Synthesis of In-Modified TiO2 Composite Materials from Waste Tobacco Stem Silk and Study of Their Catalytic Performance under Visible Light
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Materials
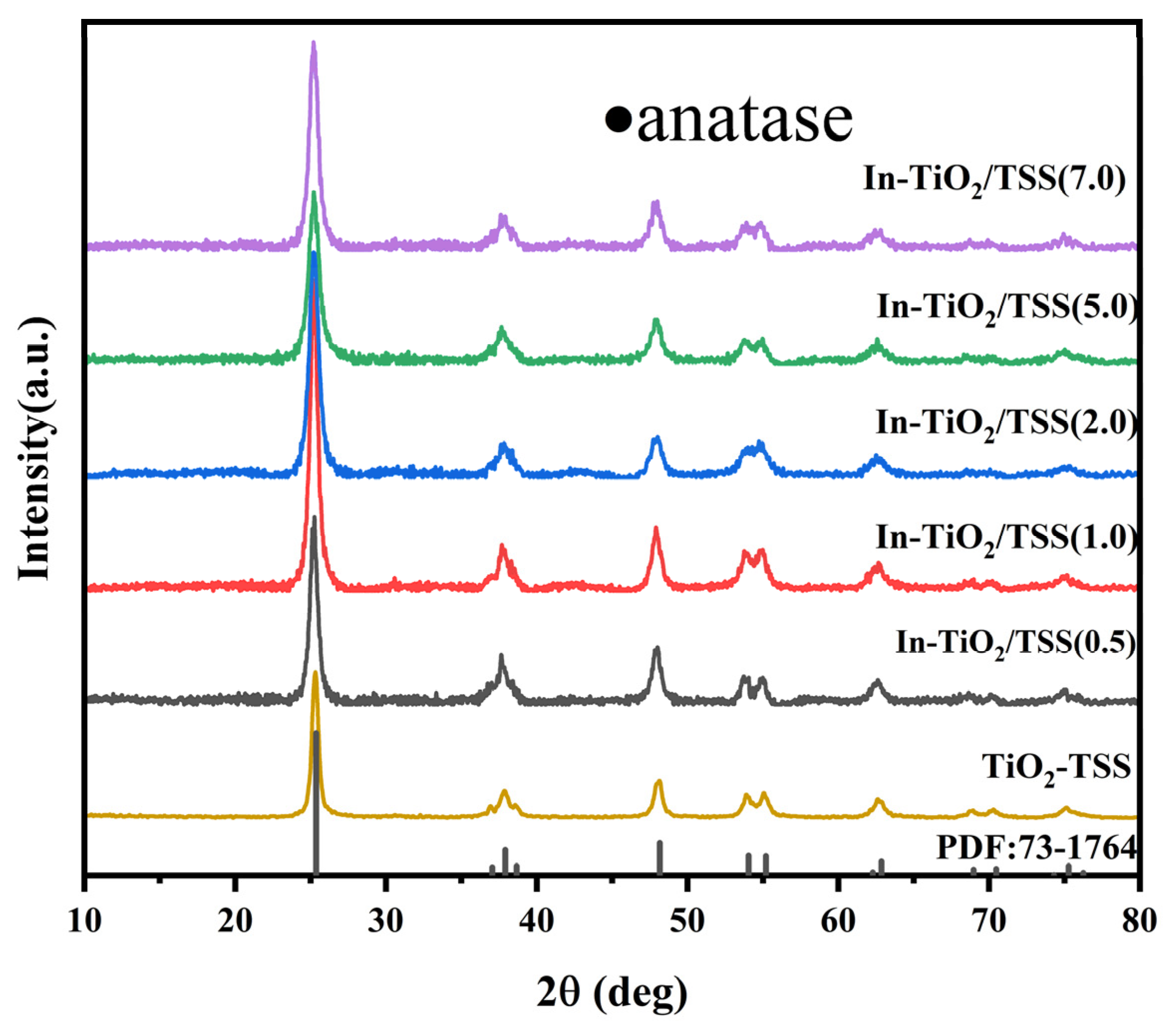
2.1.1. X-ray Diffraction (XRD) Analysis
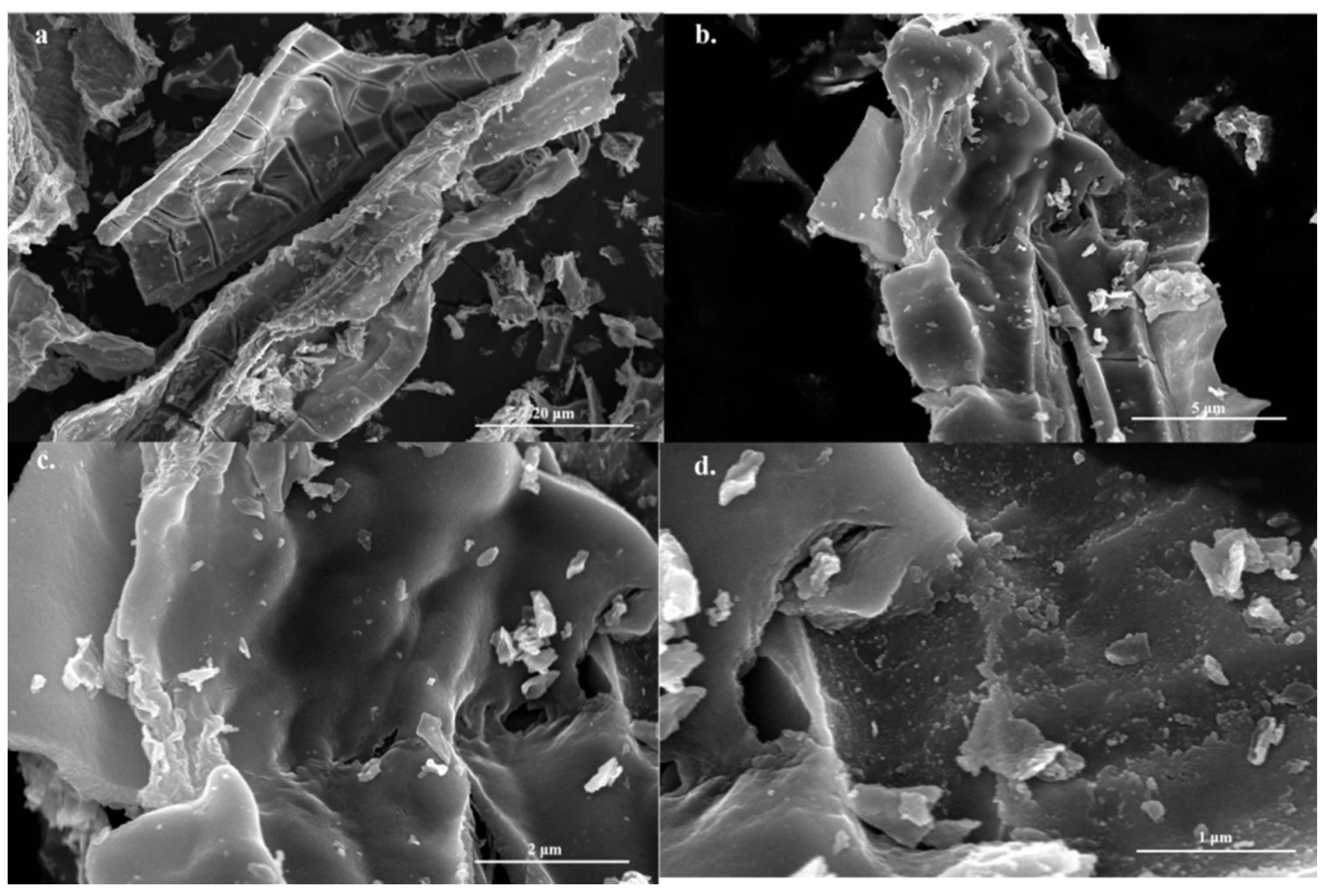
2.1.2. Scanning Electron Microscopy (SEM) Analysis of the Materials
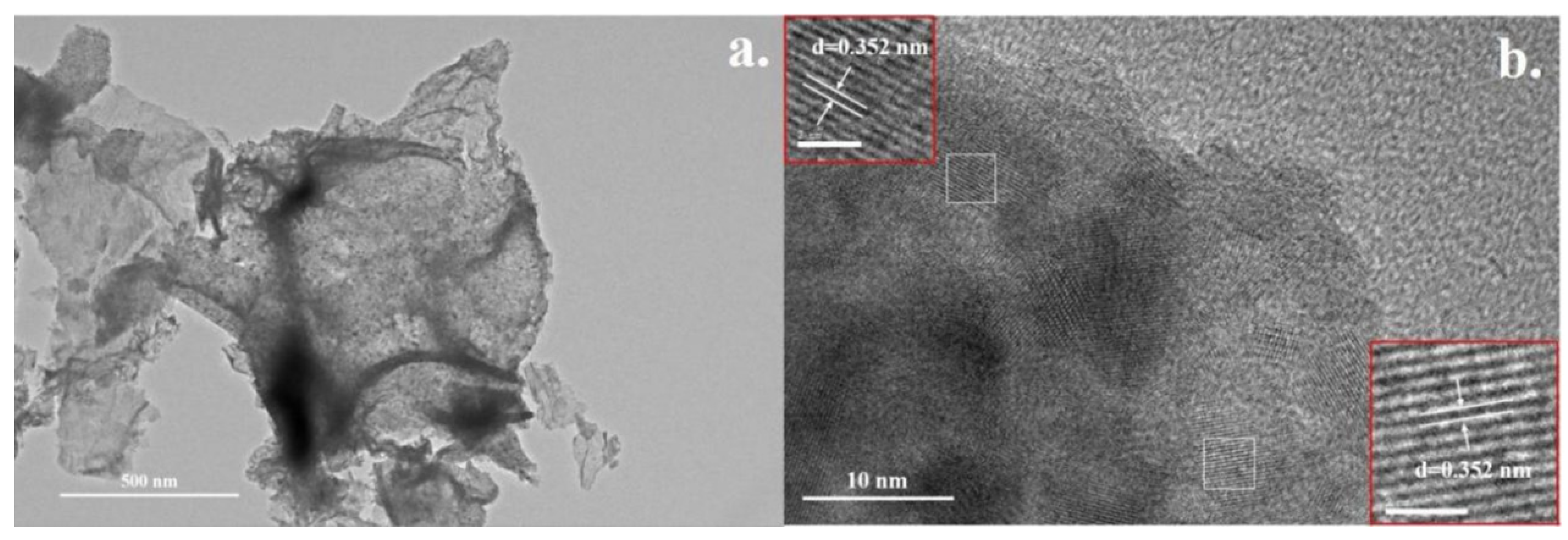
2.1.3. Transmission Electron Microscopy (TEM) Analysis of the Materials
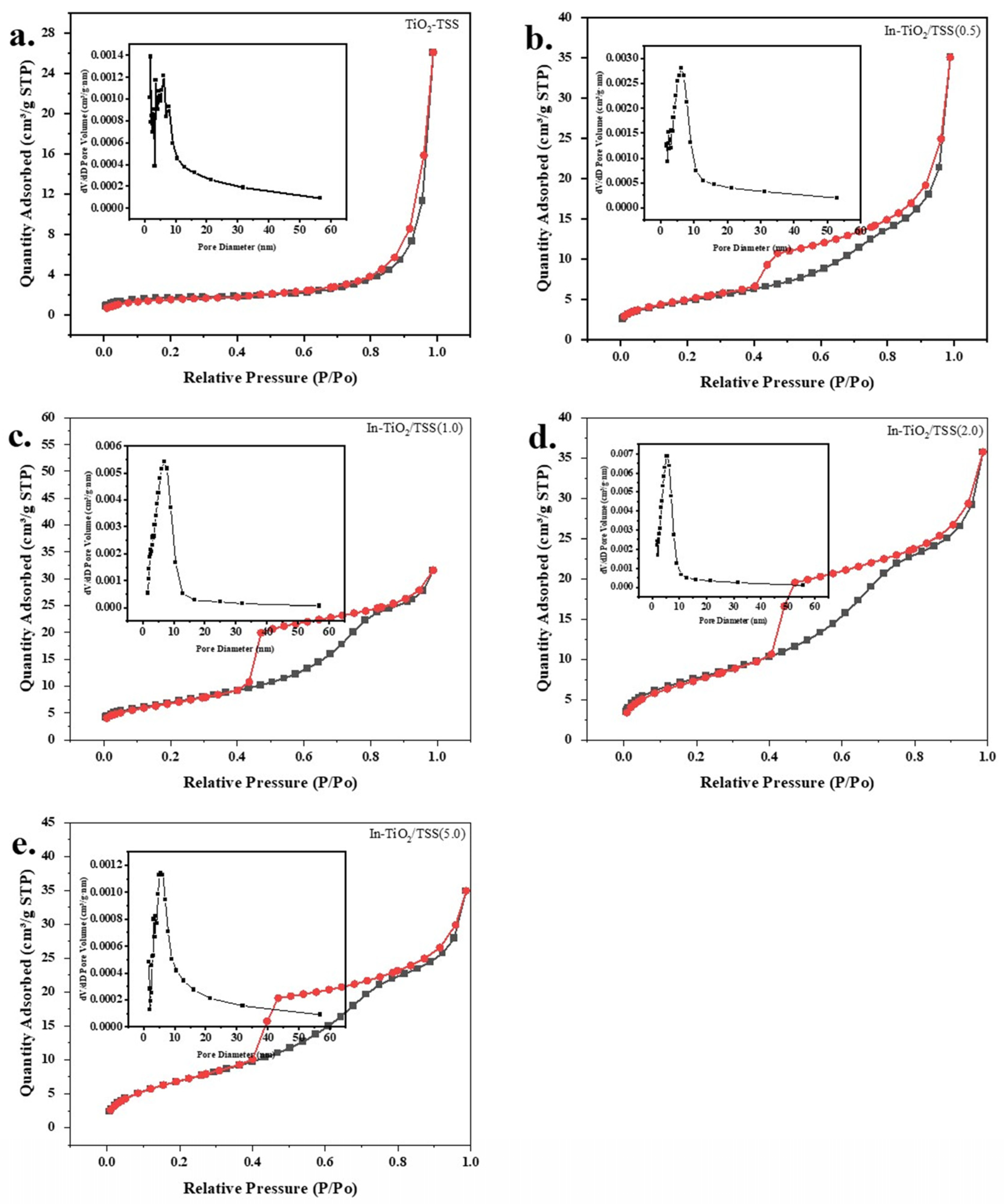
2.1.4. N2 Adsorption–Desorption Analysis of the Materials
2.2. Exploring the Performance
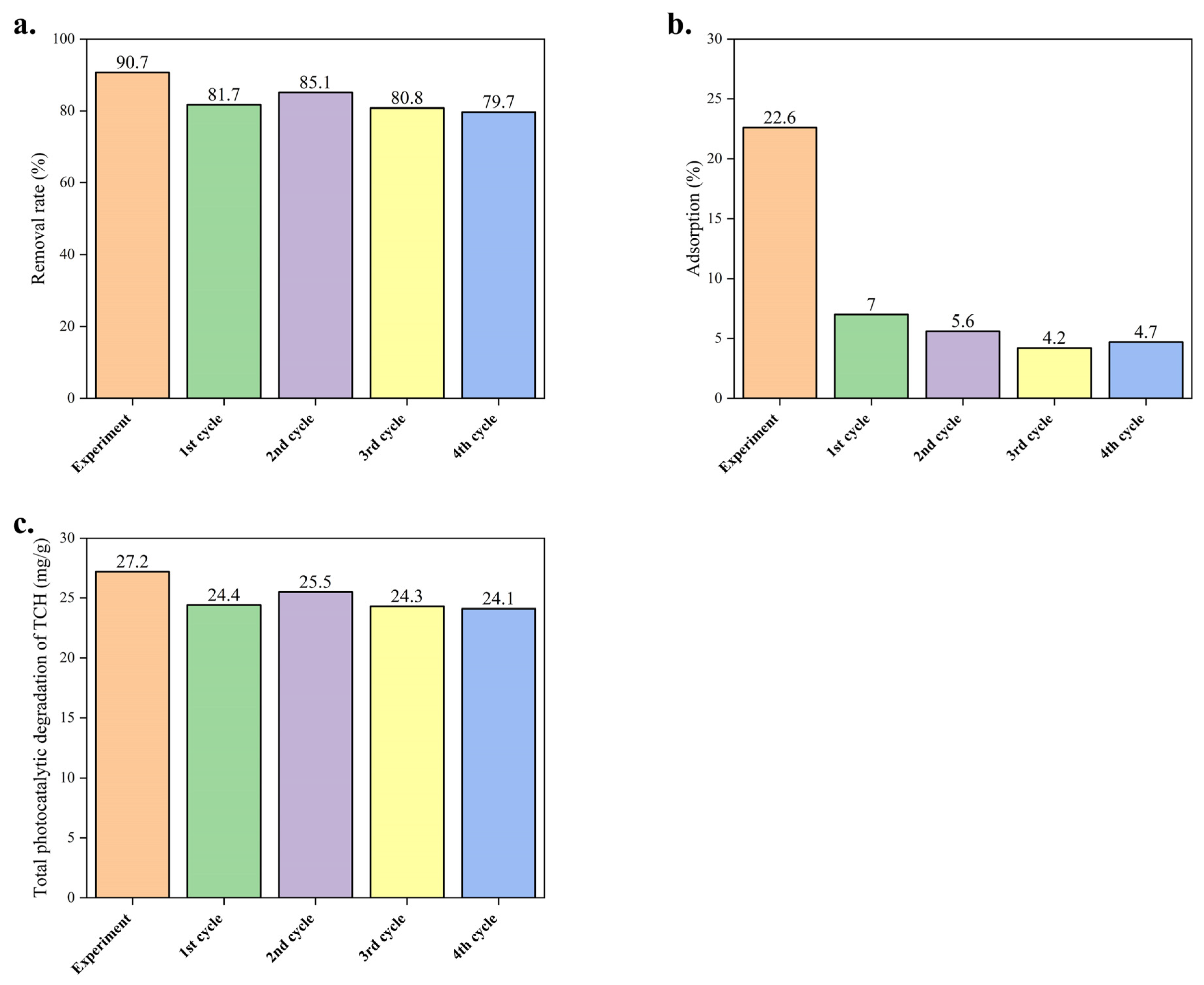
2.3. The Stability of Their Performance
2.4. Mechanistic Investigation of Enhanced Visible-Light Photocatalytic Activity
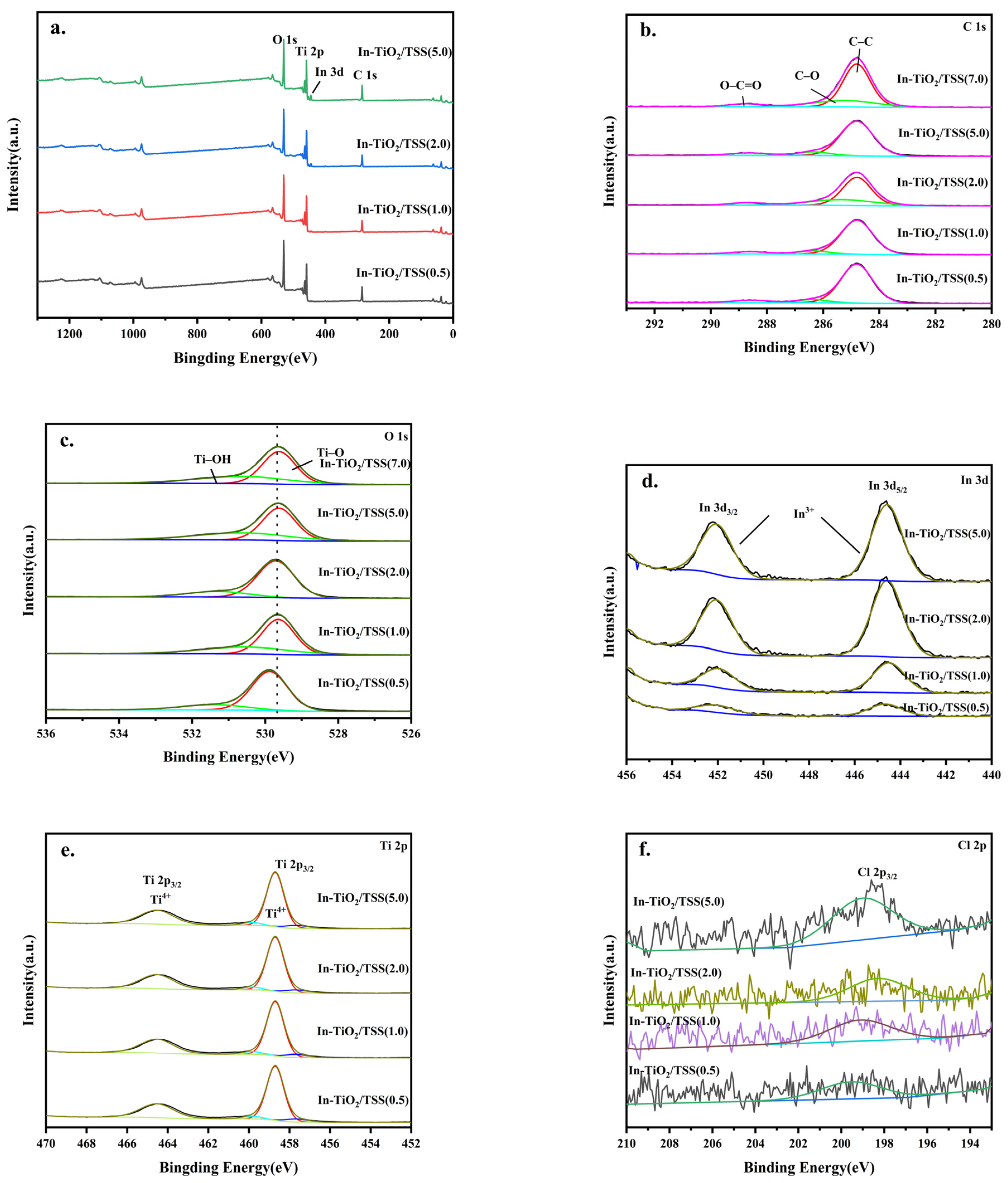
2.4.1. X-ray Photoelectron Spectroscopy (XPS) Analysis of the Materials
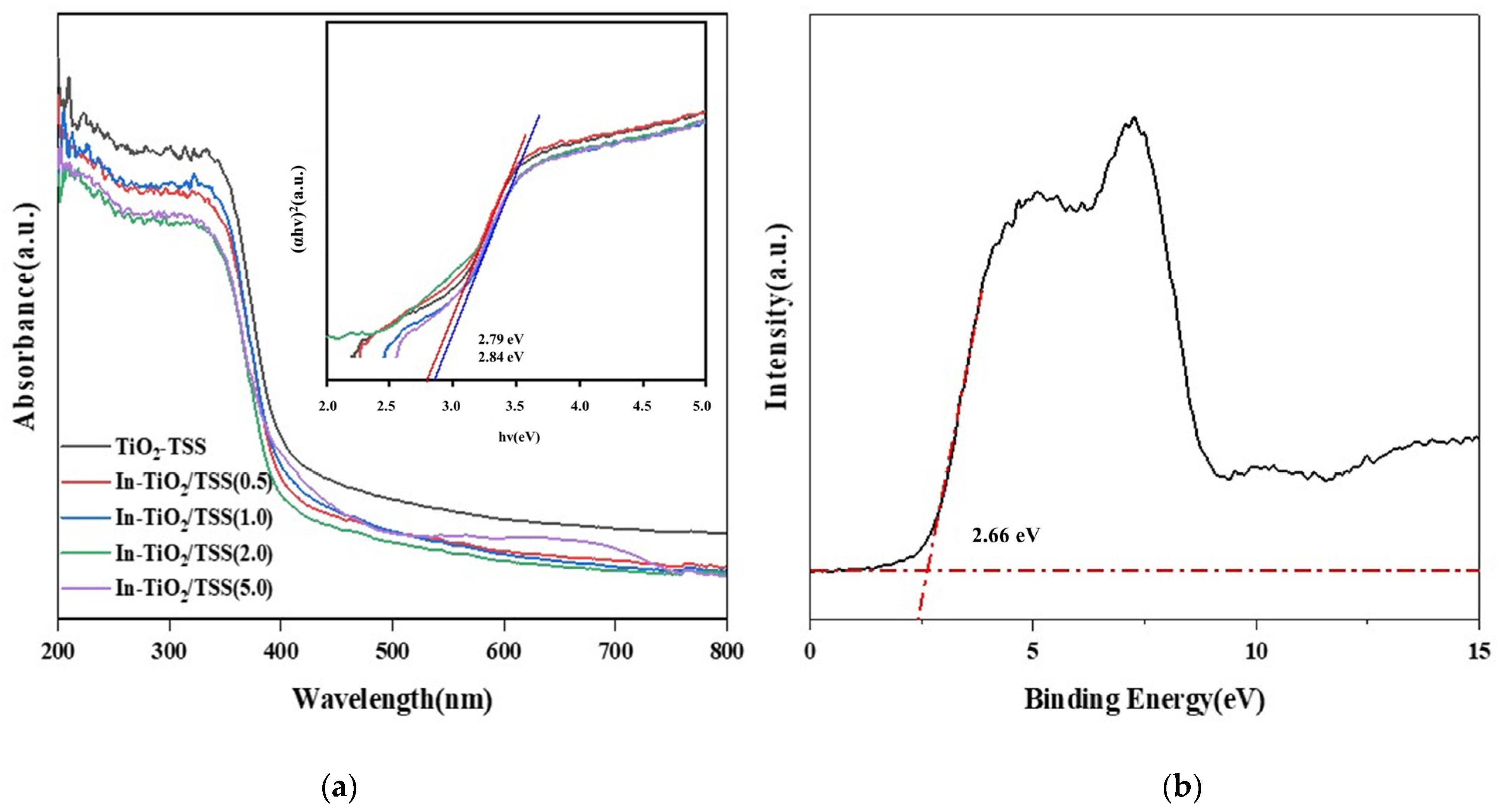
2.4.2. Analysis of Ultraviolet–Visible Diffuse Reflectance Spectroscopy (UV-Vis) and Diffuse Reflectance Spectra
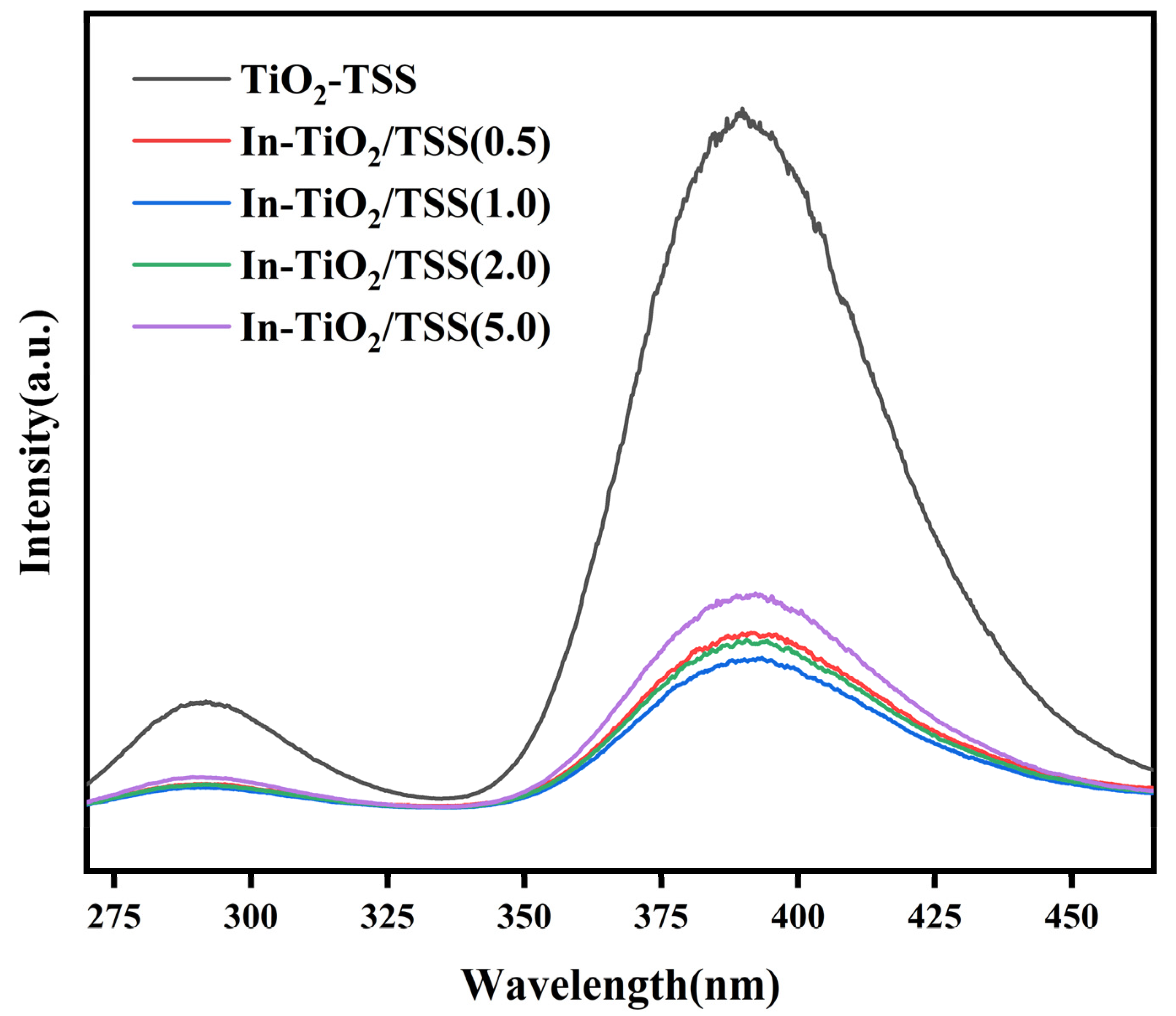
2.4.3. Analysis of Photoluminescence (PL)
2.4.4. Electrochemical Analysis
2.4.5. The Study of Active Species Trapping
3. Preparation and Structural Characterization of the Material
3.1. Materials
3.2. Sample Preparation
3.3. Testing Methods for Materials
3.4. Experimental Assessment of Material Degradation Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.Q.; Xu, H.; Cheng, T.F.; Tang, S.T.; Zhang, D.Y.; Li, M.C.; Pan, X.L. Affinity-based alleviation of dissolved organic matter (DOM) on tetracycline toxicity to photosynthesis of green algae Chlorella vulgaris: Roles of hydrophilic and hydrophobic DOM. Environ. Sci. Pollut. Res. 2023, 30, 42165–42175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ou, D.; Gu, G.; Gao, S.; Li, X.; Hu, C.; Liang, X.; Zhang, Y. Removal of tetracycline from water by catalytic photodegradation combined with the microalga Scenedesmus obliquus and the responses of algal photosynthesis and transcription. J. Environ. Manag. 2023, 326, 116693. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Wu, X.W. Microbial degradation of tetracycline in the aquatic environment: A review. Crit. Rev. Biotechnol. 2020, 40, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.W.; Li, H.; Wang, G.; Zhang, C.; Wang, Y.X.; Yang, L.J.; Li, M.M.; Lu, J.M. Adsorption, isolated electron/hole transport, and confined catalysis coupling to enhance the photocatalytic degradation performance. Appl. Catal. B Environ. 2022, 303, 120892. [Google Scholar] [CrossRef]
- Seo, Y.S.; Oh, S.G. Controlling the recombination of electron-hole pairs by changing the shape of ZnO nanorods via sol-gel method using water and their enhanced photocatalytic properties. Korean J. Chem. Eng. 2019, 36, 2118–2124. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H.G. Defect-mediated electron-hole separation in semiconductor photocatalysis. Inorg. Chem. Front. 2018, 5, 1240–1254. [Google Scholar] [CrossRef]
- Chen, T.S.; Zhang, Q.X.; Zheng, X.S.; Xie, Z.J.; Zeng, Y.Q.; Chen, P.; Liu, H.J.; Liu, Y.; Lv, W.Y.; Liu, G.G. Photocatalyst with a metal-free electron-hole pair double transfer mechanism for pharmaceutical and personal care product degradation. Environ. Sci. Nano 2019, 6, 3292–3306. [Google Scholar] [CrossRef]
- Szkoda, M.; Trzcinski, K.; Nowak, A.P.; Coy, E.; Wicikowski, L.; Lapinski, M.; Siuzdak, K.; Lisowska-Oleksiak, A. Titania nanotubes modified by a pyrolyzed metal-organic framework with zero valent iron centers as a photoanode with enhanced photoelectrochemical, photocatalytical activity and high capacitance. Electrochim. Acta 2018, 278, 13–24. [Google Scholar] [CrossRef]
- Song, K.W.; Cui, Y.; Liu, L.; Chen, B.Y.; Hirose, K.; Shahiduzzaman, M.; Umezu, S. Electro-spray deposited TiO2 bilayer films and their recyclable photocatalytic self-cleaning strategy. Sci. Rep. 2022, 12, 1582. [Google Scholar] [CrossRef]
- Gagliardi, S.; Rondino, F.; Paoletti, C.; Falconieri, M. On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial. Nanomaterials 2022, 12, 2608. [Google Scholar] [CrossRef]
- Sinha, R.; Ghosal, P.S. A comprehensive appraisal on status and management of remediation of DBPs by TiO2 based-photocatalysts: Insights of technology, performance and energy efficiency. J. Environ. Manag. 2023, 328, 117011. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, E.; Daoud, W.A.; Seyedin, S.; Wang, J.F.; Razal, J.M.; Sun, L.; Wang, X.A. Tunable photocatalytic selectivity of TiO2/SiO2 nanocomposites: Effect of silica and isolation approach. Colloid Surf. A—Physicochem. Eng. Asp. 2018, 552, 130–141. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Chandrasekaran, A. Recent advances in TiO2/ZnS-based binary and ternary photocatalysts for the degradation of organic pollutants. Sci. Total Environ. 2023, 868, 161525. [Google Scholar] [CrossRef]
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Hasan, I.; Rana, A. A review on in SITU green synthesis of titanium dioxide nanoparticles and their photocatalytic activities. Mater. Today Proc. 2023, 81, 916–918. [Google Scholar] [CrossRef]
- Hasan, I.; Alharthi, F.A. Caffeine-alginate immobilized CeTiO4 bionanocomposite for efficient photocatalytic degradation of methylene blue. J. Photochem. Photobiol. A Chem. 2022, 433, 114126. [Google Scholar] [CrossRef]
- Yang, R.J.; Fan, Y.Y.; Zhang, Y.F.; Mei, L.; Zhu, R.S.; Qin, J.Q.; Hu, J.G.; Chen, Z.X.; Ng, Y.H.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem.—Int. Ed. 2023, 135, e202218016. [Google Scholar] [CrossRef]
- Khurshid, F.; Jeyavelan, M.; Nagarajan, S. Photocatalytic dye degradation by graphene oxide doped transition metal catalysts. Synth. Met. 2021, 278, 116832. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Friedmann, D.; Caruso, R.A. Indium Oxides and Related Indium-based Photocatalysts for Water Treatment: Materials Studied, Photocatalytic Performance, and Special Highlights. Sol. RRL 2021, 5, 2100086. [Google Scholar] [CrossRef]
- Kurnaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Wang, E.J.; Yang, W.S.; Cao, Y.A. Unique Surface Chemical Species on Indium Doped TiO2 and Their Effect on the Visible Light Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 20912–20917. [Google Scholar] [CrossRef]
- Reñones, P.; Fresno, F.; Oropeza, F.E.; Gorni, G.; O’Shea, V.A.D. Structural and electronic insight into the effect of indium doping on the photocatalytic performance of TiO2 for CO2 conversion. J. Mater. Chem. A 2022, 10, 6054–6064. [Google Scholar] [CrossRef]
- Sobahi, T.R.; Amin, M.S.; Mohamed, R.M. Enlargement of photocatalytic efficiency of BaSnO3 by indium doping for thiophene degradation. Appl. Nanosci. 2018, 8, 557–565. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Kumar, A.; Hasija, V.; Singal, S.; Singh, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Nguyen, V.H. Indium sulfide-based photocatalysts for hydrogen production and water cleaning: A review. Environ. Chem. Lett. 2021, 19, 1065–1095. [Google Scholar] [CrossRef]
- Ding, J.J.; Sun, S.; Bao, J.; Luo, Z.L.; Gao, C. Synthesis of CaIn2O4 Rods and Its Photocatalytic Performance under Visible-light Irradiation. Catal. Lett. 2009, 130, 147–153. [Google Scholar] [CrossRef]
- Yu, J.G.; Dai, G.P.; Cheng, B. Effect of Crystallization Methods on Morphology and Photocatalytic Activity of Anodized TiO2 Nanotube Array Films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Qian, J.; Wang, L.; Lavoine, N.; Newman, R.; Gardner, D.J.; Li, T.; Hu, L.B.; Ragauskas, A.J.; et al. Recent Advances in Functional Materials through Cellulose Nanofiber Templating. Adv. Mater. 2021, 33, 2005538. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.X.; Zhou, J.H.; Li, X.; Wang, F. Application of Plant Viruses as a Biotemplate for Nanomaterial Fabrication. Molecules 2018, 23, 2311. [Google Scholar] [CrossRef]
- Hüttl, R.; Ullrich, F.; Wolf, G.; Kirchner, A.; Löthman, P.; Katzschner, B.; Pompe, W.; Mertig, M. Catalytic Carbon Monoxide Oxidation Using Bio-Templated Platinum Clusters. Catal. Lett. 2009, 132, 383–388. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, T.X.; Zhang, D. Biotemplated Materials for Sustainable Energy and Environment: Current Status and Challenges. ChemSusChem 2011, 4, 1344–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.K.; Shao, Q.; Ge, S.S.; Zhang, J.X.; Lin, J.; Cao, D.P.; Wu, S.D.; Dong, M.Y.; Guo, Z.H. Advances in Template Prepared Nano-oxides and Their Applications: Polluted Water Treatment, Energy, Sensing and Biomedical Drug Delivery. Chem. Rec. 2020, 20, 710–729. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Cheang, U.K.; Darvish, A.; Kim, H.; Kim, M.J. Biotemplated flagellar nanoswimmers. APL Mater. 2017, 5, 116106. [Google Scholar] [CrossRef]
- Bereczk-Tompa, É.; Vonderviszt, F.; Horváth, B.; Szalai, I.; Pósfai, M. Biotemplated synthesis of magnetic filaments. Nanoscale 2017, 9, 15062–15069. [Google Scholar] [CrossRef] [PubMed]
- Adigun, O.O.; Retzlaff-Roberts, E.L.; Novikova, G.; Wang, L.F.; Kim, B.S.; Ilavsky, J.; Miller, J.T.; Loesch-Fries, L.S.; Harris, M.T. BSMV as a Biotemplate for Palladium Nanomaterial Synthesis. Langmuir 2017, 33, 1716–1724. [Google Scholar] [CrossRef]
- Sutar, S.; Otari, S.; Jadhav, J. Biochar based photocatalyst for degradation of organic aqueous waste: A review. Chemosphere 2022, 287, 132200. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Wang, L.; Wang, Q.; Zhang, Q.; Cui, F.; Jiang, G. Synthesis of S-type heterostructure π-COF for photocatalytic tetracycline degradation. Chem. Eng. J. 2024, 479, 147534. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjorklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Wang, X.; Jia, J.; Wang, Y. Combination of photocatalysis with hydrodynamic cavitation for degradation of tetracycline. Chem. Eng. J. 2017, 315, 274–282. [Google Scholar] [CrossRef]
- Wang, B.; Wang, T.; Su, H. Hydrodynamic cavitation (HC) degradation of tetracycline hydrochloride (TC). Sep. Purif. Technol. 2022, 282, 120095. [Google Scholar] [CrossRef]
- Lan, Z.J.; Yu, Y.L.; Yan, S.; Wang, E.J.; Yao, J.H.; Cao, Y.A. Synergetic effect of N3−, In3+ and Sn4+ ions in TiO2 towards efficient visible photocatalysis. J. Photochem. Photobiol. A Chem. 2018, 356, 132–137. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhu, X.F.; Duan, J.T.; Li, H.; Xu, L.; Xiong, L.Z.; Luo, Q.; Yang, M.H.; Chang, X. Comparative study on properties of polypropylene-based composites reinforced with tobacco stalk fibers (unmodified/modified) from different parts of tobacco stalk. Ind. Crops Prod. 2023, 201, 116890. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Xie, M.; Jing, L.; Xu, H.; She, X.; Li, H.; Xie, J. Construction of novel CNT/LaVO4 nanostructures for efficient antibiotic photodegradation. Chem. Eng. J. 2019, 357, 487–497. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Xu, K.; Jiang, W.; Liu, Y.; Leng, Z.; Liu, J. Construction of fiber-shaped silver oxide/tantalum nitride p-n heterojunctions as highly efficient visible-light-driven photocatalysts. J. Colloid Interface Sci. 2017, 504, 561–569. [Google Scholar] [CrossRef]
- Jing, H.; Ou, R.; Yu, H.; Zhao, Y.; Lu, Y.; Huo, M.; Huo, H.; Wang, X. Engineering of g-C3N4 nanoparticles/WO3 hollow microspheres photocatalyst with Z-scheme heterostructure for boosting tetracycline hydrochloride degradation. Sep. Purif. Technol. 2021, 255, 117646. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Ofomaja, A.E. Facile microwave synthesis of pine cone derived C-doped TiO2 for the photodegradation of tetracycline hydrochloride under visible-LED light. J. Environ. Manag. 2018, 223, 860–867. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B Environ. 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Shi, K.P.; Wang, J.; Yin, L.; Xu, Y.; Kong, D.S.; Li, H.X.; Zhang, Y.; He, H.; Yang, S.G.; Ni, L.X.; et al. Photocatalysis Combined with Microalgae to Promote the Degradation and Detoxification of Tetracycline Hydrochloride. Bull. Environ. Contam. Toxicol. 2023, 110, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Zhang, G.A.; Luo, B.M.; Sun, D.F.; Yan, X.B.; Xue, Q.J. Surface amorphization and deoxygenation of graphene oxide paper by Ti ion implantation. Carbon 2011, 49, 3141–3147. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrogen Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Naseem, S.; Pinchuk, I.V.; Luo, Y.K.; Kawakami, R.K.; Khan, S.; Husain, S.; Khan, W. Epitaxial growth of cobalt doped TiO2 thin films on LaAlO3(100) substrate by molecular beam epitaxy and their opto-magnetic based applications. Appl. Surf. Sci. 2019, 493, 691–702. [Google Scholar] [CrossRef]
- Gao, L.K.; Gan, W.T.; Qiu, Z.; Cao, G.L.; Zhan, X.X.; Qiang, T.G.; Li, J. Biomorphic Carbon-Doped TiO2 for Photocatalytic Gas Sensing with Continuous Detection of Persistent Volatile Organic Compounds. ACS Appl. Nano Mater. 2018, 1, 1766–1775. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Zhang, Z.M.; Liu, L.; Bai, L.; Bao, R.Y.; Yang, M.B.; Yang, W. Degradable ultrathin high-performance photocatalytic hydrogen generator from porous electrospun composite fiber membrane with enhanced light absorption ability. J. Mater. Chem. A 2021, 9, 10277–10288. [Google Scholar] [CrossRef]
- Huang, D.L.; Li, J.; Zeng, G.M.; Xue, W.J.; Chen, S.; Li, Z.H.; Deng, R.; Yang, Y.; Cheng, M. Facile construction of hierarchical flower-like Z-scheme AgBr/Bi2WO6 photocatalysts for effective removal of tetracycline: Degradation pathways and mechanism. Chem. Eng. J. 2019, 375, 15. [Google Scholar] [CrossRef]
- Ma, D.D.; Wang, Z.Y.; Shi, J.W.; Zou, Y.J.; Lv, Y.X.; Ji, X.; Li, Z.H.; Cheng, Y.H.; Wang, L.Z. An ultrathin Al2O3 bridging layer between CdS and ZnO boosts photocatalytic hydrogen production. J. Mater. Chem. A 2020, 8, 11031–11042. [Google Scholar] [CrossRef]
- Wang, L.M.; Sun, Y.; Zhang, F.Y.; Hu, J.T.; Hu, W.T.; Xie, S.J.; Wang, Y.K.; Feng, J.; Li, Y.B.; Wang, G.Y.; et al. Precisely Constructed Metal Sulfides with Localized Single-Atom Rhodium for Photocatalytic C-H Activation and Direct Methanol Coupling to Ethylene Glycol. Adv. Mater. 2023, 35, 9. [Google Scholar] [CrossRef]
- Xing, W.N.; Li, C.M.; Wang, Y.; Han, Z.H.; Hu, Y.D.; Chen, D.H.; Meng, Q.Q.; Chen, G. A novel 2D/2D carbonized poly-(furfural alcohol)/g-C3N4 nanocomposites with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 2017, 115, 486–492. [Google Scholar] [CrossRef]
- Guo, C.R.; Qin, X.; Guo, R.; Lv, Y.; Li, M.R.; Wang, Z.Y.; Li, T.H. Optimization of heterogeneous Fenton-like process with Cu-Fe@CTS as catalyst for degradation of organic matter in leachate concentrate and degradation mechanism research. Waste Manag. 2021, 134, 220–230. [Google Scholar] [CrossRef]
- Wang, J.P.; Li, C.Q.; Cong, J.K.; Liu, Z.W.; Zhang, H.Z.; Liang, M.; Gao, J.K.; Wang, S.L.; Yao, J.M. Facile synthesis of nanorod-type graphitic carbon nitride/Fe2O3 composite with enhanced photocatalytic performance. J. Solid State Chem. 2016, 238, 246–251. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhao, C.C.; Wang, D.J.; Wang, Y.Q.; Liu, F. •OH− initiated heterogeneous oxidation of methyl orange using an Fe- Ce/MCM-41 catalyst. RSC Adv. 2016, 6, 18800–18808. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Nunes, B.N.; AlSalka, Y.; Hakki, A.; Curti, M.; Patrocinio, A.O.T.; Bahnemann, D.W. Application of EPR Spectroscopy in TiO2 and Nb2O5 Photocatalysis. Catalysts 2021, 11, 1514. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Zeng, B.; Li, C.; Han, H. EPR study of charge separation associated states and reversibility of surface bound superoxide radicals in SrTiO3 photocatalyst. J. Energy Chem. 2022, 70, 388–393. [Google Scholar] [CrossRef]
- Morra, E.; Giamello, E.; Chiesa, M. EPR approaches to heterogeneous catalysis. The chemistry of titanium in heterogeneous catalysts and photocatalysts. J. Magn. Reson. 2017, 280, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, D.D.; Li, H.J.; Jiang, W.; Zhou, T.Y.; Wen, Y.; Yu, B.; Che, G.B.; Wang, L. Boosting interfacial charge separation and photocatalytic activity of 2D/2D g-C3N4/ZnIn2S4S-scheme heterojunction under visible light irradiation. J. Alloys Compd. 2022, 894, 162209. [Google Scholar] [CrossRef]
- Li, J.J.; Ding, Y.; Chen, K.Y.; Li, Z.N.; Yang, H.J.; Yue, S.J.; Tang, Y.P.; Wang, Q.Z. δ-FeOOH coupled BiOBr0.5I0.5 for efficient photocatalysis-Fenton synergistic degradation of organic pollutants. J. Alloys Compd. 2022, 903, 163795. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.N.; Luo, X.B.; Luo, S.L.; Dionysiou, D.D. Highly efficient visible-light photocatalytic performance of Ag/AgIn5S8 for degradation of tetracycline hydrochloride and treatment of real pharmaceutical industry wastewater. Chem. Eng. J. 2018, 333, 423–433. [Google Scholar] [CrossRef]



| Sample | Cell Parameters (Å) | Cell Volume (Å) | |
|---|---|---|---|
| a = b | c | ||
| TiO2 | 3.776 | 9.486 | 135.253 |
| TiO2-TSS | 3.776 | 9.486 | 135.253 |
| In-TiO2/TSS(0.5) | 3.783 | 9.497 | 135.754 |
| In-TiO2/TSS(1.0) | 3.776 | 9.486 | 135.253 |
| In-TiO2/TSS(2.0) | 3.783 | 9.497 | 135.754 |
| In-TiO2/TSS(5.0) | 3.776 | 9.486 | 135.253 |
| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| TiO2-TSS | 6.3 | 0.04 | 27.0 |
| In-TiO2/TSS(0.5) | 17.3 | 0.05 | 9.01 |
| In-TiO2/TSS(1.0) | 24.9 | 0.04 | 4.5 |
| In-TiO2/TSS(2.0) | 28.0 | 0.05 | 5.2 |
| In-TiO2/TSS(5.0) | 26.9 | 0.05 | 5.2 |
| Catalyst | Concentration (mg/L) | Degradation Efficiency (%) | Refs. |
|---|---|---|---|
| CNT/LaVO4 | 10 | 84 | [43] |
| Ag2O/Ta3N5 | 10 | 78.3 | [44] |
| C3N4 NP/WO3 NHMs | 10 | 79.8 | [45] |
| C-TiO2 | 5 | 70 | [46] |
| Sn3O4/g-C3N4 | 10 | 72.2 | [47] |
| Chlorella sp. | 10 | 50 | [48] |
| TiO2-TSS | 15 | 37 | This work |
| In-TiO2/TSS(2.0) | 15 | 92.9 | This work |
| Photocatalyst | Bandgap (eV) | Photocatalyst | Band Gap (eV) |
|---|---|---|---|
| TiO2-TSS | 3.08 | In-TiO2/TSS(2.0) | 2.79 |
| In-TiO2/TSS(0.5) | 3.01 | In-TiO2/TSS(5.0) | 2.64 |
| In-TiO2/TSS(1.0) | 2.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, J.; Zhao, Y.; Zhang, J.; Bai, X.; Zhang, A.; Li, Q.; Huang, M.; Wang, J. Synthesis of In-Modified TiO2 Composite Materials from Waste Tobacco Stem Silk and Study of Their Catalytic Performance under Visible Light. Catalysts 2024, 14, 615. https://doi.org/10.3390/catal14090615
Leng J, Zhao Y, Zhang J, Bai X, Zhang A, Li Q, Huang M, Wang J. Synthesis of In-Modified TiO2 Composite Materials from Waste Tobacco Stem Silk and Study of Their Catalytic Performance under Visible Light. Catalysts. 2024; 14(9):615. https://doi.org/10.3390/catal14090615
Chicago/Turabian StyleLeng, Junyang, Yi Zhao, Jindi Zhang, Xiaoli Bai, Anlong Zhang, Quanhui Li, Mengyang Huang, and Jiaqiang Wang. 2024. "Synthesis of In-Modified TiO2 Composite Materials from Waste Tobacco Stem Silk and Study of Their Catalytic Performance under Visible Light" Catalysts 14, no. 9: 615. https://doi.org/10.3390/catal14090615
APA StyleLeng, J., Zhao, Y., Zhang, J., Bai, X., Zhang, A., Li, Q., Huang, M., & Wang, J. (2024). Synthesis of In-Modified TiO2 Composite Materials from Waste Tobacco Stem Silk and Study of Their Catalytic Performance under Visible Light. Catalysts, 14(9), 615. https://doi.org/10.3390/catal14090615







