Abstract
Fine chemicals are produced in small annual volume batch processes (often <10,000 tonnes per year), with a high associated price (usually >USD 10/kg). As a result of their usage in the production of speciality chemicals, in areas including agrochemicals, fragrances, and pharmaceuticals, the need for them will remain high for the foreseeable future. This review article assesses current methods used to produce fine chemicals with heterogeneous catalysts, including both well-established and newer experimental methods. A wide range of methods, utilising microporous and mesoporous catalysts, has been explored, including their preparation and modification before use in industry. Their potential drawbacks and benefits have been analysed, with their feasibility compared to newer, recently emerging catalysts. The field of heterogeneous catalysis for fine chemical production is a dynamic and ever-changing area of research. This deeper insight into catalytic behaviour and material properties will produce more efficient, selective, and sustainable processes in the fine chemical industry. The findings from this article will provide an excellent foundation for further exploration and a critical review in the field of fine chemical production using micro- and mesoporous heterogeneous catalysts.
1. Introduction
Fine chemicals are described as pure and complex substances, produced mainly for use in further processing to manufacture speciality, high-value chemicals. They are produced in small annual volume batch processes (often <10,000 tonnes per year) [1], with a high associated price (usually >USD 10/kg) [1]. As a result of their usage in the production of speciality chemicals, in areas including agrochemicals, fragrances, and pharmaceuticals, their necessity will remain high for the foreseeable future. As a result, the way in which fine chemicals are produced is of the utmost importance. At this moment in time, there is a scarcity of literature regarding the production of fine chemicals through the use of micro and mesoporous catalysts. Particularly in recent years, the focus has shifted away from reviewing existing catalytic methods, especially in the fine chemical industry.
The production of industrially significant complex molecules, such as fine and speciality chemicals, heavily relies on a range of organic synthetic techniques which employ several reagents and catalysts. As a result of this, significant advancements in the field of catalysis over the past two decades have been instrumental in improving precise and selective control of various reaction paths and products [2,3]. However, the main issue with soluble (typically precious metal) catalysts is often contamination of the reaction products, aside from their expense and toxicity. Additionally, these reaction promotors frequently experience moderate selectivity (towards the activation of the desired functional group), deactivation/degradation (reducing the number of turnovers or productivity), and complex product purification (from the high-added-value organic compounds) [4]. This emphasises the requirement for sophisticated catalysts to be used in various stages of organic synthesis that have the right design, reactivity, and long-term stability.
In an ideal scenario, heterogeneous catalysis provides a clearer means of recovering the reaction product (from the solid catalyst) in a liquid medium. This product can then be recovered using filtration or centrifugation, thereby reducing the likelihood of contaminating the intended synthetic product [5,6]. Furthermore, under more accommodating reaction conditions and longer reaction times, such a reactive solid could be used. It would work with a continuous flow of reactants to separate the product without requiring the reactor to be evacuated, and it would provide easy scale-up, step-economy, high yields, safety, and reproducibility [7,8]. Ordered porous solids are the most desirable among the various solids used as heterogeneous catalysts (such as polymers, crystalline metal oxides, amorphous carbon, etc.) in terms of the fundamental comprehension and control of the chemical transformation in pores (which have the same size, shape, and functionality throughout the crystal). This points towards a clear need to review existing catalytic porous solids, as well as experimentally promising materials in development.
The purpose of this review is to assess the current methods used to produce fine chemicals with heterogeneous catalysts, including both well-established methods, as well as newer experimental methods. A wide range of methods, with the utilisation of both microporous and mesoporous catalysts, will be explored, including their preparation and modification before use in industry. Their potential drawbacks, as well as benefits, will be analysed, with their feasibility compared to that of newer, recently emerging catalysts. The findings of this literature review will form a basis for a critical review of the same topic, providing a platform to further critique this field in greater detail.
The motivation for conducting this critical review on both microporous and mesoporous heterogeneous catalysts in the fine chemical industry is primarily driven by the lack of recent, comprehensive literature encapsulating this rapidly advancing field. As it stands, there is a significant amount of emerging literature surrounding individual catalysts and their applications; however, to the best of our knowledge, there are few to no concise and updated overall reviews. This paper aims to address this void by providing an updated and detailed examination of current advancements, methodologies, and applications of heterogeneous catalyst structures in the fine chemical industry.
2. Relevant Sections
2.1. What Are Fine Chemicals?
Fine chemicals are both complex and single pure chemical substances produced in relatively low annual volumes globally. These volumes are approximately <1000 MT per year, with a high price associated with them, (>USD 10 per kg) [1].
Chemicals that fall under the heading of “fine chemicals” can be found in a range of sectors, including the pharmaceutical, agrochemical, and life sciences sectors [1]. As stated by Ref. [9], fine chemicals are usually produced in batch processes, as opposed to bulk chemicals and commodities, which are produced in continuous processes. This is one of the reasons behind the smaller and more tightly controlled volume of these fine chemicals, increasing the value and thus, the market price, of these fine chemicals.
Additionally, fine chemicals are often required for very specific and complex processes. The fine chemical industry falls between the other two main sectors of chemicals: commodities and speciality chemicals, with each being the supplier and consumer of fine chemicals, respectively. As a result, fine chemicals possess uses in high-value production, as well as provide added value to cheaper chemical commodities. In 2021, fine and speciality chemicals represented approximately 17% of worldwide chemical exports, with an associated value of EUR 379 billion [10]. This is because often, there are further processing stages required for fine chemicals after their production, which leads to the final product being even greater in value. As stated by Ref. [11], fine chemicals are often produced to very exact specifications, defining what they are, as opposed to what they can do, as in the example of speciality chemicals. This is due to the fact that fine chemicals require further processing down the line for the production of other higher-value products like those speciality chemicals mentioned above.
According to Ref. [11], fine chemicals can be split into three major categories, “biocides, active pharmaceutical ingredients and speciality chemicals”. These statements are supported by Ref. [12], in which fine chemicals are referred to in a similar manner to the description above as single pure substances based on exacting specifications for further processing. An additional explanation for their high value and small production volumes would be the strict regulations to which fine chemicals are often subjected since the majority end up facing human use or consumption.
Areas in which fine chemicals are often used, once being further developed into speciality chemicals, include adhesives, agrochemicals, biocides, catalysts, dyestuffs and pigments, enzymes, electronic chemicals, flavours and fragrances, food and feed additives, pharmaceuticals and speciality polymers.
2.2. The Use of Heterogeneous Catalysts in the Production of Fine Chemicals
Why Are Catalysts Used?
Catalysis, in any form, is essential for chemical reactions. Even in those few situations where reaction speed is already sufficient, reaction economy, yield, and selectivity can be improved by the utilisation of a catalyst. An ideal catalyst is one with both a high turnover frequency (TOF), as well as an infinite amount of product produced (TON) under room temperature and atmospheric pressure. This, however, is often not feasible in practice. There are high associated costs with maintaining a reactor at room temperature, particularly during highly exothermic reactions. As a result, a compromise between the operating conditions and the desired TOF/TON is often made. Alongside this, the reaction should feature no catalyst deactivation or poisoning.
2.3. Catalyst Selection
The catalysts used in the production of fine chemicals vary drastically, being heavily dependent on the reaction and the chemicals being produced. Moreover, the choice of which catalyst to use is reliant on a range of other factors. Several catalysts could be suitable for a reaction; however, others may be more optimal in terms of cost, yield, time taken for the reaction, selectivity, ability to resist catalyst poisoning or a form of deactivation, with minimal product damage or contamination. These factors are essential for catalyst choice in terms of its economy and lifespan consideration, as explained by Ref. [13]. Additionally, it is suggested that catalysts also be considered from a practical standpoint. This reference states that in an optimal process, a catalyst should be “wide in scope, easy to perform and insensitive to oxygen and water”. It can be inferred from this that choosing the optimal catalyst is not easy. Moreover, the impact of the catalyst choice on a reaction illustrates the importance of selecting the right substance, from both an environmental and economic perspective.
Catalysts can be separated into two distinct categories, heterogeneous and homogenous. Heterogenous catalysis involves a reaction whereby the catalyst in use is in a different phase than the reactants. Often, this occurs when the catalyst is in a solid form, with the reactants being a liquid or gas. Conversely, homogenous catalysts are in the same phase as the reactants in question. Heterogenous catalysis is often favoured and used more often in the production of fine chemicals, mostly due to its ease of recovery in comparison to most homogenous catalysts.
The porosity of a catalyst is usually a concern regarding heterogeneous catalysts, unlike with homogenous catalysts. This is because fluids are unable to possess pores, whereas solids can. Since heterogeneous catalysis usually concerns a solid catalyst, it is evident why this is applicable here. In terms of porous catalytic structures, there are three major categories: microporous, mesoporous, and macroporous. Microporous catalysts feature much smaller pores than those found in mesoporous catalysts, with micropores considered to be 2 nm or smaller in diameter, whereas mesoporous materials are generally categorised as having pores with diameters between 2–50 nm, as evidenced in Ref. [14]. Often these porous materials are constructed from tetrahedral units, whereby they are commonly used in environmental remediation, as well as in treatment, purification, and separation.
2.4. Mesoporous Materials and Their Uses as Catalysts in the Production of Fine Chemicals
2.4.1. Background
Mesoporous materials have a porous structure commonly used in the field of fine chemical development. They are primarily constructed from a silica-based matrix and feature ordered, homogenous pores with a diameter range between 2–50 nm [14,15]. As a result of their extensive surface area and pore volume, accessibility to active sites within the structure is relatively uncomplicated. Moreover, any entering reactive species can undergo significantly more rapid diffusion into the structure [16].
One major advantage of mesoporous materials would be both their chemical and physical properties, which feature exceptionally high surface areas, large pore volumes, and notably, the ability to present adaptive pore sizes and shapes [17]. Having this property allows for the synthesis of catalysts that can be developed and tailored to specific reactions, improving both their overall efficiency and selectivity. In addition, their porous structure can demonstrate nanoscale effects within their internal mesochannels, which can significantly influence catalytic activity [17].
2.4.2. Types of Mesoporous Catalysts
Organo-Silica Based
Silica-based heterogeneous catalysts are typically mesoporous, with an amorphous pore structure. Their larger pore size often results in the easier embedding of functional groups and guest species, providing a high level of customisability in terms of their catalytic performance. Additionally, the use of (organo) silica-based catalysts offers a substantial reduction in the requirement of a solvent during its use in reactions, as explored during the analysis of the asymmetric aldol reaction [18]. Moreover, this study determined that the silica-based catalyst structure used can be recovered and reused several times with reactivation, providing a minimal loss of activity regarding traditionally used fresh organocatalysts. It further goes on to explain that the use of silicas as a base structure for the addition of an acid or base not only proves more affordable but shows more versatility than other solid-supported materials. This has been further supported in other literature exploring the effects of using a silica-based structure during the synthesis of fine organic chemicals [19,20].
There are two methods commonly used to bind a form of organic catalyst to a silica-based structure. The first method, “post-synthetic grafting”, consists of a set of linear steps used to build up the catalyst on the silica surface [21,22,23,24]. The use of grafting as a covalent technique has been found to provide higher stability, as well as reduced leaching, under most mild conditions [25], both of which are ultimately desirable features during the selection of a catalyst. The second method, known as the “co-condensation method”, involves the initial synthesis of any necessary precursors. Following this, the precursors can then be incorporated into the silica support structure [26,27,28]. According to Ref. [25], by using precursors, various types of active sites can be installed onto the structure in specific confined places. This is further supported and evidenced by research primarily focusing on organosilica structures and their contribution to the manufacture of value-added and fine chemicals [29,30,31]. The benefits of this method include easier modification of the catalyst, allowing its properties to be tailored to the desired outcome of the reaction. Such freedom to customise and alter reaction products is highly promising due to its usefulness in the fine chemicals industry. It is important to note that the development of silica-based catalyst structures can have a high associated production cost due to the often “costly surfactant structure directing agents”, as stated by Ref. [25]. In many cases, high production costs pose a roadblock to more elaborate and in-depth research, resulting in discouragement of their use, particularly when more cost-efficient catalyst structures exist. It is also possible to develop a hybrid–synthetic approach, using both methods, as described by Ref. [18]. This paper determined the best catalyst for the use of a silica-based structure under solvent-free conditions, with the addition of an acid, a commonly used additive during the synthesis of catalysts.
The reasoning behind utilising an acid during the synthesis of a heterogeneous catalyst is complex, with its main advantages including easier recovery of the catalyst in comparison to that required when using a base, as supported by Ref. [32]. As a result of their editable pore dimensions, the use of silica structures enables catalytic reactions involving both bulky substrates and products. This widens the potential use for them across a greater range of reactions, providing an advantage over other mesoporous materials. However, in opposition to zeolites and MOFs, mesoporous silica consists of amorphous walls, which hinders their ability and thus, the activity of embedded active sites.
As a result, further research into creating crystalline materials with ordered mesopores and inherent microporosity is required, with the end goal of expanding catalytic design possibilities and generating a solution or potential use for amorphous walls in porous solids.
Types of (Organo) Silica Structures
There is a wide range of existing mesoporous silica structures; however, not all provide utility in the context of fine chemical production. Table 1 illustrates several mesoporous silica nanoparticles (MSN) commonly used as fine chemical catalyst structures, along with their characterisation, properties, and an example of their use in the fine chemical industry.

Table 1.
Some silica structures commonly used in the fine chemical industry.
- MCM-41 (M41S) [57]
Since their discovery in 1992, mesoporous molecular sieves in the M41S family have become increasingly researched across several industries. This is mostly due to their large surface area, neatly arranged pore structure, and uniform pore size [58], all of which are appealing features from a catalytic perspective. The M41S family can be classified into three categories: hexagonal MCM-41 (a commonly used catalyst in the fine chemicals industry), with its nonintersecting channels in a honeycomb pattern; cubic MCM-48, known for its intricate three-dimensional channel system; and unstable lamellar MCM-50, which tends to collapse when the template structure is removed [59,60]. MCM-41 has gained recent industrial attention in fine chemical synthesis as a result of its advantageous properties over other silica structures. These include its particularly high surface area and thermal stability, as well as the fact that it inherently possesses mild acidic properties [61]. It is often used in reactions involving organic transformations, including acid/base catalysis and oxidative coupling. An example of MCM-41 being used in the production of a fine chemical would be its use during the manufacture of menthol, as investigated in Ref. [35]. The paper explores the continuous one-pot synthesis of menthol from citronellal, using MCM-41 as an alternative intermediary set of steps to the traditional synthetic route opted for in the industry, the Takasago five-step process. The benefits of using the Ru-modified MCM-41 structure over other multi-step traditional methods include an easier separation of the catalyst, as well as the re-use of the catalyst. Moreover, the inclination towards easier catalyst and product separation would indicate lower costs, as well as improved environmental friendliness, something the Takasago five-step process cannot easily deliver [62,63].
Similarly, the use of MCM-41, with a bi-functional powder catalyst, in batch processes (also to produce menthol) has been explored, with similar results. These studies have indicated the significant impact that an active metal modification (particularly on an aluminosilicate structure) can have on a reaction, with the various side reactions being significantly affected, depending on the metal used [64,65]. In addition to this, more reaction-specific catalysts can be derived from MCM-41 to utilise its specific properties. The use of such catalysts often results in an increased cost due to the greater complexity of catalyst production; however, the improved yield and selectivity often justify this expense. For example, a mesoporous Ce composite material has been derived from MCM-41 and has been used as a heterogeneous catalyst in the synthesis of monoterpenoid dioxinols. This study highlighted the advantages the MCM-41 structure can provide, with it producing the highest reaction selectivity in comparison to other catalyst structures (various zeolites and metal oxides) used in the procedure [66]. Potential reasons for this high selectivity include its higher nominal metal content (indicating catalytic activity from the Ce), the presence of both mild acidic and basic sites acting to minimise side reactions, and its proven stability to promote dioxinol over the backward reaction. The higher activity of the Ce-MCM-41 has been attributed to the mild acidity, high surface area, and the large mesopores of MCM-41 mesoporous materials. The synthesis route for this reaction followed a fairly unorthodox procedure in that the isopulegol underwent Prins cyclisation to first form tetrahydropyran as an intermediate, followed by its ring rearrangement to dioxinol. Minimal experimental literature on the transformation of tetrahydropyran to dioxinol currently exists. Thus, the research undertaken in this paper focuses on exploring this alternative route, which can be achieved through the utilisation of the Ce-composite MCM-41 structure. Compound (3) tetrahydropyran is an intermediate, which can be formed due to a rearrangement reaction. The associated reaction scheme is shown in Figure 1.
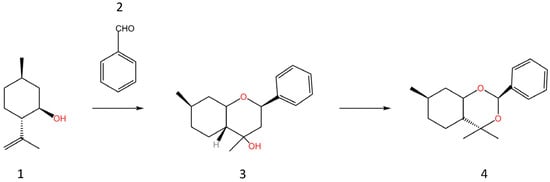
Figure 1.
A diagram showing the reaction scheme for the two-step synthesis of monoterpenoid dioxinols (4) from isopulegol (1) and benzaldehyde (2), forming the intermediate tetrahydropyran (3), adapted from Ref. [66].
A similar study investigating the synthesis of compounds using tetrahydropyran moiety with different heterogeneous catalysts yielded similar results, with a Ce-MCM-41 structure producing both the highest yield and selectivity [67]. Another prominent modification made to MCM-41 involves the addition of ruthenium (Ru) to the sieve structure. Research in this field has shown that the modification method used can itself influence the resulting structure and behaviour of the catalyst, as explored in Ref. [61], with differing conversion and activity rates experienced when subsequently used in the selective hydrogenation of cinnamaldehyde to cinnamyl alcohol, a fine chemical substance used in the production of perfumes. Additionally, Ru-modified MCM-41 has been used experimentally as a heterogeneous catalyst for the ring opening of decalin, with differing preparation methods resulting in significantly varied results in terms of overall conversion, selectivity, and activity [68].
- SBA-15 [69]
The newly discovered Santa Barbara Amorphous (SBA) family has also gained popularity because of its stronger walls, making it more stable under high-temperature and high-pressure conditions, and its larger pore size in comparison to M41S. SBA-1, which possesses a cubic assembly of rounded micelles, and SBA-15, with its highly organized hexagonal structure and larger pores compared to MCM-41, are the two most common variants in the SBA family. The large pores that SBA-15 possesses are particularly useful in accommodating larger molecules, opening the range of possible reactions for which it can be used. The synthesis of all these mesoporous silicas generally occurs in acidic or basic environments and involves using surfactants or some type of amphiphilic triblock copolymers as structure-directing agents [58].
It is also common to produce hybrid catalytic systems by functionalizing SBA-15 with differing organic or inorganic moieties, such as ferrocene in the hydroxylation of benzene to produce phenol [70]. By doing so, the selectivity of the reaction, along with the catalytic activity, can be improved. Further examples of this include the bi-functionalisation of SBA-15 in preparation for its use as a catalyst during the synthesis of 5-Hydroxymethylfurfural [71], a commonly used starting material for fine chemical production.
Metal–Organic Frameworks
Metal–organic frameworks (MOFs) are a highly studied family of porous materials regularly used in the fine chemical industry. Often used as heterogeneous catalysts, they are a form of hybrid solid, capable of being structured in either a two- or three-dimensional manner. They are effectively formed by the self-construction of cationic systems, fulfilling a node role, with polytopic organic ligands acting as a form of linkers. MOFs themselves can be categorised in several different ways, i.e., based on their synthesis method and structure, or the integration of functional composites or active precursors [72]. Table 2 illustrates these categories, along with structure examples and their uses in the fine chemical industry.

Table 2.
Classification for MOFs, along with their relevant properties and uses in the fine chemical industry.
The catalytic abilities of MOFs are dependent on the type of MOF in question. There are typically five ways in which their catalytic activity can be employed, as follows:
- Solid acid-base catalysts;
- Inherent metal framework (centres) catalyst sites;
- Metal catalyst confinement (surface anchoring);
- Post-synthesis functional composites;
- Precursors/sacrificial templates.
Solid acid–base MOFs can occur in a number of ways. To begin with, the existing metal nodes within their structure can function as Lewis and Brønsted acid sites [84]. This is due to weakly coordinated moieties which can be removed through thermal activation (leaving behind unsaturated metal sites exhibiting Lewis acid activity) and internal species bonding to metal atoms in the structure, leading to the dissociation of protons (illustrating Brønsted acid activity). Additionally, functional linkers within the structure can act as acid sites. Due to their highly customisable shapes and dimensions, additional acid functional groups can be introduced, offering Brønsted acidity [85]. Finally, catalytically active sites can be added to MOFs through the introduction of an external guest species, using the MOF as a support. This is possible due to the high pore volumes that MOFs can possess, providing a large surface area for the inclusion of an acid guest species [86]. Similarly, guest metal species can be added to either the surface (utilising the MOF as a support) or integrated into the ligand structure.
Alternatively, MOFs can act as precursors for porous carbon catalytic structures. This is often achieved through pyrolysis, whereby the MOF is exposed to high temperatures under an inert atmosphere, producing carbons that can be impregnated with existing metal species within the MOF [87,88].
MOFs have gained popularity recently within the catalyst industry due to their customisable structures, allowing for more control over the catalyst’s properties. As stated by Ref. [89], metal–organic frameworks hold several advantages over traditional zeolites. The number of possible zeolites for catalytic use is limited, whereas the number of potential metal–organic framework structures is almost infinite. Moreover, because of their high porosity and surface area, they can retain up to 50–150 wt% of occluded solvent [89], meaning that they can, in some cases, hold ten times as much solvent by weight as a zeolite. Additionally, their high surface area allows for greater exposure of active sites, facilitating improved catalytic efficiency and selectivity. This characteristic is particularly beneficial in fine chemical synthesis, where precise control over the reaction conditions and product quality is essential. Table 3, comparing relevant properties (within the context of fine chemical production) between MOFs and zeolites, is shown below.

Table 3.
Comparison of relevant (generic) properties between MOFs and zeolites in the context of fine chemical production.
In terms of their synthesis and development, MOFs can be tailored to incorporate various functional groups and metal centres, creating a platform for the design of catalysts with more specific activities and selectivities. This flexible aspect has widened the possibilities for catalytic development and provided stability in challenging catalytic processes. According to Ref. [90], newer self-assembly synthesis methods for MOFs have been studied in recent years, including hydro- or solvothermal processes, in a bid to develop more effective methods. With the benefits of utilising MOFs as heterogeneous catalysts, it is evident why research efforts have been pursued in this field.
However, the practical implementation of MOFs as heterogeneous catalysts also presents challenges related to their potential structural degradation over prolonged use, leading to a decrease in catalytic activity. Moreover, MOFs can express stability issues in particular situations, such as organic transformation under extreme conditions (acid/basic environments, high temperatures, etc.), as has been explained by Ref. [90]. Additionally, the potential diffusion limitations arising from their intricate pore structures can impact mass transport and reaction kinetics, influencing overall catalytic performance. Diffusion limitation often leads to further catalyst deactivation from pore blockages and poisoning. However, this becomes less of a problem in liquid-phase reactions with MOFs as a heterogeneous catalyst structure. Unlike traditional zeolites, which often experience diffusion limitations in liquid phase reactions and excel in gas phase reactions, MOFs traditionally possess a greater number of available pores and pore sizes, increasing their validity and uses in liquid phase reactions, which are used more regularly in fine chemical production [91].
Additionally, the advantages of MOFs (with regards to other micro/mesoporous materials) remain true for oxidation reactions, with the added benefit that MOFs contain a large amount of transition metals that are considered the conventional type used for oxidation sites. This is a major point of consideration since zeolites and other mesoporous aluminosilicates are null in terms of activity for oxidation reactions.
Some studies have explored the removal of metal from MOFs, leading to the development of carbon-based metal-free catalysts. This is achieved through carbonisation at high temperatures under inert atmospheric conditions, which can lead to an increased surface area and a larger pore volume. There is significant existing literature on such cases, focusing on the removal of metal from MOFs to form a carbon-based structure used for a range of reactions, such as Suzuki–Miyaura coupling reactions, including Refs. [92,93,94,95,96].
An additional field of research with high potential for MOFs includes the development of non-noble metal-based catalysts such as Ni and Fe. This is due to their intrinsic magnetic properties, which can promote easier and more successful recycling of the catalysts, as well as improve their life cycle, with predominant use in the oxidation of alcohols to esters [97,98,99].
Zeolites
Zeolites are crystalline aluminosilicates that are well known for their widespread uses as catalysts in the manufacturing of fine chemicals. Occurring naturally or resulting from chemical synthesis, the main reason behind their utility is their distinct molecular-scale structure of organised, linked channels and consistent pore size. Zeolites are generally represented by the following empirical formula [100]:
M2/nO·Al2O3·xSiO2·yH2O
Equation (1)—A general empirical formula representing a zeolite structure.
The x in this formula is typically greater than or equal to 2. This is because the AlO4 tetrahedra is joined to the SiO4 molecules within the structure. In this context, n represents the available cation valence (available cation ability to form chemical bonds). It is important to note that although zeolites are typically microporous, the recent emergence and development of mesoporous zeolites have significantly increased their utility, particularly in reactions involving big molecules. They are used in a vast range of reactions, with ion-exchanged zeolites being one of the most frequently used catalysts in history [101]. Depending on the form taken, their uses vary significantly. For example, in their proton exchange form, they fulfil a large role in the oil refining industry because of their strong acidic and shape-selective properties [102,103]. Further potential uses for zeolites include their application in various types of water purification, in large biomolecule separation, and the removal of some radioactive contaminants [104,105,106]. The use of zeolites for large molecule separation features across a vast range of reactions during fine chemical production, often capitalising upon the nature of their hierarchical structure. They act to increase the effective hydrogen-to-carbon ratio of chemical products through a range of intermediate dehydrogenation/hydrogenation, oligomerisation, and cracking reactions. Subsequent carbon bond-forming reactions, such as aldol condensation and Diels–Alder reactions, can then occur on the Zeolite structure to form larger molecules and aromatics [107,108,109]. An example of utilising the hierarchical nature of zeolites during processes involving large organic molecules includes such reactions as the pyrolysis of wood polymer to produce aromatics [110]. ZSM-5 is discussed as one of the more widely used zeolite catalysts in aromatics production due to its increased efficiency, with utilisation as fuel additives [111], solvents [112] or types of polymer synthesis [113]. Its microporous structure, however, is noted as a major limiting factor as a result of the reduced diffusivity for larger molecules. This further highlights the advantage of hierarchical zeolite structures, illustrating a strong case for the use of mesoporosity in zeolites.
Since the orientation of individual pores is usually random, and the sizes and shapes of the mesopores have no impact on the zeolite’s crystal structure, the mesopore system in mesoporous zeolites can be considered a non-crystallographic pore system. Since this pore system is not atomically ordered, mesoporous zeolites are regarded as hierarchical porous materials, i.e., they possess more than two pore size distributions [114,115]. Hierarchical zeolite materials can be separated into three categories: hierarchical zeolite crystals, nanosized zeolite crystals, and supported zeolite crystals, with each type varying in regard to pore size and structure. Hierarchical zeolite crystals exhibit extra pores, either mesopores (under 50 nm) or macropores (over 50 nm), within each crystal and include an additional mesopore system alongside the standard micropores. Nanosized zeolite crystals are smaller, and their mesoporous system comes from their packing structure. Supported zeolite crystals are essentially dispersed in another material’s pore system, leading to a mix of micro- and mesopores, (depending on the support structure). To alter a zeolite structure into a mesoporous form, the zeolite crystals themselves must be altered, with either a top-down or bottom-up approach [116]. The top-down method, known as post-treatment, involves the use of an acid or base component to remove any Si or Al species from the template. This method, known as demetalation, is commonly achieved via the use of dealumination and desilication, both of which have been studied in Refs. [117,118,119]. Conversely, the bottom-up approach, known as direct templating, entails the direct synthesis of mesoporous zeolites in the presence of mesoscale organic porogens and organic directing agents. Various bottom-up methods exist, with one common approach being the use of some type of solid templating. For example, templating can be completed through a range of carbon nanomaterials [120,121,122,123] to produce a variety of common zeolites such as ZSM-5, zeolite-β, zeolite-X, zeolite-A, and zeolite-Y. Carbon-based templates produced through carbonisation can also be built upon to produce different types of zeolites [124], as can the use of aerogel [125,126], polymer [127], resin [128,129], and solid biological templates [130].
Other templating approaches for producing hierarchical zeolites include delamination, the process of synthesizing layered precursors as lamellar precursors with an additionally intercalated surfactant. This surfactant can then be removed, leading to the collapse of the structure and the formation of an accessible zeolite material that exhibits mesoporosity [131,132].
Whilst the development of zeolites possessing mesoporosity exhibits advantages, the negative environmental drawbacks that their synthesis produces remain a sizeable issue. Significant emissions are produced during their manufacture due to the application of different multifunctional templates, their subsequent removal, or the release of acids and alkalis used in zeolite dealumination and desiccation [133]. Furthermore, even the processes currently in use to create microporous zeolites are not environmentally friendly because they all require the use of artificial chemicals containing silicon and aluminium, which are derived from natural silicate or aluminosilicate minerals. These derivations occur through labour-intensive procedures that result in significant waste production and energy consumption [134].
Types of Zeolites
The classification of a zeolite is governed by the silica:aluminium ratio within the structure [135]. For example, a zeolite labelled as high-silica would have a large Si:Al ratio, whereas low-silica or Al-rich would indicate that the structure has a low Si:Al ratio. In terms of the ratio number itself, its classification is dependent on the type of zeolite. For BEA zeolites, low-silica refers to a ratio less than 5, whereas high-silica refers to a ratio greater than 10 [136,137]. For X and Y zeolites, high silica indicates a ratio greater than 3, while low silica would correspond to ratios between 3 and 1.5 [138,139,140]. For ZSM-5 zeolites, high-silica corresponds to a ratio above 20, whilst low-silica indicates a ratio below 15 [136,141,142]. Table 4 shows several zeolites commonly used in the fine chemical industry, including their classification, relevant properties, and examples of their uses as catalytic materials in fine chemical production.

Table 4.
Zeolite structures commonly utilised as heterogeneous catalysts in the fine chemical industry, along with their relevant classifications, properties, and examples of their uses.
ZIFs
Zeolitic imidazolate frameworks (ZIFs), a popular porous hybrid structure developed in recent years, have become a focus in the heterogeneous catalytic industry for several reasons. Possessing a crystalline structure with the ability to express hierarchical porosity, they are a subclass of MOFs, combining some of the most desirable properties of both MOFs and zeolites. They differ in structure from traditional MOFs, since they are composed primarily of Zi (ii), Co (ii), and imidazolate linkers, as opposed to the wide range of metals and organic linkers of which MOFs can be composed. When compared to traditional MOFs, ZIFs typically exhibit much greater stability in terms of thermal, hydrothermal, and chemical properties [150,151]. This includes their ability to be boiled in various organic and alkaline solutions without the loss of crystallinity or a reduction in porosity [152].
In addition, the tetrahedral crystalline structure of a ZIF is like that of a zeolite, with the aluminium and/or silicon being replaced by either zinc or cobalt (transition metals). This mixed structure is one of the main advantages they hold over zeolites—they can be exposed to a greater range of surface modifications. Along with this, their tunable pores (due to their metal ions) and their high porosity make them extremely suitable as catalyst support structures for a range of reactions [153]. The main routes for the manufacture of ZIFs include the use of solvent-based or solvent-free synthesis [153]. The most commonly used solvents include water, methanol, or ethanol. It is not uncommon, however, to employ other solvents, such as dimethylformamide or diethyl formamide, with the solvent chosen to be dependent on the specific process. From here, the chemical route taken can vary, with a wide range of methods available, subject to the solvent chosen. Older methods include solvothermal synthesis, whereby organic solvents are used in the formation of a ZIF. As discussed above, the preliminary solvents used in experimental work include various alcohols, as explored in Ref. [152]. More recent works include the incorporation of bases to deprotonate specific linkers and provide a higher yield and greater rate of reaction. Such cases in the literature include pyridine [154] and triethylamine [155]. Findings from both of these papers provided a strong argument for the use of a basic solvent in conjunction with ZIF structures, with its main benefit being highlighted as the reusability of the ZIF structure (due to its retention of catalytic activity). However, both studies noted the impact that the reaction solvent can have on its catalytic performance, indicating some drawbacks in terms of the reactions for which they are suitable.
Whilst solvothermal synthesis can offer a wider range of choices regarding the type of ZIF developed and greater flexibility in terms of the solubility of the precursors, the associated drawbacks of organic solvents often reduce their industrial popularity. The negative environmental impacts, in addition to the high costs and in some cases, toxicity to humans, is driving research in ZIF development towards cleaner alternatives. Research shows that the use of aqueous mediums (hydrothermal synthesis) can produce higher yields than organic solvents in reduced timeframes, but they require additional linkers, such as in the case of the development of ZIF-8 nanocrystals [156], or even nano-sized ZIF-67 crystals [157]. Further, more recent research in this field has been conducted in a bid to develop this greener method, with various modifications to the process. These include the use of surfactants to regulate the size of the crystals in a ZIF structure. Successful examples include research conducted whereby the diameter of the crystals produced could be controlled [158], or whereby the impact of the surfactant used on the hierarchical structure of the ZIF was explored [159].
Newer solvent-based methods of ZIF production include microwave and ionothermal synthesis. The former involves the use of microwave-assisted heating technology and has been shown to drastically shorten the synthesis time, as well as to produce a higher yield whilst reducing the number of ligands present, without the use of deprotonating agents [159,160]. The latter, ionothermal synthesis, involves the use of ionic liquids (as solvents) in an open system. There has been minimal research in this field thus far, despite the possibilities it has been shown to possess. These include using the ionic solvent as a template to prevent competitive interaction, the wide range of novel structures that could be developed with further experimental work (as explored by Martins et Al in [161]), and its possibilities to be incorporated with other synthesis methods, such as microwave synthesis [162].
A small number of promising novel solvent-free synthesis routes exist. Despite their lower cost and their significantly eco-friendlier nature, very little research has been conducted in this area. Some studies include the synthesis of ZIFs using a dry-gel conversion, with the synthesised ZIF structures showing promising results. These include excellent reactivity and catalytic activity, as well as strong reusability [152,163].
The use of ZIFs as catalysts in the fine chemical industry is gaining traction, with catalysis roles in various scientific sectors, including pharmaceuticals [164,165], organic chemical production (hydrocarbon separation) [166], aromatics, and biomass conversion [167]. Notably, all the research conducted on ZIF structures as catalysts are remarkably novel, with their use appearing earlier in other fields, such as gas and energy storage [168,169,170], as well as drug delivery [171,172]. Whilst minimal, the positive findings from this early research are indicative of the potential uses of ZIFs as catalyst structures, and it can be anticipated that further investigation into their catalytic benefits will prove popular and essential as the fine chemical industry continues to evolve and seek cleaner and more efficient production methods.
Carbon-Based
Mesoporous carbon-based heterogeneous catalytic structures possess highly desirable physical and chemical features, particularly regarding the synthesis of products in the fine chemical industry. Notable properties include a high surface area (contributing to higher catalytic activity), significant pore volume, good thermostability due to their composition of carbon atoms linked in a framework, enhanced mass transfer, and relatively uncomplicated diffusion [173]. Due to their excellent conductive abilities, carbon-based catalysts are often used in electrochemical-based reactions [174,175]. The processes for the synthesis of carbon-based catalysts have changed over time due to the development of novel methods, with greater simplicity and fewer environmental drawbacks. Typically, carbon-based structures are microporous, so methods to introduce mesoporosity have been created. Initially, mesopores in carbon structures were created in the spaces between carbon particles, such as in carbon aerogels, or by enlarging micropores via oxidation during the activation process, such as in activated carbons [176]. These methods, however, resulted in difficulties such as the struggle to regulate the structure’s shape or a loss in carbon yield.
There are three major categories of methods for the synthesis of carbon-based structures, including:
- Activation methods;
- Catalytic activation methods (using metal ions);
- Template methods.
Activation methods are regarded to be the most widely used techniques [173], with two subcategories: chemical methods and physical methods. In the physical methods, materials are heated to between 400–900 °C in an oxygen-free environment (using gases like nitrogen or helium) to create a carbon-rich “char”. This char is then exposed to higher temperatures (800–1000 °C) and oxidising agents (such as steam or carbon dioxide) to develop a mesoporous structure [177,178]. Chemical activation, on the other hand, streamlines this process by integrating the heating and exposure to oxidising chemicals into a single phase. Usually, chemical activation entails impregnating a carbon source with specific compounds and heating it to 400–700 °C [179] to cause it to thermally breakdown. Zinc chloride (ZnCl2), aluminium chloride (AlCl3), magnesium chloride (MgCl2), potassium hydroxide (KOH), sodium carbonate (Na2CO3), phosphoric acid (H3PO4), and sodium hydroxide (NaOH) are common chemicals utilised in this procedure [179,180,181]. The choice of chemical is very important because it has a big impact on the activation process’s mechanism and necessary temperature.
Chemical activation offers several advantages over physical activation, including lower energy consumption and activation temperatures, higher carbon yields, and faster processing times. However, in some cases, physical activation is preferred due to its relatively smaller impact on the environment, its readily available activating agents, and its simple technology [182,183,184]. In some cases, combining both types of activation methods produces better activation; thus, it is employed regularly.
Catalytic activation utilising metal ions can also be employed to create mesoporous carbon-based catalysts. By acting as catalysts to activate carbon precursors, metal ions such as iron (Fe), nickel (Ni), and cobalt (Co) can speed up the activation process. They can help provide stability and control the development of the mesopores. These metal ions are particularly efficient at accelerating the char activation process, which leads to a greater volume of mesopores [185]. In some cases, where any water-based solutions are used, the metal ions can leach out of the structure, reducing its lifespan and in turn, its activity.
The third method, templating, has two distinct sub-categories; hard and soft templating. Hard templating uses pre-made inorganic or organic templates such as colloidal silica or mesoporous silica. These templates are filled with carbon precursors and then carbonised. The template is then removed by chemical etching, using an acid or base, leaving behind the carbon-based structure [176].
The soft-templating method uses self-assembling molecules, such as metal–organic frameworks and surfactants, as templates. These materials are then bonded (often through hydrogen bonding) with carbon precursors. A calcination process is then used at temperatures up to 900 °C [186,187] to remove the templates, leaving behind the mesoporous structure [188]. One reason behind the development of mesoporous molecular sieves over zeolites is their ability to process heavy oil fractions, something other mesoporous structures cannot deliver [189,190,191]. Moreover, mesoporous carbon structures can exhibit high selectivity, and research has proved their ability to increase the reaction rate, as evidenced by literature investigating the dehydrogenation of ethanol to acetaldehyde [192]. Their excellent selectivity was determined to be significantly greater than that produced using SBA-15 as a catalyst support. These findings result from the inert nature of the carbon structure, inhibiting any additional secondary or side reactions. Whilst acetaldehyde itself is not strictly a fine chemical, such findings prove particularly beneficial in the fine chemical industry, where high selectivity of desired products is paramount to increase the efficiency of valuable and expensive processes. Additionally, the natural hydrophobic properties of the carbon structure provide an enrichment potential for organic compounds, expanding its uses as a highly selective catalyst support for organic reactions. An additional study utilising mesoporous carbon structures incorporated with the catalyst (as opposed to supporting it, as traditional research investigates) highlighted their reusability in the production of γ-valerolactone [193]. In addition, the study illustrates their stability in an acidic aqueous medium. It is this high stability that proves that the development of mesoporous carbon structures directly incorporating catalysts carries excellent potential, drawing further attention to carbon-based structures and the increased benefits that can be evoked through additional research.
2.5. Microporous Materials and Their Uses as Catalysts in the Production of Fine Chemicals
2.5.1. Background
Microporous materials contain crystalline structures with interconnected cages or channels. Their classification as microporous is determined by their smaller pore diameter of less than 2 nm [14].
The two main types of microporous structures used in industry are crystalline zeolites (aluminosilicates) and activated carbons [194]. Both types of structures, along with other less common microporous structures such as metal–organic frameworks (MOFs), can be modified to be mesoporous. Further types of microporous structures such as metal oxides and aluminophosphate will be reviewed below. Typically, microporous materials are synthesised under solvothermal conditions, with the individual parameters being altered, depending on the reaction. The most altered parameters when developing microporous structures include the starting compound, any solvent used, the temperature, the pressure, and any additional charge-compensating ions [195].
2.5.2. Types of Microporous Catalysts
Zeolites
Microporous zeolites offer similar advantages to those of mesoporous/hierarchal zeolites, however, with less methodology and fewer steps in terms of their development. As described previously, zeolites can occur naturally or can be synthetically developed.
Worldwide, both academic and industrial facilities have conducted extensive research on differing types of microporous zeolites. The reasoning behind this includes their desirable abilities and properties, such as consistent channel systems, tunable Brønsted and Lewis acidic sites, coke resistance, ion-exchange, and thermal stability features. The procedure for developing synthetic zeolites varies slightly from the method mentioned above to produce mesoporous zeolites. Since zeolites are generally microporous beforehand, with further processing required to make them mesoporous, there are usually fewer steps involved in this process.
There are a range of different methods available for the synthesis of zeolites, with the most common ones listed below, as taken from Ref. [196]:
- Hydrothermal synthesis;
- Solvothermal synthesis;
- Ionothermal synthesis;
- F-synthesis;
- Microwave-assisted hydrothermal synthesis;
- Microemulsion-based hydrothermal synthesis;
- Dry-gel conversion synthesis;
- Combinational synthesis.
Despite the desired properties of microporous zeolites, there is a current drive for extensive research around modifying and developing them further. The reasoning behind this is to enhance their uses and resolve their inability to catalyse reactions with larger particles. One potential route for doing so would be their modification to a hierarchal zeolite, as described above in the mesoporous zeolite section. There are several alternative routes, however. In the editorial section prepared for microporous zeolites and their applications [197], Kumar discusses the approach of embedding microporous zeolites in amorphous silica, referring to a study conducted on selective catalysts for the methanol to olefin process [198]. Whilst olefins themselves are not strictly fine chemicals, they are often used as building blocks in further reactions to produce various aromatics and detergents. The cause for this methodology lies in the potential for customising and specifically tailoring available acid sites in the catalyst structure. The paper revealed the higher catalytic activity of the synthesised zeolite, as well as the much higher selectivity ratio. These findings support explanations in wider research in the literature proposing that the strength, availability, and type of acid sites available in a catalyst can alter the propylene selectivity and rate of formation of intermediary products.
The impact that modifications of a zeolite structure can have on a reaction is analysed in Ref. [199], with its influence and impacts explored. The paper explains how preparation, precursors, or pre-treatment can impact and alter the physicochemical and catalytic properties of a material. It also elects to take an environmentally friendly approach during the preparation of the catalyst by choosing to leave out aqueous or organic solvents and instead using a solid-state ion exchange method. One notable finding from the paper regarding zeolite performance, however, concerned the deactivation of the zeolite structures with extended reaction times caused by carbonaceous deposits, resulting in pore blockages in the zeolite. These findings suggest that an avenue for future research could incorporate exploration into the reduction or prevention of deactivation to extend the lifespan of zeolite structures and reap their full selectivity benefits.
Similarly, Kumar has assessed the impact of the chosen preparation method on the catalytic behaviour of modified zeolite extrudates [200]. The study revealed that the different methods of platinum deposition onto the structure impact the metal-to-acid site ratio, subsequently altering the acidity and strength of the catalyst structure. It also concluded that the choice of the synthesis method impacts the overall conversion, with zeolite extrudates prepared via in situ synthesis producing the highest conversion. Alternatively, zeolite catalysts can be prepared via the use of various impregnation methods. In Ref. [201], H-Y5.1 zeolite catalyst, prepared via wetness impregnation, was used across a range of reactions in the production of upgraded fuels and aromatics, with the ratio between Fe and Ni in the catalyst being varied. This involved the co-processing of hexadecane with isoeugenol (2-methoxy-4-propenylphenol), with the isoeugenol undergoing hydrodeoxygenation and the hexadecane undergoing hydroisomerisation–hydrocracking. The reaction schemes for this process can be seen in Figure 2 and Figure 3 below:

Figure 2.
The reaction scheme for n-hexadecane hydroisomerisation–hydrocracking, adapted from Ref. [201].

Figure 3.
The reaction scheme for the hydrodeoxygenation of isoeugenol, adapted from Ref. [201].
Findings from the study showed a reduction in specific surface area and a decrease in pore volume due to a blockage in the larger pores in the material. The study also showed a change in the acid sites, dependent on the catalyst structure used, with Fe leading to a decrease in strong acid sites and an increase in medium Brønsted sites, whilst Ni increased the weak and medium Lewis sites. This change in acid site presence can be attributed to interactions between the metal ions with the zeolite’s acidic surface, leading to coordination with any present oxygen/hydroxyl groups and the formation of metal clusters. This in turn can block or inhibit the active sites. The balance between both Lewis and Brønsted acid sites can heavily impact the selectivity of a reaction. For example, a greater number of Lewis acid sites (because of the nickel impregnation) contributes towards the hydrodeoxygenation (HDO) of isoeugenol. This is a result of their ability to promote the adsorption of oxygenated compounds by forming a complex with present oxygen atoms. This in turn makes the C-O bond more susceptible to cleavage in the HDO process. Additionally, these papers illustrate the effect of the preparation method and support design on a reaction and highlight the drawbacks of zeolites in terms of pore blockage tendencies. A clear link between the acidity and ending selectivity of a reaction can be seen, indicating the importance of correct catalyst preparation. This is especially important in the fine chemical industry, where the economy of reactions and selectivity to desired products can dictate the financial outcome and viability of a particular product. It could be argued that zeolites are the most commonly used porous catalyst in both the fine chemical industry, as well as in wider chemical production. Extensive literature detailing their vast applications exists, with zeolite-β featuring heavily in small-scale fine chemical production. Further examples of the use of zeolite-β include:
- The use of β zeolites in the cyclization of citronellal [202,203];
- The use of H-Beta-25 in the transformation of glucose to methyl levulinate [204];
- The use of Sn-Beta zeolite in the production of methyl lactate from glucose [205];
- The use of H-beta zeolite catalysts in the prins cyclization of (−)-isopulegol [206];
- The use of H and Fe-modified beta zeolites in the production of trans-carveol from α-pinene oxide [207].
Activated Carbons
Carbon-based structures, which are mesoporous, can also possess micropores. Whilst their ability to adsorb large organic materials is reduced in comparison to mesoporous carbon-based structures, they feature an enlarged specific surface area. This aids their adsorption of volatile molecules, as well as their hydrogen storage and CO2 capture abilities [208,209].
One common approach to developing activated carbon-based catalysts involves the use of developing biomass as a precursor [210,211,212]. Once the carbon has been obtained, it can be activated using pyrolysis in an inert atmosphere, just as mesoporous carbon is activated. It is then developed through physical or chemical activation to improve its porosity [213]. Several studies have found that the use of biomass as a precursor provides a high oxygen content, leading to greater porosity and surface area [214].
The surface reactions on carbon can be complex due to the interaction of numerous oxygen groups. These can be divided into two main groups, the basic and acidic oxygen groups [215,216]. The surface activity and catalytic behaviour of the structure are usually dependent on these surface groups, with studies conducted regarding the impact these groups have [216,217].
Like mesoporous carbon-based catalysts, they are often modified, or “doped” with additional materials, to alter the available specific surface area and increase micropore development, i.e., through nitrogen doping [218,219,220,221]. There are countless other examples of such experimental work, including the modification of carbon nanospheres with phosphorus [222], the synthesis of a dual-modified phosphomolybdic acid/silver carbon composite [223], the production of jet fuel through the hydrodeoxygenation of isoeugenol over a carbon-supported platinum catalyst [224], and many more [225,226]. One common drawback that occurs when a microporous carbon structure is utilised is the frequency of pore blockage. This is a common occurrence in microporous structures due to their smaller pore size, resulting in a subsequent loss of activity and catalytic deactivation. Whilst microporous carbon structures possess several advantages over their mesoporous counterparts, it is clear that the pore blockage tendencies are much higher, illustrating a major hindrance to their wider use.
A study conducted on the kinetics and deactivation of catalysts in the hydrogenation of β-sitosterol to β-sitostanol, utilising both micro and mesoporous carbon supports [227], reiterated catalyst deactivation as a prominent issue when using carbon-based catalyst structures, suggesting the utilisation of a larger amount of the catalyst to combat this problem. The reasoning behind this is to counter the catalysts’ adsorbent properties so that the activity can remain higher for prolonged periods. Additionally, the differing conversions obtained between the micro- and mesoporous carbon were analysed, whereby it was found that the mesoporous carbon structure provided higher overall conversions than its counterpart. In addition, deactivation occurred at a slower rate for the mesoporous carbon catalyst than for the microporous example. This difference can be credited to the fact that the mesoporous structure contained larger pores, meaning that the effects of coking are less significant and require a longer time span to build up.
In the fine chemical industry, where activity and selectivity are paramount, the use of microporous carbon catalysts carries several advantages but, in most cases, these are outweighed by its disadvantages. Their large specific surface area is particularly beneficial for the adsorption of smaller molecules, which can prove useful in purification or pollutant particle-capturing processes. Despite these advantages, however, the challenges posed by pore blockage are significant and cannot be overlooked, particularly when blockages can lead to a loss of activity and catalytic deactivation. The comparison with mesoporous carbon structures further underlines this issue, as it becomes clear that these larger pores offer higher overall conversions and slower deactivation rates. Considering these factors, it can be concluded that mesoporous carbon structures are generally more beneficial for applications in the fine chemical industry in processes in which longer catalyst life and higher resistance to catalyst fouling and deactivation are required.
Aluminophosphate
There are several other microporous structures used as heterogeneous catalysts for both the production of fine chemicals and the chemical industry as a whole.
One of these commonly used structures is aluminophosphate. Aluminophosphate is usually expressed in a crystalline structure formed by connecting oxygen atoms between alternating phosphorus tetrahedra and aluminium polyhedra. Aluminophosphates come in a variety of polymorphs that can create pore frameworks with varying dimensions. These crystalline aluminophosphates often feature uniform pores with diameters above 1 nm, placing them in the category of extra-large microporous crystalline materials [228]. The structure of these frameworks provides a number of sites for chemical reactions to occur, which is essential for their activity and effectiveness as acid catalysts [229]. Often, aluminophosphates are doped with specific metals, whereby the metal ions replace the aluminium ions within the framework. This process is frequently used in the manufacture of single-site catalysts [230], allowing for its use in particular reactions, such as in the oxidation of hydrocarbons [231] or the esterification of acetic acid [232]. One notable example of the use of the metal aluminophosphate being used in the fine chemical industry features in a research paper on the production of biphenyl urea [233]. Findings from the paper indicated cobalt-aluminophosphate as the catalyst providing the highest yield of biphenyl urea. The paper also concluded that under mild conditions, metal-aluminophosphate catalysts are a viable alternative to traditionally used environmentally hazardous and costly alternatives.
When compared to their crystalline counterparts, amorphous aluminophosphates are a more affordable and often more thermally stable catalyst [234]. They only require a straightforward co-precipitation step in their manufacture, whereas the synthesis of crystalline aluminophosphates requires the use of organic agents that direct structure, like trialkyl amines or copolymers, which are then burned off after the synthesis is complete.
MOFs
Microporous MOFS, like mesoporous MOFs, consist of self-constructing cationic systems, with polytopic organic ligands acting as linkers. In comparison to mesoporous MOFs, they possess a much larger specific surface area, making them more suitable for interactions requiring high surface contact [235]. Along with this, their smaller pore size becomes useful in situations where their ability to process and discriminate between smaller molecules proves advantageous. A notable feature of microporous MOFs is their ability to adsorb carbon dioxide [236]. An article produced by Pal et al. investigated a co-MOF and assessed its selectivity in terms of CO2 sorption through physisorption, as opposed to a chemical reaction involving Aryl C-H⋯O=C=O Interactions. The study presents the use of microporous MOFs as an effective carbon capture solution, illustrating the co-MOFs’ ability to selectively predominantly adsorb carbon dioxide over other gases in the mixture. This can be explained by the use of grand canonical Monte Carlo (GCMC) computer simulations performed on the Co-MOF structure, which showed how the CO2 molecules had been electrostatically trapped as a result of interactions between the oxygen atoms in the CO2 molecules and the hydrogen atoms attached to the pyridine rings in the framework’s spacers. These results are supported by additional literature in this field of research, such as in this study similarly investigating the selective adsorption properties of microporous MOFs [237]. These findings prove the value of microporous MOFs within the field of green chemistry, laying a foundation for further investigation into their CO2 adsorption abilities, given their potential to actively reduce atmospheric greenhouse gas emissions.
In terms of their catalytic applications, particularly in the fine chemical industry, they can be employed in the Hantzsch reaction to produce polyhydroquinolines, whereby their derivates are utilised in the pharmaceutical industry due to their medicinal functions, as discussed in Ref. [238]. The reaction scheme for this process is shown in Figure 4.

Figure 4.
A reaction scheme illustrating the organocatalysed (4a–4f) unsymmetric Hantzsch reaction using dimedone (1), substituted aldehyde (2), and acetoacetate ester or acetylacetone (3) to produce polyhydroquinoline derivatives (5), adapted from Ref. [238].
In this paper [239], the Zn-MOF conveyed high efficiency as a result of its hierarchical microporous structure, which provided a large surface area for reactions to occur on the structure. Along with this, the paper also discussed how the Zn-MOF catalyst could be reused multiple times, with only a moderate decrease in performance, demonstrating its stability and potential long-term financial viability. Notably, however, the intricacies of the potential deactivation mechanisms (such as coking) were not extensively discussed. The potential for deactivation of the catalyst is a pivotal factor in terms of its suitability over traditional catalysts for such a reaction, where such drawbacks would be crucial on an industrial scale. Similar experimental work conducted on the use of microporous Zn-MOFs as heterogeneous catalysts has been conducted by Roy et al., whereby its impact on various organic transformations has been explored [240]. The Zn-MOF studied in this paper exhibited high catalytic efficiency during reactions such as the cycloaddition of CO2 with epoxides to form cyclic carbonates (a valuable intermediate in the fine chemical industry). One advantage of using the MOF structure was highlighted as its ability to catalyse reactions under mild conditions. This could prove particularly beneficial for large-scale industrial reactions as the sector shifts towards more sustainable and eco-friendly chemical processes. Moreover, the paper addresses the low price associated with zinc metal, linking this to its large surface area-to-volume ratio and divalent oxidation state. This presents the Zn-MOF catalyst as an overall suitable alternative for several organic processes, including high-temperature reactions and the involvement of hazardous materials.
Metal Oxides
Microporous metal oxide structures feature metal centres of either single ions, clusters, or mixed-metal clusters, linked together through oxygen atoms. These linkages, which contribute towards its stability, can then form a variety of structures such as rings, chains, and layers, which lead to the formation of its porous structure. Similar to other microporous structures, they can be organised in either a crystalline or amorphous structure. Zeolites are a type of metal oxide; however, other types of metal oxides do exist, yet these are used much less frequently in the fine chemical industry. Other types of metal oxides used as microporous catalysts include MoVNbTeOx [241] and surface-phosphate nickel oxide [242], which can be used in the dehydrogenation of ethane to ethylene.
An alternative avenue for future exploration would be the use of heterogeneous transition metal oxide catalysts, which have applications in several areas, including the conversion of hydrocarbons to value-added chemicals and geochemical redox processes [243]. Their production has been explored in Ref. [244], where MoO3 and WO3 catalysts were synthesised. The paper draws comparisons between them and traditional zeolites, explaining how these transition metal oxides combine the molecular-sieve attributes of Si-zeolites with the enhanced acidic strength and redox properties of metal cations. Combining these properties into one structure provides versatility in terms of catalytic performance, which is highly sought after in the fine chemical industry. The drawbacks of transition metal oxide catalysts were discussed, with the most significant being structural stability issues and synthesis complexity. These issues provide a hindrance to the practical implementation of transition metal oxides, indicating that significant further validation, both experimentally and practically, would be required to fully leverage their advantages and to result in widespread industrial implementation.
Polymer-Supported Catalysts
Porous polymer structures are a class of amorphous, crosslinked structures capable of featuring a range of pore diameters, with the ability to possess hierarchical porosity in the majority of cases [245]. This cross-linked porous structure provides an extensive surface area, with the ability to incorporate additional functional groups and embed a range of active catalytic sites. Additionally, their extensive crosslinking imparts high thermal and chemical stability, giving rise to a range of potential uses as catalytic support structures. An additional feature of the majority of these polymer structures is their recyclability and the opportunities to recover and reuse these structures. In relation to fine chemical production, high recyclability offers a major advantage in terms of reducing production costs [246].
Alkene epoxidation is a vital chemical reaction utilised in the fine chemical industry to produce various compounds, including epoxides, a valuable intermediate in the synthesis of pharmaceutical and agrochemical products [247]. The potential use of polymer-based structures as catalyst supports for these reactions has been the subject of focus in recent years, as they have exhibited greater potential in terms of stability, as well as in regard to providing a potentially greener production route. Subsequently, new, modern methods have been developed and patented, including several patents regarding liquid phase epoxidation and incorporated heterogeneous catalysts [248,249]. These polymer structures provide a support matrix that facilitates and subsequently immobilises the catalyst.
A study conducted on the epoxidation of alkenes using a polymer-supported Mo(VI) catalyst [250] evaluated the stability and activity, as well as the catalytic leaching, under a range of conditions. It highlighted current industrial concerns regarding the potential leaching during the use of long-term heterogeneous catalysts, mentioning the recent research conducted concerning the use of alternative inorganic catalyst structures and their lack of suitability [251,252,253], underlining the need to develop and scale-up a stable method utilising a polymer-based structure instead. Whilst this research employed a batch process, the focus was to investigate the catalyst structure in regard to its future uses in continuous processes. The findings from the paper include enhanced reaction stability and efficiency, as well as the potential for the reuse of the catalyst. One drawback to note included the importance of utilising optimal conditions in the process to ensure that efficiency was optimal. However, such a drawback exists for almost all chemical reactions regardless of sector, outlining the importance of weighing the benefits of using a polymer structure as a heterogeneous catalyst over any negative aspects. Similar studies investigating the preparation of epoxides using a polybenzimidazole-supported Mo(VI) catalyst [254,255] produced similar results. Whilst the alkene being epoxidised varied between the studies, the findings illustrated that a polymer-based catalyst structure (in this case, polybenzimidazole) is active in these reactions, and it exhibits particularly high activity under reaction-specific optimised conditions. Both studies showed greater reaction efficiency, with a notable catalyst leaching recorded in Ref. [255]. However, these studies were conducted under batch conditions, and it has been suggested that when transferred to a continuous process, leaching and loss in catalytic activity would no longer be an issue. This is of great importance in terms of the scale-up and adoption of these processes on a larger scale, particularly in the fine chemical industry, wherein both prolonged catalyst activity and lifespan are highly desirable.
Various studies in the literature portray polymers as suitable catalyst supports as a result of them being inert, insoluble, and non-toxic [256,257]. In particular, being inert is an essential characteristic to maximise the efficiency of catalytic reactions. This reduces the risk of catalyst poisoning and facilitates the reuse and recovery of the catalyst, providing a potential economic advantage in the long run in terms of cost savings in regard to process scale-up. Their non-toxic properties are essential in the fine chemical industry in terms of safety assurance and regulatory compliance, particularly in industries where products may subsequently face human consumption. These findings are further supported in Ref. [258], wherein a Ps·AMP·Mo catalyst was used in the epoxidation of 1-hexene and 4-vinyl-1-cyclohexene. Continuous and batch operations were analysed, with the catalyst exhibiting high activity and selectivity in both circumstances. In addition to this, epoxidation in a FlowSyn continuous flow reactor illustrated noticeable time savings, highlighting the benefits and potential streamlining of operations provided by the use of a polymer-based catalyst structure.
The importance of optimising these processes to fully reap their benefits has been a consistent theme throughout most of the literature regarding the epoxidation of alkenes using polymer-based catalyst structures. The increased catalytic activity and efficiency are diminished under non-ideal reaction conditions, regardless of the polymer structure used, as evidenced in Ref. [259]. In this research paper, the catalyst used was a polystyrene 2-(aminomethyl) pyridine-supported Mo(VI) catalyst, yet the importance of optimising the reaction conditions was further stressed, regardless of whether it was a batch or continuous process. Additionally, the optimisation of a process reduces its environmental impact, as well as its operating and energy consumption costs. To further verify the results produced during the physical optimisation of a polymer-based catalytic epoxidation reaction, an artificial neural network model has been employed, and the results of this model were compared to those produced experimentally [260]. This has highlighted not only the importance of optimising a process in terms of cost savings and efficiency but also regarding the benefits it supplies in terms of continuous epoxidation experiments as an elaboration of those carried out in batch reactors. The importance of greener epoxidation processes has been explored in Ref. [261], with the main benefits of developing greener methods including reduced waste, further potential savings in regard to energy and material costs, as well as exhibiting progressive movements towards more environmentally conscious processes and tighter emissions regulations.
2.6. Comparison between Mesoporous and Microporous Structures and Their Uses as Catalysts in the Production of Fine Chemicals
After reviewing the existing literature concerning various types of mesoporous and microporous catalysts, one key takeaway would be that many of these structures can alternate between mesoporous, microporous, or can sometimes exhibit both (hierarchical). For example, both micro- and mesoporous zeolites exist, yet their uses differ, depending on the reaction or process in question. A similar case exists for MOFs and carbon-based structures, along with many others. However, in the context of fine chemicals, some pore structures, along with specific materials, are significantly better suited to this application. A clear example of this would be the carbon-based structures. According to the definition provided by the International Federation of Pure and Applied Chemistry (IUPAC), porous carbon-based materials can be split into three categories, based on their pore diameter size: microporous carbon (<2 nm), mesoporous carbon (between 2 and 50 nm), and macroporous carbon (>50 nm) [262]. Much of the literature on microporous carbon-based structures falls into the field of electrochemistry, as opposed to the industry of fine chemicals. One explanation for this may be due to its strong conductive abilities over other heterogeneous catalysts. Apart from this, many of the other advantages that it provides are shared with other catalytic structures. Despite this, its properties are not often harnessed in fine chemical production, yet in its mesoporous form, its use is significantly more common.
Therefore, several materials explored in this review, such as microporous MOFs, can possess either a crystalline or amorphous structure. However, the crystalline structure often features more prominently in catalytic research, whereas in industry, amorphous structures are typically used more often due to their lower cost and greater flexibility [263,264,265].
In terms of determining the most efficient or suitable catalyst, it could be argued that this is nothing but a trivial matter. Despite some structures exhibiting more advantages than others, it is solely a matter of the process in question. For example, compared with the most used catalyst support structures, such as silica and zeolites, carbon-based materials possess several advantages, such as stability in both basic and acidic mediums. Along with this, activated carbon obtained from biochar can yield a highly porous structure, with surface areas reaching as high as 2000 m2/g (depending on the source and synthesis/activation method). This is significantly larger in comparison to other common material-specific surface areas; for example, silica can only reach 750 m2/g and zeolites up to 800 m2/g [211]. However, in the case of an acid catalysis reaction, the use of a zeolite structure would be strongly preferred due to its strong Brønsted and Lewis acid sites, something that a carbon-based catalyst could not offer on its own.
2.7. Synthesis and Modification
Often, during the production of fine chemicals, a large number of preparation steps are required. This situation has been reviewed above for specific processes with certain materials. This section, however, will analyse these modification and preparation methods more generically. Often, the reactants being used must be altered or treated before use. Additionally, the catalyst being used will often require some prior steps, be it modification with alternative compounds, thermal treatment, or the application of support [266]. According to Ref. [61], the approach taken to synthesise and prepare the catalyst structure will heavily influence the formed structure in terms of the dispersion of the catalyst, as well as its surface area and concentration. These findings are also supported by Refs. [267,268,269], indicating that the method used in catalyst development and preparation is crucial in dictating the reaction’s performance and outcome. The additional costs incurred during any preparation, in terms of additional materials and energy requirements, further add to the value of the final product being manufactured; hence, the choice of a catalyst is not to be taken lightly.
The majority of the literature in this field often focuses on the effectiveness of the catalyst used for a specific process, with the type of processes being analysed varying heavily. Often, the processes being investigated consist of either hydrogenation, dehydrogenation, hydrocracking, and decarbonylation (which occur on metal sites); or dehydration, isomerization, and hydrogenolysis (which occur on acid sites) [270]. A significant amount of literature has been produced in recent years with a focus on modifying and enhancing existing micro and mesoporous catalysts to improve performance. The primary focus of research in this field has been the synthesis, characterisation, and applications of acidic, noble, and transition metal modification.
In the context of heterogeneous catalysts, modification occurs when the base catalyst substance is combined or added to an alternative substance, often a liquid or solid, to alter and extrude-specific properties that may be desired for a reaction. Unlike the direct synthesis of a catalyst, it is not being generated solely from reactants, but already exists and is being both chemically and physically altered. For example, in the case of the synthesis of florol, as explored by Ref. [271], the H-USY-30 catalyst was modified via treatment with an aqueous NaOH alkaline solution. This modification consisted of combining the two in an Easymax 106 reactor for a specific period, followed by ion exchange to convert it to its protonic form. The NaOH treatment is vital for improving the overall catalytic ability of the H-USY-30. The NaOH acts as a baseleaching agent, leading to the dissolution of the amorphous silica and aluminium within the structure. This increases porosity and leads to an alteration in the distribution and strength of the acid sites. This then minimizes side reactions and increases overall reaction selectivity. The associated reaction scheme of the desilication alkaline treatment can be seen below in Figure 5.
Figure 5.
A diagram illustrating the role that an alkaline treatment can play in the modification and preparation of an aluminosilicate structure, adapted from Ref. [271].
There are countless other examples of catalyst modification across all aspects of the chemical industry; for example, in Ref. [68], Kumar investigates the ring opening of decalin using MCM-41, modified with Ru. Subsequently, according to Ref. [61], the most common methods of mesoporous catalyst preparation or modification (zeolites, mesoporous silicas, etc.) are ion exchange and impregnation, with in situ synthesis being scarcely used, owing to it being a relatively new method.
There are a range of ways in which catalysts can be modified, depending on their existing structure and the desired outcome. These include ion exchange, impregnation of a catalyst, as well as in situ and composite methods. These will be explored in further detail below, with their relevance in the production of fine chemicals assessed and reviewed across the existing literature and experimental papers/journals.
2.7.1. Ion-Exchange
The ion exchange method is a widely used technique in catalysis, particularly for materials with micro- and mesoporous structures. It involves the replacement of ions within the material’s framework with other ions from a solution. This method is often employed for catalysts with low metal loadings, as detailed in Ref. [68], wherein MCM-41 was utilised for the ring-opening decalin. This method then allows for the controlled introduction of specific ions or molecules to modify the catalytic properties of the material. In the context of catalyst preparation, the ion exchange method helps put certain metal ions into the molecular sieve’s structure. This exchange process, in most cases, is crucial for tailoring the surface properties, as well as for enhancing the catalytic activity of the material. The reasoning behind using ion exchange in this way is to control the types and concentrations of ions introduced so that the acidity and selectivity of the catalyst can be managed, enabling it to be used more efficiently in specific chemical reactions. As mentioned previously, preparing a catalyst in this way often requires many steps to ensure that the properties of the catalyst are controlled. Even slight deteriorations from the correct process can lead to a change in the catalyst, rendering it inefficient or perhaps no longer viable. Moreover, ion exchange can be an energy-intensive process, leading to the conclusion that an ion exchange method possesses both pros and cons. This explains its popularity in the use of heterogeneous catalysts but points towards the use of other modifications and preparation steps to obtain a more ideal catalyst.
2.7.2. Evaporation Impregnation
Metal precursors are dissolved in distilled water and added to the microporous zeolites or mesoporous materials in a flask. Metal modifications are carried out for 24 h in a rotatory evaporator equipped with a water bath. After completion of the synthesis, the aqueous phase is evaporated, and the catalyst is removed from the flask, followed by drying and calcination in a muffle oven [272].
2.7.3. Deposition Precipitations
In the deposition precipitation method, the pH of the dissolved metal precursor and the microporous zeolites or mesoporous materials is enhanced to 9–10, and synthesis is carried out for 24 h in alkaline media. After completion of the synthesis, the catalyst is filtered, washed with distilled water, dried, and calcined in a muffle oven.
2.7.4. Physicochemical Characterisation
The physicochemical characterisation of catalytic materials applied in the production of fine chemicals is of immense importance for understanding the reaction mechanism. Furthermore, physicochemical characterisation results are utilized in the development of new efficient and stable catalysts with high selectivity to desired fine chemicals. Instruments and techniques applied in the physicochemical characterisations are as follows: X-ray powder diffraction (XRD), used for structure and phase purity determination; scanning electron microscopy (SEM), for determination of the morphological features, such as crystal shape, size, and distributions; energy dispersive X-ray analyses (EDXA), for measurement of the chemical compositions. Textural properties such as surface area, pore volume, and pore size distributions, of the pristine solid acid, as well as the transition and noble metal modified heterogeneous catalysts, are determined using N2-physisorption. The number and strength of the Brønsted and Lewis acid sites in the catalytic materials are measured using FTIR-Pyridine. The transition and noble metal nanoparticle size, shape, and distributions are measured using transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM). Furthermore, HRTEM and TEM are also applied for the measurement of pore dimensions, the periodicity of pores, and the channel systems in the structured microporous and mesoporous materials utilised for fine chemical synthesis. Framework Al and extra-framework Al, important for the creation of Brønsted and Lewis acid sites, are measured using 27Al-NMR and 29Si MAS NMR spectroscopies. The oxidation states of the transition and noble metals are important to produce fine chemicals using heterogeneous catalysts. X-ray photoelectron spectroscopy (XPS) is used for the determination of the oxidation states of the metal-modified catalysts.
2.7.5. Acid Preparation
Acids can play a crucial role in the development, modification, or synthesis of catalysts. They can perform several roles, with the main objective being to chemically alter the catalyst to increase conversion and promote greater selectivity towards the desired products of a reaction. Common ways in which acids are used in this process include template removal, pore diameter adjustment, and forms of surface modification.
Template removal incorporates the use of an acid to remove organic templates or surfactants from the synthesized catalysts. The acids are used to dissolve or remove the organic components from the synthesized material, leaving behind the desired porous structure. It must be noted, however, that paying close attention to the removal process is crucial to maintaining the material’s structure and desired qualities. Incomplete or improper removal could lead to structural flaws, blocked pores, or unwanted contaminants, ultimately impacting the catalyst’s performance.
Pore size adjustment allows for the diameter of the pores within the material to be altered. By doing so, the ability of reactant molecules to enter the structure and contact active sites can be modified, providing the ability to change the catalyst’s conversion and selectivity.
Altering the surface of the material using an acid can not only change its structure and the number of active sites available for a reaction, but it can also enhance the catalyst’s stability and reduce the risk of catalyst contamination or deactivation. This is vital in extending the life of the catalyst, meaning it can be recovered and reused more often, reducing catalytic costs in the long term.
2.7.6. Base Preparation
Utilising a base, either in the preparation or synthesis of a catalyst, generally achieves similar objectives as using an acid; however, the preparation progresses along a different chemical route. Bases can be used to control the pH of the catalyst, as well as to perform surface adjustments on the catalytic material. This includes adding or removing specific functional groups from the catalyst surface, which can in turn lead to a different chemical structure and to altered reaction parameters. Bases effectively allow for the customization of the structural characteristics of the catalyst, providing an enhancement to its efficiency, selectivity, and conversion. In comparison to the route followed by an acid, the alkaline route features both hydrolysis and condensation, leading to a significantly more compact and refined structure [273].
The use of either a base or acid as a heterogeneous catalyst has recently been featured in extensive research, with attempts made to generate a hybrid catalyst featuring both. Such a catalyst has been growing in popularity due to its faster reaction times and lower corrosion or deactivation potential, as evidenced in Refs. [274,275].
2.7.7. Comparison
A comparison between using an acid, a base, and an ion exchange resin as a heterogeneous catalyst during the production of biodiesel has been explored in Ref. [32]. The advantages of using an acid heterogeneous catalyst have been listed as the recovery and reuse aspect of the catalyst, with the main drawback being a loss of the catalyst due to leaching, as well as the fact that a high temperature is required, along with a prolonged reaction time. This has also been mentioned in a similar context in Refs. [276,277,278]. Conversely, the paper suggests that using a base as a heterogeneous catalyst produces a higher reaction rate. However, a loss of the catalyst also occurred due to leaching, as well as to the fact that it was sensitive to the materials used during the reaction. Finally, the paper compares these catalysts to the use of an ion-exchange resin. The ion-exchange resin featured the complete removal of by-products, as well as easy product separation from the reactants and catalyst, in addition to the ability to catalyse the reaction as either a base or an acid. Its drawbacks, however, included the fact that its resin active sites suffered from deactivation, and it exhibited a slow reaction rate.
2.7.8. Impregnation
The technique of impregnation features the absorption and/or penetration of an active catalyst species into the pores of a solid support material, such as activated carbon, alumina, or silica. The impregnated catalyst can be in the form of a solution, dispersion, or suspension, which is deposited onto the supporting structure through several physicochemical interactions.
This method of preparation allows for a relatively high concentration of active species on the support material, providing greater catalytic activity and selectivity. This is achieved through careful control of the active species and the impregnation time.
A key advantage of the impregnation method is its flexibility in accommodating a range of active species, including metals, metal oxides, and metal complexes.
Potential drawbacks regarding the use of impregnation as a catalyst synthesis method include the possible uneven distribution of the active catalyst within the porous structure of the support material, which can affect the overall catalytic performance and stability. One proposal to solve this issue would be to employ careful control of the impregnation parameters and post-impregnation treatments, e.g., drying and calcination. The use of some form of post-treatment would ensure uniform distribution, as well as strong adhesion of the active species within the structure of the support material.
The porous structure undergoing impregnation can vary, depending on the specific reaction. Often, the structure must undergo a form of preparation or treatment, usually calcination, before the impregnation step [279,280]. Following this, the impregnation occurs either through wet impregnation or incipient wetness impregnation. In some specific cases, for example, Ref. [279], impregnation of a catalyst occurs in a solventless environment, leading to what is known as “precursor dry impregnation”. Alternatively, evaporation-impregnation can be used, such as in the preparation of ceria-supported catalysts [281]. Here, evaporation–impregnation was achieved by stirring in a rotary evaporator, followed by water evaporation under vacuum.
One important aspect of the impregnation method to note is the preparation of a precursor solution and the following drying and/or calcination that occurs. This pre-step is similar to the methodology used in the ion-exchange method. However, the impregnation method is regarded to be more straightforward and is claimed to be one of the most popular methods, as per Ref. [282].
2.7.9. Oxygen Doping
Oxygen doping is an approach used on mesoporous carbon bases to improve the hydrophilicity of the surface. The surface can then provide active sites for catalytic reactions or the selective adsorption of cationic compounds. Additionally, it can aid in the dispersion of metals within the structure [283,284]. In general, oxygen doping can introduce additional active sites to the structure, increasing activity, as well as thermal stability, which in some cases, can prevent sintering, extending the life of the catalyst.
3. Conclusions
Overall, this literature review explored the complex and wide-ranging field of microporous and mesoporous catalysts within the fine chemical industry. The efficiency, selectivity, and sustainability of the chemical reactions involved are strongly influenced by the catalyst of choice, regardless of its mesoporous or microporous nature. Because their pores are smaller (<2 nm), microporous materials—like zeolites, aluminophosphate, and some metal–organic frameworks (MOFs)—offer excellent selectivity and are especially well-suited for reactions involving smaller molecules. They often require significantly fewer steps during their manufacture and synthesis, resulting in fewer harmful emissions and a lower cost of production. The most-used microporous structure, as a heterogeneous catalyst, is zeolites. However, the rise of MOFs and other alternatives that provide a wider range of customisability in terms of pore size and volume are under development every day, with their significance rapidly growing as a result of their potential promise as better heterogeneous catalysts.
Mesoporous materials, including various silica-based structures, zeolites, ZIFs and some MOFs, provide the advantage of accommodating larger molecules and demonstrating enhanced diffusion. This makes them ideal for a broader range of reactions. Moreover, they possess the ability to express adaptive pore shapes and sizes, meaning that the catalytic structure can be tailored to the needs of individual reactions, massively increasing its effectiveness due to its high selectivity and activity. The most popular mesoporous catalysts in the industry include organo-silica-based MCM-41, as well as mesoporous zeolites. In a similar fashion to microporous materials, the use of MOFs in place of traditional mesoporous catalysts is growing. The reason for this trend includes the almost infinite number of possible structures that MOFs can exhibit, meaning that they can be tailored for almost every reaction, and there is always the possibility of developing a catalytically improved structure. Moreover, the industrial catalytic use of ZIF structures as heterogeneous catalysts is beginning to rise, not just in the fine chemical industry but across a range of chemical sectors. This is a result of their high specific surface areas, coordination topology features, and in some cases, nitrogen-rich structures, leading to a high density, as well as a uniform distribution, of active sites.
This review has also demonstrated the importance of catalyst modification and preparation, showing how techniques like ion exchange, impregnation, and oxygen doping can significantly alter the properties of these catalysts, making them suitable for certain uses in the fine chemical industry. These modifications can enhance properties such as surface area, pore volume, and chemical functionality, therefore improving the catalytic activity and selectivity of a reaction.
In conclusion, the field of heterogeneous catalysis for fine chemical production is a dynamic and ever-changing area of research. Continued research regarding micro- and mesoporous catalyst design, combined with a clearer understanding of structural impact on activity, will significantly advance the field. This deeper insight into catalytic behaviour and material properties will lead to the production of more efficient, selective, and sustainable processes in the fine chemical industry. It is noteworthy to mention that solid acid, transition, and noble metal-modified heterogeneous catalysts undergo catalyst deactivation during the production of fine and speciality chemicals. However, most of the spent catalytic materials after the reaction could be regenerated and reused.
The findings from this comprehensive literature review provide a solid foundation for further exploration and a critical review in the field of fine chemical production using micro- and mesoporous heterogeneous catalysts.
4. Future Directions
There is a rapidly growing interest in the use of both microporous and mesoporous heterogeneous catalysts in the fine chemical industry. As it stands, the current work in this field is predominantly focused on small-scale investigation of these structures and their individual applications. A key area for future research in this field would involve investigations into bridging the gap between lab-scale experiments and industrial-level applications of meso- and microporous heterogeneous catalysts. It is evident through a review of the existing literature that a case for the use of various porous catalyst structures, including MOFs and ZIFs, exists. However, a lack of experimental work focused on increasing the utilisation scale and production volumes remains, indicating that further work in this field is required before the widespread adoption of porous catalysts in the fine chemical industry becomes a possibility.
Additionally, a future exploration into innovative synthesis methods to enhance selectivity, stability, and efficiency, as well as additional work on the alteration of catalyst structures, both physically and chemically, to modify specific properties is highly recommended. Doing so would open a gateway into not only a wider adoption of the catalyst structures reviewed but also into the use of more environmentally and economically beneficial processes, which is something that would be highly valuable in a sector such as the fine chemicals industry, wherein even marginal gains and advancements would prove beneficial.
Several solid acids, transition, and noble metal-modified microporous and mesoporous heterogeneous catalysts are currently applied in the production of fine chemicals, as described in this paper. Plausible explanations for the advantages of using these catalytic materials include the possibility of easy separation from the reaction media, as well as its regeneration and reuse in the production of fine chemicals. However, taking into consideration the sustainable development of human society and the efficient use of limited natural resources, new environmentally friendly catalytic materials need to be discovered and utilized for industrial processes. Furthermore, new green process technology should be developed for the rational utilisation of natural resources and the minimal generation of industrial residuals.
Finally, the incorporation of computational modelling and machine learning into catalyst design would provide additional optimisation of reactions and chemical processes, providing the opportunity to enhance the efficiency and sustainability of fine chemical production. Such optimisation could lead to reduced chemical waste, experimentation costs, and development times, all of which are critical for maintaining competitiveness and meeting regulatory standards in the fine chemical industry.
Author Contributions
J.L.: writing—original draft preparation, formal analysis, reference collections, and revision; N.K.: conceptualization, formal analysis, writing—review and editing, consultations, and comments; B.S.: conceptualization, supervision, formal analysis, consultations, writing—review and editing, resources, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work is a result of the cooperative activities between the Laboratory of Industrial Chemistry and Reaction Engineering, the Faculty of Science and Engineering, Johan Gadolin Process Chemistry Center, Åbo Akademi University, Turku, Finland, and the School of Engineering, Lancaster University, United Kingdom.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
MT—megatonnes; TOF—turnover frequency; TON—turnover number; MOF—metal–organic framework; ZIF—zeolitic imidazolate framework; GCMC—grand canonical Monte Carlo; IUPAC—International Federation of Pure and Applied Chemistry.
References
- Panizza, M. Chapter 13–Fine chemical industry, pulp and paper industry, petrochemical industry and pharmaceutical industry. In Electrochemical Water and Wastewater Treatment; Martínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Studer, M. The role of catalysis for the clean production of fine chemicals. Appl. Catal. A Gen. 1999, 189, 191–204. [Google Scholar] [CrossRef]
- Busacca, C.A.; Fandrick, D.R.; Song, J.J.; Senanayake, C.H. The Growing Impact of Catalysis in the Pharmaceutical Industry. Adv. Synth. Catal. 2011, 353, 1825–1864. [Google Scholar] [CrossRef]
- Hayler, J.D.; Leahy, D.K.; Simmons, E.M. A Pharmaceutical Industry Perspective on Sustainable Metal Catalysis. Organometallics 2019, 38, 36–46. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- de Sousa, L.B.; Speranza, J.T.; Condotta, R.; Cella, R. Heterogeneous catalyzed isomerization of turpentine oil by ordered mesoporous materials like M41S structures. Can. J. Chem. Eng. 2023, 101, 4106–4117. [Google Scholar] [CrossRef]
- Malet-Sanz, L.; Susanne, F. Continuous Flow Synthesis. A Pharma Perspective. J. Med. Chem. 2012, 55, 4062–4098. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process. Res. Dev. 2016, 20, 2–25. [Google Scholar] [CrossRef]
- Bulk Chemicals vs. Fine Chemicals: The Difference. Available online: https://capitalresin.com/bulk-chemicals-vs-fine-chemicals-the-difference/#:~:text=Fine%20chemicals%20are%20more%20complex,surface%2Dlevel%20differences%2C%20however (accessed on 11 May 2023).
- Anonymous. Global Chemicals Export Value by Segment. Available online: https://www.statista.com/statistics/1380198/global-chemicals-export-value-by-segment/ (accessed on 11 May 2023).
- Things You Should Know about Fine Chemicals. Available online: https://www.qinmuchem.com/news/things-you-should-know-about-fine-chemicals.html (accessed on 11 May 2023).
- Pollak, P. Fine Chemicals the Industry and the Business, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ballini, R. Eco-Friendly Synthesis of Fine Chemicals; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Schwanke, A.J.; Balzer, R.; Pergher, S. Microporous and mesoporous materials from natural and inexpensive sources. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Mallick, K.K.; Winnett, J. 6-3D bioceramic foams for bone tissue engineering. In Bone Substitute Biomaterials; Mallick, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts 2021, 11, 1541. [Google Scholar] [CrossRef]
- Zu, L.; Zhang, W.; Qu, L.; Liu, L.; Li, W.; Yu, A.; Zhao, D. Mesoporous Materials for Electrochemical Energy Storage and Conversion. Adv. Energy Mater. 2020, 10, 2152. [Google Scholar] [CrossRef]
- Sánchez-Antonio, O.; Romero-Sedglach, K.A.; Vázquez-Orta, E.C.; Juaristi, E. New Mesoporous Silica-Supported Organocatalysts Based on (2S)-(1,2,4-Triazol-3-yl)-Proline: Efficient, Reusable, and Heterogeneous Catalysts for the Asymmetric Aldol Reaction. Molecules 2020, 25, 4532. [Google Scholar] [CrossRef]
- Xie, Y.; Sharma, K.K.; Anan, A.; Wang, G.; Biradar, A.V.; Asefa, T. Efficient solid-base catalysts for aldol reaction by optimizing the density and type of organoamine groups on nanoporous silica. J. Catal. 2009, 265, 131–140. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Silica-Bound Homogenous Catalysts as Recoverable and Reusable Catalysts in Organic Synthesis. Adv. Synth. Catal. 2006, 348, 1391–1412. [Google Scholar] [CrossRef]
- Scatena, G.S.; de la Torre, A.F.; Cass, Q.B.; Rivera, D.G.; Paixão, M.W. Multicomponent Approach to Silica-Grafted Peptide Catalysts: A 3 D Continuous-Flow Organocatalytic System with On-line Monitoring of Conversion and Stereoselectivity. ChemCatChem 2014, 6, 3208–3214. [Google Scholar] [CrossRef]
- Puglisi, A.; Benaglia, M.; Annunziata, R.; Chiroli, V.; Porta, R.; Gervasini, A. Chiral Hybrid Inorganic–Organic Materials: Synthesis, Characterization, and Application in Stereoselective Organocatalytic Cycloadditions. J. Org. Chem. 2013, 78, 11326–11334. [Google Scholar] [CrossRef] [PubMed]
- Brühwiler, D. Postsynthetic functionalization of mesoporous silica. Nanoscale 2010, 2, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Gao, J.S.; Yang, Q.H.; Li, C. Large-pore mesoporous ethane-silicas as efficient heterogeneous asymmetric catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 170, pp. 1252–1259. [Google Scholar] [CrossRef]
- Martín, N.; Cirujano, F.G. Organic synthesis of high added value molecules with MOF catalysts. Org. Biomol. Chem. 2020, 18, 8058–8073. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.R.; Park, J.-W.; Kim, H.; Ji, H.; Lim, S.H.; Jun, C.-H. A one-step co-condensation method for the synthesis of well-defined functionalized mesoporous SBA-15 using trimethallylsilanes as organosilane sources. Chem. Commun. 2015, 51, 17084–17087. [Google Scholar] [CrossRef] [PubMed]
- Ferré, M.; Pleixats, R.; Man, M.W.C.; Cattoën, X. Recyclable organocatalysts based on hybrid silicas. Green Chem. 2016, 18, 881–922. [Google Scholar] [CrossRef]
- Putz, A.-M.; Almásy, L.; Len, A.; Ianăşi, C. Functionalized silica materials synthesized via co-condensation and post-grafting methods. Full-Nanotub. Carbon Nanostruct. 2019, 27, 323–332. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal–Organic Frameworks. Adv. Mater. 2017, 29, 1139. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Brunel, D.; Blanc, A.C.; Galarneau, A.; Fajula, F. New trends in the design of supported catalysts on mesoporous silicas and their applications in fine chemicals. Catal. Today 2002, 73, 139–152. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Balasubramani, R.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Schumacher, K.; von Hohenesche, C.D.F.; Grün, M.; Unger, K. MCM-41, MCM-48 and related mesoporous adsorbents: Their synthesis and characterisation. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 109–116. [Google Scholar] [CrossRef]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Vajglová, Z.; Kumar, N.; Peurla, M.; Eränen, K.; Mäki-Arvela, P.; Murzin, D.Y. Cascade transformations of (±)-citronellal to menthol over extruded Ru-MCM-41 catalysts in a continuous reactor. Catal. Sci. Technol. 2020, 10, 8108–8119. [Google Scholar] [CrossRef]
- Endud, S.; Wong, K.-L. Mesoporous silica MCM-48 molecular sieve modified with SnCl2 in alkaline medium for selective oxidation of alcohol. Microporous Mesoporous Mater. 2007, 101, 256–263. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Feng, P.; Gier, T.E.; Sieger, P.; Leon, R.; Petroff, P.M.; Schüth, F.; Stucky, G.D. Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 1994, 368, 317–321. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Demuth, D.G.; Feng, P.; Gier, T.E.; Sieger, P.; Firouzi, A.; Chmelka, B.F. Organization of Organic Molecules with Inorganic Molecular Species into Nanocomposite Biphase Arrays. Chem. Mater. 1994, 6, 1176–1191. [Google Scholar] [CrossRef]
- Parida, N.; Badamali, S.K. Facile synthesis and catalytic activity of nanoporous SBA-1. J. Porous Mater. 2022, 29, 161–167. [Google Scholar] [CrossRef]
- Huo, Q.; Leon, R.; Petroff, P.M.; Stucky, G.D. Mesostructure Design with Gemini Surfactants: Supercage Formation in a Three-Dimensional Hexagonal Array. Science 1995, 268, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Kot, M.; Kiderys, A.; Janiszewska, E.; Pietrowski, M.; Yang, C.-M.; Zieliński, M. Hydrogenation of toluene over nickel nanoparticles supported on SBA-3 and AlSBA-3 materials. Catal. Today 2020, 356, 64–72. [Google Scholar] [CrossRef]
- Lu, G.Q.; Zhao, X.S. Nanoporous Materials: Science and Engineering; World Scientific Publishing: Singapore, 2004. [Google Scholar]
- Ge, S.; Geng, W.; He, X.; Zhao, J.; Zhou, B.; Duan, L.; Wu, Y.; Zhang, Q. Effect of framework structure, pore size and surface modification on the adsorption performance of methylene blue and Cu2+ in mesoporous silica. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 154–162. [Google Scholar] [CrossRef]
- Mayoral, A.; Blanco, R.M.; Diaz, I. Location of enzyme in lipase-SBA-12 hybrid biocatalyst. J. Mol. Catal. B Enzym. 2013, 90, 23–25. [Google Scholar] [CrossRef]
- Kumar, A.; Nepak, D.; Srinivas, D. Direct synthesis of amides from amines using mesoporous Mn-SBA-12 and Mn-SBA-16 catalysts. Catal. Commun. 2013, 37, 36–40. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Di Renzo, F.; Ryoo, R.; Choi, M.; Fajula, F. Microporosity and connections between pores in SBA-15 mesostructured silicas as a function of the temperature of synthesis. New J. Chem. 2003, 27, 73–79. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, X.; Dai, X.; Qin, Y.; Duan, A.; Yu, Y.; Zhuo, H.; Zhao, H.; Zhang, P.; Jiang, Y.; et al. Morphology-selective synthesis of active and durable gold catalysts with high catalytic performance in the reduction of 4-nitrophenol. Nano Res. 2016, 9, 3099–3115. [Google Scholar] [CrossRef]
- Sankar, E.S.; Reddy, K.S.; Jyothi, Y.; Raju, B.D.; Rao, K.S.R. Alcoholysis of Furfuryl Alcohol into n-Butyl Levulinate Over SBA-16 Supported Heteropoly Acid Catalyst. Catal. Lett. 2017, 147, 2807–2816. [Google Scholar] [CrossRef]
- Kleitz, F.; Liu, D.; Anilkumar, G.M.; Park, I.-S.; Solovyov, L.A.; Shmakov, A.N.; Ryoo, R. Large Cage Face-Centered-Cubic Fm3m Mesoporous Silica: Synthesis and Structure. J. Phys. Chem. B 2003, 107, 14296–14300. [Google Scholar] [CrossRef]
- Kalbasi, R.J.; Zirakbash, A. Synthesis, characterization and drug release studies of poly(2-hydroxyethyl methacrylate)/KIT-5 nanocomposite as an innovative organic–inorganic hybrid carrier system. RSC Adv. 2015, 5, 12463–12471. [Google Scholar] [CrossRef]
- Bejblová, M.; Procházková, D.; Čejka, J. Acylation Reactions over Zeolites and Mesoporous Catalysts. ChemSusChem 2009, 2, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Palanichamy, M. Mesoporous cubic Ia3d materials for the preparation of fine chemicals: Synthesis of jasminaldehyde. Microporous Mesoporous Mater. 2013, 168, 126–131. [Google Scholar] [CrossRef]
- Kim, S.-S.; Pauly, T.R.; Pinnavaia, T.J. Non-ionic surfactant assembly of ordered, very large pore molecular sieve silicas from water soluble silicates. Chem. Commun. 2000, 17, 1661–1662. [Google Scholar] [CrossRef]
- Kleitz, F. Ordered mesoporous materials. In Handbook of Heterogeneous Catalysis Anonymous; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Shimizu, T.; Ota, M.; Sato, Y.; Inomata, H. Effect of pore structure on catalytic properties of mesoporous silica supported rhodium catalysts for the hydrogenation of cinnamaldehyde. Chem. Eng. Res. Des. 2015, 104, 174–179. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, L.; Zhang, J.; Anpo, M. Novel synthesis of high hydrothermal stability and long-range order MCM-48 with a convenient method. Microporous Mesoporous Mater. 2005, 86, 314–322. [Google Scholar] [CrossRef]
- Sayari, A. Catalysis by Crystalline Mesoporous Molecular Sieves. Chem. Mater. 1996, 8, 1840–1852. [Google Scholar] [CrossRef]
- Vinu, A.; Murugesan, V.; Hartmann, M. Pore Size Engineering and Mechanical Stability of the Cubic Mesoporous Molecular Sieve SBA-1. Chem. Mater. 2003, 15, 1385–1393. [Google Scholar] [CrossRef]
- Kumar, N.; Mäki-Arvela, P.; Hajek, J.; Salmi, T.; Murzin, D.; Heikkilä, T.; Laine, E.; Laukkanen, P.; Väyrynen, J. Physico-chemical and catalytic properties of Ru–MCM-41 mesoporous molecular sieve catalyst: Influence of Ru modification methods. Microporous Mesoporous Mater. 2004, 69, 173–179. [Google Scholar] [CrossRef]
- da Silva, K.A.; Robles-Dutenhefner, P.A.; Sousa, E.M.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Cyclization of (+)-citronellal to (−)-isopulegol catalyzed by H3PW12O40/SiO2. Catal. Commun. 2004, 5, 425–429. [Google Scholar] [CrossRef]
- Makiarvela, P.; Mäki-Arvela, P.; Kumar, N.; Nieminen, V.; Sjöholm, R.; Salmi, T.; Murzin, D.Y. Cyclization of citronellal over zeolites and mesoporous materials for production of isopulegol. J. Catal. 2004, 225, 155–169. [Google Scholar] [CrossRef]
- Nie, Y.; Niah, W.; Jaenicke, S.; Chuah, G.-K. A tandem cyclization and hydrogenation of (±)-citronellal to menthol over bifunctional Ni/Zr-beta and mixed Zr-beta and Ni/MCM-41. J. Catal. 2007, 248, 1–10. [Google Scholar] [CrossRef]
- Balu, A.M.; Campelo, J.M.; Luque, R.; Romero, A.A. One-step microwave-assisted asymmetric cyclisation/hydrogenation of citronellal to menthols using supported nanoparticles on mesoporous materials. Org. Biomol. Chem. 2010, 8, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Stekrova, M.; Mäki-Arvela, P.; Leino, E.; Valkaj, K.M.; Eränen, K.; Aho, A.; Smeds, A.; Kumar, N.; Volcho, K.P.; Salakhutdinov, N.F.; et al. Two-step synthesis of monoterpenoid dioxinols exhibiting analgesic activity from isopulegol and benzaldehyde over heterogeneous catalysts. Catal. Today 2017, 279, 56–62. [Google Scholar] [CrossRef]
- Stekrova, M.; Mäki-Arvela, P.; Kumar, N.; Behravesh, E.; Aho, A.; Balme, Q.; Volcho, K.P.; Salakhutdinov, N.F.; Murzin, D.Y. Prins cyclization: Synthesis of compounds with tetrahydropyran moiety over heterogeneous catalysts. J. Mol. Catal. A Chem. 2015, 410, 260–270. [Google Scholar] [CrossRef]
- Kumar, N.; Kubicka, D.; Garay, A.L.; Mäki-Arvela, P.; Heikkilä, T.; Salmi, T.; Murzin, D.Y. Synthesis of Ru-modified MCM-41 Mesoporous Material, Y and Beta Zeolite Catalysts for Ring Opening of Decalin. Top. Catal. 2009, 52, 380–386. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, J.-L.; Yan, J.-N.; Zhao, X.-G.; Chen, H.-G. Mesoporous SBA-15 material functionalized with ferrocene group and its use as heterogeneous catalyst for benzene hydroxylation. Appl. Catal. A Gen. 2004, 263, 213–217. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Chatterjee, S.; Bhaumik, A. Bifunctionalized Mesoporous SBA-15: A New Heterogeneous Catalyst for the Facile Synthesis of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2017, 5, 2763–2773. [Google Scholar] [CrossRef]
- Tong, P.; Liang, J.; Jiang, X.; Li, J. Research Progress on Metal-Organic Framework Composites in Chemical Sensors. Crit. Rev. Anal. Chem. 2020, 50, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef] [PubMed]
- Nuri, A.; Vucetic, N.; Smått, J.-H.; Mansoori, Y.; Mikkola, J.-P.; Murzin, D.Y. Pd Supported IRMOF-3: Heterogeneous, Efficient and Reusable Catalyst for Heck Reaction. Catal. Lett. 2019, 149, 1941–1951. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef]
- Pendashteh, A.; Vilela, S.M.; Krivtsov, I.; Ávila-Brande, D.; Palma, J.; Horcajada, P.; Marcilla, R. Bimetal zeolitic imidazolate framework (ZIF-9) derived nitrogen-doped porous carbon as efficient oxygen electrocatalysts for rechargeable Zn-air batteries. J. Power Sources 2019, 427, 299–308. [Google Scholar] [CrossRef]
- Li, Q.; Kim, H. Hydrogen production from NaBH4 hydrolysis via Co-ZIF-9 catalyst. Fuel Process. Technol. 2012, 100, 43–48. [Google Scholar] [CrossRef]
- Nguyen, L.T.L.; Le, K.K.A.; Truong, H.X.; Phan, N.T.S. Metal–organic frameworks for catalysis: The Knoevenagel reaction using zeolite imidazolate framework ZIF-9 as an efficient heterogeneous catalyst. Catal. Sci. Technol. 2012, 2, 521–528. [Google Scholar] [CrossRef]
- Babarao, R.; Jiang, Y.; Medhekar, N.V. Postcombustion CO2 Capture in Functionalized Porous Coordination Networks. J. Phys. Chem. C 2013, 117, 26976–26987. [Google Scholar] [CrossRef]
- Goswami, S.; Miller, C.E.; Logsdon, J.L.; Buru, C.T.; Wu, Y.-L.; Bowman, D.N.; Islamoglu, T.; Asiri, A.M.; Cramer, C.J.; Wasielewski, M.R.; et al. Atomistic Approach toward Selective Photocatalytic Oxidation of a Mustard-Gas Simulant: A Case Study with Heavy-Chalcogen-Containing PCN-57 Analogues. ACS Appl. Mater. Interfaces 2017, 9, 19535–19540. [Google Scholar] [CrossRef]
- García-García, P.; Corma, A. Hf-based Metal-Organic Frameworks in Heterogeneous Catalysis. Isr. J. Chem. 2018, 58, 1062–1074. [Google Scholar] [CrossRef]
- Hamon, L.; Heymans, N.; Llewellyn, P.L.; Guillerm, V.; Ghoufi, A.; Vaesen, S.; Maurin, G.; Serre, C.; De Weireld, G.; Pirngruber, G.D. Separation of CO2–CH4 mixtures in the mesoporous MIL-100(Cr) MOF: Experimental and modelling approaches. Dalton Trans. 2012, 41, 4052–4059. [Google Scholar] [CrossRef] [PubMed]
- Opanasenko, M.; Dhakshinamoorthy, A.; Hwang, Y.K.; Chang, J.; Garcia, H.; Čejka, J. Superior Performance of Metal–Organic Frameworks over Zeolites as Solid Acid Catalysts in the Prins Reaction: Green Synthesis of Nopol. ChemSusChem 2013, 6, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Dissegna, S.; Epp, K.; Heinz, W.R.; Kieslich, G.; Fischer, R.A. Defective metal-organic frameworks. Adv. Mater. 2018, 30, 1704501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.-W.; Tian, H.-R.; Li, X.-H.; Liu, S.-M.; Lu, Y.; Sun, Z.-X.; Liu, T. Polyoxometalate-Based Metal-Organic Framework Fractal Crystals. Matter 2020, 2, 250–260. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef]
- Hao, M.; Qiu, M.; Yang, H.; Hu, B.; Wang, X. Recent advances on preparation and environmental applications of MOF-derived carbons in catalysis. Sci. Total. Environ. 2021, 760, 143333. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mayoral, E.; Godino-Ojer, M.; Matos, I.; Bernardo, M. Opportunities from Metal Organic Frameworks to Develop Porous Carbons Catalysts Involved in Fine Chemical Synthesis. Catalysts 2023, 13, 541. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F. Heterogeneous Catalysts for Clean Technology: Spectroscopy, Design, and Monitoring; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.; Shieh, F.-K.; Wu, K.C.-W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Opanasenko, M.; Čejka, J.; Garcia, H. Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal. Sci. Technol. 2013, 3, 2509–2540. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y. Nanoporous carbons derived from MOFs as metal-free catalysts for selective aerobic oxidations. J. Mater. Chem. A 2016, 4, 5247–5257. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Cai, G.; Wang, Y.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. Palladium nanoparticles stabilized with N-doped porous carbons derived from metal–organic frameworks for selective catalysis in biofuel upgrade: The role of catalyst wettability. Green Chem. 2016, 18, 1212–1217. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, L.; Wang, C.; Feng, C.; Shang, N.; Gao, S.; Wang, C. Palladium nanoparticles embedded in metal–organic framework derived porous carbon: Synthesis and application for efficient Suzuki–Miyaura coupling reactions. RSC Adv. 2016, 6, 37118–37123. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Boo, J.R.; Liu, C.-H.; Ahamad, T.; Alshehri, S.M.; Matsagar, B.M.; Wu, K.C.-W. Oxidation of biomass-derived furans to maleic acid over nitrogen-doped carbon catalysts under acid-free conditions. Catal. Sci. Technol. 2020, 10, 1498–1506. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Bai, C.; Liao, S.; Li, Y. Base-Free Oxidation of Alcohols to Esters at Room Temperature and Atmospheric Conditions using Nanoscale Co-Based Catalysts. ACS Catal. 2015, 5, 1850–1856. [Google Scholar] [CrossRef]
- Yao, X.; Bai, C.; Chen, J.; Li, Y. Efficient and selective green oxidation of alcohols by MOF-derived magnetic nanoparticles as a recoverable catalyst. RSC Adv. 2016, 6, 26921–26928. [Google Scholar] [CrossRef]
- Tang, B.; Song, W.-C.; Yang, E.-C.; Zhao, X.-J. MOF-derived Ni-based nanocomposites as robust catalysts for chemoselective hydrogenation of functionalized nitro compounds. RSC Adv. 2017, 7, 1531–1539. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use. J. Chromatogr. Sci. 1974, 13, 18A. [Google Scholar]
- Rosas-Arbelaez, W.; Fijneman, A.J.; Friedrich, H.; Palmqvist, A.E.C. Hierarchical micro-/mesoporous zeolite microspheres prepared by colloidal assembly of zeolite nanoparticles. RSC Adv. 2020, 10, 36459–36466. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Wang, X.; Zhang, Y.; Tang, Y.; Yang, P.; Xu, F.; Wang, Y.; Wang, X.; Zhang, Y.; et al. A Novel Hierarchical Nanozeolite Composite as Sorbent for Protein Separation in Immobilized Metal-Ion Affinity Chromatography. Adv. Mater. 2003, 15, 1751–1753. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- García-Martínez, J.; Li, K.; Davis, M.E. Mesoporous Zeolites–Preparation, Characterization and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Huber, G.W.; Corma, A.; Huber, G.W.; Corma, A. Synergies between Bio- and Oil Refineries for the Production of Fuels from Biomass. Angew. Chem. Int. Ed. 2007, 46, 7184–7201. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; Oconnor, P. Processing biomass-derived oxygenates in the oil refinery: Catalytic cracking (FCC) reaction pathways and role of catalyst. J. Catal. 2007, 247, 307–327. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jeong, J.; Ryu, S.; Lee, H.W.; Jung, J.S.; Siddiqui, M.Z.; Jung, S.-C.; Jeon, J.-K.; Jae, J.; Park, Y.-K. Catalytic pyrolysis of wood polymer composites over hierarchical mesoporous zeolites. Energy Convers. Manag. 2019, 195, 727–737. [Google Scholar] [CrossRef]
- Carlson, T.R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Aromatic Production from Catalytic Fast Pyrolysis of Biomass-Derived Feedstocks. Top. Catal. 2009, 52, 241–252. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I.; Farmer, T.J. The integration of green chemistry into future biorefineries. Biofuels, Bioprod. Biorefining 2008, 3, 72–90. [Google Scholar] [CrossRef]
- Erdmenger, T.; Guerrero-Sanchez, C.; Vitz, J.; Hoogenboom, R.; Schubert, U.S. Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev. 2010, 39, 3317–3333. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Ji, Y.; Chen, F.; Xiao, F.-S. Mesoporous zeolites for biofuel upgrading and glycerol conversion. Front. Chem. Sci. Eng. 2018, 12, 132–144. [Google Scholar] [CrossRef]
- Egeblad, K.; Christensen, C.H.; Kustova, M.; Christensen, C.H. Templating Mesoporous Zeolites. Chem. Mater. 2008, 20, 946–960. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, H.; Yuan, P.; Li, T.; Yu, C.; Bi, H.; Bao, X. From natural aluminosilicate minerals to hierarchical ZSM-5 zeolites: A nanoscale depolymerization–reorganization approach. J. Catal. 2014, 319, 200–210. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Desilication: On the controlled generation of mesoporosity in MFI zeolites. J. Mater. Chem. 2006, 16, 2121–2131. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-Modified Zeolites: Preparation, Characterization, and Applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Madsen, C.; Jacobsen, C.J.H. Nanosized zeolite crystals—Convenient control of crystal size distribution by confined space synthesis. Chem. Commun. 1999, 8, 673–674. [Google Scholar] [CrossRef]
- Schmidt, I.; Madsen, C.; Jacobsen, C.J.H. Confined Space Synthesis. A Novel Route to Nanosized Zeolites. Inorg. Chem. 2000, 39, 2279–2283. [Google Scholar] [CrossRef]
- Jacobsen, C.J.; Madsen, C.; Janssens, T.V.; Jakobsen, H.J.; Skibsted, J. Zeolites by confined space synthesis—Characterization of the acid sites in nanosized ZSM-5 by ammonia desorption and 27Al/29Si-MAS NMR spectroscopy. Microporous Mesoporous Mater. 2000, 39, 393–401. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. Uniform Mesopore-Donated Zeolite Y Using Carbon Aerogel Templating. J. Phys. Chem. B 2003, 107, 10974–10976. [Google Scholar] [CrossRef]
- Kim, S.-S.; Shah, J.; Pinnavaia, T.J. Colloid-Imprinted Carbons as Templates for the Nanocasting Synthesis of Mesoporous ZSM-5 Zeolite. Chem. Mater. 2003, 15, 1664–1668. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. ZSM-5 Monolith of Uniform Mesoporous Channels. J. Am. Chem. Soc. 2003, 125, 6044–6045. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Hanzawa, Y.; Kaneko, K. Template synthesis and characterization of mesoporous zeolites. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 75–80. [Google Scholar] [CrossRef]
- Xiao, F.S.; Wang, L.; Yin, C.; Lin, K.; Di, Y.; Li, J.; Xu, R.; Su, D.S.; Schlögl, R.; Yokoi, T.; et al. Catalytic properties of hierarchical mesoporous zeolites templated with a mixture of small organic ammonium salts and mesoscale cationic polymers. Angew. Chem.-Int. Ed. 2006, 45, 3090–3093. [Google Scholar] [CrossRef]
- Wang, H.; Pinnavaia, T.J. MFI Zeolite with Small and Uniform Intracrystal Mesopores. Angew. Chem. 2006, 118, 7765–7768. [Google Scholar] [CrossRef]
- Naydenov, V.; Tosheva, L.; Sterte, J. Vanadium modified AlPO-5 spheres through resin macrotemplating. Microporous Mesoporous Mater. 2003, 66, 321–329. [Google Scholar] [CrossRef]
- Valtchev, V.; Smaihi, M.; Faust, A.C.; Vidal, L. Dual templating function of Equisetum arvense in the preparation of zeolite macrostructures. Stud. Surf. Sci. Catal. 2004, 154, 588–592. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Rey, F. Delaminated Zeolites: An Efficient Support for Enzymes. Adv. Mater. 2002, 14, 71–74. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Pergher, S.B.; Maesen, T.L.M.; Buglass, J.G. Delaminated zeolite precursors as selective acidic catalysts. Nature 1998, 396, 353–356. [Google Scholar] [CrossRef]
- Nielsen, M.; Brogaard, R.Y.; Falsig, H.; Beato, P.; Swang, O.; Svelle, S. Kinetics of Zeolite Dealumination: Insights from H-SSZ-13. ACS Catal. 2015, 5, 7131–7139. [Google Scholar] [CrossRef]
- Poliakoff, M.; Licence, P. Green chemistry. Nature 2007, 450, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, M.; Yan, W.; Yu, J. Regulation of the Si/Al ratios and Al distributions of zeolites and their impact on properties. Chem. Sci. 2023, 14, 1935–1959. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Xu, H.; Xue, T.; Jiang, J.-G.; Wu, H.-H.; He, M.; Wu, P. Postsynthesis of high silica beta by cannibalistic dealumination of OSDA-free beta and its catalytic applications. Inorg. Chem. Front. 2021, 8, 1574–1587. [Google Scholar] [CrossRef]
- Pilar, R.; Moravkova, J.; Sadovska, G.; Sklenak, S.; Brabec, L.; Pastvova, J.; Sazama, P. Controlling the competitive growth of zeolite phases without using an organic structure-directing agent. Syn-thesis of Al-rich*BEA. Microporous Mesoporous Mater. 2022, 333, 111726. [Google Scholar] [CrossRef]
- Agostini, G.; Lamberti, C.; Palin, L.; Milanesio, M.; Danilina, N.; Xu, B.; Janousch, M.; van Bokhoven, J.A. In Situ XAS and XRPD Parametric Rietveld Refinement To Understand Dealumination of Y Zeolite Catalyst. J. Am. Chem. Soc. 2010, 132, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Oleksiak, M.D.; Muraoka, K.; Hsieh, M.F.; Conato, M.T.; Shimojima, A.; Okubo, T.; Chaikittisilp, W.; Rimer, J.D. Back Cover: Organic-Free Synthesis of a Highly Siliceous Faujasite Zeolite with Spatially Biased Q4 (nAl) Si Speciation. Angew. Chem. Int. Ed. 2017, 56, 13532. [Google Scholar] [CrossRef]
- Meng, B.; Ren, S.; Li, Z.; Nie, S.; Zhang, X.; Song, W.; Guo, Q.; Shen, B. A facile organic-free synthesis of high silica zeolite Y with small crystal in the presence of Co2+. Microporous Mesoporous Mater. 2021, 323, 111248. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, C.; Song, M.; Lu, T.; Wang, W.; Wang, Z.; Yan, W.; Cheng, P.; Zhao, Z. Accelerated synthesis of Al-rich zeolite beta via different radicalized seeds in the absence of organic templates. Microporous Mesoporous Mater. 2021, 310, 110633. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. J. Earth Planet. Mater. 1954, 39, 92–96. [Google Scholar]
- He, Z.; Wu, J.; Gao, B.; He, H. Hydrothermal Synthesis and Characterization of Aluminum-Free Mn-β Zeolite: A Catalyst for Phenol Hydroxylation. ACS Appl. Mater. Interfaces 2015, 7, 2424–2432. [Google Scholar] [CrossRef]
- Grass, J.-P.; Klühspies, K.; Reiprich, B.; Schwieger, W.; Inayat, A. Layer-Like Zeolite X as Catalyst in a Knoevenagel Condensation: The Effect of Different Preparation Pathways and Cation Exchange. Catalysts 2021, 11, 474. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, C.; Wang, J.; Kong, Y.; Zhu, H.; Wang, C. Synthesis of fructone over dealmuinated USY supported heteropoly acid and its salt catalysts. J. Mol. Catal. A Chem. 2006, 247, 130–137. [Google Scholar] [CrossRef]
- Wang, Q.; Han, W.; Lyu, J.; Zhang, Q.; Guo, L.; Li, X. In situ encapsulation of platinum clusters within H-ZSM-5 zeolite for highly stable benzene methylation catalysis. Catal. Sci. Technol. 2017, 7, 6140–6150. [Google Scholar] [CrossRef]
- Kostrab, G.; Mravec, D.; Bajus, M.; Janotka, I.; Sugi, Y.; Cho, S.; Kim, J. tert-Butylation of toluene over mordenite and cerium-modified mordenite catalysts. Appl. Catal. A Gen. 2006, 299, 122–130. [Google Scholar] [CrossRef]
- Pellet, R.; Casey, D.; Huang, H.; Kessler, R.; Kuhlman, E.; Oyoung, C.; Sawicki, R.; Ugolini, J. Isomerization of n-Butene to Isobutene by Ferrierite and Modified Ferrierite Catalysts. J. Catal. 1995, 157, 423–435. [Google Scholar] [CrossRef]
- da Silva, J.F.; Ferracine, E.D.d.S.; Cardoso, D. Improved accessibility of Na-LTA zeolite catalytic sites for the Knoevenagel condensation reaction. Microporous Mesoporous Mater. 2022, 331, 111640. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Accounts Chem. Res. 2009, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Kouser, S.; Hezam, A.; Khadri, M.J.N.; Khanum, S.A. A review on zeolite imidazole frameworks: Synthesis, properties, and applications. J. Porous Mater. 2022, 29, 663–681. [Google Scholar] [CrossRef]
- Yang, T.; Chung, T.-S. Room-temperature synthesis of ZIF-90 nanocrystals and the derived nano-composite membranes for hydrogen separation. J. Mater. Chem. A 2013, 1, 6081–6090. [Google Scholar] [CrossRef]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Pan, Y.; Heryadi, D.; Zhou, F.; Zhao, L.; Lestari, G.; Su, H.; Lai, Z. Tuning the crystal morphology and size of zeolitic imidazolate framework-8 in aqueous solution by surfactants. CrystEngComm 2011, 13, 6937–6940. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, Q. Surfactants as Promising Media for the Preparation of Crystalline Inorganic Materials. Angew. Chem. Int. Ed. 2015, 54, 11616–11623. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Masel, R.I. Rapid Production of Metal−Organic Frameworks via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef]
- Martins, G.A.V.; Byrne, P.J.; Allan, P.; Teat, S.J.; Slawin, A.M.Z.; Li, Y.; Morris, R.E. The use of ionic liquids in the synthesis of zinc imidazolate frameworks. Dalton Trans. 2010, 39, 1758–1762. [Google Scholar] [CrossRef]
- Yang, L.; Lu, H. Microwave-assisted Ionothermal Synthesis and Characterization of Zeolitic Imidazolate Framework-8. Chin. J. Chem. 2012, 30, 1040–1044. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam-Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. 2011, 50, 672–675. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, M.; Zhou, M.; Du, Y.; Chen, R. Hierarchical Pd@ZIFs as Efficient Catalysts for p-Nitrophenol Reduction. Ind. Eng. Chem. Res. 2021, 60, 15045–15055. [Google Scholar] [CrossRef]
- Luo, Z.; Chaemchuen, S.; Zhou, K.; Verpoort, F. Ring-Opening Polymerization of l-Lactide to Cyclic Poly(Lactide) by Zeolitic Imidazole Framework ZIF-8 Catalyst. ChemSusChem 2017, 10, 4135–4139. [Google Scholar] [CrossRef]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Chizallet, C.; Quoineaud, A.-A.; Pirngruber, G.D. Comparison of the Behavior of Metal–Organic Frameworks and Zeolites for Hydrocarbon Separations. J. Am. Chem. Soc. 2012, 134, 8115–8126. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of Transesterification by a Nonfunctionalized Metal−Organic Framework: Acido-Basicity at the External Surface of ZIF-8 Probed by FTIR and ab Initio Calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef]
- Thomas, A.; Prakash, M. The Role of Binary Mixtures of Ionic Liquids in ZIF-8 for Selective Gas Storage and Separation: A Perspective from Computational Approaches. J. Phys. Chem. C 2020, 124, 26203–26213. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Methane Hydrate Growth Promoted by Microporous Zeolitic Imidazolate Frameworks ZIF-8 and ZIF-67 for Enhanced Methane Storage. ACS Sustain. Chem. Eng. 2021, 9, 9001–9010. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, T.; Chen, Y.; Wang, Q. The Ni/Ni3S2 nanocomposite derived from Ni-ZIF with superior energy storage performance as cathodes for asymmetric supercapacitor and rechargeable aqueous zinc ion battery. J. Alloys Compd. 2022, 891, 161935. [Google Scholar] [CrossRef]
- Dou, J.; Bian, W.; Zheng, X.; Yue, Q.; Song, Q.; Deng, S.; Wang, L.; Tan, W.; Li, W.; Zhou, B. A ZIF-based drug delivery system as three-in-one platform for joint cancer therapy. Mater. Chem. Phys. 2023, 297, 127345. [Google Scholar] [CrossRef]
- Shi, L.; Wu, J.; Qiao, X.; Ha, Y.; Li, Y.; Peng, C.; Wu, R. In Situ Biomimetic Mineralization on ZIF-8 for Smart Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 4595–4603. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Aram, E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts 2023, 13, 2. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, S.; Yang, C.; Zhang, J.Y.; Su, Y.; Zheng, G.; Fang, X. Controllable States and Porosity of Cu-Carbon for CO2 Electroreduction to Hydrocarbons. Small 2022, 18, 2238. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019, 31, e1804257. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Mui, E.L.; Ko, D.C.; McKay, G. Production of active carbons from waste tyres—A review. Carbon 2004, 42, 2789–2805. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Bergna, D.; Hu, T.; Prokkola, H.; Romar, H.; Lassi, U. Effect of Some Process Parameters on the Main Properties of Activated Carbon Produced from Peat in a Lab-Scale Process. Waste Biomass-Valorization 2020, 11, 2837–2848. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Xu, B.; Chen, Y.; Wei, G.; Cao, G.; Zhang, H.; Yang, Y. Activated carbon with high capacitance prepared by NaOH activation for supercapacitors. Mater. Chem. Phys. 2010, 124, 504–509. [Google Scholar] [CrossRef]
- Guo, Y.; Rockstraw, D.A. Physical and chemical properties of carbons synthesized from xylan, cellulose, and Kraft lignin by H3PO4 activation. Carbon 2006, 44, 1464–1475. [Google Scholar] [CrossRef]
- Xing, Z.; Qi, Y.; Tian, Z.; Xu, J.; Yuan, Y.; Bommier, C.; Lu, J.; Tong, W.; Jiang, D.-E.; Ji, X. Identify the Removable Substructure in Carbon Activation. Chem. Mater. 2017, 29, 7288–7295. [Google Scholar] [CrossRef]
- Smith, M.R.; Bittner, E.W.; Shi, W.; Johnson, J.K.; Bockrath, B.C. Chemical Activation of Single-Walled Carbon Nanotubes for Hydrogen Adsorption. J. Phys. Chem. B 2003, 107, 3752–3760. [Google Scholar] [CrossRef]
- Kyotani, T. Control of pore structure in carbon. Carbon 2000, 38, 269–286. [Google Scholar] [CrossRef]
- Tanaka, S.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Synthesis of ordered mesoporous carbons with channel structure from an organic–organic nanocomposite. Chem. Commun. 2005, 16, 2125–2127. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Katayama, Y.; Tate, M.P.; Hillhouse, H.W.; Miyake, Y. Fabrication of continuous mesoporous carbon films with face-centered orthorhombic symmetry through a soft templating pathway. J. Mater. Chem. 2007, 17, 3639–3645. [Google Scholar] [CrossRef]
- Javed, H.; Pani, S.; Antony, J.; Sakthivel, M.; Drillet, J.-F. Synthesis of mesoporous carbon spheres via a soft-template route for catalyst supports in PEMFC cathodes. Soft Matter 2021, 17, 7743–7754. [Google Scholar] [CrossRef] [PubMed]
- Bonneviot, L.; Béland, F.; Danumah, C.; Giasson, S.; Kaliaguine, S. Mesoporous Molecular Sieves; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Corma, A.; Iborra, S.; Miquel, S.; Primo, J. Catalysts for the Production of Fine Chemicals: Production of Food Emulsifiers, Monoglycerides, by Glycerolysis of Fats with Solid Base Catalysts. J. Catal. 1998, 173, 315–321. [Google Scholar] [CrossRef]
- Jaroniec, C.P.; Kruk, M.; Jaroniec, M.; Sayari, A. Tailoring Surface and Structural Properties of MCM-41 Silicas by Bonding Organosilanes. J. Phys. Chem. B 1998, 102, 5503–5510. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, L.; Lu, A. Highly Selective Copper Catalyst Supported on Mesoporous Carbon for the Dehydrogenation of Ethanol to Acetaldehyde. ChemCatChem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Liu, X.; Lan, G.; Su, P.; Qian, L.; Reina, T.R.; Wang, L.; Li, Y.; Liu, J. Highly stable Ru nanoparticles incorporated in mesoporous carbon catalysts for production of γ-valerolactone. Catal. Today 2020, 351, 75–82. [Google Scholar] [CrossRef]
- Budd, P.M.; Makhseed, S.M.; Ghanem, B.S.; Msayib, K.J.; Tattershall, C.; McKeown, N.B. Microporous polymeric materials. Mater. Today 2004, 7, 40–46. [Google Scholar] [CrossRef]
- Attfield, M.P. Microporous Materials. Sci. Prog. 2002, 85, 319–345. [Google Scholar] [CrossRef]
- CejkaM, J. Introduction to Zeolite Science and Practice, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Kumar, N. Microporous Zeolites and Related Nanoporous Materials: Synthesis, Characterization and Application in Catalysis. Catalysts 2021, 11, 382. [Google Scholar] [CrossRef]
- Kamaluddin, H.S.; Basahel, S.N.; Narasimharao, K.; Mokhtar, M. H-ZSM-5 Materials Embedded in an Amorphous Silica Matrix: Highly Selective Catalysts for Propylene in Methanol-to-Olefin Process. Catalysts 2019, 9, 364. [Google Scholar] [CrossRef]
- Kumar, N.; Mäki-Arvela, P.; Diáz, S.F.; Aho, A.; Demidova, Y.; Linden, J.; Shepidchenko, A.; Tenhu, M.; Salonen, J.; Laukkanen, P.; et al. Isomerization of α-Pinene Oxide Over Iron-Modified Zeolites. Top. Catal. 2013, 56, 696–713. [Google Scholar] [CrossRef]
- Vajglová, Z.; Kumar, N.; Peurla, M.; Hupa, L.; Semikin, K.; Sladkovskiy, D.A.; Murzin, D.Y. Effect of the Preparation of Pt-Modified Zeolite Beta-Bentonite Extrudates on Their Catalytic Behavior in n-Hexane Hydroisomerization. Ind. Eng. Chem. Res. 2019, 58, 10875–10885. [Google Scholar] [CrossRef]
- Vajglová, Z.; Gauli, B.; Mäki-Arvela, P.; Kumar, N.; Eränen, K.; Wärnå, J.; Lassfolk, R.; Simakova, I.L.; Prosvirin, I.P.; Peurla, M.; et al. Interactions between Iron and Nickel in Fe–Ni Nanoparticles on Y Zeolite for Co-Processing of Fossil Feedstock with Lignin-Derived Isoeugenol. ACS Appl. Nano Mater. 2023, 6, 10064–10077. [Google Scholar] [CrossRef]
- Vajglová, Z.; Kumar, N.; Mäki-Arvela, P.; Eränen, K.; Peurla, M.; Hupa, L.; Nurmi, M.; Toivakka, M.; Murzin, D.Y. Synthesis and Physicochemical Characterization of Shaped Catalysts of β and Y Zeolites for Cyclization of Citronellal. Ind. Eng. Chem. Res. 2019, 58, 18084–18096. [Google Scholar] [CrossRef]
- Vajglová, Z.; Kumar, N.; Mäki-Arvela, P.; Eränen, K.; Peurla, M.; Hupa, L.; Murzin, D.Y. Effect of Binders on the Physicochemical and Catalytic Properties of Extrudate-Shaped Beta Zeolite Catalysts for Cyclization of Citronellal. Org. Process. Res. Dev. 2019, 23, 2456–2463. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eränen, K.; Mäki-Arvela, P.; Salmi, T.; Peurla, M.; Angervo, I.; Hietala, J.; Murzin, D.Y. Catalytic conversion of glucose to methyl levulinate over metal-modified Beta zeolites. React. Kinet. Catal. Lett. 2022, 135, 1971–1986. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eränen, K.; Lassfolk, R.; Mäki-Arvela, P.; Salmi, T.; Peurla, M.; Angervo, I.; Hietala, J.; Murzin, D.Y. Improving the methyl lactate yield from glucose over Sn–Al-Beta zeolite by catalyst promoters. Microporous Mesoporous Mater. 2023, 351, 112483. [Google Scholar] [CrossRef]
- Zaykovskaya, A.O.; Kumar, N.; Kholkina, E.A.; Li-Zhulanov, N.S.; Mäki-Arvela, P.; Aho, A.; Peltonen, J.; Peurla, M.; Heinmaa, I.; Kusema, B.T.; et al. Synthesis and physico-chemical characterization of Beta zeolite catalysts: Evaluation of catalytic properties in Prins cyclization of (−)-isopulegol. Microporous Mesoporous Mater. 2020, 302, 110236. [Google Scholar] [CrossRef]
- Stekrova, M.; Kumar, N.; Díaz, S.; Mäki-Arvela, P.; Murzin, D.Y. H- and Fe-modified zeolite beta catalysts for preparation of trans-carveol from α-pinene oxide. Catal. Today 2015, 241, 237–245. [Google Scholar] [CrossRef]
- Ma, H.; Chen, J.-J.; Tan, L.; Bu, J.-H.; Zhu, Y.; Tan, B.; Zhang, C. Nitrogen-Rich Triptycene-Based Porous Polymer for Gas Storage and Iodine Enrichment. ACS Macro Lett. 2016, 5, 1039–1043. [Google Scholar] [CrossRef]
- Hu, X.; Radosz, M.; Cychosz, K.A.; Thommes, M. CO2-Filling Capacity and Selectivity of Carbon Nanopores: Synthesis, Texture, and Pore-Size Distribution from Quenched-Solid Density Functional Theory (QSDFT). Environ. Sci. Technol. 2011, 45, 7068–7074. [Google Scholar] [CrossRef]
- Yahya, M.A.; Mansor, M.H.; Zolkarnaini, W.A.A.W.; Rusli, N.S.; Aminuddin, A.; Mohamad, K.; Sabhan, F.A.M.; Atik, A.A.A.; Ozair, L.N. A brief review on activated carbon derived from agriculture by-product. In Proceedings of the International Conference on Recent Advancements in Science and Technology 2017 (ICoRAST2017), Melaka, Malaysia, 7–8 November 2017; p. 030023. [Google Scholar]
- Adeleye, A.T.; Akande, A.A.; Odoh, C.K.; Philip, M.; Fidelis, T.T.; Amos, P.I.; Banjoko, O.O. Efficient synthesis of bio-based activated carbon (AC) for catalytic systems: A green and sustainable approach. J. Ind. Eng. Chem. 2021, 96, 59–75. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Gheni, S.A.; Hamad, K.I.; Mohammed, A.E.; Hmood, H.M.; Mahomood, M.A.; Mohammed, H.R.; Abdulwahab, Z.T.; Ahmed, S.M.; Hassan, A.A. Microporous activated carbon catalyst for an efficient and deactivation resistive supercritical water upgrading process of sour crude oil. Diam. Relat. Mater. 2023, 135, 109887. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef]
- Al-Qayim, K.; Nimmo, W.; Hughes, K.; Pourkashanian, M. Kinetic parameters of the intrinsic reactivity of woody biomass and coal chars via thermogravimetric analysis. Fuel 2017, 210, 811–825. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Structural characterization of carbon nanofibers formed from different carbon-containing gases. Carbon 2006, 44, 3255–3262. [Google Scholar] [CrossRef]
- Kvande, I.; Zhu, J.; Zhao, T.-J.; Hammer, N.; Rønning, M.; Raaen, S.; Walmsley, J.C.; Chen, D. Importance of Oxygen-Free Edge and Defect Sites for the Immobilization of Colloidal Pt Oxide Particles with Implications for the Preparation of CNF-Supported Catalysts. J. Phys. Chem. C 2010, 114, 1752–1762. [Google Scholar] [CrossRef]
- Kvande, I.; Chen, D.; Zhao, T.-J.; Skoe, I.M.; Walmsley, J.C.; Rønning, M. Hydrogen Oxidation Catalyzed by Pt Supported on Carbon Nanofibers with Different Graphite Sheet Orientations. Top. Catal. 2009, 52, 664–674. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Wang, M.-R.; Lai, Y.-Q.; Li, X.-Y. Nitrogen-doped microporous carbon: An efficient oxygen reduction catalyst for Zn-air batteries. J. Power Sources 2017, 359, 71–79. [Google Scholar] [CrossRef]
- Abushawish, A.; Almanassra, I.W.; Backer, S.N.; Jaber, L.; Khalil, A.K.; Abdelkareem, M.A.; Sayed, E.T.; Alawadhi, H.; Shanableh, A.; Atieh, M.A. High-efficiency removal of hexavalent chromium from contaminated water using nitrogen-doped activated carbon: Kinetics and isotherm study. Mater. Chem. Phys. 2022, 291, 126758. [Google Scholar] [CrossRef]
- Quan, C.; Jia, X.; Gao, N. Nitrogen-doping activated biomass carbon from tea seed shell for CO2 capture and supercapacitor. Int. J. Energy Res. 2020, 44, 1218–1232. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Ding, C.; Meng, Y.; Wang, J.; Zhang, K.; Liu, P. Nickel–copper catalysts supported by boron and nitrogen co-doped activated carbon for gas phase carbonylation of ethanol. J. Porous Mater. 2023, 30, 1575–1585. [Google Scholar] [CrossRef]
- Pan, S.-F.; Yin, J.-L.; Zhu, X.-L.; Guo, X.-J.; Hu, P.; Yan, X.; Lang, W.-Z.; Guo, Y.-J. P-modified microporous carbon nanospheres for direct propane dehydrogenation reactions. Carbon 2019, 152, 855–864. [Google Scholar] [CrossRef]
- Jiao, C.; Xu, J.L.; Chen, X.Y.; Zhang, Z.J. Design and synthesis of phosphomolybdic acid/silver dual-modified microporous carbon composite for high performance supercapacitors. J. Alloys Compd. 2019, 791, 1005–1014. [Google Scholar] [CrossRef]
- Martínez-Klimov, M.E.; Mäki-Arvela, P.; Vajglova, Z.; Alda-Onggar, M.; Angervo, I.; Kumar, N.; Eränen, K.; Peurla, M.; Calimli, M.H.; Muller, J.; et al. Hydrodeoxygenation of Isoeugenol over Carbon-Supported Pt and Pt–Re Catalysts for Production of Renewable Jet Fuel. Energy Fuels 2021, 35, 17755–17768. [Google Scholar] [CrossRef]
- Wang, D.; Huang, B.; Shi, Z.; Long, H.; Li, L.; Yang, Z.; Dai, M. Influence of cerium doping on Cu–Ni/activated carbon low-temperature CO-SCR denitration catalysts. RSC Adv. 2021, 11, 18458–18467. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Mustafa-Alsultan, G.; Lee, H.V.; Wilson, K.; Taufiq-Yap, Y.H. Efficient deoxygenation of waste cooking oil over Co3O4–La2O3-doped activated carbon for the production of diesel-like fuel. RSC Adv. 2020, 10, 4996–5009. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Martin, G.; Simakova, I.; Tokarev, A.; Wärnå, J.; Hemming, J.; Holmbom, B.; Salmi, T.; Murzin, D. Kinetics, catalyst deactivation and modeling in the hydrogenation of β-sitosterol to β-sitostanol over microporous and mesoporous carbon supported Pd catalysts. Chem. Eng. J. 2009, 154, 45–51. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Pastore, H.; Coluccia, S.; Marchese, L. Porous aluminophosphates: From Molecular Sieves to Designed Acid Catalysts. Annu. Rev. Mater. Res. 2005, 35, 351–395. [Google Scholar] [CrossRef]
- Corà, F.; Gómez-Hortigüela, L.; Catlow, C.R.A. Aerobic oxidation of hydrocarbons in Mn-doped aluminophosphates: A computational perspective to understand mechanism and selectivity. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2053–2069. [Google Scholar] [CrossRef]
- Gómez-Hortigüela, L.; Corà, F.; Catlow, C.R.A. Complementary mechanistic properties of Fe- and Mn-doped aluminophosphates in the catalytic aerobic oxidation of hydrocarbons. Phys. Chem. Chem. Phys. 2013, 15, 6870–6874. [Google Scholar] [CrossRef] [PubMed]
- Dawaymeh, F.; Elmutasim, O.; Gaber, D.; Gaber, S.; Reddy, K.S.K.; Basina, G.; Polychronopoulou, K.; Al Wahedi, Y.; Karanikolos, G.N. Metal substitution effects of aluminophosphate AlPO4-5 as solid acid catalyst for esterification of acetic acid with ethanol. Mol. Catal. 2021, 501, 111371. [Google Scholar] [CrossRef]
- Nagaraju, N.; Kuriakose, G. A new catalyst for the synthesis of N,N-biphenylurea from aniline and dimethyl carbonate. Green Chem. 2002, 4, 269–271. [Google Scholar] [CrossRef]
- Vijayasankar, A.V.; Mahadevaiah, N.; Bhat, Y.S.; Nagaraju, N. Mesoporous aluminophosphate materials: Influence of method of preparation and iron loading on textural properties and catalytic activity. J. Porous Mater. 2011, 18, 369–378. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ebrahimnia, M.; Shahbazi, M.-A.; Keçili, R.; Ghorbani-Bidkorbeh, F. Microporous metal–organic frameworks: Synthesis and applications. J. Ind. Eng. Chem. 2022, 115, 1–11. [Google Scholar] [CrossRef]
- Pal, A.; Chand, S.; Madden, D.G.; Franz, D.; Ritter, L.; Johnson, A.; Space, B.; Curtin, T.; Das, M.C. A Microporous Co-MOF for Highly Selective CO2 Sorption in High Loadings Involving Aryl C–H···O═C═O Interactions: Combined Simulation and Breakthrough Studies. Inorg. Chem. 2019, 58, 11553–11560. [Google Scholar] [CrossRef]
- Pal, A.; Chand, S.; Elahi, S.M.; Das, M.C. A microporous MOF with a polar pore surface exhibiting excellent selective adsorption of CO2from CO2–N2and CO2–CH4gas mixtures with high CO2loading. Dalton Trans. 2017, 46, 15280–15286. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A. Synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction using organocatalysts. Tetrahedron 2007, 63, 1946–1952. [Google Scholar] [CrossRef]
- Ramish, S.M.; Ghorbani-Choghamarani, A.; Mohammadi, M. Microporous hierarchically Zn-MOF as an efficient catalyst for the Hantzsch synthesis of polyhydroquinolines. Sci. Rep. 2022, 12, 1479. [Google Scholar] [CrossRef]
- Roy, D.; Kumar, P.; Soni, A.; Nemiwal, M. A versatile and microporous Zn-based MOFs as a recyclable and sustainable heterogeneous catalyst for various organic transformations: A review (2015-present). Tetrahedron 2023, 138, 133408. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, B.; Cheng, Y. Microporous exposure on catalytic performance of MoVNbTeOx mixed metal oxides in the oxidative dehydrogenation of ethane. J. Catal. 2023, 426, 308–318. [Google Scholar] [CrossRef]
- Ivan, B.; Popescu, I.; Fechete, I.; Garin, F.; Pârvulescu, V.I.; Marcu, I.-C. The effect of phosphorus on the catalytic performance of nickel oxide in ethane oxidative dehydrogenation. Catal. Sci. Technol. 2016, 6, 6953–6964. [Google Scholar] [CrossRef]
- Kung, H.H. Transition Metal Oxides: Surface Chemistry and Catalysis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 45. [Google Scholar] [CrossRef]
- Corà, F.; Catlow, C.; Lewis, D. Design of microporous transition metal oxide catalysts and investigation of their synthesis conditions. J. Mol. Catal. A Chem. 2001, 166, 123–134. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Xiao, F.-S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef]
- Widersten, M.; Gurell, A.; Lindberg, D. Structure–function relationships of epoxide hydrolases and their potential use in biocatalysis. Biochim. Biophys. Acta 2010, 1800, 316–326. [Google Scholar] [CrossRef]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R.A. A Continuous Process for the Liquid Phase Epoxidation of an Olefinic; London South Bank University: London, UK, 2018. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Liquid Phase Epoxidation Process. European Patent Number EP2459545, 28 February 2019. [Google Scholar]
- Ambroziak, K.; Mbeleck, R.; He, Y.; Saha, B.; Sherrington, D.C. Investigation of Batch Alkene Epoxidations Catalyzed by Polymer-Supported Mo(VI) Complexes. Ind. Eng. Chem. Res. 2009, 48, 3293–3302. [Google Scholar] [CrossRef]
- Bakala, P.C.; Briot, E.; Salles, L.; Brégeault, J.-M. Comparison of liquid-phase olefin epoxidation over MoOx inserted within mesoporous silica (MCM-41, SBA-15) and grafted onto silica. Appl. Catal. A Gen. 2006, 300, 91–99. [Google Scholar] [CrossRef]
- Bruno, S.M.; Fernandes, J.A.; Martins, L.S.; Gonçalves, I.S.; Pillinger, M.; Ribeiro-Claro, P.; Rocha, J.; Valente, A.A. Dioxomolybdenum(VI) modified mesoporous materials for the catalytic epoxidation of olefins. Catal. Today 2006, 114, 263–271. [Google Scholar] [CrossRef]
- Sakthivel, A.; Zhao, J.; Kühn, F.E. Cyclopentadienyl molybdenum complexes grafted on zeolites—Synthesis and catalytic application. Catal. Lett. 2005, 102, 115–119. [Google Scholar] [CrossRef]
- Bhuiyan, M.R.; Mohammed, M.L.; Saha, B. Greener and Efficient Epoxidation of 1,5-Hexadiene with tert-Butyl Hydroperoxide (TBHP) as an Oxidising Reagent in the Presence of Polybenzimidazole Supported Mo(VI) Catalyst. Reactions 2022, 3, 537–552. [Google Scholar] [CrossRef]
- Mbeleck, R.; Mohammed, M.L.; Ambroziak, K.; Sherrington, D.C.; Saha, B. Efficient epoxidation of cyclododecene and dodecene catalysed by polybenzimidazole supported Mo(VI) complex. Catal. Today 2015, 256, 287–293. [Google Scholar] [CrossRef]
- Saha, B. Catalytic Reactors; De Gruyter: Berlin, Germany, 2016; pp. 1–338. ISBN 978-3-11-033296-4. [Google Scholar]
- Mbeleck, R.; Ambroziak, K.; Saha, B.; Sherrington, D.C. Stability and recycling of polymer-supported Mo(VI) alkene epoxidation catalysts. React. Funct. Polym. 2007, 67, 1448–1457. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Saha, B. Efficient and selective molybdenum based heterogeneous catalyst for alkene epoxidation using batch and continuous reactors. Polym. Chem. 2015, 6, 7308–7319. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Patel, D.; Niyogi, D.; Sherrington, D.C.; Saha, B. Greener and efficient epoxidation of 4-vinyl-1-cyclohexene with polystyrene 2-(aminomethyl)pyridine supported Mo(VI) catalyst in batch and continuous reactors. Chem. Eng. Res. Des. 2015, 94, 194–203. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Patel, D.; Mbeleck, R.; Niyogi, D.; Sherrington, D.C.; Saha, B. Optimisation of alkene epoxidation catalysed by polymer supported Mo(VI) complexes and application of artificial neural network for the prediction of catalytic performances. Appl. Catal. A Gen. 2013, 466, 142–152. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Saha, B. Recent Advances in Greener and Energy Efficient Alkene Epoxidation Processes. Energies 2022, 15, 2858. [Google Scholar] [CrossRef]
- Li, B.; Xiong, H.; Xiao, Y. Progress on Synthesis and Applications of Porous Carbon Materials. Int. J. Electrochem. Sci. 2020, 15, 1363–1377. [Google Scholar] [CrossRef]
- Annath, H.; Manayil, J.C.; Thompson, J.; Marr, A.C.; Raja, R. Contrasting structure-property relationships in amorphous, hierarchical and microporous aluminophosphate catalysts for Claisen-Schmidt condensation reactions. Appl. Catal. A Gen. 2021, 627, 118376. [Google Scholar] [CrossRef]
- Zallen, R. The Physics of Amorphous Solids; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Goldsmith, B.R.; Peters, B.; Johnson, J.K.; Gates, B.C.; Scott, S.L. Beyond Ordered Materials: Understanding Catalytic Sites on Amorphous Solids. ACS Catal. 2017, 7, 7543–7557. [Google Scholar] [CrossRef]
- Sá, J.; Medlin, J.W. On-the-fly Catalyst Modification: Strategy to Improve Catalytic Processes Selectivity and Understanding. ChemCatChem 2019, 11, 3355–3365. [Google Scholar] [CrossRef]
- Hartmann, M.; Bischof, C.; Luan, Z.; Kevan, L. Preparation and characterization of ruthenium clusters on mesoporous supports. Microporous Mesoporous Mater. 2001, 44–45, 385–394. [Google Scholar] [CrossRef]
- Mercadante, L.; Neri, G.; Milone, C.; Donato, A.; Galvagno, S. Hydrogenation of α,β-unsaturated aldehydes over Ru/Al2O3 catalysts. J. Mol. Catal. A Chem. 1996, 105, 93–101. [Google Scholar] [CrossRef]
- Consonni, M.; Jokic, D.; Murzin, D.Y.; Touroude, R. High Performances of Pt/ZnO Catalysts in Selective Hydrogenation of Crotonaldehyde. J. Catal. 1999, 188, 165–175. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Murzin, D.Y. Hydrodeoxygenation of Lignin-Derived Phenols: From Fundamental Studies towards Industrial Applications. Catalysts 2017, 7, 265. [Google Scholar] [CrossRef]
- Lasne, B.; Mäki-Arvela, P.; Aho, A.; Vajglova, Z.; Eränen, K.; Kumar, N.; Sánchez-Velandia, J.E.; Peurla, M.; Mondelli, C.; Pérez-Ramírez, J.; et al. Synthesis of Florol via Prins cyclization over heterogeneous catalysts. J. Catal. 2022, 405, 288–302. [Google Scholar] [CrossRef]
- Saeid, S.; Kråkström, M.; Tolvanen, P.; Kumar, N.; Eränen, K.; Peurla, M.; Mikkola, J.-P.; Maël, L.; Kronberg, L.; Eklund, P.; et al. Synthesis and Characterization of Metal Modified Catalysts for Decomposition of Ibuprofen from Aqueous Solutions. Catalysts 2020, 10, 786. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol−Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Ramadhas, A.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Dey, B.; Balasubramanian, P. Algal biodiesel production with engineered biochar as a heterogeneous solid acid catalyst. Bioresour. Technol. 2020, 310, 123392. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking OilAn Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Guidotti, M. Grafted non-ordered niobium-silica materials: Versatile catalysts for the selective epoxidation of various unsaturated fine chemicals. Catal. Today 2014, 235, 49–57. [Google Scholar] [CrossRef]
- Vilé, G.; Liu, J.; Zhang, Z. Surface engineering of a Cu-based heterogeneous catalyst for efficient azide–alkyne click cycloaddition. React. Chem. Eng. 2021, 6, 1878–1883. [Google Scholar] [CrossRef]
- Leino, E.; Kumar, N.; Mäki-Arvela, P.; Rautio, A.-R.; Dahl, J.; Roine, J.; Mikkola, J.P. Synthesis and characterization of ceria-supported catalysts for carbon dioxide transformation to diethyl carbonate. Catal. Today 2018, 306, 128–137. [Google Scholar] [CrossRef]
- Yamada, Y.; Akita, T.; Ueda, A.; Shioyama, H.; Kobayashi, T. Instruments for preparation of heterogeneous catalysts by an impregnation method. Rev. Sci. Instrum. 2005, 76, 062226. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of Porous Carbon Materials with Designed Pore Architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Wang, J.; Han, W. A Review of Heteroatom Doped Materials for Advanced Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).