Ag-Cu Nanoparticles as Cathodic Catalysts for an Anion Exchange Membrane Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
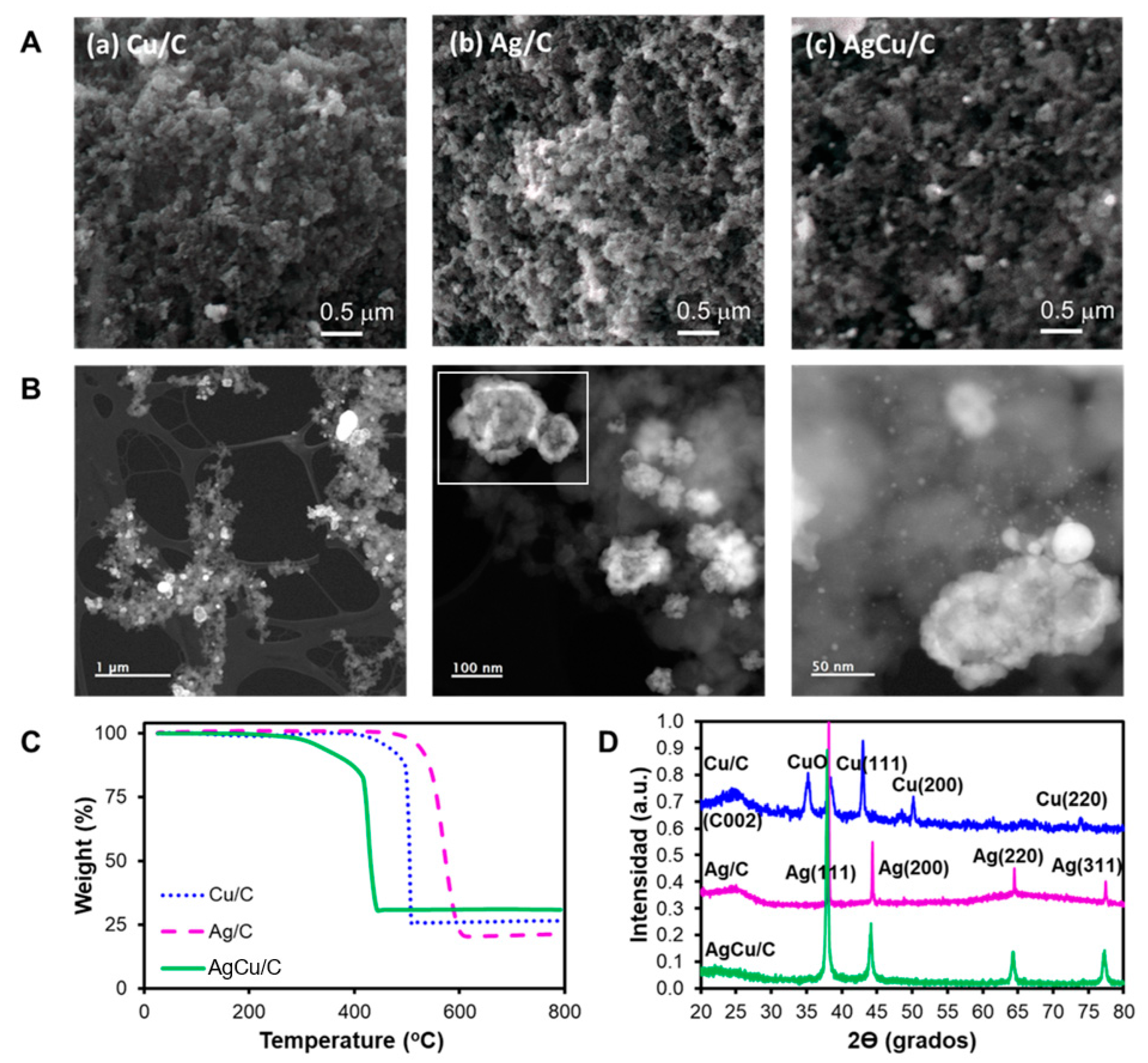
2.1. Physicochemical Characterization
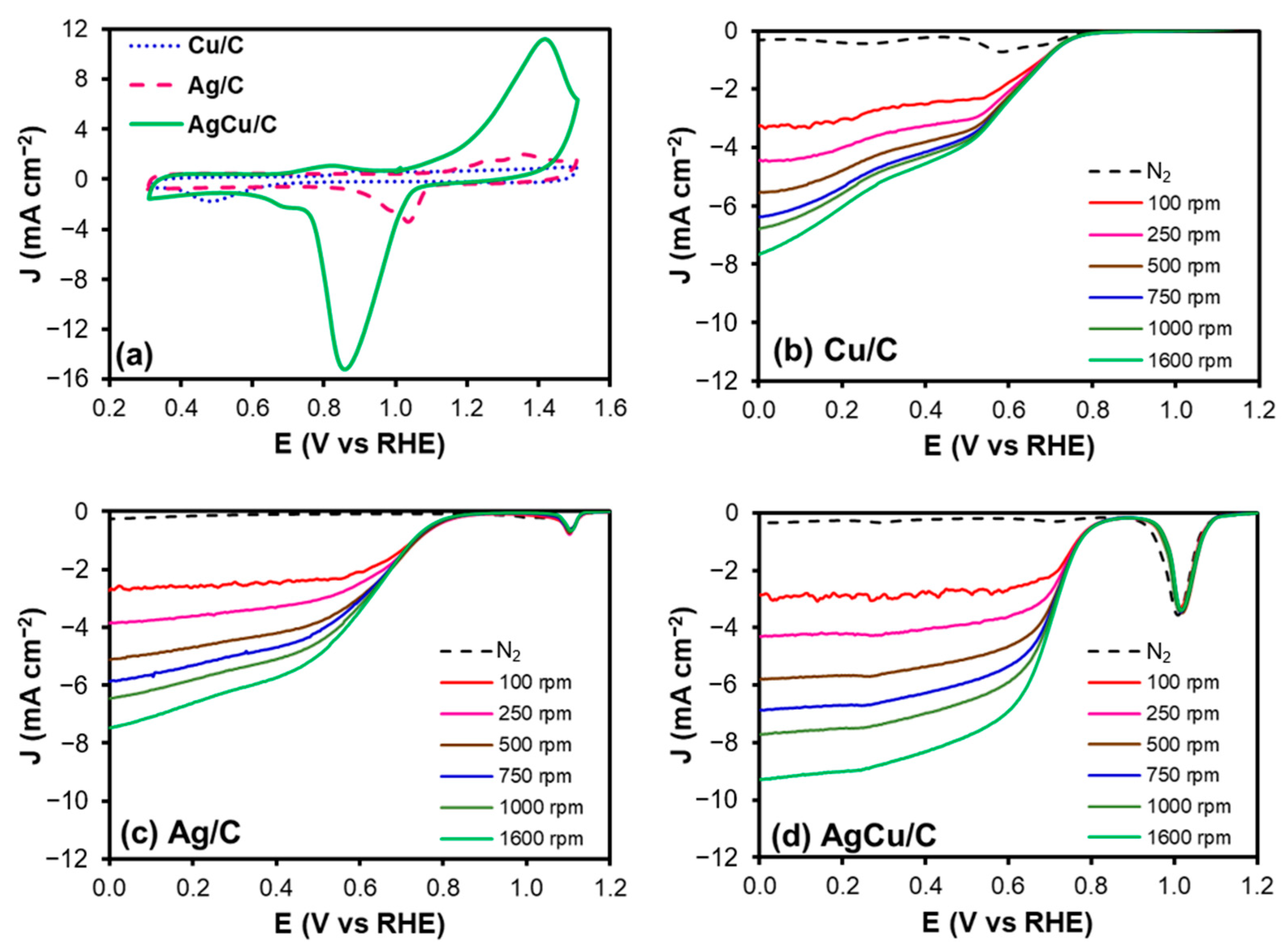
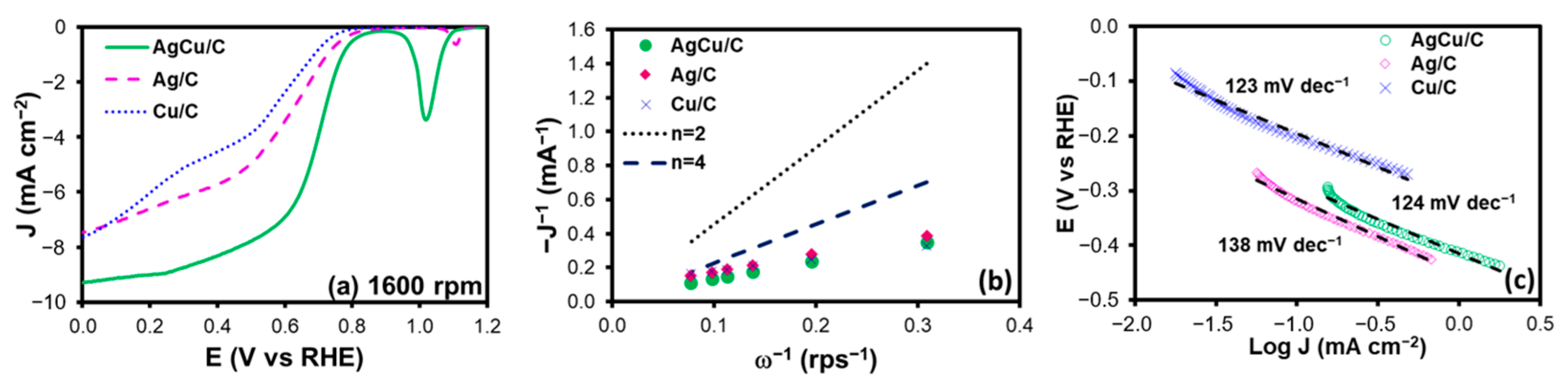
2.2. Electrochemical Characterization
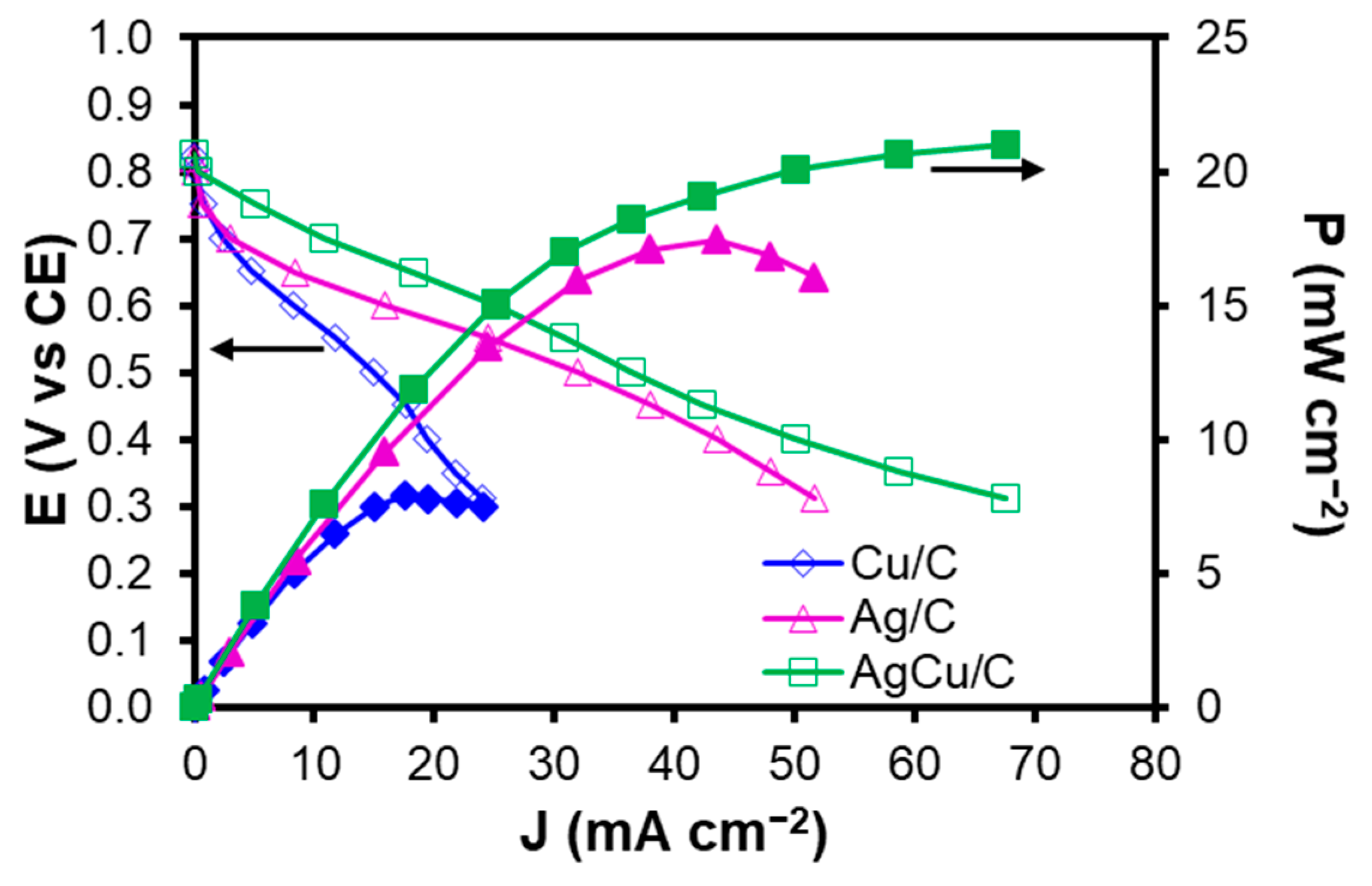
2.3. Single Fuel Cell Testing
2.3.1. Structure and Thickness of the Cathodic Catalytic Layer
2.3.2. Performance of the Cathodic Electrocatalyst in the AEMFC
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of the Nanomaterial AgCu/C
3.3. Physicochemical Characterization
3.4. Oxygen Reduction Reaction Activity
3.4.1. Cell Description and Working Electrode Preparation
3.4.2. Electrochemical Measurements
3.5. Hydrogen Fuel Cell
MEA Preparation and Fuel Cell Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, K.; Lan, Y.; Zhang, X.; Jiang, J.; Sun, C.; Yang, G.; Yang, F.; Lan, H. A Review on Interoperability of Wireless Charging Systems for Electric Vehicles. Energies 2023, 16, 1653. [Google Scholar] [CrossRef]
- Ortiz-Restrepo, J.E.; Loaiza, O.A.; Diel Urresta, J.; Velasquez, J.D.; Pastor, E.; Chaur, M.N.; Lizcano-Valbuena, W.H. A comparative study of different carbon materials as metal-free catalysts for oxygen reduction and hydrogen evolution reactions in alkaline media. Diam. Relat. Mater. 2021, 117, 108464. [Google Scholar]
- Sgarbi, R.; Kumar, K.; Jaouen, F.; Zitolo, A.; Ticianelli, E.A.; Maillard, F. Oxygen reduction reaction mechanism and kinetics on M-NxCy and M@N-C active sites present in model M-N-C catalysts under alkaline and acidic conditions. J. Solid State Electrochem. 2021, 25, 45–56. [Google Scholar]
- Zhang, H.; Sun, C.; Ge, M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202. [Google Scholar]
- Park, S.H.; Cho, Y.W.; Kim, C.S.; Park, S.B. Improved interfacial adhesion of membrane electrode assemblies using polymer binders and pore-filling membranes in alkaline membrane fuel cells. J. Solid State Electrochem. 2013, 17, 1247–1254. [Google Scholar] [CrossRef]
- Cai, S.; Jin, J.; Qiao, X.; Wang, W.; Jiang, P.; Guo, J.; Fan, H. Rapid precipitation-reduction synthesis of carbon-supported silver for efficient oxygen reduction reaction in alkaline solution. J. Solid State Electrochem. 2019, 23, 2601–2607. [Google Scholar] [CrossRef]
- de Oliveira, M.A.C.; Ficca, V.C.A.; Gokhale, R.; Santoro, C.; Mecheri, B.; D’Epifanio, A.; Licoccia, S.; Atanassov, P. Iron(II) phthalocyanine (FePc) over carbon support for oxygen reduction reaction electrocatalysts operating in alkaline electrolyte. J. Solid State Electrochem. 2021, 25, 93–104. [Google Scholar]
- Anaya-Castro, F.J.; Beltrán-Gastélum, M.; Morales Soto, O.; Pérez-Sicairos, S.; Lin, S.W.; Trujillo-Navarrete, B.; Paraguay-Delgado, F.; Salazar-Gastélum, L.J.; Romero-Castañón, T.; Reynoso-Soto, E.; et al. Ultra-Low Pt Loading in PtCo Catalysts for the Hydrogen Oxidation Reaction: What Role Do Co Nanoparticles Play? Nanomaterials 2021, 11, 3156. [Google Scholar] [CrossRef]
- Lei, H.; Siwal, S.S.; Zhang, X.; Zhang, Q. Compositional and morphological engineering of in-situ-grown Ag nanoparticles on Cu substrate for enhancing oxygen reduction reaction activity: A novel electrochemical redox tuning approach. J. Colloid Interface Sci. 2020, 571, 1–12. [Google Scholar]
- Xu, G.; Huang, J.; Li, X.; Chen, Q.; Xie, Y.; Liu, Z.; Kajiyoshi, K.; Wu, L.; Cao, L.; Feng, L. Heterostructured Cu/CuO Nanoparticles Embedded within N-Doped Carbon Nanosheets for Efficient Oxygen Reduction Reaction. Catalysts 2023, 13, 255. [Google Scholar]
- Zapata-Fernández, J.R.; Gochi-Ponce, Y.; Salazar-Gastélum, M.I.; Reynoso-Soto, E.A.; Paraguay-Delgado, F.; Lin, S.W.; Félix-Navarro, R.M. Ultrasonic-assisted galvanic displacement synthesis of Pt-Pd/MWCNT for enhanced oxygen reduction reaction: Effect of Pt concentration. Int. J. Hydrogen Energy 2017, 42, 9806–9815. [Google Scholar] [CrossRef]
- Balkan, T.; Küçükkeçeci, H.; Zarenezhad, H.; Kaya, S.; Metin, O. One pot synthesis of monodisperse copper–silver alloy nanoparticles and their composition-dependent electrocatalytic activity for oxygen reduction reaction. J. Alloys Compd. 2020, 831, 154787. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Mao, J.; Liu, C.; Chen, Y.; Lu, Z.; Feng, S.P. Bimetallic Ag–Cu nanosheets assembled flower-like structure for oxygen reduction reaction. J. Alloys Compd. 2021, 856, 157379. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Matin, M.A.; Tarlochan, F. Probing the effect of combustion controlled surface alloying in silver and copper towards ORR and OER in alkaline medium. J. Electroanal. Chem. 2019, 844, 66–77. [Google Scholar]
- Shin, K.; Kim, D.H.; Lee, H.M. Catalytic Characteristics of AgCu Bimetallic Nanoparticles in the Oxygen Reduction Reaction. ChemSusChem 2013, 6, 1044–1049. [Google Scholar] [CrossRef]
- Wu, X.; Chen, F.; Zhang, N.; Qaseem, A.; Johnston, R.L. Silver-Copper Metallic Glass Electrocatalyst with High Activity and Stability Comparable to Pt/C for Zinc–Air Batteries. J. Mater. Chem. A 2016, 4, 3527–3537. [Google Scholar] [CrossRef]
- Vega-Cartagena, M.; Rojas-Pérez, A.S.; Colón-Quintana, G.; Blasini Pérez, D.A.; Peña-Duarte, A.; Larios-Rodríguez, E.; De Jesús, M.A.; Cabrera, C.R. Potential dependent Ag nanoparticle electrodeposition on Vulcan XC-72R carbon support for alkaline oxygen reduction reaction. J. Electroanal. Chem. 2021, 891, 115242. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; You, X.; Wei, H.; Liu, S. Anodic behavior of carbon supported Cu@Ag coreeshell nanocatalysts in direct borohydride fuel cells. J. Power Sources 2015, 293, 292–300. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar]
- Higgins, D.; Wette, M.; Gibbons, B.M.; Siahrostami, S.; Hahn, C.; Escudero-Escribano, M.; García-Melchor, M.; Ulissi, Z.; Davis, R.C.; Mehta, A.; et al. Copper silver thin films with metastable miscibility for oxygen reduction electrocatalysis in alkaline electrolytes. ACS Appl. Energy Mater. 2018, 1, 1990–1999. [Google Scholar] [CrossRef]
- Nubla, K.; Sandhyarani, N. Ag nanoparticles anchored Ag2WO4 nanorods: An efficient methanol tolerant and durable Pt free electro-catalyst toward oxygen reduction reaction. Electrochim. Acta 2020, 340, 135942. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Li, Y.; Hu, X.; Huang, Y. The synthesis of highly active Cu and N co-doped electrocatalysts via strong electrostatic adsorption method for oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 23694–23701. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Goya, M.C.; Arévalo, M.C.; Rodríguez, J.L.; Pastor, E. Carbon supported Ag and Ag-Co catalysts tolerant to methanol and ethanol for the oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2016, 41, 19789–19798. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.; Wang, Z.; Qi, J.; Li, W. Carbon supported Ag nanoparticles as high performance cathode catalyst for H2/O2 anion exchange membrane fuel cell. Front. Chem. 2013, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Truong, V.M.; Yang, M.K.; Yang, H. Functionalized carbon black supported silver (Ag/C) catalysts in cathode electrode for alkaline anion exchange membrane fuel cells. Int. J. Pr. Eng. Manuf.-Gt. 2019, 6, 711–721. [Google Scholar]
- Zeng, L.; Zhao, T.S.; An, L. A high-performance supportless silver nanowire catalyst for anion exchange membrane fuel cells. J. Mater. Chem. A 2015, 3, 1410–1416. [Google Scholar] [CrossRef]
- Truong, V.M.; Tolchard, J.R.; Svendby, J.; Manikandan, M.; Miller, H.A.; Sunde, S.; Yang, H.; Dekel, D.R.; Oyarce Barnett, A. Platinum and platinum group metal-free catalysts for anion exchange membrane fuel cells. Energies 2020, 13, 582. [Google Scholar] [CrossRef] [Green Version]
- Gutru, R.; Turtayeva, Z.; Xu, F.; Maranzana, G.; Vigolo, B.; Desforges, A. A comprehensive review on water management strategies and developments in anion exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 19642–19663. [Google Scholar]
- Rivera-Lugo, Y.; Salazar-Gastélum, M.I.; López-Rosas, D.M.; Reynoso-Soto, E.A.; Pérez-Sicairos, S.; Velraj, S.; Flores-Hernández, J.R.; Félix-Navarro, R.M. Effect of template, reaction time and platinum concentration in the synthesis of PtCu/CNT catalyst for PEMFC applications. Energy 2018, 148, 561–570. [Google Scholar]
- Qaseem, A.; Chen, F.; Wu, X.; Johnston, R.L. Pt-free silver nanoalloy electrocatalysts for oxygen reduction reaction in alkaline media. Catal. Sci. Technol. 2016, 6, 3317–3340. [Google Scholar]
- Liu, Z.W.; Peng, F.; Wang, H.J.; Yu, H.; Zheng, W.X.; Yang, J. Phosphorus-doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium. Angew. Chem. 2011, 123, 3315–3319. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, F.; Jin, Y.; Liu, Z. Ag-Cu nanoalloyed film as a high-performance cathode electrocatalytic material for zinc-air battery. Nanoscale Res. Lett. 2015, 10, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, T.; Heliso Dolla, T.; Surprise Gwebu, S.; Mashola, T.A.; Tshepiso Dlamini, L.; Carleschi, E.; Ndungu, P.; Maxakato, N.W. Mn-Ni-Co-O Spinel Oxides towards Oxygen Reduction Reaction in Alkaline Medium: Mn0.5Ni0.5Co2O4/C Synergism and Cooperation. Catalysts 2021, 11, 1059. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Catalyst | E1/2 (V vs. RHE) | J0.2 V (mA cm−2) | Jk (mA cm−2) | Tafel Slope (mV dec−1) | α | J0 (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|---|
| Cu/C | 0.56 | −6.0 | 9.1 | 123 | 0.48 | 2.57 | This work |
| Ag/C | 0.60 | −6.6 | 13.6 | 138 | 0.43 | 0.52 | This work |
| AgCu/C | 0.69 | −9.0 | 29.2 | 124 | 0.47 | 0.47 | This work |
| Ag | 0.73 | −4.5 a | - | 113 | - | - | [12] |
| Cu30Ag70 | 0.76 | −5.0 a | - | 85 | - | - | [12] |
| Cu40Ag60 | 0.76 | −5.3 a | - | 84 | - | - | [12] |
| Cu60Ag40 | 0.76 | −5.5 a | - | 78 | - | - | [12] |
| Ag | 0.75 | −4.3 b | −0.55 | 101 | - | - | [13] |
| AgCu | 0.82 | −4.5 b | −1.35 | 70 | - | - | [13] |
| Cu | 0.33 | −5.3 c | - | - | - | - | [20] |
| Ag | 0.73 | −5.8 c | 0.38 | 90 | - | - | [20] |
| Ag50Cu50 | 0.70 | −6.0 c | 0.40 | 45 | - | - | [20] |
| Ag/C | 0.62 | −3.0 d | - | −81 | - | - | [21] |
| n-Cu/NC | 0.83 | −5.5 | - | 55 | - | - | [22] |
| Ag/C | 0.67 | −5.6 e | - | 112 | - | - | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Gastélum, M.; Portillo-Fuentes, S.G.; Flores-Hernández, J.R.; Salazar-Gastélum, M.I.; Trujillo-Navarrete, B.; Romero-Castañón, T.; Silva-Carrillo, C.; Reynoso-Soto, E.A.; Félix-Navarro, R.M. Ag-Cu Nanoparticles as Cathodic Catalysts for an Anion Exchange Membrane Fuel Cell. Catalysts 2023, 13, 1050. https://doi.org/10.3390/catal13071050
Beltrán-Gastélum M, Portillo-Fuentes SG, Flores-Hernández JR, Salazar-Gastélum MI, Trujillo-Navarrete B, Romero-Castañón T, Silva-Carrillo C, Reynoso-Soto EA, Félix-Navarro RM. Ag-Cu Nanoparticles as Cathodic Catalysts for an Anion Exchange Membrane Fuel Cell. Catalysts. 2023; 13(7):1050. https://doi.org/10.3390/catal13071050
Chicago/Turabian StyleBeltrán-Gastélum, Mara, Samantha Goretti Portillo-Fuentes, José Roberto Flores-Hernández, Moisés Israel Salazar-Gastélum, Balter Trujillo-Navarrete, Tatiana Romero-Castañón, Carolina Silva-Carrillo, Edgar Alonso Reynoso-Soto, and Rosa María Félix-Navarro. 2023. "Ag-Cu Nanoparticles as Cathodic Catalysts for an Anion Exchange Membrane Fuel Cell" Catalysts 13, no. 7: 1050. https://doi.org/10.3390/catal13071050






