Application of Laccase Catalysis in Bond Formation and Breakage: A Review
Abstract
1. Introduction
2. The Effect of the Mediator System on Laccase Catalysis
3. Laccase-Catalyzed C–N, C–C, and C–O Bond Breakage
3.1. C–N Bond Breakage
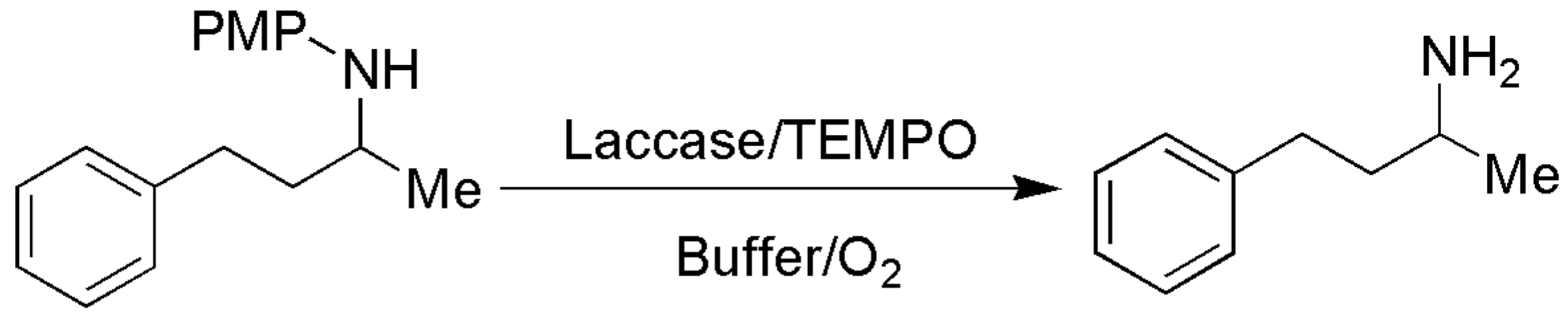
3.1.1. N-PMP Removal
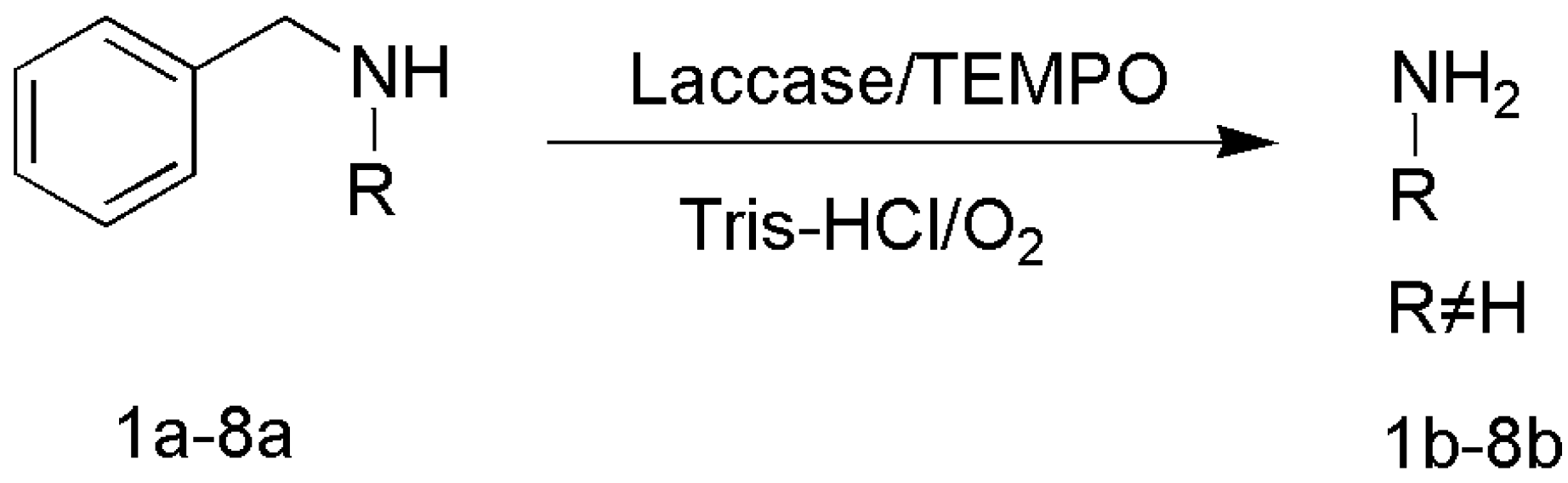
3.1.2. N-Benzyl Removal
3.2. C–C Bond Breakage
3.3. C–O Bond Breakage
4. Laccase-Catalyzed C–N, C–C, C–O Bond Formation
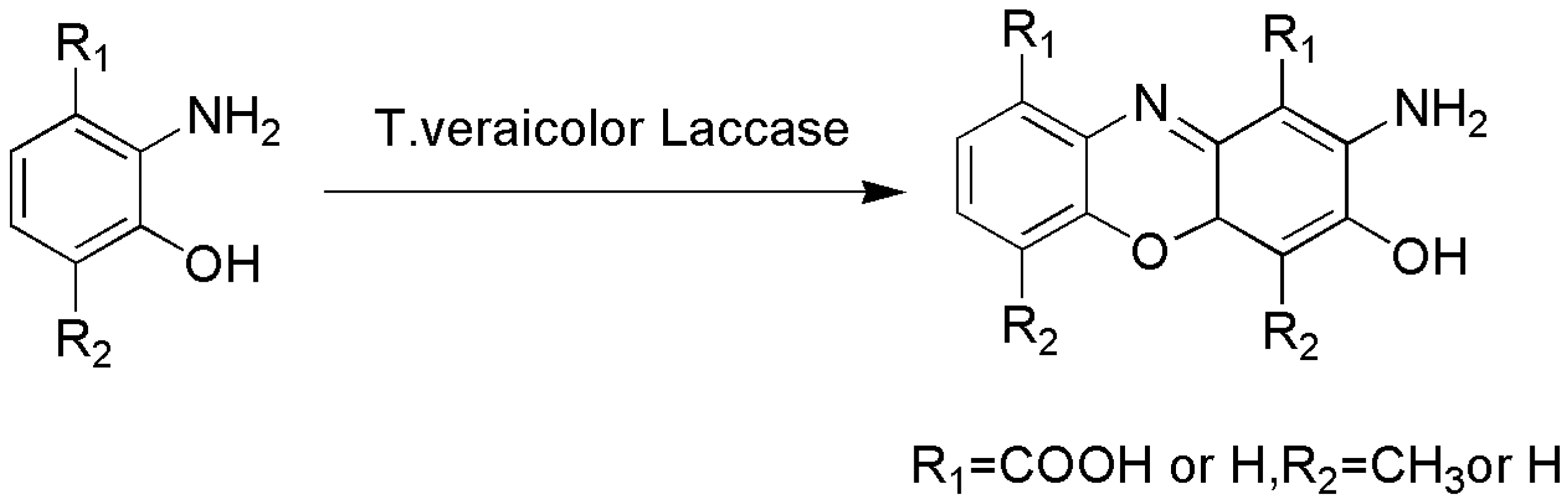
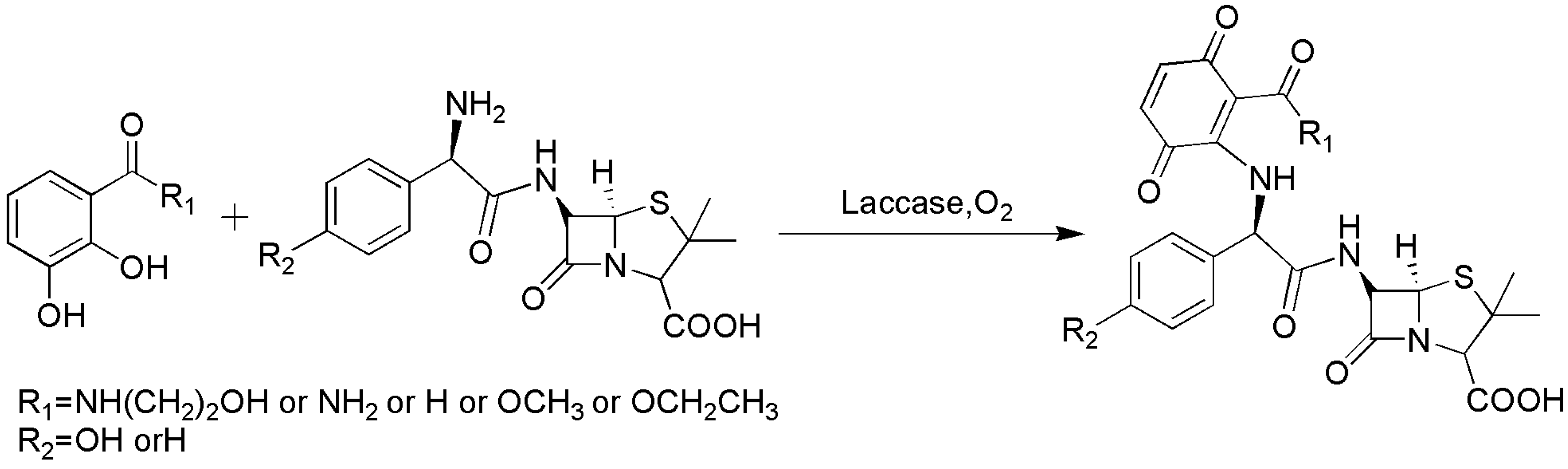
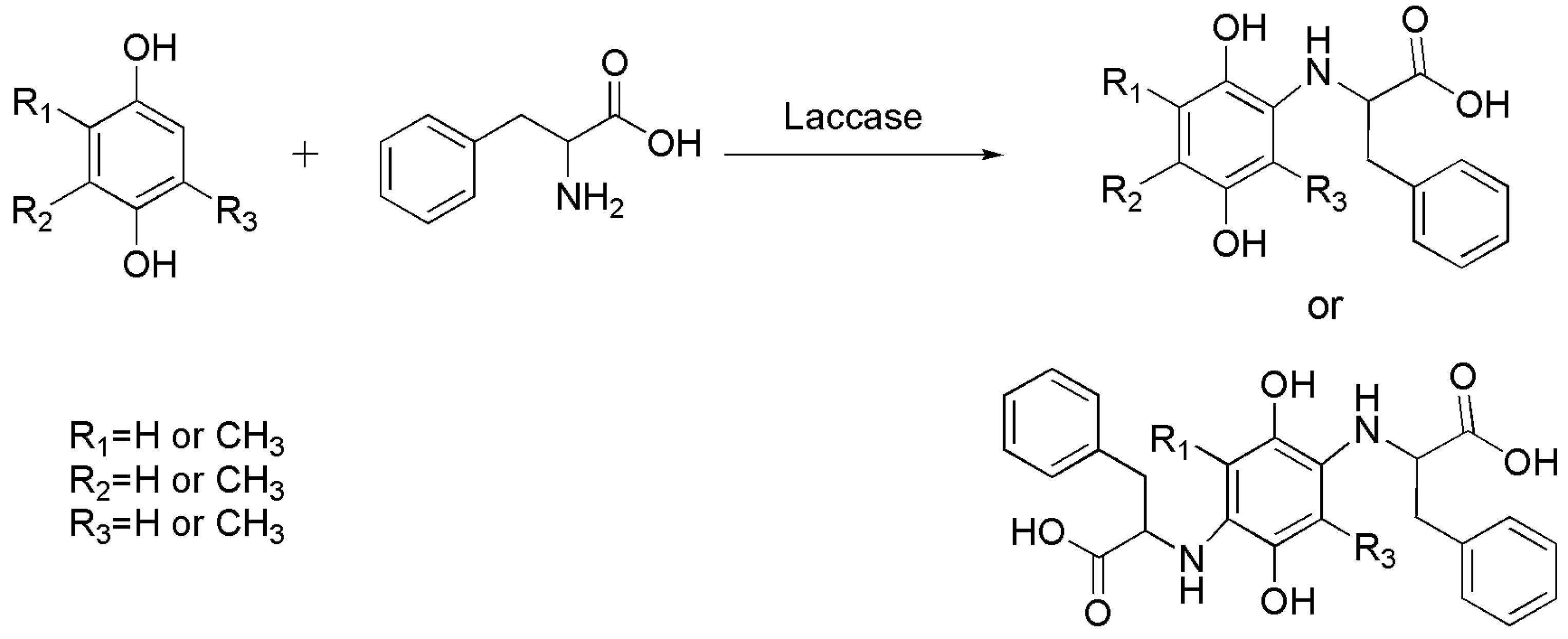
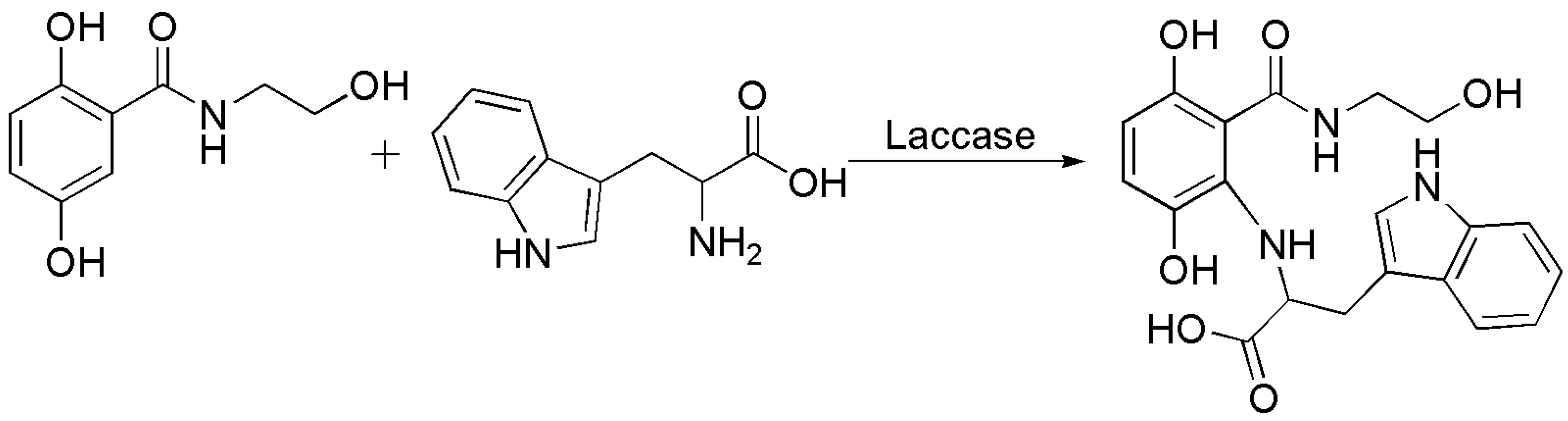
4.1. C–N Bond Formation
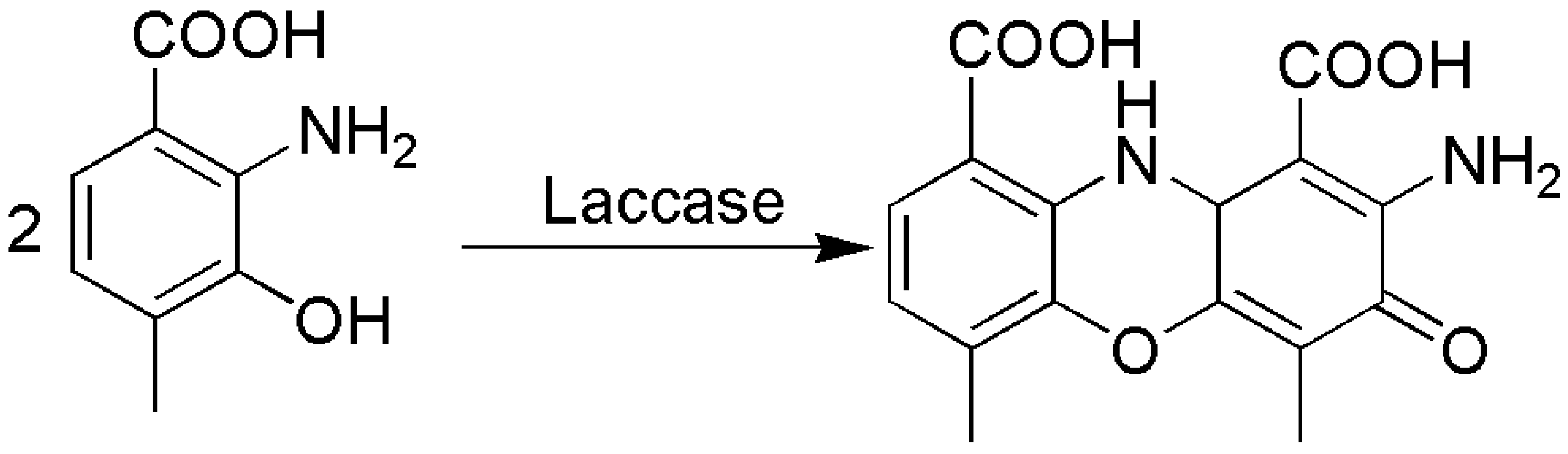
4.2. C–C Bond Formation
4.3. C–O Bond Formation
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozer, A.; Ay Sal, F.; Belduz, A.O.; Kirci, H.; Canakci, S. Use of feruloyl esterase as laccase-mediator system in paper bleaching. Appl. Biotech. Biochem. 2020, 190, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Go, N. Function and molecular evolution of multicopper blue proteins. Cell. Mol. Life Sci. 2005, 62, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Arya, S.K. Utility of laccase in pulp and paper industry: A progressive step towards the green technology. Int. J. Biol. Macromol. 2019, 134, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Claus, H. Laccases: Structure, reactions, distribution. Micron 2004, 35, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kagalkar, A.N.; Khandare, R.V.; Govindwar, S.P. Textile dye degradation potential of plant laccase significantly enhances upon augmentation with redox mediators. RSC Adv. 2015, 5, 80505–80517. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial laccase: Recent update on production, properties and industrial applications. 3 Biotech 2017, 7, 323. [Google Scholar] [CrossRef]
- Balaes, T. Ligninolytic enzyme system in ecological adaptation of lignicolous macrofungi. Appl. Ecol. Env. Res. 2017, 15, 207–224. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkolazka, A.; Paszczynski, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Guan, Z.B.; Luo, Q.; Wang, H.R.; Chen, Y.; Liao, X.R. Bacterial laccases: Promising biological green tools for industrial applications. Cell. Mol. Life Sci. 2018, 75, 3569–3592. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, N. Microbial laccase: A robust enzyme and its industrial applications. Biologia 2020, 75, 1183–1193. [Google Scholar] [CrossRef]
- Piscitelli, A.; Del Vecchio, C.; Faraco, V.; Giardina, P.; Macellaro, G.; Miele, A.; Pezzella, C.; Sannia, G. Fungal laccases: Versatile tools for lignocellulose transformation. Comptes Rendus Biol. 2011, 334, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Martins, L.O.; Robalo, M.P. Laccases: Versatile biocatalysts for the synthesis of heterocyclic cores. Molecules 2021, 26, 3719. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, J.; Jia, W.; Chang, S.; Li, S.; Li, Y. Lignin engineering through laccase modification: A promising field for energy plant improvement. Biotechnol. Biofuels 2015, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Taoka, M.; Yamauchi, Y.; Craig Everroad, R.; Seto, Y.; Isobe, T.; Kamo, M.; Chosa, N. Re-examination of a α-chymotrypsin-solubilized laccase in the pupal cuticle of the silkworm, Bombyx mori: Insights into the regulation system for laccase activation during the ecdysis process. Insect. Biochem. Mol. Biol. 2014, 55, 61–69. [Google Scholar] [CrossRef]
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal laccase discovered but yet undiscovered. Bioresour. Bioprocess. 2018, 5, 4. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef]
- Malmstrom, B.G.; Agro, A.F.; Antonini, E. The mechanism of laccase-catalyzed oxidations: Kinetic evidence for the involvement of several electron-accepting sites in the enzyme. Eur. J. Biochem. 1969, 9, 383–391. [Google Scholar] [CrossRef]
- Dong, J.L.; Zhang, Y.Z. Purification and characterization of two laccase isoenzymes from a ligninolytic fungus Trametes gallica. Prep. Biochem. Biotech. 2004, 34, 179–194. [Google Scholar] [CrossRef]
- Chakroun, H.; Mechichi, T.; Martinez, M.J.; Dhouib, A.; Sayadi, S. Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: Application on bioremediation of phenolic compounds. Process Biochem. 2010, 45, 507–513. [Google Scholar] [CrossRef]
- Giovanelli, G.; Ravasini, G. Apple juice stabilization by combined enzyme-membrane filtration process. LWT Food Sci. Technol. 1993, 26, 1–7. [Google Scholar] [CrossRef]
- Marques de Souza, C.G.; Peralta, R.M. Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium. J. Basic Microbiol. 2003, 43, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Hayashi, Y.; Hibi, T.; Hosono, K.; Beppu, T.; Ueda, K. Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J. Biochem. 2003, 133, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Molina-Guijarro, J.M.; Pérez Torres, J.; Muñoz Dorado, J.; Guillén Carretero, F.; Moya Lobo, R.; Hernández Cutuli, M.; Arias Fernández, M.E. Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int. Microbiol. 2009, 12, 13–21. [Google Scholar]
- Niladevi, K.N.; Jacob, N.; Prema, P. Evidence for a halotolerant-alkaline laccase in Streptomyces psammoticus: Purification and characterization. Process Biochem. 2008, 43, 654–660. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2001, 286, 902–908. [Google Scholar] [CrossRef]
- Ranocha, P.; McDougall, G.; Hawkins, S.; Sterjiades, R.; Borderies, G.; Stewart, D.; Cabanes-Macheteau, M.; Boudet, A.M.; Goffner, D. Biochemical characterization, molecular cloning and expression of laccases–a divergent gene family–in poplar. Eur. J. Biochem. 1999, 259, 485–495. [Google Scholar] [CrossRef]
- Sato, Y.; Bao, W.; Sederoff, R.; Whetten, R. Molecular cloning and expression of eight laccase cDNAs in loblolly pine (Pinus taeda)*. J. Plant. Res. 2001, 114, 147–155. [Google Scholar] [CrossRef]
- Hüttermann, A.; Mai, C.; Kharazipour, A. Modification of lignin for the production of new compounded materials. Appl. Microbiol. Biotechnol. 2001, 55, 387–394. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Suderman, R.J.; Jiang, H.; Zhu, Y.C.; Gorman, M.J.; Kramer, K.J.; Kanost, M.R. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2004, 34, 29–41. [Google Scholar] [CrossRef]
- Niu, B.L.; Shen, W.F.; Liu, Y.; Weng, H.B.; He, L.H.; Mu, J.J.; Wu, Z.L.; Jiang, P.; Tao, Y.Z.; Meng, Z.Q. Cloning and RNAi-mediated functional characterization of MaLac2 of the pine sawyer, Monochamus alternatus. Insect Mol. Biol. 2008, 17, 303–312. [Google Scholar] [CrossRef]
- Lang, M.; Kanost, M.R.; Gorman, M.P.O. Multicopper oxidase-3 is a laccase associated with the peritrophic matrix of Anopheles gambiae. PLoS ONE 2012, 7, e33985. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P. Radical or electron-transfer mechanism of oxidation with some laccase/mediator systems. J. Mol. Catal. B-enzmy. 2002, 18, 169–171. [Google Scholar] [CrossRef]
- Kunamneni, A.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Laccases and their applications: A patent review. Recent Pat. Biotechnol. 2008, 2, 10–24. [Google Scholar] [CrossRef]
- Sigoillot, C.; Record, E.; Belle, V.; Robert, J.L.; Levasseur, A.; Punt, P.J.; van den Hondel, C.A.M.J.J.; Fournel, A.; Sigoillot, J.C.; Asther, M. Natural and recombinant fungal laccases for paper pulp bleaching. Appl. Microbiol. Biotechnol. 2004, 64, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Mayolo-Deloisa, K.; Gonzalez-Gonzalez, M.; Rito-Palomares, M. Laccases in food industry: Bioprocessing, potential industrial and biotechnological applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Lassouane, F.; Ait-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2019, 271, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Parra Guardado, A.L.; Belleville, M.-P.; Rostro Alanis, M.d.J.; Parra Saldivar, R.; Sanchez-Marcano, J. Effect of redox mediators in pharmaceuticals degradation by laccase: A comparative study. Process Biochem. 2019, 78, 123–131. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase–mediator systems and their applications: A Review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Martínez-Montero, L.; Díaz-Rodríguez, A.; Gotor, V.; Gotor-Fernández, V.; Lavandera, I. Broadening the chemical scope of laccases: Selective deprotection of N-benzyl groups. Green Chem. 2015, 17, 2794–2798. [Google Scholar] [CrossRef]
- Verkade, J.M.M.; van Hemert, L.J.C.; Quaedflieg, P.J.L.M.; Schoemaker, H.E.; Schürmann, M.; van Delft, F.L.; Rutjes, F.P.J.T. Laccase-mediated deprotection of para-methoxyphenyl (PMP)-protected amines. Adv. Synth. Catal. 2007, 349, 1332–1336. [Google Scholar] [CrossRef]
- Freitas, E.D.; Bubna, G.A.; Brugnari, T.; Kato, C.G.; Nolli, M.; Rauen, T.G.; Regina, D.; Peralta, R.A.; Bracht, A.; Souza, C.D. Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products. Chem. Eng. J. 2017, 330, 1361–1369. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates: An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Könst, P.; Kara, S.; Kochius, S.; Holtmann, D.; Arends, I.W.; Ludwig, R.; Hollmann, F. Expanding the scope of laccase-mediator systems. ChemCatChem 2013, 5, 3027–3032. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.; Freiermuth, B.; Bodie, E.; Borneman, S. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl. Environ. Microbiol. 1997, 63, 4627–4632. [Google Scholar] [CrossRef] [PubMed]
- Eggert, C.; Temp, U.; Dean, J.F.; Eriksson, K.-E.L. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996, 391, 144–148. [Google Scholar] [CrossRef]
- Kawai, S.; Umezawa, T.; Higuchi, T. < original> Oxidation of methoxylated benzyl alcohols by laccase of Coriolus versicolor in the presence of syringaldehyde. Wood Res. Bull. Wood Res. Inst. Kyoto Univ. 1989, 76, 10–16. [Google Scholar]
- Park, S.; Jung, D.; Do, H.; Yun, J.; Lee, D.; Hwang, S.; Lee, S.H. Laccase-mediator system using a natural mediator as a whitening agent for the decolorization of melanin. Polymers 2021, 13, 3671. [Google Scholar] [CrossRef]
- Valls, C.; Colom, J.F.; Baffert, C.; Gimbert, I.; Roncero, M.B.; Sigoillot, J.-C. Comparing the efficiency of the laccase–NHA and laccase–HBT systems in eucalyptus pulp bleaching. Biochem. Engin. J. 2010, 49, 401–407. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Jia, L.; Xue, P.; Yao, H. Recent developments of a co-immobilized laccase–mediator system: A review. RSC Adv. 2021, 11, 29498–29506. [Google Scholar] [CrossRef] [PubMed]
- Witayakran, S.; Ragauskas, A.J. Synthetic applications of laccase in green chemistry. Adv. Synth. Catal. 2009, 351, 1187–1209. [Google Scholar] [CrossRef]
- Johannes, C.; Majcherczyk, A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 2000, 66, 524–528. [Google Scholar] [CrossRef]
- Matsumura, E.; Yamamoto, E.; Numata, A.; Kawano, T.; Shin, T.; Murao, S. Structures of the laccase-catalyzed oxidation products of hydroxybenzoic acids in the presence of ABTS [2,2′-azinodi-(3-ethylbenzothiazoline-6-sulfonic acid)]. Agric. Biol. Chem. 1986, 50, 1355–1357. [Google Scholar]
- Zhao, Q.; Huang, W.; Luo, Z.; Liu, L.; Lu, Y.; Li, Y.; Li, L.; Hu, J.; Ma, H.; Chen, J. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018, 4, eaao1761. [Google Scholar] [CrossRef]
- Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Burton, S. Potential applications of laccase-mediated coupling and grafting reactions: A review. Enzym. Microb. Technol. 2011, 48, 195–208. [Google Scholar] [CrossRef]
- Li, K.; Helm, R.F.; Eriksson, K.E.L. Mechanistic studies of the oxidation of a non-phenolic lignin model compound by the laccase/1-hydroxybenzotriazole redox system. Biotechnol. Appl. Biochem. 1998, 27, 239–243. [Google Scholar] [CrossRef]
- d’Acunzo, F.; Galli, C. First evidence of catalytic mediation by phenolic compounds in the laccase-induced oxidation of lignin models. Eur. J. Biochem. 2003, 270, 3634–3640. [Google Scholar] [CrossRef]
- Blanquez, A.; Rodriguez, J.; Brissos, V.; Mendes, S.; Martins, L.O.; Ball, A.S.; Arias, M.E.; Hernandez, M. Decolorization and detoxification of textile dyes using a versatile Streptomyces laccase-natural mediator system. Saudi J. Biol. Sci. 2019, 26, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Vandertol-Vanier, H.; Vazquez-Duhalt, R.; Tinoco, R.; Pickard, M. Enhanced activity by poly (ethylene glycol) modification of Coriolopsis gallica laccase. J. Ind. Microbiol. Biotechnol. 2002, 29, 214–220. [Google Scholar] [CrossRef]
- Han, M.-J.; Park, H.-T.; Song, H.-G. Degradation of phenanthrene by Trametes versicolor and its laccase. J. Microbiol. 2004, 42, 94–98. [Google Scholar] [PubMed]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Crit. Rev. Biotechnol. 2018, 38, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Kim, E.J.; Murugesan, K.; Park, H.K.; Kim, Y.M.; Kwon, J.H.; Kim, W.G.; Lee, J.Y.; Chang, Y.S. Laccase-catalysed polymeric dye synthesis from plant-derived phenols for potential. Microb. Biotechnol. 2010, 3, 324–335. [Google Scholar] [CrossRef]
- Sun, K.; Li, S.; Si, Y.; Huang, Q. Advances in laccase-triggered anabolism for biotechnology applications. Crit. Rev. Biotechnol. 2021, 41, 969–993. [Google Scholar] [CrossRef]
- Bull, S.D.; Davies, S.G.; Fenton, G.; Mulvaney, A.W.; Prasad, R.S.; Smith, A.D. Chemoselective debenzylation of N-benzyl tertiary amines with ceric ammonium nitrate. J. Chem. Soc. Perkin Trans. 2000, 22, 3765–3774. [Google Scholar] [CrossRef]
- Bull, S.D.; Davies, S.G.; Mulvaney, A.W.; Prasad, R.S.; Smith, A.D.; Fenton, G. Chemoselective oxidative debenzylation of tertiary N-benzyl amines. Chem. Commun. 2000, 5, 337–338. [Google Scholar] [CrossRef]
- Cernova, M.; Cerna, I.; Pohl, R.; Hocek, M. Regioselective direct CH arylations of protected uracils. synthesis of 5- and 6-aryluracil bases. J. Org. Chem. 2011, 76, 5309–5319. [Google Scholar] [CrossRef]
- Chi, L.-P.; Li, X.-M.; Wan, Y.-P.; Li, X.; Wang, B.-G. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus aspergillus insuetus SD-512. J. Nat. Prod. 2020, 83, 3652–3660. [Google Scholar] [CrossRef]
- Davies, S.G.; Fletcher, A.M.; Hughes, D.G.; Lee, J.A.; Price, P.D.; Roberts, P.M.; Russell, A.J.; Smith, A.D.; Thomson, J.E.; Williams, O.M.H. Asymmetric synthesis of piperidines and octahydroindolizines using a one-pot ring-closure/N-debenzylation procedure. Tetrahedron 2011, 67, 9975–9992. [Google Scholar] [CrossRef]
- Sonobe, T.; Oisaki, K.; Kanai, M. Catalytic aerobic production of imines en route to mild, green, and concise derivatizations of amines. Chem. Sci. 2012, 3, 3249–3255. [Google Scholar] [CrossRef]
- Davies, S.G.; Fletcher, A.M.; Foster, E.M.; Houlsby, I.T.; Roberts, P.M.; Schofield, T.M.; Thomson, J.E. The asymmetric syntheses of pyrrolizidines, indolizidines and quinolizidines via two sequential tandem ring-closure/N-debenzylation processes. Org. Biomol. Chem. 2014, 12, 9223–9235. [Google Scholar] [CrossRef] [PubMed]
- Mateljak, I.; Monza, E.; Lucas, M.F.; Guallar, V.; Aleksejeva, O.; Ludwig, R.; Leech, D.; Shleev, S.; Alcalde, M. Increasing redox potential, redox mediator activity, and stability in a fungal laccase by computer-guided mutagenesis and directed evolution. ACS Catal. 2019, 9, 4561–4572. [Google Scholar] [CrossRef]
- Mateljak, I.; Alcalde, M. Engineering a highly thermostable high-redox potential laccase. ACS Sustain. Chem. Eng. 2021, 9, 9632–9637. [Google Scholar] [CrossRef]
- Gomez-Fernandez, B.J.; Risso, V.A.; Sanchez-Ruiz, J.M.; Alcalde, M. Consensus design of an evolved high-redox potential laccase. Front. Bioeng. Biotechnol. 2020, 8, 354. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental effects and estrogenicity of bisphenol A alternatives in a zebrafish embryo model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef]
- Lv, Y.-Z.; Yao, L.; Wang, L.; Liu, W.-R.; Zhao, J.-L.; He, L.-Y.; Ying, G.-G. Bioaccumulation, metabolism, and risk assessment of phenolic endocrine disrupting chemicals in specific tissues of wild fish. Chemosphere 2019, 226, 607–615. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Lazim, Z.M.; Hadibarata, T.; Puteh, M.H.; Yusop, Z. Adsorption characteristics of bisphenol A onto low-cost modified phyto-waste material in aqueous solution. Water Air Soil Pollut. 2015, 226, 34. [Google Scholar] [CrossRef]
- Huang, Q.; Chai, K.; Zhou, L.; Ji, H. A phenyl-rich beta-cyclodextrin porous crosslinked polymer for efficient removal of aromatic pollutants: Insight into adsorption performance and mechanism. Chem. Eng. J. 2020, 387, 124020. [Google Scholar] [CrossRef]
- Hongyan, L.; Zexiong, Z.; Shiwei, X.; He, X.; Yinian, Z.; Haiyun, L.; Zhongsheng, Y. Study on transformation and degradation of bisphenol A by Trametes versicolor laccase and simulation of molecular docking. Chemosphere 2019, 224, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Waigi, M.G.; Li, S.; Sun, K.; Si, Y. Fungal laccase-mediated humification of estrogens in aquatic ecosystems. Water Res. 2019, 166, 115040. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Fukuda, T.; Miyamoto, H.; Kawabata, T.; Suzuki, M.; Uwajima, T. Polymerization of bisphenol A by purified laccase from Trametes villosa. Biochem. Biophys. Res. Commun. 2001, 287, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.A.; Anne, F.T. A novel acid-stable intracellular laccase from Aureobasidium pullulans: Purification, characterization and application in the removal of bisphenol A from solutions. Biocatal. Agric. Biotechnol. 2021, 33, 101966. [Google Scholar] [CrossRef]
- Daâssi, D.; Prieto, A.; Zouari-Mechichi, H.; Martínez, M.J.; Nasri, M.; Mechichi, T. Degradation of bisphenol A by different fungal laccases and identification of its degradation products. Int. Biodeterior. Biodegrad. 2016, 110, 181–188. [Google Scholar] [CrossRef]
- Zeng, S.; Zhao, J.; Xia, L. Simultaneous production of laccase and degradation of bisphenol A with Trametes versicolor cultivated on agricultural wastes. Bioprocess. Biosyst. Eng. 2017, 40, 1237–1245. [Google Scholar] [CrossRef]
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; de Freitas, E.N.; Contato, A.G.; Corrêa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; de Moraes, M.d.L.T.; Bracht, A. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351. [Google Scholar] [CrossRef]
- Fujisawa, M.; Hirai, H.; Nishida, T. Degradation of polyethylene and nylon-66 by the laccase-mediator system. J. Polym. Environ. 2001, 9, 103–108. [Google Scholar] [CrossRef]
- Yao, C.; Xia, W.; Dou, M.; Du, Y.; Wu, J. Oxidative degradation of UV-irradiated polyethylene by laccase-mediator system. J. Hazard. Mater. 2022, 440, 129709. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hirai, H.; Murata, H.; Nishida, T. Removal of estrogenic activities of 17beta-estradiol and ethinylestradiol by ligninolytic enzymes from white rot fungi. Water Res. 2003, 37, 1972–1975. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; An, Q.; Meng, G.; Wu, X.J.; Dai, Y.C.; Si, J.; Cui, B.K. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int. J. Biol. Macromol. 2017, 102, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Nadaroglu, H.; Mosber, G.; Gungor, A.A.; Adıguzel, G.; Adiguzel, A. Biodegradation of some azo dyes from wastewater with laccase from Weissella viridescens LB37 immobilized on magnetic chitosan nanoparticles. J. Water Process Eng. 2019, 31, 100866. [Google Scholar] [CrossRef]
- Taghizadeh, T.; Talebian-Kiakalaieh, A.; Jahandar, H.; Amin, M.; Tarighi, S.; Faramarzi, M.A. Biodegradation of bisphenol A by the immobilized laccase on some synthesized and modified forms of zeolite Y. J. Hazard. Mater. 2020, 386, 121950. [Google Scholar] [CrossRef]
- Latif, A.; Maqbool, A.; Sun, K.; Si, Y. Immobilization of Trametes Versicolor laccase on Cu-alginate beads for biocatalytic degradation of bisphenol A in water: Optimized immobilization, degradation and toxicity assessment. J. Environ. Chem. Eng. 2022, 10, 107089. [Google Scholar] [CrossRef]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M.N. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Fu, M.; Xing, J.; Ge, Z. Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci. Total Environ. 2019, 651, 2857–2865. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.; Ghobadi Nejad, Z.; Ghasemi, S.; Khafaji, M.; Borghei, S.M. Removal of bisphenol A in aqueous solution using magnetic cross-linked laccase aggregates from Trametes hirsuta. Bioresour. Technol. 2020, 306, 123169. [Google Scholar] [CrossRef]
- Dar, O.I.; Aslam, R.; Pan, D.; Sharma, S.; Andotra, M.; Kaur, A.; Jia, A.-Q.; Faggio, C. Source, bioaccumulation, degradability and toxicity of triclosan in aquatic environments: A review. Environ. Technol. Innov. 2022, 25, 102122. [Google Scholar] [CrossRef]
- Reiss, R.; Lewis, G.; Griffin, J. An ecological risk assessment for triclosan in the terrestrial environment. Environ. Toxicol. Chem. 2009, 28, 1546–1556. [Google Scholar] [CrossRef]
- Guo, J.; Iwata, H. Risk assessment of triclosan in the global environment using a probabilistic approach. Ecotoxicol. Environ. Saf. 2017, 143, 111–119. [Google Scholar] [CrossRef]
- Milanovic, M.; Duric, L.; Milosevic, N.; Milic, N. Comprehensive insight into triclosan—From widespread occurrence to health outcomes. Environ. Sci. Pollut. Res. Int. 2021, 30, 25119–25140. [Google Scholar] [CrossRef]
- Anger, C.T.; Sueper, C.; Blumentritt, D.J.; McNeill, K.; Engstrom, D.R.; Arnold, W.A. Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores. Environ. Sci. Technol. 2013, 47, 1833–1843. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, P.; Saharan, V.; Kapoor, R.K. Simultaneous laccase production and transformation of bisphenol-A and triclosan using Trametes versicolor. 3 Biotech 2019, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.N.; Wang, J.H.; Chen, Y.C.; Hu, Y.Y. The transformation of triclosan by laccase: Effect of humic acid on the reaction kinetics, products and pathway. Environ. Pollut. 2018, 234, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Seto, Y.; Hashimoto, K.; Kurushima, H. Mini-review an insect-specific system for terrestrialization: Laccase mediated cuticle formation. Insect Biochem. Mol. Biol. 2019, 108, 61–70. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Thakur, S.; Sharma, S.; Yadav, N.; Rastegari, A.A.; Yadav, A.N.; Saxena, A.K. Disruption of protease genes in microbes for production of heterologous proteins. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–75. [Google Scholar] [CrossRef]
- Theerachat, M.; Guieysse, D.; Morel, S.; Remaud-Simeon, M.; Chulalaksananukul, W. Laccases from marine organisms and their applications in the biodegradation of toxic and environmental pollutants: A Review. Appl. Biochem. Biotechnol. 2019, 187, 583–611. [Google Scholar] [CrossRef]
- Ciecholewski, S.; Hammer, E.; Manda, K.; Bose, G.; Nguyen, V.T.; Langer, P.; Schauer, F. Laccase-catalyzed carbon–carbon bond formation: Oxidative dimerization of salicylic esters by air in aqueous solution. Tetrahedron 2005, 61, 4615–4619. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.; Conrad, J.; Harms, K.; Nohr, D.; Beifuss, U. Laccase-catalyzed green synthesis and cytotoxic activity of novel pyrimidobenzothiazoles and catechol thioethers. RSC Adv. 2017, 7, 17427–17441. [Google Scholar] [CrossRef]
- Osiadacz, J.; Al-Adhami, A.J.; Bajraszewska, D.; Fischer, P.; Peczyñska-Czoch, W. On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J. Biotechnol. 1999, 72, 141–149. [Google Scholar] [CrossRef]
- Pilati, S.; Malacarne, G.; Navarro-Payá, D.; Tomè, G.; Riscica, L.; Cavecchia, V.; Matus, J.T.; Moser, C.; Blanzieri, E. Vitis oneGenE: A causality-based approach to generate gene networks in Vitis vinifera sheds light on the laccase and dirigent gene families. Biomolecules 2021, 11, 1744. [Google Scholar] [CrossRef]
- Mogharabi, M.; Faramarzi, M.A. Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with laccases: An Updated Overview. Catalysts 2020, 11, 26. [Google Scholar] [CrossRef]
- Hahn, V.; Mikolasch, A.; Manda, K.; Gördes, D.; Thurow, K.; Schauer, F. Derivatization of amino acids by fungal laccases: Comparison of enzymatic and chemical methods. J. Mol. Catal. B Enzym. 2009, 60, 76–81. [Google Scholar] [CrossRef]
- Rouhani, S.; Azizi, S.; Kibechu, R.W.; Mamba, B.B.; Msagati, T. Laccase immobilized Fe3O4-graphene oxide nanobiocatalyst improves stability and immobilization efficiency in the green preparation of sulfa drugs. Catalysts 2020, 10, 459. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Panossian, A.; Leroux, F.R.; Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. [Google Scholar] [CrossRef] [PubMed]
- Emirdağ-Öztürk, S.; Hajdok, S.; Conrad, J.; Beifuss, U. Laccase-catalyzed reaction of 3-tert-butyl-1H-pyrazol-5 (4H)-one with substituted catechols using air as an oxidant. Tetrahedron 2013, 69, 3664–3668. [Google Scholar] [CrossRef]
- Wei, T.B.; Chen, Y.; Hu, Q.; Yang, J.K.; Huo, Z.Q.; Duan, R.F.; Wang, J.X.; Zeng, Y.P.; Li, J.M.; Liao, Y.X. Hydride vapor phase epitaxy of strain-reduced GaN film on nano-island template produced using self-assembled CsCl nanospheres. Mater. Lett. 2012, 68, 327. [Google Scholar] [CrossRef]
- Xie, H.; Guo, R.; Zhong, H.; Feng, Q.; Lan, Z.; Qin, B.; Ward, K.J.; Jackson, M.A.; Xia, Y.; Chen, X. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. 2016, 3, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Yang, Y.; Zhao, J.; Zhang, L.; Xu, L.; Chu, X.; Liu, X.; Tian, J.; Wu, N. Identification of bacterial laccase CueO mutation from the metagenome of chemical plant sludge. Bioresour. Bioprocess. 2017, 4, 48. [Google Scholar] [CrossRef]
- Manda, K.; Gördes, D.; Mikolasch, A.; Hammer, E.; Schmidt, E.; Thurow, K.; Schauer, F. Carbon-oxygen bond formation by fungal laccases: Cross-coupling of 2, 5-dihydroxy-N-(2-hydroxyethyl)-benzamide with the solvents water, methanol, and other alcohols. Appl. Biochem. Microbiol. 2007, 76, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mikolasch, A.; Matthies, A.; Lalk, M.; Schauer, F. Laccase-induced C–N coupling of substituted p-hydroquinones with p-aminobenzoic acid in comparison with known chemical routes. Appl. Microbiol. Biotechnol. 2008, 80, 389–397. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Jeon, J.-R.; Chang, Y.-S. Laccase-mediated oxidation of small organics: Bifunctional roles for versatile applications. Trends Biotechnol. 2013, 31, 335–341. [Google Scholar] [CrossRef] [PubMed]
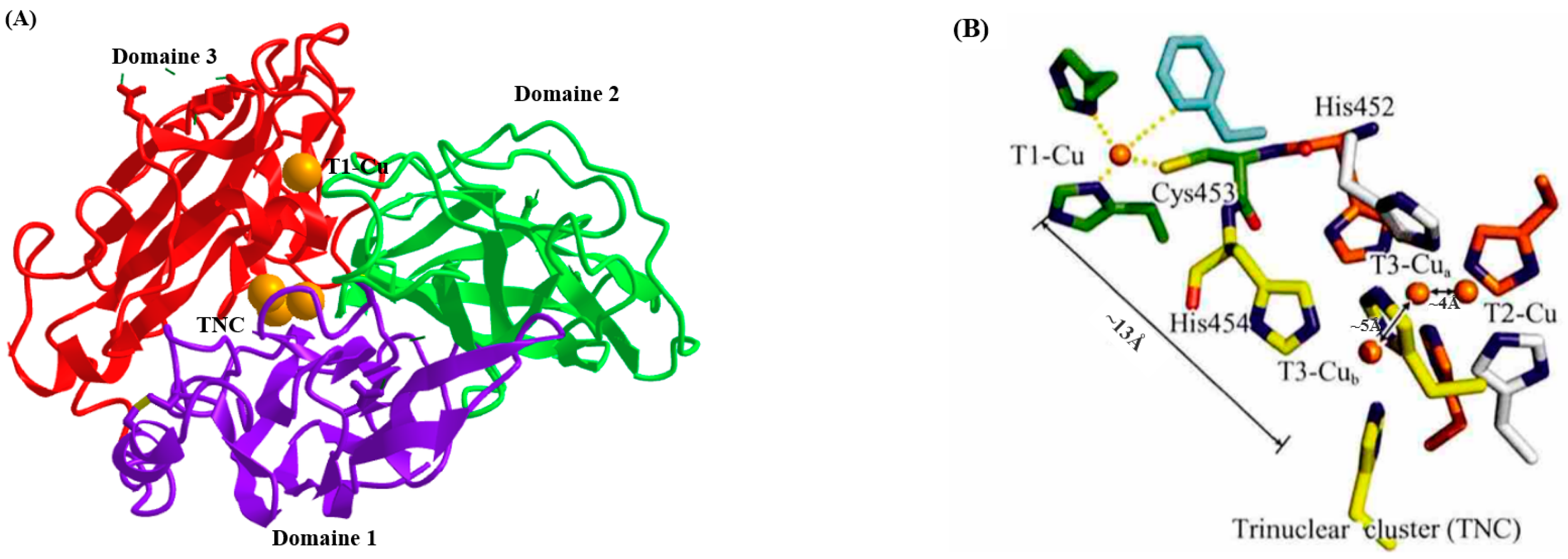









| Source | Molecular Mass | Optimal pH | Optimal Temperature (°C) | Ref. |
|---|---|---|---|---|
| Fungal | 46–80 kD | 2.2–6 | 30–55 | [18,19,20,21] |
| Bacteria | 43–114 kD | 4.0–8.0 | 40–75 | [22,23,24,25,26] |
| Plant | 59.2–140 kD | 6.7 | 20 | [27,28,29] |
| Animal | 73–110 kD | 6.5–8.0 | - | [30,31,32] |
| Entry | PMP Amine | Laccase | Product and Yield |
|---|---|---|---|
| 1 2 | 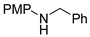 Substrate 1 | T AB |  Product 1 0% 0% |
| 3 4 | 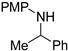 Substrate 2 | T AB | 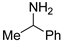 Product 2 31% 56% |
| 5 6 |  Substrate 3 | T AB |  Product 3 67% 71% |
| 7 8 | 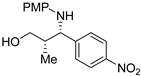 Substrate 4 | T AB |  Product 4 64% 74% |
| 9 10 |  Substrate 5 | T AB |  Product 5 89% 52% |
| 11 12 |  Substrate 6 | T AB |  Product 6 34% 41% |
| 13 14 |  Substrate 7 | T AB |  Product 7 30% 47% |
| Entry | Amine | Product and Yield |
|---|---|---|
| 1 |  1a |  1b 7% |
| 2 |  2a |  2b 97% |
| 3 |  3a |  3b 99% |
| 4 |  4a |  4b 99% |
| 5 |  5a | 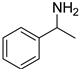 5b 97% |
| 6 |  6a | 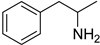 6b 99% |
| 7 |  7a |  7b 99% |
| 8 |  8a |  8b <1% |
| Entry | Amine | Product and Yield |
|---|---|---|
| 1 | 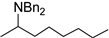 9a | 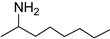 9b 4% |
| 2 |  10a |  10b <1% |
| 3 | 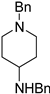 11a | 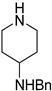 11b <1% |
 | |||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | Yield% | Number of Products |
| H | H | H | H | 0 | 0 |
| Me | H | H | H | 0 | 0 |
| Me | Me | Me | Me | 84 | 1 |
| Me | Me | (CH2)2Cl | Me | 85 | 1 |
| Me | Me | (CH2)2Cl | Me | 85 | 5 |
| Et | Me | (CH2)2Cl | Me | 74 | 5 |
| Et | Me | (CH2)2Cl | H | 0 | >7 |
| Me | H | Me | H | 78 | 3 |
| Me | Me | H | Me | 0 | >7 |
 | |||
|---|---|---|---|
| R | Regioisomer | Ratio | Yield% |
| H | a | - | 95 |
| Me | a,b | 91:9 | 98 |
| OMe | a | - | 80 |
| F | a,b | 60:40 | 98 |
| Br | b | - | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Yu, Z.; Wang, Q.; Liu, Y.; Jiang, L.; Xu, C.; Xian, M. Application of Laccase Catalysis in Bond Formation and Breakage: A Review. Catalysts 2023, 13, 750. https://doi.org/10.3390/catal13040750
Lin H, Yu Z, Wang Q, Liu Y, Jiang L, Xu C, Xian M. Application of Laccase Catalysis in Bond Formation and Breakage: A Review. Catalysts. 2023; 13(4):750. https://doi.org/10.3390/catal13040750
Chicago/Turabian StyleLin, Huan, Zongjiang Yu, Qian Wang, Yaojie Liu, Long Jiang, Chao Xu, and Mo Xian. 2023. "Application of Laccase Catalysis in Bond Formation and Breakage: A Review" Catalysts 13, no. 4: 750. https://doi.org/10.3390/catal13040750
APA StyleLin, H., Yu, Z., Wang, Q., Liu, Y., Jiang, L., Xu, C., & Xian, M. (2023). Application of Laccase Catalysis in Bond Formation and Breakage: A Review. Catalysts, 13(4), 750. https://doi.org/10.3390/catal13040750






