Photocatalytic Production of Hydrogen Peroxide from Covalent-Organic-Framework-Based Materials: A Mini-Review
Abstract
:1. Introduction
2. Principles of Photocatalytic COFs for H2O2 Production
2.1. Photocatalytic COFs Mainly via ORR Pathway
2.2. Photocatalytic COFs via Dual-Channel Pathway
3. Strategies to Improve Photocatalytic H2O2 Production
3.1. Functional Groups Modification
3.2. Design of Spatially Separated Redox Centers
3.3. COF Nanohybrids
3.4. Linkage Chemistry
3.5. Dimensions Control
4. Perspective and Outlook
4.1. Clarify the Reaction Mechanism and the Effect of Structure on Catalytic Performance
4.2. Improve Catalytic Performance and Applicability
4.3. Standardize Performance Judgement Criteria
4.4. Combine with Other Fields
4.5. Assess the Environmental and Safety Performance of COFs
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.; Gu, H.; Wang, X.; Li, L.; Zhang, J.; Zhang, H.; Li, Y.-F.; Dai, W.-L. Robust hollow tubular ZnIn2S4 modified with embedded metal-organic-framework-layers: Extraordinarily high photocatalytic hydrogen evolution activity under simulated and real sunlight irradiation. Appl. Catal. B Environ. 2021, 298, 120632. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, H.; Huang, Y.; Wang, X.; Gao, L.; Li, Q.; Li, Y.; Zhang, Y.; Cui, Y.; Gao, R.; et al. Rational design of covalent organic frameworks/NaTaO3 S-scheme heterostructure for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2024, 664, 916–927. [Google Scholar] [CrossRef]
- Jose, M.C.-M.; Gema, B.-B.; Jose, L.G.F. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.; Cao, J.; Sun, Y.; Liao, F.; Liu, Y.; Huang, H.; Shao, M.; Kang, Z. A function-switchable metal-free photocatalyst for the efficient and selective production of hydrogen and hydrogen peroxide. J. Mater. Chem. A 2020, 8, 11773–11780. [Google Scholar] [CrossRef]
- Teong, S.P.; Li, X.; Zhang, Y. Hydrogen peroxide as an oxidant in biomass-to-chemical processes of industrial interest. Green Chem. 2019, 21, 5753–5780. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.-M.; Nam, W. Recent progress in production and usage of hydrogen peroxide. Chin. J. Catal. 2021, 42, 1241–1252. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, D.; Chu, C.; Mao, S. Photocatalytic H2O2 production Systems: Design strategies and environmental applications. Chem. Eng. J. 2023, 451, 138489. [Google Scholar] [CrossRef]
- García-Serna, J.; Moreno, T.; Biasi, P.; Cocero, M.J.; Mikkola, J.-P.; Salmi, T.O. Engineering in direct synthesis of hydrogen peroxide: Targets, reactors and guidelines for operational conditions. Green Chem. 2014, 16, 2320–2343. [Google Scholar] [CrossRef]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Gao, G.; Tian, Y.; Gong, X.; Pan, Z.; Yang, K.; Zong, B. Advances in the production technology of hydrogen peroxide. Chin. J. Catal. 2020, 41, 1039–1047. [Google Scholar] [CrossRef]
- Edwards, J.K.; Solsona, B.N.E.N.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Switching off Hydrogen Peroxide Hydrogenation in the Direct Synthesis Process. Science 2009, 323, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Hou, H.; Zeng, X.; Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Han, L.; Strasser, P. A comparative perspective of electrochemical and photochemical approaches for catalytic H2O2 production. Chem. Soc. Rev. 2020, 49, 6605. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, N.; Fan, J.; Lu, C.; Lv, K. Carbon nitride for photocatalytic water splitting to produce hydrogen and hydrogen peroxide. Mater. Today Chem. 2022, 26, 101028. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.; Kim, H.; Jung, G.Y.; Kim, M.-G. Solid-Phase Photocatalysts: Physical Vapor Deposition of Au Nanoislands on Porous TiO2 Films for Millimolar H2O2 Production within a Few Minutes. ACS Catal. 2019, 9, 9206–9211. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, B.; Zhang, Y.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Wang, L. S-scheme ZnO/WO3 heterojunction photocatalyst for efficient H2O2 production. J. Mater. Sci. Technol. 2022, 124, 193–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Wang, L.; Zhang, X.; Zhu, Z.; Wang, J.; Yu, W.; Zhang, Y. Cooperation of congenital and acquisitus sulfur vacancy for excellent photocatalytic hydrogen peroxide evolution of CdS nanorods from air. Chem. Eng. J. 2022, 454, 140420. [Google Scholar] [CrossRef]
- Li, F.; Tang, X.; Hu, Z.; Li, X.; Li, F.; Xie, Y.; Jiang, Y.; Yu, C. Boosting the hydrogen peroxide production over In2S3 crystals under visible light illumination by gallium ions doping and sulfur vacancies modulation. Chin. J. Catal. 2023, 55, 253–264. [Google Scholar] [CrossRef]
- Liu, C.; Bao, T.; Yuan, L.; Zhang, C.; Wang, J.; Wan, J.; Yu, C. Semiconducting MOF@ZnS Heterostructures for Photocatalytic Hydrogen Peroxide Production: Heterojunction Coverage Matters. Adv. Funct. Mater. 2021, 32, 2111404. [Google Scholar] [CrossRef]
- Kondo, Y.; Hino, K.; Kuwahara, Y.; Mori, K.; Yamashita, H. Photosynthesis of hydrogen peroxide from dioxygen and water using aluminium-based metal–organic framework assembled with porphyrin- and pyrene-based linkers. J. Mater. Chem. A 2023, 11, 9530. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Sekhar Jena, H.; Bourda, L.; Laemont, A.; Pachfule, P.; Roeser, J.; Chandran, C.V.; Borgmans, S.; Rogge, S.M.J.; Leus, K.; et al. Strongly Reducing (Diarylamino)benzene-Based Covalent Organic Framework for Metal-Free Visible Light Photocatalytic H2O2 Generation. J. Am. Chem. Soc. 2020, 142, 20107–20116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Dai, D.; Yao, J. Tailoring metal–organic frameworks for photocatalytic H2O2 production. Coord. Chem. Rev. 2023, 501, 215597. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Sugano, Y.; Tsukamoto, D.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Highly selective production of hydrogen peroxide on graphitic carbon nitride (g-C3N4) photocatalyst activated by visible light. ACS Catal. 2014, 4, 774–780. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Z.; Zhu, Y.; Yang, C.; Wu, J.; Hu, W. 2D covalent organic frameworks: From synthetic strategies to advanced optical-electrical-magnetic functionalities. Adv. Mater. 2022, 34, 2102290. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Takii, T.; Hagi, T.; Mori, S.; Kofuji, Y.; Kitagawa, Y.; Tanaka, S.; Ichikawa, S.; Hirai, T. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 2019, 18, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, M.; Zhu, Y.; Li, P.; Zhang, Y.; Wang, M.; Shen, Y. A thioether-decorated triazine-based covalent organic framework towards overall H2O2 photosynthesis without sacrificial agents. Appl. Catal. B Environ. 2023, 334, 122862. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Liu, C.; Xia, D.; Ou, X.; Cai, Y.; Zhou, Y.; Jiang, J.; Han, B. Sulfone-Modified Covalent Organic Frameworks Enabling Efficient Photocatalytic Hydrogen Peroxide Generation via One-Step Two-Electron O2 Reduction. Angew. Chem. Int. Ed. 2023, 62, e202305355. [Google Scholar] [CrossRef]
- Liao, Q.; Sun, Q.; Xu, H.; Wang, Y.; Xu, Y.; Li, Z.; Hu, J.; Wang, D.; Li, H.; Xi, K. Regulating Relative Nitrogen Locations of Diazine Functionalized Covalent Organic Frameworks for Overall H2O2 Photosynthesis. Angew. Chem. Int. Ed. 2023, 62, e202310556. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Wan, Y.; Zhang, Y.; Qi, Z.; Wu, X.; Xu, H. Acetylene and Diacetylene Functionalized Covalent Triazine Frameworks as Metal-Free Photocatalysts for Hydrogen Peroxide Production: A New Two-Electron Water Oxidation Pathway. Adv. Mater. 2019, 32, 1904433. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-N.; Li, Q.; Shi, J.-W.; Zhang, M.; Zhang, L.; Li, S.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Oxidation-Reduction Molecular Junction Covalent Organic Frameworks for Full Reaction Photosynthesis of H2O2. Angew. Chem. Int. Ed. 2023, 62, e202218868. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.-Y.; Song, L.-P.; Fan, Y.-F.; Pan, Z.-X.; Yang, P.; Ma, Y.; Xu, Q.; Tang, B. Thiophene-Containing Covalent Organic Frameworks for Overall Photocatalytic H2O2 Synthesis in Water and Seawater. Angew. Chem. Int. Ed. 2023, 62, e202309624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Li, L.; Tan, H.; Ye, N.; Gu, Y.; Zhao, S.Q.; Zhang, S.P.; Luo, M.C.; Guo, S.J. Fluorination of Covalent Organic Framework Reinforcing the Confinement of Pd Nanoclusters Enhances Hydrogen Peroxide Photosynthesis. J. Am. Chem. Soc. 2023, 145, 19877–19884. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, W.; Wu, Y.; Wang, L.; Wu, X.; Xu, H.; Chen, L. Covalent Organic Frameworks Containing Dual O2 Reduction Centers for Overall Photosynthetic Hydrogen Peroxide Production. Angew. Chem. Int. Ed. 2022, 62, e202217479. [Google Scholar] [CrossRef]
- Qin, C.; Wu, X.; Tang, L.; Chen, X.; Li, M.; Mou, Y.; Su, B.; Wang, S.; Feng, C.; Liu, J.; et al. Dual donor-acceptor covalent organic frameworks for hydrogen peroxide photosynthesis. Nat. Commun. 2023, 14, 5238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; Lu, J. Built-in electric field and oxygen absorption synergistically optimized an organic/inorganic heterojunction for high-efficiency photocatalytic hydrogen peroxide production. J. Colloid Interface Sci. 2023, 648, 664–673. [Google Scholar] [CrossRef]
- Wu, M.; Shan, Z.; Wang, J.; Liu, T.; Zhang, G. Three-dimensional covalent organic framework with tty topology for enhanced photocatalytic hydrogen peroxide production. Chem. Eng. J. 2023, 454, 140121. [Google Scholar] [CrossRef]
- Isaka, Y.; Kondo, Y.; Kawase, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Photocatalytic production of hydrogen peroxide through selective two-electron reduction of dioxygen utilizing amine-functionalized MIL-125 deposited with nickel oxide nanoparticles†. Chem. Commun. 2018, 54, 9270–9273. [Google Scholar] [CrossRef]
- Isaka, Y.; Kawase, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Two-Phase System Utilizing Hydrophobic Metal–Organic Frameworks (MOFs) for Photocatalytic Synthesis of Hydrogen Peroxide. Angew. Chem. Int. Ed. 2019, 58, 5402–5406. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, B.; Yu, J.; Wang, L.; Ho, W. TiO2/In2S3 S-scheme photocatalyst with enhanced H2O2-production activity. Nano Res. 2021, 16, 4506–4514. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Cao, J.; Wang, H.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. Efficient production of H2O2 via two-channel pathway over ZIF-8/C3N4 composite photocatalyst without any sacrificial agent. Appl. Catal. B Environ. 2020, 278, 119289. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.; Park, S.O.; Hwang, J.M.; Hong, Y.; Sharma, P.; Jeon, W.C.; Cho, Y.; Yang, C.; Kwak, S.K.; et al. High performance H2O2 production achieved by sulfur-doped carbon on CdS photocatalyst via inhibiting reverse H2O2 decomposition. Appl. Catal. B Environ. 2021, 284, 119690. [Google Scholar] [CrossRef]
- He, T.; Zhao, Y. Covalent Organic Frameworks for Energy Conversion in Photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202303086. [Google Scholar] [CrossRef]
- Yong, Z.; Ma, T. Solar-to-H2O2 Catalyzed by Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202308980. [Google Scholar] [CrossRef]
- Freese, T.; Meijer, J.T.; Feringa, B.L.; Beil, S.B. An organic perspective on photocatalytic production of hydrogen peroxide. Nat. Catal. 2023, 6, 553–558. [Google Scholar] [CrossRef]
- Wu, S.; Quan, X. Design Principles and Strategies of Photocatalytic H2O2 Production from O2 Reduction. ACS ES&T Eng. 2022, 2, 1068–1079. [Google Scholar] [CrossRef]
- Sheng, B.; Deng, C.; Li, Y.; Xie, S.; Wang, Z.; Sheng, H.; Zhao, J. In Situ Hydroxylation of a Single-Atom Iron Catalyst for Preferential 1O2 Production from H2O2. ACS Catal. 2022, 12, 14679–14688. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Hu, Z.; Yu, J.C. Enhanced Mass Transfer of Oxygen through a Gas–Liquid–Solid Interface for Photocatalytic Hydrogen Peroxide Production. Adv. Funct. Mater. 2021, 31, 2106120. [Google Scholar] [CrossRef]
- Tan, D.; Zhuang, R.; Chen, R.; Ban, M.; Feng, W.; Xu, F.; Chen, X.; Wang, Q. Covalent Organic Frameworks Enable Sustainable Solar to Hydrogen Peroxide. Adv. Funct. Mater. 2024, 34, 2311655. [Google Scholar] [CrossRef]
- Li, L.; Lv, X.; Xue, Y.; Shao, H.; Zheng, G.; Han, Q. Custom-Design of Strong Electron/Proton Extractor on COFs for Efficient Photocatalytic H2O2 Production. Angew. Chem. Int. Ed. 2024, 63, e202320218. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tao, Y.; Wang, D.; Li, C.; Jiang, Q.; Shi, Y.; Wang, J.; Qin, J.; Zhou, S.; Kong, Y. Rational modification of hydroxy-functionalized covalent organic frameworks for enhanced photocatalytic hydrogen peroxide evolution. J. Colloid Interface Sci. 2022, 629, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, Y.; Li, X.; Wang, X.; Guo, D.; Zhang, S.; Zhang, K.; Wu, J.; Zheng, J.; Zheng, S.; et al. Postmodification of an Amine-Functionalized Covalent Organic Framework for Enantioselective Adsorption of Tyrosine. ACS Appl. Mater. Interfaces 2023, 15, 24836–24845. [Google Scholar] [CrossRef] [PubMed]
- Pachfule, P.; Acharjya, A.; Roeser, J.; Langenhahn, T.; Schwarze, M.; Schomäcker, R.; Thomas, A.; Schmidt, J. Diacetylene Functionalized Covalent Organic Framework (COF) for Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2018, 140, 1423–1427. [Google Scholar] [CrossRef]
- Hou, Y.; Zhou, P.; Liu, F.; Lu, Y.; Tan, H.; Li, Z.; Tong, M.; Ni, J. Efficient Photosynthesis of Hydrogen Peroxide by Cyano-Containing Covalent Organic Frameworks from Water, Air and Sunlight. Angew. Chem. Int. Ed. 2024, 63, e202318562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lv, H.; Cheng, J.; Wang, L.; Wu, X.; Xu, H. Rational Design of Covalent Heptazine Frameworks with Spatially Separated Redox Centers for High-Efficiency Photocatalytic Hydrogen Peroxide Production. Adv. Mater. 2021, 34, 2107480. [Google Scholar] [CrossRef]
- Zhai, L.; Xie, Z.; Cui, C.-X.; Yang, X.; Xu, Q.; Ke, X.; Liu, M.; Qu, L.-B.; Chen, X.; Mi, L. Constructing Synergistic Triazine and Acetylene Cores in Fully Conjugated Covalent Organic Frameworks for Cascade Photocatalytic H2O2 Production. Chem. Mater. 2022, 34, 5232–5240. [Google Scholar] [CrossRef]
- Chai, S.; Chen, X.; Zhang, X.; Fang, Y.; Sprick, R.S.; Chen, X. Rational design of covalent organic frameworks for efficient photocatalytic hydrogen peroxide production. Environ. Sci. Nano 2022, 9, 2464. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, Y.; Ai, J.; Yan, Y.; Yang, X.; Wang, D.; Liao, G. Embedding Au nanoclusters into the pores of carboxylated COF for the efficient photocatalytic production of hydrogen peroxide. J. Mater. Chem. A 2023, 11, 21109–21122. [Google Scholar] [CrossRef]
- Chen, H.; Gao, S.; Huang, G.; Chen, Q.; Gao, Y.; Bi, J. Built-in electric field mediated S-scheme charge migration in COF/In2S3 heterojunction for boosting H2O2 photosynthesis and sterilization. Appl. Catal. B Environ. 2024, 343, 123545. [Google Scholar] [CrossRef]
- Feng, Y.; Li, J.; Ye, S.; Gao, S.; Cao, R. Growing COFs in situ on CdS nanorods as core–shell heterojunctions to improve the charge separation efficiency. Sustain. Energy Fuels 2022, 6, 5089. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Zhang, H.; Li, H.; Yang, S. Uncovering Original Z Scheme Heterojunctions of COF/MOx (M = Ti, Zn, Zr, Sn, Ce, and Nb) with Ascendant Photocatalytic Selectivity for Virtually 99.9% NO-to-NO3− Oxidation. Adv. Funct. Mater. 2023, 33, 2303851. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Zhu, B.; Fedin, M.V.; Cheng, B.; Yu, J.; Zhang, L. ZnO/COF S-scheme heterojunction for improved photocatalytic H2O2 production performance. Chem. Eng. J. 2022, 444, 136584. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.-L.; Dong, Y.-B. Construction of covalent organic frameworks via multicomponent reactions. J. Am. Chem. Soc. 2023, 145, 1475–1496. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Cui, J.; Li, X.; Zhang, Y.; Wang, C.; Yu, X.; Ye, J. Defective g-C3N4/covalent organic framework van der Waals heterojunction toward highly efficient S-scheme CO2 photoreduction. Appl. Catal. B Environ. 2021, 301, 120814. [Google Scholar] [CrossRef]
- Cong, Y.; Li, X.; Zhang, S.; Zheng, Q.; Zhang, Y.; Lv, S.-W. Embedding carbon quantum dots into crystalline polyimide covalent organic frameworks to enhance water oxidation for achieving dual-channel photocatalytic H2O2 Generation in a wide pH range. ACS Appl. Mater. Interfaces 2023, 15, 43799–43809. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, J.; Sham, Y.-T.; Gao, S.; Pan, M.; Chen, Q.; Huang, G.; Wong, P.K.; Bi, J. Efficient hydrogen peroxide photosynthesis over CdS/COF for water disinfection: The S-Scheme pathway, oxygen adsorption, and reactor design. ACS Sustain. Chem. Eng. 2023, 11, 17552–17563. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Zhang, Y.; Cheng, B.; Zhu, B.; Wang, L. BiOBr/COF S-scheme photocatalyst for H2O2 production via concerted two-electron pathway. J. Mater. Sci. Technol. 2023, 166, 241–249. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2845–3234. [Google Scholar] [CrossRef]
- Campbell, N.L.; Clowes, R.; Ritchie, L.K.; Cooper, A.I. Rapid Microwave Synthesis and Purification of Porous Covalent Organic Frameworks. Chem. Mater. 2009, 21, 204–206. [Google Scholar] [CrossRef]
- Yue, J.-Y.; Song, L.-P.; Pan, Z.-X.; Yang, P.; Ma, Y.; Xu, Q.; Tang, B. Regulating the H2O2 Photosynthetic Activity of Covalent Organic Frameworks through Linkage Orientation. ACS Catal. 2024, 14, 4728–4737. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, D.; Kong, A.; Zou, Y.; Yuan, L.; Liu, C.; Luo, S.; Wei, G.; Yu, C. Robust Covalent Organic Framework Photocatalysts for H2O2 Production: Linkage Position Matters. Angew. Chem. Int. Ed. 2024, 63, e202404077. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, Y.; Li, N.; Zhang, H.; Liu, X.; Yang, X.; Pan, H.; Wang, T.; Wang, K.; Qi, D.; et al. 12 Connecting Sites Linked Three-dimensional Covalent Organic Frameworks with Intrinsic Non-interpenetrated shp Topology for Photocatalytic H2O2 Synthesis. Angew. Chem. Int. Ed. 2024, 63, e202401014. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Fang, Q.; Yan, Y.; Qiu, S. Functional Regulation and Stability Engineering of Three-Dimensional Covalent Organic Frameworks. Acc. Chem. Res. 2022, 55, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Lin, G.; Ding, H.; Gao, C.; Mal, A.; Wang, C. Three-Dimensional Covalent Organic Frameworks: From Topology Design to Applications. Acc. Chem. Res. 2020, 53, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Luo, Y.; Shi, J.-L.; Ding, H.; Lang, X.; Chen, W.; Zheng, A.; Sun, J.; Wang, C. 2D and 3D Porphyrinic Covalent Organic Frameworks: The Influence of Dimensionality on Functionality. Angew. Chem. Int. Ed. 2020, 59, 3624–3629. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Yang, Z.; Chen, C.; Chu, C.; Chen, B. Enhanced photocatalytic hydrogen peroxide production at a solid-liquid-air interface via microenvironment engineering. Appl. Catal. B Environ. 2022, 305, 121066. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Liu, S.; Zhang, D.; Cai, B.; Liang, Y.; Wu, M.; Liao, Y.; Zhao, X. Pyridine-based Covalent Organic Frameworks with Pyridyl-Imine Structures for Boosting Photocatalytic H2O2 Production via One-Step 2e− Oxygen Reduction. Angew. Chem. Int. Ed. 2024, 63, e202404563. [Google Scholar] [CrossRef]
- Xu, C.; Zou, R.; Peng, Y.; Liu, Q.; Ruan, S.; Hu, J. In situ transmission electron microscope studies on one-dimensional nanomaterials: Manipulation, properties and applications. Prog. Mater. Sci. 2020, 113, 100674. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; He, S.; Chen, X.; Huang, X.; Zhou, H. Dynamic Imine Exchange Reactions for Facile Synthesis of Imine-Linked Covalent Organic Frameworks. Chem. Mater. 2023, 35, 10070–10077. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Xiao, Y.; Chen, X.; Huang, X.; Zhou, H. Facile Solution-Refluxing Synthesis and Photocatalytic Dye Degradation of a Dynamic Covalent Organic Framework. Molecules 2022, 27, 8002. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhou, P.; Hou, Y.; Tan, H.; Liang, Y.; Liang, J.; Zhang, Q.; Guo, S.; Tong, M.; Ni, J. Covalent organic frameworks for direct photosynthesis of hydrogen peroxide from water, air and sunlight. Nat. Commun. 2023, 14, 4344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, S.; Chen, C.; Wen, X.; Miao, J.; Zhou, B.; Long, M.; Zhang, L. Keto-anthraquinone covalent organic framework for H2O2 photosynthesis with oxygen and alkaline water. Nat. Commun. 2024, 15, 2649. [Google Scholar] [CrossRef] [PubMed]



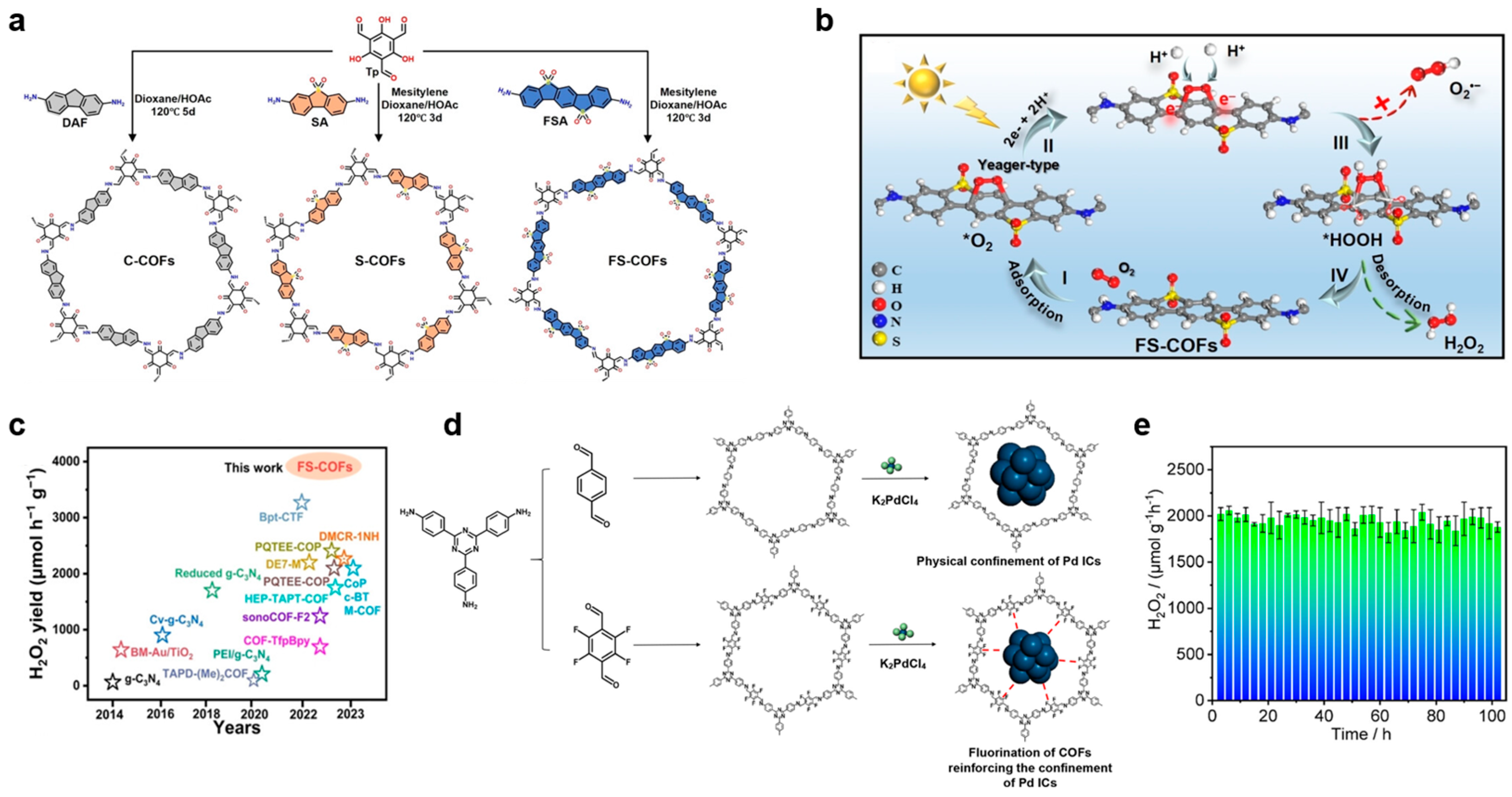

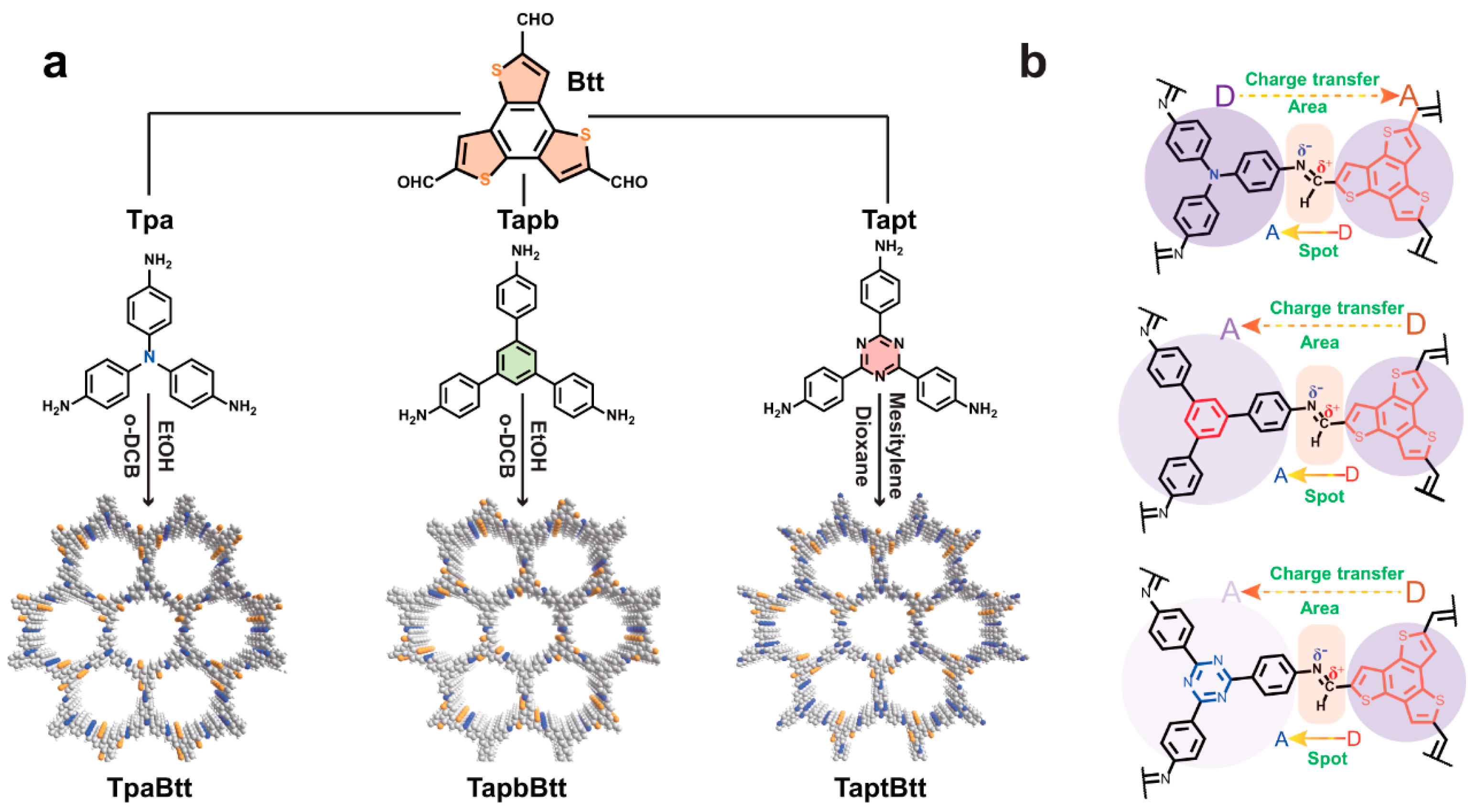
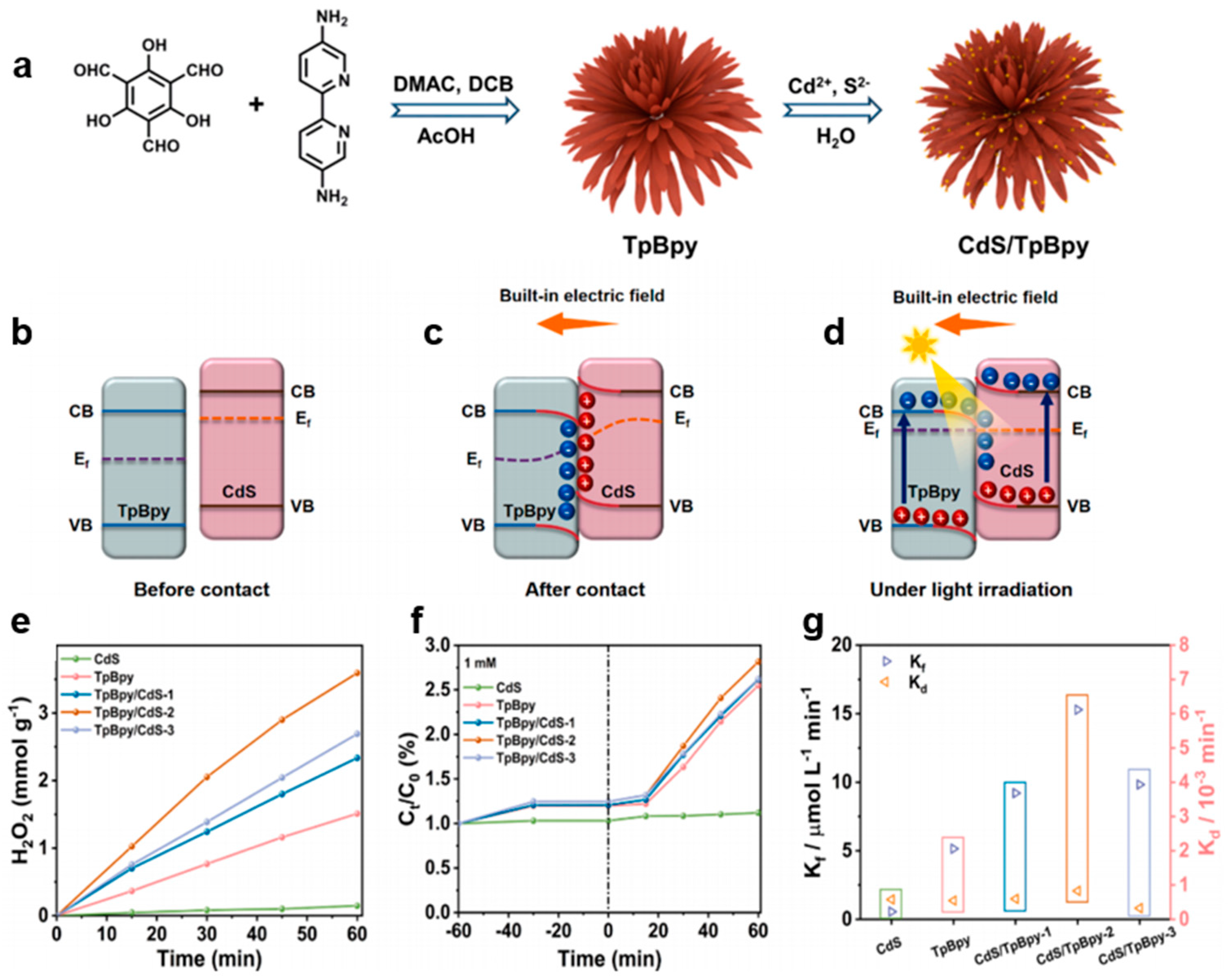

| Photocatalysts | Irradiation Condition | Catalysts Amount | Reaction Solution | Reaction Pathway | H2O2 Generation Rate | SCC Efficiency (%) | AQY (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| TDB-COF | Simulated AM 1.5 G illumination | 10 mg | 10 mL water | Two-step 1e− ORR and two-step 1e− WOR | 723.5 μmol h−1 g−1 | 0.39% | 1.4% | [28] |
| FS-COFs | 300 W Xe lamp (λ > 420 nm) | 5 mg | 20 mL water | 2e− ORR | 3904.2 μmol h−1 g−1 | 6.21% | [29] | |
| TpDz-COF | 300 W Xe lamp (λ > 420 nm) | 3 mg | 18 mL water | 2e− ORR | 7327 μmol h−1 g−1 | 0.62% | 11.9% | [30] |
| CTF-EDDBN | 300 W Xe lamp (λ > 420 nm) | 30 mg | 50 mL water | 2e− ORR and 2e− WOR | 54 μmol h−1 g−1 | 0.07% | [31] | |
| CTF-BDDBN | 300 W Xe lamp (λ > 420 nm) | 30 mg | 50 mL water | 2e− ORR and 2e− WOR | 97 μmol h−1 g−1 | 0.14% | [31] | |
| TTF-BT-COF | 300 W Xe lamp (λ > 420 nm) | 5 mg | 10 mL water | Two-step 1e− ORR and two-step 1e− WOR | 2760 μmol h−1 g−1 | 0.49% | 11.19% | [32] |
| TD-COF | LED (400–700 nm, 100 mW cm−2) | 1 mg | 4 mL seawater | Two-step 1e− ORR and 2e− WOR | 3364 μmol h−1 g−1 | 0.15% | [33] | |
| TT-COF | LED (400–700 nm, 100 mW cm−2) | 1 mg | 4 mL seawater | Two-step 1e− ORR and 2e− WOR | 2890 μmol h−1 g−1 | 0.14% | [33] | |
| TAPT-TFPA COFs@Pd-ICs | 300 W Xe lamp (λ > 400 nm) | 10 mg | 20 mL 10% ethanol water | Two-step 1e− ORR | 2143 μmol h−1 g−1 | 0.82% | 6.5% | [34] |
| HEP-TAPT-COF | 300 W Xe lamp (λ > 420 nm) | 50 mg | 100 mL water | 2e− ORR | 87.50 μmol h−1 | 0.65% | 15.35% | [35] |
| HEP-TAPB-COF | 300 W Xe lamp (λ > 420 nm) | 50 mg | 100 mL water | 2e− ORR | 49.50 μmol h−1 | 0.38% | 9.98% | [35] |
| TaptBtt-COF | 300 W Xe lamp (λ > 420 nm) | 15 mg | 50 mL water | Two-step 1e− ORR and 2e− WOR | 1407 μmol h−1 g−1 | 0.296% | 4.6% | [36] |
| CdS/TpBpyCOF | 300 W Xe lamp (λ > 420 nm) | 10 mg | 50 mL water | Two-step 1e− ORR | 3600 μmol h−1 g−1 | 13.4% | [37] | |
| COF- NUST -16 | 300 W Xe lamp (λ > 420 nm) | 5 mg | 50 mL 10% ethanol water | Two-step 1e− ORR and Two-step 1e− WOR | 1081 μmol h−1 g−1 | [38] | ||
| MIL-125-NH2 | 500 W Xe lamp (λ > 420 nm) | 5 mg | Acetonitrile/water (4:1) 5 mL | Two-step 1e− ORR | 8 mM h−1 | [39] | ||
| MIL-125-R7 | visible light (λ > 420 nm) | 5 mg | Water/benzyl alcohol (2:5) 7 mL | Two-step 1e− ORR | about 400 μM h−1 | [40] | ||
| TiO2/In2S3 | 300 W Xe lamp | 20 mg | 40 mL 10% ethanol water | Two-step 1e− ORR | 376 μmol h−1 L−1 | 3.42% | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, J.; Huang, Y.; Wang, X.; Liao, Y.; Zhang, H.; Dai, W. Photocatalytic Production of Hydrogen Peroxide from Covalent-Organic-Framework-Based Materials: A Mini-Review. Catalysts 2024, 14, 429. https://doi.org/10.3390/catal14070429
Meng J, Huang Y, Wang X, Liao Y, Zhang H, Dai W. Photocatalytic Production of Hydrogen Peroxide from Covalent-Organic-Framework-Based Materials: A Mini-Review. Catalysts. 2024; 14(7):429. https://doi.org/10.3390/catal14070429
Chicago/Turabian StyleMeng, Jiayi, Yamei Huang, Xinglin Wang, Yifan Liao, Huihui Zhang, and Weilin Dai. 2024. "Photocatalytic Production of Hydrogen Peroxide from Covalent-Organic-Framework-Based Materials: A Mini-Review" Catalysts 14, no. 7: 429. https://doi.org/10.3390/catal14070429






