The Potential of Apricot Tree Resin as a Viable Feedstock for High-Value Chemicals via Hydrothermal Gasification
Abstract
1. Introduction
2. Results and Discussion
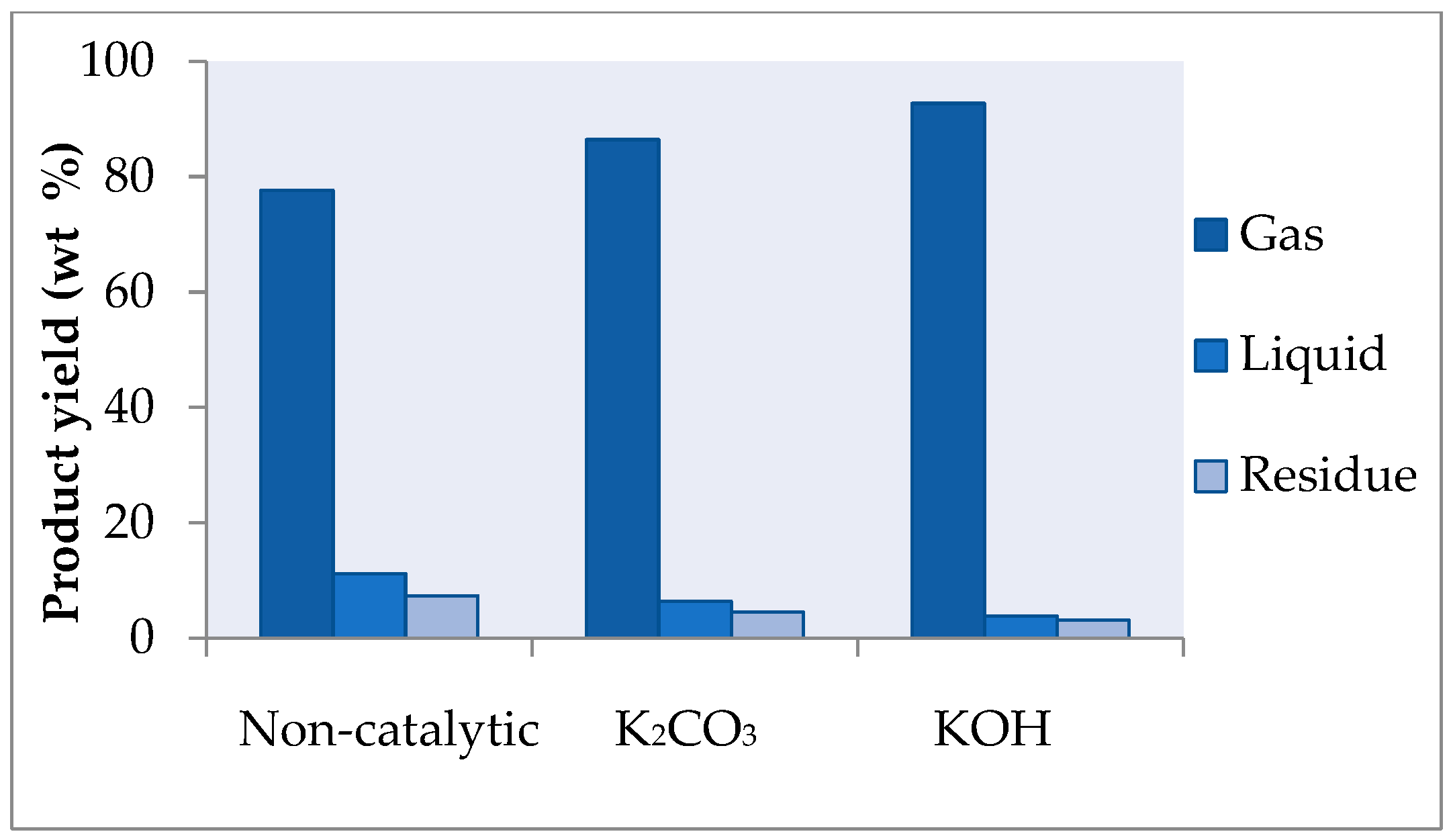
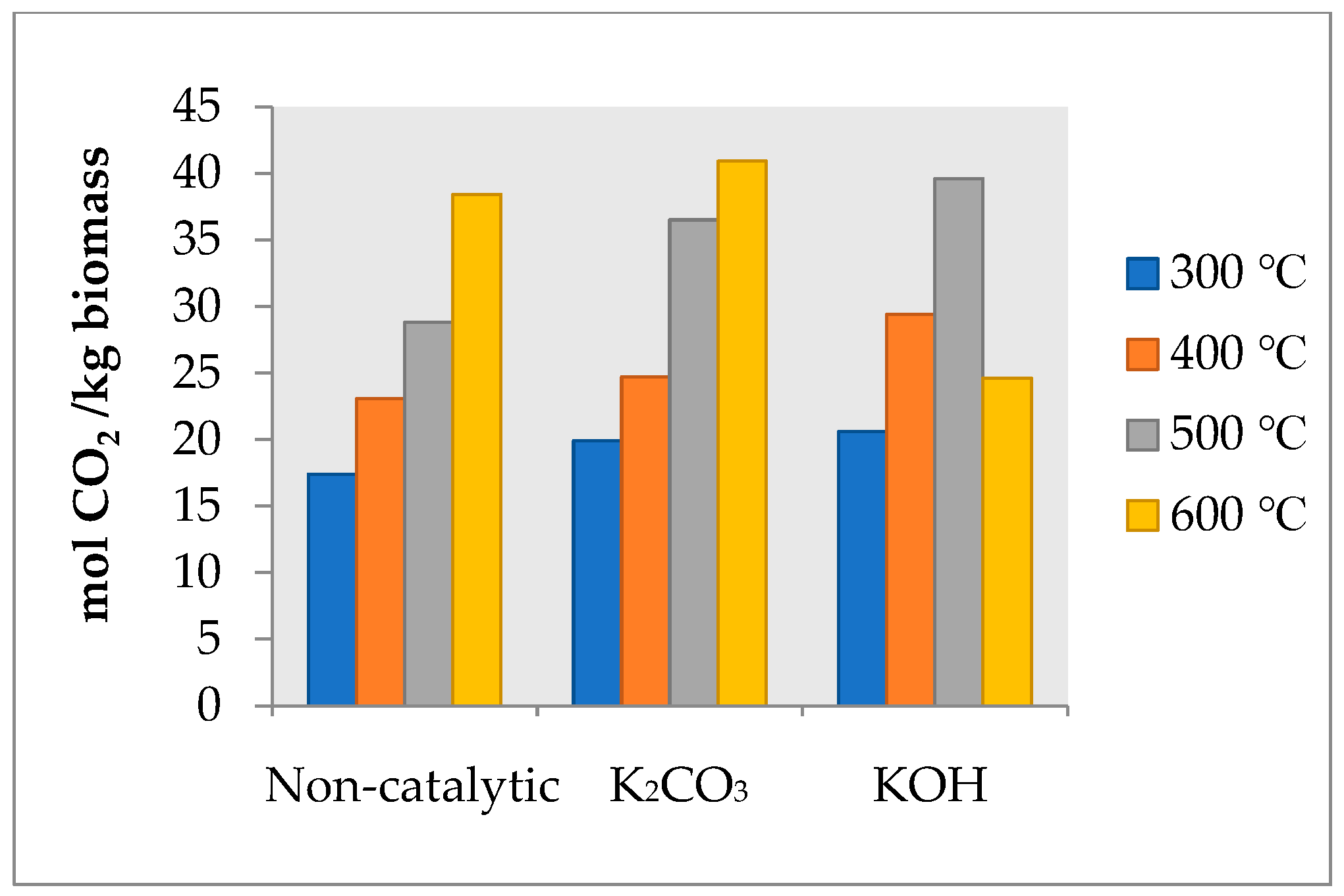
2.1. Influence of Temperature and Catalyst on the Distribution of Products and Gas Composition
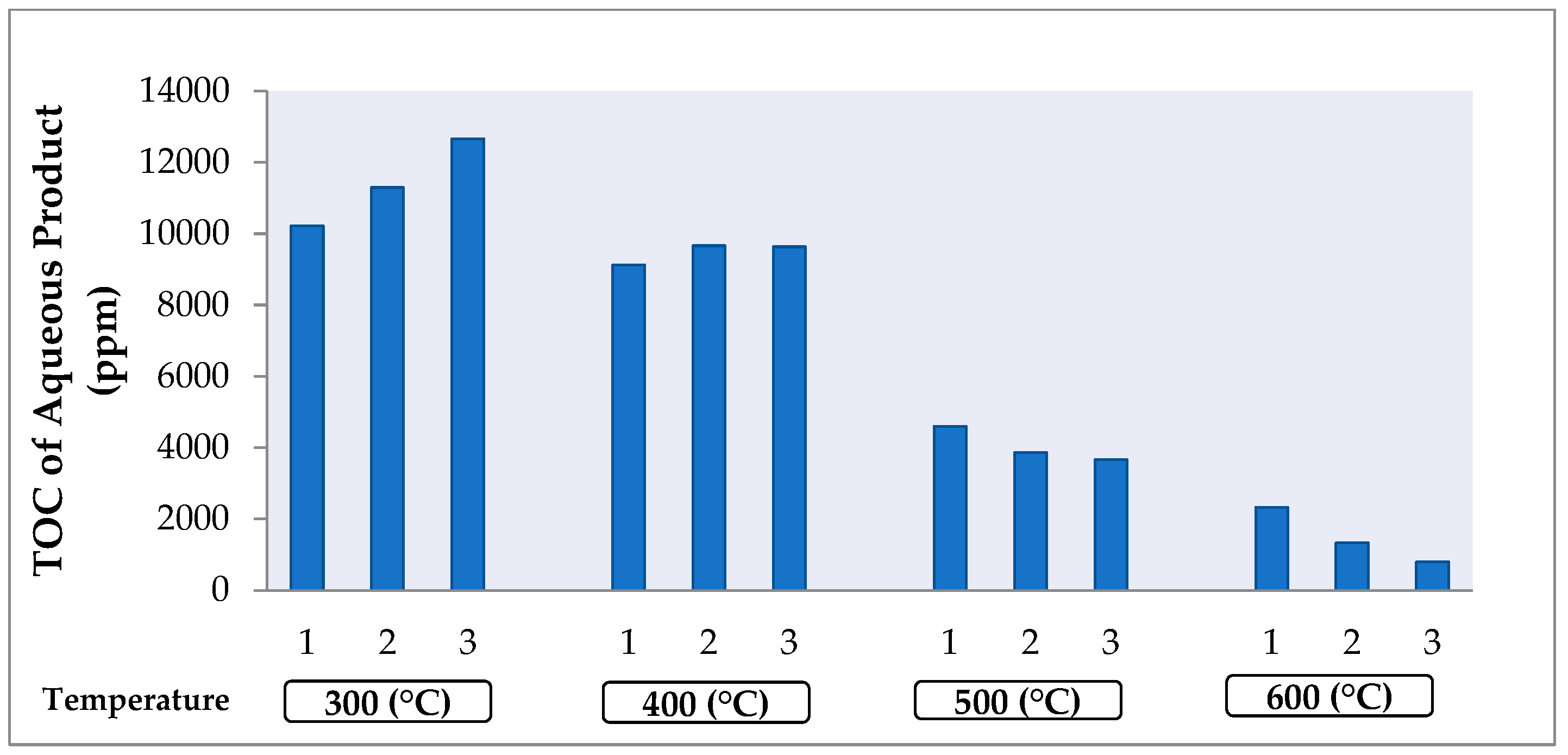
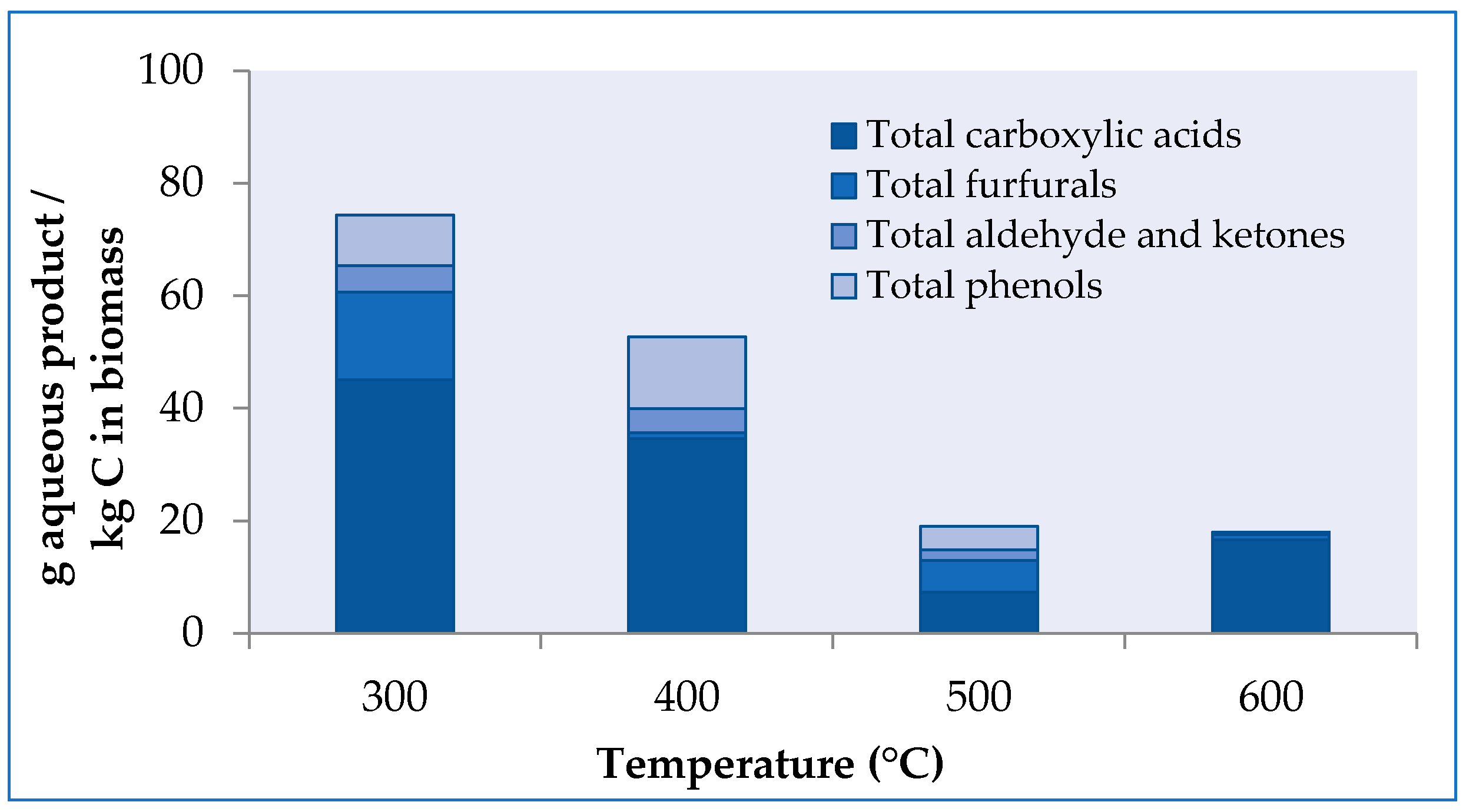
2.2. Influence of Temperature and Catalyst on the Aqueous Product Efficiency and Aqueous Composition
3. Materials and Methods
3.1. Materials
3.2. Experimental System
3.3. Experimental Procedure
3.4. Analysis of Gaseous, Aqueous, and Solid Products
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTG | Hydrothermal Gasification |
| CWG | Catalytic Wet Gasification |
| SCWG | Supercritical Water Gasification |
| WGSR | Water Gas Shift Reaction |
| TOC | Total Organic Carbon |
| HPLC | High-performance liquid chromatography |
| GC | Gas chromatography |
| SSM | Solid Sample Module |
| PID | Proportional-Integral-Derivative |
References
- Poyraz, S.; Gül, M. The development of apricot production and foreign trade in the world and in Turkey. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2022, 22, 601–616. [Google Scholar]
- Fathi, M.; Mohebbi, M.; Koocheki, A. Some physicochemical properties of Prunus armeniaca L. gum exudates. Int. J. Biol. Macromol. 2016, 82, 82744–82750. [Google Scholar] [CrossRef]
- Habib, M.A.; Gubran, A.Q.A.; Alquaity, A.B.S.; Qasem, N.A.A. Hydrogen combustion, production, and applications: A review. Alex. Eng. J. 2024, 100, 182–207. [Google Scholar] [CrossRef]
- Khandelwal, K.; Boahene, P.; Nanda, S.; Dalai, A.K. A Review of the Design and Performance of Catalysts for Hydrothermal Gasification of Biomass to Produce Hydrogen-Rich Gas Fuel. Molecules 2023, 28, 5137. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yan, M.; Wang, S. Recent advances in supercritical water gasification of biowaste catalyzed by transition metal-based catalysts for hydrogen production. Renew. Sustain. Energy Rev. 2022, 154, 111831. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, X.; Wang, Y.; Wang, L. Methane Production from Biomass by Thermochemical Conversion: A Review. Catalysts 2023, 13, 771. [Google Scholar] [CrossRef]
- Nanda, S.; Gong, M.; Hunter, H.N.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. An assessment of pinecone gasification in subcritical, near-critical and supercritical water. Fuel Process. Technol. 2017, 168, 84–96. [Google Scholar] [CrossRef]
- Borges, A.C.P.; Onwudili, J.A.; Andrade, H.M.C.; Alvesa, C.T.; Ingrame, A.; Vieira de Meloa, S.A.B.; Torres, E.A. Catalytic supercritical water gasification of eucalyptus wood chips in a batch reactor. Fuel 2019, 255, 115804. [Google Scholar] [CrossRef]
- Seraj, S.; Azargohar, R.; Bougadda, V.B.; Dalai, A.K. Energy recovery from agro-forest wastes through hydrothermal carbonization coupled with hydrothermal co-gasification: Effects of succinic acid on hydrochars and H2 production. Chemosphere 2023, 337, 139390. [Google Scholar] [CrossRef]
- Zeng, B.; Shimuzu, N. Hydrogen Generation from Wood Chip and Biochar by Combined Continuous Pyrolysis and Hydrothermal Gasification. Energies 2021, 14, 3793. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Dalai, A.K. Hydrogen-rich gas production from hydrothermal gasification of fuel pellets obtained from co-pelletization of agricultural residues. Int. J. Hydrogen Energy 2024, 52, 80–93. [Google Scholar] [CrossRef]
- Salimi, M.; Nejati, B.; Karimi, A.; Tavasoli, A. Hydrothermal gasification performance of Iranian rice straw in supercritical water media for hydrogen-rich gas production. BioResources 2016, 11, 6362–6377. [Google Scholar] [CrossRef][Green Version]
- Nanda, S.; Reddy, S.N.; Dalai, A.K.; Kozinski, J.A. Subcritical and supercritical water gasification of lignocellulosic biomass impregnated with nickel nanocatalyst for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 4907–4921. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Huang, J.; Williams, P.T. Pyrolysis/gasification of cellulose, hemicellulose and lignin for hydrogen production in the presence of various nickel-based catalysts. Fuel 2013, 106, 697–706. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Acevedo, S.; Riazi, M.; Ocampo-Pérez, R.; Cortés, F.B.; Franco, C.A. Catalytic conversion of n-C7 asphaltenes and resins II into hydrogen using CeO2-based nanocatalysts. Nanometerials 2021, 11, 1301. [Google Scholar] [CrossRef]
- Demirel, E.; Erkey, C.; Ayas, N. Supercritical water gasification of fruit pulp for hydrogen production: Effect of reaction parameters. J. Supercrit. Fluids 2021, 177, 105329. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Li, Z.; Chen, D. Characteristics of tar formation during cellulose, hemicellulose and lignin gasification. Fuel 2014, 118, 250–256. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, S.; Russell, C.K.; Hu, J.; Rony, A.H.; Tan, G.; Chen, A. Improvement of H2-rich gas production with tar abatement from pine wood conversion over bi-functional Ca2Fe2O5 catalyst: Investigation of inner-looping redox reaction and promoting mechanisms. Appl. Energy 2018, 212, 931–943. [Google Scholar] [CrossRef]
- Popelier, G.; Dossche, G.; Kulkarni, S.P.; Vermeire, F.; Sabbe, M.; Van Geem, K.M. A critical review of the influence of supercritical water on the pyrolysis of plastic waste: Modelling approaches and process effects. J. Anal. Appl. Pyrolysis 2024, 183, 106805. [Google Scholar] [CrossRef]
- Kumar, P.; Dave, A.; Reddy, S.N.; Nanda, S. Hydrothermal gasification of waste biomass and plastics into hydrogen-rich syngas: A review. Environ. Chem. Lett. 2025, 23, 117–138. [Google Scholar] [CrossRef]
- Okolie, J.A.; Rana, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass: A state-of-the-art Review of process parameters, reaction mechanisms and catalysis. Sustain. Energy Fuels 2019, 3, 578–598. [Google Scholar] [CrossRef]
- Castello, D.; Kruse, A.; Fiori, L. Low temperature supercritical water gasification of biomass constituents: Glucose/phenol mixtures. Biomass Bioenergy 2015, 73, 84–94. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, L. Treatment of municipal sewage sludge in supercritical water: A review. Water Res. 2016, 89, 118–131. [Google Scholar] [CrossRef]
- Smith, J.; Brown, L.; Nguyen, T. Sustainable biohydrogen production from lignocellulosic biomass sources. Environ. Sci. Pollut. Res. 2023, 30, 62914–62930. [Google Scholar]
- Chen, X.; Li, Y.; Zhang, H. Catalytic influence of alkali and alkali earth metals in black liquor gasification. Biomass Convers. Biorefinery 2022, 12, 4171–4183. [Google Scholar]
- Jones, M.; Patel, D.; Lee, S. Comprehensive review of biomass utilization and gasification for sustainable bioenergy development. Environ. Dev. Sustain. 2021, 23, 14938–14958. [Google Scholar]
- Kumar, R.; Singh, S.; Verma, D. Advancing lignocellulosic biomass pretreatment with nanotechnology: A review. Cellulose 2024, 32, 1235–1250. [Google Scholar]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage fiber analysis. In Agriculture Handbook, Superintendent of Documents; US Government Printing Office: Washington, DC, USA, 1970; pp. 829–835. [Google Scholar]
- Gökkaya, D.S.; Saglam, M.; Yuksel, M.; Ballice, L. Hydrothermal gasificationof xylose: Effects of reaction temperature, pressure, and K2CO3 as a catalyston product distribution. Biomass Bioenergy 2016, 91, 26–36. [Google Scholar] [CrossRef]






| Biomass | |
|---|---|
| Proximate analysis (wt%) | |
| Moisture | 9.20 |
| Ash | 2.35 |
| Protein | 2.05 |
| Ultimate analysis (dry, wt%) | |
| C | 36.80 |
| H | 6.70 |
| N | 0.40 |
| S | 0.04 |
| O (from difference) | 42.46 |
| Components (daf, wt%) | Biomass |
|---|---|
| Cellulose | 36.55 |
| Lignin | 16.24 |
| Hemicellulose | 41.45 |
| Extractives | 4.76 |
| Non-Catalytic | K2CO3 | KOH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction Temp. (°C) | 300 | 400 | 500 | 600 | 300 | 400 | 500 | 600 | 300 | 400 | 500 | 600 |
| Reactor Pres. (MPa) | 12.0 | 24.0 | 36.0 | 45.0 | 11.5 | 24.5 | 35.5 | 45.5 | 12.5 | 24.0 | 36.0 | 45.5 |
| Product yield (C %) | ||||||||||||
| Gas | 24.4 | 32.3 | 58.4 | 77.6 | 27.0 | 35.7 | 66.2 | 86.4 | 28.2 | 43.9 | 76.1 | 92.7 |
| Liquid | 49.1 | 43.8 | 22.1 | 11.2 | 54.2 | 46.4 | 18.6 | 6.4 | 60.8 | 46.2 | 17.6 | 3.8 |
| Residue | 22.8 | 20.5 | 16.7 | 7.4 | 15.1 | 13.5 | 11 | 4.5 | 8.7 | 7.4 | 4.5 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvi Gökkaya, D. The Potential of Apricot Tree Resin as a Viable Feedstock for High-Value Chemicals via Hydrothermal Gasification. Catalysts 2025, 15, 425. https://doi.org/10.3390/catal15050425
Selvi Gökkaya D. The Potential of Apricot Tree Resin as a Viable Feedstock for High-Value Chemicals via Hydrothermal Gasification. Catalysts. 2025; 15(5):425. https://doi.org/10.3390/catal15050425
Chicago/Turabian StyleSelvi Gökkaya, Dilek. 2025. "The Potential of Apricot Tree Resin as a Viable Feedstock for High-Value Chemicals via Hydrothermal Gasification" Catalysts 15, no. 5: 425. https://doi.org/10.3390/catal15050425
APA StyleSelvi Gökkaya, D. (2025). The Potential of Apricot Tree Resin as a Viable Feedstock for High-Value Chemicals via Hydrothermal Gasification. Catalysts, 15(5), 425. https://doi.org/10.3390/catal15050425







