Electrocatalytic Hydrogen Evolution Reaction from Acetic Acid over Gold Immobilized Glassy Carbon Surface
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Fabrication of Electrode and Electrochemical Measurements
3. Results and Discussion
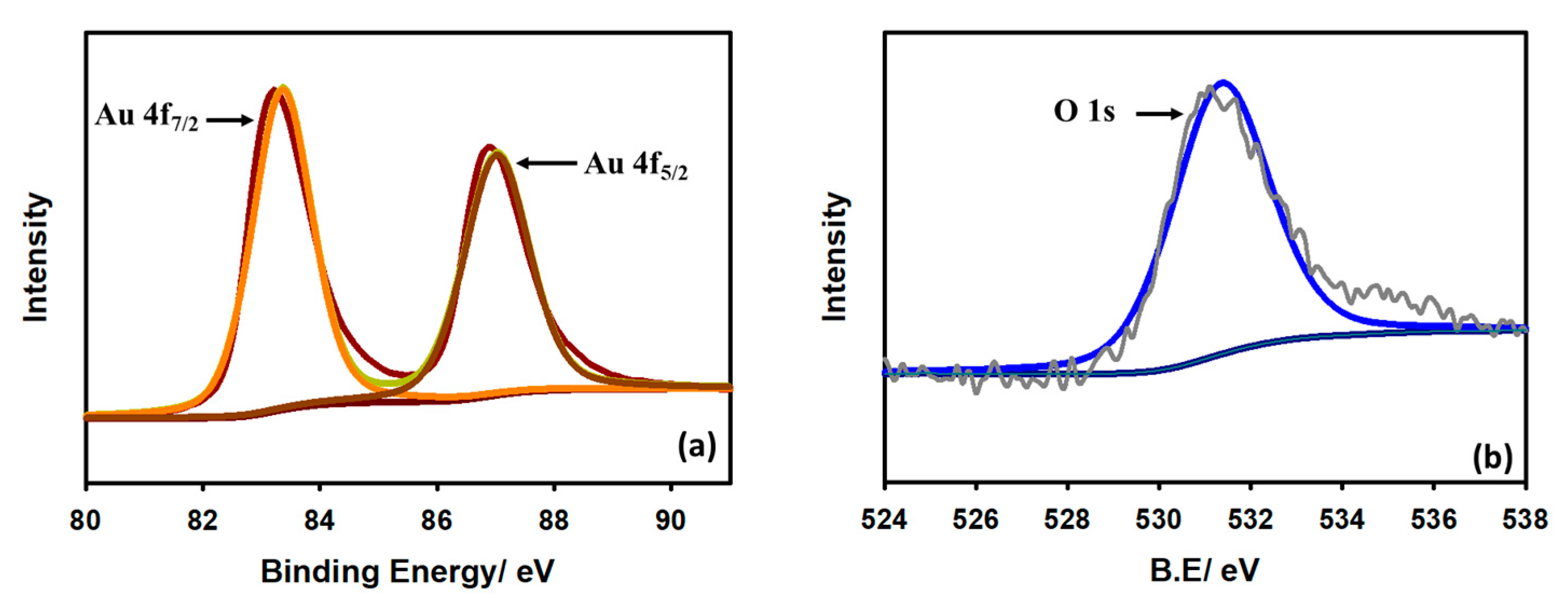
3.1. Surface Characterization
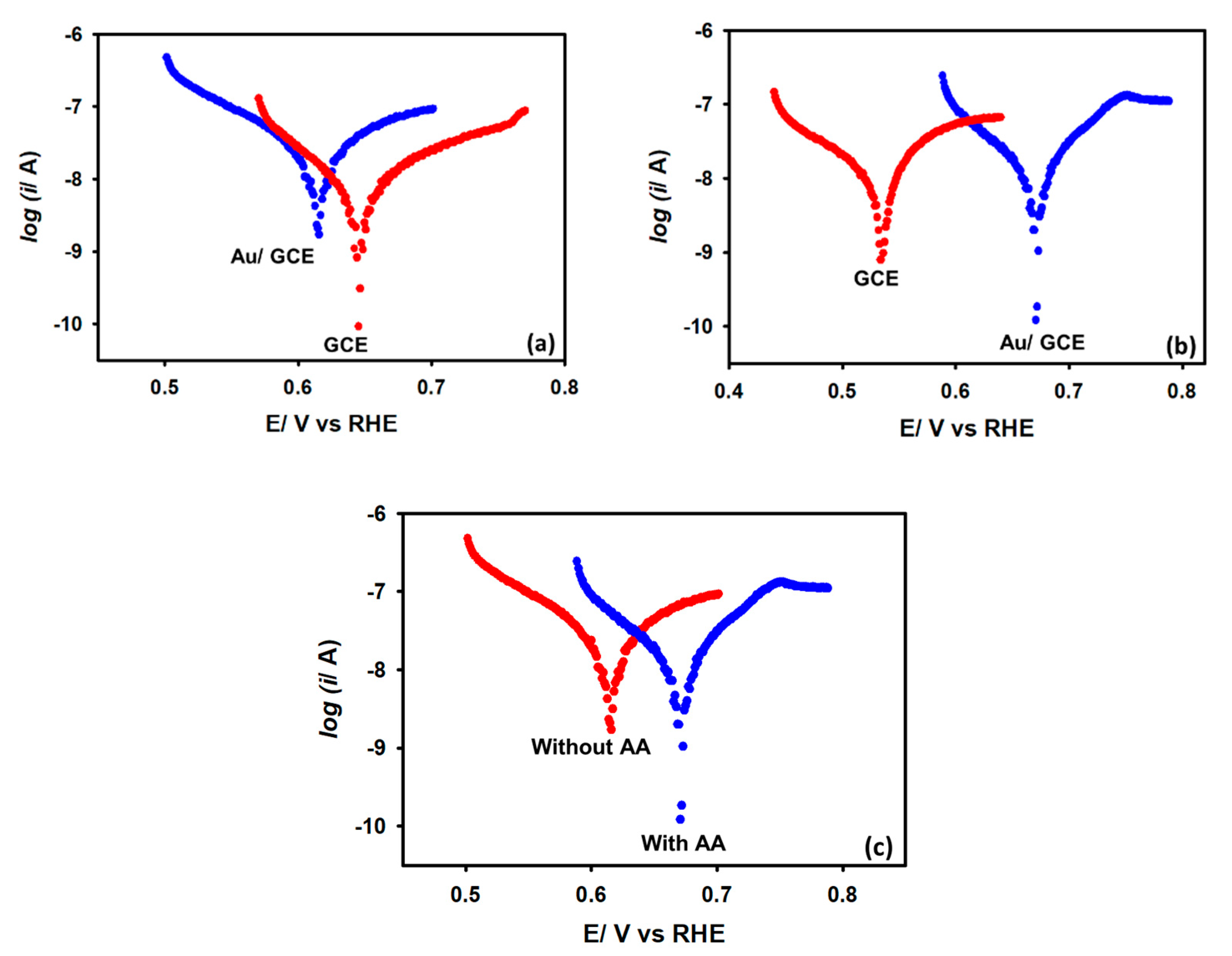
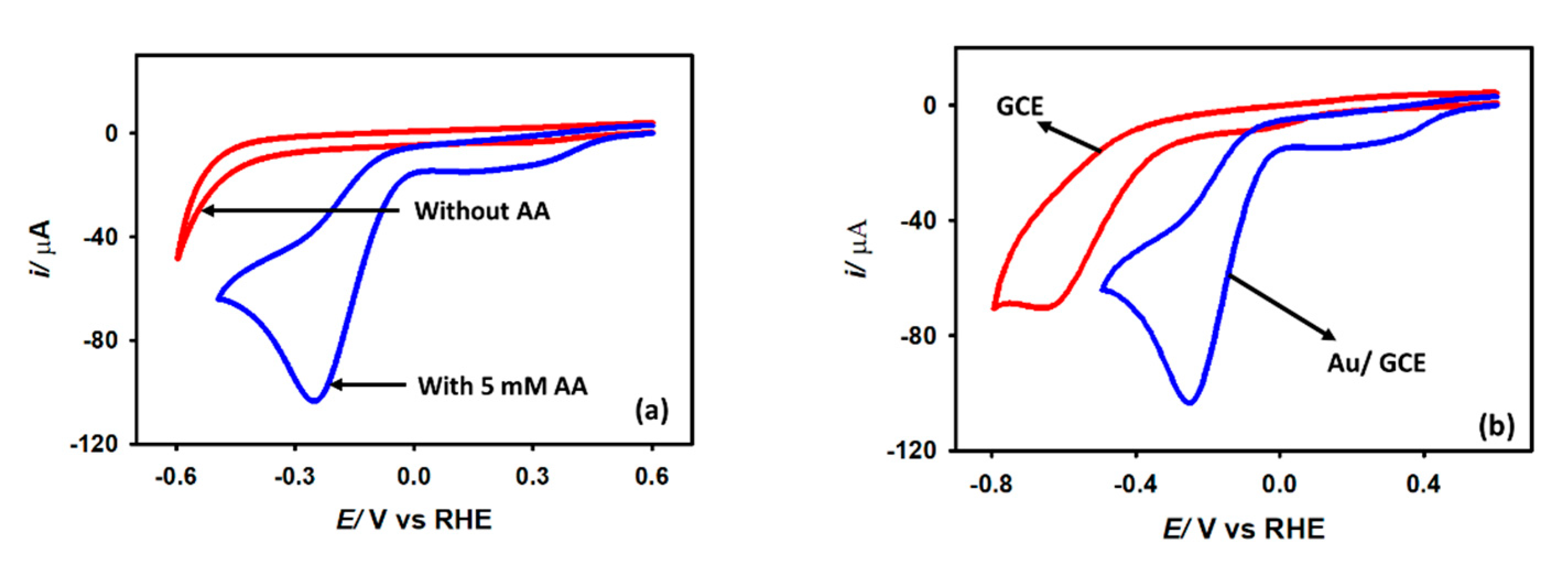
3.2. Electrochemical Characterization
3.3. HER Studies
3.3.1. Catalysis
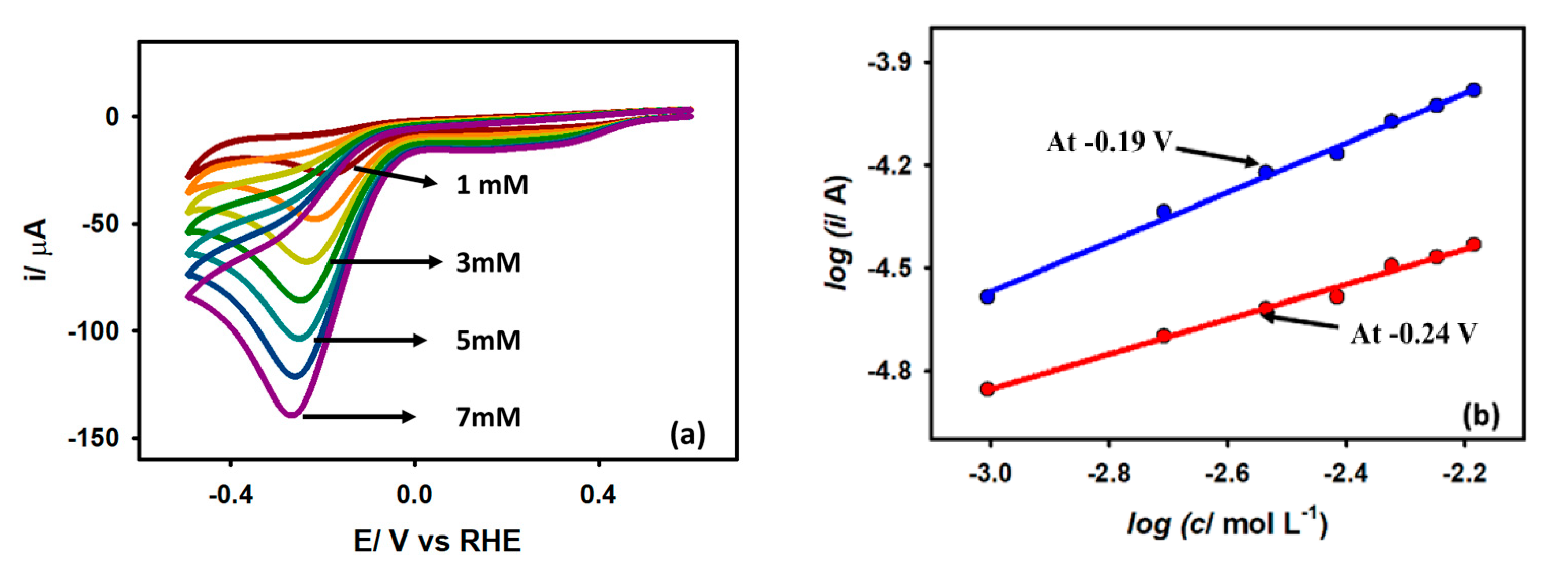
3.3.2. Effect of Acetic Acid Concentration
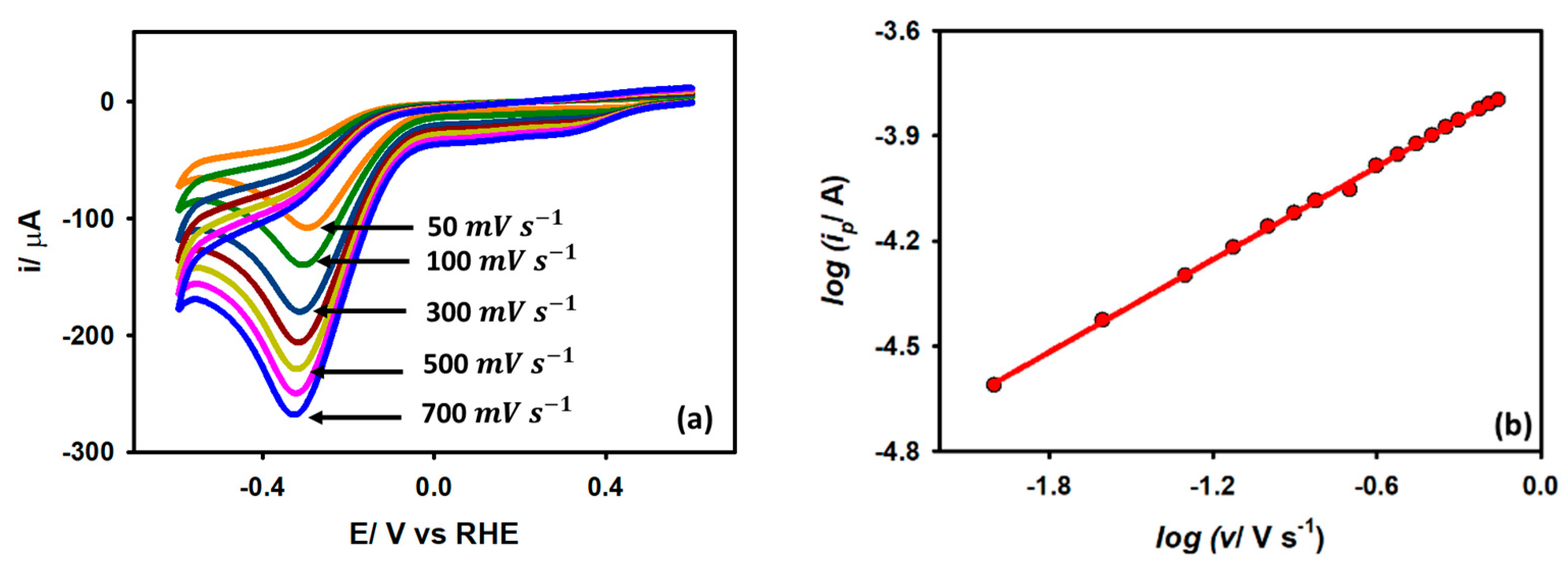
3.3.3. Effect of Scan Rate
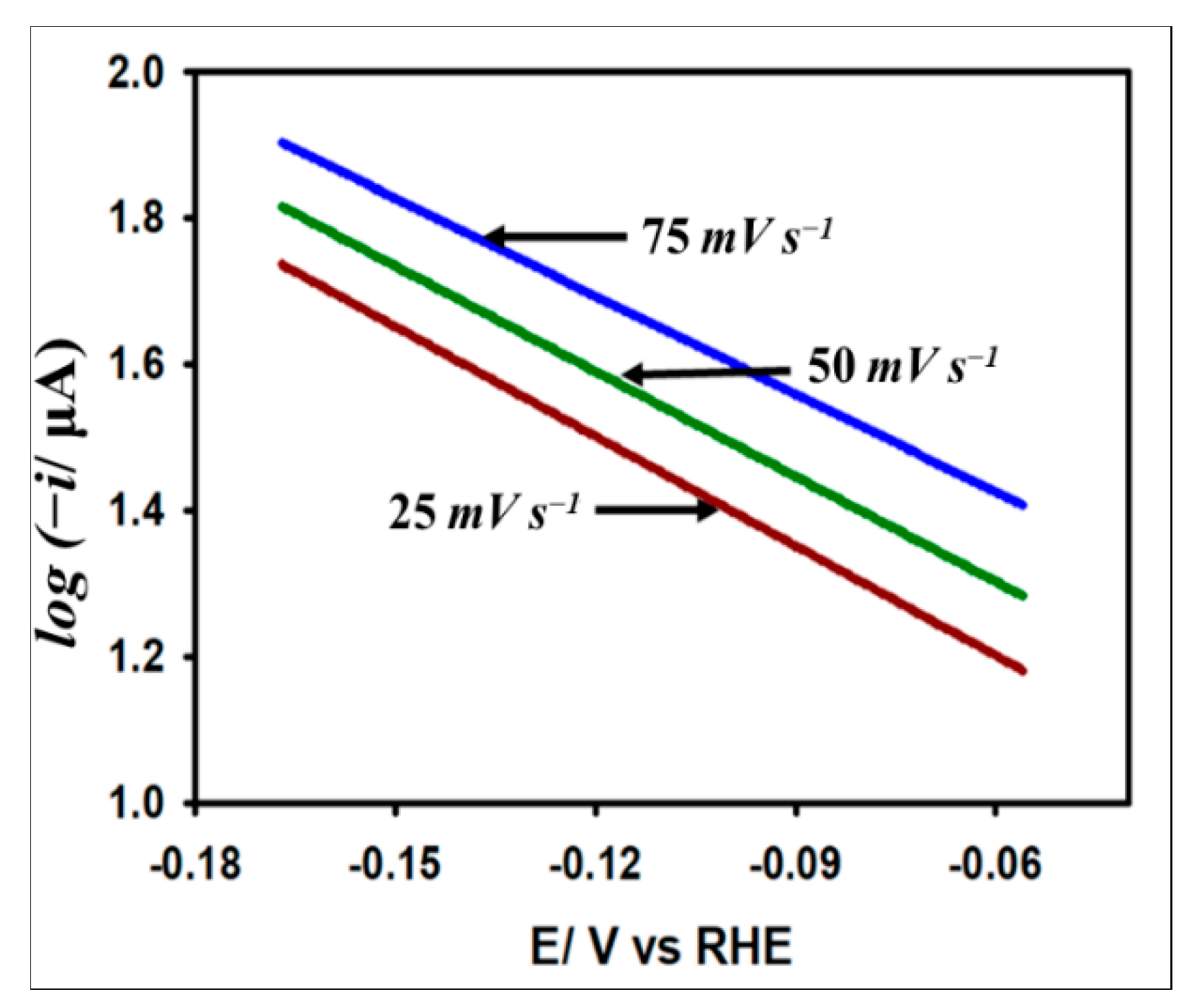
3.3.4. Tafel Analysis
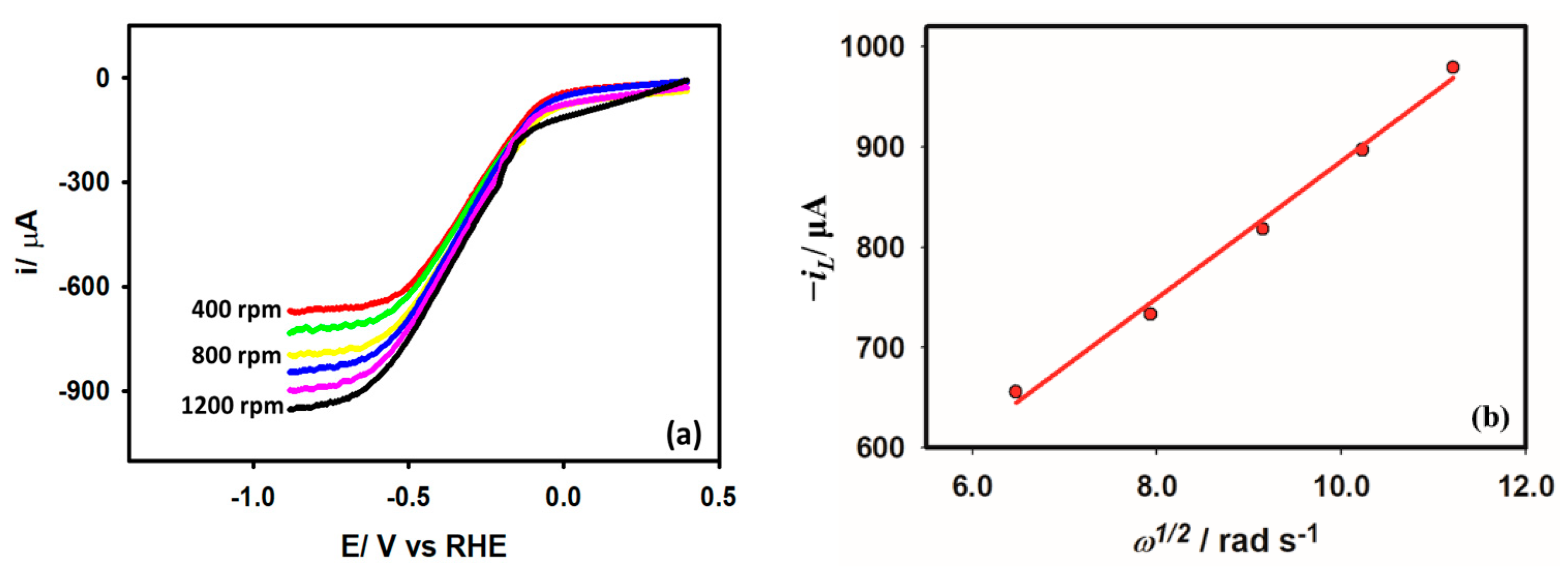
3.3.5. Diffusion Coefficient
3.3.6. Turnover Frequency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef] [Green Version]
- Gyamfi, B.A.; Bein, M.A.; Bekun, F.V. Investigating the nexus between hydroelectricity energy, renewable energy, nonrenewable energy consumption on output: Evidence from E7 countries. Environ. Sci. Pollut. Res. 2020, 27, 25327–25339. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, T.; Zahedi, A. Solar energy and wind power supply supported by storage technology: A review. Sustain. Energy Technol. Assess. 2019, 35, 25–31. [Google Scholar] [CrossRef]
- Li, Z.; Siddiqi, A.; Anadon, L.D.; Narayanamurti, V. Towards sustainability in water-energy nexus: Ocean energy for seawater desalination. Renew. Sustain. Energy Rev. 2018, 82, 3833–3847. [Google Scholar] [CrossRef] [Green Version]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Askari, M.B.; Beheshti-Marnani, A.; Seifi, M.; Rozati, S.M.; Salarizadeh, P. Fe3O4@MoS2/RGO as an Effective Nano-Electrocatalyst Toward Electrochemical Hydrogen Evolution Reaction and Methanol Oxidation in Two Settings for Fuel Cell Application; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 537. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Choi, S.; Davenport, T.C.; Haile, S.M. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: Exceptional reversibility, stability, and demonstrated faradaic efficiency. Energy Environ. Sci. 2019, 12, 206–215. [Google Scholar] [CrossRef]
- Chen, M.; Hu, J.; Wang, Y.; Wang, C.; Tang, Z.; Li, C.; Liang, D.; Cheng, W.; Yang, Z.; Zhang, H. Hydrogen production from acetic acid steam reforming over Ti-modified Ni/Attapulgite catalysts. Int. J. Hydrogen Energy 2021, 46, 3651–3668. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Chen, L.; Yang, S.; Xie, X.; Gao, M.; Li, T.; Zhao, B.; Si, H.; Hua, D. Steam reforming of acetic acid for hydrogen production over Ni/CaxFeyO catalysts. Int. J. Hydrogen Energy 2021, 46, 33132–33142. [Google Scholar] [CrossRef]
- Junior, R.B.S.; Rabelo-Neto, R.C.; Gomes, R.S.; Noronha, F.B.; Fréty, R.; Brandão, S.T. Steam reforming of acetic acid over Ni-based catalysts derived from La1−xCaxNiO3 perovskite type oxides. Fuel 2019, 254, 115714. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, Z.; Zhao, X.; Yan, E.; Li, X.; Guo, J. A critical review on limitations and enhancement strategies associated with biohydrogen production. Int. J. Hydrogen Energy 2021, 46, 16565–16590. [Google Scholar] [CrossRef]
- Xing, Z.; Zong, X.; Pan, J.; Wang, L. On the engineering part of solar hydrogen production from water splitting: Photoreactor design. Chem. Eng. Sci. 2013, 104, 125–146. [Google Scholar] [CrossRef]
- Rajamathi, C.R.; Gupta, U.; Pal, K.; Kumar, N.; Yang, H.; Sun, Y.; Shekhar, C.; Yan, B.; Parkin, S.; Waghmare, U.V.; et al. Photochemical Water Splitting by Bismuth Chalcogenide Topological Insulators. ChemPhysChem 2017, 17, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Bahnemann, A.A.I.D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Zhang, X.Z.Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Sultan, S.; Myung, C.W.; Yoon, T.; Li, N.; Ha, M.; Harzandi, A.M.; Park, H.J.; Kim, D.Y.; Chandrasekaran, S.S.; et al. Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 2018, 3, 773–782. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Xu, T.; Lu, Z.; Wu, X.; Wan, P.; Sun, X.; Jiang, L. Under-Water Superaerophobic Pine-Shaped Pt Nanoarray Electrode for Ultrahigh-Performance Hydrogen Evolution. Adv. Funct. Mater. 2015, 25, 1737–1744. [Google Scholar] [CrossRef]
- Xie, C.; Chen, W.; Du, S.; Yan, D.; Zhang, Y.; Chen, J.; Liu, B.; Wang, S. In-situ phase transition of WO3 boosting electron and hydrogen transfer for enhancing hydrogen evolution on Pt. Nano Energy 2020, 71, 104653. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kobayashi, H.; Wu, D.; Okazoe, S.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; et al. Significant Enhancement of Hydrogen Evolution Reaction Activity by Negatively Charged Pt through Light Doping of W. J. Am. Chem. Soc. 2020, 142, 17250–17254. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Y.; She, S.; Miao, S.; Ni, M.; Zhou, W.; Liu, M.; Shao, Z. Bigger is Surprisingly Better: Agglomerates of Larger RuP Nanoparticles Outperform Benchmark Pt Nanocatalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, e1800047. [Google Scholar] [CrossRef] [PubMed]
- Callejas, J.F.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Synthesis, Characterization, and Properties of Metal Phosphide Catalysts for the Hydrogen-Evolution Reaction. Chem. Mater. 2016, 28, 6017–6044. [Google Scholar] [CrossRef]
- Skúlason, Y.A.E. Hydrogen Evolution Reaction Catalyzed by Transition-Metal Nitrides. J. Phys. Chem. C 2017, 121, 24036–24045. [Google Scholar] [CrossRef]
- Jin, H.; Gu, Q.; Chen, B.; Tang, C.; Zheng, Y.; Zhang, H.; Jaroniec, M.; Qiao, S.-Z. Molten Salt-Directed Catalytic Synthesis of 2D Layered Transition-Metal Nitrides for Efficient Hydrogen Evolution. Chem 2020, 6, 2382–2394. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Shi, Z.; Yang, L.; Tang, Y. Structural Design and Electronic Modulation of Transition-Metal-Carbide Electrocatalysts toward Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1802880. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-Activated Transition Metal Carbides for Efficient Hydrogen Evolution in Acidic and Alkaline Solutions. Adv. Energy Mater. 2020, 10, 2002260. [Google Scholar] [CrossRef]
- Chen, W.-F.; Sasaki, K.; Ma, C.; Frenkel, A.I.; Marinkovic, N.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Hydrogen-Evolution Catalysts Based on Non-Noble Metal Nickel-Molybdenum Nitride Nanosheets. Angew. Chemie 2012, 124, 6235–6239. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Ge, C.; Cui, W.; Pu, Z.; Asiri, A.M.; Sun, X. CoP nanostructures with different morphologies: Synthesis, characterization and a study of their electrocatalytic performance toward the hydrogen evolution reaction. J. Mater. Chem. A 2014, 2, 14634–14640. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.J.; Zhu, J.Y.; Li, Y.; Zhao, J.; Li, F.T. Fabrication of two-dimensional Ni2P/ZnIn2S4 heterostructures for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2018, 353, 15–24. [Google Scholar] [CrossRef]
- Raoof, J.B.; Hosseini, S.R.; Ojani, R.; Mandegarzad, S. MOF-derived Cu/nanoporous carbon composite and its application for electro-catalysis of hydrogen evolution reaction. Energy 2015, 90, 1075–1081. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, G.; Ren, Z.; Pan, K.; Shi, Y.; Wang, J.; Fu, H. Hierarchical core-shell carbon Nanofiber@ZnIn2S4 composites for enhanced hydrogen evolution performance. ACS Appl. Mater. Interfaces 2014, 6, 13841–13849. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jin, J.; Zhang, H.; Lu, M.; Peng, Y.; Huang, B.; Xi, P.; Yan, C. Atomic Arrangement in Metal-Doped NiS2 Boosts the Hydrogen Evolution Reaction in Alkaline Media. Angew. Chem.-Int. Ed. 2019, 58, 18676–18682. [Google Scholar] [CrossRef] [PubMed]
- Dinh, C.-T.; Jain, A.; de Arquer, F.P.G.; De Luna, P.; Li, J.; Wang, N.; Zheng, X.; Cai, J.; Gregory, B.Z.; Voznyy, O.; et al. Multi-site electrocatalysts for hydrogen evolution in neutral media by destabilization of water molecules. Nat. Energy 2019, 4, 107–114. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Hu, G.; Gul, S.; Yano, J.; Jiang, D.-E.; Sun, Y. Universal Surface Engineering of Transition Metals for Superior Electrocatalytic Hydrogen Evolution in Neutral Water. J. Am. Chem. Soc. 2017, 139, 12283–12290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z.J.; Jaroniec, M.; Qiao, S.Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.W.; Pan, L.; Zhang, X.; Zou, J.J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Tahira, A.; Tang, P.; Liu, X.; Morante, J.R.; Fahlman, M.; Arbiol, J.; Vagin, M.; Vomiero, A. MoS x @NiO Composite Nanostructures: An Advanced Nonprecious Catalyst for Hydrogen Evolution Reaction in Alkaline Media. Adv. Funct. Mater. 2019, 29, 1807562. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Chen, Y.; Cheng, G.; Chen, S.; Luo, W. Ultrathin Nitrogen-Doped Carbon Coated with CoP for Efficient Hydrogen Evolution. ACS Catal. 2017, 7, 3824–3831. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, C.; Zhang, J.; Wang, W.; Zhou, X.; Pan, B.; Tang, K.; Zuo, J.; Yang, Q. Synthesis of FeP2/C nanohybrids and their performance for hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 499–503. [Google Scholar] [CrossRef]
- Oh, S.J.; Choi, G.G.; Kim, J.S. Production of acetic acid-rich bio-oils from the fast pyrolysis of biomass and synthesis of calcium magnesium acetate deicer. J. Anal. Appl. Pyrolysis. 2017, 124, 122–129. [Google Scholar] [CrossRef]
- Thoi, V.S.; Karunadasa, H.I.; Surendranath, Y.; Long, J.R.H.; Chang, C.J. Electrochemical generation of hydrogen from acetic acid using a molecular molybdenum-oxo catalyst. Energy Environ. Sci. 2012, 5, 7762–7770. [Google Scholar] [CrossRef]
- Tang, Y.; Dong, L.; Wu, H.B.; Yu, X.Y. Tungstate-modulated Ni/Ni(OH)2interface for efficient hydrogen evolution reaction in neutral media. J. Mater. Chem. A 2021, 9, 1456–1462. [Google Scholar] [CrossRef]
- Lu, X.; Yu, T.; Wang, H.; Luo, R.; Liu, P.; Yuan, S.; Qian, L. Self-supported nanoporous gold with gradient tin oxide for sustainable and efficient hydrogen evolution in neutral media. J. Renew. Mater. 2020, 8, 133–151. [Google Scholar] [CrossRef]
- Song, S.; Zang, J.; Zhou, S.; Gao, H.; Tian, H.; Yuan, Y.; Li, W.; Wang, Y. Self-supported amorphous nickel-iron phosphorusoxides hollow spheres on Ni-Fe foam for highly efficient overall water splitting. Electrochim. Acta 2021, 392, 138996. [Google Scholar] [CrossRef]



| Electrode | OCP/V | Rs/Ω | Rct/kΩ | |
|---|---|---|---|---|
| Without AA | With 5 mM AA | |||
| GCE | 0.651 | 0.542 | 126 | 9.78 |
| Au/GCE | 0.622 | 0.675 | 130 | 4.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, B.H.; Begum, H.; Ibrahim, F.A.; Hamdy, M.S.; Oyshi, T.A.; Khatun, N.; Hasnat, M.A. Electrocatalytic Hydrogen Evolution Reaction from Acetic Acid over Gold Immobilized Glassy Carbon Surface. Catalysts 2023, 13, 744. https://doi.org/10.3390/catal13040744
Alshammari BH, Begum H, Ibrahim FA, Hamdy MS, Oyshi TA, Khatun N, Hasnat MA. Electrocatalytic Hydrogen Evolution Reaction from Acetic Acid over Gold Immobilized Glassy Carbon Surface. Catalysts. 2023; 13(4):744. https://doi.org/10.3390/catal13040744
Chicago/Turabian StyleAlshammari, Basmah H., Humayra Begum, Fatma A. Ibrahim, Mohamed S. Hamdy, Tahamida A. Oyshi, Nazia Khatun, and Mohammad A. Hasnat. 2023. "Electrocatalytic Hydrogen Evolution Reaction from Acetic Acid over Gold Immobilized Glassy Carbon Surface" Catalysts 13, no. 4: 744. https://doi.org/10.3390/catal13040744
APA StyleAlshammari, B. H., Begum, H., Ibrahim, F. A., Hamdy, M. S., Oyshi, T. A., Khatun, N., & Hasnat, M. A. (2023). Electrocatalytic Hydrogen Evolution Reaction from Acetic Acid over Gold Immobilized Glassy Carbon Surface. Catalysts, 13(4), 744. https://doi.org/10.3390/catal13040744









