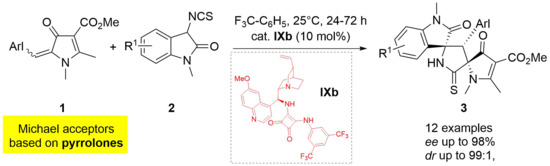
Double Spirocyclization of Arylidene-?2-Pyrrolin-4-Ones with 3-Isothiocyanato Oxindoles
Abstract
:1. Introduction
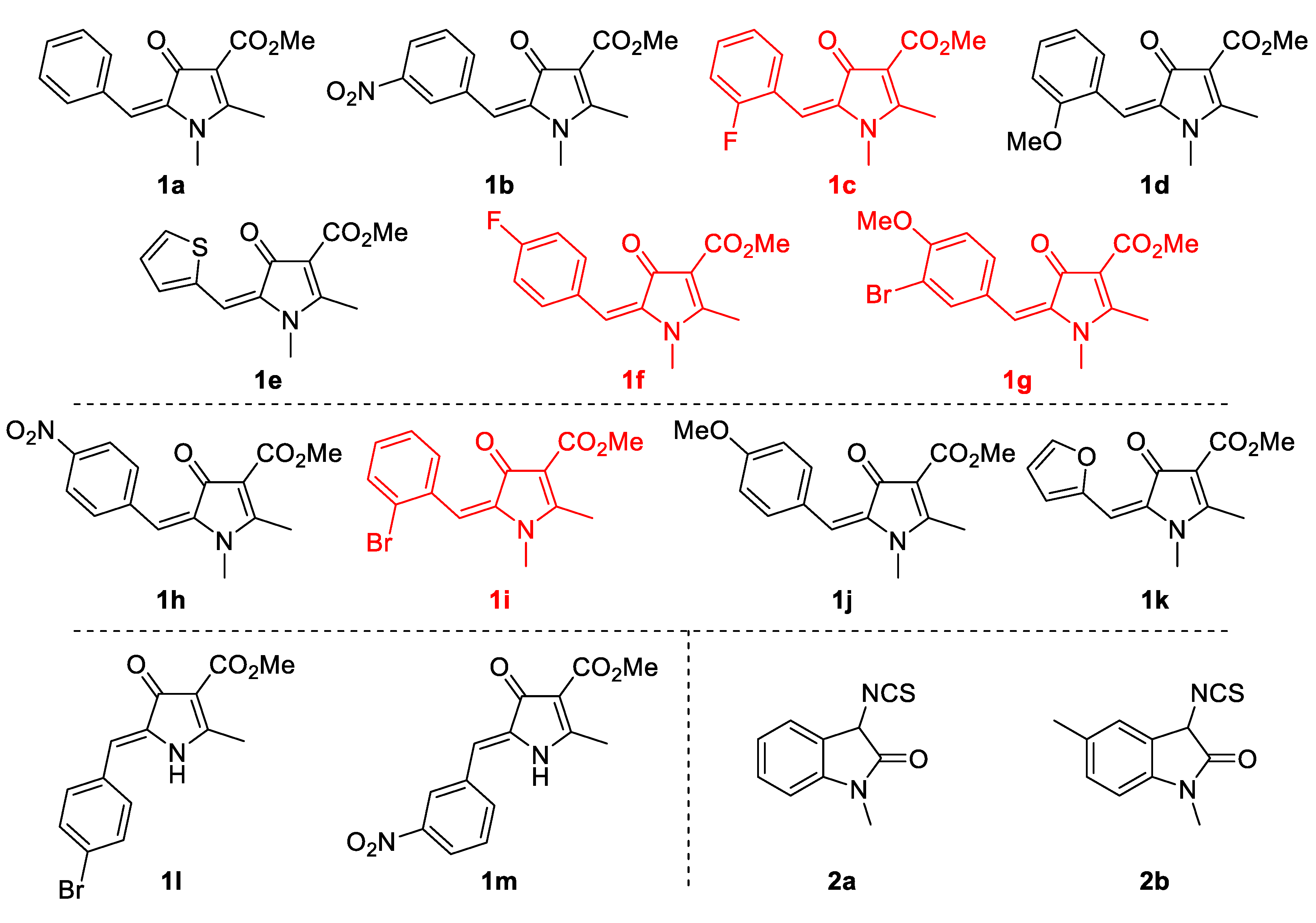
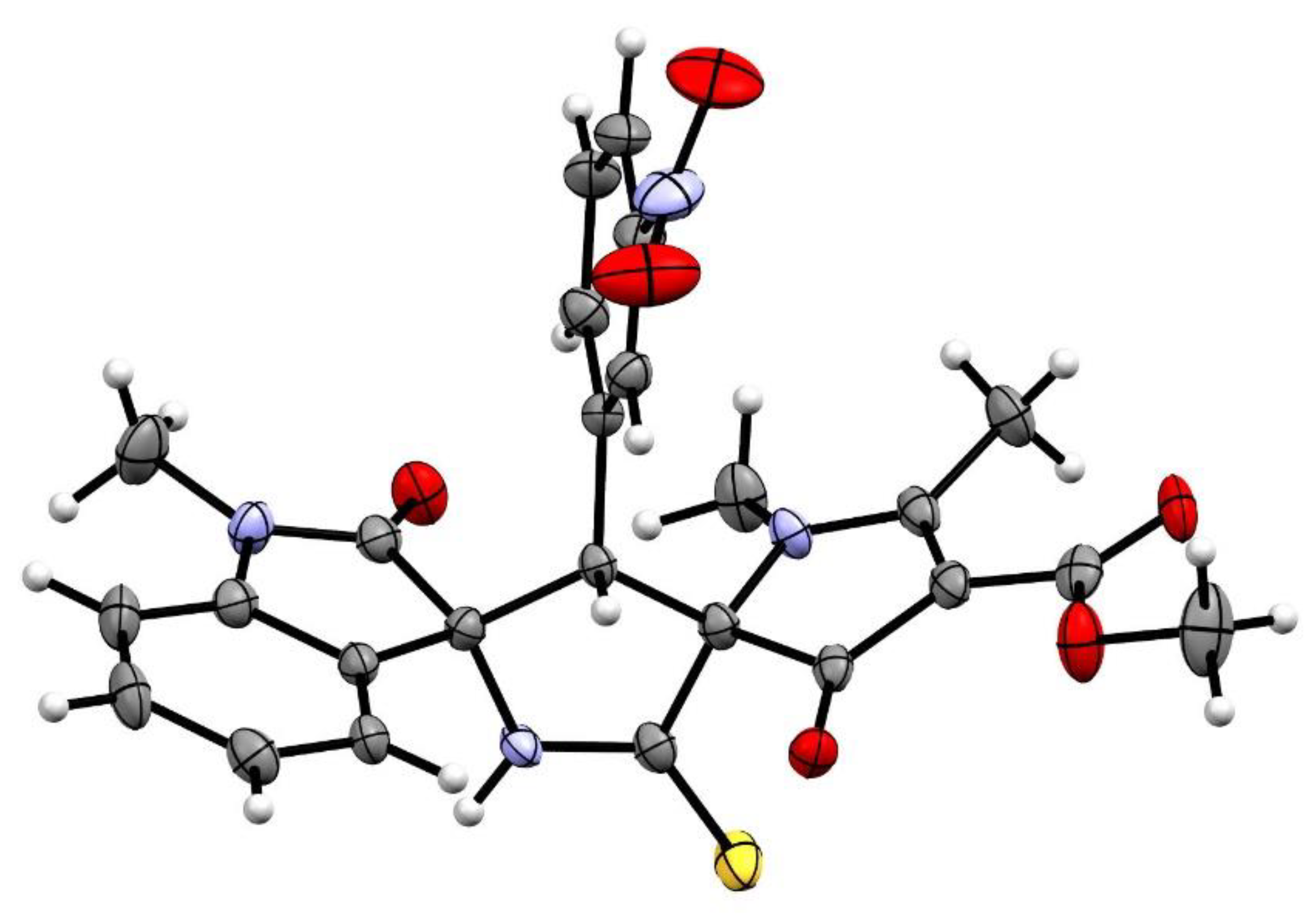
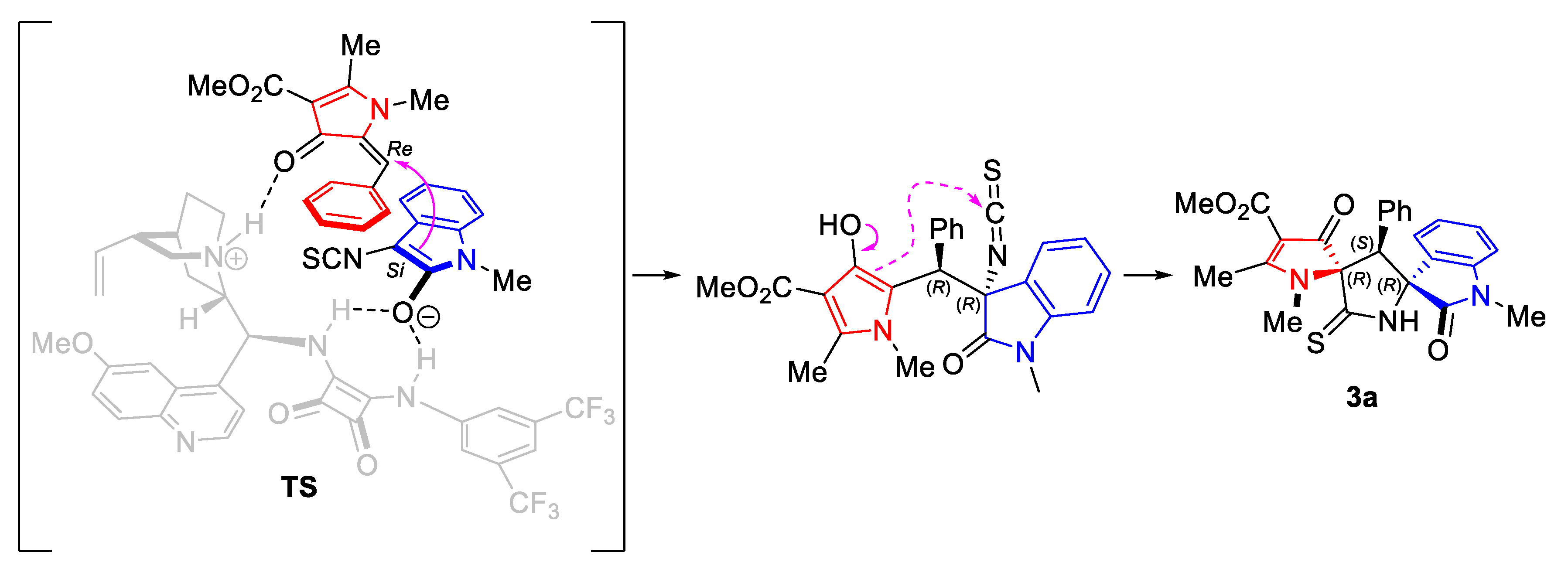
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Methods, Syntheses, and Characterization
3.2. Synthesis of (E)-Methyl 5-Arylidene-1,2-Dimethyl-4-oxo-4,5-Dihydro-1H-Pyrrole-3-Carboxylate-General Procedure 1 (GP1)
3.3. Organocatalyzed Bis-Spiroheterocyclization-Preparation of Racemic Mixtures-General Procedure 2 (GP2)
3.4. Organocatalyzed Stereoselective Bis-Spiroheterocyclization-General Procedure 3 (GP3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panda, S.S.; Mohapatra, P.P.; Jones, R.A.; Bachawala, P. Spirooxindoles as Potential Pharmacophores. Mini Rev. Med. Chem. 2017, 17, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Sun, W.; Liu, L.; Lu, J.; McEachern, D.; Shargary, S.; Bernard, D.; Li, X.; Zhao, T.; et al. A Potent Small-Molecule Inhibitor of the MDM2-p53 Interaction (MI-888) Achieved Complete and Durable Tumor Regression in Mice. J. Med. Chem. 2013, 56, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, S.; Page, P.; Missotten, M.; Colovray, V.; Cleva, C.; Arrighi, J.-F.; Atherall, J.; Macritchie, J.; Martin, T.; Humbert, Y.; et al. Discovery of a New Class of Potent, Selective, and Orally Bioavailable CRTH2 (DP2) Receptor Antagonists for the Treatment of Allergic Inflammatory Diseases. J. Med. Chem. 2008, 51, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.; Danishefsky, S.J.; Sepp-Lorenzino, L.; Rosen, N. Total Synthesis of Spirotryprostatin A, Leading to the Discovery of Some Biologically Promising Analogs. J. Am. Chem. Soc. 1999, 121, 2147–2155. [Google Scholar] [CrossRef]
- Yeung, B.K.S.; Zou, B.; Rottmann, M.; Lakshminarayana, S.B.; Ang, S.H.; Leong, S.Y.; Tan, J.; Wong, J.; Keller-Maerki, S.; Fischli, C.; et al. Spirotetrahydro β-Carbolines (Spiroindolones): A New Class of Potent and Orally Efficacious Compounds for the Treatment of Malaria. J. Med. Chem. 2010, 53, 5155–5164. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, P.; Jian, J.; Chen, Y.; Xu, J. Nine-step total synthesis of (-)-strychnofoline. Chem. Commun. 2018, 54, 1125–1128. [Google Scholar] [CrossRef]
- Ye, N.; Chen, H.; Wold, E.A.; Shi, P.-Y.; Zhou, J. Therapeutic Potential of Spirooxindoles as Antiviral Agents. ACS Infect. Dis. 2016, 2, 382–392. [Google Scholar] [CrossRef]
- Torres, R.R. (Ed.) Stereoselective Organocatalysis: Bond Formation Methodologies and Activation Modes, 1st ed.; JohnWiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Jakab, G.; Schreiner, P.R. Brønsted Acids: Chiral (Thio)urea Derivatives. In Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, 1st ed.; Dalko, P.I., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 315–342. [Google Scholar]
- Krištofíková, D.; Modrocká, V.; Mečiarová, M.; Šebesta, R. Green Asymmetric Organocatalysis. ChemSusChem 2020, 13, 2828–2858. [Google Scholar] [CrossRef]
- Chanda, T.; Zhao, J.C.G. Recent Progress in Organocatalytic Asymmetric Domino Transformations. Adv. Synth. Catal. 2018, 360, 2–79. [Google Scholar] [CrossRef]
- Held, F.E.; Tsogoeva, S.B. Asymmetric cycloaddition reactions catalyzed by bifunctional thiourea and squaramide organocatalysts: Recent advances. Catal. Sci. Technol. 2016, 6, 645–667. [Google Scholar] [CrossRef] [Green Version]
- Holland, M.C.; Gilmour, R. Deconstructing Covalent Organocatalysis. Angew. Chem. Int. Ed. 2015, 54, 3862–3871. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Serdyuk, O.V.; Heckel, C.M.; Tsogoeva, S.B. Bifunctional primary amine-thioureas in asymmetric organocatalysis. Org. Biomol. Chem. 2013, 11, 7051–7071. [Google Scholar] [CrossRef] [Green Version]
- Malerich, J.P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef] [Green Version]
- Alemán, J.; Parra, A.; Jiang, H.; Jørgensen, K.A. Squaramides: Bridging from Molecular Recognition to Bifunctional Organocatalysis. Chem. Eur. J. 2011, 17, 6890–6899. [Google Scholar] [CrossRef]
- Enders, D.; Huttl, M.R.M.; Grondal, C.; Raabe, G. Control of four stereocentres in a triple cascade organocatalytic reaction. Nature 2006, 441, 861–863. [Google Scholar] [CrossRef]
- Tan, F.; Cheng, H.-G. Recent advances in catalytic asymmetric cascade reactions of 3-isothiocyanato oxindoles for synthesis of spirooxindoles. Targets Heterocycl. Syst. 2017, 21, 158–180. [Google Scholar]
- Mei, G.-J.; Shi, F. Catalytic asymmetric synthesis of spirooxindoles: Recent developments. Chem. Commun. 2018, 54, 6607–6621. [Google Scholar] [CrossRef]
- Gasperi, T.; Miceli, M.; Campagne, J.-M.; Marcia de Figueiredo, R. Non-covalent organocatalyzed domino reactions involving oxindoles: Recent advances. Molecules 2017, 22, 1636. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C.F. Organocatalytic Asymmetric Assembly Reactions: Synthesis of Spirooxindoles via Organocascade Strategies. ACS Catal. 2014, 4, 743–762. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Dou, P.-H.; You, Y.; Wang, Z.-H.; Zhou, M.-Q.; Xu, X.-Y.; Yuan, W.-C. Organocatalytic asymmetric [3+2]-cycloaddition of 3-isothiocyanato oxindoles with 1,3,5-trisubstituted-hexahydro-1,3,5-triazines to access spiro-imidazolidinethione-oxindoles. Tetrahedron 2019, 75, 130571. [Google Scholar] [CrossRef]
- Gui, H.-Z.; Wei, Y.; Shi, M. Construction of spirothioureas having an amino quaternary stereogenic center via a [3+2] annulation of 3-isothiocyanato oxindoles with 2-aminoacrylates. Org. Biomol. Chem. 2018, 16, 9218–9222. [Google Scholar] [CrossRef]
- Du, D.; Xu, Q.; Li, X.-G.; Shi, M. Construction of Spirocyclic Oxindoles through Regio- and Stereoselective [3+2] or [3+2]/[4+2] Cascade Reaction of α,β-Unsaturated Imines with 3-Isothiocyanato Oxindole. Chem. Eur. J. 2016, 22, 4733–4737. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Cui, B.-D.; Zuo, J.; Zhao, J.-Q.; You, Y.; Chen, Y.-Z.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Quinine-catalyzed asymmetric domino Mannich-cyclization reactions of 3-isothiocyanato oxindoles with imines for the synthesis of spirocyclic oxindoles. Tetrahedron 2015, 71, 949–955. [Google Scholar] [CrossRef]
- Cai, H.; Zhou, Y.; Zhang, D.; Xu, J.; Liu, H. A Mannich/cyclization cascade process for the asymmetric synthesis of spirocyclic thioimidazolidineoxindoles. Chem. Commun. 2014, 50, 14771–14774. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Dou, P.-H.; Zhao, J.-Q.; Yuan, W.-C. Organocatalyzed asymmetric cascade Mannich/cyclization of 3-isothiocyanato oxindoles with cyclic ketimines for the synthesis of polycyclic spiro-thioimidazolidine-oxindoles. Tetrahedron 2020, 76, 131116. [Google Scholar] [CrossRef]
- Zhao, B.-L.; Du, D.-M. Asymmetric Synthesis of Spirooxindoles with Seven Stereocenters via Organocatalyzed One-pot Three-component Sequential Cascade Reactions. Adv. Synth. Catal. 2019, 361, 3412–3419. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.-L.; Xu, S.-W.; Tian, M.-Y.; Zhang, M.; Liu, X.-L.; Yuan, W.-C. Regio- and stereoselective [3+2] cycloaddition reaction: Access to isoxazole-dispirobisoxindoles featuring three contiguous stereocenters. Org. Biomol. Chem. 2019, 17, 6551–6556. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Chen, Y.-Z.; Cui, B.-D.; Xu, X.-Y.; Yuan, W.-C. Thiourea-catalyzed asymmetric domino Michael-cyclization reaction of 3-isothiocyanato oxindoles with β,γ-unsaturated α-keto esters for the synthesis of spirocyclic oxindoles. Tetrahedron 2019, 75, 2155–2161. [Google Scholar] [CrossRef]
- Zhu, W.-R.; Chen, Q.; Lin, N.; Chen, K.-B.; Zhang, Z.-W.; Fang, G.; Weng, J.; Lu, G. Organocatalytic Michael/cyclization cascade reactions of 3-isothiocyanato oxindoles with 3-trifluoroethylidene oxindoles: An approach for the synthesis of 3′-trifluoromethyl substituted 3,2′-pyrrolidine-bispirooxindoles. Org. Chem. Front. 2018, 5, 1375–1380. [Google Scholar] [CrossRef]
- Song, Y.-X.; Du, D.-M. Squaramide-Catalyzed Asymmetric Michael/Cyclization Cascade Reaction of Unsaturated Thiazolidinones and 3-Isothiocyanato Oxindoles: Synthesis of New Bispirocyclic Heterocycles. Synthesis 2018, 50, 1535–1545. [Google Scholar]
- Lin, N.; Long, X.-w.; Chen, Q.; Zhu, W.-r.; Wang, B.-c.; Chen, K.-b.; Jiang, C.-w.; Weng, J.; Lu, G. Highly efficient construction of chiral dispirocyclic oxindole/thiobutyrolactam/chromanone complexes through Michael/cyclization cascade reactions with a rosin-based squaramide catalyst. Tetrahedron 2018, 74, 3734–3741. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; Du, D.-M. Squaramide-catalyzed asymmetric Michael/cyclization cascade reaction of 3-isothiocyanato oxindoles with chalcones for synthesis of pyrrolidinyl spirooxindoles. Org. Chem. Front. 2017, 4, 1229–1238. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Tian, T.; Pang, H.-L.; Li, B.; Chen, X.-Q.; Yang, Z.; Meng, W.; Song, X.-Q.; Zhao, Y.-D.; Liu, Y.-Y. Organocatalytic [3+2] Cycloadditions of Barbiturate-Based Olefins with 3-Isothiocyanato Oxindoles: Highly Diastereoselective and Enantioselective Synthesis of Dispirobarbiturates. Adv. Synth. Catal. 2016, 358, 2619–2630. [Google Scholar] [CrossRef]
- Wu, C.; Jing, L.; Qin, D.; Yin, M.; He, Q. Organocatalytic asymmetric synthesis of trans-configured trispirooxindoles through a cascade Michael-cyclization reaction. Tetrahedron Lett. 2016, 57, 2857–2860. [Google Scholar] [CrossRef]
- Kayal, S.; Mukherjee, S. Catalytic enantioselective cascade Michael/cyclization reaction of 3-isothiocyanato oxindoles with exocyclic α,β-unsaturated ketones en route to 3,2′-pyrrolidinyl bispirooxindoles. Org. Biomol. Chem. 2016, 14, 10175–10179. [Google Scholar] [CrossRef]
- Chowdhury, R.; Kumar, M.; Ghosh, S.K. Organocatalyzed enantioselective Michael addition/cyclization cascade reaction of 3-isothiocyanato oxindoles with arylidene malonates. Org. Biomol. Chem. 2016, 14, 11250–11260. [Google Scholar] [CrossRef]
- Du, D.; Jiang, Y.; Xu, Q.; Tang, X.-Y.; Shi, M. Enantioselective [3+2]-Cyclization of 3-Isothiocyanato Oxindoles with Trifluoromethylated 2-Butenedioic Acid Diesters. ChemCatChem 2015, 7, 1366–1371. [Google Scholar] [CrossRef]
- Kayal, S.; Mukherjee, S. Catalytic Asymmetric Michael Addition/Cyclization Cascade Reaction of 3-Isothiocyanatooxindoles with Nitro Olefins. Eur. J. Org. Chem. 2014, 2014, 6696–6700. [Google Scholar] [CrossRef]
- Cui, B.-D.; Li, S.-W.; Zuo, J.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Quinine-catalyzed asymmetric domino Michael-cyclization reaction for the synthesis of spirocyclic oxindoles bearing two spiro quaternary centers and three consecutive stereocenters. Tetrahedron 2014, 70, 1895–1902. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.-L.; Tian, Z.-Q.; Huang, Y.-D.; Wang, Y.-M. Highly Efficient Enantioselective Construction of Bispirooxindoles Containing Three Stereocenters through an Organocatalytic Cascade Michael-Cyclization Reaction. Chem. Eur. J. 2013, 19, 1747–1753. [Google Scholar] [CrossRef]
- Tan, F.; Cheng, H.-G.; Feng, B.; Zou, Y.-Q.; Duan, S.-W.; Chen, J.-R.; Xiao, W.-J. Highly Enantioselective Organocatalytic Michael Addition/Cyclization Cascade Reaction of Ylideneoxindoles with Isothiocyanato Oxindoles: A Formal [3+2] Cycloaddition Approach to Optically Active Bispirooxindole Derivatives. Eur. J. Org. Chem. 2013, 2013, 2071–2075. [Google Scholar] [CrossRef]
- Liu, X.-L.; Han, W.-Y.; Zhang, X.-M.; Yuan, W.-C. Highly Efficient and Stereocontrolled Construction of 3,3′-Pyrrolidonyl Spirooxindoles via Organocatalytic Domino Michael/Cyclization Reaction. Org. Lett. 2013, 15, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-Y.; Li, S.-W.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. 3-Isothiocyanato oxindoles serving as powerful and versatile precursors to structurally diverse dispirocyclic thiopyrrolidineoxindoles through a cascade Michael/cyclization process with amino-thiocarbamate catalysts. Chem. Eur. J. 2013, 19, 5551–5556. [Google Scholar] [CrossRef]
- Du, D.; Jiang, Y.; Xu, Q.; Shi, M. Enantioselective Construction of Spirooxindole Derivatives: Asymmetric [3+2] Cyclization of Isothiocyanatooxindoles with Allenic Esters or 2-Butynedioic Acid Diesters. Adv. Synth. Catal. 2013, 355, 2249–2256. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, J.; Wang, S.; Wang, D.; Wang, R. An asymmetric approach toward chiral multicyclic spirooxindoles from isothiocyanato oxindoles and unsaturated pyrazolones by a chiral tertiary amine thiourea catalyst. Chem. Commun. 2013, 49, 1657–1659. [Google Scholar] [CrossRef]
- Cao, Y.-M.; Shen, F.-F.; Zhang, F.-T.; Wang, R. Catalytic asymmetric Michael addition/cyclization of isothiocyanato oxindoles: Highly efficient and versatile approach for the synthesis of 3,2′-pyrrolidinyl mono- and bi-spirooxindole frameworks. Chem. Eur. J. 2013, 19, 1184–1188. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Da, B.-C.; Xiang, S.-H.; Zhu, S.; Yuan, Z.-Y.; Guo, Z.; Tan, B. Organocatalytic double arylation of 3-isothiocyanato oxindoles: Stereocontrolled synthesis of complex spirooxindoles. Tetrahedron 2019, 75, 1689–1696. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, B.-L.; Du, D.-M. Organocatalytic Asymmetric Michael/Cyclization Cascade Reaction of 3-Isothiocyanato Oxindoles with Maleimides for the Efficient Construction of Pyrrolidonyl Spirooxindoles. Eur. J. Org. Chem. 2016, 2016, 4711–4718. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Zhou, M.-Q.; Wu, Z.-J.; Wang, Z.-H.; Yue, D.-F.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Asymmetric Michael/Cyclization Cascade Reaction of 3-Isothiocyanato Oxindoles and 3-Nitroindoles with Amino-Thiocarbamate Catalysts: Enantioselective Synthesis of Polycyclic Spirooxindoles. Org. Lett. 2015, 17, 2238–2241. [Google Scholar] [CrossRef]
- Fu, Z.-K.; Pan, J.-Y.; Xu, D.-C.; Xie, J.-W. Organocatalytic domino Michael/cyclization reaction: Efficient synthesis of multi-functionalized tetracyclic spirooxindoles with multiple stereocenters. RSC Adv. 2014, 4, 51548–51557. [Google Scholar] [CrossRef]
- Kayal, S.; Mukherjee, S. Catalytic Aldol-Cyclization Cascade of 3-Isothiocyanato Oxindoles with α-Ketophosphonates for the Enantioselective Synthesis of β-Amino-α-hydroxyphosphonates. Org. Lett. 2015, 17, 5508–5511. [Google Scholar] [CrossRef]
- Chen, W.-B.; Han, W.-Y.; Han, Y.-Y.; Zhang, X.-M.; Yuan, W.-C. Highly efficient synthesis of spiro[oxazolidine-2-thione-oxindoles] with 3-isothiocyanato oxindoles and aldehydes via an organocatalytic cascade aldol-cyclization reaction. Tetrahedron 2013, 69, 5281–5286. [Google Scholar] [CrossRef]
- Chen, W.-B.; Wu, Z.-J.; Hu, J.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Organocatalytic direct asymmetric aldol reactions of 3-isothiocyanato oxindoles to ketones: Stereocontrolled synthesis of spirooxindoles bearing highly congested contiguous tetrasubstituted stereocenters. Org. Lett. 2011, 13, 2472–2475. [Google Scholar] [CrossRef]
- Jiang, Y.; Pei, C.-K.; Du, D.; Li, X.-G.; He, Y.-N.; Xu, Q.; Shi, M. Enantioselective Synthesis of Spirooxindoles: Asymmetric [3+2] Cycloaddition of (3-Isothiocyanato)oxindoles with Azo Dicarboxylates. Eur. J. Org. Chem. 2013, 2013, 7895–7901. [Google Scholar] [CrossRef]
- Williams, R.M.; Kwast, E.; Coffman, H.; Glinka, T. Remarkable, enantio-divergent biogenesis of brevianamide A and B. J. Am. Chem. Soc. 1989, 111, 3064–3065. [Google Scholar] [CrossRef]
- Bowen, C.; Malcolm, S.; Melnick, L.; Xie, L. Modulators of Opioid Receptors and Methods of Use Thereof. WO 2013070659 A1, 16 May 2013. [Google Scholar]
- Murugesan, D.; Mital, A.; Kaiser, M.; Shackleford, D.M.; Morizzi, J.; Katneni, K.; Campbell, M.; Hudson, A.; Charman, S.A.; Yeates, C.; et al. Discovery and Structure-Activity Relationships of Pyrrolone Antimalarials. J. Med. Chem. 2013, 56, 2975–2990. [Google Scholar] [CrossRef]
- Murugesan, D.; Kaiser, M.; White, K.L.; Norval, S.; Riley, J.; Wyatt, P.G.; Charman, S.A.; Read, K.D.; Yeates, C.; Gilbert, I.H. Structure-Activity Relationship Studies of Pyrrolone Antimalarial Agents. ChemMedChem 2013, 8, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.B., III; Hirschmann, R.; Pasternak, A.; Yao, W.; Sprengeler, P.A.; Holloway, M.K.; Kuo, L.C.; Chen, Z.; Darke, P.L.; Schleif, W.A. An Orally Bioavailable Pyrrolinone Inhibitor of HIV-1 Protease: Computational Analysis and X-ray Crystal Structure of the Enzyme Complex. J. Med. Chem. 1997, 40, 2440–2444. [Google Scholar] [CrossRef]
- Angermann, A.; Lehr, S.; Fischer, R.; Bojack, G.; Helmke, H.; Schmutzler, D.; Dietrich, H.; Gatzweiler, E.; Rosinger, C.H. New alkynyl-substituted 3-phenylpyrrolidine-2,4-diones and Use Thereof as Herbicides. WO 2017060203 A1, 13 April 2017. [Google Scholar]
- Grošelj, U.; Žorž, M.; Golobič, A.; Stanovnik, B.; Svete, J. α-Amino acid derived enaminones and their application in the synthesis of N-protected methyl 5-substituted-4-hydroxypyrrole-3-carboxylates and other heterocycles. Tetrahedron 2013, 69, 11092–11108. [Google Scholar] [CrossRef]
- Ričko, S.; Meden, A.; Ciber, L.; Štefane, B.; Požgan, F.; Svete, J.; Grošelj, U. Construction of Vicinal Tetrasubstituted Stereogenic Centers via a Mannich-Type Organocatalyzed Addition of Δ2-Pyrrolin-4-ones to Isatin Imines. Adv. Synth. Catal. 2018, 360, 1072–1076. [Google Scholar] [CrossRef]
- Ričko, S.; Meden, A.; Ivančič, A.; Perdih, A.; Štefane, B.; Svete, J.; Grošelj, U. Organocatalyzed Deracemization of Δ2-Pyrrolin-4-ones. Adv. Synth. Catal. 2017, 359, 2288–2296. [Google Scholar] [CrossRef]
- Ričko, S.; Svete, J.; Štefane, B.; Perdih, A.; Golobič, A.; Meden, A.; Grošelj, U. 1,3-Diamine-Derived Bifunctional Organocatalyst Prepared from Camphor. Adv. Synth. Catal. 2016, 358, 3786–3796. [Google Scholar]
- Grošelj, U.; Ciber, L.; Gnidovec, J.; Testen, Ž.; Požgan, F.; Štefane, B.; Tavčar, G.; Svete, J.; Ričko, S. Synthesis of Spiro-Δ2-Pyrrolin-4-One Pseudo Enantiomers via an Organocatalyzed Sulfa-Michael/Aldol Domino Sequence. Adv. Synth. Catal. 2019, 361, 5118–5126. [Google Scholar] [CrossRef]
- Grayson, M.N. Mechanism and Origins of Stereoselectivity in the Cinchona Thiourea- and Squaramide-Catalyzed Asymmetric Michael Addition of Nitroalkanes to Enones. J. Org. Chem. 2017, 82, 4396–4401. [Google Scholar] [CrossRef]
- Ričko, S.; Izzo, J.A.; Jørgensen, K.A. Insights on the Pseudo-Enantiomeric Properties of Bifunctional Cinchona Alkaloid Squaramide-derived Organocatalyst. Chem. Eur. J. 2020. Accepted Author Manuscript. [Google Scholar] [CrossRef]
- Ričko, S.; Požgan, F.; Štefane, B.; Svete, J.; Golobič, A.; Grošelj, U. Stereodivergent Synthesis of Camphor-Derived Diamines and Their Application as Thiourea Organocatalysts. Molecules 2020, 25, 2978. [Google Scholar] [CrossRef]
- Mailhol, D.; Duque, M.d.M.S.; Raimondi, W.; Bonne, D.; Constantieux, T.; Coquerel, Y.; Rodriguez, J. Enantioselective Organocatalytic Michael Addition of Cyclobutanones to Nitroalkenes. Adv. Synth. Catal. 2012, 354, 3523–3532. [Google Scholar] [CrossRef]
- Konishi, H.; Lam, T.Y.; Malerich, J.P.; Rawal, V.H. Enantioselective α-Amination of 1,3-Dicarbonyl Compounds Using Squaramide Derivatives as Hydrogen Bonding Catalysts. Org. Lett. 2010, 12, 2028–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baran, R.; Veverková, E.; Škvorcová, A.; Šebesta, R. Enantioselective Michael addition of 1,3-dicarbonyl compounds to a nitroalkene catalyzed by chiral squaramides—A key step in the synthesis of pregabalin. Org. Biomol. Chem. 2013, 11, 7705–7711. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Du, D.-M. Highly Enantioselective Michael Addition of Nitroalkanes to Chalcones Using Chiral Squaramides as Hydrogen Bonding Organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef]
- Badiola, E.; Fiser, B.; Gómez-Bengoa, E.; Mielgo, A.; Olaizola, I.; Urruzuno, I.; García, J.M.; Odriozola, J.M.; Razkin, J.; Oiarbide, M.; et al. Enantioselective Construction of Tetrasubstituted Stereogenic Carbons through Brønsted Base Catalyzed Michael Reactions: α′-Hydroxy Enones as Key Enoate Equivalent. J. Am. Chem. Soc. 2014, 136, 17869–17881. [Google Scholar] [CrossRef]
- Zhao, B.-L.; Du, D.-M. Catalytic asymmetric conjugate addition of various α-mercaptoketones to α,β-unsaturated N-acylated oxazolidin-2-ones with bifunctional organocatalyst. RSC Adv. 2014, 4, 27346–27353. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly Enantioselective Conjugate Addition of Nitromethane to Chalcones Using Bifunctional Cinchona Organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef]



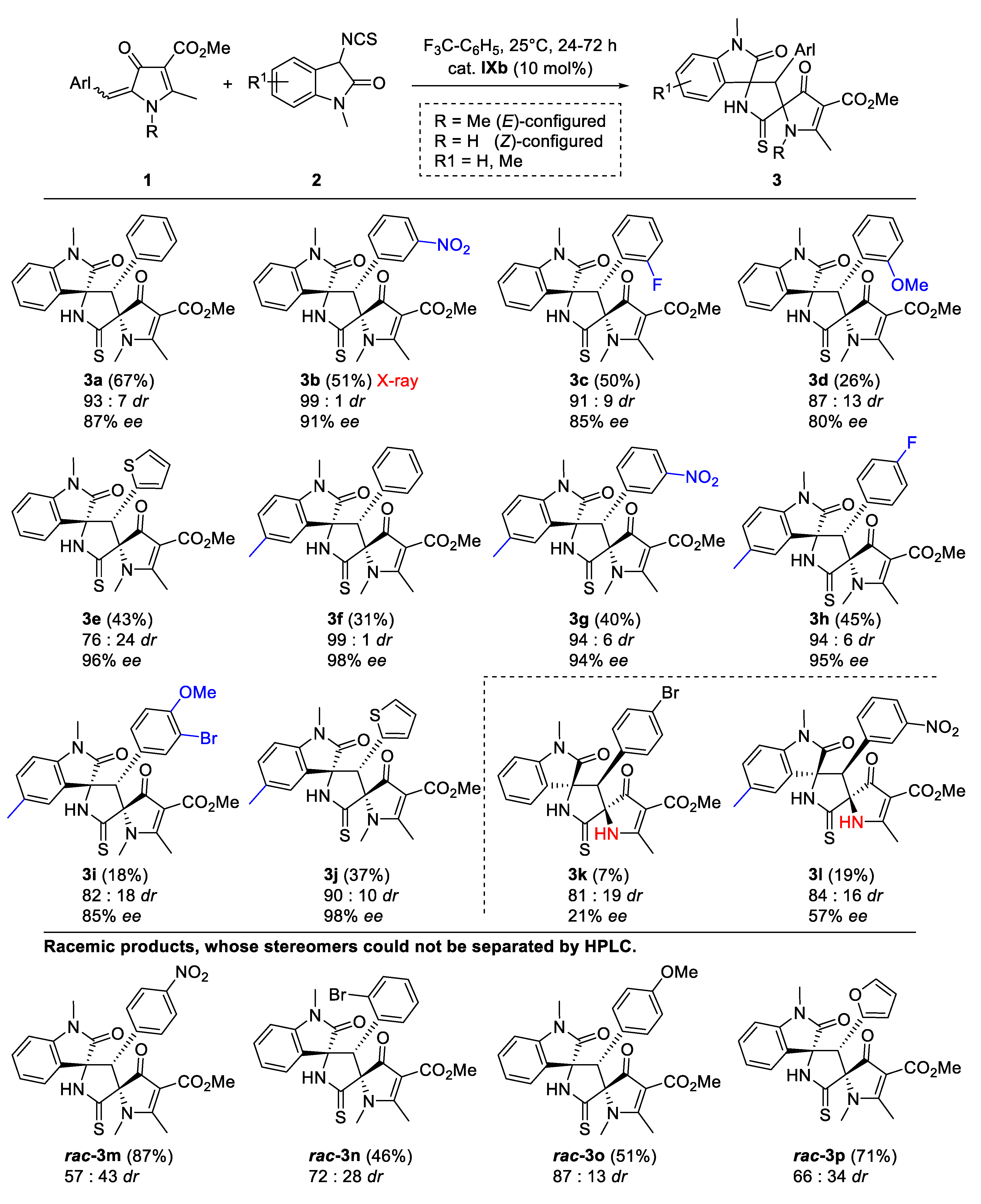

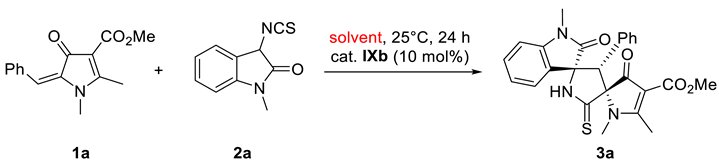
| Solvent | Yield (%) | dr | ee (%) | |
|---|---|---|---|---|
| 1 | toluene | 60 | 95:5 | 79 |
| 2 | 1,4-dioxane | 46 | 93:7 | 83 |
| 3 | Et2O | 35 | 80:20 | 75 |
| 4 | 1,2-dimethoxyethane | 49 | 95:5 | 78 |
| 5 | THF | 55 | 94:6 | 82 |
| 6 | CH2Cl2 | 61 | 93:7 | 6 |
| 7 | PhCF3 | 67 | 93:7 | 87 |
| 8 | acetone | 44 | 94:6 | 87 |
| 9 | t-butyl methyl ketone | 41 | 95:5 | 87 |
| 10 | MeCN | 30 | 79:21 | 52 |
| 11 | MeOH | 34 | 86:14 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ričko, S.; Testen, Ž.; Ciber, L.; Požgan, F.; Štefane, B.; Brodnik, H.; Svete, J.; Grošelj, U. Double Spirocyclization of Arylidene-?2-Pyrrolin-4-Ones with 3-Isothiocyanato Oxindoles. Catalysts 2020, 10, 1211. https://doi.org/10.3390/catal10101211
Ričko S, Testen Ž, Ciber L, Požgan F, Štefane B, Brodnik H, Svete J, Grošelj U. Double Spirocyclization of Arylidene-?2-Pyrrolin-4-Ones with 3-Isothiocyanato Oxindoles. Catalysts. 2020; 10(10):1211. https://doi.org/10.3390/catal10101211
Chicago/Turabian StyleRičko, Sebastijan, Žan Testen, Luka Ciber, Franc Požgan, Bogdan Štefane, Helena Brodnik, Jurij Svete, and Uroš Grošelj. 2020. "Double Spirocyclization of Arylidene-?2-Pyrrolin-4-Ones with 3-Isothiocyanato Oxindoles" Catalysts 10, no. 10: 1211. https://doi.org/10.3390/catal10101211
APA StyleRičko, S., Testen, Ž., Ciber, L., Požgan, F., Štefane, B., Brodnik, H., Svete, J., & Grošelj, U. (2020). Double Spirocyclization of Arylidene-?2-Pyrrolin-4-Ones with 3-Isothiocyanato Oxindoles. Catalysts, 10(10), 1211. https://doi.org/10.3390/catal10101211