Personalized Lifestyle Interventions for Prevention and Treatment of Obesity-Related Cancers: A Call to Action
Simple Summary
Abstract
1. Introduction
2. Cancer and Obesity
3. Lifestyle Modifications and Cancer
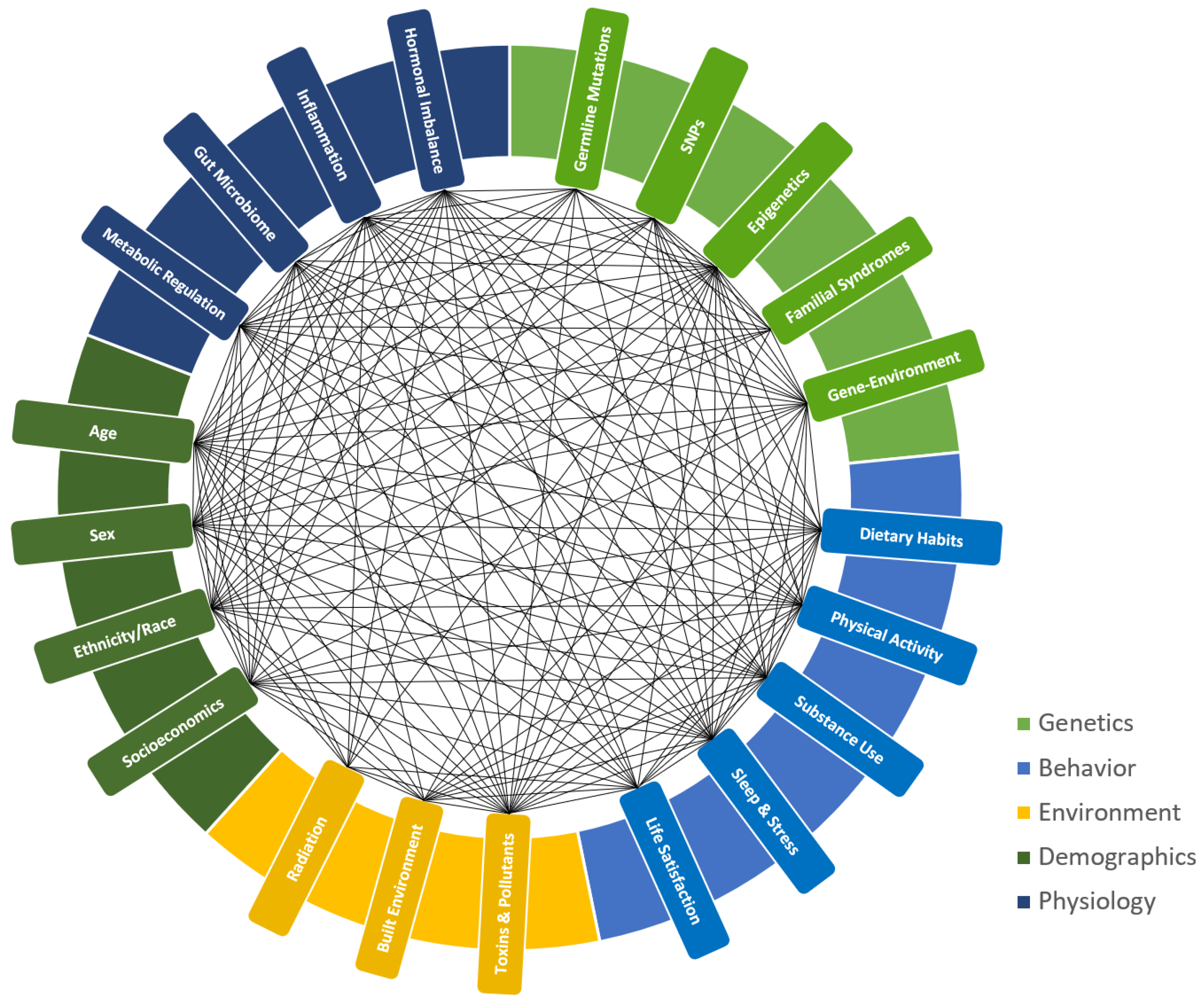
4. Personalized Lifestyle Approaches in Cancer Prevention and Treatment
5. A Call to Action
5.1. Researchers and Scientists
5.2. Clinicians and Healthcare Providers in Cancer Care
5.3. Policy- and Decisionmakers in Public Health
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Peterson, C.; Denlinger, N.; Yang, Y. Recent Advances and Challenges in Cancer Immunotherapy. Cancers 2022, 14, 3972. [Google Scholar] [CrossRef]
- Barot, S.; Patel, H.; Yadav, A.; Ban, I. Recent Advancement in Targeted Therapy and Role of Emerging Technologies to Treat Cancer. Med. Oncol. 2023, 40, 324. [Google Scholar] [CrossRef]
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The Global Burden of Cancer: Priorities for Prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar] [CrossRef]
- Goddard, K.A.B.; Feuer, E.J.; Umar, A.; Castle, P.E. Accelerating Progress to Reduce the Cancer Burden through Prevention and Control in the United States. JNCI J. Natl. Cancer Inst. 2025, 117, 20–28. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Renzi, C.; Rubin, G.P.; Lyratzopoulos, G. The Prevalence of Chronic Conditions in Patients Diagnosed with One of 29 Common and Rarer Cancers: A Cross-Sectional Study Using Primary Care Data. Cancer Epidemiol. 2020, 69, 101845. [Google Scholar] [CrossRef]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef]
- Sharman, R.; Harris, Z.; Ernst, B.; Mussallem, D.; Larsen, A.; Gowin, K. Lifestyle Factors and Cancer: A Narrative Review. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 166–183. [Google Scholar] [CrossRef]
- Ma, J.; Rosas, L.G.; Lv, N. Precision Lifestyle Medicine: A New Frontier in the Science of Behavior Change and Population Health. Am. J. Prev. Med. 2016, 50, 395–397. [Google Scholar] [CrossRef]
- Byrne, S.; Boyle, T.; Ahmed, M.; Lee, S.H.; Benyamin, B.; Hyppönen, E. Lifestyle, Genetic Risk and Incidence of Cancer: A Prospective Cohort Study of 13 Cancer Types. Int. J. Epidemiol. 2023, 52, 817–826. [Google Scholar] [CrossRef]
- Martins, F.O.; Conde, S.V. Impact of Diet Composition on Insulin Resistance. Nutrients 2022, 14, 3716. [Google Scholar] [CrossRef]
- Karoly, H.C.; Stevens, C.J.; Magnan, R.E.; Harlaar, N.; Hutchison, K.E.; Bryan, A.D. Genetic Influences on Physiological and Subjective Responses to an Aerobic Exercise Session among Sedentary Adults. J. Cancer Epidemiol. 2012, 2012, 540563. [Google Scholar] [CrossRef]
- Leońska-Duniec, A.; Ahmetov, I.I.; Zmijewski, P. Genetic Variants Influencing Effectiveness of Exercise Training Programmes in Obesity—An Overview of Human Studies. Biol. Sport 2016, 33, 207–214. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 February 2025).
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Obesity and Carcinogenesis: Historical Perspective. Am. J. Clin. Nutr. 1987, 45 (Suppl. S1), 271–276. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- National Cancer Institute. Obesity and Cancer Risk. 2022. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet (accessed on 17 February 2025).
- Hidayat, K.; Du, X.; Shi, B.M. Body Fatness at a Young Age and Risks of Eight Types of Cancer: Systematic Review and Meta-Analysis of Observational Studies. Obes. Rev. 2018, 19, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Fontvieille, E.; Viallon, V.; Recalde, M.; Freisling, H.; Manczuk, M.; Eriksen, A.K.; Olsen, A.; Ward, H.A.; Tjønneland, A.; Overvad, K.; et al. Body Mass Index and Cancer Risk Among Adults with and Without Cardiometabolic Diseases: Evidence from the EPIC and UK Biobank Prospective Cohort Studies. BMC Med. 2023, 21, 418. [Google Scholar] [CrossRef]
- Luo, J.; Hendryx, M.; Manson, J.E.; Figueiredo, J.C.; LeBlanc, E.S.; Barrington, W.; Rohan, T.E.; Howard, B.V.; Reding, K.; Ho, G.Y.; et al. Intentional Weight Loss and Obesity-Related Cancer Risk. JNCI Cancer Spectr. 2019, 3, pkz054. [Google Scholar] [CrossRef]
- Parker, E.D.; Folsom, A.R. Intentional Weight Loss and Incidence of Obesity-Related Cancers: The Iowa Women’s Health Study. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Neuhouser, M.L.; Agurs-Collins, T.; Zanetti, K.A.; Cadmus-Bertram, L.; Dean, L.T.; Drake, B.F. Impact of Obesity on Cancer Survivorship and the Potential Relevance of Race and Ethnicity. J. Natl. Cancer Inst. 2013, 105, 1344–1354. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients with Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.; Kim, M.; Cea, V.; Gong, G.; Cinelli, M.A.; Mercer, J.; Lokman, N.A.; Morrison, J.; Hagemann, T.; et al. Obesity and Cancer—Mechanisms Underlying Tumour Progression and Recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef]
- Tang, C.; Castillon, V.J.; Waters, M.; Fong, C.; Park, T.; Boscenco, S.; Kim, S.; Pekala, K.; Carrot-Zhang, J.; Hakimi, A.A.; et al. Obesity-Dependent Selection of Driver Mutations in Cancer. Nat. Genet. 2024, 56, 2318–2321. [Google Scholar] [CrossRef]
- Godsland, I.F. Insulin Resistance and Hyperinsulinaemia in the Development and Progression of Cancer. Clin. Sci. 2009, 118, 315–332. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Hyperinsulinaemia in Cancer. Nat. Rev. Cancer 2020, 20, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huo, D. Circulating Insulin-Like Growth Factor-1 and Risk of Total and 19 Site-Specific Cancers: Cohort Study Analyses from the UK Biobank. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic Inflammation and Oxidative Stress in Human Carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA Damage, Mutations, and Cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Wang, B.; Fang, W.; Liu, J.; Yuan, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and Cancer: Advances and New Agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Bocian-Jastrzębska, A.; Malczewska-Herman, A.; Kos-Kudła, B. Role of Leptin and Adiponectin in Carcinogenesis. Cancers 2023, 15, 4250. [Google Scholar] [CrossRef]
- Orzołek, I.; Sobieraj, J.; Domagała-Kulawik, J. Estrogens, Cancer and Immunity. Cancers 2022, 14, 2265. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered Macrophage Differentiation and Immune Dysfunction in Tumor Development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Díaz-Tejedor, A.; Lorenzo-Mohamed, M.; Puig, N.; García-Sanz, R.; Mateos, M.-V.; Garayoa, M.; Paíno, T. Immune System Alterations in Multiple Myeloma: Molecular Mechanisms and Therapeutic Strategies to Reverse Immunosuppression. Cancers 2021, 13, 1353. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Sarma, D.K.; Kumawat, M.; Tiwari, R.; Verma, V.; Nagpal, R.; Kumar, M. Implication of Obesity and Gut Microbiome Dysbiosis in the Etiology of Colorectal Cancer. Cancers 2023, 15, 1913. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, J.; Khatiwada, S.; Akon, A.C.; Yu, K.L.; Shen, S.; Zekry, A. Unveiling the Complex Relationship Between Gut Microbiota and Liver Cancer: Opportunities for Novel Therapeutic Interventions. Gut Microbes 2023, 15, 2240031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, F.J.; Jiang, Q.R.; Gao, H.J.; Song, X.; Zhu, H.Q.; Zhou, X.; Lu, J. Association between Gut Microbiota and Hepatocellular Carcinoma and Biliary Tract Cancer: A Mendelian Randomization Study. World J. Clin. Cases 2024, 12, 3497–3504. [Google Scholar] [CrossRef]
- Minot, S.S.; Li, N.; Srinivasan, H.; Ayers, J.L.; Yu, M.; Koester, S.T.; Stangis, M.M.; Dominitz, J.A.; Halberg, R.B.; Grady, W.M.; et al. Colorectal Cancer-Associated Bacteria Are Broadly Distributed in Global Microbiomes and Drivers of Precancerous Change. Sci. Rep. 2024, 14, 23646. [Google Scholar] [CrossRef] [PubMed]
- Crudele, L.; Piccinin, E.; Moschetta, A. Visceral Adiposity and Cancer: Role in Pathogenesis and Prognosis. Nutrients 2021, 13, 2101. [Google Scholar] [CrossRef]
- Luo, J.; Margolis, K.L.; Adami, H.O.; LaCroix, A.; Ye, W.; Women’s Health Initiative Investigators. Obesity and Risk of Pancreatic Cancer among Postmenopausal Women: The Women’s Health Initiative (United States). Br. J. Cancer 2008, 99, 527–531. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and Heritable Factors in the Causation of Cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer Is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Irigaray, P.; Newby, J.A.; Clapp, R.; Hardell, L.; Howard, V.; Montagnier, L.; Epstein, S.; Belpomme, D. Lifestyle-Related Factors and Environmental Agents Causing Cancer: An Overview. Biomed. Pharmacother. 2007, 61, 640–658. [Google Scholar] [CrossRef]
- Jumba, K.K. The Impact of Lifestyle and Environmental Factors on Cancer Risk and Prevention. IDOSR J. Appl. Sci. 2024, 9, 98–101. [Google Scholar] [CrossRef]
- Kaaks, R. Post-Diagnosis Adiposity, Physical Activity, Dietary Factors and Cancer Survival: A Systematic Review of the Evidence Base (WCRF Global Cancer Update Programme). Int. J. Cancer 2024, 155, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Alorayf, A.E.A.; Alhamamah, M.A.M.; Alhamamah, S.A.M.; Alhamamah, H.A.M.; Almasabi, F.M.; Al Jamish, Y.A.A.; Al-Aorif, F.M.A.; Allajam, M.M.M.; Alsoma, M.A.H.; Al Abbas, A.M.H. Evaluating the Effectiveness of Lifestyle Interventions in Preventing Chronic Diseases: A Systematic Review. J. Ecohumanism 2024, 3, 4651–4659. [Google Scholar] [CrossRef]
- Fung, T.; Giovannucci, E.; Anderson, C.A.M.; Booth, S.; Deierlein, A.; Gardner, C.; Hoelscher, D.M.; Raynor, H.; Stanford, F.C.; Talegawkar, S.; et al. Dietary Patterns and Risk of Breast Cancer: A Systematic Review [Internet]; USDA Nutrition Evidence Systematic Review: Alexandria, VA, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK611673/ (accessed on 5 April 2025).
- de Rezende, L.F.M.; de Sá, T.H.; Markozannes, G.; Rey-López, J.P.; Lee, I.M.; Tsilidis, K.K.; Ioannidis, J.P.A.; Eluf-Neto, J. Physical Activity and Cancer: An Umbrella Review of the Literature Including 22 Major Anatomical Sites and 770,000 Cancer Cases. Br. J. Sports Med. 2018, 52, 826–833. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Cook, L.S.; Wang, Q.; Kokts-Porietis, R.L.; McNeil, J.; Ryder-Burbidge, C.; Courneya, K.S. Prospective Cohort Study of Pre- and Postdiagnosis Physical Activity and Endometrial Cancer Survival. J. Clin. Oncol. 2020, 38, 4107–4117. [Google Scholar] [CrossRef] [PubMed]
- Amirsasan, R.; Akbarzadeh, M.; Akbarzadeh, S. Exercise and Colorectal Cancer: Prevention and Molecular Mechanisms. Cancer Cell Int. 2022, 22, 247. [Google Scholar] [CrossRef]
- Xie, F.; You, Y.; Huang, J.; Guan, C.; Chen, Z.; Fang, M.; Yao, F.; Han, J. Association between Physical Activity and Digestive-System Cancer: An Updated Systematic Review and Meta-Analysis. J. Sport Health Sci. 2021, 10, 4–13. [Google Scholar] [CrossRef]
- Chan, J.; Ng, D.W.L.; Fielding, R.; Lam, W.W.T. Comparing the Experiences of Cancer Survivors Living with Sleep Disturbances between Differing Levels of Psychological Distress: A Qualitative Study. BMC Psychiatry 2024, 24, 869. [Google Scholar] [CrossRef]
- Calvo-Schimmel, A.; Paul, S.M.; Cooper, B.A.; Harris, C.; Shin, J.; Oppegaard, K.; Hammer, M.J.; Cartwright, F.; Conley, Y.P.; Kober, K.M.; et al. Various Types of Stress and Greater Use of Disengagement Coping Are Associated with Worse Sleep Disturbance in Oncology Patients Undergoing Chemotherapy. Stress Health 2024, 40, e3279. [Google Scholar] [CrossRef]
- Krueger, E.; Secinti, E.; Stewart, J.C.; Rand, K.L.; Mosher, C.E. Cognitive-Behavioral and Mindfulness-Based Interventions for Distress in Patients with Advanced Cancer: A Meta-Analysis. Psychooncology 2024, 33, e6259. [Google Scholar] [CrossRef]
- Danhauer, S.C.; Addington, E.L.; Cohen, L.; Sohl, S.J.; Van Puymbroeck, M.; Albinati, N.K.; Culos-Reed, S.N. Yoga for Symptom Management in Oncology: A Review of the Evidence Base and Future Directions for Research. Cancer 2019, 125, 1979–1989. [Google Scholar] [CrossRef]
- Roos, E.T.; Lahti, J.M.; Rahkonen, O. Lifestyle and Cancer—A Joint Pairwise Association of Lifestyle Habits with Subsequent Cancer Diagnosis. Eur. J. Public Health 2019, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Diez de Los Rios de la Serna, C.; Fernández-Ortega, P.; Lluch-Canut, T. Lifestyle Behavior Interventions for Preventing Cancer in Adults with Inherited Cancer Syndromes: Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 14098. [Google Scholar] [CrossRef]
- Luu, N.M.; Bui, T.T.; Tran, T.P.T.; Nguyen, T.H.T.; Oh, J.K. Combinations of Lifestyle Behaviors and Cancer Risk among Korean Adults. Sci. Rep. 2023, 13, 13765. [Google Scholar] [CrossRef] [PubMed]
- Swain, C.T.V.; Boyle, T.; Mahmood, S.; Lynch, B.M. Sedentary Behaviour and Cancer. In Sedentary Behaviour Epidemiology; Leitzmann, M.F., Jochem, C., Schmid, D., Eds.; Springer Series on Epidemiology and Public Health; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Millstein, R.A.; Winters, L.; Sullivan, C.; Eisenstat, S.; Sorg, E.; Comander, A. Implementing a Multidisciplinary Lifestyle Medicine Clinic for Cancer Survivorship. Oncology 2024, 38, 421–425. [Google Scholar] [CrossRef]
- Ergas, I.J.; Baltrushes, R.Z.; Dzubnar, J.M.; Farhady, P.; Shim, V. Lifestyle Medicine Program for Patients with Breast Cancer at Kaiser Permanente Oakland. JCO Oncol. Pract. 2024, 20, 359. [Google Scholar] [CrossRef]
- Storz, M.A.; Brommer, M.; Jeitler, M. Adherence to Selected Pillars of Lifestyle Medicine in the United States: A Cross-Sectional Analysis. Am. J. Lifestyle Med. 2023. [Google Scholar] [CrossRef]
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision Medicine: Concept and Tools. Med. J. Armed Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Rossi, T.; Bandini, E.; Balzi, W.; Fabbri, F.; Massa, I.; Maltoni, R. Obesity and Dose of Anti-Cancer Therapy: Are We Sure to Be on the Right Track in the Precision Medicine Era? Front. Med. 2021, 8, 725346. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Ubago-Guisado, E.; Petrova, D.; Amiano, P.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. The Role of Diet, Alcohol, BMI, and Physical Activity in Cancer Mortality: Summary Findings of the EPIC Study. Nutrients 2021, 13, 4293. [Google Scholar] [CrossRef]
- National Cancer Institute. Scoring Standards for the 2018 WCRF/AICR Score. Available online: https://epi.grants.cancer.gov/wcrf-aicr-score/details.html (accessed on 17 February 2025).
- World Cancer Research Fund. About Our Recommendations. Available online: https://www.wcrf.org/research-policy/evidence-for-our-recommendations/about-our-recommendations (accessed on 17 February 2025).
- Foley, P.J. Effect of Low Carbohydrate Diets on Insulin Resistance and the Metabolic Syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Haskins, C.; Cohen, J.; Kotecha, R.; Kaiser, A. Low Carbohydrate Diets in Cancer Therapeutics: Current Evidence. Front. Nutr. 2021, 8, 662952. [Google Scholar] [CrossRef]
- Lewandowska, A.; Religioni, U.; Czerw, A.; Deptała, A.; Karakiewicz, B.; Partyka, O.; Pajewska, M.; Sygit, K.; Cipora, E.; Kmieć, K.; et al. Nutritional Treatment of Patients with Colorectal Cancer. Int. J. Environ. Res. Public Health 2022, 19, 6881. [Google Scholar] [CrossRef]
- Laguna, T.; Piette-Gomez, O.; Garranzo, M.; de Cedrón, M.G.; de Molina, A.R.; de Santa Pau, E.C. Epigenomic Variability and Transcriptomics as a Novel Multiomic Complementary Approach for Personalized Nutrition in Colorectal Cancer Patients. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rein, M.; Dadiani, M.; Godneva, A.; Bakalenik-Gavry, M.; Morzaev-Sulzbach, D.; Lotan-Pompan, M.; Weinberger, A.; Segal, E.; Gal-Yam, E. BREAst Cancer Personalized NuTrition (BREACPNT): Dietary Intervention in HR+ Breast Cancer Patients Treated with Endocrine Therapy. Cancer Res. 2024, 84 (Suppl. S9), PO2-02-02. [Google Scholar]
- Chen, G.; Leary, S.; Niu, J.; Perry, R.; Papadaki, A. The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials. Nutrients 2023, 15, 2099. [Google Scholar] [CrossRef]
- Jun, S.; Park, H.; Kim, U.J.; Choi, E.J.; Lee, H.A.; Park, B.; Lee, S.Y.; Jee, S.H.; Park, H. Cancer Risk Based on Alcohol Consumption Levels: A Comprehensive Systematic Review and Meta-Analysis. Epidemiol. Health 2023, 45, e2023092. [Google Scholar] [CrossRef]
- Esser, M.B.; Sherk, A.; Liu, Y.; Henley, S.J.; Naimi, T.S. Reducing Alcohol Use to Prevent Cancer Deaths: Estimated Effects Among U.S. Adults. Am. J. Prev. Med. 2024, 66, 725–729. [Google Scholar] [CrossRef]
- Hendershot, C.S.; Neighbors, C.; George, W.H.; McCarthy, D.M.; Wall, T.L.; Liang, T.; Larimer, M.E. ALDH2, ADH1B, and Alcohol Expectancies: Integrating Genetic and Learning Perspectives. Psychol. Addict. Behav. 2009, 23, 452–463. [Google Scholar] [CrossRef]
- Chang, J.S.; Hsiao, J.R.; Chen, C.H. ALDH2 Polymorphism and Alcohol-Related Cancers in Asians: A Public Health Perspective. J. Biomed. Sci. 2017, 24, 19. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, H.L.; Yang, W.; Hsieh, M.L.; Liu, C.C.; Lee, T.Y.; Huang, J.Y.; Nong, J.Y.; Li, F.A.; Chuang, H.-L.; et al. A Common East-Asian ALDH2 Mutation Causes Metabolic Disorders and the Therapeutic Effect of ALDH2 Activators. Nat. Commun. 2023, 14, 5971. [Google Scholar] [CrossRef]
- American Cancer Society. Physical Activity and the Person with Cancer. Available online: https://www.cancer.org/cancer/survivorship/be-healthy-after-treatment/physical-activity-and-the-cancer-patient.html (accessed on 17 February 2025).
- World Health Organization (WHO). Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 17 February 2025).
- Diao, X.; Ling, Y.; Zeng, Y.; Wu, Y.; Guo, C.; Jin, Y.; Chen, X.; Feng, S.; Guo, J.; Ding, C.; et al. Physical Activity and Cancer Risk: A Dose-Response Analysis for the Global Burden of Disease Study 2019. Cancer Commun. 2023, 43, 1229–1243. [Google Scholar] [CrossRef]
- Misiąg, W.; Piszczyk, A.; Szymańska-Chabowska, A.; Chabowski, M. Physical Activity and Cancer Care—A Review. Cancers 2022, 14, 4154. [Google Scholar] [CrossRef]
- Martin Ginis, K.A.; van der Ploeg, H.P.; Foster, C.; Lai, B.; McBride, C.B.; Ng, K.; Pratt, M.; Shirazipour, C.H.; Smith, P.B.; Vásquez, P.M.; et al. Participation of People Living with Disabilities in Physical Activity: A Global Perspective. Lancet 2021, 398, 443–455. [Google Scholar] [CrossRef] [PubMed]
- van der Ploeg, H.P.; van der Beek, A.J.; van der Woude, L.H.; van Mechelen, W. Physical Activity for People with a Disability: A Conceptual Model. Sports Med. 2004, 34, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Minton, J.; Dimairo, M.; Everson-Hock, E.; Scott, E.; Goyder, E. Exploring the Relationship Between Baseline Physical Activity Levels and Mortality Reduction Associated with Increases in Physical Activity: A Modelling Study. BMJ Open 2013, 3, e003509. [Google Scholar] [CrossRef]
- Mustian, K.M.; Sprod, L.K.; Janelsins, M.; Peppone, L.J.; Mohile, S. Exercise Recommendations for Cancer-Related Fatigue, Cognitive Impairment, Sleep Problems, Depression, Pain, Anxiety, and Physical Dysfunction: A Review. Oncol. Hematol. Rev. 2012, 8, 81–88. [Google Scholar] [CrossRef]
- Papadopetraki, A.; Giannopoulos, A.; Maridaki, M.; Zagouri, F.; Droufakou, S.; Koutsilieris, M.; Philippou, A. The Role of Exercise in Cancer-Related Sarcopenia and Sarcopenic Obesity. Cancers 2023, 15, 5856. [Google Scholar] [CrossRef]
- Yu, X.; Pu, H.; Voss, M. Overview of Anti-Inflammatory Diets and Their Promising Effects on Non-Communicable Diseases. Br. J. Nutr. 2024, 132, 898–918. [Google Scholar] [CrossRef]
- Bilal, I.; Chowdhury, A.; Davidson, J.; Whitehead, S. Phytoestrogens and Prevention of Breast Cancer: The Contentious Debate. World J. Clin. Oncol. 2014, 5, 705–712. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Campbell, K.L.; Hayes, S.C. Weight Management and Its Role in Breast Cancer Rehabilitation. Cancer 2012, 118, 2277–2287. [Google Scholar] [CrossRef]
- Joaquim, A.; Leão, I.; Antunes, P.; Capela, A.; Viamonte, S.; Alves, A.J.; Helguero, L.A.; Macedo, A. Impact of Physical Exercise Programs in Breast Cancer Survivors on Health-Related Quality of Life, Physical Fitness, and Body Composition: Evidence from Systematic Reviews and Meta-Analyses. Front. Oncol. 2022, 12, 955505. [Google Scholar] [CrossRef]
- Groarke, J.M.; Richmond, J.; Kelly, M.G.; McSharry, J.; Groarke, A.; Kerr, T.; Singaroyan, N.; Harney, O.; Haughey, C.; Glynn, L.; et al. Examining the Impact of a Personalized Self-Management Lifestyle Program Using Mobile Technology on the Health and Well-Being of Cancer Survivors: Protocol and Rationale for a Randomized Controlled Trial (The Moving On Study). JMIR Res. Protoc. 2019, 8, e13214. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Kumar, N.; Xu, J.Y.; Patel, J.; Damaraju, S.; Shen-Tu, G.; Greiner, R. Personalized Breast Cancer Onset Prediction from Lifestyle and Health History Information. PLoS ONE 2022, 17, e0279174. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Pan, P.; Zhang, J.; Hou, X.; Wang, Y.; Chen, G.; Muhammad, P.; Reis, R.L.; Ding, L.; et al. Humanistic Health Management and Cancer: Associations of Psychology, Nutrition, and Exercise with Cancer Progression and Pathogenesis. Adv. Sci. 2024, 11, e2400665. [Google Scholar] [CrossRef]
- Masson, G.; Mills, K.; Griffin, S.J.; Sharp, S.J.; Klein, W.M.P.; Sutton, S.; Usher-Smith, J.A. A Randomized Controlled Trial of the Effect of Providing Online Risk Information and Lifestyle Advice for the Most Common Preventable Cancers. Prev. Med. 2020, 138, 106154. [Google Scholar] [CrossRef]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated with BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving Personalized Medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef]
- Estapé, T. Cancer in the Elderly: Challenges and Barriers. Asia Pac. J. Oncol. Nurs. 2018, 5, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.X.; Reichman, M.E.; Miller, B.A.; Hankey, B.F.; Singh, G.K.; Lin, Y.D.; Goodman, M.T.; Lynch, C.F.; Schwartz, S.M.; Chen, V.W.; et al. Impact of Socioeconomic Status on Cancer Incidence and Stage at Diagnosis: Selected Findings from the Surveillance, Epidemiology, and End Results: National Longitudinal Mortality Study. Cancer Causes Control 2009, 20, 417–435. [Google Scholar] [CrossRef]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., III; Prada, D.; Samet, J.; Thurston, G.; Cohen, A.; et al. Outdoor Air Pollution and Cancer: An Overview of the Current Evidence and Public Health Recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Torkamani, A.; Butte, A.J.; Glicksberg, B.S.; Schuller, B.; Rodriguez, B.; Ting, D.S.W.; Bates, D.; Schaden, E.; Peng, H.; et al. The Promise of Digital Healthcare Technologies. Front. Public Health 2023, 11, 1196596. [Google Scholar] [CrossRef]
- Bevel, M.S.; Tsai, M.H.; Parham, A.; Andrzejak, S.E.; Jones, S.; Moore, J.X. Association of Food Deserts and Food Swamps with Obesity-Related Cancer Mortality in the US. JAMA Oncol. 2023, 9, 909–916. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motevalli, M.; Stanford, F.C. Personalized Lifestyle Interventions for Prevention and Treatment of Obesity-Related Cancers: A Call to Action. Cancers 2025, 17, 1255. https://doi.org/10.3390/cancers17081255
Motevalli M, Stanford FC. Personalized Lifestyle Interventions for Prevention and Treatment of Obesity-Related Cancers: A Call to Action. Cancers. 2025; 17():1255. https://doi.org/10.3390/cancers17081255
Chicago/Turabian StyleMotevalli, Mohamad, and Fatima Cody Stanford. 2025. "Personalized Lifestyle Interventions for Prevention and Treatment of Obesity-Related Cancers: A Call to Action" Cancers 17, no. : 1255. https://doi.org/10.3390/cancers17081255
APA StyleMotevalli, M., & Stanford, F. C. (2025). Personalized Lifestyle Interventions for Prevention and Treatment of Obesity-Related Cancers: A Call to Action. Cancers, 17(), 1255. https://doi.org/10.3390/cancers17081255






