Dependence of the Fluidizing Condition on Operating Parameters for Sorption-Enhanced Methanol Synthesis Catalyst and Adsorbent
Abstract
:1. Introduction
2. Results
2.1. Solid Characterization
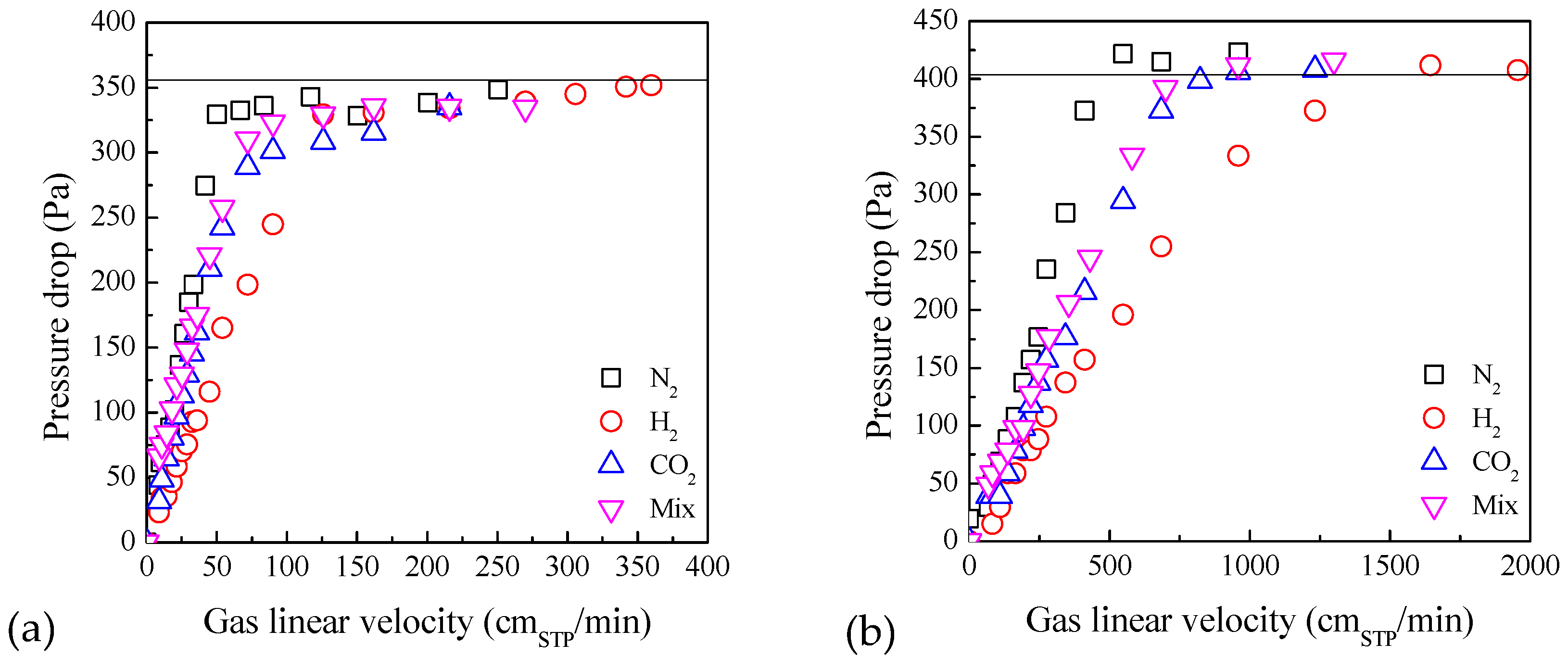
2.2. Influence of Gas Type on Fluidization
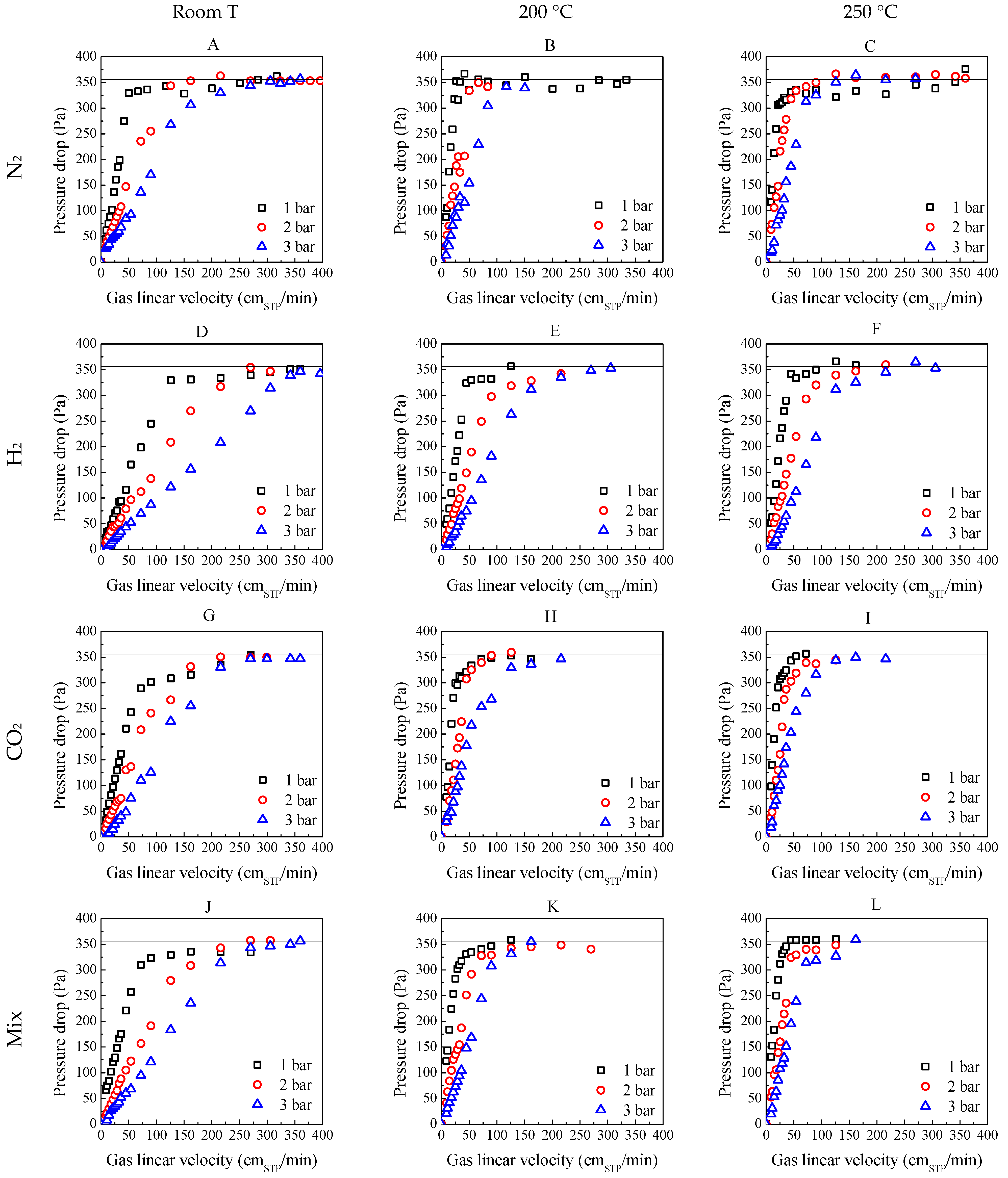
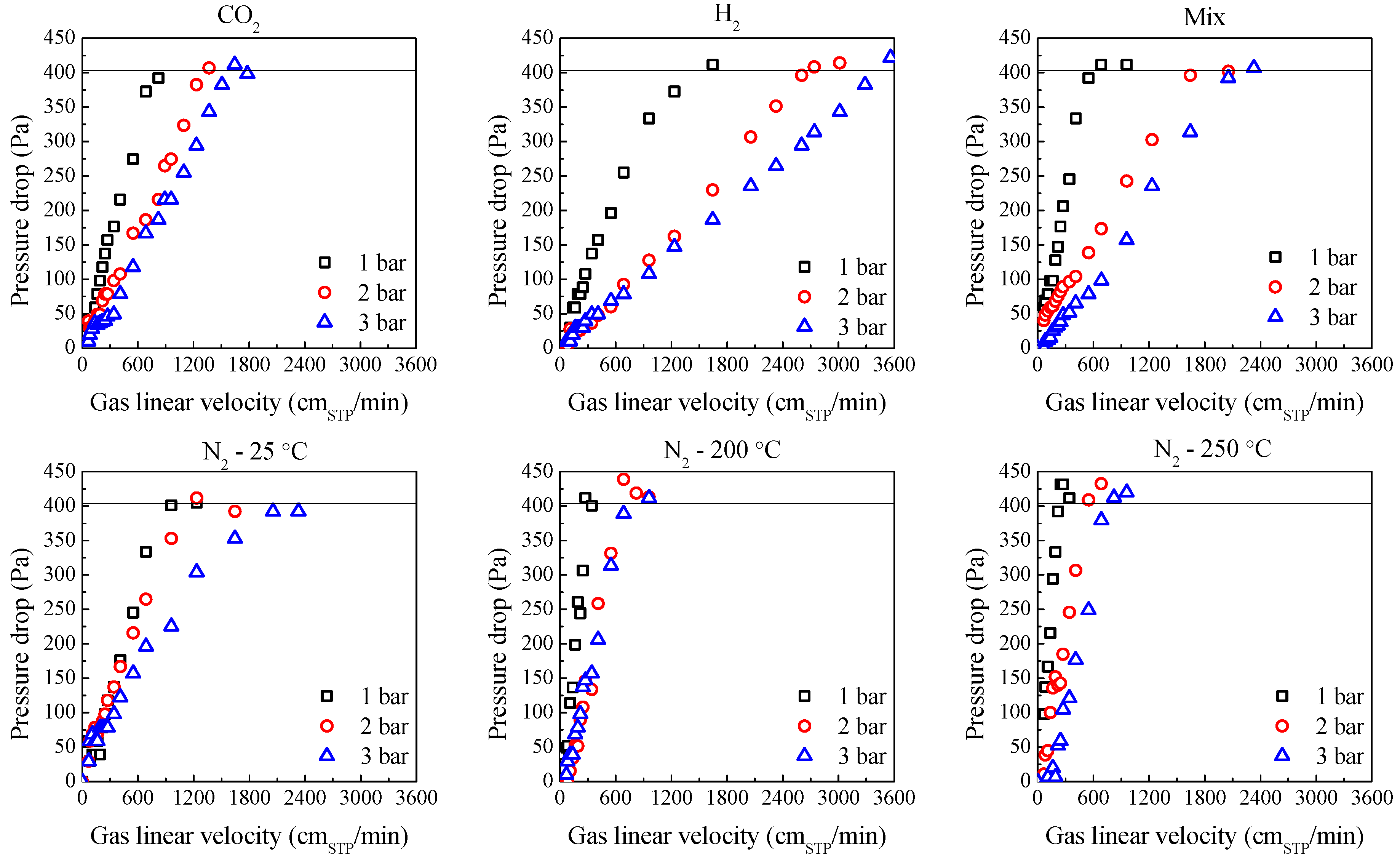
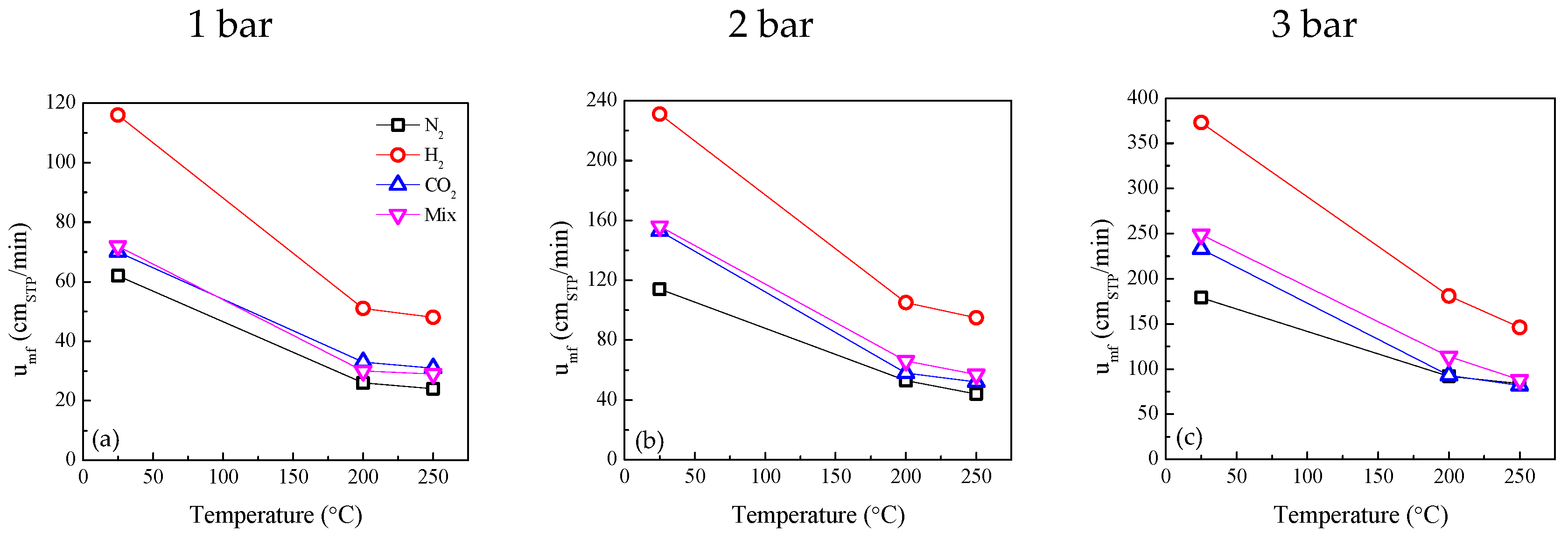
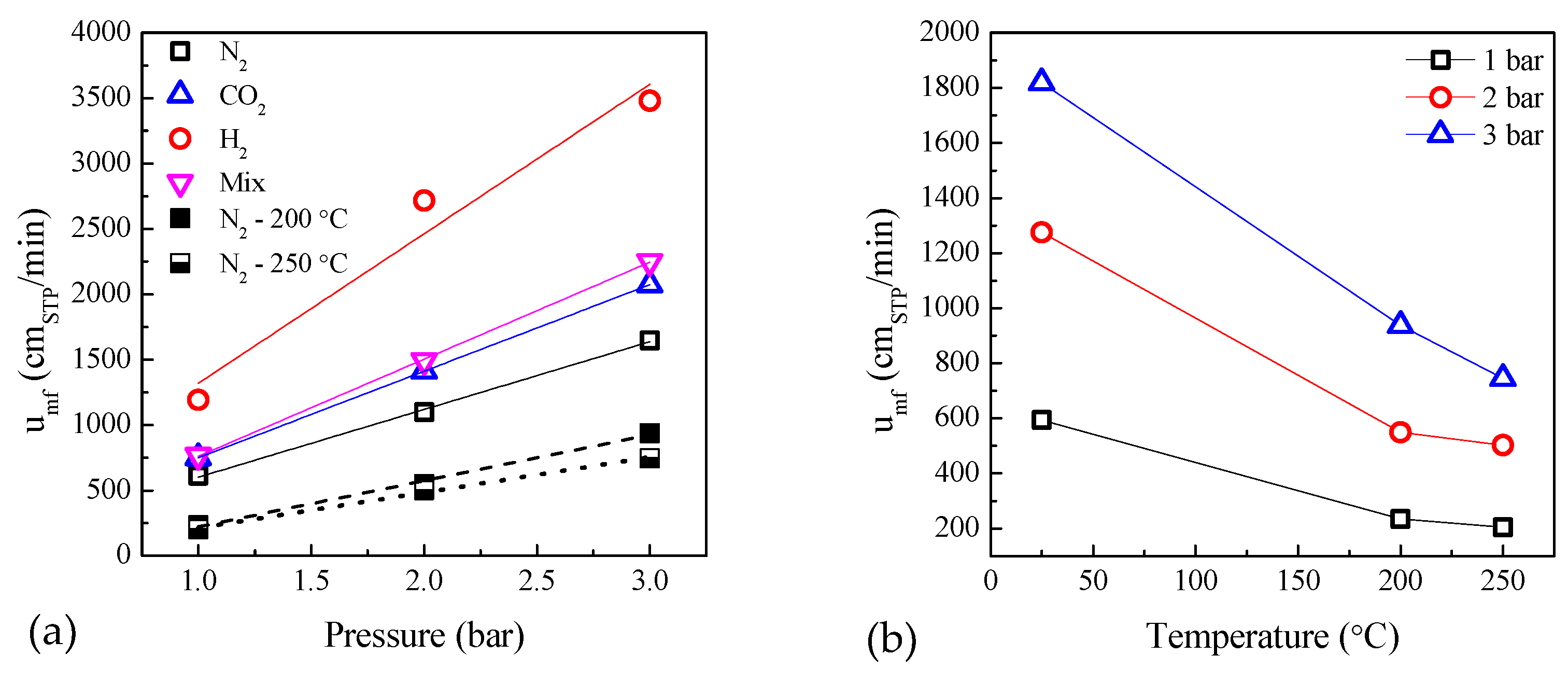
2.3. Influence of Temperature and Pressure on Fluidization
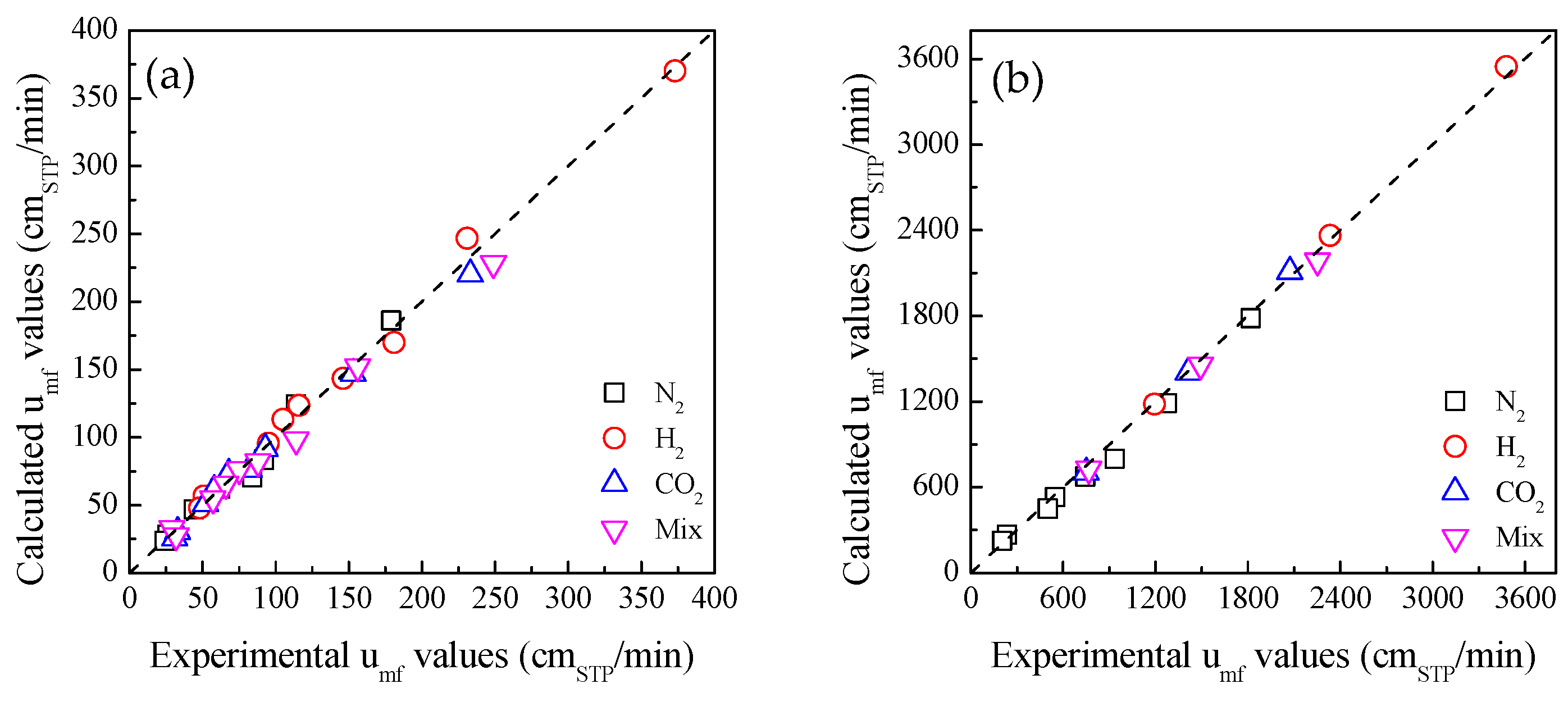
3. Discussion and Theoretical Analysis
Example Application
4. Materials and Methods
4.1. Solid Preparation
4.2. Solid Characterization
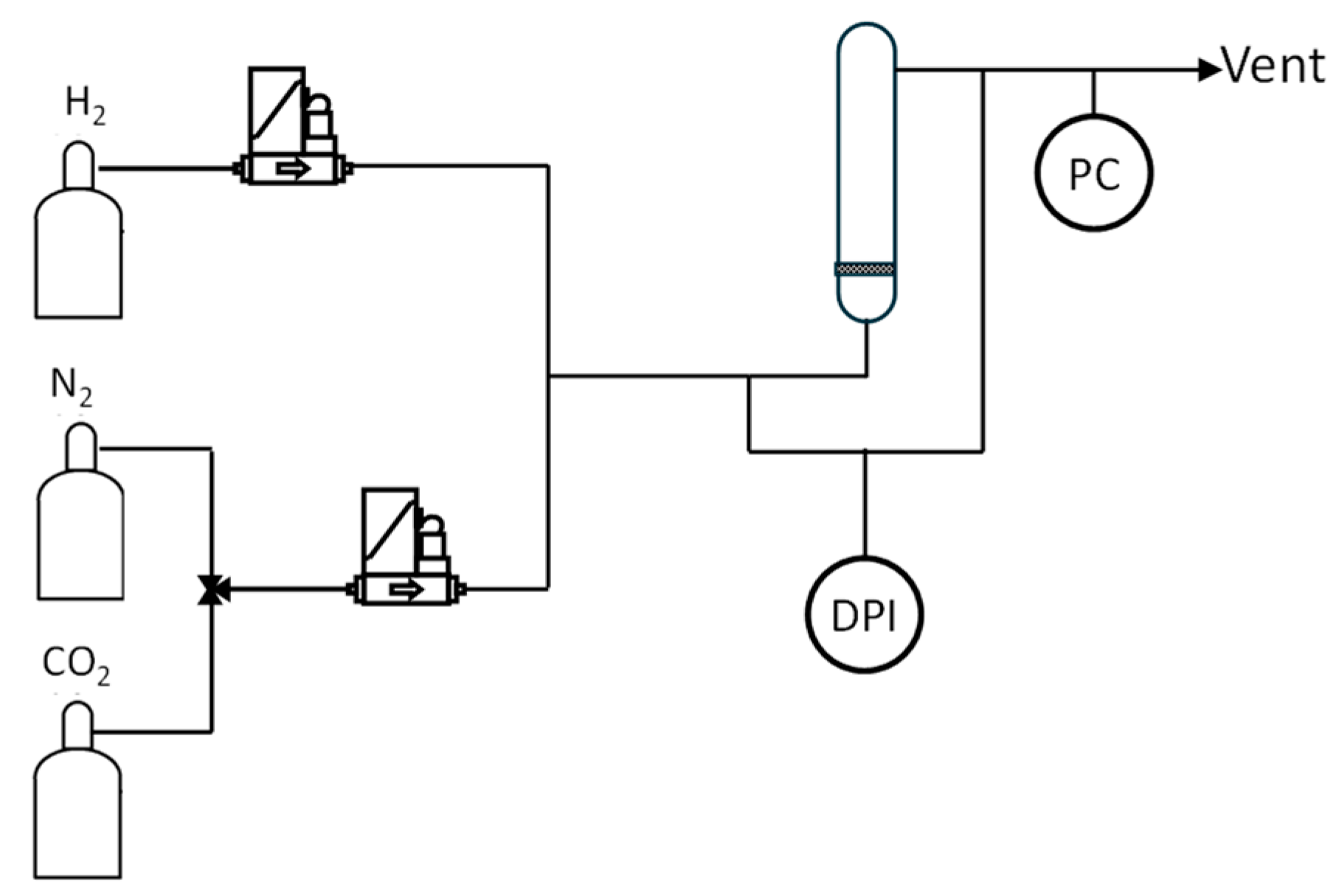
4.3. Experimental Setup
4.4. Experimental Procedure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Symbols
| εmf | void fraction at minimum fluidization | - |
| ΔP | pressure drop | Pa |
| φ | particle sphericity | - |
| μ | fluid viscosity | mPa *s |
| ρp | particle density | g/cm3 |
| ρf | fluid density | g/cm3 |
| ρc | fluid density at critical point | g/cm3 |
| ρr | relative density ρf/ρc | - |
| dp | particle diameter | cm |
| g | gravity acceleration | m/s2 |
| Hmf | expanded bed height at minimum fluidization | cm |
| Hpack | packed bed height | cm |
| M | molecular weight | g/mol |
| Pc | critical pressure | - |
| Remf | Reynolds number at minimum fluidization | - |
| T | temperature | K * |
| Tc | critical temperature | - |
| umf | minimum fluidization velocity | cm/s |
| Zc | acentric factor at critical point | - |
| * if not indicated otherwise | ||
| subscripts | ||
| 0 | actual condition (u0 indicates the considered value of gas velocity) | |
| c | critical conditions | |
| i, j | generic rotation index; indicates i species, j species | |
| f | fluid (of a property relative to the fluid) | |
| mf | minimum fluidization | |
| p | particle (of a property relative to the particle) | |
| pack | in packed bed conditions | |
| r | relative property (property at defined T,P/property at critical conditions) | |
| Ref | reference conditions | |
| s | standard conditions (273 K and 100 kPa) | |
List of Abbreviations
| DPI | differential pressure indicator |
| HBP | heavy-big-packed |
| ID | internal diameter |
| LSF | light-small-fluid |
| PC | pressure controller |
| PSD | particle size distribution |
| STP | standard temperature and pressure |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
Appendix A
| Zeolite dp (μm) | Catalyst dp (μm) | |
| Mass average diameter | 118.6 | 330.3 |
| Surface average diameter | 104.5 | 333.9 |
| Arithmetic mean | 112.5 | 325 |
| Weighted arithmetic mean | 120.8 | 333.2 |
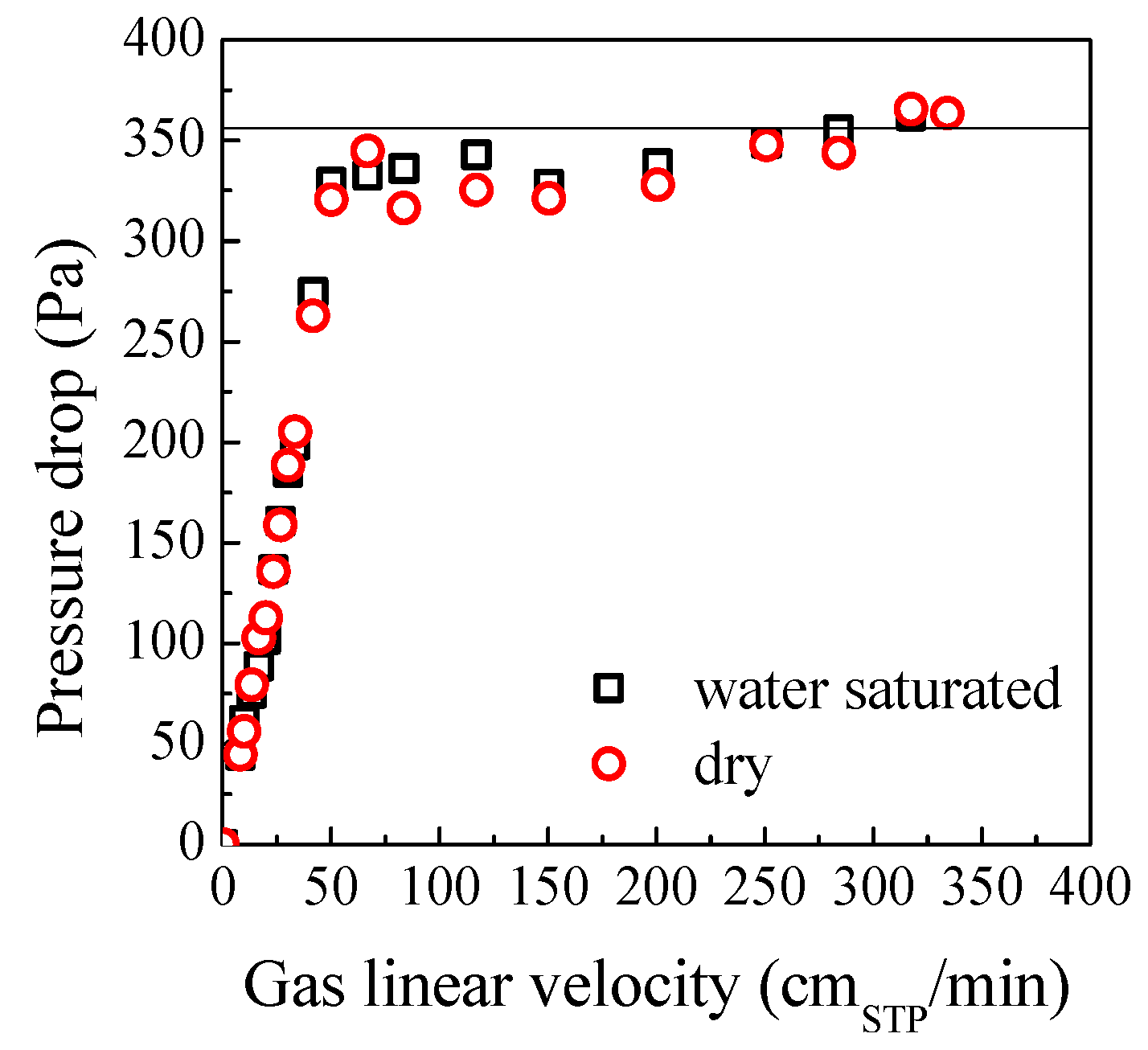
| Experimental | Calculated | |||||||
|---|---|---|---|---|---|---|---|---|
| P (bar) | P (bar) | |||||||
| T (°C) | 1 | 2 | 3 | T (°C) | 1 | 2 | 3 | |
| N2 | 25 | 62 | 114 | 179 | 24 | 124 | 186 | |
| 200 | 26 | 53 | 92 | 200 | 28 | 56 | 83 | |
| 250 | 24 | 44 | 84 | 250 | 23 | 47 | 70 | |
| CO2 | 25 | 68 | 153 | 233 | 24 | 73 | 147 | 220 |
| 200 | 33 | 58 | 93 | 200 | 31 | 61 | 92 | |
| 250 | 31 | 52 | 82 | 250 | 25 | 51 | 76 | |
| H2 | 25 | 116 | 231 | 373 | 24 | 123 | 247 | 370 |
| 200 | 51 | 105 | 181 | 200 | 57 | 113 | 170 | |
| 250 | 48 | 95 | 146 | 250 | 48 | 96 | 143 | |
| Mix | 25 | 75 | 156 | 249 | 24 | 76 | 152 | 228 |
| 200 | 29 | 66 | 114 | 200 | 33 | 65 | 98 | |
| 250 | 32 | 57 | 88 | 250 | 27 | 55 | 82 | |
| Experimental | Calculated | |||||||
|---|---|---|---|---|---|---|---|---|
| P (bar) | P (bar) | |||||||
| T (°C) | 1 | 2 | 3 | T (°C) | 1 | 2 | 3 | |
| N2 | 25 | 594 | 1275 | 1819 | 25 | 1188 | 1782 | |
| 200 | 235 | 549 | 937 | 200 | 266 | 533 | 799 | |
| 250 | 204 | 502 | 745 | 250 | 224 | 449 | 673 | |
| CO2 | 25 | 750 | 1412 | 2073 | 25 | 703 | 1406 | 2109 |
| H2 | 25 | 1193 | 2334 | 3479 | 25 | 1182 | 2363 | 3545 |
| Mix | 25 | 767 | 1492 | 2251 | 25 | 728 | 1456 | 2185 |
References
- Menéndez, M.; Herguido, J.; Bérard, A.; Patience, G.S. Experimental Methods in Chemical Engineering: Reactors—Fluidized Beds. Can. J. Chem. Eng. 2019, 97, 2383–2394. [Google Scholar] [CrossRef]
- Acosta-López, J.G.; de Lasa, H. Artificial Intelligence for Hybrid Modeling in Fluid Catalytic Cracking (FCC). Processes 2024, 12, 61. [Google Scholar] [CrossRef]
- Bin Sulayman, A.; Torres Brauer, N.; de Lasa, H. A Fluidizable Catalyst for N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions. Catalysts 2023, 13, 1462. [Google Scholar] [CrossRef]
- Rojas Chaves, F.; Torres Brauer, N.; Torres, C.; de Lasa, H. Conversion of Biomass-Derived Tars in a Fluidized Catalytic Post-Gasification Process. Catalysts 2024, 14, 202. [Google Scholar] [CrossRef]
- Kaushal, P.; Abedi, J. A Simplified Model for Biomass Pyrolysis in a Fluidized Bed Reactor. J. Ind. Eng. Chem. 2010, 16, 748–755. [Google Scholar] [CrossRef]
- Pang, L.; Shao, Y.; Zhong, W.; Liu, H. Experimental Investigation on the Coal Combustion in a Pressurized Fluidized Bed. Energy 2018, 165, 1119–1128. [Google Scholar] [CrossRef]
- Basu, P. Combustion of Coal in Circulating Fluidized-Bed Boilers: A Review. Chem. Eng. Sci. 1999, 54, 5547–5557. [Google Scholar] [CrossRef]
- Rüdisüli, M.; Schildhauer, T.J.; Biollaz, S.M.A.; Van Ommen, J.R. Scale-up of Bubbling Fluidized Bed Reactors—A Review. Powder Technol. 2012, 217, 21–38. [Google Scholar] [CrossRef]
- Kim, S.D.; Kangt, Y. Heat and Mass Transfer in Three-Phase Fluidized-Bed Reactors-an Overview. Chem. Eng. Sci. 1997, 52, 3639–3660. [Google Scholar] [CrossRef]
- Khadilkar, A.; Rozelle, P.L.; Pisupati, S.V. Models of Agglomerate Growth in Fluidized Bed Reactors: Critical Review, Status and Applications. Powder Technol. 2014, 264, 216–228. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Stoneham, MA, USA, 1991; ISBN 0409902330. [Google Scholar]
- Wu, S.Y.; Baeyens, J. Effect of Operating Temperature on Minimum Fluidization Velocity. Powder Technol. 1991, 67, 217–220. [Google Scholar] [CrossRef]
- Bi, H.T.; Grace, J.R. Effects of Pressure and Temperature on Flow Regimes in Gas-Solid Fluidization Systems. Can. J. Chem. Eng. 1996, 74, 1025–1027. [Google Scholar] [CrossRef]
- Liu, Z.; Kawaguchi, T.; Tanaka, T.; Tsuji, Y. The Effect of Temperature on the Minimum Fluidization Velocity Calculated by Distinct Element Method. JSME Int. J. 2002, 45, 66–71. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Effect of Temperature on Fluidization of Geldart’s Group A and C Powders: Role of Interparticle Forces. Ind. Eng. Chem. Res. 2017, 56, 12811–12821. [Google Scholar] [CrossRef]
- Gosavi, S.; Kulkarni, N.; Mathpati, C.S.; Mandal, D. CFD Modeling to Determine the Minimum Fluidization Velocity of Particles in Gas-Solid Fluidized Bed at Different Temperatures. Powder Technol. 2018, 327, 109–119. [Google Scholar] [CrossRef]
- Chirone, R.; Poletto, M.; Barletta, D.; Lettieri, P. The Effect of Temperature on the Minimum Fluidization Conditions of Industrial Cohesive Particles. Powder Technol. 2020, 362, 307–322. [Google Scholar] [CrossRef]
- Gorbe, J.; Lasobras, J.; Francés, E.; Herguido, J.; Menéndez, M.; Kumakiri, I.; Kita, H. Preliminary Study on the Feasibility of Using a Zeolite A Membrane in a Membrane Reactor for Methanol Production. Sep. Purif. Technol. 2018, 200, 164–168. [Google Scholar] [CrossRef]
- Raso, R.; Tovar, M.; Lasobras, J.; Herguido, J.; Kumakiri, I.; Araki, S.; Menéndez, M. Zeolite Membranes: Comparison in the Separation of H2O/H2/CO2 Mixtures and Test of a Reactor for CO2 Hydrogenation to Methanol. Catal. Today 2021, 364, 270–275. [Google Scholar] [CrossRef]
- Menéndez, M.; Herguido, J.; Soler, J.; Lasobras, J. Reactor System for Sorption-Enhanced Catalytic Reactions with Continuous Regeneration of Sorbent and Related Methods. Patent Application EP23382685.8, 2023. [Google Scholar]
- Trifan, B.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Modifications in the Composition of CuO/ZnO/Al2O3 Catalyst for the Synthesis of Methanol by CO2 Hydrogenation. Catalysts 2021, 11, 774. [Google Scholar] [CrossRef]
- Mohamed, S.H. Transparent Conductive Gallium-Doped Indium Oxide Nanowires for Optoelectronic Applications. J. Korean Phys. Soc. 2013, 62, 902–905. [Google Scholar] [CrossRef]
- Ergun, S.; Orning, A.A. Fluid Flow through Randomly Packed Columns and Fluidized Beds. Ind. Eng. Chem. 1949, 41, 1179. [Google Scholar] [CrossRef]
- Li, L.; Duan, Y.; Duan, L.; Xu, C.; Anthony, E.J. Flow Characteristics in Pressurized Oxy-Fuel Fluidized Bed under Hot Condition. Int. J. Multiph. Flow 2018, 108, 1–10. [Google Scholar] [CrossRef]
- Sidorenko, I.; Rhodes, M.J. Influence of Pressure on Fluidization Properties. Powder Technol. 2004, 141, 137–154. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Z.; Zhong, W.; Bian, Z.; Yu, A. Minimum Fluidization Velocity of Particles with Different Size Distributions at Elevated Pressures and Temperatures. Chem. Eng. Sci. 2020, 216, 115555. [Google Scholar] [CrossRef]
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Jossi, J.A.; Stiel, L.I.; Thodos, G. The Viscosity of Pure Substances in the Dense Gaseous and Liquid Phases. AIChE J. 1962, 8, 59. [Google Scholar] [CrossRef]
- Davidson, T.A. A Simple and Accurate Method for Calculating Viscosity of Gaseous Mixtures; United States Department of the Interior, Bureau of Mines: Washington, DC, USA, 1993. [Google Scholar]
- Wilke, C.R. A Viscosity Equation for Gas Mixtures. J. Chem. Phys. 1950, 18, 517–519. [Google Scholar] [CrossRef]
- Johnson, C.A.; Solomon, E.; Rubin, L.C.; Gerster, J.A.; Colburn, A.P.; Wohl, K.; Wang, Y.; Eng, Z.; Wen, C.Y.; Yu, Y.H. A Generalized Method for Predicting the Minimum Fluidization Velocity. Wohl K. Trans. Am. Inst. Chem. Eng. 1953, 47, 271–272. [Google Scholar]
- Grace, J.R. Modelling and Simulation of Two-Phase Fluidized Bed Reactors. In Chemical Reactor Design and Technology; Springer: Dordrecht, The Netherlands, 1986; p. 245. [Google Scholar]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. 2016, 128, 6369–6373. [Google Scholar] [CrossRef]
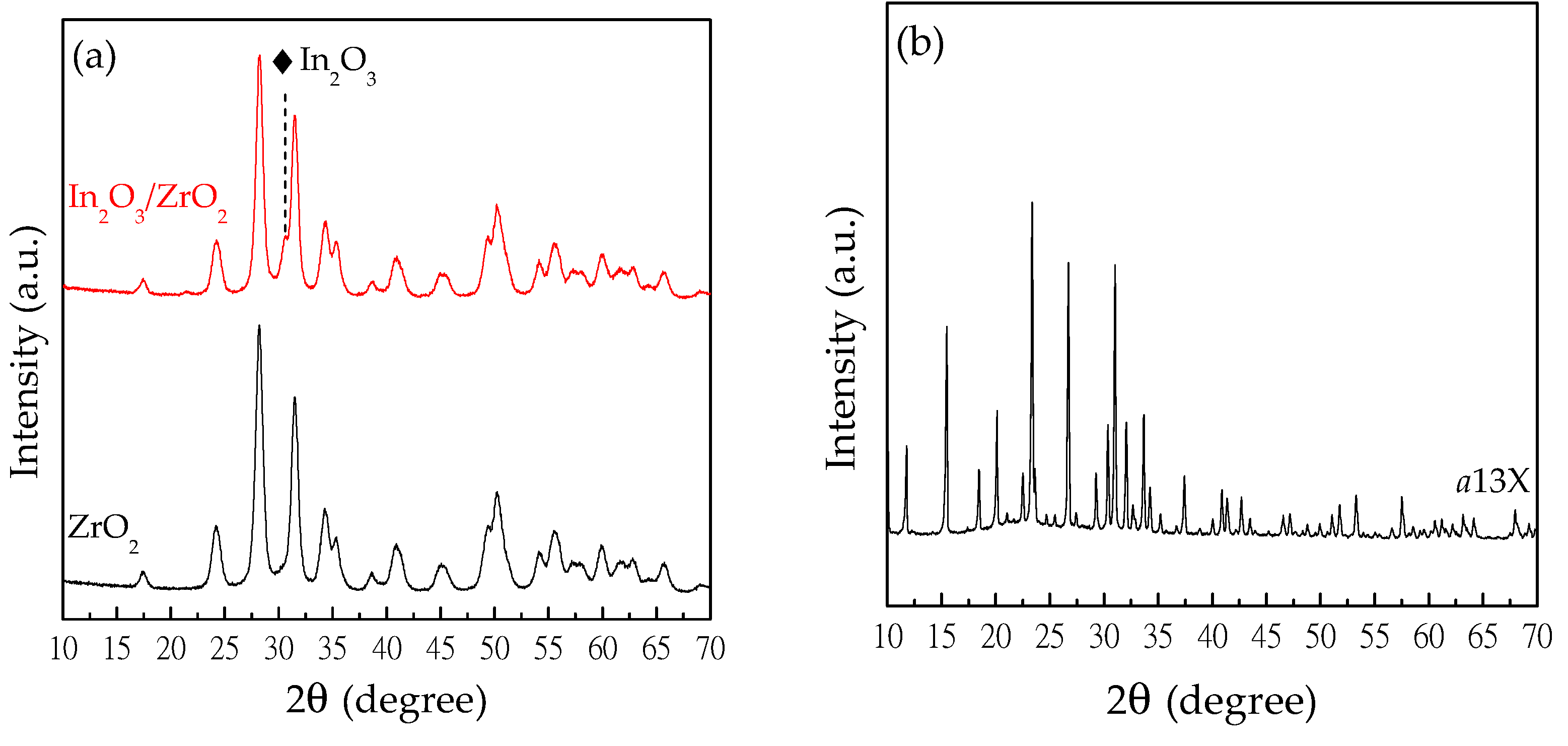

| 13X | a13X | |
|---|---|---|
| SiO2 | 49.91 | 60.92 |
| Al2O3 | 31.42 | 26.25 |
| Na2O | 16.97 | 13.94 |
| Si/Al ratio | 1.40 | 2.05 |
| Added Si (g/100 g) | 8.98 | |
| Added SiO2 (g/100 g) | 19.2 |
| umf (cmSTP/min) | εmf * | φp * | ρapp a (g/cm3) | ρp * (g/cm3) | ||||
|---|---|---|---|---|---|---|---|---|
| N2 | H2 | CO2 | Mix | |||||
| Cat. | 594 | 1148 | 750 | 767 | 0.32 | 0.99 | 1.05 | 1.41 |
| a13X | 62 | 116 | 68 | 75 | 0.56 | 0.87 | 0.53 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renda, S.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Dependence of the Fluidizing Condition on Operating Parameters for Sorption-Enhanced Methanol Synthesis Catalyst and Adsorbent. Catalysts 2024, 14, 432. https://doi.org/10.3390/catal14070432
Renda S, Lasobras J, Soler J, Herguido J, Menéndez M. Dependence of the Fluidizing Condition on Operating Parameters for Sorption-Enhanced Methanol Synthesis Catalyst and Adsorbent. Catalysts. 2024; 14(7):432. https://doi.org/10.3390/catal14070432
Chicago/Turabian StyleRenda, Simona, Javier Lasobras, Jaime Soler, Javier Herguido, and Miguel Menéndez. 2024. "Dependence of the Fluidizing Condition on Operating Parameters for Sorption-Enhanced Methanol Synthesis Catalyst and Adsorbent" Catalysts 14, no. 7: 432. https://doi.org/10.3390/catal14070432











