Abstract
Developing highly active and available, environmentally friendly, and low-cost photocatalytic materials is one of the most popular topics in photocatalytic degradation systems. In the present study, a series of BiOCl/Sepiolite composite photocatalysts were prepared (in the range of 5%BiOCl/Sepiolite–30%BiOCl/Sepiolite). Their characterization was conducted using X-ray diffraction, diffuse reflectance spectroscopy, scanning electron microscopy, nitrogen physical physisorption at the temperature of liquid nitrogen (77 K), and attenuated total reflectance-Fourier transform infrared spectroscopy. Results showed that composite photocatalysts possess superior efficiency than the parent materials for losartan, an antihypertensive agent, degradation in water, with the sample with only 10%wt. BiOCl shows the highest performance. The beneficial effect of the addition of sepiolite to BiOCl is derived from the increase in surface area, the prevention of particle aggregation, and the efficient separation of photogenerated species. Increasing catalyst concentration from 125 mg/L up to 500 mg/L was accompanied by an increase in the apparent kinetic constant from 0.077 min−1 to 0.197 min−1 while varying losartan concentration from 0.25 to 5.00 mg/L slowed down the removal efficiency. In addition, losartan degradation was only partially hampered in the case of bottled water, whereas it was practically stopped in a secondary wastewater effluent. Overall, this study serves as a useful guide for using geopolymers in photocatalytic applications.
1. Introduction
Even though photocatalytic efficiencies have not improved over the past ten years, interest in an entirely green technology such as photocatalysis remains unchanged. This is evident if one takes a quick look at the number of published papers, which shows an ever-increasing trend [1,2]. In specific, driven by the need for efficient and sustainable solutions for environmental and energy challenges, scientists developed innovative photocatalytic materials with tunable physicochemical properties. Semiconductor heterojunctions with graphitic carbon nitride [3] and incorporation of MOF-derived carbon materials [4] and plasmonic nanorods [5] in photocatalytic synthesis are only some characteristic examples. In addition, special emphasis has been placed on the development and integration of low-cost and widely available materials into photocatalytic systems so that they are also compatible with the principles imposed by the circular economy. Such materials include biochar [6], natural organic pigments [7], or geopolymers [8]. On this basis, sepiolite has been incorporated into alternative photocatalytic material synthesis [9]. Sepiolite is a fibrous clay mineral primarily composed of hydrated magnesium silicate. Its unique fibrous structure and high surface area make it useful in various environmental applications, including photocatalysis. Sepiolite’s porous structure not only increases the surface available catalytic sites but also improves the optical properties of the semiconductors that are usually conjugated with [10]. Recently, its presence in composite photocatalytic systems has gained significant interest as it can contribute to the overall enhancement of photocatalytic performance. For this, researchers have investigated different techniques for integrating photocatalytic materials, like titanium dioxide or alternative metal oxides, onto sepiolite surfaces [11,12]. Additionally, photocatalysts based on sepiolite provide benefits such as stability, reusability, and low-cost synthesis compared to using solely photocatalytic substances [13]. These sepiolite/TiO2 or sepiolite/metal oxides composite materials have demonstrated improved efficiency in photocatalytic degradation systems for water purification.
In recent years, special emphasis has been placed on upgrading the existing water treatment systems in order to be effective in terms of emerging pollutants (EMPs) degradation. EMPs are a class of natural or synthetic compounds detected in very low amounts (nanograms or micrograms per liter) in the aquatic environment, such as pharmaceuticals, personal care products, pesticides, industrial chemicals, and even microplastics [14,15]. Wastewater treatment plants are probably the primary source of EMPs in aqueous media, with non-point resources also playing a significant role. On this basis, photocatalysis belonging to the group of Advanced oxidation processes (AOPs) has come out with great efficiencies [16]. For example, in quinolones, a class of wide-spectrum antibiotics, degradation efficiency reached 95.1% after three hours of visible light irradiation using metal-organic frameworks based on Fe as the photocatalytic material [17]. In addition, S. Mergenbayeva et al. studied sulfamethoxazole degradation in water in the presence of TiO2/zeolite composite materials with very promising results [18]. Ceftriaxone, another antibiotic agent that has been regularly found in surface water and groundwater, degradation in water was systematically studied using a series of supercritical antisolvent-micronized ZnO coupled with commercially available TiO2 under both visible and UV irradiation [19]. Results showed that Ceftriaxone was completely degraded after a very short irradiation time under UV irradiation, while a longer period was required under visible. In addition, X. Wei et al. chose tetracycline as the target compound. They reported the synthesis of Cr2O3/ZrO2 photocatalysts by a coprecipitation method, achieving greater than 97% removal with hydroxyl and superoxide radicals as the main oxidative species.
One common type of medication that has been widely used to treat high blood pressure in humans is losartan (LOS) [20]. As a result, surface water, untreated wastewater, treatment facilities, and groundwater have all been shown to contain LOS in significant amounts, ranging from nano to micrograms per liter [21,22]. Taking into consideration recent data reported by the World Health Organization (WHO), showing that up to 1.5 billion persons suffer from hypertension, it is evident that unless more efficient water purification systems are used immediately, LOS amount in water will increase dramatically in the next few years.
In this study, a batch of BiOCl/Sepiolite composite photocatalysts was prepared, varying the amount of sepiolite and BiOCl in the resulting material (5%BiOCl/Sepiolite, 10%BiOCl/Sepiolite, 20%BiOCl/Sepiolite, 30%BiOCl/Sepiolite). Their morphological and optical properties were determined in detail, and their efficiency was tested towards LOS degradation not only in ultrapure water but also in real and synthetic aqueous media of environmental concern. BiOCl has been extensively studied by our group, considering its photocatalytic properties, and it has shown very promising results [23,24,25]. BiOCl, a bismuth oxyhalide, is a highly researched photocatalytic material due to its advantageous properties. These include a unique layered structure, intrinsic defects like oxygen vacancies, chemical stability, and low toxicity. BiOCl possesses a layered structure characterized by [Bi2O2] slabs interleaved by double slabs of halogen atoms, which ensures sufficient space to polarize the relevant atoms and orbitals [26]. The conjunction with sepiolite is expected not only to lower synthesis costs but also to improve its performance. As far as we know, this is the first time that such a composite photocatalyst has been used for LOS degradation in water. The effect of many experimental parameters, as well as the photocatalyst’s stability and the possible photocatalytic mechanism, are discussed.
2. Results and Discussion
2.1. Crystallographic, Morphological and Surface Properties
The specific surface area (SSA) of the as-prepared samples was evaluated using the N2 adsorption-desorption method, employing the Brunauer-Emmett-Teller (BET) analysis. The results are presented in Table 1. The SSA value of pure sepiolite is significantly high (133 m2/g), indicating its porous nature [27]. In contrast, the specific surface area of BiOCl is considerably lower, estimated to be equal to 18 m2/g. As expected, the gradual deposition of BiOCl onto the sepiolite surface resulted in a progressive decrease in SSA values, mainly due to the partial coverage of sepiolite’s pores with BiOCl particles. This blockage reduces the effective surface area of the composites, as the pores of sepiolite that contribute to the high surface area are no longer accessible. Moreover, as the concentration of BiOCl increases, its particles may aggregate, leading to larger clusters characterized by lower SSA compared to dispersed particles. This can also lead to larger crystallite size with a smaller surface area-to-volume ratio.

Table 1.
Surface and structural analysis of the samples.
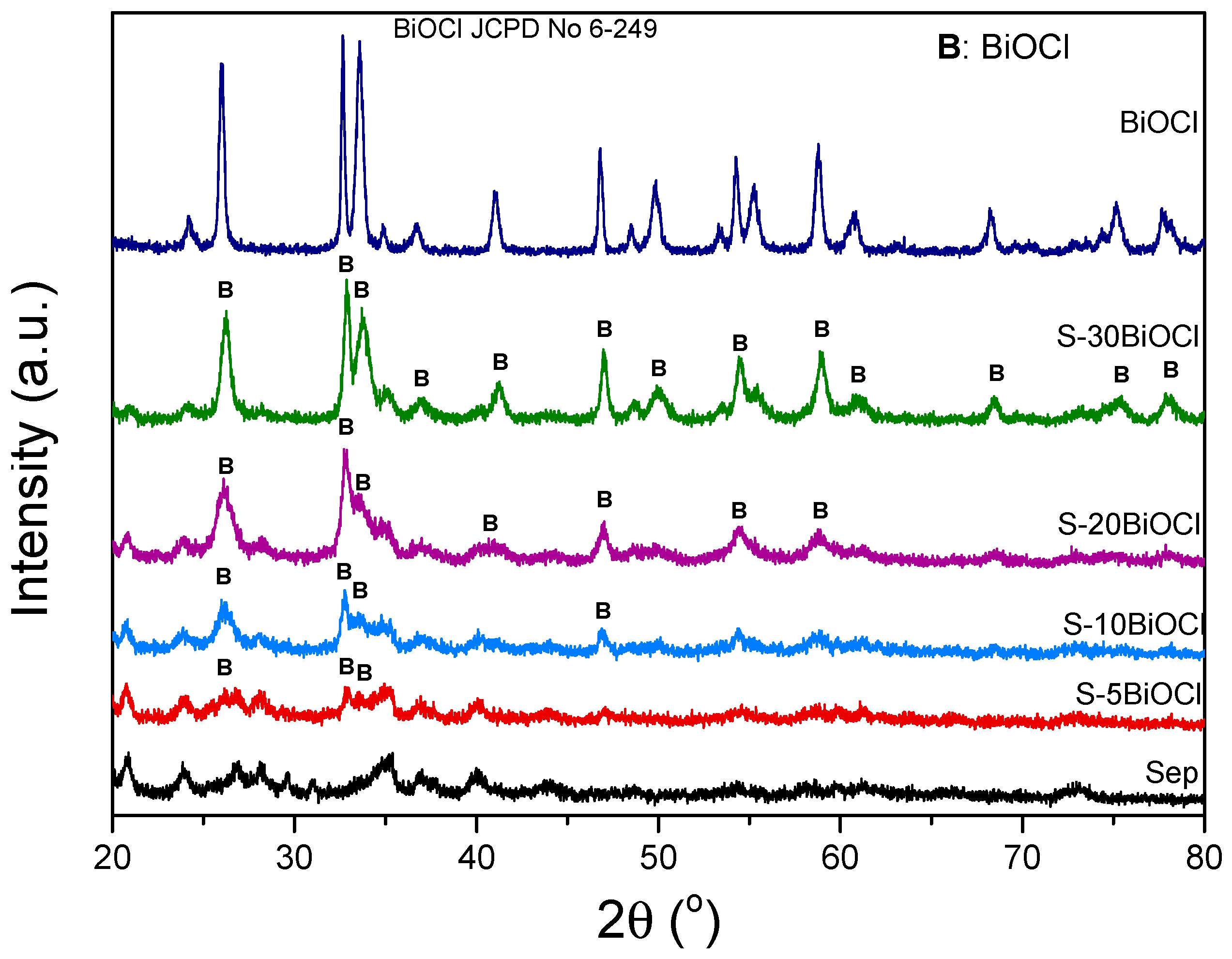
The crystalline structure of the pristine sepiolite, BiOCl, and composite BiOCl/Sepiolite photocatalysts was investigated by X-ray Diffraction (XRD) analysis, as illustrated in Figure 1. The XRD pattern of pure sepiolite exhibits distinct peaks located at 2θ = 20.45°, 24.12°, 27.38°, 34.72°, 36.72°, and 40.10° which correspond to the orthorhombic structure of the clay (JCPDS No. 29-1492). It should be noted that sepiolite exhibits another main peak at 2θ = 7.3° [28]. The XRD pattern of pristine BiOCl displays sharp and intense peaks (JCPDS No. 6-249), validating the formation of highly crystalline material. The deposition of BiOCl on the surface of the sepiolite led to the development of characteristic peaks positioned at 2θ = 25.95°, 32.60°, 33.60°, 46.00°, corresponding to (101), (110), (102) and (200) crystal planes. These observations corroborate the hypothesis of the successful incorporation of BiOCl on the sepiolite fibers. In addition, the primary crystallite size of BiOCl was calculated using the Debye–Scherrer equation, utilizing the line broadening of the (101) peak positioned at 2θ = 25.95°, and the values are summarized in Table 1. It is observed that while pure BiOCl consists of crystallites of approximately 25 nm diameter, the primary crystallite size of the S-10BiOCl sample is only ~6 nm. In general, it is perceived as a trend of larger crystallite size with higher BiOCl content.

Figure 1.
XRD patterns of sepiolite, BiOCl, and S–%x BiOCl (x = 5, 10, 20, 30 wt.%).
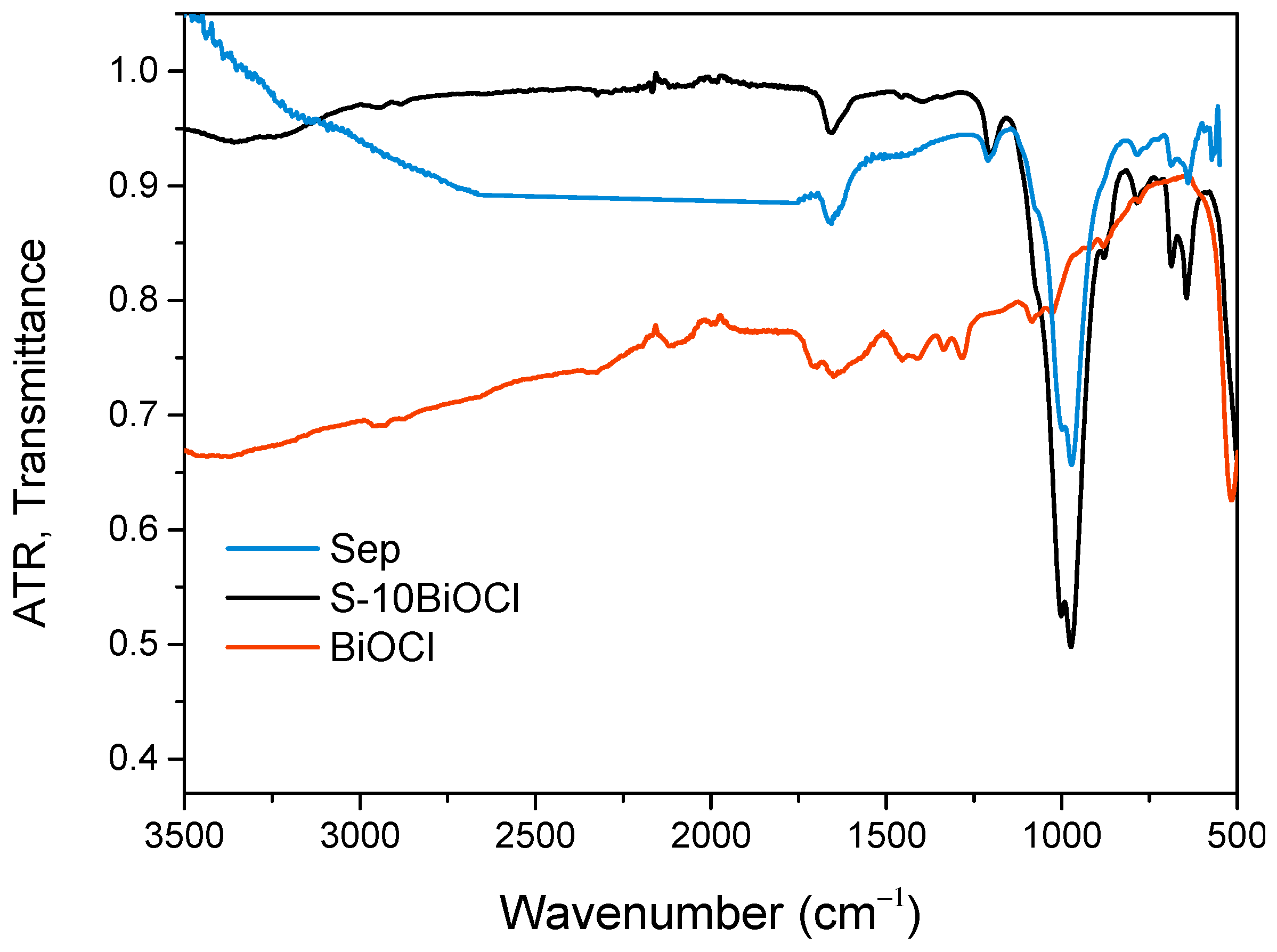
The ATR-FTIR spectra of sepiolite, BiOCl, and S-10BiOCl samples are presented in Figure 2. It should be noted that the ATR-FTIR spectra of the pure sepiolite have been published in a previous investigation [28]. From the ATR-FTIR spectra, it is evident that there is a low-intensity band at about 3680 cm−1 that corresponds to stretching (νOH) vibrations of hydroxyl groups in the octahedral sheet of sepiolite [29,30]. The band around 1010 cm−1 is due to the stretching of Si–O in the Si–O–Si groups of the tetrahedral sheet of sepiolite [31,32]. The bands at 680 cm−1 [29,30] and 780 cm−1 [30,33] are due to the vibration of OH and contract vibration of Si–O, respectively, of sepiolite.

Figure 2.
ATR-FTIR spectra of sepiolite, BiOCl, and S-10BiOCl.
The results indicate that the structure of sepiolite did not change significantly after the treatment (Figure 2), as expected, and the S structure remained largely unaffected. The intensity of all S bands is weakened after the treatment, as expected by Beer’s law [34]. Consequently, the ATR-FTIR study confirmed the XRD observations that S was not significantly affected by the nanocomposite synthesis.
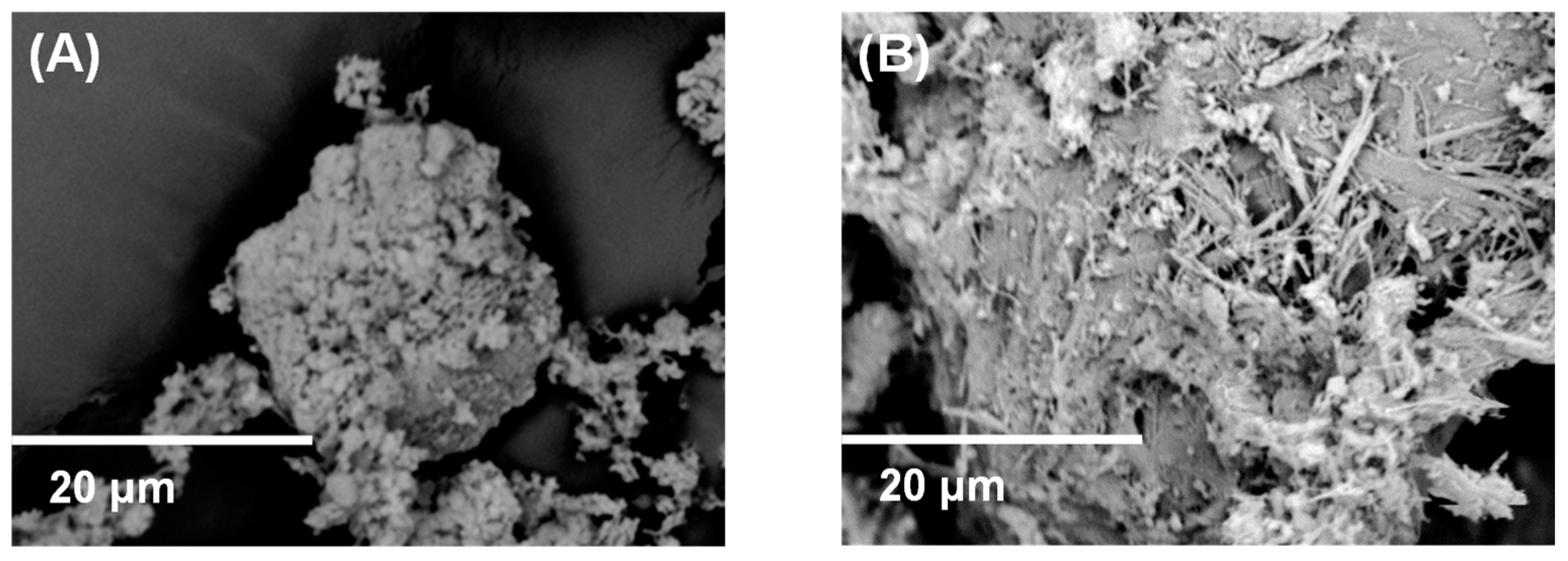
From the SEM study of the samples, there are several observations that are worthy of mention (Figure 3). Even though in the BiOCl sample, the magnification of the SEM was not high enough to reveal the exact particle size and morphology of BiOCl particles, it is evident that for this reason, the size of the particles is low enough to be characterized as nano. In the BiOCl/Sepiolite sample, the sepiolite nanofibers are observable, and their length is up to 10 microns or more, while their other dimensions are in the nanoscale. In some cases, over the sepiolite’s nanofibers, more or less spherical nanoparticles are observed that correspond to BiOCl nanoparticles.

Figure 3.
SEM Images of (A) BiOCl and (B) BiOCl/Sepiolite.
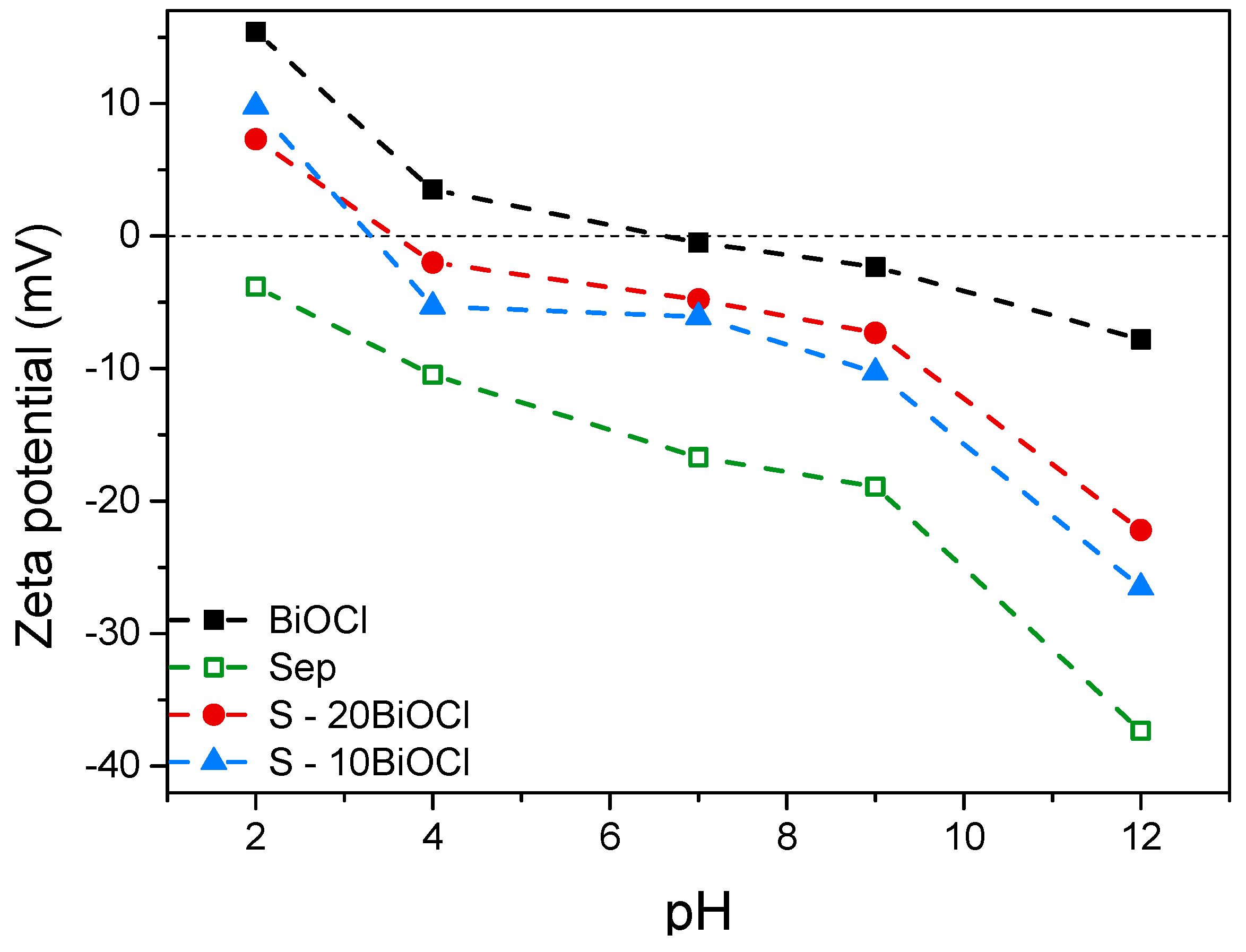
Zeta potential is a key parameter in understanding the surface charge properties of particles in colloidal systems, including photocatalytic materials, as it indicates the surface charge of the photocatalyst particles, which can influence the adsorption of pollutants on the surface during photocatalytic reactions. At pH values above the point of zero zeta potential (pzp), the surface typically becomes negatively charged, and below this point, it becomes positively charged. In the present study, the zeta potential of the photocatalysts as a function of pH was determined employing the Smoluchowski equation, and the results are presented in Figure 4 [35]. It is observed that BiOCl has a pzp ~6.70. As expected for a clay mineral, sepiolite exhibited a strong negative zeta potential across the entire pH range investigated, confirming the inherent negative charge associated with the sepiolite surface, likely arising from the deprotonation of the silanol (Si–OH) groups [27,28,36]. Results indicated that a reduced BiOCl content caused a significant shift of pzp to lower pH values. Specifically, the pzc was observed to alter from pH 6.70 for pure BiOCl to pH 3.30 for S-10ΒiOCl (Figure 4).

Figure 4.
Surface charge of sepiolite, BiOCl, S-20BiOCl, and S-10BiOCl as a function of pH.
2.2. Optical Properties
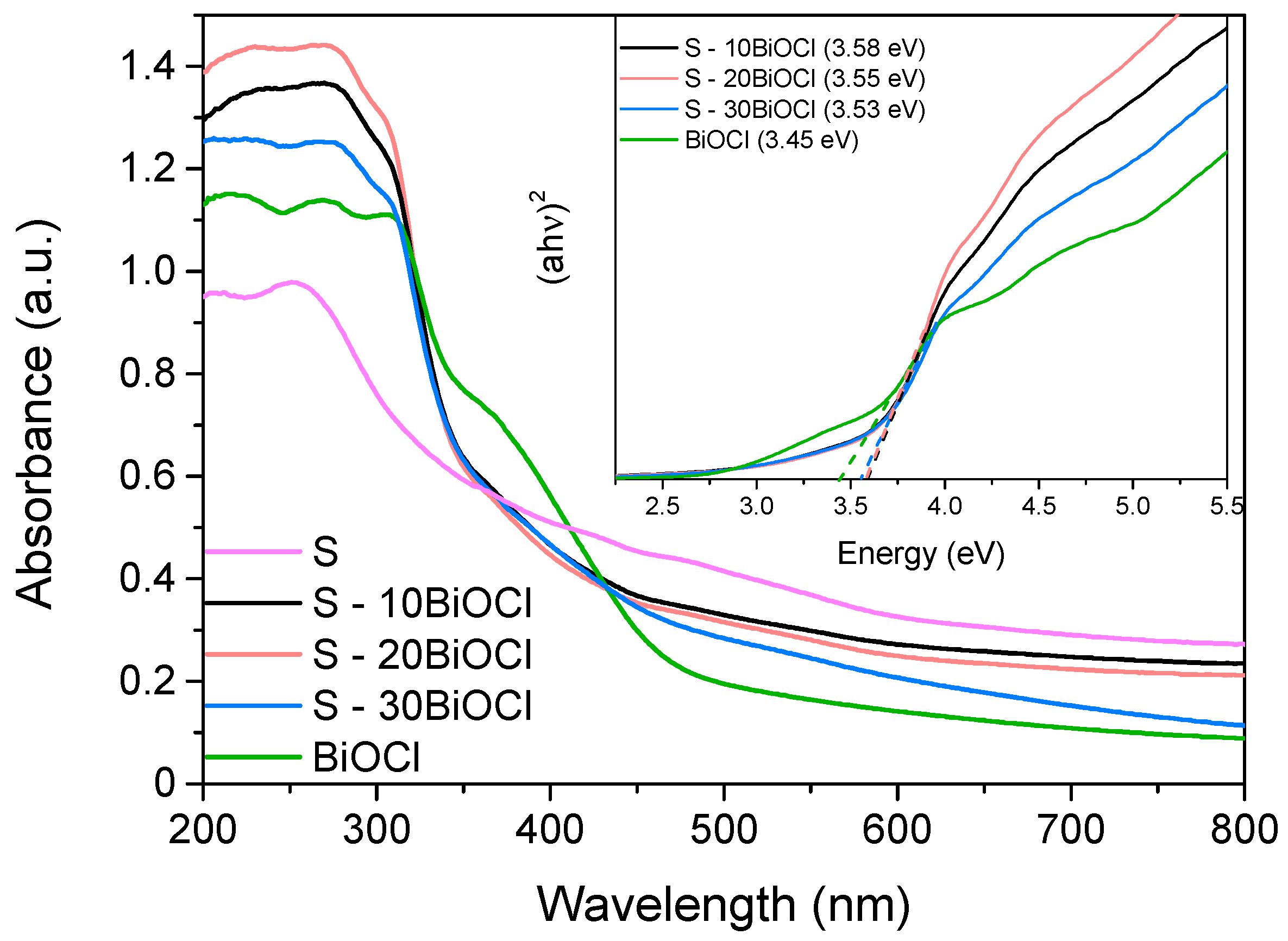
To assess the optical properties of the samples and determine the band gap energy (Ebg), diffuse reflectance spectroscopy in the UV and visible regions was utilized. The recorded spectra are depicted in Figure 5. It is observed that pure BiOCl is characterized by an absorption “tail” located at 495 nm, implying that it can absorb light mainly in the UV region and only at a small portion of the visible part of the solar spectrum. For the composite samples, this absorption “tail” is shifted to higher wavelengths approximately equal to 580 nm. Moreover, it is evident that coupling BiOCl with sepiolite resulted in enhanced absorption not only in the near UV and UV region (200–400 nm) but also in the visible part. These results may be attributed to the fact that the addition of sepiolite benefitted the formation of a framework that led to the development of BiOCl nanoparticles of smaller particle sizes that were highly distributed, thus, favoring the interaction with light [37]. This is in accordance with the primary crystallite size of the prepared samples as calculated with Debye–Scherrer equation (Table 1).

Figure 5.
UV-vis diffuse reflectance spectra of sepiolite, BiOCl, and the composite photocatalysts. Inset graph: Corresponding band gaps (Eg) estimated employing Tauc plot.
Despite this enhancement, the band gap energy (Eg) of both pristine BiOCl and composite materials, estimated using the Tauc plot method (inset graph), remained unchanged at approximately 3.50 eV, implying that the electronic properties of BiOCl are practically unaffected [38]. This behavior was expected since, in the composite samples, BiOCl and Sepiolite retained their intrinsic properties, with interfaces formed due to the fact that they were in close contact. In contrast, significant changes in Ebg take place only when new energy states are formed in the bulk of the composite photocatalyst due to “intense” conditions (high temperature or pressure, doping) taking place during the synthesis procedure [39].
2.3. Photocatalytic Activity
2.3.1. Screening Experiments
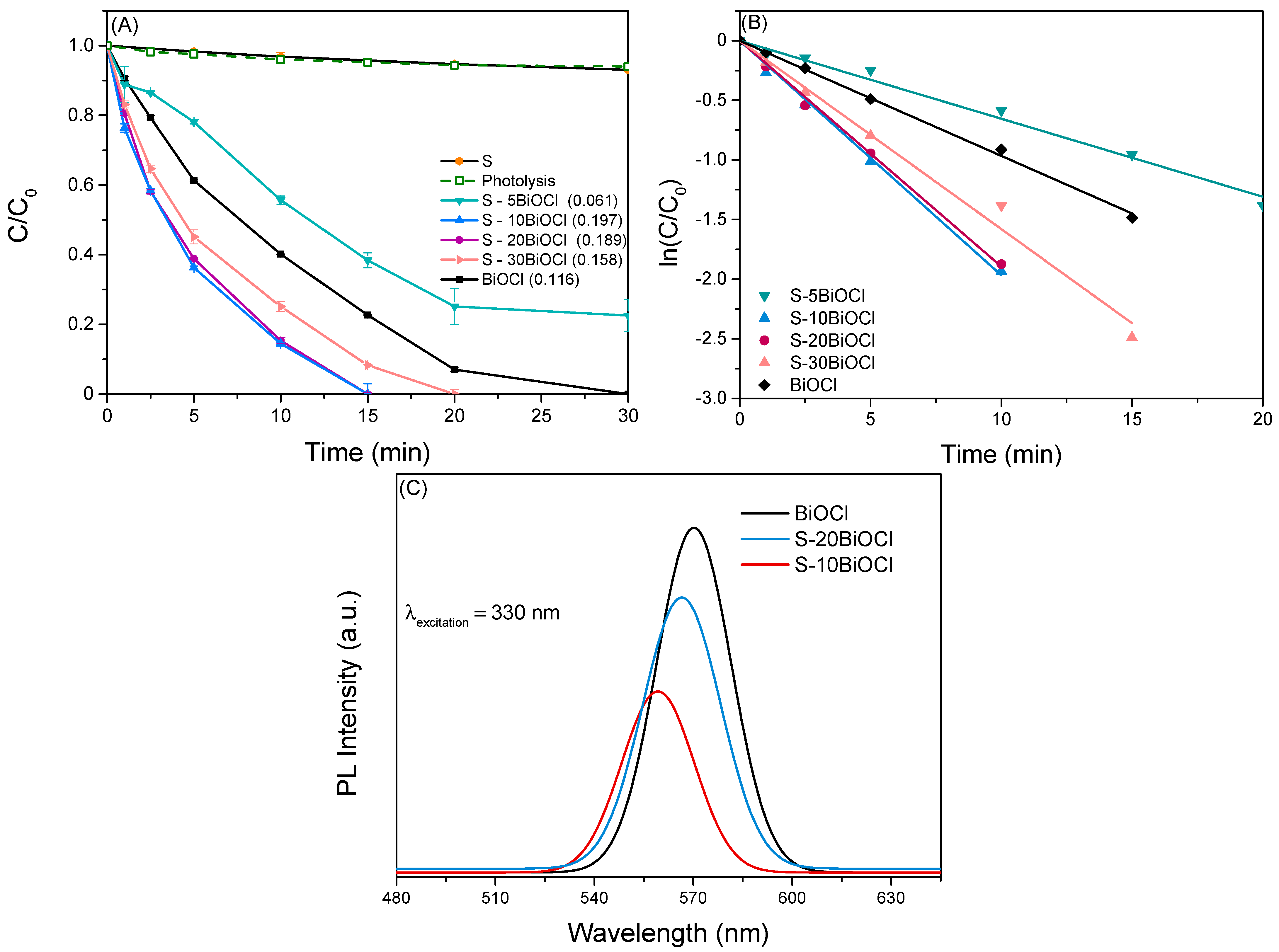
This study investigates the efficiency of BiOCl/Sepiolite composite photocatalysts for the removal of the pharmaceutical LOS in water under simulated solar irradiation. A series of clay-based photocatalysts with variable BiOCl content (5–30 wt%) was synthesized in order to assess the influence of BiOCl loading on the photocatalytic process. The preliminary experiments were carried out in ultrapure water (UPW) at ambient temperature and inherent pH. Figure 6A depicts the normalized concentration of LOS plotted as a function of irradiation time. Error bars in the figures depict the standard deviation of the mean for two independent experiments.

Figure 6.
(A) Photocatalytic degradation of LOS using BiOCl/Sepiolite composite catalysts, and (B) Estimation of the observed kinetic constant (kobs). Experimental parameters: Ccatalyst = 500 mg/L, CLOS = 0.5 mg/L, and pH = neutral. (C) Photoluminescence spectra of pure BiOCl, S-10BiOCl, and S-20BiOCl composite photocatalysts.
According to the literature, similar photocatalytic systems follow pseudo-first-order reaction kinetics with respect to the pollutant concentration, described by the following equation [40,41,42,43]:
where C0 represents the initial concentration of LOS, C is the concentration of LOS at a given irradiation time t, and kobs (min−1) is the observed reaction rate constant. Hence, kobs can be computed from the slope of the plot ln(C0/Ct) vs. irradiation time as shown in Figure 6B and are presented in brackets in each graph. The linear fit of this kinetic plot yields a coefficient of determination (R2) greater than 0.99, confirming a strong agreement with the pseudo-first-order kinetic model.
Composite photocatalysts with BiOCl content of 10% w.t or higher demonstrated significantly enhanced degradation capacity compared to pure BiOCl. Notably, optimal results were recorded in the case of S-10BiOCl, where complete elimination of LOS occurred within 15 min of solar irradiation. Further increase of BiOCl loading caused a slight drop in the observed oxidation rate. On the other hand, sepiolite alone proved to be photocatalytically inactive, consistent with previous studies [9,44,45].
The superior activity of composite catalysts is firmly associated with the synergistic interplay between sepiolite and BiOCl. The unique fibrous morphology of sepiolite offers a well-suited platform for uniform BiOCl dispersion, preventing aggregation and maximizing the utilization of photoactive sites. Furthermore, sepiolite’s high porosity and SSA contribute to a strong adsorption capacity for organic molecules, bringing them in close proximity to the photocatalytic sites [9,46,47]. However, it is crucial to strike a balance between the content of sepiolite and the content of BiOCl since incorporating the high amount of BiOCl will reduce the SSA of the composite material. It is worth noting that the best-performing photocatalyst (S-10BiOCl) in the present study is characterized by a significantly lower amount of the active component compared to other similar scientific studies [28,42].
To further explore the enhanced photocatalytic activity of the composite photocatalysts, photoluminescence (PL) spectroscopy was employed. PL technique is utilized in order to investigate the emission of light from a material after optical excitation and, by extension, to evaluate the recombination rate of photogenerated electron-hole pairs within the photocatalyst [48,49,50]. Figure 6C depicts the PL spectra of pure BiOCl, S-10BiOCl, which exhibited the highest photocatalytic activity, and S-20BiOCl, with an excitation wavelength of 330 nm [51]. The luminescence intensities of the samples increased in order: S-10BiOCl < S-20BiOCl < BiOCl. It is evident that pure BiOCl showed the highest PL intensity, signifying a higher recombination rate of electron-hole pairs compared to composite materials. The PL peak intensities of the composites were lower than those of BiOCl, revealing that the introduction of sepiolite could inhibit the complexation of electron/hole pair. The observed low PL intensity can likely be attributed to the relatively small crystallite size of BiOCl within the composite catalyst, as detailed in Table 1. In smaller crystallites, the distance that charge carriers need to travel to reach the surface is significantly reduced, minimizing the chance of encountering recombination sites in the bulk of the material. S-10BiOCl showed the lowest PL intensity among the investigated samples, which aligns perfectly with the photocatalytic results. The improvement in the separation efficiency of the photogenerated charge carrier could be caused by the interfacial charge transfer between sepiolite and BiOCl [52]. Moreover, the degree of order–disorder in S-BiOCl samples could be responsible for the presence of vacancies as well as surface and bulk structural defects, which in turn play an important role in the visible emission spectra and in the photocatalytic activity. This phenomenon was well studied by former studies in the field [50,53].
Subsequent experiments utilized S-10BiOCl as the photocatalyst to delve deeper into the effect of the operational parameters as well as the photocatalyst’s stability.
2.3.2. Evaluation of the Effect of Photocatalyst Dosage, LOS Concentration and pH Solution
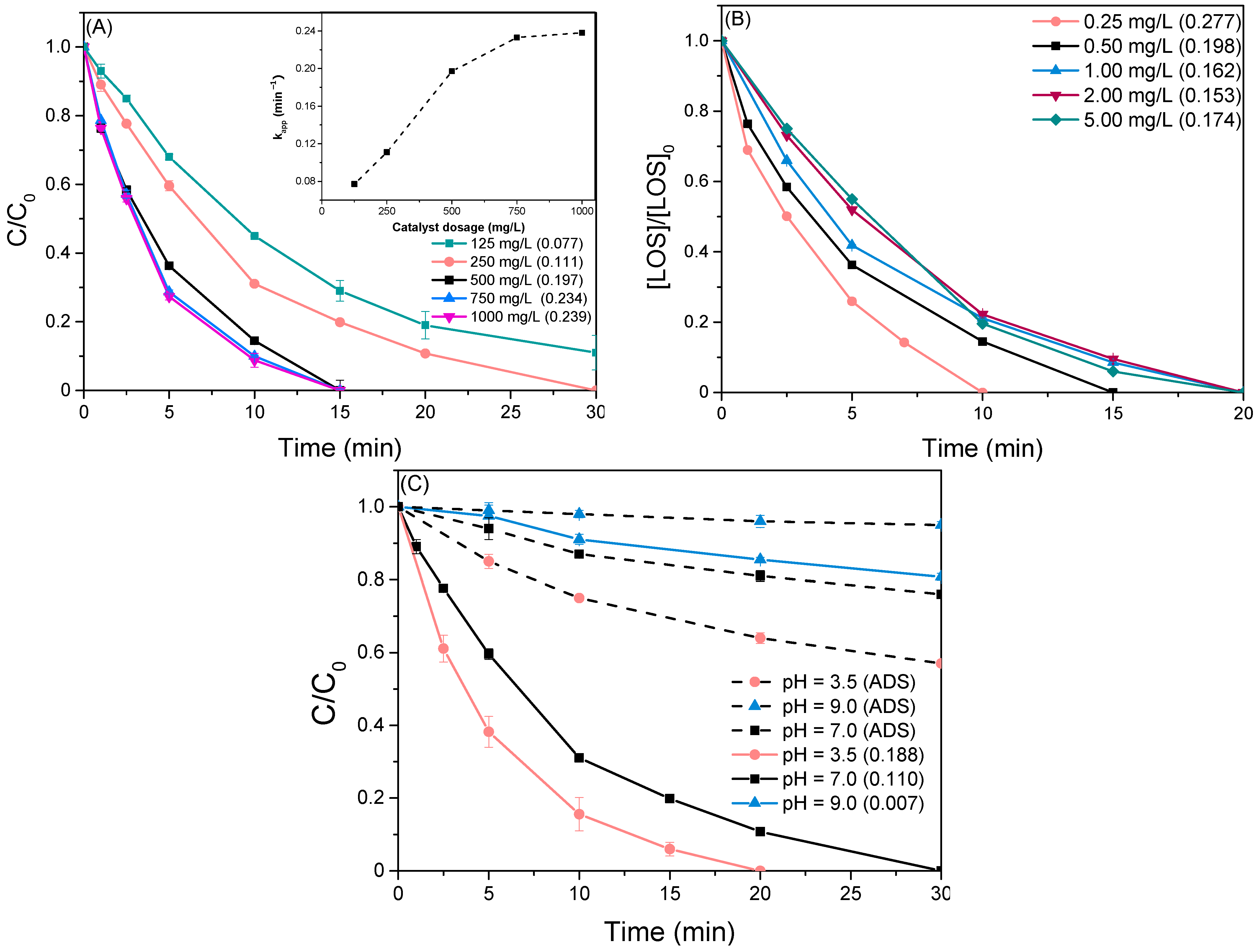
The effect of catalyst loading was assessed by varying the dosage from 125 to 1000 mg/L while maintaining all other parameters constant (Figure 7A). The observed trend suggests a remarkable boost in the degradation rate with increasing catalyst loading from 125 mg/L up to a critical value of 500 mg/L, as reflected by an increase in the kobs value from 0.077 min−1 to 0.197 min−1. This behavior can be attributed to the elevated availability of active sites on the catalyst surface, facilitating efficient photon adsorption and subsequent generation of electron-hole pairs (e−/h+). Above this value, the kobs appears to reach a plateau (inset graph), likely due to the formation of a turbid solution by the excessive amount of photocatalyst, which impedes efficient light penetration. Therefore, it is essential to select the optimal catalyst load to ensure sufficient photon adsorption while avoiding excessive catalyst usage [54,55].

Figure 7.
(A) Degradation of 0.5 mg/L LOS varying catalyst loading S-10BiOCl. Inset: Observed kinetic constant as a function of catalyst dosage, (B) Effect of LOS initial concentration with a catalyst loading of 500 mg/L, and (C) Normalized concentration of LOS as a function of time at different pH values in the presence of 500 mg/L S-10BiOCl.
Subsequent experiments were performed with initial LOS concentration spanning from 0.25 to 5.00 mg/L, maintaining a constant S-10BiOCl dosage of 500 mg/L. The corresponding normalized concentration profiles are summarized in Figure 7B. Within the range of environmental interest (0.25–1.00 mg/L), a gradual increase in LOS concentration considerably hampered the photocatalytic rate, decreasing the removal efficiency from 100% to 80% for a concentration of 1.00 mg/L at the 30-minute mark. From the standpoint of kinetic, the corresponding kobs values declined from 0.277 to 0.162 min−1, supporting the initial assumption that the process is governed by pseudo-first-order kinetics. Interestingly, when the concentration was further increased from 1.00 to 5.00 mg/L, kobs remained practically unchanged, suggesting a possible transition from pseudo-first to first-order kinetics in agreement with the literature [56,57,58].
The efficacy of the photocatalytic process hinges on maintaining an appropriate solution pH, as it crucially dictates the surface charge of the catalyst, the oxidation capacity of the oxidizing species, and the speciation of the organic compound [59,60]. In this regard, experiments were conducted by adjusting the solution pH at distinct values (pH = 3.5, 7, 9), and the corresponding results are presented in Figure 7C. At acidic pH, LOS concentration dropped rapidly, achieving complete abatement within approximately 20 min of irradiation. In comparison, the oxidation rate was slightly slower at a neutral pH, as evidenced by a decline in kobs from 0.188 to 0.110 min−1. On the contrary, the behavior at alkaline pH (9.0) differed markedly from the acidic and neutral conditions since the degradation rate exhibited a sharp decline, with LOS decomposition not exceeding 20%, revealing the presence of inhibitory phenomena. Due to the fact that the photodegradation process is concomitantly implicated with adsorption and oxidation reactions, the adsorption capacity of the photocatalysts was investigated at the same pH values (dashed lines). Adsorption profiles presented a similar trend with photocatalytic reactions, suggesting that the explanation lies in the type of electrostatic interactions between the catalytic charged surface and the target pollutant.
Losartan is a weak carboxylic acid with notable stability across a wide pH range (3–9) and features both an acidic and a basic center. Scientific studies report a measured pKa value of 4.9 for LOS and, across a pH range from 1–7, participates in a dynamic equilibrium between its neutral form and a dipolar ionic form [20,61]. The speciation profile of LOS undergoes a pronounced shift under alkaline conditions (pH > 8), favoring the formation of negatively charged anionic form (COO−) through deprotonation [62]. The observed inhibitory phenomena can be interpreted considering the surface charge of the S-10BiOCl catalyst and LOS speciation at pH 8. Strong electrostatic repulsions between negatively charged catalyst surfaces according to zeta potential measurements (Figure 4) and LOS anions can prevent LOS from effectively adhering to the catalyst surface and initiating the photocatalytic process. Consequently, photocatalytic degradation is hindered, and the process becomes dominated by mass transfer limitations. Additionally, the effectiveness of the generated •OH is strongly diminished in the basic environment since they are distinguished by shorter lifetime and lower oxidation potential [63,64,65,66]. On the other hand, under neutral and acidic conditions, the repulsive electrostatic forces formed between the photocatalyst and LOS are insignificant as the photocatalyst surface, and the pollutant tends to be oppositely charged.
2.3.3. Influence of Water Matrix
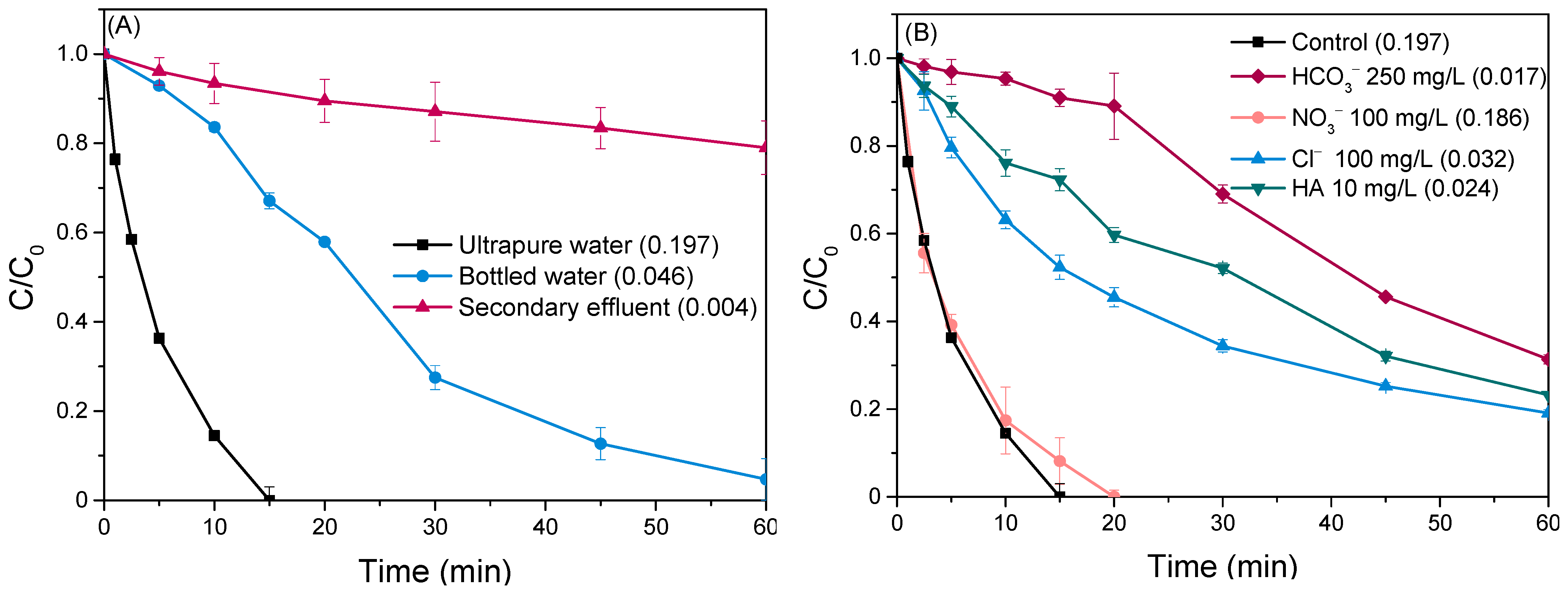
Estimating the efficacy of photocatalytic degradation processes in real-world scenarios requires investigating their performance beyond controlled laboratory environments. Dissolved organic matter (DOM) and inorganic constituents prevalent in natural waters and treated wastewater effluents can significantly alter photocatalytic performance through a multitude of mechanisms [56,67]. In this regard, experiments were carried out in bottled mineral water and the effluent of the secondary treatment process (Figure 8A). Inorganic ions, prevalent in bottled water, can potentially quench the photogenerated oxidizing species and compete with the target pollutant, thereby reducing the oxidation rate. The observed rate in the secondary effluent is even lower (kobs = 0.004 min−1) due to the presence of organic compounds, which likely act as a barrier by absorbing light irradiation and potentially clogging the active sites of the catalyst [68,69,70]. Additionally, the diminished removal efficiencies in these environmental water bodies can be partially attributed to the slightly alkaline pH (~8) of these matrices, which disfavor pollutant adsorption, leading to mass transfer limitations (as discussed in Section 2.3.2).

Figure 8.
(A) Removal profile of LOS in various water bodies, and (B) Impact of water matrix constituents on the degradation of LOS. Operational conditions: Ccatalyst = 500 mg/L and CLOS = 0.5 mg/L.
To shed more light on this observation, the impact of specific constituents was investigated. Specifically, a set of experiments was performed in synthetic matrices spiked with individual ions (HCO3−, Cl−, NO3−) commonly found in mineral water and humic acid (HA), a surrogate for organic matter present in wastewater (Figure 8B).
Notably, the addition of NO3− exhibited a negligible impact on the reaction rate since the degradation profile of LOS was similar to the control experiment with corresponding kobs of 0.188 min−1 and 0.197 min−1, respectively. On the other hand, adding chloride anions to the system posed severe limitations to the elimination of LOS, leading to 80% removal after 60 min of irradiation. Cl− can capture the oxidizing agents (h+ and •OH) and lead to the formation of chlorine radicals (Cl•) with distinctly less reactivity [71,72,73]. The inhibitory effects were even more pronounced in the case of bicarbonate ions (HCO3−). The presence of HCO3− altered the pH of the solution from 7 to 8.3, developing mass transfer limitations as discussed previously (Section 2.3.2). This is evidenced by the almost negligible degradation of LOS (~10%) during the first 20 min of the experiment, indicating a hindered accessibility of LOS to the catalyst surface. The reaction kinetics exhibited a noteworthy shift after this initial period, implying that an extended time period is required for the mass transfer reactions to equilibrate. Despite this shift, the overall LOS removal remained limited, not exceeding 80% even after 60 min due to the competitive interaction of bicarbonates with the target pollutant for harnessing the reactive species. Interactions of bicarbonates with the holes and •OH lead to the formation of secondary species of lower oxidational capacity, e.g., CO3•− [74,75,76]. In addition, LOS degradation was slowed down when humic acid was added to the solution, probably due to the adsorption of the latter to the photocatalyst active sites and the limited light penetration.
2.3.4. Determination of Reactive Species
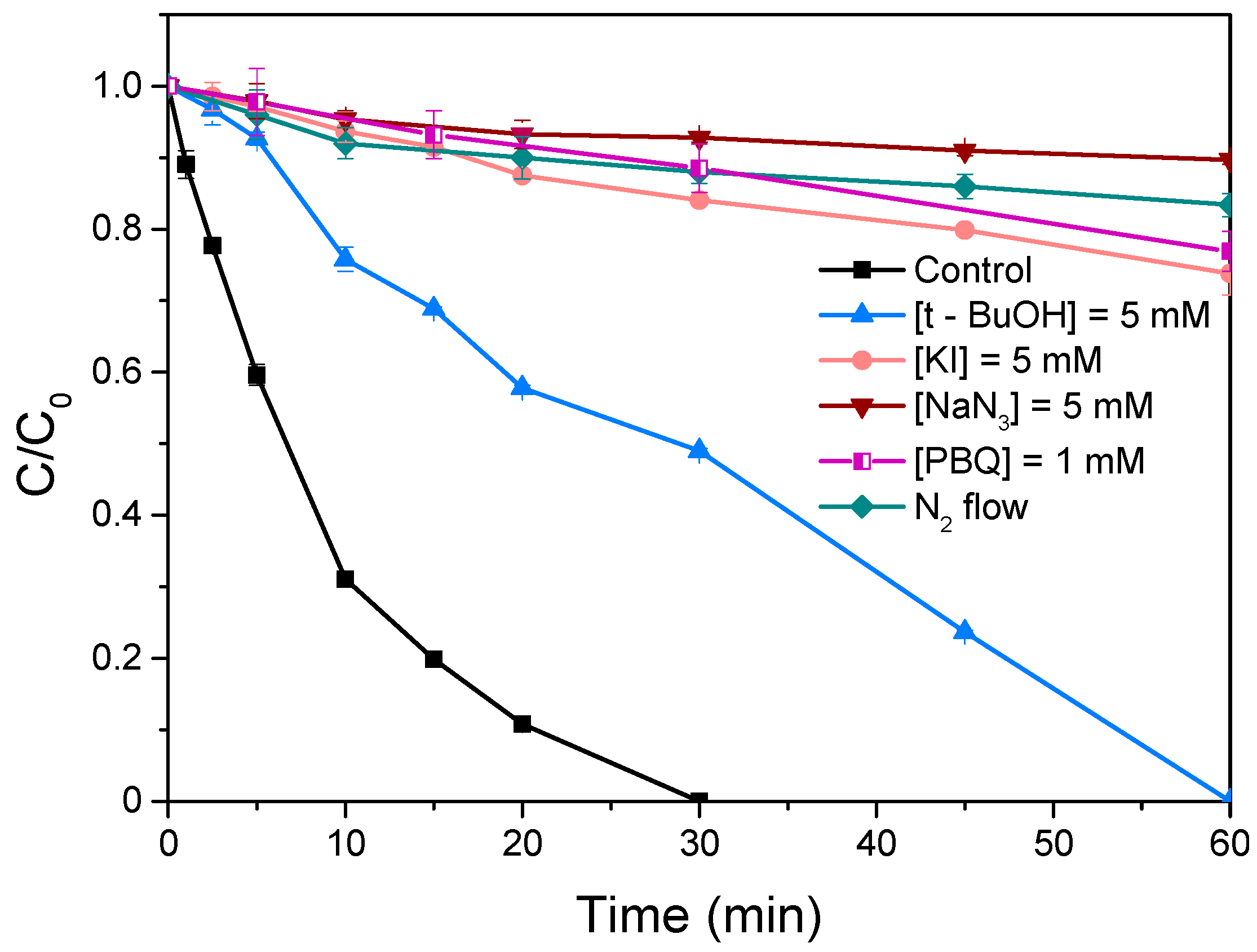
Based on the above results, it is perceptible that the formation of a binary heterostructure (S-BiOCl) could effectively boost the oxidation potential of the system. To identify the primary, photogenerated, oxidizing species, appropriate trapping agents were introduced to the solution. Tert-butanol (TBA, k•OH = 5.0 × 108 L/(mol s)), potassium iodide (KI, kh+ = 3.0 × 109 L/(mol s)), sodium azide (NaN3, k1O2 = 1.0 × 109 L/(mol s)) and p-benzoquinone (p-BQ, kO2•− = 2.9 × 109 L/(mol s)) were employed to selectively capture hydroxyl radicals (•OH), photoinduced holes (h+), singlet molecular oxygen (1O2) and superoxide radicals (O2•−), respectively [25,77,78,79,80,81]. All probe reagents were maintained at a concentration of 5 mM, except for p-benzoquinone (0.02 mM), due to its solubility and chromatographic analysis limitations.
As shown in Figure 9, the introduction of TBA into the solution resulted in a reduction of LOS removal efficiency from 100% to 50% after 30 min of irradiation, highlighting the partial contribution of •OH to the degradation process. Conversely, the degradation rate was totally suppressed in the presence of NaN3, p-BQ, and KI, indicating that 1O2, O2•−, and h+ serve as the primary reactive species. Additionally, the role of dissolved oxygen in O2•− and 1O2 generation was investigated by purging the solution with purified nitrogen (N2) to create an oxygen-depleted environment [82]. The recorded removal profile of the target compound was nearly identical to that observed after the addition of NaN3, underscoring the critical role of molecular oxygen as a key precursor for O2•− and 1O2 generation.

Figure 9.
Removal profiles of LOS in the presence of different trapping agents. Operational conditions: Ccatalyst = 250 mg/L, CLOS = 0.5 mg/L, Cscavengers = 5 mM, and Initial pH = neutral.
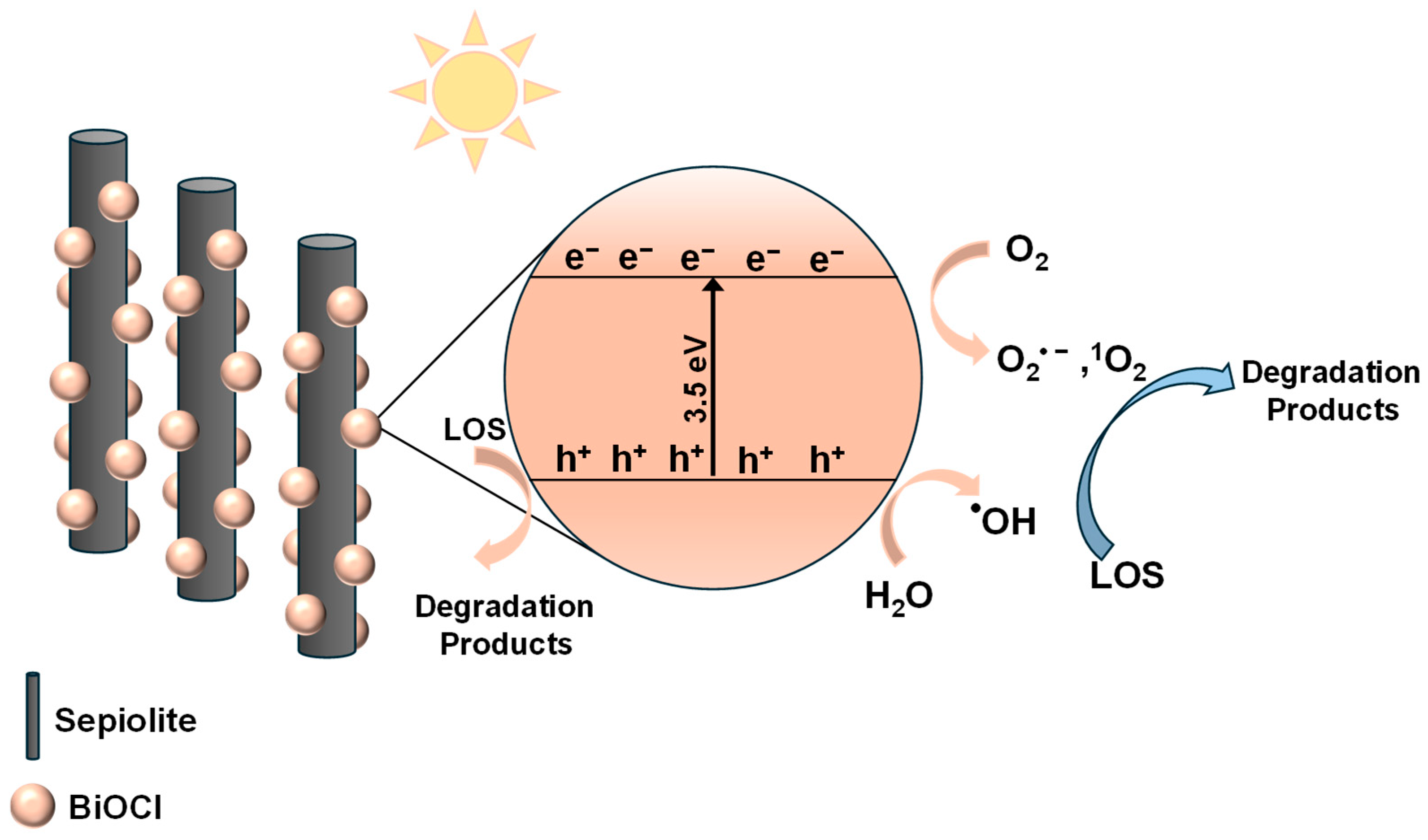
Based on the above observations, the radical pathway can be outlined as follows: Firstly, the addition of sepiolite increased the specific surface area of S-10BiOCl, allowing LOS molecules to be readily adsorbed onto the composite, thereby promoting the subsequent degradation process. Under simulated solar irradiation, BiOCl-absorbed photons become excited and generate photoinduced electrons and holes. Photoinduced holes located at the valence band (VB) either oxidized directly LOS by abstracting electrons or interacted with water molecules and hydroxyl anions, forming highly reactive •OH, which further reacted with LOS via hydroxylation [83,84]. Concurrently, the photoexcited electrons located at the conduction band (CB) reduced the dissolved oxygen, producing O2•− which subsequently reacted with available holes to form 1O2 [85,86]. This comprehensive mechanism highlights the involvement of both h+ and ROS (•OH, O2•−, and 1O2) in the overall degradation of LOS and its intermediate products. The main reactions can be described as follows (Equations (2)–(9)):
Summarizing, the enhanced photocatalytic activity of S-10BiOCl was primarily attributed to the formation of uniformly distributed, smaller BiOCl nanoparticles on the sepiolite surface after combining sepiolite with BiOCl. Additionally, the increased number of exposed active sites in S-10BiOCl shortened the carrier migration path and improved carrier separation efficiency [87]. The proposed photocatalytic mechanism is schematically illustrated in Scheme 1.

Scheme 1.
Schematic illustration of a possible photocatalytic mechanism for LOS degradation under simulated solar irradiation over the S-10BiOCl photocatalyst.
2.3.5. Reusability Tests
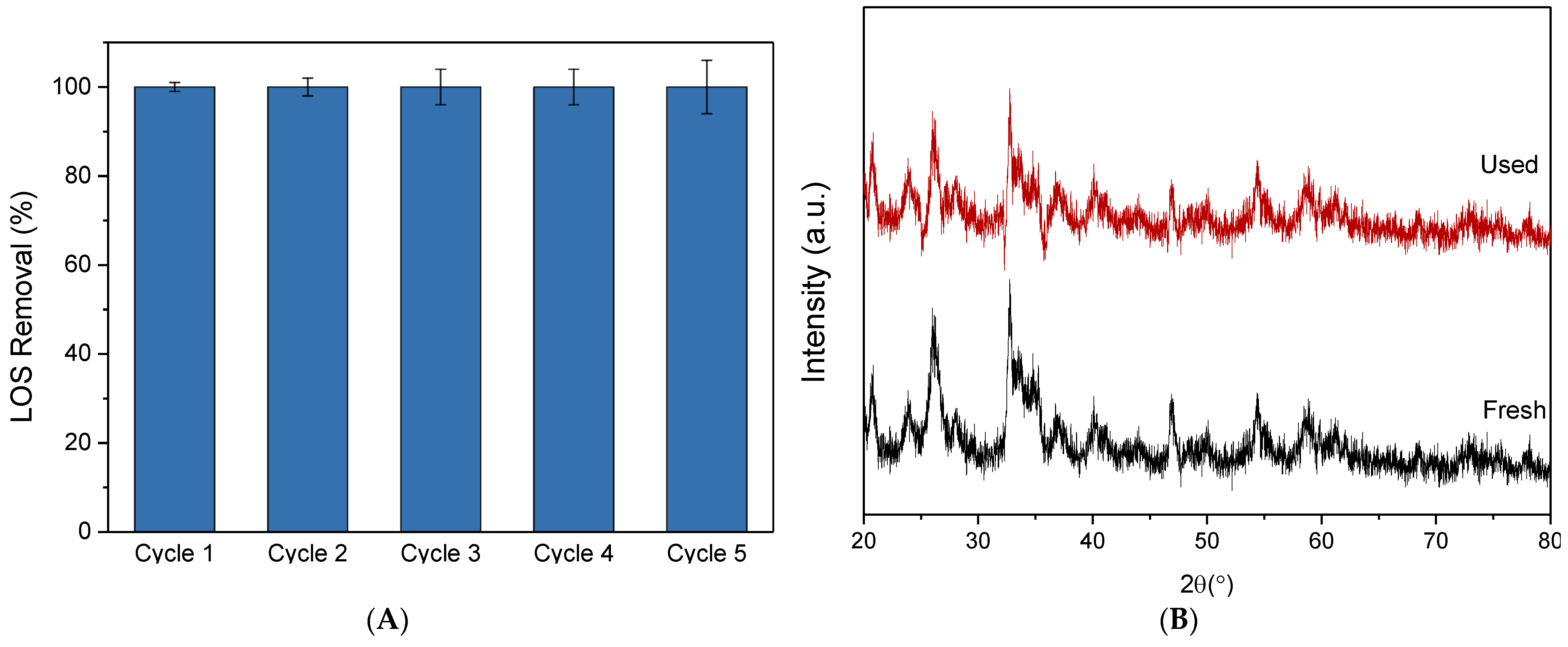
The photocatalytic stability of the composite catalyst was evaluated through five sequential 30-minute experimental runs. After each run, the catalyst was recovered using vacuum filtration, washed, and dried overnight to remove excess moisture before being reused without any additional treatment. In order to maintain a constant catalyst dosage, several parallel experiments were conducted under identical conditions. As depicted in Figure 10A, the composite catalyst exhibited high photocatalytic stability, as evidenced by the complete degradation of LOS after 30 min of irradiation in every cycle. Additionally, the XRD pattern of the used sample remained unchanged compared to the fresh one, aside from a minor decrease in peak intensity, indicating that the catalyst maintained its structural integrity throughout the degradation process (Figure 10B).

Figure 10.
(A) Stability tests of S-10BiOCl on the degradation of 0.5 mg/L LOS. Operational condition: [S-10BiOCl] = 30 mg/L, Volume = 120 mL, and Initial pH = 7. (B) XRD pattern of the fresh and used samples.
3. Materials and Methods
3.1. Chemical Reagents
Losartan was supplied from Sigma Aldrich (St. Louis, MO, USA). For photocatalyst preparation: Bismuth (III) nitrate pentahydrate (Bi(NO3)3·5H2O), acetic acid (CH3COOH), potassium chloride (KCl), thiourea (CH4N2S) and ethylene glycol (HOCH2CH2OH) were are supplied by Sigma-Aldrich. The sepiolite used in the present study was discovered by D. Papoulis and I. Koukouvelas in Corinth [28].
For the synthetic water matrices: Sodium chloride (NaCl), sodium bicarbonate (NaHCO3) and humic acid (HA) were purchased from Alfa Aesar (Haverhill, MA, USA). In addition, sodium hydroxide (NaOH) and sulfuric acid (H2SO4) were used for pH alteration, and tert-Butyl alcohol (TBA), sodium azide (NaN3), and potassium iodide (KI) used as radical scavengers, were also obtained from the same company.
For HPLC analysis, methanol (MeOH) and acetonitrile (ACN) were supplied by Sigma Aldrich.
The primary characteristics of the real water matrices are: (a) Bottled water (BW): pH = 7.6, conductivity = 350 mS/cm, total dissolved solids = 250 mg/L, total hardness = 220 mg/L, chlorides = 4.1 mg/L and bicarbonates = 240 mg/L; (b) secondary treated wastewater (WW): pH = 8.5, total suspended solids = 22 mg/L, chemical oxygen demand = 47 mg/L, total organic carbon = 4.5 mg/L, chlorides = 280 mg/L and bicarbonates = 290 mg/L.
3.2. Procedure for Sepiolite/BiOCl Synthesis
First, 100 mL of ethylene glycol:water solution (1:1 v/v ratio) containing a pre-determined amount of sepiolite was mixed with 4.85 g of Bi (NO3)3·5H2O, and the mixture was left to stir for 30 min at room temperature. Next, 10 mL of the CH4N2S solution (1.0 mol/L) and 0.01 mol of KCl were added. Ultimately, 50 milliliters of 2% (v/v) CH3COOH was gradually added. For one hour, the solution was stirred continuously. Vacuum filtration was used to separate the final product, which was then dried overnight at 60 °C.
3.3. Physicochemical Characterization
Crystallographic analysis was carried out using an X-ray diffractometer (Brucker D8 Advance, Billerica, MA, USA). The obtained spectra were used to calculate the primary crystallite size according to the Scherrer equation [88]. Diffuse reflectance spectroscopy using a Varian Cary 3E device (Palo Alto, CA, USA) was used to determine the optical properties of sepiolite/BiOCl photocatalysts. Then, the Attenuated Total Reflection infrared (ATR/FTIR) measurements were conducted by using an ATR Miracle accessory of PIKE technologies attached to the EQUINOX 55 FT-IR spectrometer (BRUKER). Brunauer–Emmett–Teller (BET) method was used to determine the specific surface area of the samples based on data acquired by nitrogen physisorption at the temperature of liquid nitrogen (77 K) (Micromeritics, Gemini III 2375, Norcross, GA, USA). Nanocomposite morphology was determined at the laboratory of Electron Microscopy and Microanalysis of the University of Patras using a Scanning Electron Microscope (SEM) (JEOL 6300, USA, Inc., Peabody, MA, USA) equipped with an energy dispersive spectrometer (EDS). In addition, the laser Doppler micro-electrophoresis method was adopted for zeta potential estimation (Malvern Zetasizer, Malvern Instruments, Surrey, UK) [89].
3.4. Photocatalytic Activity Test
The photocatalytic efficiency of the as-prepared materials was assessed under simulated solar light irradiation (100 W Xenon lamp). According to chemical actinometry, the light intensity entering the photoreactor was equal to 1.3 × 10−4 einstein/(m2·s). Initially, 60 mg of the photocatalyst was added to 60 mL of LOS solution (0.5 mg/L) and stirred magnetically in darkness for 15 min to establish adsorption-desorption equilibrium. Subsequently, the suspensions were exposed to light, and throughout the experiment, samples were collected and filtered through 0.45 μm membrane filters. The concentration of remaining LOS was measured by means of high-performance liquid chromatography (HPLC). All experiments were performed in duplicate to ensure accurate results. Details can be found elsewhere [25]. Furthermore, the impact of reactive species—superoxide radicals, hydroxyl radicals, and holes (h+)—in the photocatalytic breakdown of LOS was investigated via radical scavenger tests (NaN3, TBA, KI, and Ar flow). For the consecutive experimental runs, the photocatalyst was collected, filtered, and repeatedly rinsed with deionized water. After that, it was dried for ~12 h at 60 °C and used in the next cycle.
4. Conclusions
The present study constitutes a significant contribution to the field of photocatalytic degradation of persistent organic micropollutants in water, with emphasis given to pharmaceuticals. The main goal was the development of a sustainable, low-cost, and highly active photocatalytic material, and, thus, a series of BiOCl/Sepiolite composite samples of variable sepiolite content was synthesized. The coupling of sepiolite with BiOCl had a beneficial effect in all cases, but the shortest degradation time periods were achieved in the case of the S-10BiOCl sample. The fact that BiOCl content in the composite photocatalyst is relatively low significantly increases the scientific value and novelty of the proposed material and paves the way for a more cost-effective and sustainable approach in clay-based photocatalysts. The excellent stability of the proposed material further enhances this finding. The superior activity of the composite samples was mainly attributed to the interface formed between the sepiolite and the semiconductor, which prevented the recombination of photogenerated species as well as provided a larger surface area for the degradation reactions. In addition, the present system showed high efficiency both in acidic and neutral conditions. Considering results obtained in real water matrices of environmental concern, LOS degradation kinetics in BW was lower but still acceptable, in contrast to wastewater, where it was obvious that longer irradiation time and higher catalyst dosage are necessary to retain the efficiency. Considering the photocatalytic mechanism, it was found that photogenerated holes, hydroxyl radicals, and singlet molecular oxygen play a crucial role. However, further investigation is needed for the corresponding quantification.
Author Contributions
Conceptualization, K.K. and A.P.; methodology, K.K., E.E.K., D.P. (Dionisios Panagiotaras) and D.P. (Dimitrios Papoulis); formal analysis, A.P. and Z.F.; investigation, K.K., E.E.K., D.P. (Dionisios Panagiotaras) and D.P. (Dimitrios Papoulis); resources Z.F. and A.P.; data curation, K.K. and D.P. (Dimitrios Papoulis); writing-original draft preparation K.K. and A.P.; supervision, A.P. and Z.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors wish to thank K. Govatsi staff of the Laboratory of Electron Microscopy and Microanalysis (L.E.M.M.) at the University of Patras for SEM images.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, R.; Ansari, M.O.; Taleb, M.A.; Oves, M.; Barakat, M.A.; Alghamdi, M.A.; Al Makishah, N.H. Integrated Adsorption-Photocatalytic Decontamination of Oxytetracycline from Wastewater Using S-Doped TiO2/WS2/Calcium Alginate Beads. Catalysts 2022, 12, 1676. [Google Scholar] [CrossRef]
- Sharma, N.; Hernadi, K. The Emerging Career of Strontium Titanates in Photocatalytic Applications: A Review. Catalysts 2022, 12, 1619. [Google Scholar] [CrossRef]
- Budiarso, I.J.; Dabur, V.A.; Rachmantyo, R.; Judawisastra, H.; Hu, C.; Wibowo, A. Carbon Nitride- and Graphene-Based Materials for the Photocatalytic Degradation of Emerging Water Pollutants. Mater. Adv. 2024, 5, 2668–2688. [Google Scholar] [CrossRef]
- Ramadhan Ikreedeegh, R.; Arif Hossen, M.; Sherryna, A.; Tahir, M. Recent Advances on Synthesis and Photocatalytic Applications of MOF-Derived Carbon Materials: A Review. Coord. Chem. Rev. 2024, 510, 215834. [Google Scholar] [CrossRef]
- Slapničar, Š.; Žerjav, G.; Zavašnik, J.; Finšgar, M.; Pintar, A. Synthesis and Characterization of Plasmonic Au/TiO2 Nanorod Solids for Heterogeneous Photocatalysis. J. Environ. Chem. Eng. 2023, 11, 109835. [Google Scholar] [CrossRef]
- Wu, R.; Liu, W.; Bai, R.; Zheng, D.; Tian, X.; Lin, W.; Ke, Q.; Li, L. Waste Biomass-Mediated Synthesis of TiO2/P, K-Containing Grapefruit Peel Biochar Composites with Enhanced Photocatalytic Activity. Molecules 2024, 29, 2090. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Yerien, D.E.; Postigo, A. Bioinspired Photocatalyzed Organic Synthetic Transformations. The Use of Natural Pigments and Vitamins in Photocatalysis. ChemCatChem 2022, 14, e202200623. [Google Scholar] [CrossRef]
- Fan, X.; Liu, X.; Wang, Y. Low-Cost and Resource-Efficient Monolithic Photocatalyst with Enhanced Solar Light Utilization for the Photocatalytic Treatment of Organic Wastewater. Chemosphere 2023, 312, 137052. [Google Scholar] [CrossRef]
- Wang, P.; Qi, C.; Hao, L.; Wen, P.; Xu, X. Journal of Materials Science & Technology Sepiolite/Cu2O/Cu Photocatalyst: Preparation and High Performance for Degradation of Organic Dye. J. Mater. Sci. Technol. 2019, 35, 285–291. [Google Scholar] [CrossRef]
- Du, Y.Y.; Fang, H.H.; Zheng, P.W. Porous Sepiolite/Starch Composites: Preparation, Structure and Absorption Properties. Adv. Mater. Res. 2013, 634–638, 1937–1942. [Google Scholar] [CrossRef]
- Song, J.; Ren, X.; Hu, G.; Wang, L.; Hu, X. Enhanced Photocatalytic Degradation of Indoor Formaldehyde by Sepiolite Decorated with TiO2 Nanoparticles: Effects of Key Preparation Parameters. Microporous Mesoporous Mater. 2023, 353, 112515. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Mayoral, A.; García-Hernández, M.; Ben Haj Amara, A.; Ruiz-Hitzky, E. Sepiolite Nanoplatform for the Simultaneous Assembly of Magnetite and Zinc Oxide Nanoparticles as Photocatalyst for Improving Removal of Organic Pollutants. J. Hazard. Mater. 2017, 340, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Hu, G.; Guo, Q.; Gao, D.; Wang, L.; Hu, X. Ag/Ag3PO4 Nanoparticles Assembled on Sepiolite Nanofibers: Enhanced Visible-Light-Driven Photocatalysis and the Important Role of Ag Decoration. Mater. Sci. Semicond. Process. 2023, 156, 107272. [Google Scholar] [CrossRef]
- Bencsik, D.; Wadhawan, T.; Házi, F.; Karches, T. Plant-Wide Models for Optimizing the Operation and Maintenance of BTEX-Contaminated Wastewater Treatment and Reuse. Environments 2024, 11, 88. [Google Scholar] [CrossRef]
- Kaprara, E.; Psaltou, S.; Salapasidou, M.; Kalandaridis, S.; Palasantza, P.-A.; Germanidis, G.; Diamantopoulos, P.; Mitrakas, M.; Zouboulis, A. Evaluation of Heterogeneous Catalytic Ozonation for Micropollutants Removal from Wastewater: Application of a Pre-Industrial-Scale Unit. Catalysts 2024, 14, 227. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Xia, G.; Tang, Y.; Wu, Z.; Yao, J. Competitive Coordination Initiated One-Pot Synthesis of Core–Shell Bi-MOF@BiOX (X = I, Br and Cl) Heterostructures for Photocatalytic Elimination of Mixed Pollutants. Sep. Purif. Technol. 2023, 316, 123819. [Google Scholar] [CrossRef]
- Chang, H.; Xu, G.; Huang, X.; Xu, W.; Luo, F.; Zang, J.; Lin, X.; Huang, R.; Yu, H.; Yu, B. Photocatalytic Degradation of Quinolones by Magnetic MOFs Materials and Mechanism Study. Molecules 2024, 29, 2294. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Abitayev, Z.; Batyrbayeva, M.; Vakros, J.; Mantzavinos, D.; Atabaev, T.S.; Poulopoulos, S.G. TiO2/Zeolite Composites for SMX Degradation under UV Irradiation. Catalysts 2024, 14, 147. [Google Scholar] [CrossRef]
- Mancuso, A.; Mottola, S.; Sacco, O.; Vaiano, V.; De Marco, I. Photocatalytic Degradation of Ceftriaxone Using TiO2 Coupled with ZnO Micronized by Supercritical Antisolvent Route. Nanomaterials 2023, 13, 3130. [Google Scholar] [CrossRef]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Ávila-Torres, Y.; Torres-Palma, R.A.; Co, E.A.S. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water 2020, 12, 3398. [Google Scholar] [CrossRef]
- Zaouak, A.; Noomen, A.; Jelassi, H. Degradation Mechanism of Losartan in Aqueous Solutions under the Effect of Gamma Radiation. Radiat. Phys. Chem. 2021, 184, 109435. [Google Scholar] [CrossRef]
- Kaur, B.; Dulova, N. UV-Assisted Chemical Oxidation of Antihypertensive Losartan in Water. J. Environ. Manag. 2020, 261, 110170. [Google Scholar] [CrossRef] [PubMed]
- Ioannidi, A.A.; Giannakopoulos, S.; Petala, A.; Frontistis, Z.; Mantzavinos, D. Fabrication of a Novel MoB/BiOCl Photocatalyst for Losartan and Escherichia Coli Removal. Catal. Today 2024, 430, 114510. [Google Scholar] [CrossRef]
- Ioannidi, A.A.; Zappa, J.; Petala, A.; Souliotis, M.; Mantzavinos, D.; Frontistis, Z. Solar Light-Induced Photocatalytic Degradation of Sulfamethoxazole by Cobalt Phosphide-Promoted Bismuth Vanadate. Water 2023, 15, 1370. [Google Scholar] [CrossRef]
- Kouvelis, K.; Ioannidi, A.A.; Petala, A.; Souliotis, M.; Frontistis, Z. Photocatalytic Degradation of Losartan with Bismuth Oxychloride: Batch and Pilot Scale Demonstration. Catalysts 2023, 13, 1175. [Google Scholar] [CrossRef]
- Guo, T.; Fan, X.; Jiang, X.; Qi, Y.; Du, J.; Zhang, A.; Wang, H. Engineering Shape of BiOCl Nanosheets with Improved Visible-Light Response for Superior Photocatalytic Degradation of Rhodamine B. J. Alloys Compd. 2023, 948, 169586. [Google Scholar] [CrossRef]
- Papoulis, D.; Somalakidi, K.; Todorova, N.; Trapalis, C.; Panagiotaras, D.; Sygkridou, D.; Stathatos, E.; Gianni, E.; Mavrikos, A.; Komarneni, S. Sepiolite/TiO2 and Metal Ion Modified Sepiolite/TiO2 Nanocomposites: Synthesis, Characterization and Photocatalytic Activity in Abatement of NOx Gases. Appl. Clay Sci. 2019, 179, 105156. [Google Scholar] [CrossRef]
- Papoulis, D.; Panagiotaras, D.; Tsigrou, P.; Christoforidis, K.C.; Petit, C.; Apostolopoulou, A.; Stathatos, E.; Komarneni, S.; Koukouvelas, I. Halloysite and Sepiolite–TiO2 Nanocomposites: Synthesis Characterization and Photocatalytic Activity in Three Aquatic Wastes. Mater. Sci. Semicond. Process. 2018, 85, 1–8. [Google Scholar] [CrossRef]
- Uğurlu, M. Adsorption of a Textile Dye onto Activated Sepiolite. Microporous Mesoporous Mater. 2009, 119, 276–283. [Google Scholar] [CrossRef]
- Zhang, G.; Xiong, Q.; Xu, W.; Guo, S. Synthesis of Bicrystalline TiO2 Supported Sepiolite Fibers and Their Photocatalytic Activity for Degradation of Gaseous Formaldehyde. Appl. Clay Sci. 2014, 102, 231–237. [Google Scholar] [CrossRef]
- Sabah, E.; Çelik, M.S. Interaction of Pyridine Derivatives with Sepiolite. J. Colloid Interface Sci. 2002, 251, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Shoval, S.; Michaelian, K.H.; Boudeulle, M.; Panczer, G.; Lapides, I.; Yariv, S. Study of Thermally Treated Dickite by Infrared and Micro-Raman Spectroscopy Using Curve-Fitting Technique. J. Therm. Anal. Calorim. 2002, 69, 205–225. [Google Scholar] [CrossRef]
- Vicente-Rodríguez, M.A.; Suarez, M.; Bañares-Muñoz, M.A.; de Dios Lopez-Gonzalez, J. Comparative FT-IR Study of the Removal of Octahedral Cations and Structural Modifications during Acid Treatment of Several Silicates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1996, 52, 1685–1694. [Google Scholar] [CrossRef]
- Madejová, J.; Gates, W.P.; Petit, S. Chapter 5—IR Spectra of Clay Minerals. In Infrared and Raman Spectroscopies of Clay Minerals; Gates, W.P., Kloprogge, J.T., Madejová, J., Bergaya, F.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. ISBN 1572-4352. [Google Scholar]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-Potential Measurement Using the Smoluchowski Equation and the Slope of the Current-Time Relationship in Electroosmotic Flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Velde, B. Composition and Mineralogy of Clay Minerals. In Origin and Mineralogy of Clays: Clays and the Environment; Velde, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 8–42. ISBN 978-3-662-12648-6. [Google Scholar]
- Su, G.; Liu, L.; Kuang, Q.; Liu, X.; Dong, W.; Niu, M.; Tang, A.; Xue, J. Enhanced Visible-Light Photocatalytic Activity and Recyclability of Magnetic Core-Shell Fe3O4@SiO2@BiFeO3–Sepiolite Microspheres for Organic Pollutants Degradation. J. Mol. Liq. 2021, 335, 116566. [Google Scholar] [CrossRef]
- Qiu, J.; Li, M.; Xu, J.; Zhang, X.-F.; Yao, J. Bismuth Sulfide Bridged Hierarchical Bi2S3/BiOCl@ZnIn2S4 for Efficient Photocatalytic Cr(VI) Reduction. J. Hazard. Mater. 2020, 389, 121858. [Google Scholar] [CrossRef]
- Petala, A.; Tsikritzis, D.; Kollia, M.; Ladas, S.; Kennou, S.; Kondarides, D.I. Synthesis and Characterization of N-Doped TiO2 Photocatalysts with Tunable Response to Solar Radiation. Appl. Surf. Sci. 2014, 305, 281–291. [Google Scholar] [CrossRef]
- Min, S.; Wang, F.; Jin, Z.; Xu, J. Cu2O Nanoparticles Decorated BiVO4 as an Effective Visible-Light-Driven p-n Heterojunction Photocatalyst for Methylene Blue Degradation. Superlattices Microstruct. 2014, 74, 294–307. [Google Scholar] [CrossRef]
- Landge, V.K.; Sonawane, S.H.; Sivakumar, M.; Sonawane, S.S.; Uday Bhaskar Babu, G.; Boczkaj, G. S-Scheme Heterojunction Bi2O3-ZnO/Bentonite Clay Composite with Enhanced Photocatalytic Performance. Sustain. Energy Technol. Assess. 2021, 45, 101194. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Song, J.; Zhang, G.; Li, C.; Zheng, S. Journal of Colloid and Interface Science Synthesis of Novel Ternary Heterogeneous BiOCl/TiO 2/Sepiolite Composite with Enhanced Visible-Light-Induced Photocatalytic Activity towards Tetracycline. J. Colloid Interface Sci. 2019, 533, 238–250. [Google Scholar] [CrossRef]
- Velegraki, T.; Hapeshi, E.; Fatta-Kassinos, D.; Poulios, I. Solar-Induced Heterogeneous Photocatalytic Degradation of Methyl-Paraben. Appl. Catal. B Environ. 2015, 178, 2–11. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Bouberka, Z.; Roussel, P.; Volkringer, C.; Addad, A.; Ouddane, B.; Pierlot, C.; Maschke, U. Development of a TiO2/Sepiolite Photocatalyst for the Degradation of a Persistent Organic Pollutant in Aqueous Solution. Nanomaterials 2022, 12, 3313. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.; Sasaki, K. Dye-Sensitized Photocatalyst of Sepiolite for Organic Dye Degradation. Catalysts 2019, 9, 235. [Google Scholar] [CrossRef]
- Aranda, P.; Kun, R.; Martín-luengo, M.A.; Letaïef, S.; Dékány, I.; Ruiz-hitzky, E. Titania—Sepiolite Nanocomposites Prepared by a Surfactant Templating Colloidal Route. Chem. Mater. 2008, 20, 84–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhang, G. Photocatalytic Degradation of Organic Contaminants by TiO2/Sepiolite Composites Prepared at Low Temperature. Chem. Eng. J. 2011, 173, 1–10. [Google Scholar] [CrossRef]
- Anpo, M.; Dzwigaj, S.; Che, M. Chapter 1 Applications of Photoluminescence Spectroscopy to the Investigation of Oxide-Containing Catalysts in the Working State. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2009; Volume 52, pp. 1–42. ISBN 0360-0564. [Google Scholar]
- Abdi-Jalebi, M.; Ibrahim Dar, M.; Sadhanala, A.; Johansson, E.M.J.; Pazoki, M. Chapter 3—Optical Absorption and Photoluminescence Spectroscopy. In Micro and Nano Technologies; Pazoki, M., Hagfeldt, A., Edvinsson, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–79. ISBN 978-0-12-814727-6. [Google Scholar]
- Li, Q.; Anpo, M.; Wang, X. Application of Photoluminescence Spectroscopy to Elucidate Photocatalytic Reactions at the Molecular Level. Res. Chem. Intermed. 2020, 46, 4325–4344. [Google Scholar] [CrossRef]
- Ning, S.; Shi, X.; Zhang, H.; Lin, H.; Zhang, Z.; Long, J.; Li, Y.; Wang, X. Reconstructing Dual-Induced {0 0 1} Facets Bismuth Oxychloride Nanosheets Heterostructures: An Effective Strategy to Promote Photocatalytic Oxygen Evolution. Sol. RRL 2019, 3, 1900059. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Zhu, G.; Wang, M.; Chen, Y.; Zhu, J.; Xi, Y.; He, H. Plasmonic Ag Coated Zn/Ti-LDH with Excellent Photocatalytic Activity. Appl. Surf. Sci. 2018, 433, 458–467. [Google Scholar] [CrossRef]
- Pinatti, I.M.; Tello, A.C.M.; Pereira, P.F.S.; Trench, A.B.; Teodoro, M.D.; Rosa, I.L.V.; da Silva, A.B.F.; Longo, E.; Andrés, J.; Simões, A.Z. Towards a Relationship between Photoluminescence Emissions and Photocatalytic Activity of Ag2SeO4: Combining Experimental Data and Theoretical Insights. Dalt. Trans. 2022, 51, 11346–11362. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis: Recent Overview and Trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Duta, A.; Malato, S.; Bogatu, C.; Covei, M.; Polo-l, M.I. Novel ZnO Photocatalysts for Pollutants ’ Abatement under Solar Radiation at Pilot Plant Scale. Catal. Today 2023, 413–415, 113947. [Google Scholar] [CrossRef]
- Rioja, N.; Zorita, S.; Peñas, F.J. Effect of Water Matrix on Photocatalytic Degradation and General Kinetic Modeling. Appl. Catal. B Environ. 2016, 180, 330–335. [Google Scholar] [CrossRef]
- Djebbari, C.; Ammouchi, N.; Nakib, C.; Zouied, D.; Dob, K. Degradation of Malachite Green Using Heterogeneous Nanophotocatalysts (NiO/TiO2, CuO/TiO2) under Solar and Microwave Irradiation. SN Appl. Sci. 2021, 3, 255. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, Mineralization and Antibiotic Inactivation of Amoxicillin by UV-A/TiO2 Photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Abedini, F.; Omidyan, R.; Salehi, M. Theoretical Insights on Nonradiative Deactivation Mechanisms of Protonated Xanthine. J. Photochem. Photobiol. A Chem. 2019, 385, 112067. [Google Scholar] [CrossRef]
- Zielinska, M.; Wojnowska-Baryla, I.; Cydzik-Kwiatkowska, A. Bisphenol A Removal from Water and Wastewater; Springer: Cham, Switzerland, 2018; ISBN 9783319923611. [Google Scholar] [CrossRef]
- Foley, L.; Toney, T.; Barlow, J.W.; O’Connor, M.; Fitzgerald-Hughes, D.; Ramtoola, Z. Investigation of the Physical, Chemical and Microbiological Stability of Losartan Potassium 5 mg/mL Extemporaneous Oral Liquid Suspension. Molecules 2021, 26, 301. [Google Scholar] [CrossRef] [PubMed]
- Rolando, B.; Fruttero, R.; Henchoz, Y.; Martel, S. Physicochemical Profiling of Sartans: A Detailed Study of Ionization Constants and Distribution Coefficients. Helv. Chim. Acta 2008, 91, 468–482. [Google Scholar]
- Ma, J.; Wang, F.; Mostafavi, M. Ultrafast Chemistry of Water Radical Cation, H2O•+, in Aqueous Solutions. Molecules 2018, 23, 244. [Google Scholar] [CrossRef]
- Castellote, M. Cement and Concrete Research Quanti Fi Cation of Hydroxyl Radicals on Cementitious Materials by Fl Uorescence Spectrophotometry as a Method to Assess the Photocatalytic Activity. Cem. Concr. Res. 2015, 74, 108–115. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Nosaka, Y. The PH Dependence of OH Radical Formation in Photo-Electrochemical Water Oxidation with Rutile. Phys. Chem. Chem. Phys. 2015, 17, 30570–30576. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Comment on “Singlet Oxygen 1O2 in Photocatalysis on TiO2. Where Does It Come From?”. J. Phys. Chem. C 2019, 123, 27993–27995. [Google Scholar] [CrossRef]
- Petala, A.; Mantzavinos, D.; Frontistis, Z. Impact of Water Matrix on the Photocatalytic Removal of Pharmaceuticals by Visible Light Active Materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Ahmad, S.; Almehmadi, M.; Janjuhah, H.T.; Kontakiotis, G.; Abdulaziz, O.; Saeed, K.; Ahmad, H.; Allahyani, M.; Aljuaid, A.; Alsaiari, A.A.; et al. The Effect of Mineral Ions Present in Tap Water on Photodegradation of Organic Pollutants: Future Perspectives. Water 2023, 15, 175. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.-C.; Juang, R.-S. Effects of Water Matrix Components on Degradation Efficiency and Pathways of Antibiotic Metronidazole by UV/TiO2 Photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Rajca, M. Impact of the Water Composition on the Degradation Kinetics of Natural Organic Matter in Photocatalytic Membrane Reactors. Environ. Prot. Eng. 2015, 41, 29–39. [Google Scholar] [CrossRef]
- Tavakoli Joorabi, F.; Kamali, M.; Sheibani, S. Effect of Aqueous Inorganic Anions on the Photocatalytic Activity of CuO–Cu2O Nanocomposite on MB and MO Dyes Degradation. Mater. Sci. Semicond. Process. 2022, 139, 106335. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.; Lou, L.-P.; Wu, X. Involvement of Chloride Anion in Photocatalytic Process. J. Environ. Sci. 2005, 17, 761–765. [Google Scholar]
- Gao, X.; Guo, Q.; Tang, G.; Peng, W.; Luo, Y.; He, D. Effects of Inorganic Ions on the Photocatalytic Degradation of Carbamazepine. J. Water Reuse Desalin. 2019, 9, 301–309. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Boczkaj, G.; Aubry, O.; Aravind, U.K.; Aravindakumar, C.T. Advanced Oxidation Processes for Degradation of Water Pollutants—Ambivalent Impact of Carbonate Species: A Review. Water 2023, 15, 1615. [Google Scholar] [CrossRef]
- Tolić Čop, K.; Mutavdžić Pavlović, D.; Gazivoda Kraljević, T. Photocatalytic Activity of TiO2 for the Degradation of Anticancer Drugs. Nanomaterials 2022, 12, 3532. [Google Scholar] [CrossRef] [PubMed]
- Bancirova, M. Sodium Azide as a Specific Quencher of Singlet Oxygen during Chemiluminescent Detection by Luminol and Cypridina Luciferin Analogues. Luminescence 2011, 26, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, N.; Nguyet, B.T.M.; Nghi, N.H.; Khieu, D.Q. Photocatalytic Degradation of Methylene Blue by Using ZnO/Longan Seed Activated Carbon Under Visible-Light Region. J. Inorg. Organomet. Polym. Mater. 2021, 31, 446–459. [Google Scholar] [CrossRef]
- Guo, R.; Xi, B.; Guo, C.; Liu, W.; Lv, N.; Xu, J. Comprehensive Insight into Heterogeneous Persulfate Activation for Environmental Pollutants Degradation: Approaches and Mechanism. Environ. Funct. Mater. 2022, 1, 239–252. [Google Scholar] [CrossRef]
- Tizaoui, C.; Grima, N.M.; Derdar, M.Z. Effect of the Radical Scavenger T-Butanol on Gas–Liquid Mass Transfer. Chem. Eng. Sci. 2009, 64, 4375–4382. [Google Scholar] [CrossRef]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-Hydroquinone Based Determination of Electron and Superoxide Radical Formed in Heterogeneous Photocatalytic Systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Q.; Tang, G.; Zhu, W.; Luo, Y. Controllable Synthesis of Solar-Light-Driven BiOCl Nanostructures for Highly Efficient Photocatalytic Degradation of Carbamazepine. J. Solid State Chem. 2019, 277, 133–138. [Google Scholar] [CrossRef]
- Kobielusz, M.; Mikrut, P.; Macyk, W. Photocatalytic Synthesis of Chemicals. Adv. Inorg. Chem. 2018, 72, 93–144. [Google Scholar] [CrossRef]
- Shan, A.; Lu, Y.; Cheng, L.; Hou, Z.; Xili, D.; Li, Y.; Liu, J.; Ma, H.; Yang, J. Materials Today Sustainability Enhanced Direct Degradation of Photogenerated Holes Induced by Dissolved O2 Chemisorbed on Oxygen-de Fi Cient Nano-TiO2. Mater. Today Sustain. 2023, 22, 100391. [Google Scholar] [CrossRef]
- Demyanenko, A.V.; Bogomolov, A.S.; Dozmorov, N.V.; Svyatova, A.I.; Pyryaeva, A.P.; Goldort, V.G.; Kochubei, S.A.; Baklanov, A.V. Singlet Oxygen 1O2 in Photocatalysis on TiO2. Where Does It Come From? J. Phys. Chem. C 2019, 123, 2175–2181. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Boczkaj, G.; Aravindakumar, C.T. Singlet Oxygen in the Removal of Organic Pollutants: An Updated Review on the Degradation Pathways Based on Mass Spectrometry and DFT Calculations. Chemosphere 2023, 345, 140203. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, X.; Li, T.; Gao, S.; Gao, D.; Guo, Q.; Wang, L.; Hu, X. Facile Synthesis of Nano CeO2/Sepiolite Composite as Visible-Light-Driven Photocatalyst for Rapid Tetracycline Removal. J. Environ. Chem. Eng. 2024, 12, 112829. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 02, 154–160. [Google Scholar] [CrossRef]
- Dimitriadou, S.; Frontistis, Z.; Petala, A.; Bampos, G.; Mantzavinos, D. Carbocatalytic Activation of Persulfate for the Removal of Drug Diclofenac from Aqueous Matrices. Catal. Today 2020, 355, 937–944. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).