Some Insights into the Use of Heterogeneous Copper Catalysts in the Hydroprocessing of Levulinic Acid
Abstract
1. Introduction
1.1. Importance of LA/LEs and Scope of the Review
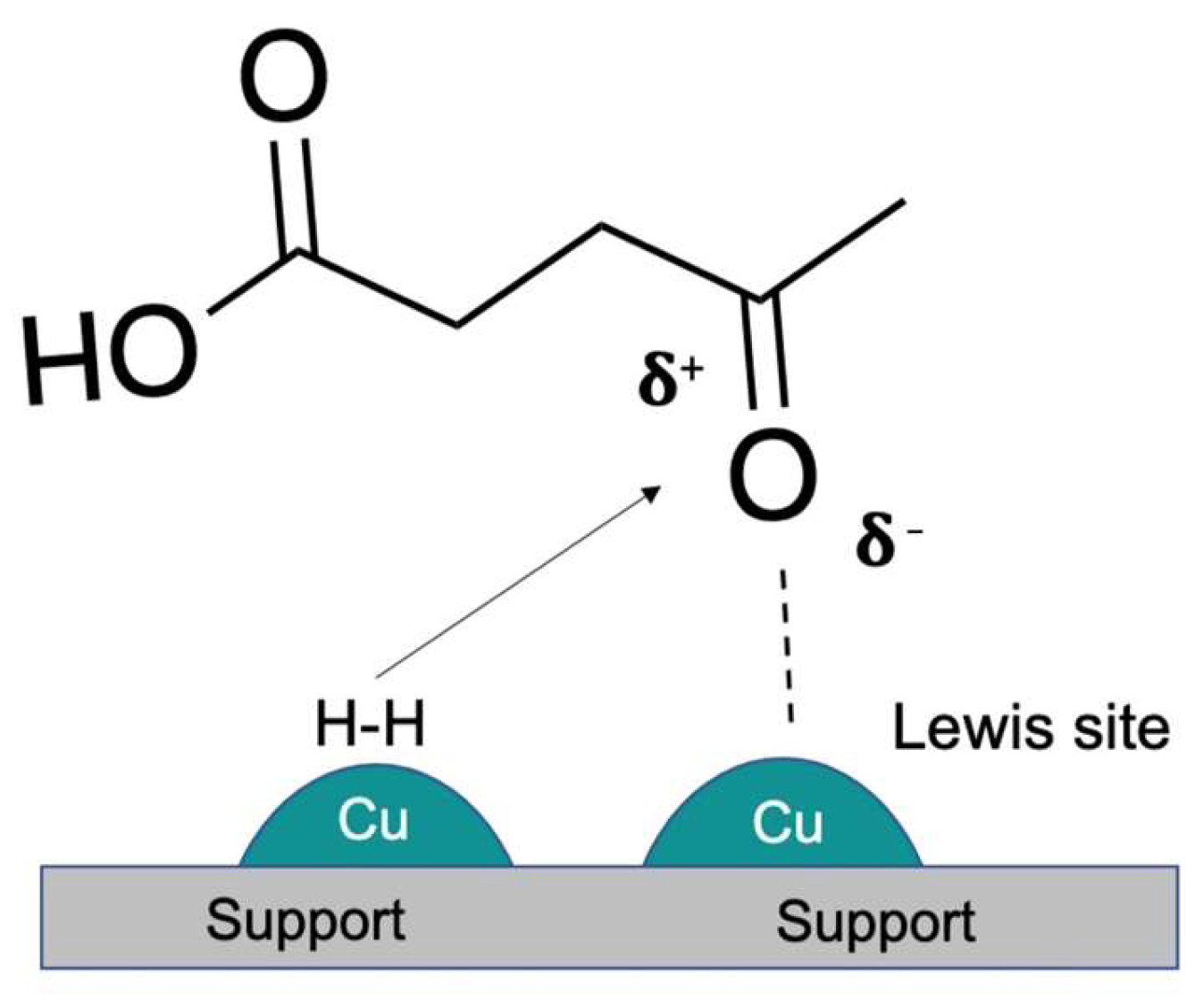
1.2. General Considerations about Copper Catalysts
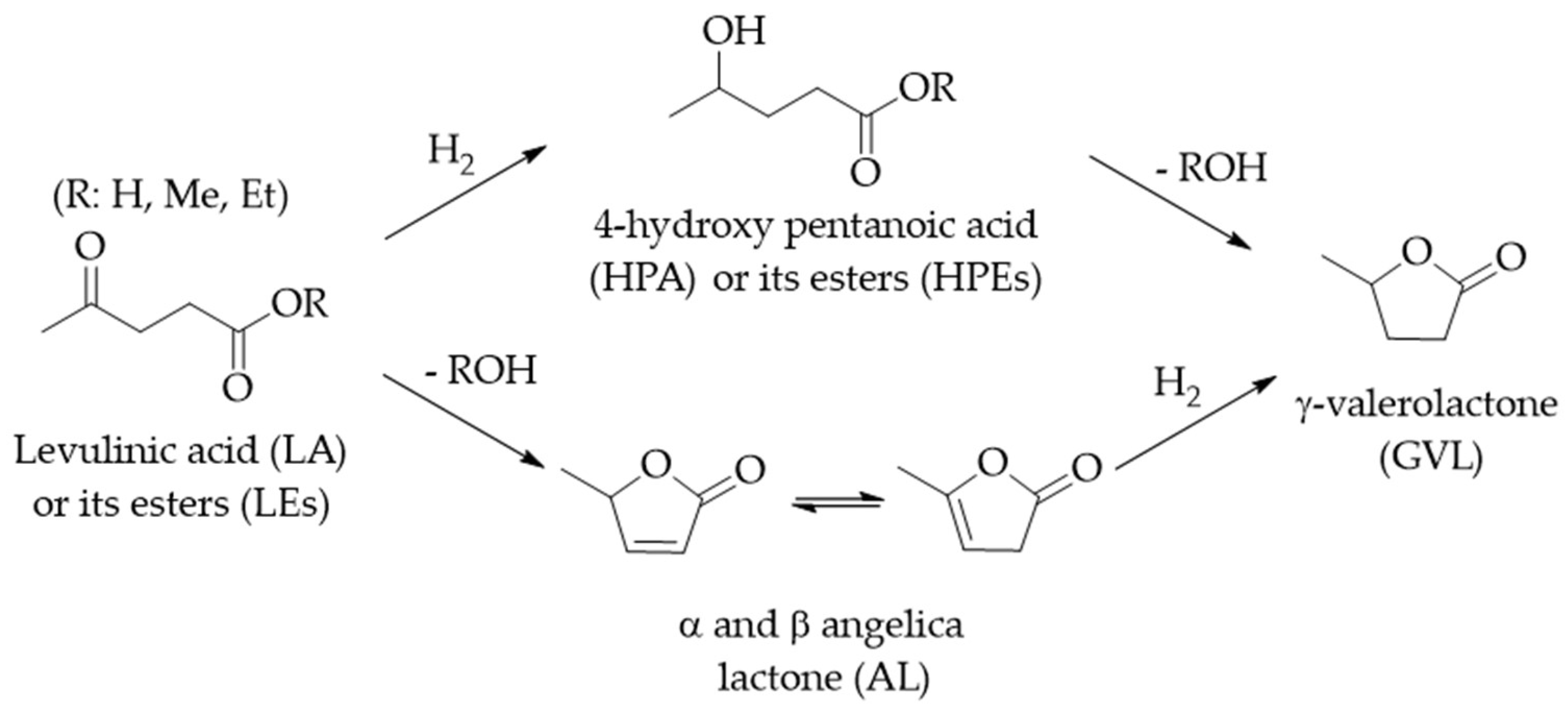
2. LA/LE Hydrogenation to GVL and Valerates
2.1. Hydrogenation of LA/LEs to GVL with Molecular Hydrogen
| Batch | ||||
|---|---|---|---|---|
| Catalyst | Preparation Procedure | Reaction Conditions | Catalytic Performances (%) | Ref. |
| Cu/ZrO2 | CP | LA/LEs, H2O or MeOH, molLA/gcat = 0.086 mol g−1 in H2O, molLE/gcat = 0.069 mol g−1 in MeOH, molLA/gcat = 0.086 mol g−1 in MeOH, 200 °C, 5 h, P(H2) = 35 bar, batch | C = 100 S > 90 | [5] |
| Cu-Ni/γAl2O3 | WI SG | LA, H2O, molLA/gcat = 0.086 mol g−1, 250 °C, 6 h, P(H2) = 65 bar, batch | C = 100 S = 96 | [87] |
| (1) Cu-WO3/ZrO2 (2) Cu/ZrO2 | CP OG | LA, (1) EtOH or (2) H2O, molLA/gcat = 0.022 mol g−1, 200 °C, 6 h, P(H2) = 50 bar, batch | C = 100 (1) S = 94 (2) S = 99 | [84] |
| Cu/ZrO2 | CP | LA, H2O, molLA/gcat = 0.086 mol g−1, 200 °C, 2 h, P(H2) = 35 bar, batch. | C = 100 S = 80 | [76] |
| Cu/ZrO2 | OG DP MT | LA, H2O, molLA/gcat = 0.086 mol g−1, 200 °C, 1 h, P(H2) = 35 bar, batch | C = 90 S = 100 | [77] |
| Cu-Ag/Al2O3 | WI | LA, THF, molLA/gcat = 0.017 mol g−1, 180 °C, 1 h, P(H2) = 14 bar, batch | C = 100 S = 100 | [11] |
| Cu-Ni organosilica nanospheres | ST WI CP | LA, 2-PrOH, molLA/gcat = 0.031 mol g−1, 120 °C, 13 h, P(H2) = 40 bar, batch | C = 99 S = 97 | [70] |
| Cu-Al2O3 | CP | LA, EtOH, molLA/gcat = 0.0086 mol g−1, 110 °C, 2 h, P(H2) = 30 bar, batch | C = 100 S = 95 | [71] |
| Al2O3-Cu/ZrO2 | CP | LA, H2O, molLA/gcat = 0.086 mol g−1, 200 °C, 2 h, P(H2) = 30 bar, batch | C = 100 S = 100 | [82] |
| Cu-Ni/γ-Al2O3 | WI | LA, solvent-free, molLA/gcat = 0.196 mol g−1, 220 °C, 6 h, P(H2) = 30 bar, batch | C = 100 S = 99 | [86] |
| Cu-Ni/Al2O3 | CI | LEs, n-HEX, molLE/gcat = 0.069 mol g−1, 180 °C, 6 h, P(H2) = 25 bar, batch | C = 100 S = 98 | [61] |
| B2O3-Cu/ZrO2 | CP for Cu/ZrO2, then boric acid | LA, H2O, molLA/gcat = 0.069 mol g−1, 150 °C, 5 h, P(H2) = 30 bar, batch | C = 100 S = 100 | [30] |
| Flow | ||||
| Catalyst | Preparation Procedure | Reaction Conditions | Catalytic Performances (%) | Ref. |
| Cu/γAl2O3 | WI | LA, H2O, 265 °C, P(H2) = 1 bar, H2 = 30 mL min−1, WHSV = 0.169 h−1, H2/LA molar ratio = 201, flow | C = 98 S = 87 | [74] |
| Cu/ZrO2 | WI | LA, H2O, 265 °C, P(H2) = 1 bar, H2 = 30 mL min−1, WHSV = 0.169 h−1, H2/LA molar ratio = 201, flow | C = 81 S = 83 | [73] |
| Cu/Al2O3-SiO2 | CP | LEs, EtOH, 140 °C, 1000 h, P(H2) = 30 bar, WHSV = 0.6 h−1, H2/LE molar ratio = 50, flow | C = 79 S = 96 | [72] |
| Cu-Ni/SiO2 | WI with citric acid | LA, solvent-free, 250 °C, P(H2) = 1 bar, H2 = 30 mL min−1, WHSV = 13.2 h−1, H2/LA molar ratio = 6, flow | C = 98 S = 98 | [68] |
| Cu-Ni/KIT6/ ZSM-5 | WI | LA, 250 °C, P(H2) = 1 bar, H2 = 30 mL min−1, time on stream = 24 h, H2/LA molar ratio = 0.0024, flow | C = 100 S = 80 | [75] |
| B2O3-Cu/ZrO2 | CP for Cu/ZrO2, then boric acid | LA, H2O, 200 °C, P(H2) = 30 bar, H2 = 40 mL min−1, H2/LA molar ratio = 2711, flow | C = 100 S = 100 | [30] |
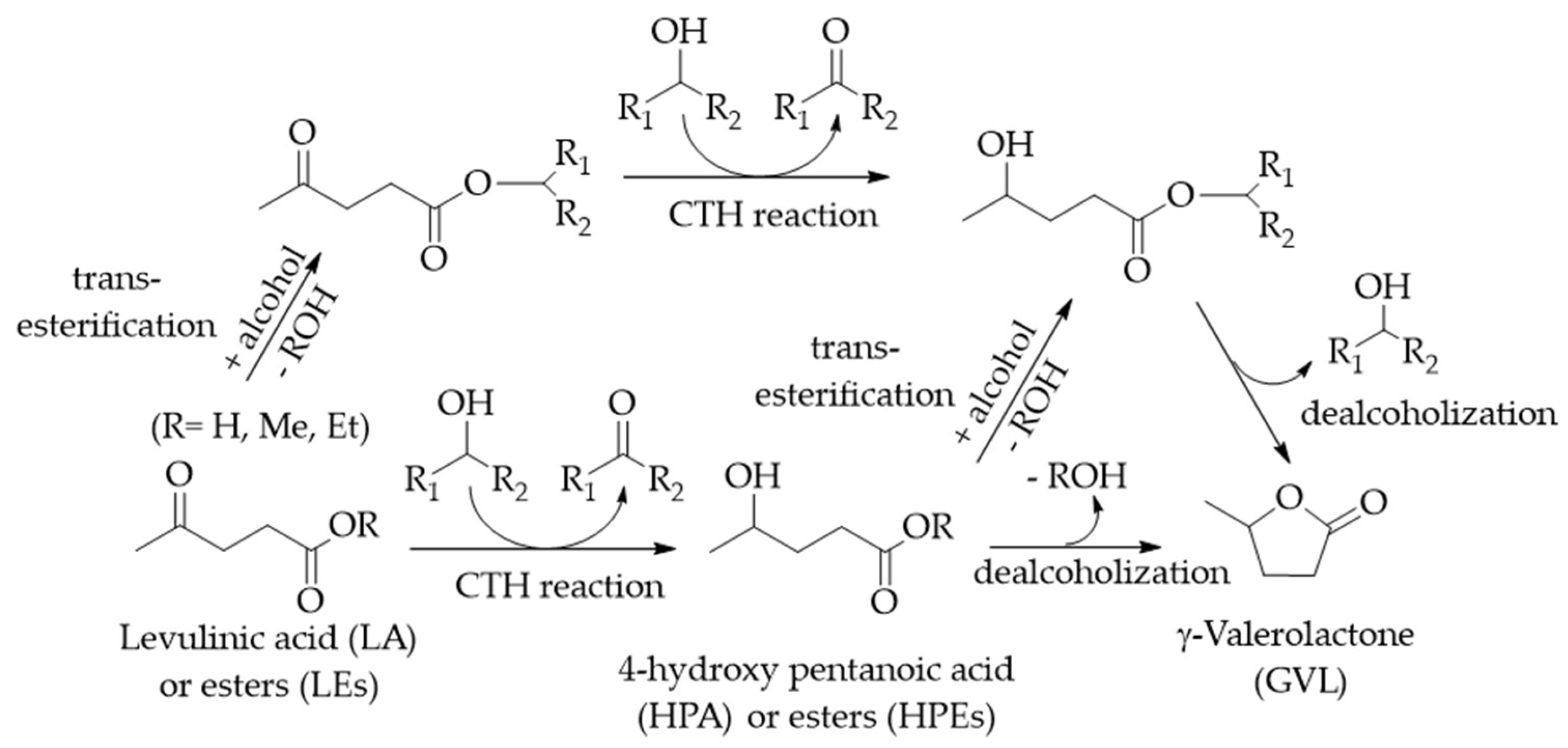
2.2. Catalytic Transfer Hydrogenation (CTH) of LA/LEs to GVL
2.3. Transformation of LA/LEs and GVL to VE
3. Hydrogenation of LA/LEs and GVL to Give 1,4-PDO
4. Hydrogenation of LA/LEs and GVL to Give 2-MTHF
5. Conclusions and Future Directions
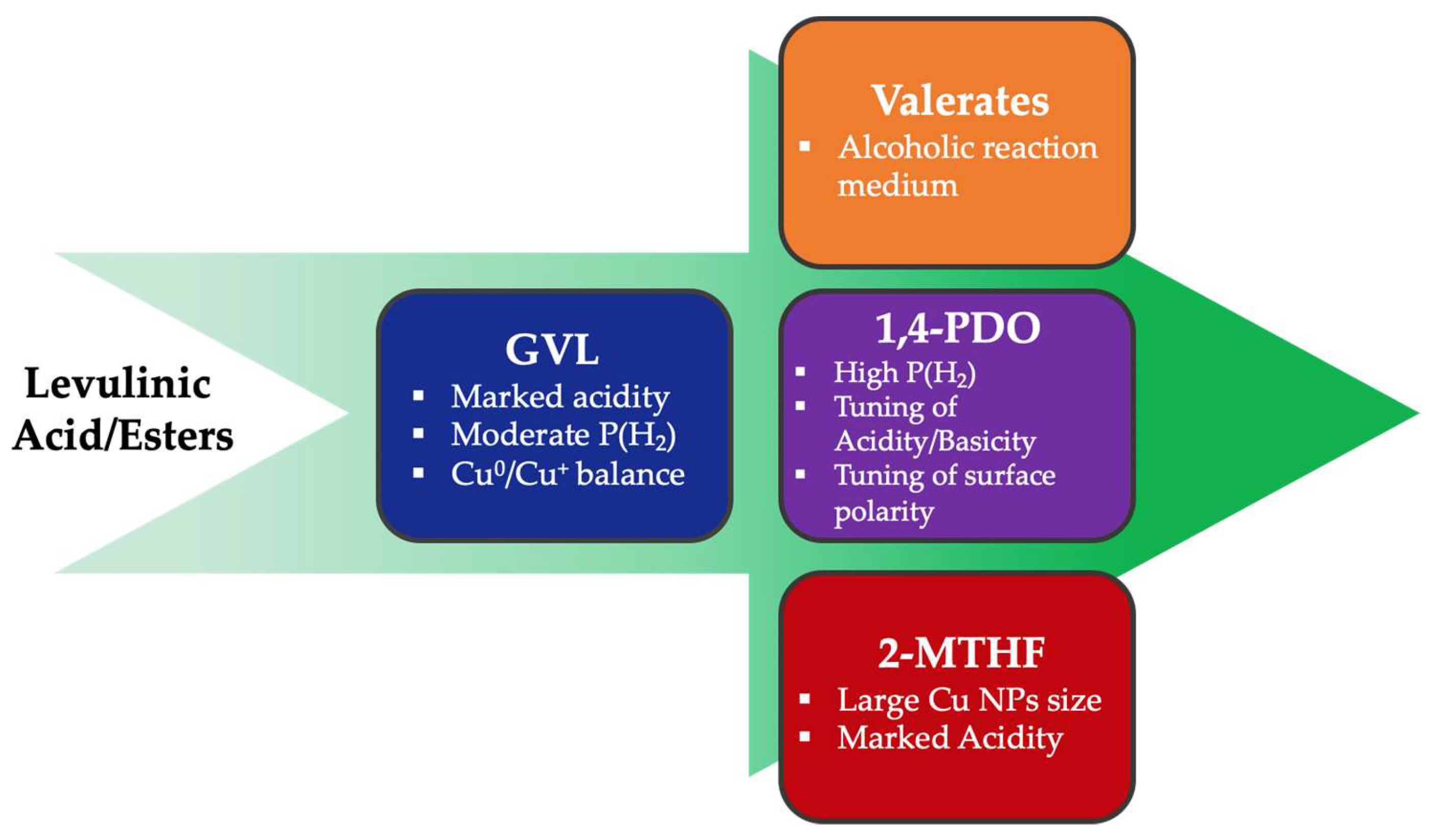
5.1. General Strategies to Selectively Obtain the Desired Product
- -
- GVL and valerates are preferentially obtained when a catalyst with a pronounced acidic character is used. Clearly, the presence of an alcoholic medium is required for the preparation of valerates.
- -
- By tuning the acid/base properties, the reaction is more prone to proceed through the ring opening, leading to the formation of 1,4-PDO [71]. 1,4-PDO dehydration can be avoided by limiting catalyst acidity or polarity. High H2 pressure is usually needed to reach high yields.
- -
- The yield in 2-MTHF can be optimized by increasing the acidity of the catalyst and the copper particle size.
5.2. Catalyst Deactivation and Stability Considerations
5.3. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Pileidis, F.D.; Titirici, M.-M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic Acid Production from Renewable Waste Resources: Bottlenecks, Potential Remedies, Advancements and Applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- De Vries, J.G. Industrial Implementation of Chemical Biomass Conversion. Curr. Opin. Green Sustain. Chem. 2023, 39, 100715. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.-B.; Guo, Q.-X.; Fu, Y. RANEY® Ni Catalyzed Transfer Hydrogenation of Levulinate Esters to γ-Valerolactone at Room Temperature. Chem. Commun. 2013, 49, 5328–5330. [Google Scholar] [CrossRef]
- Hengne, A.M.; Rode, C.V. Cu-ZrO2 Nanocomposite Catalyst for Selective Hydrogenation of Levulinic Acid and Its Ester to γ-Valerolactone. Green Chem. 2012, 14, 1064–1072. [Google Scholar] [CrossRef]
- Di Menno Di Bucchianico, D.; Wang, Y.; Buvat, J.-C.; Pan, Y.; Casson Moreno, V.; Leveneur, S. Production of Levulinic Acid and Alkyl Levulinates: A Process Insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Ahmad, E.; Alam, M.D.I.; Pant, K.K.; Haider, M.A. Catalytic and Mechanistic Insights into the Production of Ethyl Levulinate from Biorenewable Feedstocks. Green Chem. 2016, 18, 4804–4823. [Google Scholar] [CrossRef]
- Lange, J.-P.; van de Graaf, W.D.; Haan, R.J. Conversion of Furfuryl Alcohol into Ethyl Levulinate Using Solid Acid Catalysts. ChemSusChem 2009, 2, 437–441. [Google Scholar] [CrossRef]
- Tiwari, M.S.; Gawade, A.B.; Yadav, G.D. Magnetically Separable Sulfated Zirconia as Highly Active Acidic Catalysts for Selective Synthesis of Ethyl Levulinate from Furfuryl Alcohol. Green Chem. 2017, 19, 963–976. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Brønsted and Lewis Acid Sites for the Production of γ-Valerolactone from Furfural. Angew. Chem. Int. Ed. 2013, 52, 8022–8025. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, J.; Li, S.; Yin, J.; Sun, X.; Guo, X.; Song, C.; Zhou, J. Hydrogenation of Levulinic Acid into Gamma-Valerolactone over in Situ Reduced CuAg Bimetallic Catalyst: Strategy and Mechanism of Preventing Cu Leaching. Appl. Catal. B 2018, 232, 1–10. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, Q.; Wang, J.; Mu, T. Valorization of Levulinic Acid over Non-Noble Metal Catalysts: Challenges and Opportunities. Green Chem. 2018, 20, 4391–4408. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Raspolli Galletti, A.M. New Frontiers in the Catalytic Synthesis of Levulinic Acid: From Sugars to Raw and Waste Biomass as Starting Feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Melchiorre, M.; Lentini, D.; Cucciolito, M.E.; Taddeo, F.; Hmoudah, M.; di Serio, M.; Ruffo, F.; Russo, V.; Esposito, R. Sustainable Ketalization of Glycerol with Ethyl Levulinate Catalyzed by the Iron (III)-Based Metal-Organic Framework MIL-88A. Molecules 2022, 27, 7229. [Google Scholar] [CrossRef]
- Zaccheria, F.; Ravasio, N.; Ercoli, M.; Allegrini, P. Heterogeneous Cu-Catalysts for the Reductive Deoxygenation of Aromatic Ketones without Additives. Tetrahedron Lett. 2005, 46, 7743–7745. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Methanol Economy and Net Zero Emissions: Critical Analysis of Catalytic Processes, Reactors and Technologies. Green Chem. 2021, 23, 8361–8405. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.; Ding, M.; Zhao, J. Heterogeneous Cu Catalyst in Organic Transformations. Nano Res. 2022, 15, 2810–2833. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y. Copper-Based Non-Precious Metal Heterogeneous Catalysts for Environmental Remediation. Chin. J. Catal. 2018, 39, 566–582. [Google Scholar] [CrossRef]
- Zaccheria, F.; Ravasio, N.; Chan-Thaw, C.E.; Scotti, N.; Bondioli, P. A Bifunctional Copper Catalyst for the One Pot-One Step Esterification + Hydrogenation of Tall Oil Fatty Acids. Top. Catal. 2012, 55, 631–636. [Google Scholar] [CrossRef]
- Zaccheria, F.; Shaikh, N.I.; Scotti, N.; Psaro, R.; Ravasio, N. New Concepts in Solid Acid Catalysis: Some Opportunities Offered by Dispersed Copper Oxide. Top. Catal. 2014, 57, 1085–1093. [Google Scholar] [CrossRef]
- Zaccheria, F.; Mariani, M.; Scotti, N.; Psaro, R.; Ravasio, N. Catalytic Upgrading of Lactose: A Rest Raw Material from the Dairy Industry. Green Chem. 2017, 19, 1904–1910. [Google Scholar] [CrossRef]
- Cavuoto, D.; Ravasio, N.; Scotti, N.; Gervasini, A.; Campisi, S.; Marelli, M.; Cappelletti, G.; Zaccheria, F. A Green Solvent Diverts the Hydrogenation of γ–Valerolactone to 1,4—Pentandiol over Cu/SiO2. Mol. Catal. 2021, 516, 111936. [Google Scholar] [CrossRef]
- Lange, J.-P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Mui, Y.F.; Lo, S.W.; Lui, M.Y.; Akien, G.R.; Horváth, I.T. Catalytic Conversion of Fructose, Glucose, and Sucrose to 5-(Hydroxymethyl)Furfural and Levulinic and Formic Acids in γ-Valerolactone as a Green Solvent. ACS Catal. 2014, 4, 1470–1477. [Google Scholar] [CrossRef]
- Du, X.-L.; Bi, Q.-Y.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Tunable Copper-Catalyzed Chemoselective Hydrogenolysis of Biomass-Derived γ-Valerolactone into 1,4-Pentanediol or 2-Methyltetrahydrofuran. Green Chem. 2012, 14, 935–939. [Google Scholar] [CrossRef]
- Lange, J.-P.; Vestering, J.Z.; Haan, R.J. Towards ‘Bio-Based’ Nylon: Conversion of γ-Valerolactone to Methyl Pentenoate under Catalytic Distillation Conditions. Chem. Commun. 2007, 33, 3488–3490. [Google Scholar] [CrossRef] [PubMed]
- Weng, R.; Yu, Z.; Xiong, J.; Lu, X. Effects of water in the heterogeneous catalytic valorization of levulinic acid into γ-valerolactone and its derivatives. Green Chem. 2020, 22, 3013–3027. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The Physical Chemistry and Materials Science behind Sinter-Resistant Catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhao, L.; Li, J.; Li, M.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Highly Selective Synthesis of γ-Valerolactone from Levulinic Acid at Mild Conditions Catalyzed by Boron Oxide Doped Cu/ZrO2 Catalysts. Appl. Catal. A Gen. 2019, 587, 117244. [Google Scholar] [CrossRef]
- Adkins, H.; Folkers, K. The Catalytic Hydrogenation of Esters to Alcohols. J. Am. Chem. Soc. 1931, 53, 1095–1097. [Google Scholar] [CrossRef]
- Folkers, K.; Adkins, H. The Catalytic Hydrogenation of Esters to Alcohols. II. J. Am. Chem. Soc. 1932, 54, 1145–1154. [Google Scholar] [CrossRef]
- Christian, R.V., Jr.; Brown, H.D.; Hixon, R.M. Derivatives of γ-Valerolactone, 1,4-Pentanediol and 1,4-Di-(β-Cyanoethoxy)-Pentane1. J. Am. Chem. Soc. 1947, 69, 1961–1963. [Google Scholar] [CrossRef]
- Bonrath, W.; Castelijns, A.M.C.F.; de Vries, J.G.; Guit, R.P.M.; Schütz, J.; Sereinig, N.; Vaessen, H.W.L.M. Gas Phase Hydrogenation of Levulinic Acid to γ-Valerolactone. Catal. Lett. 2016, 146, 28–34. [Google Scholar] [CrossRef]
- Li, Z.; Zuo, M.; Jiang, Y.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Lin, L. Stable and Efficient CuCr Catalyst for the Solvent-Free Hydrogenation of Biomass Derived Ethyl Levulinate to γ-Valerolactone as Potential Biofuel Candidate. Fuel 2016, 175, 232–239. [Google Scholar] [CrossRef]
- Karam, L.; Neumann, C.N. Heterogeneously Catalyzed Carboxylic Acid Hydrogenation to Alcohols. ChemCatChem 2022, 14, e202200953. [Google Scholar] [CrossRef]
- Bond, J.Q.; Martin Alonso, D.; West, R.M.; Dumesic, J.A. γ-Valerolactone Ring-Opening and Decarboxylation over SiO2/Al2O3 in the Presence of Water. Langmuir 2010, 26, 16291–16298. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic Routes for the Conversion of Biomass into Liquid Hydrocarbon Transportation Fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic Reactions of Gamma-Valerolactone: A Platform to Fuels and Value-Added Chemicals. Appl. Catal. B 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Horváth, I.T. Solvents from Nature. Green Chem. 2008, 10, 1024–1028. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of γ-Valerolactone by Hydrogenation of Biomass-Derived Levulinic Acid over Ru/C Catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of γ-Valerolactone to Liquid Alkenes for Transportation Fuels. Science (1979) 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Fábos, V.; Lui, M.Y.; Mui, Y.F.; Wong, Y.Y.; Mika, L.T.; Qi, L.; Cséfalvay, E.; Kovács, V.; Szűcs, T.; Horváth, I.T. Use of Gamma-Valerolactone as an Illuminating Liquid and Lighter Fluid. ACS Sustain. Chem. Eng. 2015, 3, 1899–1904. [Google Scholar] [CrossRef]
- Kon, K.; Onodera, W.; Shimizu, K. Selective Hydrogenation of Levulinic Acid to Valeric Acid and Valeric Biofuels by a Pt/HMFI Catalyst. Catal. Sci. Technol. 2014, 4, 3227–3234. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Dzierbinski, A.; Palkovits, R. Solvent-Free γ-Valerolactone Hydrogenation to 2-Methyltetrahydrofuran Catalysed by Ru/C: A Reaction Network Analysis. Green Chem. 2014, 16, 1358–1364. [Google Scholar] [CrossRef]
- Mizugaki, T.; Nagatsu, Y.; Togo, K.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Selective Hydrogenation of Levulinic Acid to 1,4-Pentanediol in Water Using a Hydroxyapatite-Supported Pt–Mo Bimetallic Catalyst. Green Chem. 2015, 17, 5136–5139. [Google Scholar] [CrossRef]
- Li, M.; Li, G.; Li, N.; Wang, A.; Dong, W.; Wang, X.; Cong, Y. Aqueous Phase Hydrogenation of Levulinic Acid to 1,4-Pentanediol. Chem. Commun. 2014, 50, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Synthesis of γ-Valerolactone from Carbohydrates and Its Applications. ChemSusChem 2016, 9, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Goh, T.-W.; Qi, Z.; Goes, S.; Brashler, K.; Perez, C.; Huang, W. Conversion of Levulinic Acid to γ-Valerolactone over Few-Layer Graphene-Supported Ruthenium Catalysts. ACS Catal. 2016, 6, 593–599. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Jarvis, C.; Wu, G. Clean and Selective Production of γ-Valerolactone from Biomass-Derived Levulinic Acid Catalyzed by Recyclable Pd Nanoparticle Catalyst. J. Clean Prod. 2014, 72, 230–232. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, J.; Cao, Y.; Fan, K. Catalytic Conversion of Biomass-Derived Levulinic Acid into γ-Valerolactone Using Iridium Nanoparticles Supported on Carbon Nanotubes. Chin. J. Catal. 2013, 34, 993–1001. [Google Scholar] [CrossRef]
- Sun, P.; Gao, G.; Zhao, Z.; Xia, C.; Li, F. Stabilization of Cobalt Catalysts by Embedment for Efficient Production of Valeric Biofuel. ACS Catal. 2014, 4, 4136–4142. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic Synthesis of α-Methylene-γ-Valerolactone: A Biomass-Derived Acrylic Monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Primo, A.; Concepción, P.; Corma, A. Synergy between the Metal Nanoparticles and the Support for the Hydrogenation of Functionalized Carboxylic Acids to Diols on Ru/TiO2. Chem. Commun. 2011, 47, 3613–3615. [Google Scholar] [CrossRef]
- Tan, J.; Cui, J.; Ding, G.; Deng, T.; Zhu, Y.; Li, Y. Efficient Aqueous Hydrogenation of Levulinic Acid to γ-Valerolactone over a Highly Active and Stable Ruthenium Catalyst. Catal. Sci. Technol. 2016, 6, 1469–1475. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Zhang, X.; Zhang, Q.; Li, Y.; Wang, C.; Ma, L. Conversion of Levulinic Acid to γ-Valerolactone over Ru/Al2O3–TiO2 Catalyst under Mild Conditions. RSC Adv. 2018, 8, 40989–40995. [Google Scholar] [CrossRef]
- Almeida, L.D.; Rocha, A.L.A.; Rodrigues, T.S.; Robles-Azocar, P.A. Highly Selective Hydrogenation of Levulinic Acid Catalyzed by Ru on TiO2-SiO2 Hybrid Support. Catal. Today 2020, 344, 158–165. [Google Scholar] [CrossRef]
- Ruiz-Bernal, Z.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. Ru Catalysts Supported on Commercial and Biomass-Derived Activated Carbons for the Transformation of Levulinic Acid into γ-Valerolactone under Mild Conditions. Catalysts 2021, 11, 559. [Google Scholar] [CrossRef]
- Gupta, S.S.R.; Kantam, M.L. Selective Hydrogenation of Levulinic Acid into γ-Valerolactone over Cu/Ni Hydrotalcite-Derived Catalyst. Catal. Today 2018, 309, 189–194. [Google Scholar] [CrossRef]
- Li, Y.; Lan, X.; Liu, B.; Wang, T. Synthesis of γ-Valerolactone from Ethyl Levulinate Hydrogenation and Ethyl 4-Hydroxypentanoate Lactonization over Supported Cu-Ni Bimetallic, Bifunctional Catalysts. J. Ind. Eng. Chem. 2022, 107, 215–223. [Google Scholar] [CrossRef]
- Clough, S.R. Hexane. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 900–904. ISBN 978-0-12-386455-0. [Google Scholar]
- Chang, Y.C. Neurotoxic Effects of N-Hexane on the Human Central Nervous System: Evoked Potential Abnormalities in n-Hexane Polyneuropathy. J. Neurol. Neurosurg. Psychiatry 1987, 50, 269–274. [Google Scholar] [CrossRef]
- Chhabra, R.S.; Herbert, R.A.; Roycroft, J.H.; Chou, B.; Miller, R.A.; Renne, R.A. Carcinogenesis Studies of Tetrahydrofuran Vapors in Rats and Mice. Toxicol. Sci. 1998, 41, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Saito, T.; Otsuka, S.; Ozawa, T.; Yamada, Y.; Sato, S. Selective Hydrogenation of γ-Valerolactone to 2-Methyltetrahydrofuran over Cu/Al2O3 Catalyst. Appl. Catal. A Gen. 2020, 590, 117309. [Google Scholar] [CrossRef]
- Bijoy, R.; Agarwala, P.; Roy, L.; Thorat, B.N. Unconventional Ethereal Solvents in Organic Chemistry: A Perspective on Applications of 2-Methyltetrahydrofuran, Cyclopentyl Methyl Ether, and 4-Methyltetrahydropyran. Org. Process Res. Dev. 2022, 26, 480–492. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green Synthesis of Gamma-Valerolactone (GVL) through Hydrogenation of Biomass-Derived Levulinic Acid Using Non-Noble Metal Catalysts: A Critical Review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Yoshida, R.; Sun, D.; Yamada, Y.; Sato, S. Stable Cu-Ni/SiO2 Catalysts Prepared by Using Citric Acid-Assisted Impregnation for Vapor-Phase Hydrogenation of Levulinic Acid. Mol. Catal. 2018, 454, 70–76. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green Solvents for Sustainable Organic Synthesis: State of the Art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Pendem, S.; Mondal, I.; Shrotri, A.; Rao, B.S.; Lingaiah, N.; Mondal, J. Unraveling the Structural Properties and Reactivity Trends of Cu–Ni Bimetallic Nanoalloy Catalysts for Biomass-Derived Levulinic Acid Hydrogenation. Sustain. Energy Fuels 2018, 2, 1516–1529. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, K.; Li, Q.; Liu, Q.; Zhang, S.; Liu, Q.; Hu, G.; Hu, X. Copper-Based Catalysts with Tunable Acidic and Basic Sites for the Selective Conversion of Levulinic Acid/Ester to γ-Valerolactone or 1,4-Pentanediol. Green Chem. 2019, 21, 4499–4511. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, J.; Xu, X.; Wang, W.; Li, J.; Zhao, Y.; Tang, K.; Song, Q.; Qi, X.; Kong, D.; et al. Continuous Hydrogenation of Ethyl Levulinate to γ-Valerolactone and 2-Methyl Tetrahydrofuran over Alumina Doped Cu/SiO2 Catalyst: The Potential of Commercialization. Sci. Rep. 2016, 6, 28898. [Google Scholar] [CrossRef] [PubMed]
- Balla, P.; Perupogu, V.; Vanama, P.K.; Komandur, V.R.C. Hydrogenation of Biomass-Derived Levulinic Acid to γ-Valerolactone over Copper Catalysts Supported on ZrO2. J. Chem. Technol. Biotechnol. 2016, 91, 769–776. [Google Scholar] [CrossRef]
- Putrakumar, B.; Nagaraju, N.; Kumar, V.P.; Chary, K.V.R. Hydrogenation of Levulinic Acid to γ-Valerolactone over Copper Catalysts Supported on γ-Al2O3. Catal. Today 2015, 250, 209–217. [Google Scholar] [CrossRef]
- Popova, M.; Trendafilova, I.; Oykova, M.; Mitrev, Y.; Shestakova, P.; Mihályi, M.R.; Szegedi, Á. Hydrodeoxygenation of Levulinic Acid to γ-Valerolactone over Mesoporous Silica-Supported Cu-Ni Composite Catalysts. Molecules 2022, 27, 5383. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Iqbal, S.; Ishikawa, S.; Reece, C.; Thomas, L.M.; Miedziak, P.J.; Morgan, D.J.; Edwards, J.K.; Bartley, J.K.; Willock, D.J.; et al. The Conversion of Levulinic Acid into γ-Valerolactone Using Cu–ZrO2 Catalysts. Catal. Sci. Technol. 2016, 6, 6022–6030. [Google Scholar] [CrossRef]
- Ishikawa, S.; Jones, D.R.; Iqbal, S.; Reece, C.; Morgan, D.J.; Willock, D.J.; Miedziak, P.J.; Bartley, J.K.; Edwards, J.K.; Murayama, T.; et al. Identification of the Catalytically Active Component of Cu–Zr–O Catalyst for the Hydrogenation of Levulinic Acid to γ-Valerolactone. Green Chem. 2017, 19, 225–236. [Google Scholar] [CrossRef]
- Yuan, J.; Li, S.-S.; Yu, L.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Copper-Based Catalysts for the Efficient Conversion of Carbohydrate Biomass into γ-Valerolactone in the Absence of Externally Added Hydrogen. Energy Environ. Sci. 2013, 6, 3308–3313. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0–Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, X.; Zhu, S.; Dong, F.; Zheng, H.; Zhu, Y.; Li, Y.-W. Construction of Cu/ZrO2/Al2O3 Composites for Ethanol Synthesis: Synergies of Ternary Sites for Cascade Reaction. Appl. Catal. B 2015, 166–167, 551–559. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of Boric Oxide Doping on the Stability and Activity of a Cu–SiO2 Catalyst for Vapor-Phase Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- He, D.; He, Q.; Jiang, P.; Zhou, G.; Hu, R.; Fu, W. Novel Cu/Al2O3-ZrO2 Composite for Selective Hydrogenation of Levulinic Acid to γ-Valerolactone. Catal. Commun. 2019, 125, 82–86. [Google Scholar] [CrossRef]
- Fang, C.; Kuboon, S.; Khemthong, P.; Butburee, T.; Chakthranont, P.; Itthibenchapong, V.; Kasamechonchung, P.; Witoon, T.; Faungnawakij, K. Highly Dispersed NiCu Nanoparticles on SBA-15 for Selective Hydrogenation of Methyl Levulinate to γ-Valerolactone. Int. J. Hydrogen Energy 2020, 45, 24054–24065. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Pan, T.; Yu, C.; Deng, J.; Guo, Q.; Fu, Y. Supported Copper Catalysts for Highly Efficient Hydrogenation of Biomass-Derived Levulinic Acid and γ-Valerolactone. Green Chem. 2016, 18, 1287–1294. [Google Scholar] [CrossRef]
- Scotti, N.; Bossola, F.; Zaccheria, F.; Ravasio, N. Copper–Zirconia Catalysts: Powerful Multifunctional Catalytic Tools to Approach Sustainable Processes. Catalysts 2020, 10, 168. [Google Scholar] [CrossRef]
- Gebresillase, M.N.; Raguindin, R.Q.; Kim, H.; Seo, J.G. Supported Bimetallic Catalysts for the Solvent-Free Hydrogenation of Levulinic Acid to γ-Valerolactone: Effect of Metal Combination (Ni-Cu, Ni-Co, Cu-Co). Catalysts 2020, 10, 1354. [Google Scholar] [CrossRef]
- Obregón, I.; Corro, E.; Izquierdo, U.; Requies, J.; Arias, P.L. Levulinic Acid Hydrogenolysis on Al2O3-Based Ni-Cu Bimetallic Catalysts. Chin. J. Catal. 2014, 35, 656–662. [Google Scholar] [CrossRef]
- Scotti, N.; Zaccheria, F.; Evangelisti, C.; Psaro, R.; Ravasio, N. Dehydrogenative Coupling Promoted by Copper Catalysts: A Way to Optimise and Upgrade Bio-Alcohols. Catal. Sci. Technol. 2017, 7, 1386–1393. [Google Scholar] [CrossRef]
- Armelao, L.; Bertagnolli, H.; Bleiner, D.; Groenewolt, M.; Gross, S.; Krishnan, V.; Sada, C.; Schubert, U.; Tondello, E.; Zattin, A. Highly Dispersed Mixed Zirconia and Hafnia Nanoparticles in a Silica Matrix: First Example of a ZrO2–HfO2–SiO2 Ternary Oxide System. Adv. Funct. Mater. 2007, 17, 1671–1681. [Google Scholar] [CrossRef]
- Sulym, I.; Sternik, D.; Oleksenko, L.; Lutsenko, L.; Borysenko, M.; Derylo-Marczewska, A. Highly Dispersed Silica-Supported Ceria–Zirconia Nanocomposites: Preparation and Characterization. Surf. Interfaces 2016, 5, 8–14. [Google Scholar] [CrossRef]
- Zaccheria, F.; Bossola, F.; Scotti, N.; Evangelisti, C.; Dal Santo, V.; Ravasio, N. On Demand Production of Ethers or Alcohols from Furfural and HMF by Selecting the Composition of a Zr/Si Catalyst. Catal. Sci. Technol. 2020, 10, 7502–7511. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the Role of Low Coordination Sites in a Cu Metal Nanoparticle: A Step toward the Selective Synthesis of Second Generation Biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Liu, S.; Fan, G.; Yang, L.; Li, F. Highly Efficient Transformation of γ-Valerolactone to Valerate Esters over Structure-Controlled Copper/Zirconia Catalysts Prepared via a Reduction-Oxidation Route. Appl. Catal. A Gen. 2017, 543, 180–188. [Google Scholar] [CrossRef]
- Cai, B.; Zhou, X.-C.; Miao, Y.-C.; Luo, J.-Y.; Pan, H.; Huang, Y.-B. Enhanced Catalytic Transfer Hydrogenation of Ethyl Levulinate to γ-Valerolactone over a Robust Cu–Ni Bimetallic Catalyst. ACS Sustain Chem. Eng. 2017, 5, 1322–1331. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary Concept of Nickel Toxicity—An Overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef] [PubMed]
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic Transfer Hydrogenolysis as an Effective Tool for the Reductive Upgrading of Cellulose, Hemicellulose, Lignin, and Their Derived Molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Lu, Y.-M.; Liu, Y.-X.; Wu, Z.-B.; Hu, D.-Y.; Yang, S. Cascade Catalytic Transfer Hydrogenation–Cyclization of Ethyl Levulinate to γ-Valerolactone with Al–Zr Mixed Oxides. Appl. Catal. A Gen. 2016, 510, 11–19. [Google Scholar] [CrossRef]
- Tang, X.; Chen, H.; Hu, L.; Hao, W.; Sun, Y.; Zeng, X.; Lin, L.; Liu, S. Conversion of Biomass to γ-Valerolactone by Catalytic Transfer Hydrogenation of Ethyl Levulinate over Metal Hydroxides. Appl. Catal. B 2014, 147, 827–834. [Google Scholar] [CrossRef]
- Du, X.-L.; He, L.; Zhao, S.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Hydrogen-Independent Reductive Transformation of Carbohydrate Biomass into γ-Valerolactone and Pyrrolidone Derivatives with Supported Gold Catalysts. Angew. Chem. Int. Ed. 2011, 50, 7815–7819. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Blair Vásquez, P.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer Hydrogenation of Methyl and Ethyl Levulinate Promoted by a ZrO2 Catalyst: Comparison of Batch vs Continuous Gas-Flow Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-Valerolactone from Lignocellulosic Biomass for Sustainable Fuels and Chemicals Supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Komanoya, T.; Nakajima, K.; Kitano, M.; Hara, M. Synergistic Catalysis by Lewis Acid and Base Sites on ZrO2 for Meerwein–Ponndorf–Verley Reduction. J. Phys. Chem. C 2015, 119, 26540–26546. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Osada, Y.; Fujitani, T.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over ZrO2 Catalyst Supported on SBA-15 Silica. Catal. Today 2017, 281, 418–428. [Google Scholar] [CrossRef]
- Campbell, E.J.; Zhou, H.; Nguyen, S.T. Catalytic Meerwein−Pondorf−Verley Reduction by Simple Aluminum Complexes. Org. Lett. 2001, 3, 2391–2393. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Dumesic, J.A. Exploring Meerwein–Ponndorf–Verley Reduction Chemistry for Biomass Catalysis Using a First-Principles Approach. ACS Catal. 2013, 3, 2694–2704. [Google Scholar] [CrossRef]
- Zhang, M.; Li, R.; Wu, Y.; Yu, Y. Mechanistic Insights into the Meerwein–Ponndorf–Verley Reaction and Relative Side Reactions over MgO in the Process of Ethanol to 1,3-Butadiene: A DFT Study. Ind. Eng. Chem. Res. 2021, 60, 2871–2880. [Google Scholar] [CrossRef]
- Douthwaite, M.; Zhang, B.; Iqbal, S.; Miedziak, P.J.; Bartley, J.K.; Willock, D.J.; Hutchings, G.J. Transfer Hydrogenation of Methyl Levulinate with Methanol to Gamma Valerolactone over Cu-ZrO2: A Sustainable Approach to Liquid Fuels. Catal. Commun. 2022, 164, 106430. [Google Scholar] [CrossRef]
- Shimizu, K. Heterogeneous Catalysis for the Direct Synthesis of Chemicals by Borrowing Hydrogen Methodology. Catal. Sci. Technol. 2015, 5, 1412–1427. [Google Scholar] [CrossRef]
- de Graauw, J.A.; van Bekkum, H.; Huskens, J.C.F.P. Meerwein-Ponndorf-Verley Reductions and Oppenauer Oxidations: An Integrated Approach. Synth. (Stuttg.) 1994, 1994, 1007–1017. [Google Scholar] [CrossRef]
- Wei, J.; Cao, X.; Wang, T.; Liu, H.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Liu, S.; Lin, L. Catalytic Transfer Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis(Hydroxymethyl)Furan over Tunable Zr-Based Bimetallic Catalysts. Catal. Sci. Technol. 2018, 8, 4474–4484. [Google Scholar] [CrossRef]
- Yu, N.; Lu, H.; Yang, W.; Zheng, Y.; Hu, Q.; Liu, Y.; Wu, K.; Liang, B. Transfer Hydrogenation of Levulinic Acid to γ-Valerolactone over Acid Site-Modified CuNi Alloy. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Lai, D.-M.; Fu, Y.; Guo, Q.-X. Catalytic Conversion of Biomass-Derived Carbohydrates into γ-Valerolactone without Using an External H2 Supply. Angew. Chem. Int. Ed. 2009, 48, 6529–6532. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, Y.; Li, J.; Fu, Y.; Liao, B.; Guo, Q.-X. Conversion of Levulinic Acid and Formic Acid into γ-Valerolactone over Heterogeneous Catalysts. ChemSusChem 2010, 3, 1172–1175. [Google Scholar] [CrossRef]
- Scotti, N.; Zaccheria, F.; Bisio, C.; Vittoni, C.; Ravasio, N. Switching Selectivity in the Hydrogen Transfer Reduction of Furfural. ChemistrySelect 2018, 3, 8344–8348. [Google Scholar] [CrossRef]
- Wang, J.; Jaenicke, S.; Chuah, G.-K. Zirconium–Beta Zeolite as a Robust Catalyst for the Transformation of Levulinic Acid to γ-Valerolactone via Meerwein–Ponndorf–Verley Reduction. RSC Adv. 2014, 4, 13481–13489. [Google Scholar] [CrossRef]
- Hengne, A.M.; Kadu, B.S.; Biradar, N.S.; Chikate, R.C.; Rode, C.V. Transfer Hydrogenation of Biomass-Derived Levulinic Acid to γ-Valerolactone over Supported Ni Catalysts. RSC Adv. 2016, 6, 59753–59761. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Li, W.; Jiang, Y.; Hu, L.; Liu, S.; Sun, Y.; Lin, L. In Situ Generated Catalyst System to Convert Biomass-Derived Levulinic Acid to γ-Valerolactone. ChemCatChem 2015, 7, 1372–1379. [Google Scholar] [CrossRef]
- Tabanelli, T.; Vásquez, P.B.; Paone, E.; Pietropaolo, R.; Dimitratos, N.; Cavani, F.; Mauriello, F. Improved Catalytic Transfer Hydrogenation of Levulinate Esters with Alcohols over ZrO2 Catalyst. Chem. Proc. 2020, 2, 28. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Marelli, M.; Psaro, R.; Ravasio, N.; Zaccheria, F. New Generation Biofuels: γ-Valerolactone into Valeric Esters in One Pot. RSC Adv. 2013, 3, 1302–1306. [Google Scholar] [CrossRef]
- Pothu, R.; Gundeboyina, R.; Boddula, R.; Perugopu, V.; Ma, J. Recent Advances in Biomass-Derived Platform Chemicals to Valeric Acid Synthesis. New J. Chem. 2022, 46, 5907–5921. [Google Scholar] [CrossRef]
- Velisoju, V.K.; Jampaiah, D.; Gutta, N.; Bentrup, U.; Brückner, A.; Bhargava, S.K.; Akula, V. Conversion of γ-Valerolactone to Ethyl Valerate over Metal Promoted Ni/ZSM-5 Catalysts: Influence of Ni0/Ni2+ Heterojunctions on Activity and Product Selectivity. ChemCatChem 2020, 12, 1341–1349. [Google Scholar] [CrossRef]
- Yi, Z.; Hu, D.; Xu, H.; Wu, Z.; Zhang, M.; Yan, K. Metal Regulating the Highly Selective Synthesis of Gamma-Valerolactone and Valeric Biofuels from Biomass-Derived Levulinic Acid. Fuel 2020, 259, 116208. [Google Scholar] [CrossRef]
- Pan, T.; Deng, J.; Xu, Q.; Xu, Y.; Guo, Q.-X.; Fu, Y. Catalytic Conversion of Biomass-Derived Levulinic Acid to Valerate Esters as Oxygenated Fuels Using Supported Ruthenium Catalysts. Green Chem. 2013, 15, 2967–2974. [Google Scholar] [CrossRef]
- Bacchiocchi, R.; De Maron, J.; Tabanelli, T.; Bianchi, D.; Cavani, F. Supported Rhenium Catalysts for the Hydrogenation of Levulinic Acid Derivatives: Limits and Potential. Sustain. Energy Fuels 2023, 7, 671–681. [Google Scholar] [CrossRef]
- Muñoz-Olasagasti, M.; López Granados, M.; Jiménez-Gómez, C.P.; Cecilia, J.A.; Maireles-Torres, P.; Dumesic, J.A.; Mariscal, R. The Relevance of Lewis Acid Sites on the Gas Phase Reaction of Levulinic Acid into Ethyl Valerate Using CoSBA-XAl Bifunctional Catalysts. Catal. Sci. Technol. 2021, 11, 4280–4293. [Google Scholar] [CrossRef]
- Muñoz-Olasagasti, M.; Martínez-Salazar, I.; Granados, M.L.; López-Aguado, C.; Iglesias, J.; Morales, G.; Mariscal, R. Elucidating the Roles of Acid Site Nature and Strength in the Direct Conversion of Levulinic Acid into Ethyl Valerate: The Case of Zr-Modified Beta Zeolite-Supported Pd Catalysts. Sustain. Energy Fuels 2022, 6, 1164–1174. [Google Scholar] [CrossRef]
- Martínez Figueredo, K.G.; Virgilio, E.M.; Segobia, D.J.; Bertero, N.M. Production of Pentyl Valerate from γ-Valerolactone, Pentanol and H2 Using Pd and Rh-Based Bifunctional Catalysts. React. Chem. Eng. 2022, 7, 1997–2008. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Xiong, J.; Ji, N. Transformation of Levulinic Acid to Valeric Biofuels: A Review on Heterogeneous Bifunctional Catalytic Systems. ChemSusChem 2019, 12, 3915–3930. [Google Scholar] [CrossRef]
- Al-Naji, M.; Van Aelst, J.; Liao, Y.; d’Hullian, M.; Tian, Z.; Wang, C.; Gläser, R.; Sels, B.F. Pentanoic Acid from γ-Valerolactone and Formic Acid Using Bifunctional Catalysis. Green Chem. 2020, 22, 1171–1181. [Google Scholar] [CrossRef]
- Arnaud, S.P.; Wu, L.; Wong Chang, M.-A.; Comerford, J.W.; Farmer, T.J.; Schmid, M.; Chang, F.; Li, Z.; Mascal, M. New Bio-Based Monomers: Tuneable Polyester Properties Using Branched Diols from Biomass. Faraday Discuss. 2017, 202, 61–77. [Google Scholar] [CrossRef]
- Stadler, B.M.; Brandt, A.; Kux, A.; Beck, H.; de Vries, J.G. Properties of Novel Polyesters Made from Renewable 1,4-Pentanediol. ChemSusChem 2020, 13, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Macosko, C.W.; Hillmyer, M.A. Thermoplastic Polyurethane Elastomers from Bio-Based Poly(δ-Decalactone) Diols. Polym. Chem. 2014, 5, 3231–3237. [Google Scholar] [CrossRef]
- Shen, R.; Long, M.; Lei, C.; Dong, L.; Yu, G.; Tang, J. Anticorrosive Waterborne Polyurethane Coatings Derived from Castor Oil and Renewable Diols. Chem. Eng. J. 2022, 433, 134470. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhao, H.; Liu, Z.; Huang, J.; Yang, Y.; Chen, Y. Characterization of Liquefied Products from Corn Stalk in the Presence of Polyhydric Alcohols with Acid Catalysis. Bioresources 2022, 17, 4262–4279. [Google Scholar] [CrossRef]
- Samoilov, V.; Ni, D.; Goncharova, A.; Zarezin, D.; Kniazeva, M.; Ladesov, A.; Kosyakov, D.; Bermeshev, M.; Maximov, A. Bio-Based Solvents and Gasoline Components from Renewable 2,3-Butanediol and 1,2-Propanediol: Synthesis and Characterization. Molecules 2020, 25, 1723. [Google Scholar] [CrossRef]
- Piskun, A.S.; van de Bovenkamp, H.H.; Rasrendra, C.B.; Winkelman, J.G.M.; Heeres, H.J. Kinetic Modeling of Levulinic Acid Hydrogenation to γ-Valerolactone in Water Using a Carbon Supported Ru Catalyst. Appl. Catal. A Gen. 2016, 525, 158–167. [Google Scholar] [CrossRef]
- Rozenblit, A.; Avoian, A.J.; Tan, Q.; Sooknoi, T.; Resasco, D.E. Reaction Mechanism of Aqueous-Phase Conversion of γ-Valerolactone (GVL) over a Ru/C Catalyst. J. Energy Chem. 2016, 25, 1008–1014. [Google Scholar] [CrossRef]
- Bababrik, R.M.; Wang, B.; Resasco, D.E. Reaction Mechanism for the Conversion of γ-Valerolactone (GVL) over a Ru Catalyst: A First-Principles Study. Ind. Eng. Chem. Res. 2017, 56, 3217–3222. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Fan, G.; Yang, L.; Li, F. Controlling Product Selectivity by Surface Defects over MoOx-Decorated Ni-Based Nanocatalysts for γ-Valerolactone Hydrogenolysis. J. Catal. 2019, 379, 100–111. [Google Scholar] [CrossRef]
- Sun, D.; Saito, T.; Yamada, Y.; Chen, X.; Sato, S. Hydrogenation of γ-Valerolactone to 1,4-Pentanediol in a Continuous Flow Reactor. Appl. Catal. A Gen. 2017, 542, 289–295. [Google Scholar] [CrossRef]
- Bucciol, F.; Tabasso, S.; Grillo, G.; Menegazzo, F.; Signoretto, M.; Manzoli, M.; Cravotto, G. Boosting Levulinic Acid Hydrogenation to Value-Added 1,4-Pentanediol Using Microwave-Assisted Gold Catalysis. J. Catal. 2019, 380, 267–277. [Google Scholar] [CrossRef]
- Fan, M.; Shao, Y.; Sun, K.; Li, Q.; Zhang, S.; Wang, Y.; Xiang, J.; Hu, S.; Wang, S.; Hu, X. Switching Production of γ-Valerolactone and 1,4-Pentanediol from Ethyl Levulinate via Tailoring Alkaline Sites of CuMg Catalyst and Hydrogen Solubility in Reaction Medium. Mol. Catal. 2021, 510, 111680. [Google Scholar] [CrossRef]
- Zhai, X.; Li, C.; Di, X.; Yin, D.; Liang, C. Preparation of Cu/MgO Catalysts for γ-Valerolactone Hydrogenation to 1,4-Pentanediol by MOCVD. J. Fuel Chem. Technol. 2017, 45, 537–546. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Li, Y.; Sun, P.; Wang, J.; Li, F. Highly Chemoselective Hydrogenation of Lactone to Diol over Efficient Copper-Based Bifunctional Nanocatalysts. Appl. Catal. B 2019, 245, 251–261. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Z.; Arai, M.; Zhang, C.; Liu, K.; Shi, R.; Wu, P.; Wang, Z.; Lin, W.; Cheng, H.; et al. Transformation of γ-Valerolactone into 1,4-Pentanediol and 2-Methyltetrahydrofuran over Zn-Promoted Cu/Al2O3 Catalysts. Catal. Sci. Technol. 2020, 10, 4412–4423. [Google Scholar] [CrossRef]
- Cavuoto, D.; Ravasio, N.; Zaccheria, F.; Marelli, M.; Cappelletti, G.; Campisi, S.; Gervasini, A. Tuning the Cu/SiO2 Wettability Features for Bio-Derived Platform Molecules Valorization. Mol. Catal. 2022, 528, 112462. [Google Scholar] [CrossRef]
- Simakova, I.; Demidova, Y.; Simonov, M.; Prikhod’ko, S.; Niphadkar, P.; Bokade, V.; Dhepe, P.; Murzin, D.Y. Heterogeneously Catalyzed γ-Valerolactone Hydrogenation into 1,4-Pentanediol in Milder Reaction Conditions. Reactions 2020, 1, 54–71. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, F.-S. The Importance of Catalyst Wettability. ChemCatChem 2014, 6, 3048–3052. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Zheng, A.; Xiao, F.-S.; Dai, S. Hydrophobic Solid Acids and Their Catalytic Applications in Green and Sustainable Chemistry. ACS Catal. 2018, 8, 372–391. [Google Scholar] [CrossRef]
- Cavuoto, D.; Zaccheria, F.; Ravasio, N. Some Critical Insights into the Synthesis and Applications of Hydrophobic Solid Catalysts. Catalysts 2020, 10, 1337. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of Levulinic Acid and Use as a Platform Chemical for Derived Products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kudo, S.; Ashik, U.P.M.; Einaga, H.; Hayashi, J. Selective Hydrodeoxygenation of γ-Valerolactone over Silica-Supported Rh-Based Bimetallic Catalysts. Energy Fuels 2020, 34, 7190–7197. [Google Scholar] [CrossRef]
- Pothu, R.; Challa, P.; Rajesh, R.; Boddula, R.; Balaga, R.; Balla, P.; Perugopu, V.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. Vapour-Phase Selective Hydrogenation of γ-Valerolactone to 2-Methyltetrahydrofuran Biofuel over Silica-Supported Copper Catalysts. Nanomaterials 2022, 12, 3414. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, B.; Wu, H.; Liu, M.; Liu, H.; Zhang, J.; Yang, G.; Han, B. Highly Efficient Hydrogenation of Levulinic Acid into 2-Methyltetrahydrofuran over Ni-Cu/Al2O3-ZrO2 Bifunctional Catalysts. Green Chem. 2019, 21, 606–613. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Liu, A.-F.; Zhang, Q.; Li, K.-M.; Porterfield, W.B.; Li, L.-C.; Wang, F. Mechanistic Insights into the Solvent-Driven Adsorptive Hydrodeoxygenation of Biomass Derived Levulinate Acid/Ester to 2-Methyltetrahydrofuran over Bimetallic Cu–Ni Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 11477–11490. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Ye, L.; Zhang, M.; Xiong, J.; Zhang, R.; Li, X.; Ji, N.; Lu, X. Layered Double Hydroxide-Derived Bimetallic Ni−Cu Catalysts Prompted the Efficient Conversion of γ-Valerolactone to 2-Methyltetrahydrofuran. ChemCatChem 2022, 14, e202101441. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.-M.; Hwang, Y.K.; Hwang, D.W.; Lee, J.-H.; Halligudi, S.B.; Hwang, J.-S.; Chang, J.-S. Direct Hydrocyclization of Biomass-Derived Levulinic Acid to 2-Methyltetrahydrofuran over Nanocomposite Copper/Silica Catalysts. ChemSusChem 2011, 4, 1749–1752. [Google Scholar] [CrossRef]



| Catalyst | Preparation Procedure | Reaction Conditions | Catalytic Performances (%) | Ref. |
|---|---|---|---|---|
| Cu/ZrO2 | WI DP OG | LA, FA, H2O, LA/FA molar ratio = 1, 200 °C, 5 h, flow. | C = 100 S = 100 | [78] |
| Cu-Ni/Al2O3 | WI | LEs, 2-BuOH, molLE/gcat = 0.01mol g−1, 150 °C, 12 h, batch. | C = 100 S = 97 | [94] |
| Cu-Ni/SBA15 | glycol WI | LEs, 2-PrOH, molLE/gcat = 0.0048 mol g−1, 140 °C, 3 h, batch. | C = 91 S = 90 | [83] |
| Cu/ZrO2 | OG | LEs, MeOH, molLE/gcat = 0.019 mol g−1, 220 °C, 1 h, batch. | C = 99 S = 88 | [110] |
| Cu-Ni-Al2O3/AC | WI | LA, 2-PrOH, molLA/gcat = 0.086 mol g−1, 220 °C, 2 h, batch. | C = 100 S = 97 | [114] |
| Focus | Key Catalytic Aspects | [Ref.] |
|---|---|---|
| Bimetallic Cu-Ni |
| [87] |
| Cu-γAl2O3 |
| [74] |
| Cu-MOx |
| [73] |
| Cu/SiO2 vs. Cu/Al2O3-SiO2 |
| [72] |
| Cu/t-ZrO2 |
| [77] |
| Bimetallic Cu-Ag/Al2O3 | Effect of Ag:
| [11] |
| Cu-Ni organosilica nanospheres |
| [70] |
| Cu-Ni on KIT-6 ZSM-5 doped |
| [75] |
| B2O3-Cu/ZrO2 | Effect of B:
| [30] |
| Al2O3-Cu/ZrO2 |
| [114] |
| Cu-Ni on mesoporous high surface SBA-15 |
| [83] |
| Batch | ||||
|---|---|---|---|---|
| Catalyst | Preparation Method | Reaction Conditions | Catalytic Performances (%) | Ref. |
| Cu/MgO | CVD | 1,4-dioxane, 240 °C, 10 h, P(H2) = 100 bar, molGVL/g cat = 0.0125 mol g−1, batch | C = 91 S = 94 | [146] |
| Cu1.5/Mg1.5AlO | CP | 1,4-dioxane, 160 °C, 12 h, P(H2) = 50 bar, molGVL/g cat = 0.0625 mol g−1, batch | C = 93 S = 99 | [147] |
| Zn1.5Cu/Al2O3 | WI | 1,4-dioxane, 200 °C, 2 h, P(H2) = 40 bar, molGVL/g cat = 0.02 mol g−1, batch | C = 91 S = 97 | [148] |
| Cu/SiO2 | CH | Cyclopentyl-methylether, 160 °C, 22 h, P(H2) = 50 bar, molGVL/g cat = 0.05 mol g−1, batch | C = 78 S = 98 | [23] |
| Cu/SiO2 –10% TEOCS | CH | Cyclopentyl-methylether, 160 °C, 22 h, P(H2) = 50 bar, molGVL/g cat= 0.05 mol g−1, batch | C = 80 S = 99 | [149] |
| Flow | ||||
| Catalyst | Preparation Method | Reaction Conditions | Catalytic Performances (%) | Ref. |
| Cu/ZnO | WI | 1,4-dioxane, 140 °C, WHSV = 0.4 h−1, H2/GVL molar ratio = 810, flow | C = 82 S = 99 | [143] |
| Cu/SiO2 | WI | 1-butanol, 130 °C, WHSV = 4.61 h−1, H2/GVL molar ratio = 2485, flow | C = 32 S = 67 | [150] |
| Batch | ||||
|---|---|---|---|---|
| Catalyst | Preparation Method | Reaction Conditions | Catalytic Performances (%) | Ref. |
| Cu-Ni/Al2O3–ZrO2 | SG WI | LA, 2-BuOH, 210 °C, 10 h, P (H2) = 35 bar, molLA/gcat = 0.043 mol g−1, batch | C = 100 S = 100 | [158] |
| Cu-Ni/Al2O3 | WI | LEs, n-HEX, 180 °C, 4 h, P (H2) = 40 bar, molLE/g cat = 0.0083 mol g−1, batch | C = 100 S = 98 | [159] |
| Ni2Cu1/Al2O3 | CP | GVL, 2-PrOH, 200 °C, 5 h, P (H2) = 50 bar, molGVL/g cat = 0.04 mol g−1, batch | C = 100 S = 88 | [160] |
| Flow | ||||
| Catalyst | Preparation Method | Reaction Conditions | Catalytic Performances (%) | Ref. |
| Cu/Al2O3-SiO2 | CP | LEs, EtOH, 250 °C, 1000 h, P (H2) = 30 bar, WHSV = 0.6 h−1, H2/LE molar ratio = 50, flow | C = 100 S = 65 | [72] |
| Ni–Cu/SiO2 | CP | GVL, 1,4 dioxane, 265 °C, P (H2) = 25 bar, WHSV = 0.5 h−1 H2/LE molar ratio = 80, flow | C = 100 S = 89 | [161] |
| Cu/SiO2 | DP | GVL, H2O, 300 °C, P (H2) = 1 bar, H2 = 50 mL min−1, WHSV = 1.0 h−1, H2/GVL molar ratio = 24, flow | C = 97 S = 84 | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavuoto, D.; Ardemani, L.; Ravasio, N.; Zaccheria, F.; Scotti, N. Some Insights into the Use of Heterogeneous Copper Catalysts in the Hydroprocessing of Levulinic Acid. Catalysts 2023, 13, 697. https://doi.org/10.3390/catal13040697
Cavuoto D, Ardemani L, Ravasio N, Zaccheria F, Scotti N. Some Insights into the Use of Heterogeneous Copper Catalysts in the Hydroprocessing of Levulinic Acid. Catalysts. 2023; 13(4):697. https://doi.org/10.3390/catal13040697
Chicago/Turabian StyleCavuoto, Denise, Leandro Ardemani, Nicoletta Ravasio, Federica Zaccheria, and Nicola Scotti. 2023. "Some Insights into the Use of Heterogeneous Copper Catalysts in the Hydroprocessing of Levulinic Acid" Catalysts 13, no. 4: 697. https://doi.org/10.3390/catal13040697
APA StyleCavuoto, D., Ardemani, L., Ravasio, N., Zaccheria, F., & Scotti, N. (2023). Some Insights into the Use of Heterogeneous Copper Catalysts in the Hydroprocessing of Levulinic Acid. Catalysts, 13(4), 697. https://doi.org/10.3390/catal13040697










