Daratumumab. Adv. Sci. 2021, 8, 2001879. [Google Scholar] [CrossRef]
- Mondello, P.; Cuzzocrea, S.; Navarra, M.; Mian, M. 90 Y-Ibritumomab Tiuxetan: A Nearly Forgotten Opportunity. Oncotarget 2015, 7, 7597–7609. [Google Scholar] [CrossRef]
- Haberkorn, U.; Giesel, F.; Morgenstern, A.; Kratochwil, C. The Future of Radioligand Therapy: α, β, or Both? J. Nucl. Med. 2017, 58, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Bidkar, A.P.; Zerefa, L.; Yadav, S.; VanBrocklin, H.F.; Flavell, R.R. Actinium-225 Targeted Alpha Particle Therapy for Prostate Cancer. Theranostics 2024, 14, 2969–2992. [Google Scholar] [CrossRef]
- Ruigrok, E.A.M.; Tamborino, G.; de Blois, E.; Roobol, S.J.; Verkaik, N.; De Saint-Hubert, M.; Konijnenberg, M.W.; van Weerden, W.M.; de Jong, M.; Nonnekens, J. In Vitro Dose Effect Relationships of Actinium-225- and Lutetium-177-Labeled PSMA-I&T. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3627–3638. [Google Scholar] [CrossRef]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.-F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Dawicki, W.; Allen, K.J.H.; Jiao, R.; Malo, M.E.; Helal, M.; Berger, M.S.; Ludwig, D.L.; Dadachova, E. Daratumumab-225Actinium Conjugate Demonstrates Greatly Enhanced Antitumor Activity against Experimental Multiple Myeloma Tumors. Oncoimmunology 2019, 8, 1607673. [Google Scholar] [CrossRef]
- Juzeniene, A.; Stenberg, V.Y.; Bruland, Ø.S.; Revheim, M.-E.; Larsen, R.H. Dual Targeting with 224Ra/212Pb-Conjugates for Targeted Alpha Therapy of Disseminated Cancers: A Conceptual Approach. Front. Med. 2023, 9, 1051825. [Google Scholar] [CrossRef]
- Dahle, J.; Saidi, A.; Stallons, T.; Heyerdahl, H.; Repetto-Llamazares, A.; Torgue, J. Abstract 5432: Targeted Alpha Therapy with 212Pb-NNV003 in Treatment of NHL. Cancer Res. 2022, 82, 5432. [Google Scholar] [CrossRef]
- Durand-Panteix, S.; Monteil, J.; Sage, M.; Garot, A.; Clavel, M.; Saidi, A.; Torgue, J.; Cogne, M.; Quelven, I. Preclinical Study of 212Pb Alpha-Radioimmunotherapy Targeting CD20 in Non-Hodgkin Lymphoma. Br. J. Cancer 2021, 125, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Van Laere, C.; Koole, M.; Deroose, C.M.; de Voorde, M.V.; Baete, K.; Cocolios, T.E.; Duchemin, C.; Ooms, M.; Cleeren, F. Terbium Radionuclides for Theranostic Applications in Nuclear Medicine: From Atom to Bedside. Theranostics 2024, 14, 1720–1743. [Google Scholar] [CrossRef]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger Electrons for Cancer Therapy—A Review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef]
- Zukotynski, K.; Jadvar, H.; Capala, J.; Fahey, F. Targeted Radionuclide Therapy: Practical Applications and Future Prospects. Biomark. Cancer 2016, 8, 35–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aparicio-Pérez, C.; Carmona, M.; Benabdellah, K.; Herrera, C. Failure of ALL Recognition by CAR T Cells: A Review of CD 19-Negative Relapses after Anti-CD 19 CAR-T Treatment in B-ALL. Front. Immunol. 2023, 14, 1165870. [Google Scholar] [CrossRef]
- Patel, R.B.; Hernandez, R.; Carlson, P.; Grudzinski, J.; Bates, A.M.; Jagodinsky, J.C.; Erbe, A.; Marsh, I.R.; Arthur, I.; Aluicio-Sarduy, E.; et al. Low-Dose Targeted Radionuclide Therapy Renders Immunologically Cold Tumors Responsive to Immune Checkpoint Blockade. Sci. Transl. Med. 2021, 13, eabb3631. [Google Scholar] [CrossRef]
- Khazaei Monfared, Y.; Heidari, P.; Klempner, S.J.; Mahmood, U.; Parikh, A.R.; Hong, T.S.; Strickland, M.R.; Esfahani, S.A. DNA Damage by Radiopharmaceuticals and Mechanisms of Cellular Repair. Pharmaceutics 2023, 15, 2761. [Google Scholar] [CrossRef]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine Release Syndrome and Associated Neurotoxicity in Cancer Immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Philippa, J.; Cheetham, M.D.; Daniel, P.; Petrylak, M.D. Alpha Particles as Radiopharmaceuticals in the Treatment of Bone Metastases: Mechanism of Action of Radium-223 Chloride (Alpharadin) and Radiation. Oncology 2012, 26, 330. [Google Scholar]
- Forrer, F.; Oechslin-Oberholzer, C.; Campana, B.; Herrmann, R.; Maecke, H.R.; Mueller-Brand, J.; Lohri, A. Radioimmunotherapy with 177Lu-DOTA-Rituximab: Final Results of a Phase I/II Study in 31 Patients with Relapsing Follicular, Mantle Cell, and Other Indolent B-Cell Lymphomas. J. Nucl. Med. 2013, 54, 1045–1052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yadav, M.P.; Singla, S.; Thakral, P.; Ballal, S.; Bal, C. Dosimetric Analysis of 177Lu-DOTA-Rituximab in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma. Nucl. Med. Commun. 2016, 37, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Edamadaka, Y.; Parghane, R.V.; Sahu, S.; Lad, S.; Kamaldeep; Wanage, G.; Shanmukhaiah, C.; Kulkarni, V.; Basu, S. Internal Dosimetry and Biodistribution of Indigenously Prepared 177 Lu-DOTA-Rituximab in Lymphoma and Other Hematological Malignancies Treated with Rituximab. Nucl. Med. Commun. 2024, 45, 823–834. [Google Scholar] [CrossRef]
- Shim, K.; Longtine, M.S.; Abou, D.S.; Hoegger, M.J.; Laforest, R.S.; Thorek, D.L.J.; Wahl, R.L. Cure of Disseminated Human Lymphoma with [177Lu]Lu-Ofatumumab in a Preclinical Model. J. Nucl. Med. 2023, 64, 542–548. [Google Scholar] [CrossRef]
- Longtine, M.S.; Shim, K.; Hoegger, M.J.; Benabdallah, N.; Abou, D.S.; Thorek, D.L.J.; Wahl, R.L. Cure of Disseminated Human Lymphoma with [225Ac]Ac-Ofatumumab in a Preclinical Model. J. Nucl. Med. 2023, 64, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Rouhollahi, Z.; Aghamiri, S.M.R.; Yousefnia, H.; Alirezapour, B.; Moghaddasi, A.; Zolghadri, S. Preclinical Aspects of [89Zr]Zr-DFO-Rituximab: A High Potential Agent for Immuno-PET Imaging. Curr. Radiopharm. 2024, 18, 131–140. [Google Scholar] [CrossRef]
- Zettlitz, K.A.; Salazar, F.B.; Yamada, R.E.; Trinh, K.R.; Vasuthasawat, A.; Timmerman, J.M.; Morrison, S.L.; Wu, A.M. 89Zr-ImmunoPET Shows Therapeutic Efficacy of Anti-CD20-IFNα Fusion Protein in a Murine B-Cell Lymphoma Model. Mol. Cancer Ther. 2022, 21, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Longtine, M.; Fears, A.; Benabdallah, N.; Unnerstall, R.; Johnston, H.; Shim, K.; Hasson, A.; Zhang, H.; Ulmert, D.; et al. Evaluation of Candidate Theranostics for 227Th/89Zr Paired Radioimmunotherapy of Lymphoma. J. Nucl. Med. 2023, 64, 1062–1068. [Google Scholar] [CrossRef]
- Lee, I.; Lim, I.; Lee, K.C.; Kang, H.J.; Lim, S.M. 64 Cu-DOTA-Rituximab PET/CT of B-Cell Non-Hodgkin Lymphoma for Imaging the CD20 Expression. Clin. Nucl. Med. 2023, 48, e82–e83. [Google Scholar] [CrossRef]
- Bobrowicz, M.; Kubacz, M.; Slusarczyk, A.; Winiarska, M. CD37 in B Cell Derived Tumors—More than Just a Docking Point for Monoclonal Antibodies. Int. J. Mol. Sci. 2020, 21, 9531. [Google Scholar] [CrossRef]
- Lapalombella, R.; Yeh, Y.-Y.; Wang, L.; Ramanunni, A.; Rafiq, S.; Jha, S.; Staubli, J.; Lucas, D.M.; Mani, R.; Herman, S.E.M.; et al. Tetraspanin CD37 Directly Mediates Transduction of Survival and Apoptotic Signals. Cancer Cell 2012, 21, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Malenge, M.M.; Patzke, S.; Ree, A.H.; Stokke, T.; Ceuppens, P.; Middleton, B.; Dahle, J.; Repetto-Llamazares, A.H.V. 177Lu-Lilotomab Satetraxetan Has the Potential to Counteract Resistance to Rituximab in Non-Hodgkin Lymphoma. J. Nucl. Med. 2020, 61, 1468–1475. [Google Scholar] [CrossRef]
- Blakkisrud, J.; Løndalen, A.; Martinsen, A.C.T.; Dahle, J.; Holtedahl, J.E.; Bach-Gansmo, T.; Holte, H.; Kolstad, A.; Stokke, C. Tumor-Absorbed Dose for Non-Hodgkin Lymphoma Patients Treated with the Anti-CD37 Antibody Radionuclide Conjugate 177Lu-Lilotomab Satetraxetan. J. Nucl. Med. 2017, 58, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Blakkisrud, J.; Holtedahl, J.E.; Løndalen, A.; Dahle, J.; Bach-Gansmo, T.; Holte, H.; Nygaard, S.; Kolstad, A.; Stokke, C. Biodistribution and Dosimetry Results from a Phase 1 Trial of Therapy with the Antibody-Radionuclide Conjugate 177Lu-Lilotomab Satetraxetan. J. Nucl. Med. 2018, 59, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, A.; Illidge, T.; Bolstad, N.; Spetalen, S.; Madsbu, U.; Stokke, C.; Blakkisrud, J.; Løndalen, A.; O’Rourke, N.; Beasley, M.; et al. Phase 1/2a Study of 177Lu-Lilotomab Satetraxetan in Relapsed/Refractory Indolent Non-Hodgkin Lymphoma. Blood Adv. 2020, 4, 4091–4101. [Google Scholar] [CrossRef]
- Pichard, A.; Marcatili, S.; Karam, J.; Constanzo, J.; Ladjohounlou, R.; Courteau, A.; Jarlier, M.; Bonnefoy, N.; Patzke, S.; Stenberg, V.; et al. The Therapeutic Effectiveness of 177Lu-Lilotomab in B-Cell Non-Hodgkin Lymphoma Involves Modulation of G2/M Cell Cycle Arrest. Leukemia 2020, 34, 1315–1328. [Google Scholar] [CrossRef]
- Løndalen, A.; Blakkisrud, J.; Revheim, M.-E.; Dahle, J.; Kolstad, A.; Stokke, C. FDG PET/CT and Dosimetric Studies of 177Lu-Lilotomab Satetraxetan in a First-in-Human Trial for Relapsed Indolent Non-Hodgkin Lymphoma-Are We Hitting the Target? Mol. Imaging Biol. 2022, 24, 807–817. [Google Scholar] [CrossRef]
- Maaland, A.F.; Saidi, A.; Torgue, J.; Heyerdahl, H.; Stallons, T.A.R.; Kolstad, A.; Dahle, J. Targeted Alpha Therapy for Chronic Lymphocytic Leukaemia and Non-Hodgkin’s Lymphoma with the Anti-CD37 Radioimmunoconjugate 212Pb-NNV003. PLoS ONE 2020, 15, e0230526. [Google Scholar] [CrossRef]
- Maaland, A.F.; Heyerdahl, H.; O’Shea, A.; Eiriksdottir, B.; Pascal, V.; Andersen, J.T.; Kolstad, A.; Dahle, J. Targeting B-Cell Malignancies with the Beta-Emitting Anti-CD37 Radioimmunoconjugate 177Lu-NNV003. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2311–2321. [Google Scholar] [CrossRef]
- Giesen, D.; Hooge, M.N.L.; Nijland, M.; Heyerdahl, H.; Dahle, J.; de Vries, E.G.E.; Pool, M. 89Zr-PET Imaging to Predict Tumor Uptake of 177Lu-NNV003 Anti-CD37 Radioimmunotherapy in Mouse Models of B Cell Lymphoma. Sci. Rep. 2022, 12, 6286. [Google Scholar] [CrossRef]
- Malenge, M.M.; Maaland, A.F.; Repetto-Llamazares, A.; Middleton, B.; Nijland, M.; Visser, L.; Patzke, S.; Heyerdahl, H.; Kolstad, A.; Stokke, T.; et al. Anti-CD37 Radioimmunotherapy with 177Lu-NNV003 Synergizes with the PARP Inhibitor Olaparib in Treatment of Non-Hodgkin’s Lymphoma in Vitro. PLoS ONE 2022, 17, e0267543. [Google Scholar] [CrossRef]
- Xu, J.; Luo, W.; Li, C.; Mei, H. Targeting CD22 for B-Cell Hematologic Malignancies. Exp. Hematol. Oncol. 2023, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Schultz, L.; Gardner, R. Mechanisms of and Approaches to Overcoming Resistance to Immunotherapy. Hematol. Am. Soc. Hematol. Educ. Program. 2019, 2019, 226–232. [Google Scholar] [CrossRef]
- Yurkiewicz, I.R.; Muffly, L.; Liedtke, M. Inotuzumab Ozogamicin: A CD22 mAb–Drug Conjugate for Adult Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Drug Des. Devel Ther. 2018, 12, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Bötticher, B.; Arndt, M.A.E.; Mier, W.; Sauter, M.; Exner, E.; Keller, A.; Krämer, S.; Leotta, K.; Wischnjow, A.; et al. Preclinical Evaluation of a Diabody-Based (177)Lu-Radioimmunoconjugate for CD22-Directed Radioimmunotherapy in a Non-Hodgkin Lymphoma Mouse Model. Cancer Lett. 2016, 381, 296–304. [Google Scholar] [CrossRef]
- Weber, T.; Bötticher, B.; Mier, W.; Sauter, M.; Krämer, S.; Leotta, K.; Keller, A.; Schlegelmilch, A.; Grosse-Hovest, L.; Jäger, D.; et al. High Treatment Efficacy by Dual Targeting of Burkitt’s Lymphoma Xenografted Mice with a (177)Lu-Based CD22-Specific Radioimmunoconjugate and Rituximab. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 489–498. [Google Scholar] [CrossRef]
- Laszlo, G.S.; Sandmaier, B.M.; Kehret, A.R.; Orozco, J.J.; Hamlin, D.K.; Dexter, S.L.; Lim, S.Y.T.; Cole, F.M.; Huo, J.; Wilbur, D.S.; et al. [211At]Astatine-Based Anti-CD22 Radioimmunotherapy For B-Cell Malignancies. Leuk. Lymphoma 2023, 64, 1335–1339. [Google Scholar] [CrossRef]
- Niu, J.; Qiu, H.; Xiang, F.; Zhu, L.; Yang, J.; Huang, C.; Zhou, K.; Tong, Y.; Cai, Y.; Dong, B.; et al. CD19/CD22 Bispecific CAR-T Cells for MRD-Positive Adult B Cell Acute Lymphoblastic Leukemia: A Phase I Clinical Study. Blood Cancer J. 2023, 13, 44. [Google Scholar] [CrossRef]
- Stein, R.; Mattes, M.J.; Cardillo, T.M.; Hansen, H.J.; Chang, C.-H.; Burton, J.; Govindan, S.; Goldenberg, D.M. CD74: A New Candidate Target for the Immunotherapy of B-Cell Neoplasms. Clin. Cancer Res. 2007, 13, 5556s–5563s. [Google Scholar] [CrossRef]
- Hertlein, E.; Triantafillou, G.; Sass, E.J.; Hessler, J.D.; Zhang, X.; Jarjoura, D.; Lucas, D.M.; Muthusamy, N.; Goldenberg, D.M.; Lee, R.J.; et al. Milatuzumab Immunoliposomes Induce Cell Death in CLL by Promoting Accumulation of CD74 on the Surface of B Cells. Blood 2010, 116, 2554–2558. [Google Scholar] [CrossRef]
- Le, Q.; Tang, T.; Leonti, A.; Castro, S.; McKay, C.N.; Perkins, L.; Pardo, L.; Kirkey, D.; Hylkema, T.; Call, L.; et al. Preclinical Studies Targeting CD74 with STRO-001 Antibody-Drug Conjugate in Acute Leukemia. Blood Adv. 2023, 7, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Abrahams, C.; Yu, A.; Embry, M.; Henningsen, R.; DeAlmeida, V.; Matheny, S.; Kline, T.; Yam, A.; Stafford, R.; et al. Targeting CD74 in B-Cell Non-Hodgkin Lymphoma with the Antibody-Drug Conjugate STRO-001. Oncotarget 2023, 14, 1–13. [Google Scholar] [CrossRef]
- Michel, R.B.; Rosario, A.V.; Brechbiel, M.W.; Jackson, T.J.; Goldenberg, D.M.; Mattes, M.J. Experimental Therapy of Disseminated B-Cell Lymphoma Xenografts with 213Bi-Labeled Anti-CD74. Nucl. Med. Biol. 2003, 30, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Linenberger, M.L. CD33-Directed Therapy with Gemtuzumab Ozogamicin in Acute Myeloid Leukemia: Progress in Understanding Cytotoxicity and Potential Mechanisms of Drug Resistance. Leukemia 2005, 19, 176–182. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Schubert, M.-L.; Lauk, A.; Yao, H.; Blank, M.F.; Cui, C.; Janssen, M.; Schmidt, C.; Göllner, S.; et al. CD33-Directed Immunotherapy with Third-Generation Chimeric Antigen Receptor T Cells and Gemtuzumab Ozogamicin in Intact and CD33-Edited Acute Myeloid Leukemia and Hematopoietic Stem and Progenitor Cells. Int. J. Cancer 2022, 150, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Maakaron, J.E.; Rogosheske, J.; Long, M.; Bachanova, V.; Mims, A.S. CD33-Targeted Therapies: Beating the Disease or Beaten to Death? J. Clin. Pharmacol. 2021, 61, 7–17. [Google Scholar] [CrossRef]
- Allen, K.J.H.; Jiao, R.; Li, J.; Beckford-Vera, D.R.; Dadachova, E. In Vitro and In Vivo Characterization of 89Zirconium-Labeled Lintuzumab Molecule. Molecules 2022, 27, 6589. [Google Scholar] [CrossRef]
- Romão, E.; Krasniqi, A.; Maes, L.; Vandenbrande, C.; Sterckx, Y.G.-J.; Stijlemans, B.; Vincke, C.; Devoogdt, N.; Muyldermans, S. Identification of Nanobodies against the Acute Myeloid Leukemia Marker CD33. Int. J. Mol. Sci. 2020, 21, 310. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Wickstroem, K.; Wang, E.; Shea, A.O.; Sponheim, K.; Karlsson, J.; Bjerke, R.M.; Ryan, O.B.; Cuthbertson, A.S. In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2016, 15, 2422–2431. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Wickstroem, K.; Hammer, S.; Bjerke, R.M.; Zitzmann-Kolbe, S.; Ryan, O.B.; Karlsson, J.; Scholz, A.; Hennekes, H.; Mumberg, D.; et al. Advances in Precision Oncology: Targeted Thorium-227 Conjugates As a New Modality in Targeted Alpha Therapy. Cancer Biother. Radiopharm. 2020, 35, 497–510. [Google Scholar] [CrossRef]
- Garg, R.; Allen, K.J.H.; Dawicki, W.; Geoghegan, E.M.; Ludwig, D.L.; Dadachova, E. 225Ac-Labeled CD33-Targeting Antibody Reverses Resistance to Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia Models. Cancer Med. 2021, 10, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers 2019, 11, 1358. [Google Scholar] [CrossRef]
- El Achi, H.; Dupont, E.; Paul, S.; Khoury, J.D. CD123 as a Biomarker in Hematolymphoid Malignancies: Principles of Detection and Targeted Therapies. Cancers 2020, 12, 3087. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Konopleva, M. Approval of Tagraxofusp-Erzs for Blastic Plasmacytoid Dendritic Cell Neoplasm. Blood Adv. 2020, 4, 4020–4027. [Google Scholar] [CrossRef]
- Zanotta, S.; Galati, D.; De Filippi, R.; Pinto, A. Breakthrough in Blastic Plasmacytoid Dendritic Cell Neoplasm Cancer Therapy Owing to Precision Targeting of CD123. Int. J. Mol. Sci. 2024, 25, 1454. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.G.; Montesinos, P.; DeAngelo, D.J.; Wang, E.S.; Papadantonakis, N.; Todisco, E.; Sweet, K.L.; Pemmaraju, N.; Lane, A.A.; Torres-Miñana, L.; et al. Pivekimab Sunirine (IMGN632), a Novel CD123-Targeting Antibody-Drug Conjugate, in Relapsed or Refractory Acute Myeloid Leukaemia: A Phase 1/2 Study. Lancet Oncol. 2024, 25, 388–399. [Google Scholar] [CrossRef]
- Laszlo, G.S.; Orozco, J.J.; Kehret, A.R.; Lunn, M.C.; Huo, J.; Hamlin, D.K.; Scott Wilbur, D.; Dexter, S.L.; Comstock, M.L.; O’Steen, S.; et al. Development of [211At]Astatine-Based Anti-CD123 Radioimmunotherapy for Acute Leukemias and Other CD123+ Malignancies. Leukemia 2022, 36, 1485–1491. [Google Scholar] [CrossRef]
- Ye, N.; Cai, J.; Dong, Y.; Chen, H.; Bo, Z.; Zhao, X.; Xia, M.; Han, M. A Multi-Omic Approach Reveals Utility of CD45 Expression in Prognosis and Novel Target Discovery. Front. Genet. 2022, 13, 928328. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Nath, R.; Seropian, S.; Choe, H.; Litzow, M.R.; Abboud, C.; Koshy, N.; Stiff, P.; Tomlinson, B.; Abhyankar, S.; et al. Randomized Phase III SIERRA Trial of 131I-Apamistamab Before Allogeneic Hematopoietic Cell Transplantation Versus Conventional Care for Relapsed/Refractory AML. J. Clin. Oncol. 2025, 43, 201–213. [Google Scholar] [CrossRef]
- Tuazon, S.A.; Sandmaier, B.M.; Gooley, T.A.; Fisher, D.R.; Holmberg, L.A.; Becker, P.S.; Lundberg, S.J.; Orozco, J.J.; Gopal, A.K.; Till, B.G.; et al. 90Y-Labeled Anti-CD45 Antibody Allogeneic Hematopoietic Cell Transplantation for High-Risk Multiple Myeloma. Bone Marrow Transpl. 2021, 56, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Cassaday, R.D.; Press, O.W.; Pagel, J.M.; Rajendran, J.G.; Gooley, T.A.; Fisher, D.R.; Holmberg, L.A.; Miyaoka, R.S.; Sandmaier, B.M.; Green, D.J.; et al. Phase I Study of a CD45-Targeted Antibody-Radionuclide Conjugate for High-Risk Lymphoma. Clin. Cancer Res. 2019, 25, 6932–6938. [Google Scholar] [CrossRef] [PubMed]
- Baljinder, S.; Ankit, W.; Shekhawat, A.S.; Ashwin, S.; Malhotra, P.; Waheed, A.; Harneet, K.; Nisha, R.; Madan, R.; Arora, S.; et al. CXCR4 Theranostics: A Potential Game Changer in Solid Tumors and Hematological Malignancies. In Beyond Becquerel and Biology to Precision Radiomolecular Oncology: Festschrift in Honor of Richard P. Baum; Prasad, V., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 309–320. ISBN 978-3-031-33533-4. [Google Scholar]
- Peled, A.; Klein, S.; Beider, K.; Burger, J.A.; Abraham, M. Role of CXCL12 and CXCR4 in the Pathogenesis of Hematological Malignancies. Cytokine 2018, 109, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Kipps, T.J. CXCR4: A Key Receptor in the Crosstalk between Tumor Cells and Their Microenvironment. Blood 2006, 107, 1761–1767. [Google Scholar] [CrossRef]
- Buck, A.K.; Serfling, S.E.; Lindner, T.; Hänscheid, H.; Schirbel, A.; Hahner, S.; Fassnacht, M.; Einsele, H.; Werner, R.A. CXCR4-Targeted Theranostics in Oncology. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4133–4144. [Google Scholar] [CrossRef]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hänscheid, H.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; et al. [177Lu]Pentixather: Comprehensive Preclinical Characterization of a First CXCR4-Directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef]
- Braitsch, K.; Lorenzini, T.; Hefter, M.; Koch, K.; Nickel, K.; Peeken, J.C.; Götze, K.S.; Weber, W.; Allmann, A.; D’Alessandria, C.; et al. CXCR4-Directed Endoradiotherapy with [177Lu]Pentixather Added to Total Body Irradiation for Myeloablative Conditioning in Patients with Relapsed/Refractory Acute Myeloid Leukemia. Theranostics 2025, 15, 19–29. [Google Scholar] [CrossRef]
- Christensen, K.A.; Fath, M.A.; Ewald, J.T.; Robles-Planells, C.; Graves, S.A.; Johnson, S.S.; Zacharias, Z.R.; Houtman, J.C.D.; O’Dorisio, M.S.; Schultz, M.K.; et al. Targeting CXCR4 with [212Pb/203Pb]-Pentixather Significantly Increases Overall Survival in Small Cell Lung Cancer. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kawano, Y.; Kushima, S.; Hata, H.; Matsuoka, M. The Role of CD38 in Multiple Myeloma Cell Biology. Blood 2021, 138, 1580. [Google Scholar] [CrossRef]
- Shen, F.; Shen, W. Isatuximab in the Treatment of Multiple Myeloma: A Review and Comparison with Daratumumab. Technol. Cancer Res. Treat. 2022, 21, 15330338221106563. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Tsukada, N.; Nomura, M.; Kasuya, Y.; Oda, Y.; Sato, K.; Takei, T.; Ogura, M.; Abe, Y.; Suzuki, K.; et al. Real-World Clinical Outcomes in Patients with Multiple Myeloma Treated with Isatuximab after Daratumumab Treatment. Ann. Hematol. 2023, 102, 1477–1483. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Herrero Alvarez, N.; Michel, A.L.; Viray, T.D.; Mayerhoefer, M.E.; Lewis, J.S. 89Zr-DFO-Isatuximab for CD38-Targeted ImmunoPET Imaging of Multiple Myeloma and Lymphomas. ACS Omega 2023, 8, 22486–22495. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Lewis, J.; Landgren, O. CD38-Targeted 89Zr-DFO-Daratumumab PET of Myeloma: Immuno-PET Impacting Clinical Care. J. Nucl. Med. 2025, 66, 482. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, B.; Liu, T.; Li, L.; Hu, B.; Li, C.; Jia, B.; Wang, F. 99mTc-CD3813: A Nanobody-Based Single Photon Emission Computed Tomography Radiotracer with Clinical Potential for Myeloma Imaging and Evaluation of CD38 Expression. Mol. Pharm. 2022, 19, 2583–2594. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, D.; Wang, C.; Zhang, Y.; An, S.; Chen, Y.; Huang, G.; Liu, J. Annotating CD38 Expression in Multiple Myeloma with [18F]F-Nb1053. Mol. Pharm. 2022, 19, 3502–3510. [Google Scholar] [CrossRef]
- Huang, W.; Wang, T.; Qiu, Y.; Li, C.; Chen, B.; Song, L.; Yang, Q.; Sun, X.; Jia, B.; Kang, L. CD38-Specific immunoPET Imaging for Multiple Myeloma Diagnosis and Therapeutic Monitoring: Preclinical and First-in-Human Studies. Eur. J. Nucl. Med. Mol. Imaging 2024, 52, 1791–1804. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Hou, Y.N.; Liu, Q.; Zhang, D.; Zhao, H.; Zhang, Y.; An, S.; Li, L.; Hou, J.; et al. ImmunoPET Imaging of Multiple Myeloma with [68Ga]Ga-NOTA-Nb1053. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2749–2760. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, K.; Mishra, A.; Lofland, G.; Marsh, I.; Kumar, D.; Ghiaur, G.; Imus, P.; Rowe, S.P.; Hobbs, R.F.; et al. CD38-Specific Gallium-68 Labeled Peptide Radiotracer Enables Pharmacodynamic Monitoring in Multiple Myeloma with PET. Adv. Sci. 2024, 11, e2308617. [Google Scholar] [CrossRef]
- Zheleznyak, A.; Tang, R.; Duncan, K.; Manion, B.; Liang, K.; Xu, B.; Vanover, A.; Ghai, A.; Prior, J.; Lees, S.; et al. Development of New CD38 Targeted Peptides for Cancer Imaging. Mol. Imaging Biol. 2024, 26, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Duray, E.; Lejeune, M.; Baron, F.; Beguin, Y.; Devoogdt, N.; Krasniqi, A.; Lauwers, Y.; Zhao, Y.J.; D’Huyvetter, M.; Dumoulin, M.; et al. A Non-Internalised CD38-Binding Radiolabelled Single-Domain Antibody Fragment to Monitor and Treat Multiple Myeloma. J. Hematol. Oncol. 2021, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Quelven, I.; Monteil, J.; Sage, M.; Saidi, A.; Mounier, J.; Bayout, A.; Garrier, J.; Cogne, M.; Durand-Panteix, S. 212Pb α-Radioimmunotherapy Targeting CD38 in Multiple Myeloma: A Preclinical Study. J. Nucl. Med. 2020, 61, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Teiluf, K.; Seidl, C.; Blechert, B.; Gaertner, F.C.; Gilbertz, K.-P.; Fernandez, V.; Bassermann, F.; Endell, J.; Boxhammer, R.; Leclair, S.; et al. α-Radioimmunotherapy with 213Bi-Anti-CD38 Immunoconjugates Is Effective in a Mouse Model of Human Multiple Myeloma. Oncotarget 2015, 6, 4692–4703. [Google Scholar] [CrossRef]
- O’Steen, S.; Comstock, M.L.; Orozco, J.J.; Hamlin, D.K.; Wilbur, D.S.; Jones, J.C.; Kenoyer, A.; Nartea, M.E.; Lin, Y.; Miller, B.W.; et al. The α-Emitter Astatine-211 Targeted to CD38 Can Eradicate Multiple Myeloma in a Disseminated Disease Model. Blood 2019, 134, 1247–1256. [Google Scholar] [CrossRef]
- Minnix, M.; Adhikarla, V.; Caserta, E.; Poku, E.; Rockne, R.; Shively, J.E.; Pichiorri, F. Comparison of CD38-Targeted α- Versus β-Radionuclide Therapy of Disseminated Multiple Myeloma in an Animal Model. J. Nucl. Med. 2021, 62, 795–801. [Google Scholar] [CrossRef]
- Sammartano, V.; Franceschini, M.; Fredducci, S.; Caroni, F.; Ciofini, S.; Pacelli, P.; Bocchia, M.; Gozzetti, A. Anti-BCMA Novel Therapies for Multiple Myeloma. Cancer Drug Resist. 2023, 6, 169–181. [Google Scholar] [CrossRef]
- Xing, L.; Liu, Y.; Liu, J. Targeting BCMA in Multiple Myeloma: Advances in Antibody-Drug Conjugate Therapy. Cancers 2023, 15, 2240. [Google Scholar] [CrossRef]
- Guo, R.; Lu, W.; Zhang, Y.; Cao, X.; Jin, X.; Zhao, M. Targeting BCMA to Treat Multiple Myeloma: Updates From the 2021 ASH Annual Meeting. Front. Immunol. 2022, 13, 839097. [Google Scholar] [CrossRef]
- Swan, D.; Madduri, D.; Hocking, J. CAR-T Cell Therapy in Multiple Myeloma: Current Status and Future Challenges. Blood Cancer J. 2024, 14, 206. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Yang, N.; Shang, J.; Gao, X.; Chen, J.; Wei, H.; Li, Y.; Zeng, H.; Xu, H.; et al. Discovery of a Highly Specific Radiolabeled Antibody Targeting B-Cell Maturation Antigen: Applications in PET Imaging of Multiple Myeloma. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Comstock, M.L.; O’Steen, S.; Lin, Y.; Hamlin, D.; Wilbur, D.S.; Orozco, J.J.; Storb, R.F.; Walter, R.B.; Ataca Atilla, P.; Till, B.G.; et al. BCMA-Directed Low Dose Alpha-Emitter Therapy Eliminates Minimal Residual Disease in a Multiple Myeloma Mouse Xenograft Model. Blood 2023, 142, 53. [Google Scholar] [CrossRef]
- Malaer, J.D.; Mathew, P.A. CS1 (SLAMF7, CD319) Is an Effective Immunotherapeutic Target for Multiple Myeloma. Am. J. Cancer Res. 2017, 7, 1637–1641. [Google Scholar] [PubMed]
- Gormley, N.J.; Ko, C.-W.; Deisseroth, A.; Nie, L.; Kaminskas, E.; Kormanik, N.; Goldberg, K.B.; Farrell, A.T.; Pazdur, R. FDA Drug Approval: Elotuzumab in Combination with Lenalidomide and Dexamethasone for the Treatment of Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2017, 23, 6759–6763. [Google Scholar] [CrossRef]
- Ghai, A.; Zheleznyak, A.; Mixdorf, M.; O’Neal, J.; Ritchey, J.; Rettig, M.; DiPersio, J.; Shokeen, M.; Achilefu, S. Development of [89Zr]DFO-Elotuzumab for immunoPET Imaging of CS1 in Multiple Myeloma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1302–1311. [Google Scholar] [CrossRef]
- De Veirman, K.; Puttemans, J.; Krasniqi, A.; Ertveldt, T.; Hanssens, H.; Romao, E.; Hose, D.; Goyvaert, C.; Vlummens, P.; Muyldermans, S.; et al. CS1-Specific Single-Domain Antibodies Labeled with Actinium-225 Prolong Survival and Increase CD8+ T Cells and PD-L1 Expression in Multiple Myeloma. Oncoimmunology 2021, 10, 2000699. [Google Scholar] [CrossRef]
- Flieswasser, T.; Van den Eynde, A.; Van Audenaerde, J.; De Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 Axis in Oncology: The New Kids on the Block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef]
- Sauer, T.; Parikh, K.; Sharma, S.; Omer, B.; Sedloev, D.; Chen, Q.; Angenendt, L.; Schliemann, C.; Schmitt, M.; Müller-Tidow, C.; et al. CD70-Specific CAR T Cells Have Potent Activity against Acute Myeloid Leukemia without HSC Toxicity. Blood 2021, 138, 318–330. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Mihaylova, D.; Uran, S.R.; Borrebaek, J.; Grant, D.; Bjerke, R.M.; Karlsson, J.; Cuthbertson, A.S. Targeted Alpha Therapy Using a Novel CD70 Targeted Thorium-227 Conjugate in in Vitro and in Vivo Models of Renal Cell Carcinoma. Oncotarget 2017, 8, 56311–56326. [Google Scholar] [CrossRef]
- Sonmezoglu, K.; Vatankulu, B.; Elverdi, T.; Akyel, R.; Erkan, M.E.; Halac, M.; Ocak, M.; Demirci, E.; Aydin, Y. The Role of 68Ga-DOTA-TATE PET/CT Scanning in the Evaluation of Patients with Multiple Myeloma: Preliminary Results. Nucl. Med. Commun. 2017, 38, 76–83. [Google Scholar] [CrossRef]
- Uijen, M.J.M.; Derks, Y.H.W.; Merkx, R.I.J.; Schilham, M.G.M.; Roosen, J.; Privé, B.M.; van Lith, S.a.M.; van Herpen, C.M.L.; Gotthardt, M.; Heskamp, S.; et al. PSMA Radioligand Therapy for Solid Tumors Other than Prostate Cancer: Background, Opportunities, Challenges, and First Clinical Reports. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4350–4368. [Google Scholar] [CrossRef] [PubMed]
- Miceli, A.; Riondato, M.; D’Amico, F.; Donegani, M.I.; Piol, N.; Mora, M.; Spina, B.; Morbelli, S.; Bauckneht, M. Concomitant Prostate Cancer and Hodgkin Lymphoma: A Differential Diagnosis Guided by a Combined 68Ga-PSMA-11 and 18F-FDG PET/CT Approach. Medicina 2021, 57, 975. [Google Scholar] [CrossRef] [PubMed]
- Kanthan, G.L.; Coyle, L.; Kneebone, A.; Schembri, G.P.; Hsiao, E. Follicular Lymphoma Showing Avid Uptake on 68Ga PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2016, 41, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cong, Y.; Shi, L.; Jiang, Y.; Zhang, H. Multiple Myeloma with Diffuse Uptake on 18F-PSMA-1007 Positron Emission Tomography/Computed Tomography: A Case Description and Literature Review. Quant. Imaging Med. Surg. 2023, 13, 5369–5373. [Google Scholar] [CrossRef]
- Yang, Y.; Vedvyas, Y.; Alcaina, Y.; Son, J.Y.; Min, I.M.; Jin, M.M. Low-Dose Targeted Radionuclide Therapy Synergizes with CAR T Cells and Enhances Tumor Response. Front. Immunol. 2024, 15, 1355388. [Google Scholar] [CrossRef]
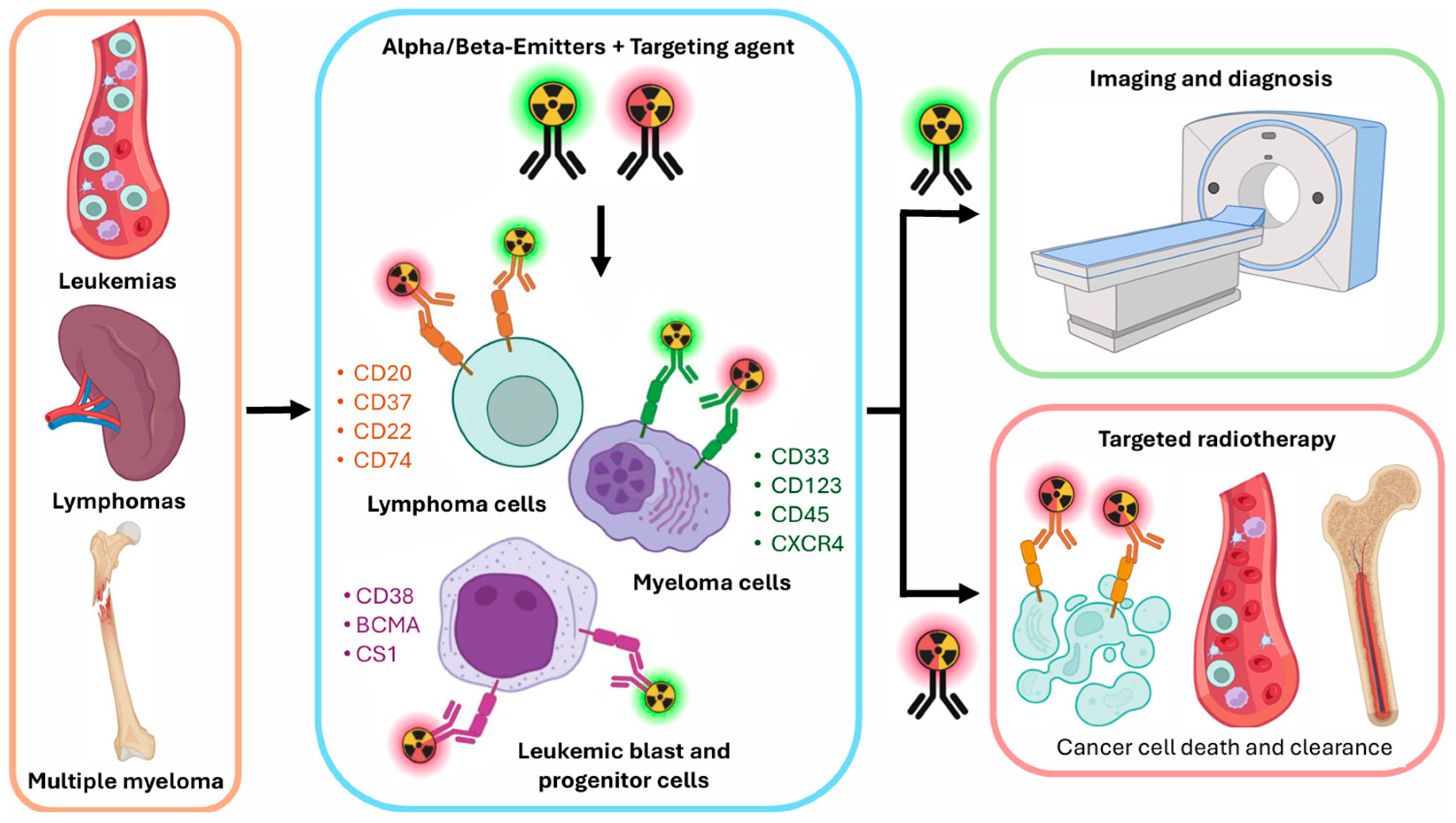
| Cancer Type | Cellular Target | Targeting Compound | Radioisotope | Tumor Model | Clinical Trial | References |
|---|---|---|---|---|---|---|
| B-cell Lymphomas | CD20 | ibritumomab tiuxetan (Zevalin) | 90Y | / | NHL patients (FDA approved in 2002) | [12,13,15,16] |
| tositumomab | 131I | / | NHL patients (FDA approved in 2003) | [13,14] | ||
| rituximab | 177Lu, 89Zr, 64Cu | / | Follicular, Mantle and Marginal Lymphoma (Phase I/II); NHL (Phase I) | [41,42,43] | ||
| ofatumumab | 177Lu, 225Ac, 89Zr | Raji-cell or 38C13-hCD20 | / | [44,45,46,47,48] | ||
| CD37 | Lilotomab satetraxetan (Betalutin®) | 177Lu | DOHH2 or Raji-cell | Indolent NHL (First in human [FIH]) | [52,53,54,55,56,57] | |
| NNV003 | 177Lu, 89Zr, 212Pb | REC-1, REC1 B, RAMOS, Daudi or MEC-2 | / | [58,59,127] | ||
| CD22 | huRFB4 | 177Lu | Raji-cell | / | [64,65] | |
| epratuzumab, G5/44 | 211At | Ramos | / | [66] | ||
| CD74 | LL1 | 213Bi | Raji-cell | / | [72] | |
| Myeloid and Lymphoid Leukemias | CD33 | lintuzumab | 89Zr, 227Th, 225Ac | OCI-AML3, HL-60 or U937 | AML patients (FIH) | [76,78,79,80,81] |
| CD33-targeting Nbs | 99mTc | THP-1 | / | [77] | ||
| CD123 | CD123-targeting mAbs | 211At | MOLM-13 | / | [87] | |
| CD45 | apamistamab | 131I | / | AML patients (Phase III) | [89] | |
| BC8 | 90Y, 131I | / | MM patients (Phase I); B-NHL, T-NHL, and HL patients (Phase I) | [90,91] | ||
| CXCR4 | pentixather | 177Lu, 90Y | Daudi | MM patients (FIH); AML patients (Phase I/II) | [95,96,97,98] | |
| Multiple Myeloma | CD38 | isatuximab | 89Zr | MM.1S or K562 | / | [104] |
| daratumumab | 89Zr, 212Pb, 225Ac, 177Lu | RPMI 8226, MOLP-8, OPM-2, NCI-H929 or MM1-S | MM patients (Phase II) | [105,113,116] | ||
| CD3813 Nb | 99mTc, 68Ga | Ramos | / | [106,108] | ||
| Nb1053 | 18F, 68Ga | MM.1S | / | [107,109] | ||
| AJ206 | 68Ga | MOLP8, MM.1S or patient cells | / | [110] | ||
| SL022-GGS | 64Cu | MM.1S | / | [111] | ||
| CD38-targeting sdAbs | 177Lu | RPMI 8226 | / | [112] | ||
| MOR03087 | 213Bi | OPM2 | / | [114] | ||
| OKT10-B10 | 211At | OPM-2 or NCI-H929 | / | [115] | ||
| BCMA | BCMAh230430 | 89Zr, 177Lu | MM.1S or KYSE520 | / | [121] | |
| BCMA-B10 | 211At | MM1R or NCI-H929 | / | [122] | ||
| CS1 | elotuzumab | 89Zr | MM.1S | / | [125] | |
| CS1-targeting sdAbs | 225Ac | 5T33MM or 5TGM1 | / | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanovic, B.; Hugonnet, F.; Montemagno, C. Theranostics in Hematological Malignancies: Cutting-Edge Advances in Diagnosis and Targeted Therapy. Cancers 2025, 17, 1247. https://doi.org/10.3390/cancers17071247
Bogdanovic B, Hugonnet F, Montemagno C. Theranostics in Hematological Malignancies: Cutting-Edge Advances in Diagnosis and Targeted Therapy. Cancers. 2025; 17(7):1247. https://doi.org/10.3390/cancers17071247
Chicago/Turabian StyleBogdanovic, Bojana, Florent Hugonnet, and Christopher Montemagno. 2025. "Theranostics in Hematological Malignancies: Cutting-Edge Advances in Diagnosis and Targeted Therapy" Cancers 17, no. 7: 1247. https://doi.org/10.3390/cancers17071247
APA StyleBogdanovic, B., Hugonnet, F., & Montemagno, C. (2025). Theranostics in Hematological Malignancies: Cutting-Edge Advances in Diagnosis and Targeted Therapy. Cancers, 17(7), 1247. https://doi.org/10.3390/cancers17071247







