One-Dimensional Tubular Carbon Nitride Embedded in Ni2P for Enhanced Photocatalytic Activity of H2 Evolution
Abstract
:1. Introduction
2. Results and Discussion
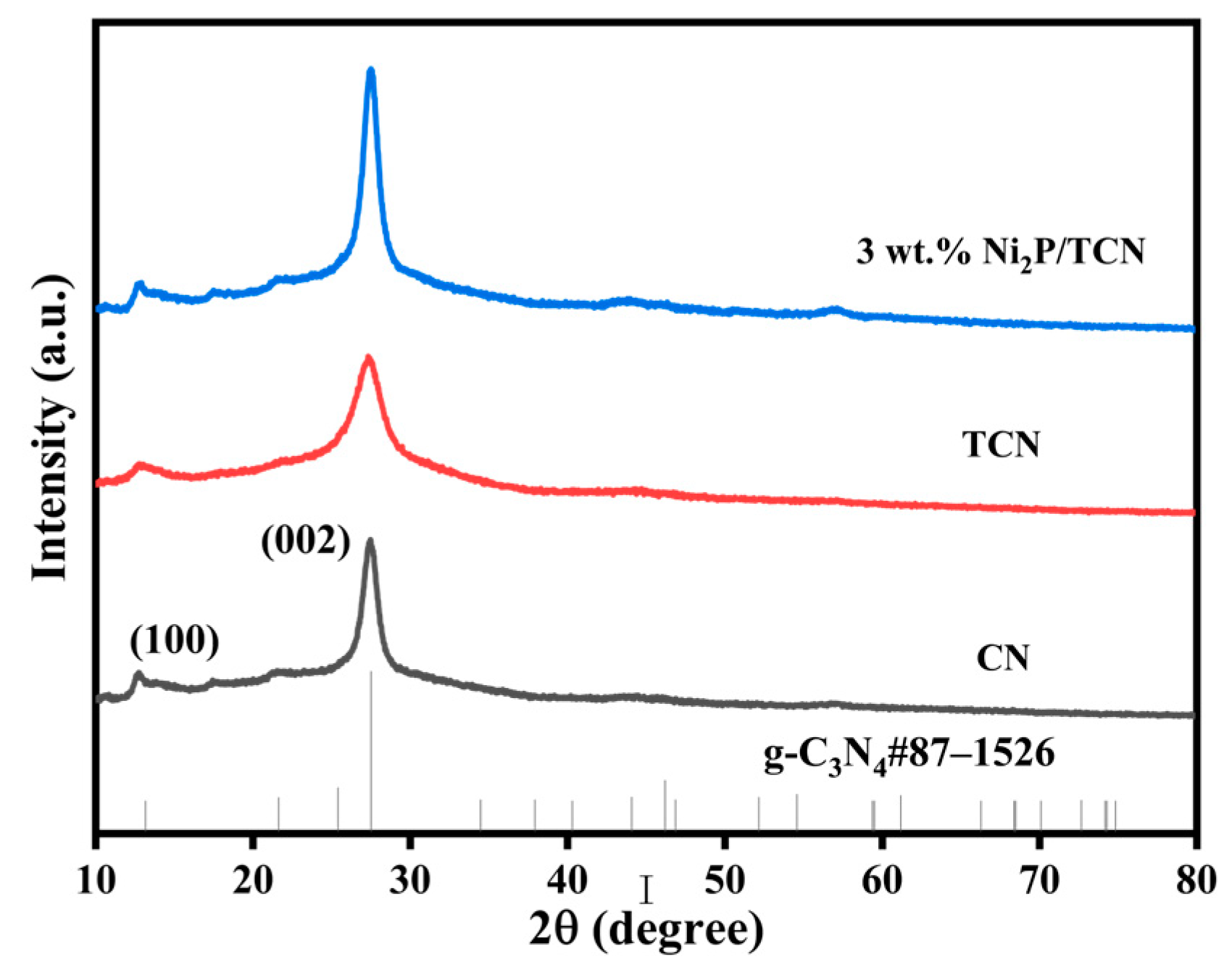
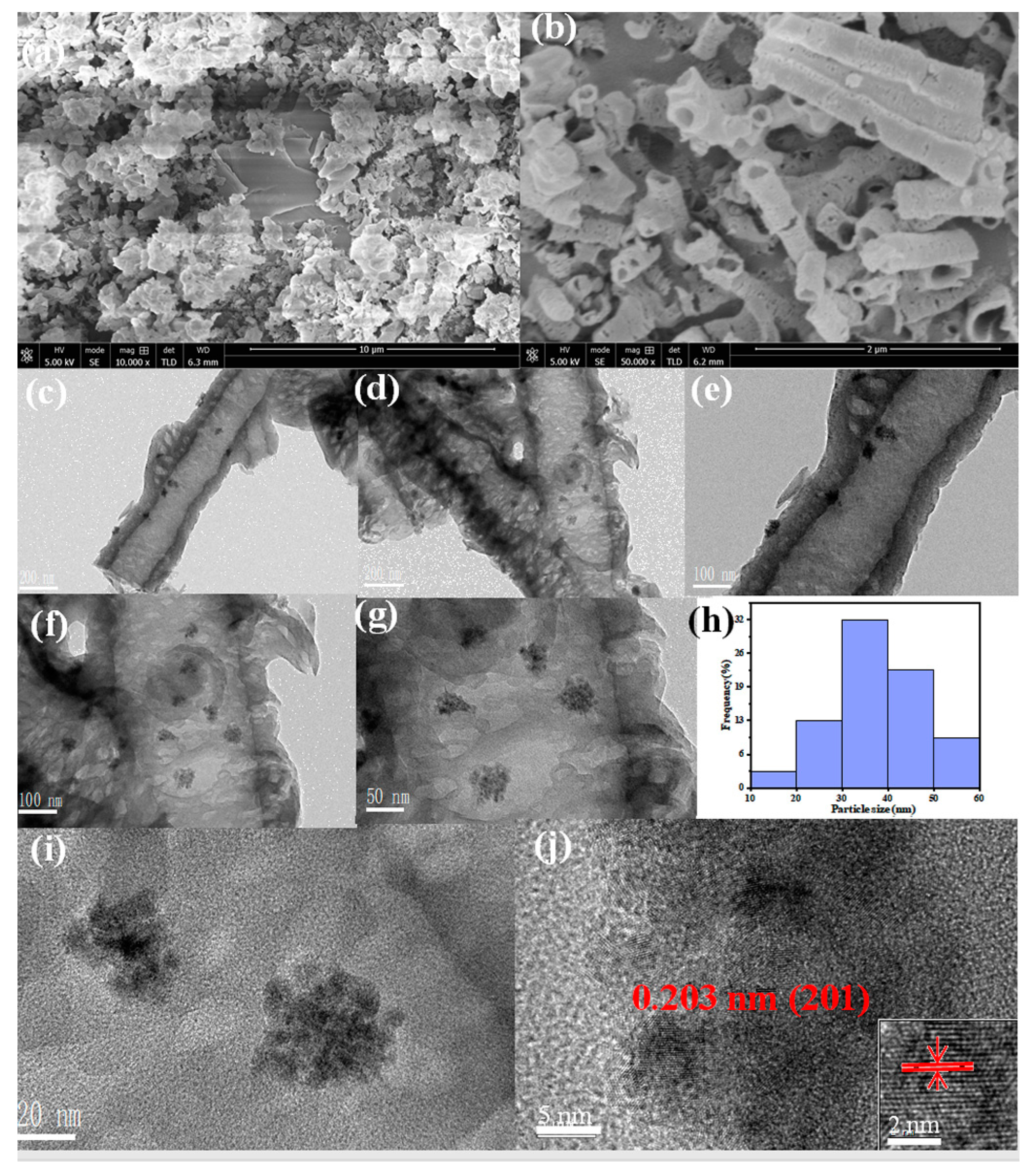
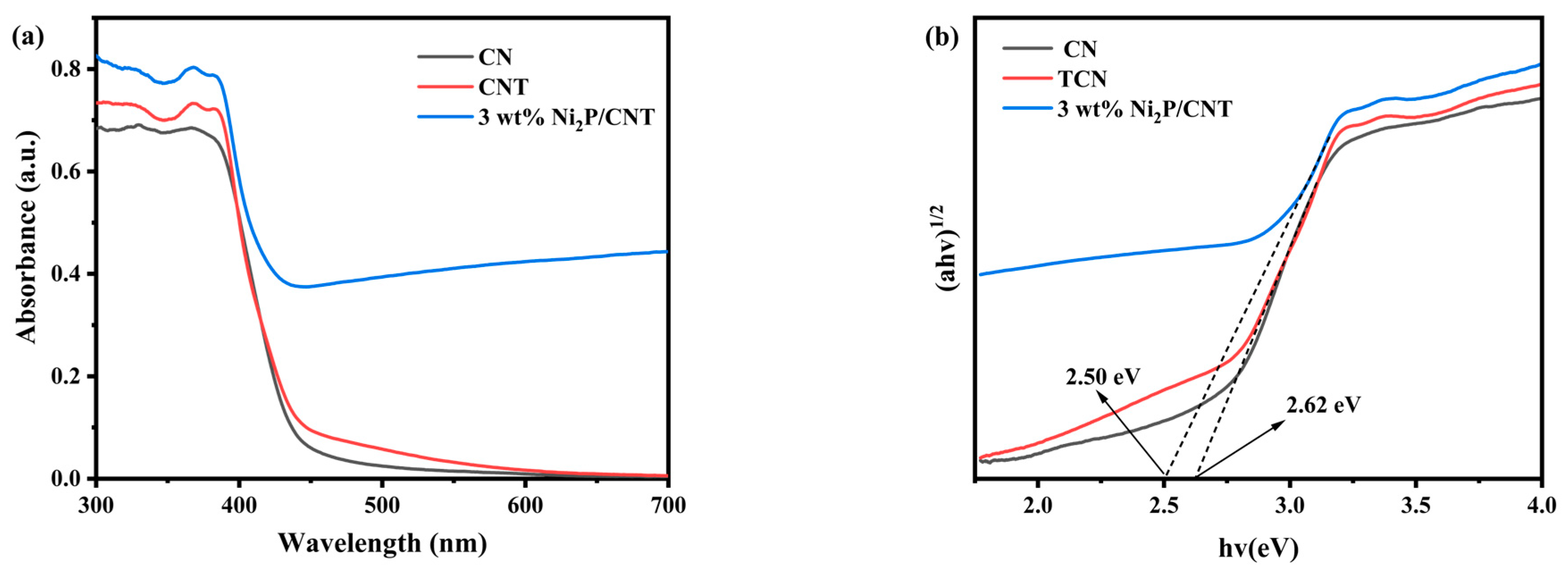
2.1. Catalyst Characterization
2.2. Performance Analysis of Photocatalysts
2.3. Reaction Mechanism Studies
3. Experimental Section
3.1. Materials
3.2. Preparation of CN
3.3. Preparation of Tubular TCN
3.4. Preparation of Ni2P/TCN
3.5. Characterization
3.6. Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development-A technological and geopolitical overview. Int. J. Hydrogen Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Voumik, L.C.; Sultana, T. Impact of urbanization, industrialization, electrification and renewable energy on the environment in BRICS: Fresh evidence from novel CS-ARDL model. Heliyon 2022, 8, e11457. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, Z.; Zou, Y.; Chen, J.; Shi, J.W. The progress of g-C3N4 in photocatalytic H2 evolution: From fabrication to modification. Coord. Chem. Rev. 2024, 500, 215489. [Google Scholar] [CrossRef]
- Dong, F.; Qin, L.; Zhang, T.; Li, X.; Kang, S.Z. A novel pathway toward efficient improvement of the photocatalytic activity and stability of CdS-based photocatalyst for light driven H2 evolution: The synergistic effect between CdS and SrWO4. Int. J. Hydrogen Energy 2023, 48, 13877–13889. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Cai, P.; Yao, W.; Lin, J. Formation of p-n Heterojunctions by Incorporation of Mn2+ into the CdS Lattice toward Highly Efficient Photocatalytic H2 Evolution. J. Phys. Chem. C 2023, 127, 4544–4552. [Google Scholar] [CrossRef]
- Dai, X.; Feng, S.; Ma, C.; Xu, L.; Fan, L.; Ye, Z.; Wang, Y. Direct Z-scheme ZnCo2S4/MOF-199 constructed by bimetallic sulfide modified MOF for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 639, 158142. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Tian, Y.; Xu, Z.; Wang, W.; Qiu, M.; Wang, H.; Li, X.; Zhu, G.; Wang, Y. Covalent Organic Framework/g-C3N4 van der Waals Heterojunction toward H2 Production. Inorg. Chem. 2023, 62, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.X.; Ma, C.; Zhang, F.G.; Cheng, Q.; Liu, Q.Y.; Yuan, Y.J.; Zhang, X. Sub-10 nm anatase TiO2 nanoparticles for rapid photocatalytic H2 production from lignocellulosic biomass. J. Mater. Chem. A 2023, 11, 7488–7497. [Google Scholar] [CrossRef]
- Khan, H.; Charles, H.; Lee, C.S. Fabrication of noble-metal-free copper-doped TiO2 nanofibers synergized with acetic acid-treated g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 607, 155068. [Google Scholar] [CrossRef]
- Yan, D.; Miao, H.; Fan, J.; Yu, Q.; Liu, E.; Sun, T. Constructing Dual Cocatalysts of Ni2P-NiS-Decorated TiO2 for Boosting Photocatalytic H2 Evolution. Langmuir 2023, 39, 16648–16656. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Chen, S.; Bai, J.; Li, J.; Zhang, Y.; Li, L.; Xia, L.; Rahim, M.; Xu, Q.; et al. Bird-nest structured ZnO/TiO2 as a direct Z-scheme photoanode with enhanced light harvesting and carriers kinetics for highly efficient and stable photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 267, 118599. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, F.; Chen, Y.; Li, J.Y.; Xu, Y.J. Photoredox-catalyzed biomass intermediate conversion integrated with H2 production over Ti3C2Tx/CdS composites. Green Chem. 2020, 22, 163–169. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.; Wang, J.; Li, J.; Wang, G. Review on S-Scheme Heterojunctions for Photocatalytic Hydrogen Evolution. Acta Phys. Chim. Sin. 2023, 39, 2212016. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, X.; Chen, Y.; Xiao, X.; Chen, T.; Wang, Y. Recent Advances in Carbon Nitride-Based S-scheme Photocatalysts for Solar Energy Conversion. Materials 2023, 16, 3745. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, C.; Qin, J.; Rajendran, S.; Zhang, X. A facile template synthesis of phosphorus-doped graphitic carbon nitride hollow structures with high photocatalytic hydrogen production activity. Mater. Chem. Phys. 2022, 275, 125299. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A Facile One-Step Synthesis of Fe-Doped g-C3N4 Nanosheets and Their Improved Visible-Light Photocatalytic Performance. Chemcatchem 2017, 9, 1708–1715. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; He, C.; Zhang, P.; Mi, H. Oxygen-doped crystalline carbon nitride with greatly extended visible-light-responsive range for photocatalytic H2 generation. Appl. Catal. B Environ. 2021, 283, 119636. [Google Scholar] [CrossRef]
- Jiang, J.; Cao, S.; Hu, C.; Chen, C. A comparison study of alkali metal-doped g-C3N4 for visible-light photocatalytic hydrogen evolution. Chin. J. Catal. 2017, 38, 1981–1989. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-Doped Carbon Nitride Tubes with a Layered Micro-nanostructure for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Edit. 2016, 55, 1830–1834. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule Self-Assembly Synthesis of Porous Few-Layer Carbon Nitride for Highly Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Cui, L.; Song, J.; McGuire, A.F.; Kang, S.; Fang, X.; Wang, J.; Yin, C.; Li, X.; Wang, Y.; Cui, B. Constructing Highly Uniform Onion-Ring-like Graphitic Carbon Nitride for Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution. ACS Nano 2018, 12, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Wang, H.; Li, Y.; Liu, Y.; Qian, Q.; Jin, X.; Wang, X.; Zhang, J.; Zhang, G. Realizing synergistic effect of electronic modulation and nanostructure engineering over graphitic carbon nitride for highly efficient visible-light H2 production coupled with benzyl alcohol oxidation. Appl. Catal. B Environ. 2020, 269, 118772. [Google Scholar] [CrossRef]
- Du, R.; Li, B.; Han, X.; Xiao, K.; Wang, X.; Zhang, C.; Arbiol, J.; Cabot, A. 2D/2D Heterojunction of TiO2 Nanoparticles and Ultrathin G-C3N4 Nanosheets for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 1557. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Li, Z.; Huang, Z.H.; Kang, F.; Yang, Q.H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Ruan, D.; Kim, S.; Fujitsuka, M.; Majima, T. Defects rich g-C3N4 with mesoporous structure for efficient photocatalytic H2 production under visible light irradiation. Appl. Catal. B Environ. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Yang, P.; Zhuzhang, H.; Wang, R.; Lin, W.; Wang, X. Carbon Vacancies in a Melon Polymeric Matrix Promote Photocatalytic Carbon Dioxide Conversion. Angew. Chem. Int. Edit. 2019, 58, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xu, W.; Wan, H.; Yuan, D.; Chen, C.; Wang, L.; Guan, G.; Dai, W.L. Nitrogen vacancy engineered graphitic C3N4-based polymers for photocatalytic oxidation of aromatic alcohols to aldehydes. Appl. Catal. B Environ. 2018, 221, 626–634. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient Photocatalytic Overall Water Splitting Induced by the Giant Internal Electric Field of a g-C3N4/rGO/PDIP Z-Scheme Heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef]
- Dang, V.D.; Adorna, J., Jr.; Annadurai, T.; Bui, T.A.N.; Tran, H.L.; Lin, L.Y.; Doong, R.A. Indirect Z-scheme nitrogen-doped carbon dot decorated Bi2MoO6/g-C3N4 photocatalyst for enhanced visible-light-driven degradation of ciprofloxacin. Chem. Eng. J. 2021, 422, 130103. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.T.F.; et al. High Efficiency Photocatalytic Water Splitting Using 2D α-Fe2O3/g-C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Bai, X.; Zong, R.; Li, C.; Liu, D.; Liu, Y.; Zhu, Y. Enhancement of visible photocatalytic activity via Ag@C3N4 core-shell plasmonic composite. Appl. Catal. B Environ. 2014, 147, 82–91. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, Y.; Xu, Y.; Wang, X. Photocatalytic hydrogen production over carbon nitride loaded with WS2 as cocatalyst under visible light. Appl. Catal. B Environ. 2014, 156, 122–127. [Google Scholar] [CrossRef]
- Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Z.; Qiu, B.; Xing, M.; Zhang, J. Emerging Cocatalysts on g-C3N4 for Photocatalytic Hydrogen Evolution. Small 2021, 17, 2101070. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Lee, S.L.J.; Zhao, X.; Lin, S. Constructing novel graphitic carbon nitride-based nanocomposites—From the perspective of material dimensions and interfacial characteristics. Chemosphere 2022, 302, 134889. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Zhu, Y. Photocatalytic Activity Enhanced via g-C3N4 Nanoplates to Nanorods. J. Phys. Chem. C 2013, 117, 9952–9961. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Fan, X.; Wu, M.; Wang, M.; Cheng, R.; Zhang, L.; Yao, H.; Shi, J. Core-shell LaPO4/g-C3N4 nanowires for highly active and selective CO2 reduction. Appl. Catal. B Environ. 2017, 201, 629–635. [Google Scholar] [CrossRef]
- Li, C.; Du, Y.; Wang, D.; Yin, S.; Tu, W.; Chen, Z.; Kraft, M.; Chen, G.; Xu, R. Unique P-Co-N Surface Bonding States Constructed on g-C3N4 Nanosheets for Drastically Enhanced Photocatalytic Activity of H2 Evolution. Adv. Funct. Mater. 2017, 27, 1604328. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Dong, Y.; Jiang, P. Noble-Metal-Free Iron Phosphide Cocatalyst Loaded Graphitic Carbon Nitride as an Efficient and Robust Photocatalyst for Hydrogen Evolution under Visible Light Irradiation. Acs Sustain. Chem. Eng. 2017, 5, 8053–8060. [Google Scholar] [CrossRef]
- Huang, Z.; Long, X.; Liu, M.; Li, X.; Du, Y.; Liu, Q.; Chen, Y.; Guo, S.; Chen, R. Constructing CoP-C/g-C3N4 nanocomposites with P-C bond bridged interface and van der Waals heterojunctions for enhanced photocatalytic H2 evolution. J. Colloid Interface Sci. 2024, 653, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Cao, S.; Yu, J. Ni-P cluster modified carbon nitride toward efficient photocatalytic hydrogen production. Chin. J. Catal. 2019, 40, 867–874. [Google Scholar] [CrossRef]
- Tang, J.; Li, X.; Ma, Y.; Xu, N.; Liu, Y.; Zhang, Q. Formation of interfacial P-Ni-P coordination to boost charge transfer of polymeric carbon nitride for enhanced photocatalytic activity of H2 evolution. Appl. Surf. Sci. 2022, 602, 154228. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, G.; Lin, Z.; Wang, X. Condensed and low-defected graphitic carbon nitride with enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B Environ. 2016, 181, 413–419. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.C.H.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Tang, Y.; Zeng, Y.; Wang, L.; Zhang, S.; Cai, T.; Liu, Y.; Luo, S.; Pei, Y.; et al. Positioning cyanamide defects in g-C3N4: Engineering energy levels and active sites for superior photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 237, 24–31. [Google Scholar] [CrossRef]
- Lv, S.; Ng, Y.H.; Zhu, R.; Li, S.; Wu, C.; Liu, Y.; Zhang, Y.; Jing, L.; Deng, J.; Dai, H. Phosphorus vapor assisted preparation of P-doped ultrathin hollow g-C3N4 sphere for efficient solar-to-hydrogen conversion. Appl. Catal. B Environ. 2021, 297, 120438. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Luo, B.; Song, R.; Jing, D. Significantly enhanced photocatalytic hydrogen generation over graphitic carbon nitride with carefully modified intralayer structures. Chem. Eng. J. 2018, 332, 499–507. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Liu, G.; Cheng, H.M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, S.; Jiang, P.; Xu, Z.J. Graphitic C3N4 modified by Ni2P cocatalyst: An efficient, robust and low cost photocatalyst for visible-light-riven H2 evolution from water. Chem. Eng. J. 2017, 315, 296–303. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Ye, Z.; Zhou, M.; Wang, H.; Ma, C.; Wang, D.; Huo, P.; Yan, Y. Fast electron transfer and enhanced visible light photocatalytic activity using multi-dimensional components of carbon quantum dots@3D daisy-like In2S3/single-wall carbon nanotubes. Appl. Catal. B Environ. 2017, 204, 224–238. [Google Scholar] [CrossRef]
- Muhammad, A.; Tahir, M.; Al-Shahrani, S.S.; Ali, A.M.; Rather, S.U. Template free synthesis of graphitic carbon nitride nanotubes mediated by lanthanum (La/g-CNT) for selective photocatalytic CO2 reduction via dry reforming of methane (DRM) to fuels. Appl. Surf. Sci. 2020, 504, 144177. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, P.; Wang, L.; Wang, S.; Shi, J.; Lan, X. Electronegativity Assisted Synthesis of Magnetically Recyclable Ni/NiO/g-C3N4 for Significant Boosting H2 Evolution. Materials 2021, 14, 2894. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhu, M.; Fujitsuka, M.; Wang, A.; Shi, C.; Majima, T. Phase Effect of NixPy Hybridized with g-C3N4 for Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2017, 9, 30583–30590. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Luo, X.; Wen, J.; Ma, S.; Li, X.; Fang, Y.; Zhang, X. Enhanced visible light photocatalytic H2 production over Z-scheme g-C3N4 nansheets/WO3 nanorods nanocomposites loaded with Ni(OH)x cocatalysts. Chin. J. Catal. 2017, 38, 240–252. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Shen, R.; Li, X.; Luo, X.; Zhang, H.; Zhang, A.; Bi, G. Markedly enhanced visible-light photocatalytic H2 generation over g-C3N4 nanosheets decorated by robust nickel phosphide (Ni12P5) cocatalysts. Dalton Trans. 2017, 46, 1794–1802. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Q.; Ma, L.; Wu, H.; Li, Y.; Han, J.; Chen, G.; Xing, W. Strengthening reactive metal-support interaction to stabilize Ni species on the nitrogen vacancies of g-C3N4 for boosting photocatalytic H2 production. Catal. Sci. Technol. 2021, 11, 7134–7140. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.; Zhang, L.; Si, R.; Shen, M.; Wang, M.; Tian, J.; Shi, J. Electron Configuration Modulation of Nickel Single Atoms for Elevated Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Edit. 2020, 59, 6827–6831. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, S.; Zhou, D.; Di, T.; Wang, T. Two-Dimensional Layered Co(OH)2/g-C3N4/Ni(OH)2 Ternary Nanocomposites for Enhanced Visible-Light Photocatalytic H2-Production Activity. ACS Appl. Energy Mater. 2021, 4, 6340–6347. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, L.J.; Pan, W.G.; Bai, S.C.; Guo, R.T. Efficient photocatalytic H2 evolution over NiS-PCN Z-scheme composites via dual charge transfer pathways. Appl. Catal. B Environ. 2021, 289, 120040. [Google Scholar] [CrossRef]
- Andreou, E.K.; Koutsouroubi, E.D.; Vamvasakis, L.; Armatas, G.S. Ni2P-Modified P-Doped Graphitic Carbon Nitride Hetero-Nanostructures for Efficient Photocatalytic Aqueous Cr(VI) Reduction. Catalysts 2023, 13, 437. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.Y.; Wang, W.J.; Xiong, W.P.; Zeng, G.M.; Huang, D.L.; Zhang, C.; Song, B.; Xue, W.J.; Li, X.P.; et al. Recent advances in application of transition metal phosphides for photocatalytic hydrogen production. Chem. Eng. J. 2021, 405, 126547. [Google Scholar] [CrossRef]
- Zhang, C.; Chu, S.P.; Liu, B.Q.; Liu, Y.; Guo, Z.M.; Lv, Z.G. MOF-mediated fabrication of coralloid Ni2P@CdS for enhanced visible-light hydrogen evolution. Appl. Surf. Sci. 2021, 569, 150987. [Google Scholar] [CrossRef]









| Sample | SBET (m2·g−1) | Total Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| CN | 74 | 0.39 | 22.74 |
| TCN | 95 | 0.50 | 21.80 |
| 3 wt.% Ni2P/TCN | 88 | 0.42 | 19.46 |
| Catalysts | Synthetic Method | Light Source | Activity (μmol·g−1·h−1) | Reference |
|---|---|---|---|---|
| Ni/NiO/g-C3N4 | solvothermal method | 300 W Xe-lamp (λ > 420 nm) | 2310 | [55] |
| NixPy/g-C3N4 | hydrothermal method | 500 W Xe-lamp (λ > 420 nm) | 162 | [56] |
| WO3/g-C3N4/ Ni(OH)x | photodeposition method | 300 W Xe-lamp (λ > 400 nm) | 576 | [57] |
| Ni12P5/g-C3N4 | mechanical grinding method | 300 W Xe-lamp (λ > 420 nm) | 126.6 | [58] |
| DCN-Ni | chemical reduction method | 300 W Xe-lamp (λ > 420 nm) | 449 | [59] |
| CN-0.2Ni-HO | high-temperature hydrogen reduction | 300 W Xe-lamp (λ > 420 nm) | 354.9 | [60] |
| Co(OH)2/g-C3N4/Ni(OH)2 | solvothermal method | 300 W Xe-lamp (λ > 420 nm) | 899 | [61] |
| NiP/g-C3N4 | chemical reduction method | 350 W Xe-lamp (λ > 420 nm) | 1506 | [43] |
| NiS/SO-PCN | solvothermal method | 300 W Xe-lamp (λ > 420 nm) | 1239 | [62] |
| Ni2P/P-PCN | high-temperature phosphating process | 300 W Xe-lamp (λ > 420 nm) | 1250 | [44] |
| Ni2P/TCN | photodeposition | 300 W Xe-lamp (λ > 400 nm) | 3715 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Jiao, Y.; Li, F.; Fang, C.; Ding, J.; Wan, H.; Zhang, P.; Guan, G. One-Dimensional Tubular Carbon Nitride Embedded in Ni2P for Enhanced Photocatalytic Activity of H2 Evolution. Catalysts 2024, 14, 243. https://doi.org/10.3390/catal14040243
Jiang C, Jiao Y, Li F, Fang C, Ding J, Wan H, Zhang P, Guan G. One-Dimensional Tubular Carbon Nitride Embedded in Ni2P for Enhanced Photocatalytic Activity of H2 Evolution. Catalysts. 2024; 14(4):243. https://doi.org/10.3390/catal14040243
Chicago/Turabian StyleJiang, Chenyong, Yiwei Jiao, Fada Li, Cheng Fang, Jing Ding, Hui Wan, Ping Zhang, and Guofeng Guan. 2024. "One-Dimensional Tubular Carbon Nitride Embedded in Ni2P for Enhanced Photocatalytic Activity of H2 Evolution" Catalysts 14, no. 4: 243. https://doi.org/10.3390/catal14040243




