Ni-Ag Catalysts for Hydrogen Production through Dry Reforming of Methane: Characterization and Performance Evaluation
Abstract
1. Introduction
2. Results
2.1. Characterization of Fresh Catalysts
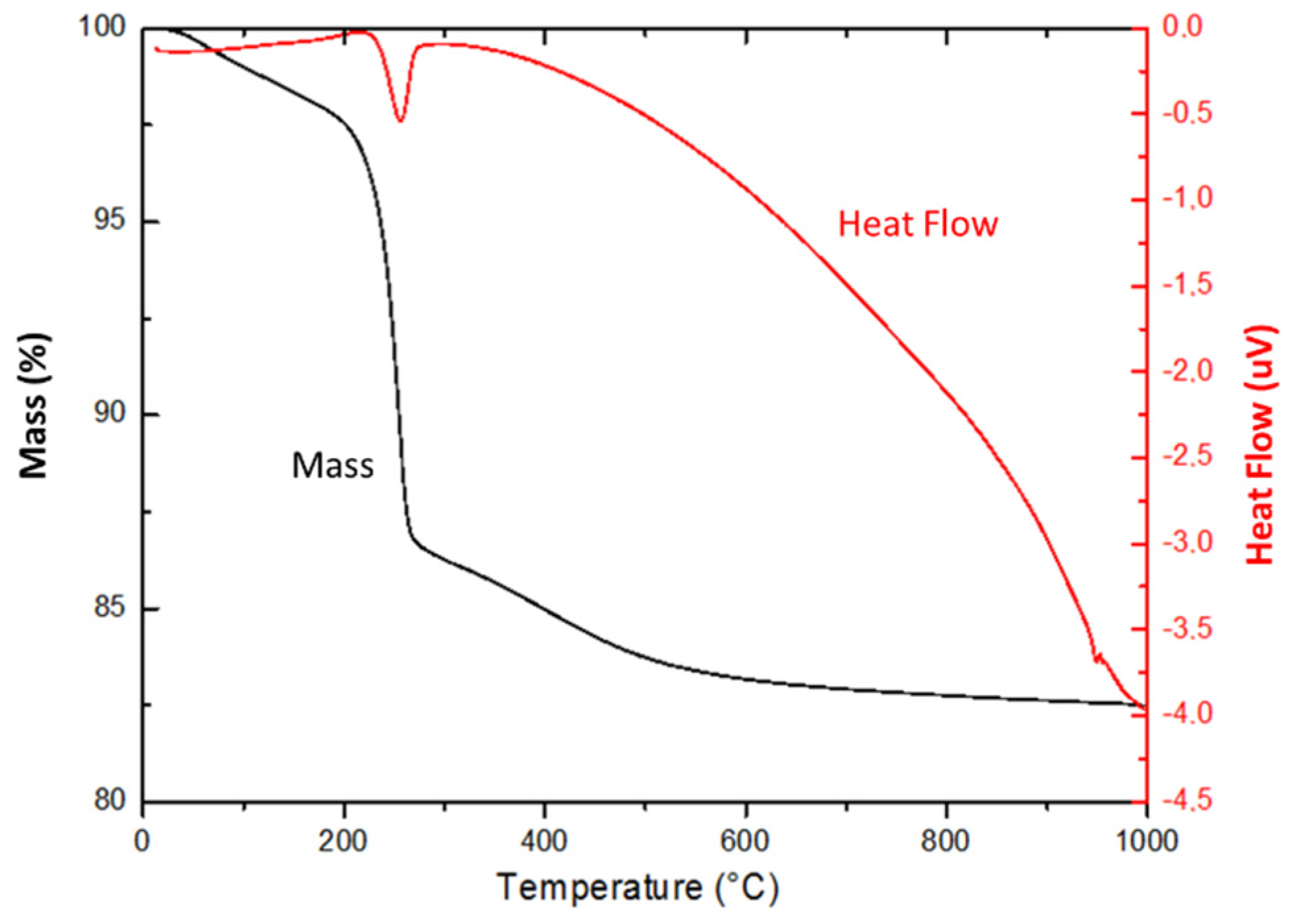
2.1.1. Thermal Study (TG-DSC-MS and XRD) of Precursors
2.1.2. Study of Stoichiometry of Fresh Catalysts (ICP-OES and EDX)
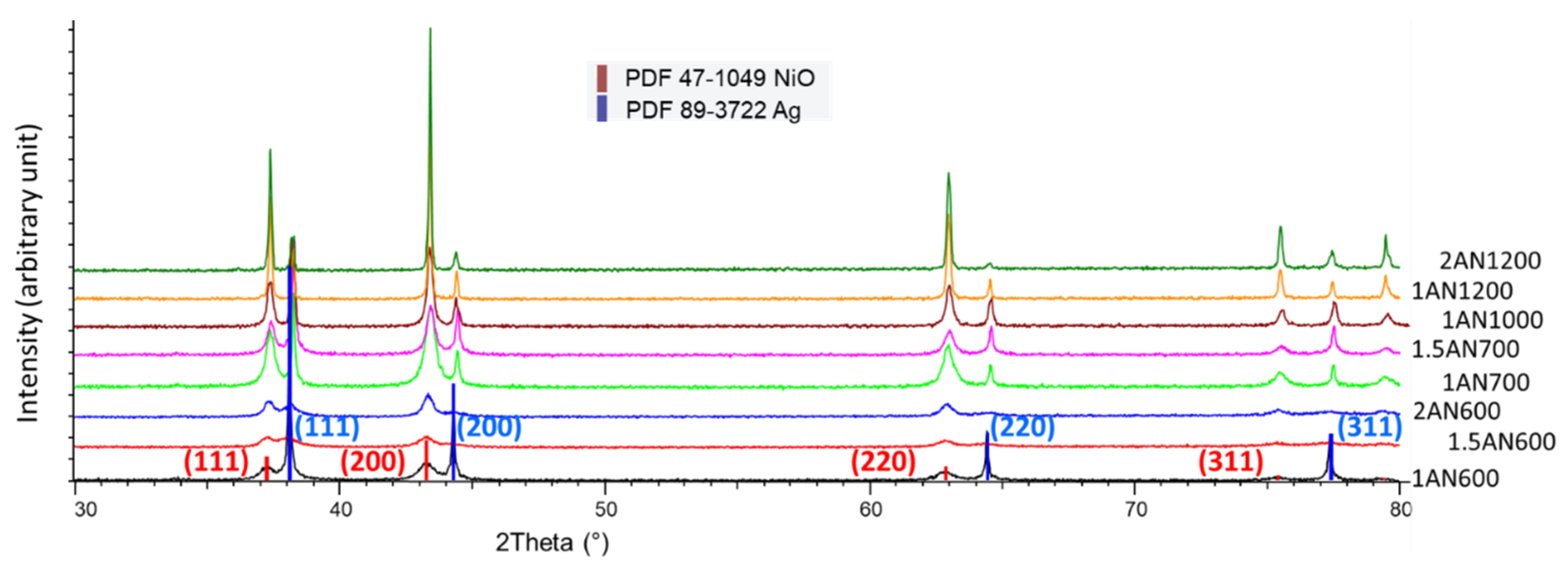
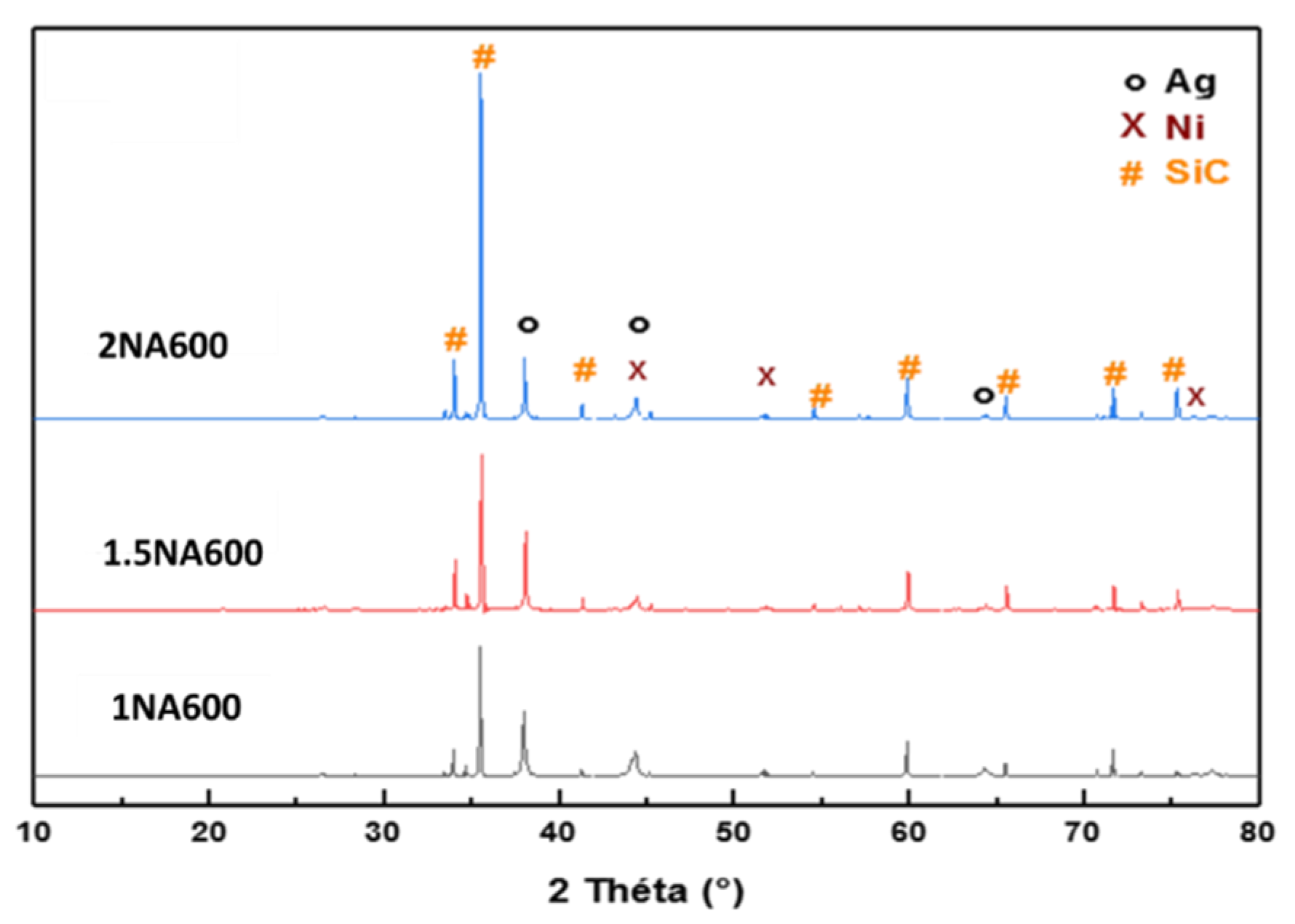
2.1.3. Structural Characterization of Fresh Catalysts (XRD)
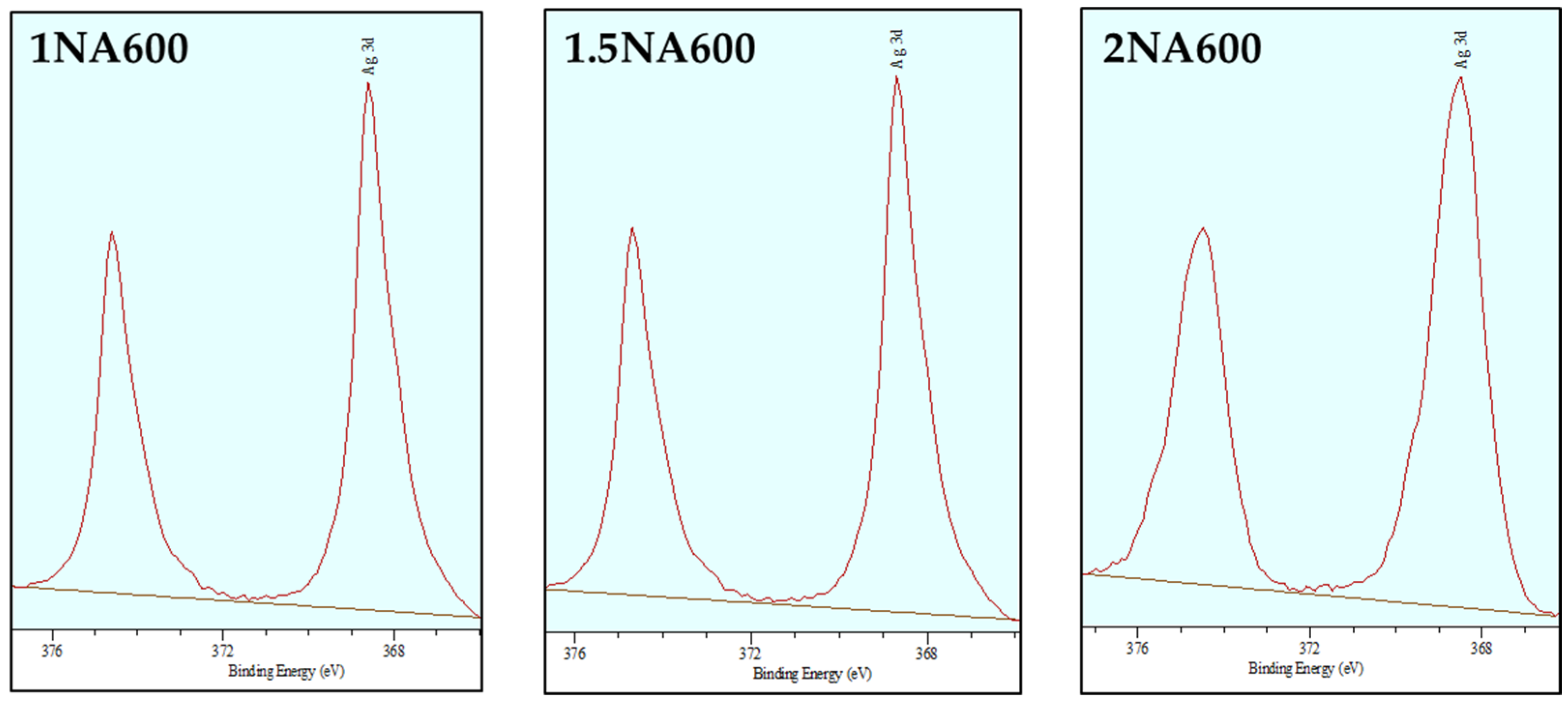
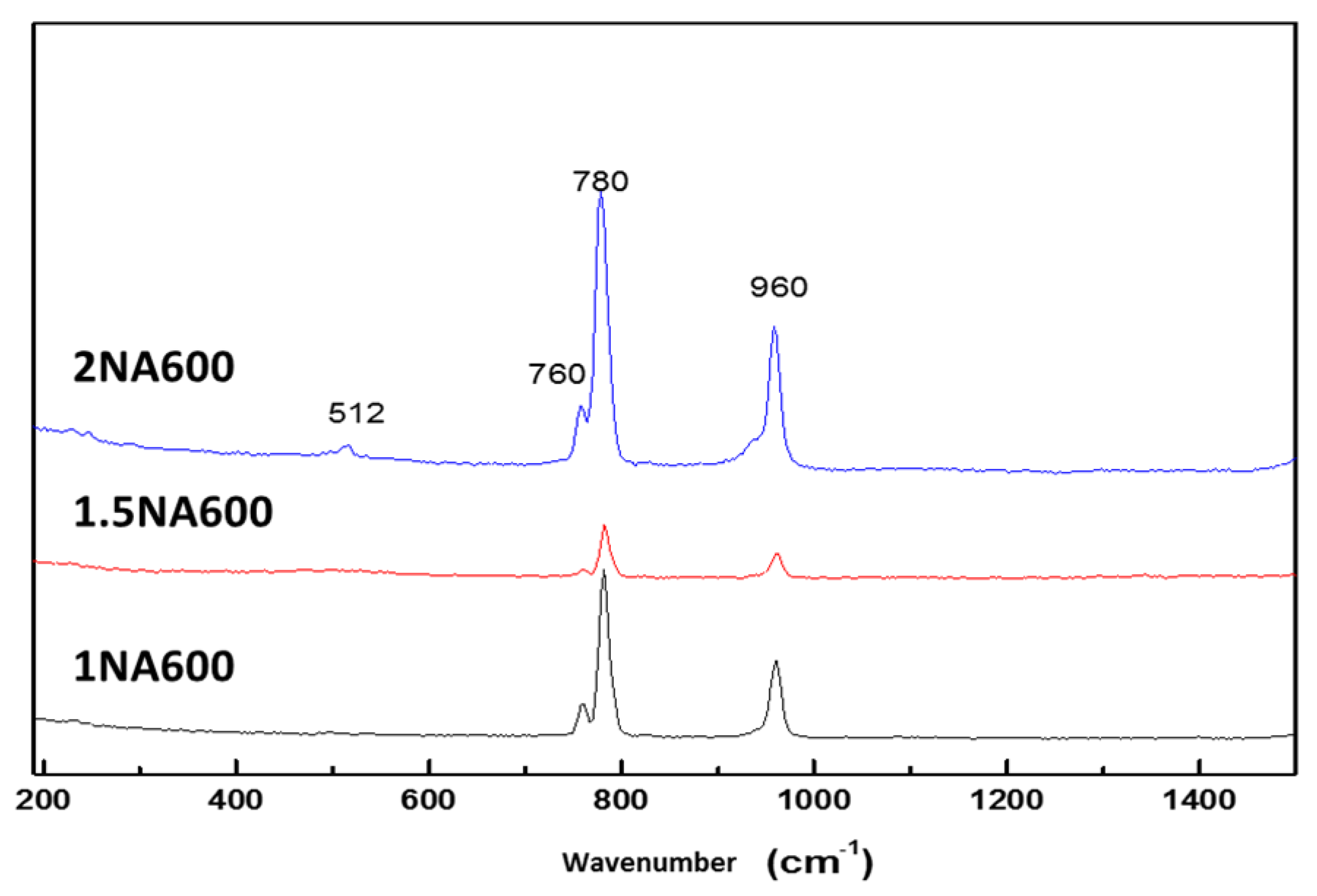
2.1.4. Textural Characterization of Fresh Catalysts (BET, SEM and XPS)
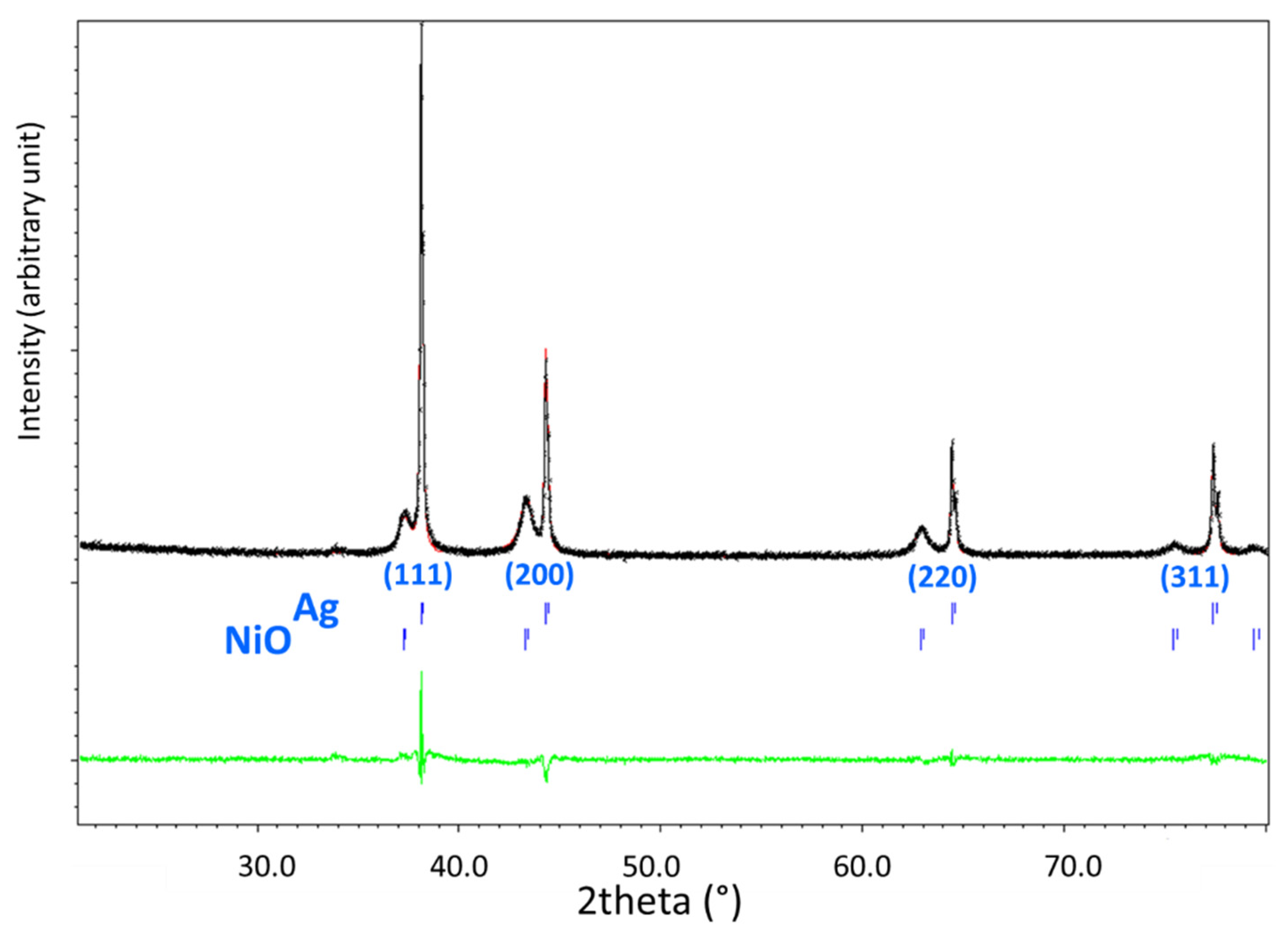
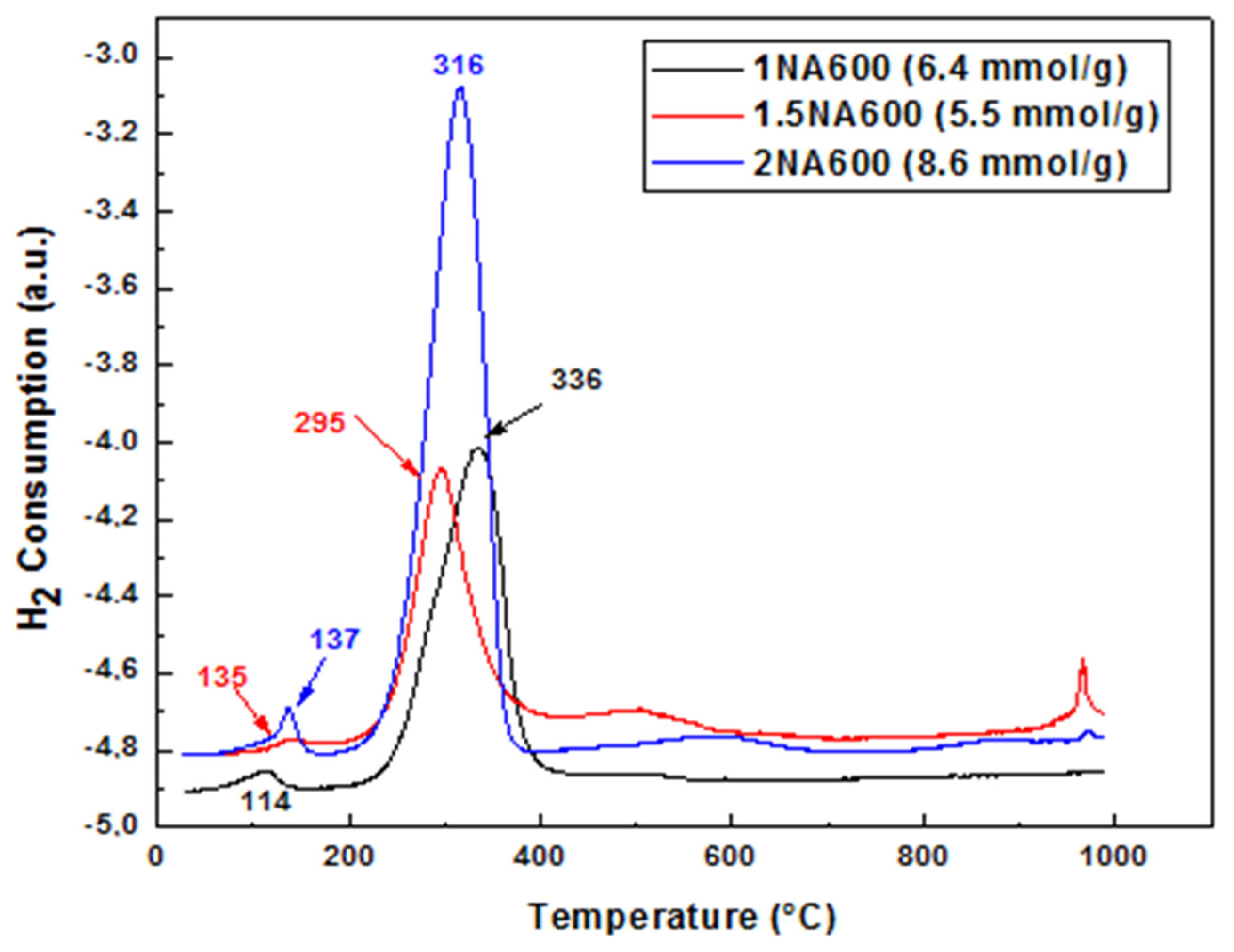
2.1.5. Reducibility Studies of Fresh Catalysts (TPR and XRD after Reduction)
2.2. Catalytic Performance in CO2 Reforming of Methane
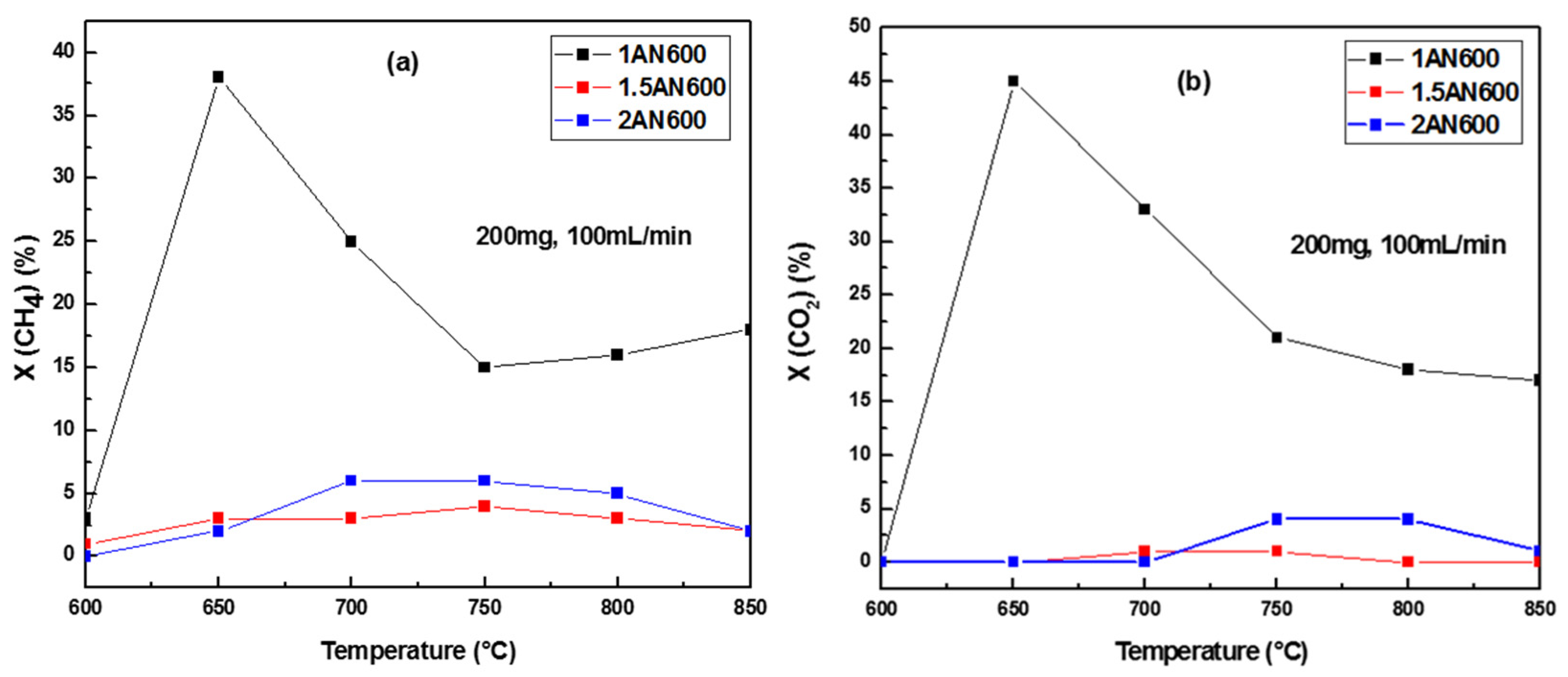
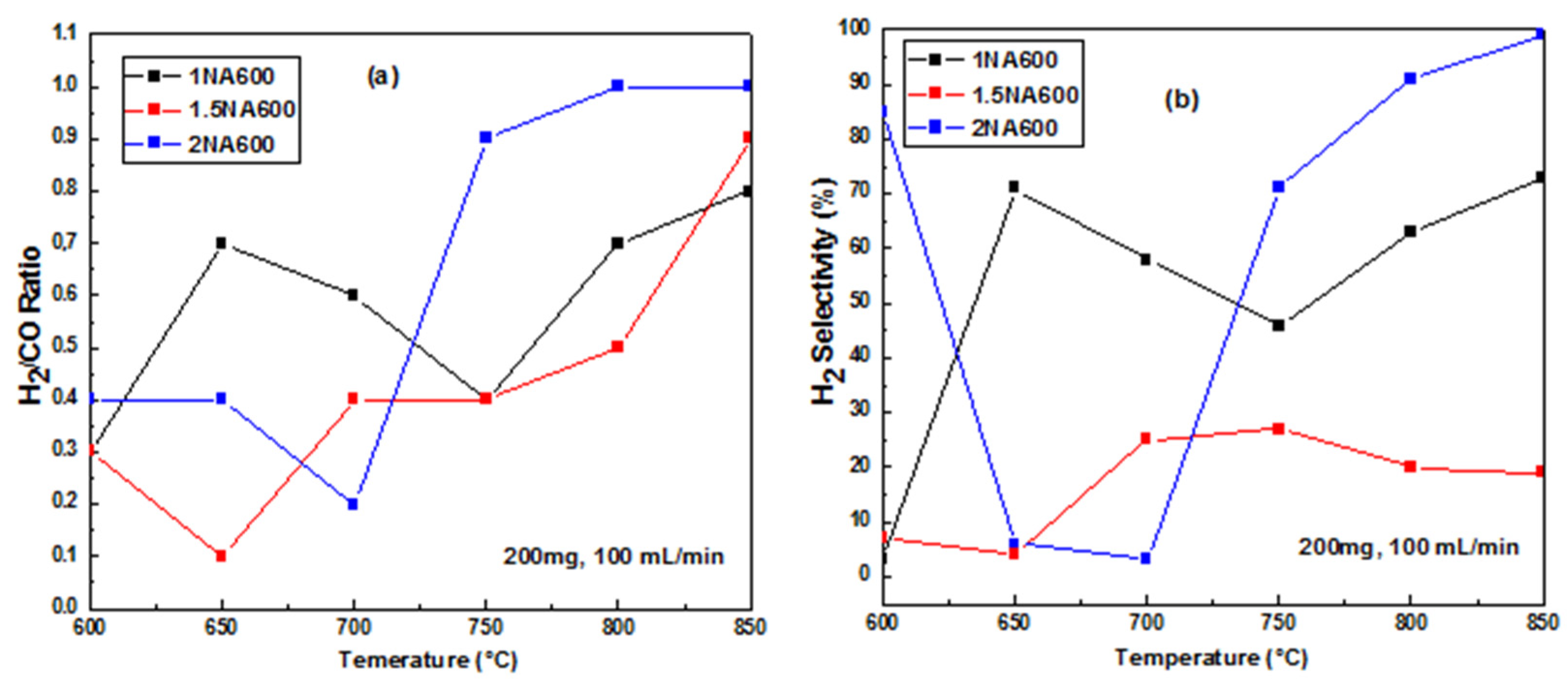
2.2.1. The Catalytic Activity versus Temperature Test
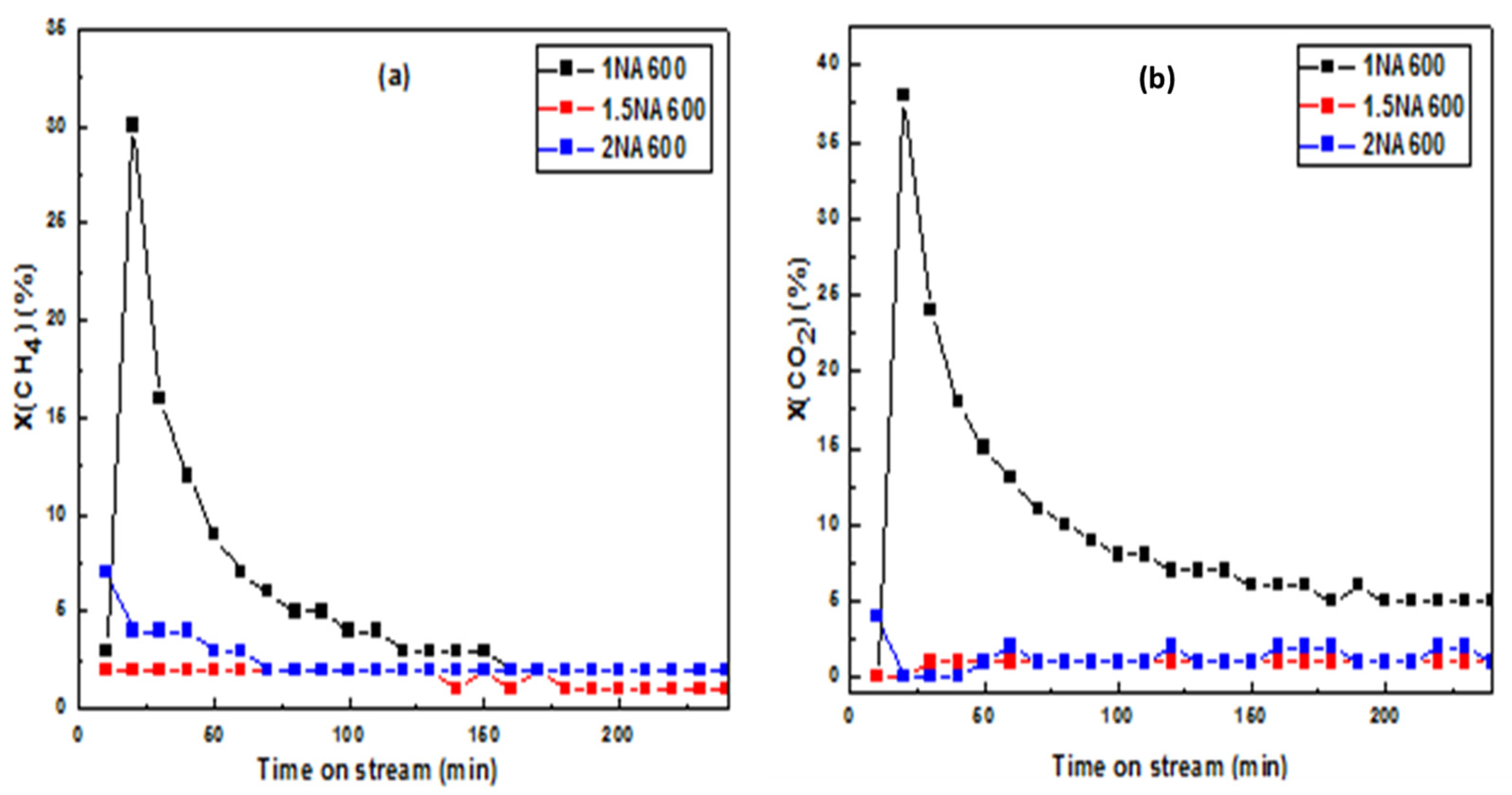
2.2.2. The Time-on-Stream Test
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Catalysts Characterization
3.4. Catalytic Reforming Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Saché, E.; Reina, T.R. Analysis of Dry Reforming as direct route for gas phase CO2 conversion. The past, the present and future of catalytic DRM technologies. Prog. Energy Combust. Sci. 2022, 89, 100970. [Google Scholar] [CrossRef]
- Fischer, F.; Tropsch, H. The Preparation of Synthetic Oil Mixture from Carbon Monoxide and Hydrogen. Brennstoff-Chem 1928, 9, 539. [Google Scholar]
- Tungatarova, S.; Xanthopoulou, G. Ni-Al Self-Propagating High-Temperature Synthesis Catalysts in Dry Reforming of Methane to Hydrogen-Enriched Fuel Mixtures. Catalysts 2022, 12, 1270. [Google Scholar] [CrossRef]
- Ashcroft, A.; Cheetham, A.K. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Liu, C.J.; Ye, J. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Hu, Y.H. Advances in catalysts for CO2 reforming of methane. Advances in CO2 conversion and utilization. ACS Publ. 2010, 1056, 155–174. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G. High carbon-resistance Ni/CeAlO3-Al2O3 catalyst for CH4/CO2 reforming. Appl. Catal. B Environ. 2013, 136, 260–268. [Google Scholar] [CrossRef]
- Aziz, M.; Setiabudi, H. A review of heterogeneous catalysts for syngas production via dry reforming. J. Taiwan Inst. Chem. Eng. 2019, 101, 139–158. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
- Lercher, J.A.; Bitter, J.H.; Steghuis, A.G.; Van Ommen, J.G.; Seshan, K. Methane utilisation via synthesis gas generation-catalytic chemistry and technology. In Environmental Catalysis; World Scientific Publishing Co Pte Ltd.: Singapore, 1999. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Y.A.; Lu, Y.; Tong, G.; Zhu, K.; Zhou, X. The promoting role of Ag in Ni-CeO2 catalyzed CH4-CO2 dry reforming reaction. Appl. Catal. B Environ. 2015, 165, 43–56. [Google Scholar] [CrossRef]
- Djinović, P.; Črnivec, I.G.O. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni–Co bimetallic catalysts. Appl. Catal. B Environ. 2012, 125, 259–270. [Google Scholar] [CrossRef]
- San-José-Alonso, D.; Juan-Juan, J. Ni, Co and bimetallic Ni–Co catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Gaur, S.; Haynes, D.J. Rh, Ni, and Ca substituted pyrochlore catalysts for dry reforming of methane. Appl. Catal. A Gen. 2011, 403, 142–151. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, J.M.; Piña, J.; El Solh, T.; De Lasa, H.I. Coke formation over a nickel catalyst under methane dry reforming conditions: Thermodynamic and kinetic models. Ind. Eng. Chem. Res. 2005, 44, 4846–4854. [Google Scholar] [CrossRef]
- Bradford, M.C.; Vannice, M.A. Catalytic reforming of methane with carbon dioxide over nickel catalysts II. Reaction kinetics. Appl. Catal. A Gen. 1996, 142, 97–122. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- James, O.O.; Maity, S. Towards reforming technologies for production of hydrogen exclusively from renewable resources. Green Chem. 2011, 13, 2272–2284. [Google Scholar] [CrossRef]
- Labinger, J.A.; Bercaw, J.E. Understanding and exploiting C–H bond activation. Nature 2002, 417, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Shi, J.; Xu, H.; Shen, W.; Hu, C. Low-temperature CO2 reforming of methane on Zr-promoted Ni/SiO2 catalyst. Fuel Process. Technol. 2016, 144, 1–7. [Google Scholar] [CrossRef]
- Tomiyama, S.; Takahashi, R.; Sato, S.; Sodesawa, T.; Yoshida, S. Preparation of Ni/SiO2 catalyst with high thermal stability for CO2-reforming of CH4. Appl. Catal. A Gen. 2003, 241, 349–361. [Google Scholar] [CrossRef]
- Uchida, T.; Ikeda, I.Y.; Takeya, S.; Kamata, Y.; Ohmura, R.; Nagao, J.; Buffett, B.A. Kinetics and stability of CH4–CO2 mixed gas hydrates during formation and long-term storage. ChemPhysChem 2005, 6, 646–654. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Amin, A.; Ibrahim, A.A.; Khan, W.U.; Soliman, M.A.; AL-Otaibi, R.L.; Fakeeha, A.H. Effect of Ce and Co addition to Fe/Al2O3 for catalytic methane decomposition. Catalysts 2016, 6, 40. [Google Scholar] [CrossRef]
- Mette, K.; Kühl, S.; Tarasov, A.; Düdder, H.; Kähler, K.; Muhler, M.; Behrens, M. Redox dynamics of Ni catalysts in CO2 reforming of methane. Catal. Today 2015, 242, 101–110. [Google Scholar] [CrossRef]
- Angeli, S.D.; Turchetti, L.; Monteleone, G.; Lemonidou, A.A. Catalyst development for steam reforming of methane and model biogas at low temperature. Appl. Catal. B Environ. 2016, 181, 34–46. [Google Scholar] [CrossRef]
- Cao, C.; Bourane, A.; Schlup, J.R.; Hohn, K.L. In situ IR investigation of activation and catalytic ignition of methane over Rh/Al2O3 catalysts. Appl. Catal. A Gen. 2008, 344, 78–87. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Highly carbon-resistant Ni–Co/SiO2 catalysts derived from phyllosilicates for dry reforming of methane. J. CO2 Util. 2017, 18, 345–352. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Ibrahim, G.; Choudhury, H.A.; Elbashir, N.O. Scaling Up the Advanced Dry Reforming of Methane (DRM) Reactor System for Multi-Walled Carbon Nanotubes and Syngas Production: An Experimental and Modeling Study. Chem. Eng. Process. Process Intensif. 2024, 197, 109693. [Google Scholar] [CrossRef]
- Pinto, D.; Hu, L.; Urakawa, A. Enabling complete conversion of CH4 and CO2 in dynamic coke-mediated dry reforming (DC-DRM) on Ni catalysts. Chem. Eng. J. 2023, 474, 145641. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; Abdullah, A.M. Engineering of Pt-based nanostructures for efficient dry (CO2) reforming: Strategy and mechanism for rich-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5901–5928. [Google Scholar] [CrossRef]
- Faroldi, B.; Múnera, J. Well-dispersed Rh nanoparticles with high activity for the dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 16127–16138. [Google Scholar] [CrossRef]
- Luisetto, I.; Sarno, C. Packed and monolithic reactors for the dry reforming of methane: Ni supported on γ-Al2O3 promoted by Ru. Adv. Sci. Lett. 2017, 23, 5977–5979. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Varitis, S.; Zhumabek, M.; Karanasios, K.; Vekinis, G.; Tungatarova, S.A.; Baizhumanova, T.S. Direct Reduction in Greenhouse Gases by Continuous Dry (CO2) Reforming of Methane over Ni-Containing SHS Catalysts. Energies 2021, 14, 6078. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Chen, L.W.; Ng, K.H. Hydrothermally synthesized tremella-like monometallic Ni/SiO2 for effective and stable dry reforming of methane (DRM) to syngas. Process Saf. Environ. Prot. 2024, 183, 244–259. [Google Scholar] [CrossRef]
- Zhao, R.; Cao, K.; Ye, R.; Tang, Y.; Du, C.; Liu, F.; Shan, B. Deciphering the stability mechanism of Pt-Ni/Al2O3 catalysts in syngas production via DRM. Chem. Eng. J. 2024, 491, 151966. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Hernández, P.S.; Anzures, F.M.; Martínez, A.G.; Galicia, G.M.; García, M.E.F.; Pérez-Hernández, R. MgO impregnation to Al2O3 supported Ni catalyst for SYNGAS production using greenhouse gases: Some aspects of chemical state of Ni species. Int. J. Hydrogen Energy 2024, 52, 1131–1140. [Google Scholar] [CrossRef]
- Alabi, W.O.; Adesanmi, B.M.; Wang, H.; Patzig, C. Correlation of MgO loading to spinel inversion, octahedral site occupancy, site generation and performance of bimetal Co–Ni catalyst for dry reforming of CH4. Int. J. Hydrogen Energy 2024, 51, 1087–1098. [Google Scholar] [CrossRef]
- Wen, F.; Xu, C.; Huang, N.; Wang, T.; Sun, X.; Li, H.; Xia, G. Exceptional stability of spinel Ni–MgAl2O4 catalyst with ordered mesoporous structure for dry reforming of methane. Int. J. Hydrogen Energy 2024, 69, 1481–1491. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, G.; Zhong, J.; Xie, J. Key features of nano NixMg1-xAl2O4 spinel for biogas bi-reforming: CO2 activation and coke elimination. Int. J. Hydrogen Energy 2024, 53, 383–393. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, J.; Rao, Q.; Gai, Z.; Li, Y.; Li, P.; Jin, H. Reversible exsolution of iron from perovskites for highly selective syngas production via chemical looping dry reforming of methane. Fuel 2024, 366, 131386. [Google Scholar] [CrossRef]
- Cao, D.; Luo, C.; Luo, T.; Shi, Z.; Wu, F.; Li, X.; Zhang, L. Dry reforming of methane by La2NiO4 perovskite oxide: B-site substitution improving reactivity and stability. Chem. Eng. J. 2024, 482, 148701. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Varun, Y.; Reddy, B.H.; Sreedhar, I.; Singh, S.A. A short review on recent advancements of dry reforming of methane (DRM) over pyrochlores. Mater. Today Proc. 2024, 72, 361–369. [Google Scholar] [CrossRef]
- Cheng, H.; Su, P.; Yuan, S.W.; Wang, Z.; Liu, M. Preparation of a green and highly active vermiculite-derived hydrotalcite and its application in methane dry reforming. Int. J. Hydrogen Energy 2024, 50, 726–733. [Google Scholar] [CrossRef]
- Maziviero, F.V.; Melo, D.M.; Medeiros, R.L.; Silva, J.C.; Araújo, T.R.; Oliveira, Â.A.; Melo, M.A. Influence of Mn, Mg, Ce and P promoters on Ni-X/Al2O3 catalysts for dry reforming of methane. J. Energy Inst. 2024, 113, 101523. [Google Scholar] [CrossRef]
- Chaudhary, K.J.; Al-Fatesh, A.S.; Ibrahim, A.A.; Osman, A.I.; Fakeeha, A.H.; Alhoshan, M.; Kumar, R. Enhanced hydrogen production through methane dry reforming: Evaluating the effects of promoter-induced variations in reducibility, basicity, and crystallinity on Ni/ZSM-5 catalyst performance. Energy Convers. Manag. 2024, 23, 100631. [Google Scholar] [CrossRef]
- Vang, R.T.; Honkala, K. Controlling the catalytic bond-breaking selectivity of Ni surfaces by step blocking. Nat. Mater. 2005, 4, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Parizotto, N.; Rocha, K. Alumina-supported Ni catalysts modified with silver for the steam reforming of methane: Effect of Ag on the control of coke formation. Appl. Catal. A Gen. 2007, 330, 12–22. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Vang, R.T. From atom-resolved scanning tunneling microscopy (STM) studies to the design of new catalysts. Catal. Today 2006, 111, 34–43. [Google Scholar] [CrossRef]
- Gavrielatos, I.; Montinaro, D. Thermogravimetric and Electrocatalytic Study of Carbon Deposition of Ag-doped Ni/YSZ Electrodes under Internal CH4 Steam Reforming Conditions. Fuel Cells 2009, 9, 883–890. [Google Scholar] [CrossRef]
- Jeong, H.; Kang, M. Hydrogen production from butane steam reforming over Ni/Ag loaded MgAl2O4 catalyst. Appl. Catal. B Environ. 2010, 95, 446–455. [Google Scholar] [CrossRef]
- Rovik, A.K.; Klitgaard, S.K. Effect of alloying on carbon formation during ethane dehydrogenation. Appl. Catal. A Gen. 2009, 358, 269–278. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, C. Effect of Ag on the control of Ni-catalyzed carbon formation: A density functional theory study. Catal. Today 2012, 186, 54–62. [Google Scholar] [CrossRef]
- Zhu, Y.A.; Chen, D. First-principles calculations of C diffusion through the surface and subsurface of Ag/Ni (1 0 0) and reconstructed Ag/Ni (1 0 0). Surf. Sci. 2010, 604, 186–195. [Google Scholar] [CrossRef]
- Boldrin, P.; Ruiz-Trejo, E.; Mermelstein, J.; Bermúdez Men´endez, J.M.; Ramírez Reina, T.; Brandon, N.P. Strategies for Carbon and Sulfur Tolerant Solid Oxide Fuel Cell Materials, Incorporating Lessons from Heterogeneous Catalysis. Chem. Rev. 2016, 116, 13633–13684. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Ruiz, S.; Zhang, Q.; Gándara-Loe, J.; Pastor-Pérez, L.; Odriozola, J.A.; Reina, T.R.; Bobadilla, L.F. H2-rich syngas production from biogas reforming: Overcoming coking and sintering using bimetallic Ni-based catalysts. Int. J. Hydrogen Energy 2023, 48, 27907–27917. [Google Scholar] [CrossRef]
- Fang, X.; Mao, L.; Xu, L.; Shen, J.; Xu, J.; Xu, X.; Wang, X. Ni-Fe/La2O3 bimetallic catalysts for methane dry reforming: Elucidating the role of Fe for improving coke resistance. Fuel 2024, 357, 129950. [Google Scholar] [CrossRef]
- Armengol-Profitós, M.; Braga, A.; Pascua-Solé, L.; Lucentini, I.; Garcia, X.; Soler, L.; Llorca, J. Enhancing the performance of a novel CoRu/CeO2 bimetallic catalyst for the dry reforming of methane via a mechanochemical process. Appl. Catal. B Environ. 2024, 345, 123624. [Google Scholar] [CrossRef]
- Diao, J.F.; Zhang, T.; Xu, Z.N.; Guo, G.C. The atomic-level adjacent NiFe bimetallic catalyst significantly improves the activity and stability for plasma-involved dry reforming reaction of CH4 and CO2. Chem. Eng. J. 2023, 467, 143271. [Google Scholar] [CrossRef]
- Pandey, S.K.; Tripathi, M.K.; Ramanathan, V.; Mishra, P.K.; Tiwary, D. Highly facile Ag/NiO nanocomposite synthesized by sol-gel method for mineralization of rhodamine B. J. Phys. Chem. Solids 2021, 159, 110287. [Google Scholar] [CrossRef]
- Zhou, L.X.; Yang, Y.Y. In situ synthesis of Ag/NiO derived from hetero-metallic MOF for supercapacitor application. Chem. Pap. 2021, 75, 1795–1807. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Ryu, K.S. Synthesis of Ag/NiO honeycomb structured nanoarrays as the electrode material for high performance asymmetric supercapacitor devices. Sci. Rep. 2019, 9, 4864. [Google Scholar] [CrossRef]
- Aydoghmish, S.M.; Hassanzadeh-Tabrizi, S. Facile synthesis and investigation of NiO–ZnO–Ag nanocomposites as efficient photocatalysts for degradation of methylene blue dye. Ceram. Int. 2019, 45, 14934–14942. [Google Scholar] [CrossRef]
- Khedkar, C.V.; Vedpathak, A.S. Synthesis, characterization, electrochemical and catalytic performance of NiO nanostructures and Ag-NiO nanocomposite. Chem. Phys. Impact 2023, 6, 100153. [Google Scholar] [CrossRef]
- Zhang, X.W.; Su, Y.X. Effect of Ag on deNOx performance of SCR-C3H6 over Fe/Al-PILC catalysts. J. Fuel Chem. Technol. 2019, 47, 1368–1378. [Google Scholar] [CrossRef]
- Predoi, D.; Jitianu, A. Study of FexOy–SiO2 nanoparticles obtained by sol-gel synthesis. Dig. J. Nanomater. Biostruct 2006, 1, 93–97. [Google Scholar]
- Singh, A.; Yadav, B.C. Green synthesized ZnO/NiO heterostructures based quick responsive LPG sensor for the detection of below LEL with DFT calculations. Results Surf. Interfaces 2023, 11, 100103. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy (XPS) reference pages. Available online: http://www.xpsfitting.com/ (accessed on 1 March 2024).
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wandekar, R.; Ali, M. Physicochemical studies of NiO–GDC composites. Mater. Chem. Phys. 2006, 99, 289–294. [Google Scholar] [CrossRef]
- Augusto, B.L.; Costa, L.O. Ethanol reforming over Ni/CeGd catalysts with low Ni content. Int. J. Hydrogen Energy 2012, 37, 12258–12270. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Galicia, G.M. Synthesis and characterization of bimetallic Cu–Ni/ZrO2 nanocatalysts: H2 production by oxidative steam reforming of methanol. Int. J. Hydrogen Energy 2008, 33, 4569–4576. [Google Scholar] [CrossRef]
- Ashok, J.; Subrahmanyam, M. Hydrotalcite structure derived Ni–Cu–Al catalysts for the production of H2 by CH4 decomposition. Int. J. Hydrogen Energy 2008, 33, 2704–2713. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol–gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M. Reduction property and catalytic activity of Ce1−XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A Gen. 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Kirumakki, S.R.; Shpeizer, B.G. Hydrogenation of naphthalene over NiO/SiO2–Al2O3 catalysts: Structure–activity correlation. J. Catal. 2006, 242, 319–331. [Google Scholar] [CrossRef]
- Joo, O.S.; Jung, K.D. CH4 dry reforming on alumina-supported nickel catalyst. Bull. Korean Chem. Soc. 2002, 23, 1149–1153. [Google Scholar] [CrossRef]
- Casenave, S.; Martinez, H. Acid–base properties of Mg–Ni–Al mixed oxides using LDH as precursors. Thermochim. Acta 2001, 379, 85–93. [Google Scholar] [CrossRef]
- Casenave, S.; Martinez, H. Acid-Base properties of MgCuAl mixed oxides. J. Therm. Anal. Calorim. 2003, 72, 191–198. [Google Scholar] [CrossRef]
- Benrabaa, R.; Löfberg, A.; Rubbens, A.; Bordes-Richard, E.; Vannier, R.N.; Barama, A. Structure, reactivity and catalytic properties of nanoparticles of nickel ferrite in the dry reforming of methane. Catal. Today 2013, 203, 188–195. [Google Scholar] [CrossRef]
- Hallassi, M.; Benrabaa, R. Characterization and Syngas Production at Low Temperature via Dry Reforming of Methane over Ni-M (M= Fe, Cr) Catalysts Tailored from LDH Structure. Catalysts 2022, 12, 1507. [Google Scholar] [CrossRef]
- Benrabaa, R.; Boukhlouf, H.; Löfberg, A.; Rubbens, A.; Vannier, R.N.; Bordes-Richard, E.; Barama, A. Nickel ferrite spinel as catalyst precursor in the dry reforming of methane: Synthesis, characterization and catalytic properties. J. Nat. Gas Chem. 2012, 21, 595–604. [Google Scholar] [CrossRef]
- Slagtern, Å.; Olsbye, U. Partial oxidation of methane to synthesis gas using La-MO catalysts. Appl. Catal. A Gen. 1994, 110, 99–108. [Google Scholar] [CrossRef]




| N° | Reaction Name | Reaction Equation | ΔH298K (kJ/mol) |
|---|---|---|---|
| Main reaction | |||
| 1 | Dry reforming of CH4 | CH4 + CO2 ↔ 2CO + 2H2 | +247 |
| Side reactions | |||
| 2 | Reverse water–gas shift | CO2 + H2 ↔ CO + H2O | +41 |
| 3 | Decomposition of CH4 | CH4 ↔ C + 2H2 | +75 |
| 4 | Disproportionation of CO | 2CO ↔ C + CO2 | −172 |
| 5 | Hydrogenation of CO2 | CO2 + 2H2 ↔ C + 2H2O | −90 |
| 6 | Hydrogenation of CO | CO + H2 ↔ C + H2O | −131 |
| Catalyst | B.E.T. | XRD Data | EDX | ICP | XPS | ||||
|---|---|---|---|---|---|---|---|---|---|
| SSA (m2/g) | Phase Ratio | Cs a (n m) | Cs b (nm) | Cs c (nm) | a d (Å) | Ni/Ag | Ni/Ag | Ni/Ag | |
| 1NA600 | 20 | NiO (69) Ag (31) | 8.7 (6) 70 (4) | Ni: 12.6 (2) Ag: 21.3 (2) NiO: 3.2 (1) | Ni: 30 (1) Ag: 20.8 (4) | 4.177 (2) 4.0875 (5) | 1.3 | 0.73 | 6.7 |
| 1.5NA600 | 3 | NiO (71) Ag (29) | 12.6 (5) 6.5 (3) | Ni: 13.1 (1) Ag: 12.7 (1) NiO: 7.4 (1) | Ni: 39 (3) Ag:21 (1) | 4.177 (2) 4.092 (2) | 1.9 | 0.96 | 6.5 |
| 2NA600 | 5 | NiO (80) Ag (20) | 16.8 (4) 7.5 (3) | Ni: 15.1 (1) Ag: 12.2 (1) NiO: 7.6 (2) | Ni: 35 (2) Ag: 16.3 (1) | 4.1801 (7) 4.0917 (9) | 3 | 1.51 | 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henni, H.; Benrabaa, R.; Roussel, P.; Löfberg, A. Ni-Ag Catalysts for Hydrogen Production through Dry Reforming of Methane: Characterization and Performance Evaluation. Catalysts 2024, 14, 400. https://doi.org/10.3390/catal14070400
Henni H, Benrabaa R, Roussel P, Löfberg A. Ni-Ag Catalysts for Hydrogen Production through Dry Reforming of Methane: Characterization and Performance Evaluation. Catalysts. 2024; 14(7):400. https://doi.org/10.3390/catal14070400
Chicago/Turabian StyleHenni, Hayat, Rafik Benrabaa, Pascal Roussel, and Axel Löfberg. 2024. "Ni-Ag Catalysts for Hydrogen Production through Dry Reforming of Methane: Characterization and Performance Evaluation" Catalysts 14, no. 7: 400. https://doi.org/10.3390/catal14070400
APA StyleHenni, H., Benrabaa, R., Roussel, P., & Löfberg, A. (2024). Ni-Ag Catalysts for Hydrogen Production through Dry Reforming of Methane: Characterization and Performance Evaluation. Catalysts, 14(7), 400. https://doi.org/10.3390/catal14070400









