Recent Advances in Catalytic Atroposelective Synthesis of Axially Chiral Quinazolinones
Abstract
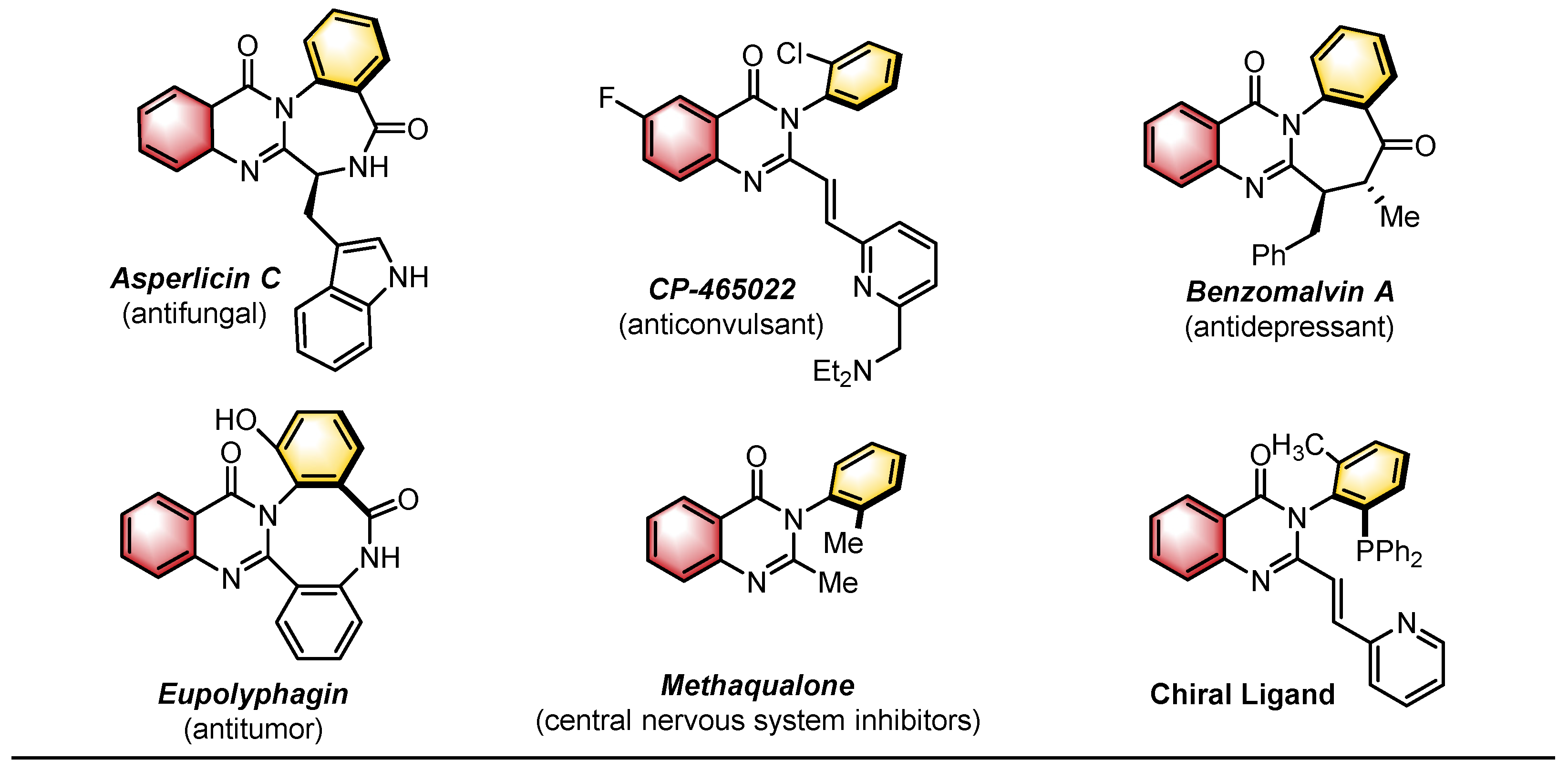
1. Introduction
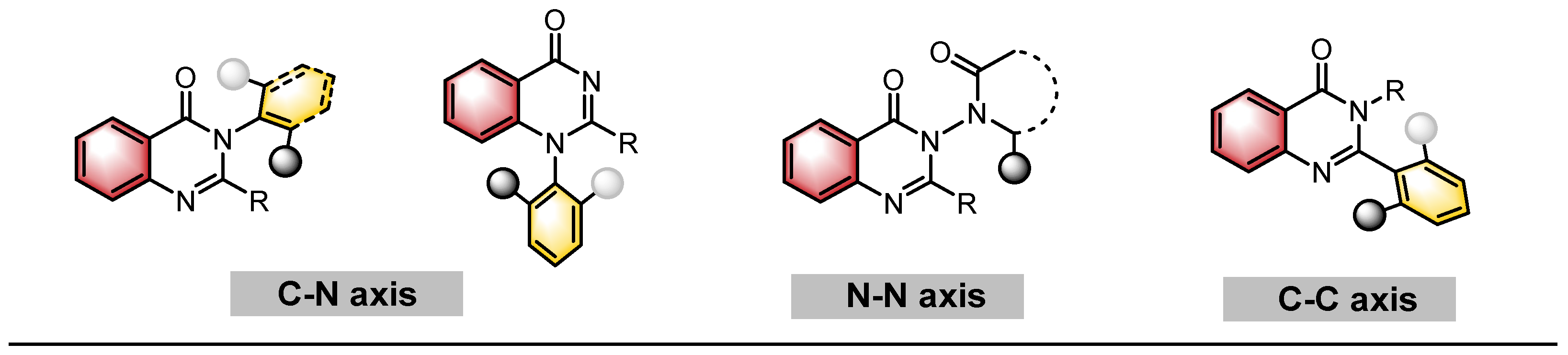
2. Catalytic Asymmetric Synthesis of Axially Chiral Quinazolinones
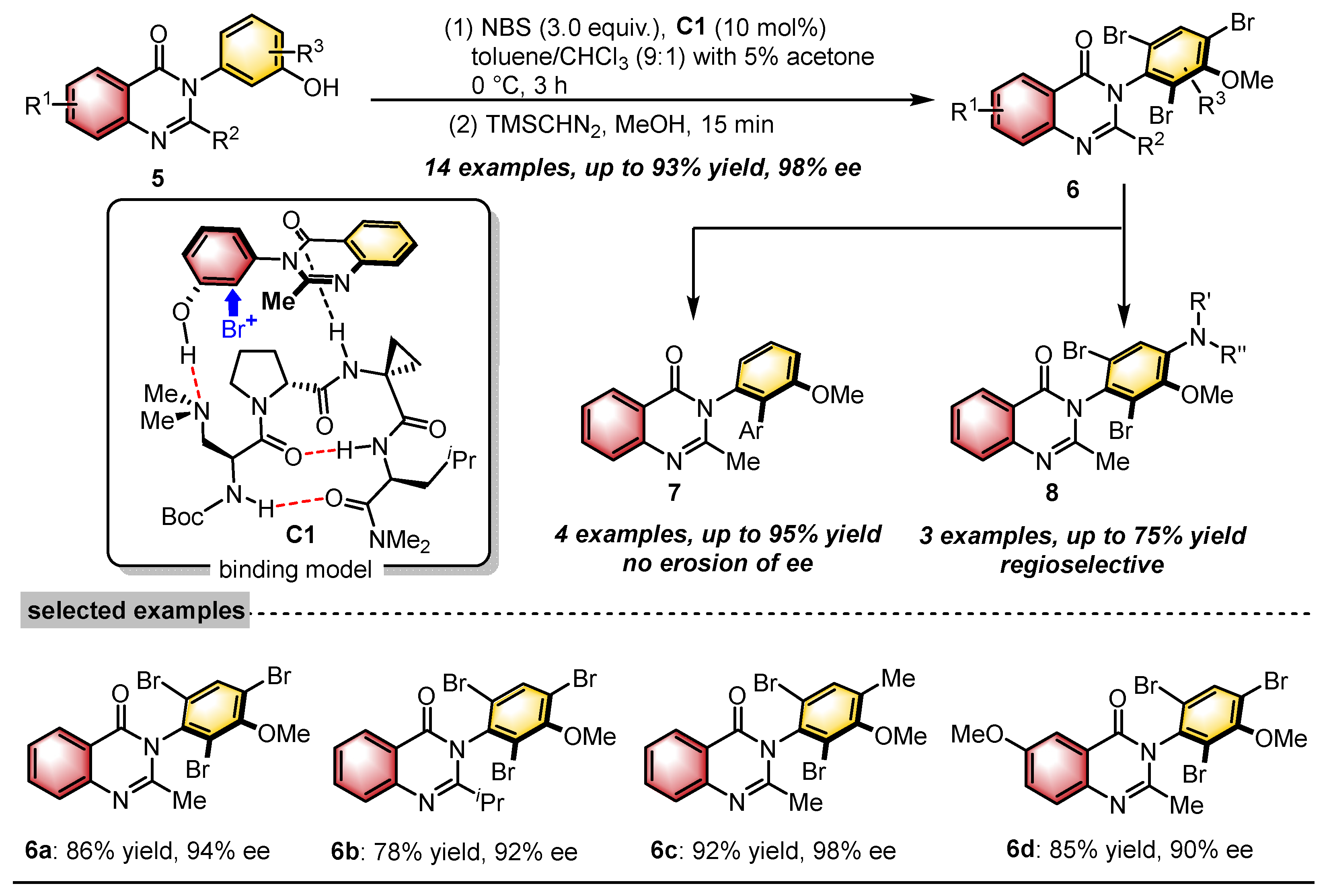
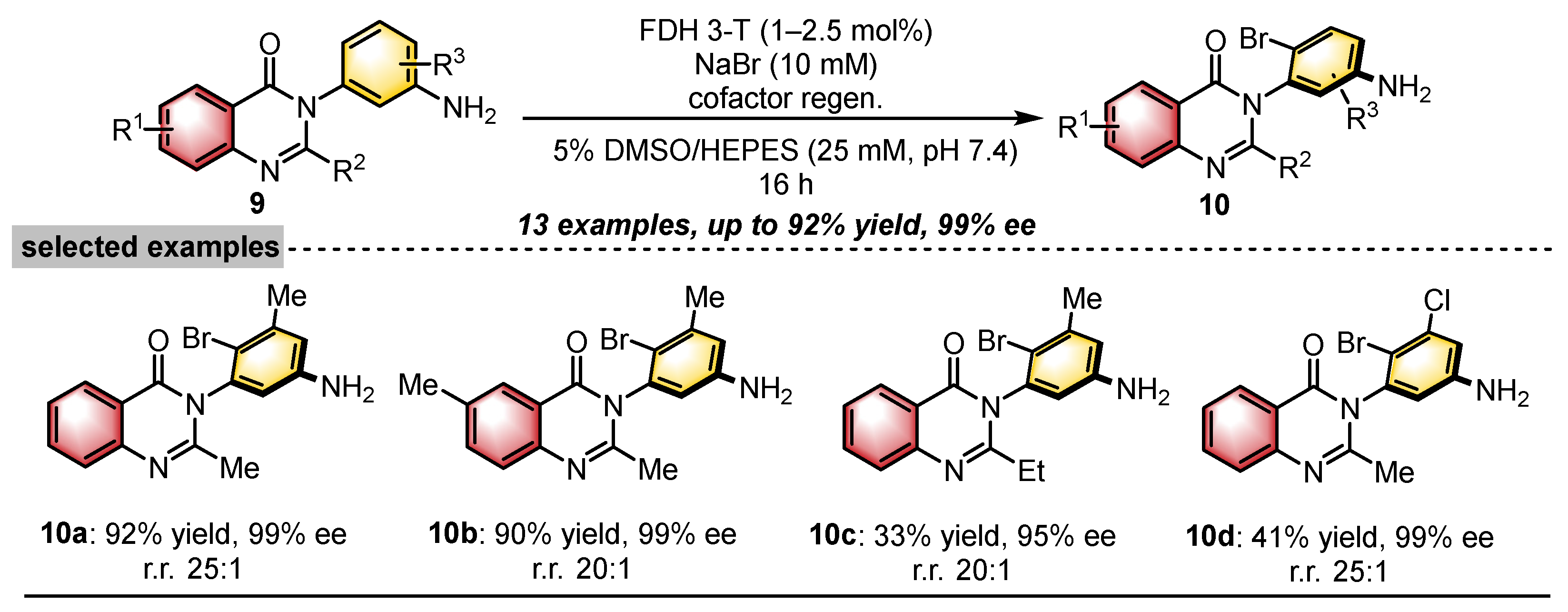
2.1. Quinazolinones with C–N Axial Chirality
2.2. Quinazolinones with N–N Axial Chirality
2.3. Quinazolinones with C–C Axial Chirality
3. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf, Z.; Iqbal, J.; Saeed, A. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: An update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 2016, 24, 2361–2381. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, U.A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef]
- Alsibaee, A.M.; Al-Yousef, H.M.; Al-Salem, H.S. Quinazolinones, the Winning Horse in Drug Discovery. Molecules 2023, 28, 978. [Google Scholar] [CrossRef]
- Gatadi, S.; Pulivendala, G.; Gour, J.; Malasala, S.; Bujji, S.; Parupalli, R.; Shaikh, M.; Godugu, C.; Nanduri, S. Synthesis and evaluation of new 4(3H)-Quinazolinone derivatives as potential anticancer agents. J. Mol. Struct. 2020, 1200, 127097. [Google Scholar] [CrossRef]
- Ghodge, B.; Kshirsagar, A.; Navghare, V. Synthesis, characterization, and investigation of the anti-inflammatory effect of 2,3-disubstituted quinazoline-4(1H)-one. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 30. [Google Scholar] [CrossRef]
- Bouley, R.; Ding, D.; Peng, Z.; Bastian, M.; Lastochkin, E.; Song, W.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Mobashery, S.; et al. Structure–Activity Relationship for the 4(3H)-Quinazolinone Antibacterials. J. Med. Chem. 2016, 59, 5011–5021. [Google Scholar] [CrossRef]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.S.; Seshadri, S. Chromophoric potential of the 4(3H)-quinazolinones: A review. Color. Technol. 2004, 120, 101–107. [Google Scholar] [CrossRef]
- Xing, Z.; Wu, W.; Miao, Y.; Tang, Y.; Zhou, Y.; Zheng, L.; Fu, Y.; Song, Z.; Peng, Y. Recent advances in quinazolinones as an emerging molecular platform for luminescent materials and bioimaging. Org. Chem. Front. 2021, 8, 1867–1889. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, J.; Wu, X. Recent advances in 4(3H)-quinazolinone syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- Tlili, A.; Blondiaux, E.; Frogneux, X.; Cantat, T. Reductive functionalization of CO2 with amines: An entry to formamide, formamidine and methylamine derivatives. Green Chem. 2015, 17, 157–168. [Google Scholar] [CrossRef]
- Rohokale, R.S.; Kshirsagar, U.A. Advanced synthetic strategies for constructing quinazolinone scaffolds. Synthesis 2016, 48, 1253–1268. [Google Scholar] [CrossRef]
- Hemalatha, K.; Madhumitha, G. Synthetic strategy with representation on mechanistic pathway for the therapeutic applications of dihydroquinazolinones. Eur. J. Med. Chem. 2016, 123, 596–630. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Sivaramakrishna, A. Remarkably flexible quinazolinones-synthesis and biological applications. J. Heterocycl. Chem. 2020, 57, 942–954. [Google Scholar] [CrossRef]
- Cheng, J.; Xiang, S.; Li, S.; Ye, L.; Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 2021, 121, 4805–4902. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.; Koay, W.; Guan, C.; Lu, Y. Atropisomers beyond the C-C axial chirality: Advances in catalytic asymmetric synthesis. Chem 2022, 8, 1855–1893. [Google Scholar] [CrossRef]
- Xiang, S.; Ding, W.; Wang, Y.; Tan, B. Catalytic atroposelective synthesis. Nat. Catal. 2024, 7, 483–498. [Google Scholar] [CrossRef]
- Zhang, H.; Li, T.; Liu, S.; Shi, F. Catalytic asymmetric synthesis of atropisomers bearing multiple chiral elements: An emerging field. Angew. Chem. Int. Ed. 2024, 63, e202311053. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, X.; Wang, X.; Yang, J.; Yao, X.; Crews, P.; Valeriote, F.A.; Wu, Q. New isocoumarins and alkaloid from Chinese insect medicine, Eupolyphaga sinensis Walker. Fitoterapia 2012, 83, 1275–1280. [Google Scholar] [CrossRef]
- Gangadhar, N.M.; Stockwell, B.R. Chemical genetic approaches to probing cell death. Curr. Opin. Chem. Biol. 2007, 11, 83–87. [Google Scholar] [CrossRef][Green Version]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J.; et al. RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 865–869. [Google Scholar] [CrossRef]
- Dai, X.; Wong, A.; Virgil, S.C. Synthesis and Resolution of Quinazolinone Atropisomeric Phosphine Ligands. J. Org. Chem. 1998, 63, 2597–2600. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Virgil, S. Asymmetric allylic alkylation catalyzed by palladium complexes with atropisomeric quinazolinone phosphine ligands. Tetrahedron Lett. 1999, 40, 1245–1248. [Google Scholar] [CrossRef]
- Tokitoh, T.; Kobayashi, T.; Nakada, E.; Inoue, T.; Yokoshima, S.; Takahashi, H.; Natsugari, H. Diastereoselective synthesis of atropisomeric 3-(2-substituted aryl)quinazolin-4-ones and their stereochemical properties. Heterocycles 2006, 70, 93–99. [Google Scholar] [CrossRef]
- Diener, M.E.; Metrano, A.J.; Kusano, S.; Miller, S.J. Enantioselective Synthesis of 3-Arylquinazolin-4(3H)-ones via Peptide-Catalyzed Atroposelective Bromination. J. Am. Chem. Soc. 2015, 137, 12369–12377. [Google Scholar] [CrossRef] [PubMed]
- Metrano, A.J.; Abascal, N.C.; Mercado, B.Q.; Paulson, E.K.; Miller, S.J. Structural studies of β-turn-containing peptide catalysts for atroposelective quinazolinone bromination. Chem. Commun. 2016, 52, 4816–4819. [Google Scholar] [CrossRef]
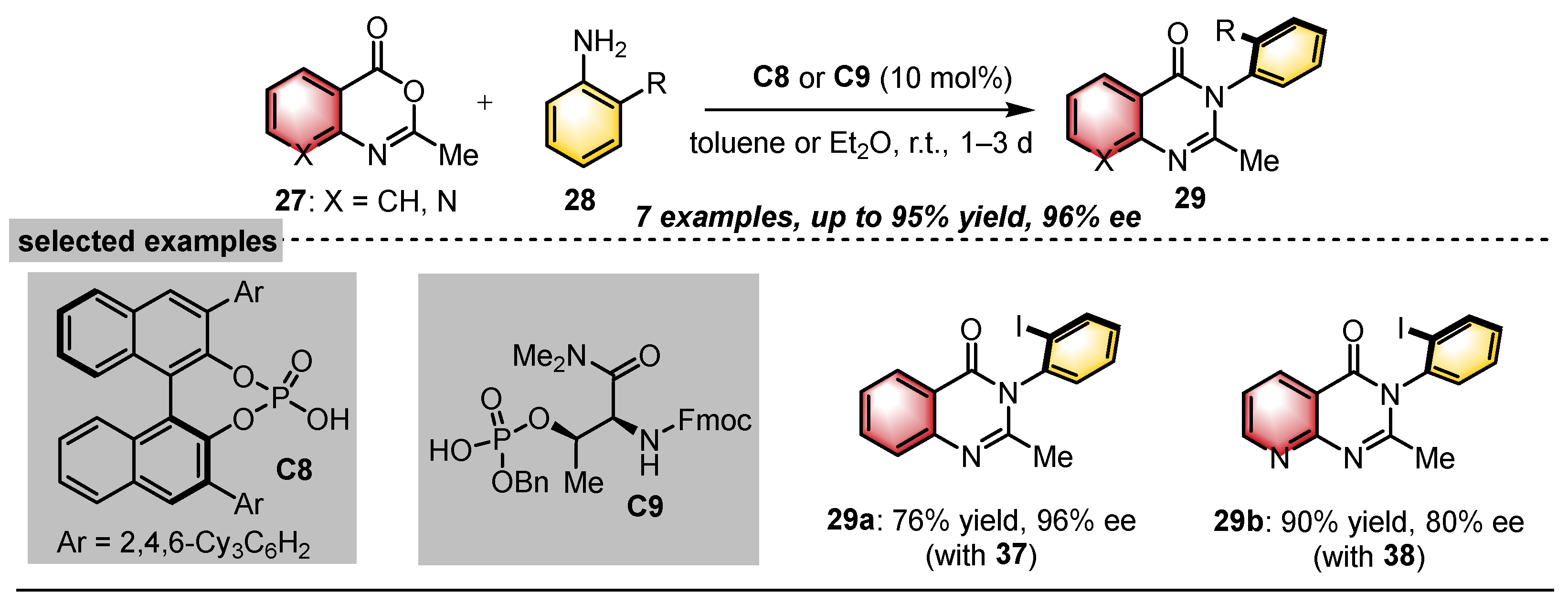
- Snodgrass, H.M.; Mondal, D.; Lewis, J.C. Directed Evolution of Flavin-Dependent Halogenases for Site- and Atroposelective Halogenation of 3-Aryl-4(3H)-Quinazolinones via Kinetic or Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2022, 144, 16676–16682. [Google Scholar] [CrossRef]
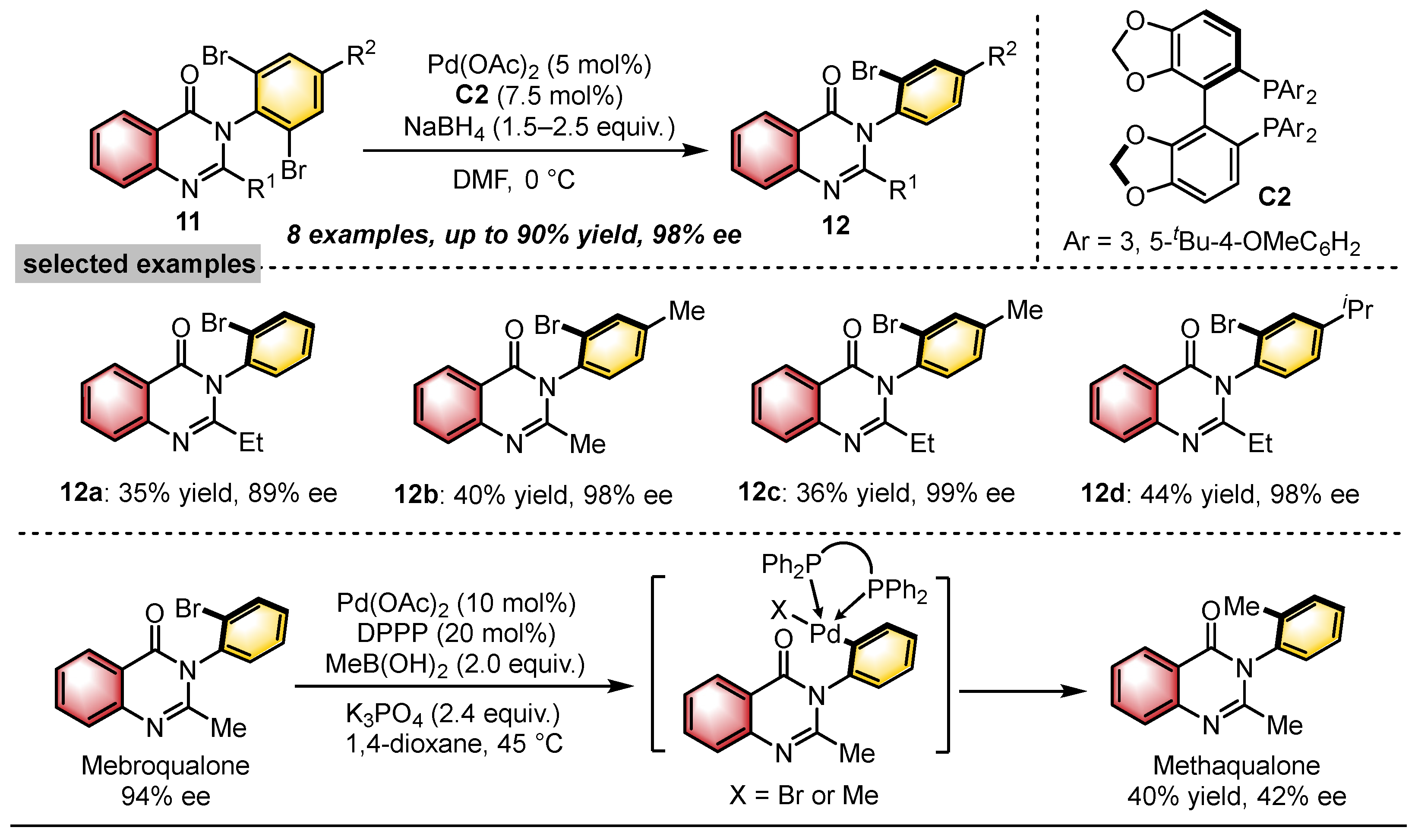
- Hirai, M.; Terada, S.; Yoshida, H.; Ebine, K.; Hirata, T.; Kitagawa, O. Catalytic Enantioselective Synthesis of N–C Axially Chiral Mebroqualone and its Derivatives through Reductive Asymmetric Desymmetrization. Org. Lett. 2016, 18, 5700–5703. [Google Scholar] [CrossRef]
- Matsuoka, M.; Iida, A.; Kitagawa, O. α-Alkylation of N-C Axially Chiral Quinazolinone Derivatives Bearing Various ortho -Substituted Phenyl Groups: Relation between Diastereoselectivity and the ortho-Substituent. Synlett 2018, 29, 2126–2130. [Google Scholar] [CrossRef]
- Iida, A.; Matsuoka, M.; Hasegawa, H.; Vanthuyne, N.; Farran, D.; Roussel, C.; Kitagawa, O. N–C Axially Chiral Compounds with an ortho-Fluoro Substituent and Steric Discrimination between Hydrogen and Fluorine Atoms Based on a Diastereoselective Model Reaction. J. Org. Chem. 2019, 84, 3169–3175. [Google Scholar] [CrossRef]
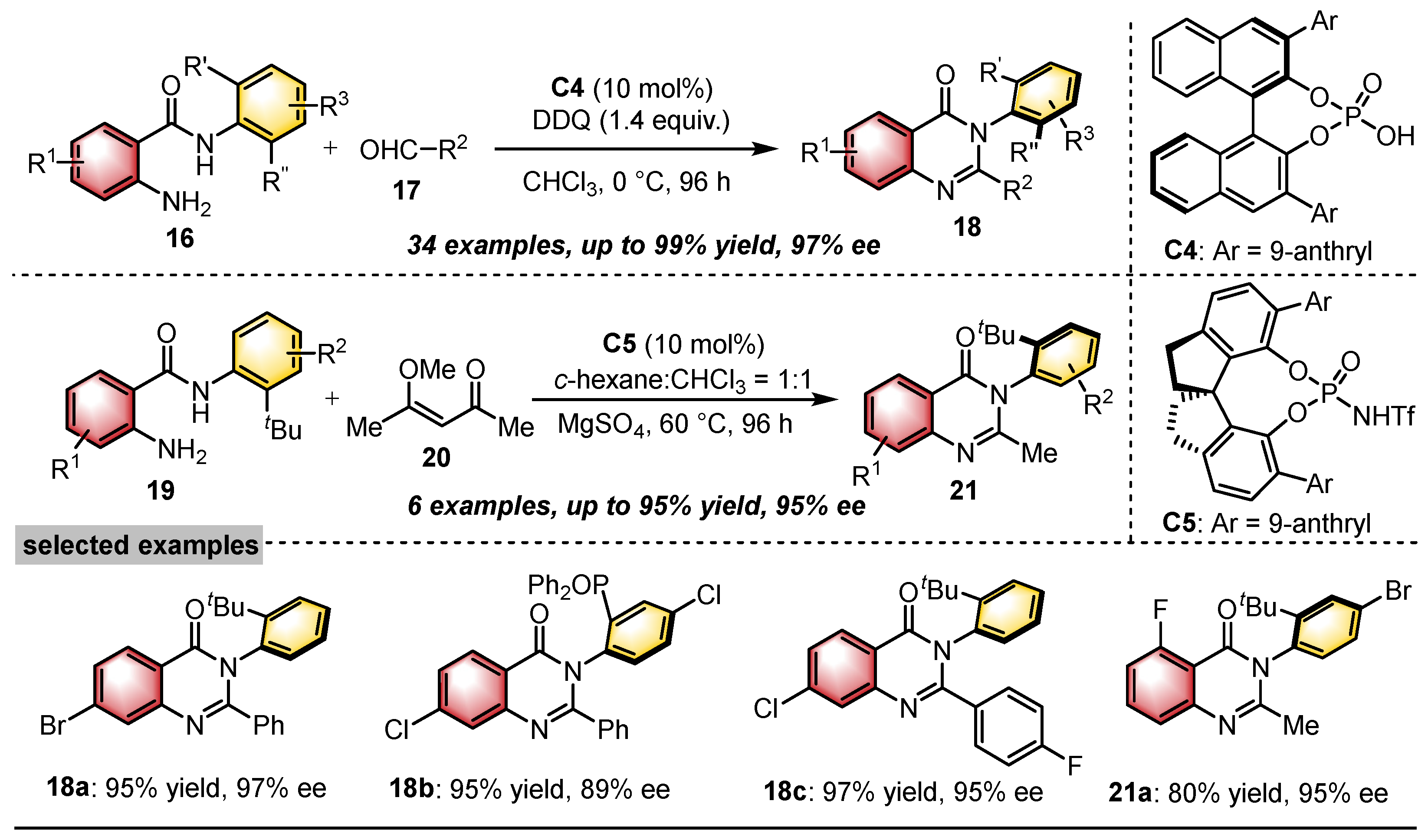
- Yoon, H.; Galls, A.; Rozema, S.D.; Miller, S.J. Atroposelective Desymmetrization of Resorcinol-Bearing Quinazolinones via Cu-Catalyzed C–O Bond Formation. Org. Lett. 2022, 24, 762–766. [Google Scholar] [CrossRef] [PubMed]
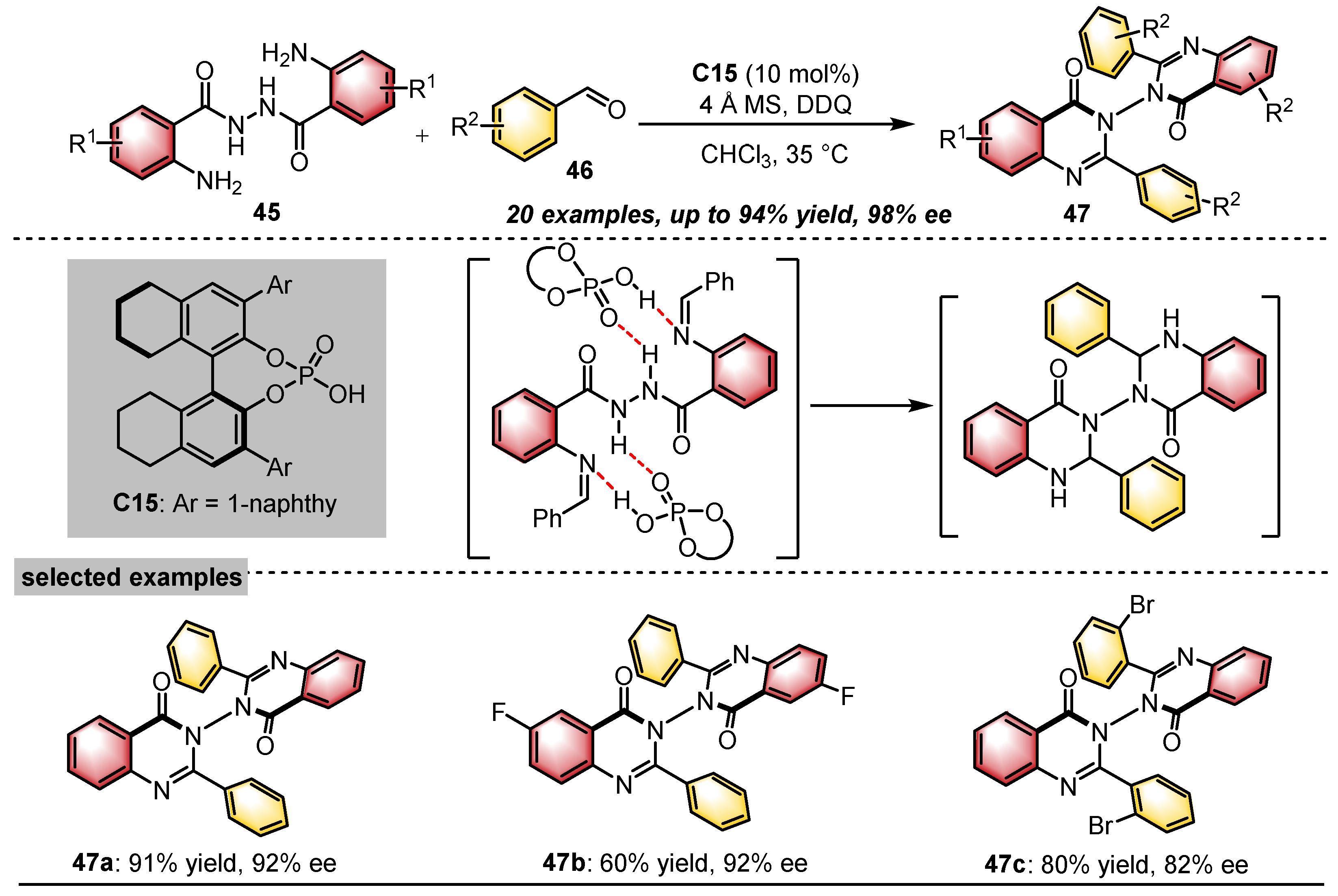
- Wang, Y.; Zheng, S.; Hu, Y.; Tan, B. Brønsted acid-catalysed enantioselective construction of axially chiral arylquinazolinones. Nat. Commun. 2017, 8, 15489. [Google Scholar] [CrossRef] [PubMed]
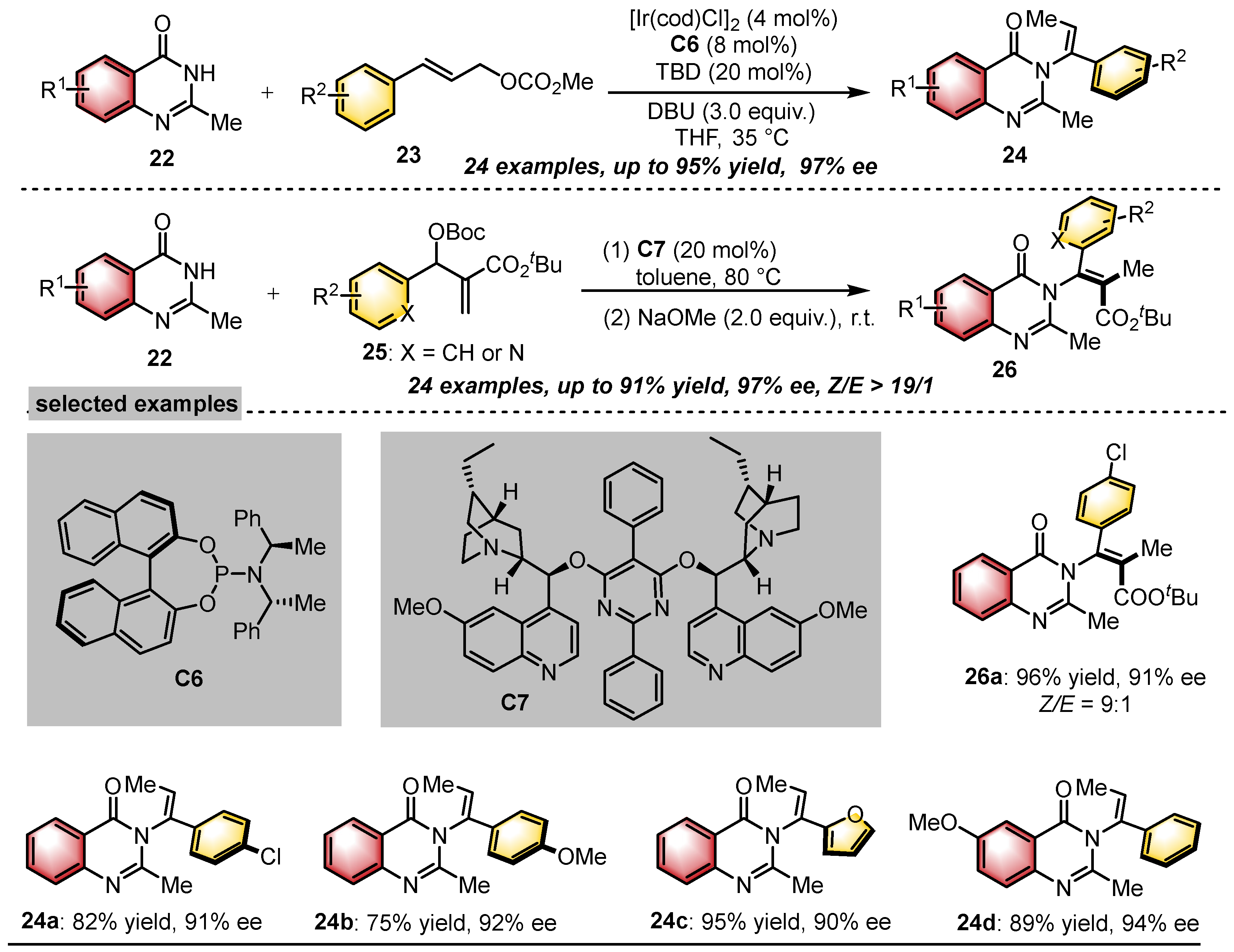
- Zou, J.Y.; Yang, Y.Y.; Gu, J.; Liu, F.; Ye, Z.; Yi, W.; He, Y. Asymmetric Allylic Substitution-Isomerization for the Modular Synthesis of Axially Chiral N-Vinylquinazolinones. Angew. Chem. Int. Ed. 2023, 62, e202310320. [Google Scholar] [CrossRef]
- Guo, M.C.; Miller, S.J. Catalyst–Substrate Pairings for Carbocyclic and Heterocyclic Systems in Atroposelective Quinazolinone Synthesis. ACS Catal. 2024, 14, 17226–17232. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, L. Construction of axially chiral molecules enabled by photoinduced enantioselective reactions. Chem. Sci. 2024, 15, 12636–12643. [Google Scholar] [CrossRef]
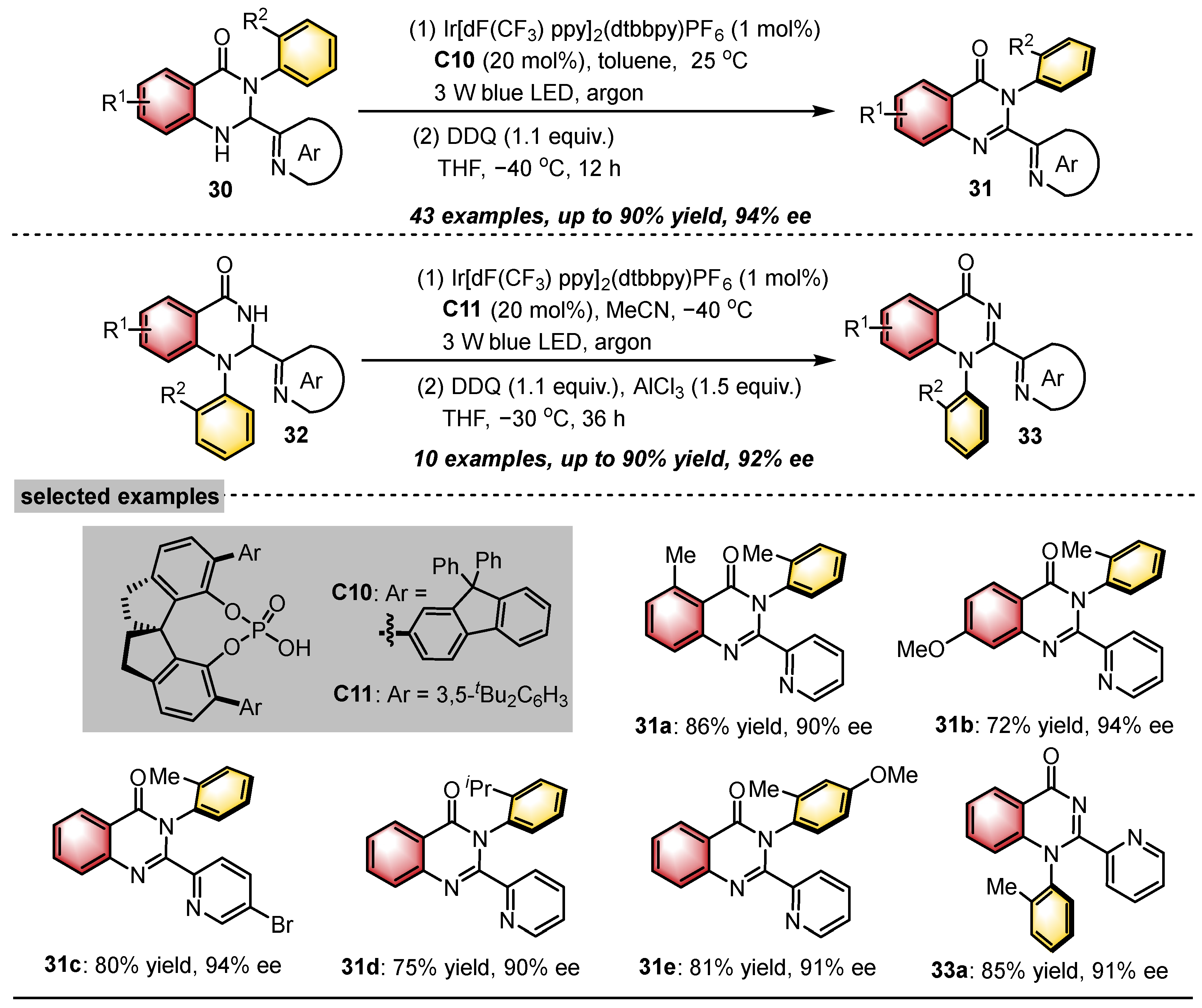
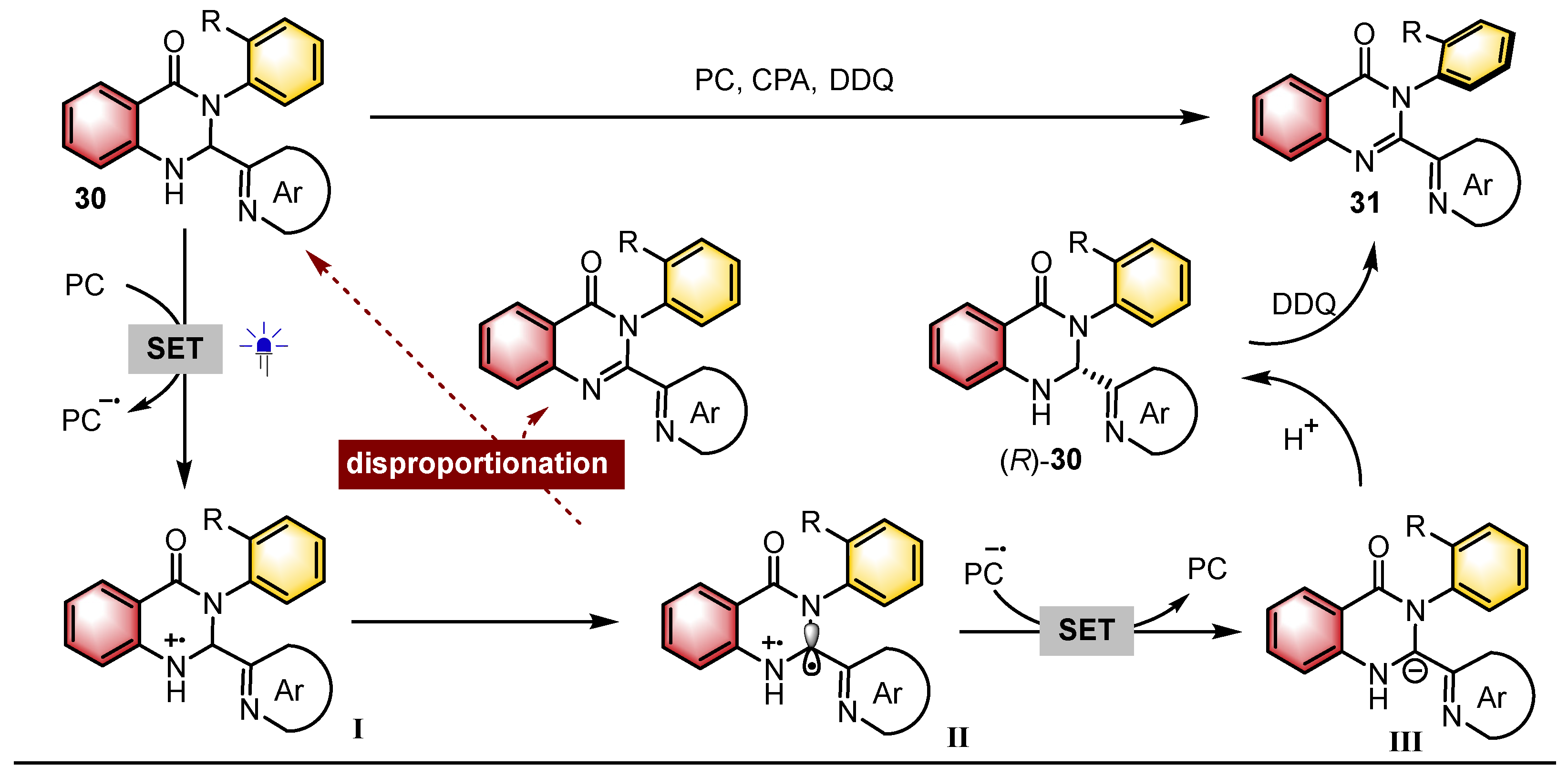
- Liu, Y.; Chu, M.; Li, X.; Cao, Z.; Zhao, X.; Yin, Y.; Jiang, Z. Photoredox Catalytic Deracemization Enabled Enantioselective and Modular Access to Axially Chiral N-Arylquinazolinones. Angew. Chem. Int. Ed. 2024, 63, e202411236. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Xu, Q.; Guo, C.; Zhang, D.; Lu, C.; Liu, R. Enantioselective Synthesis of Nitrogen–Nitrogen Biaryl Atropisomers via Copper-Catalyzed Friedel–Crafts Alkylation Reaction. J. Am. Chem. Soc. 2021, 143, 15005–15010. [Google Scholar] [CrossRef]
- Mei, G.; Wong, J.J.; Zheng, W.; Nangia, A.A.; Houk, K.N.; Lu, Y. Rational design and atroposelective synthesis of N–N axially chiral compounds. Chem 2021, 7, 2743–2757. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Q.; Li, Y.; Pan, M.; Yang, C.; Yang, G.; Li, X. Asymmetric synthesis of N–N axially chiral compoundsvia organocatalytic atroposelective N-acylation. Chem. Sci. 2021, 13, 141–148. [Google Scholar] [CrossRef]
- Pan, M.; Shao, Y.; Zhao, Q.; Li, X. Asymmetric Synthesis of N–N Axially Chiral Compounds by Phase-Transfer-Catalyzed Alkylations. Org. Lett. 2022, 24, 374–378. [Google Scholar] [CrossRef]
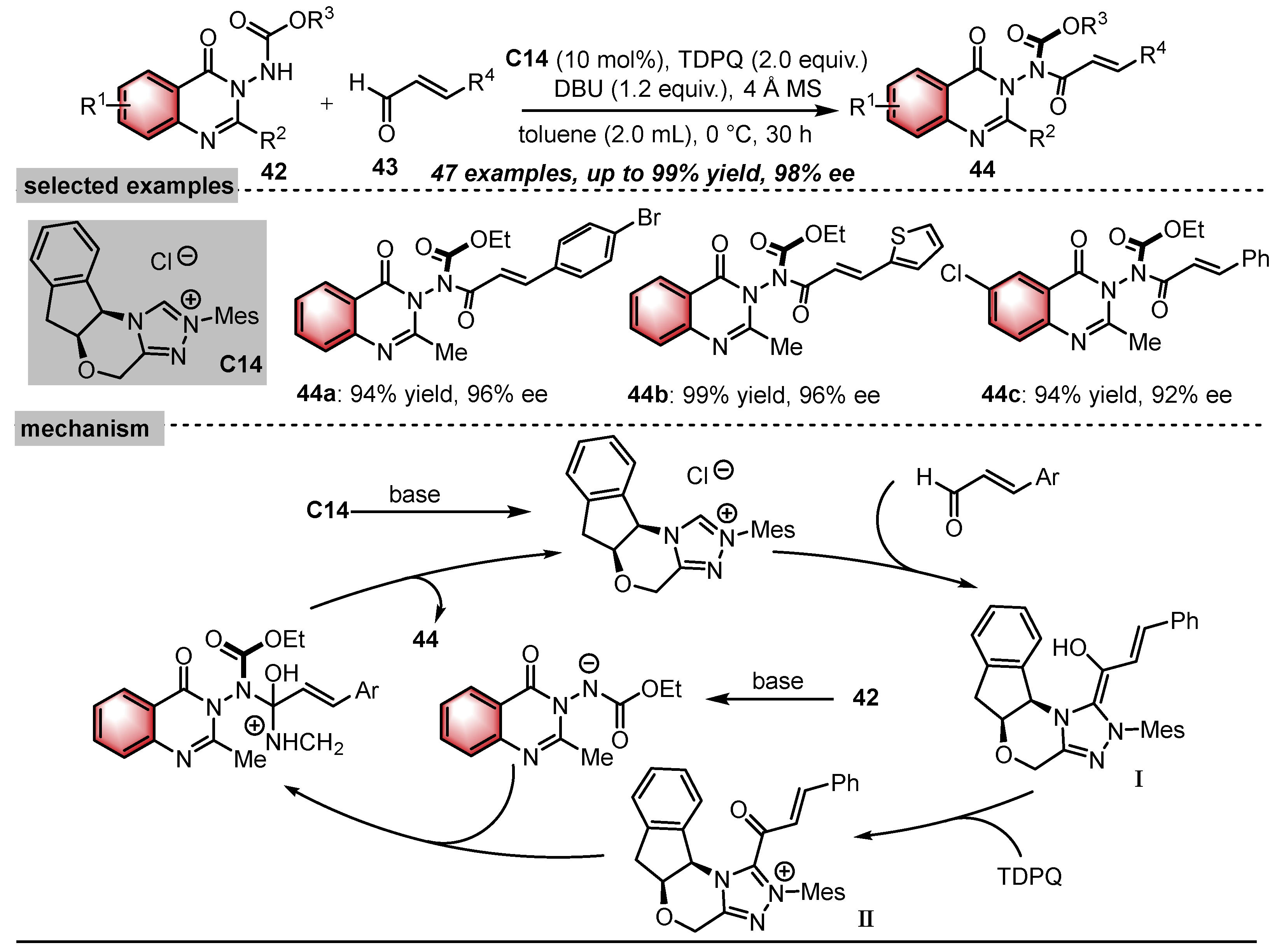
- Balanna, K.; Barik, S.; Barik, S.; Shee, S.; Manoj, N.; Gonnade, R.G.; Biju, A.T. N-Heterocyclic Carbene-Catalyzed Atroposelective Synthesis of N–N Axially Chiral 3-Amino Quinazolinones. ACS Catal. 2023, 13, 8752–8759. [Google Scholar] [CrossRef]
- Pu, L.; Zhang, Y.; Liu, W.; Teng, F. Chiral phosphoric acid-catalyzed dual-ring formation for enantioselective construction of N–N axially chiral 3,3′-bisquinazolinones. Chem. Commun. 2022, 58, 13131–13134. [Google Scholar] [CrossRef] [PubMed]
- Wen-Tao, W.; Zhang, S.; Lin, W.; Zhang-Hong, L.; Hu, D.; Huang, F.; Bai, R.; Yu, L.; Qian, L.; Jia-Yu, L. Catalytic stereodivergent and simultaneous construction of axial and point chirality. Org. Chem. Front. 2024, 11, 3308–3319. [Google Scholar] [CrossRef]
- Wang, N.; Gao, S.; Shu, Z.; Cheng, B.; Ma, C.; Zhang, Y.; Shi, F. Catalytic atroposelective synthesis of indolyl quinazolinones bearing N–N/C–C diaxes. Sci. China Chem. 2025, 68. [Google Scholar] [CrossRef]
- Teng, F.; Yu, T.; Peng, Y.; Hu, W.; Hu, H.; He, Y.; Luo, S.; Zhu, Q. Palladium-Catalyzed Atroposelective Coupling–Cyclization of 2-Isocyanobenzamides to Construct Axially Chiral 2-Aryl- and 2,3-Diarylquinazolinones. J. Am. Chem. Soc. 2021, 143, 2722–2728. [Google Scholar] [CrossRef]
- Gao, Z.; Qian, J.; Yang, H.; Zhang, J.; Jiang, G. Enantioselective Construction of C–C Axially Chiral Quinazolinones via Chirality Exchange and Phase-Transfer Catalysis. Org. Lett. 2021, 23, 1731–1737. [Google Scholar] [CrossRef]







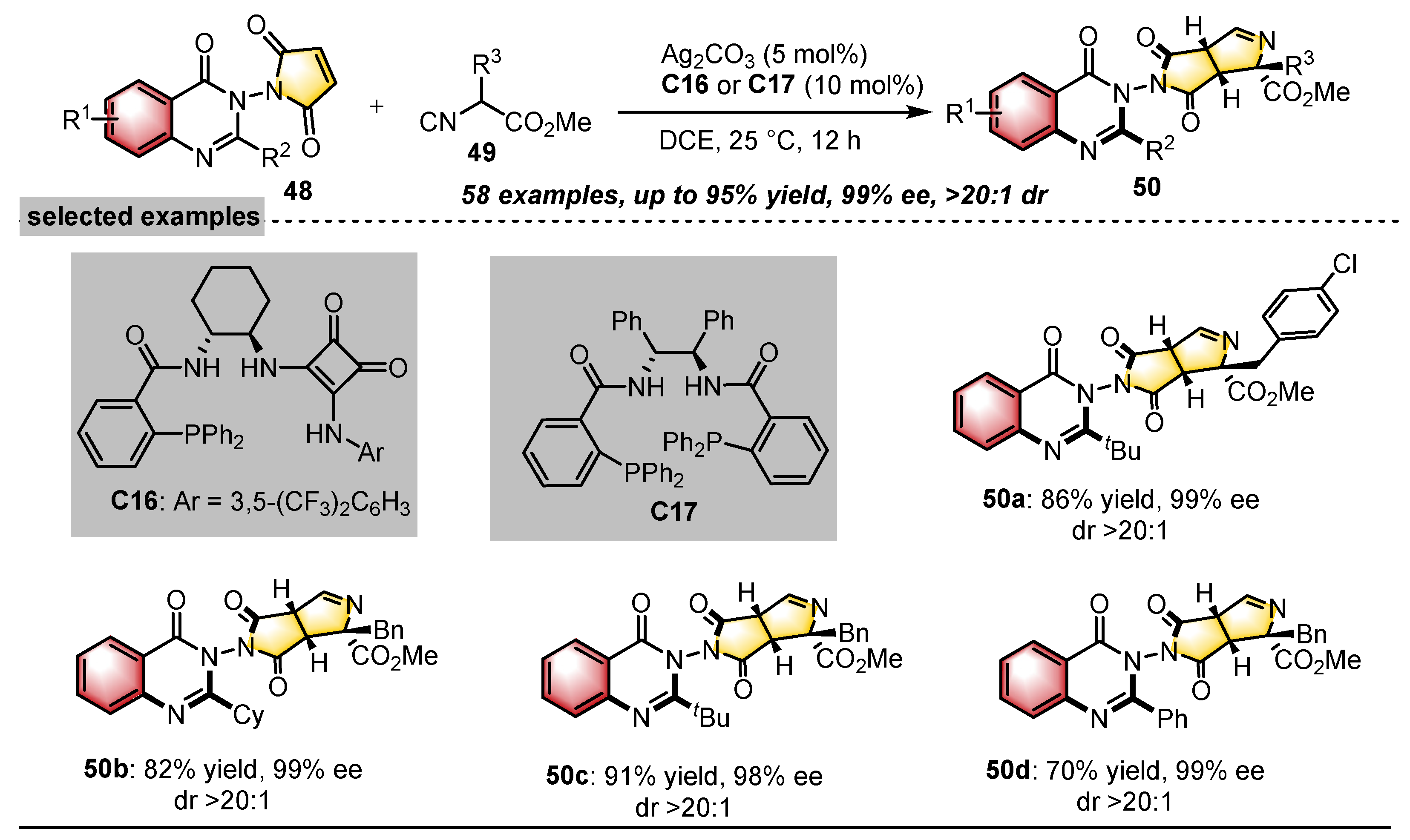
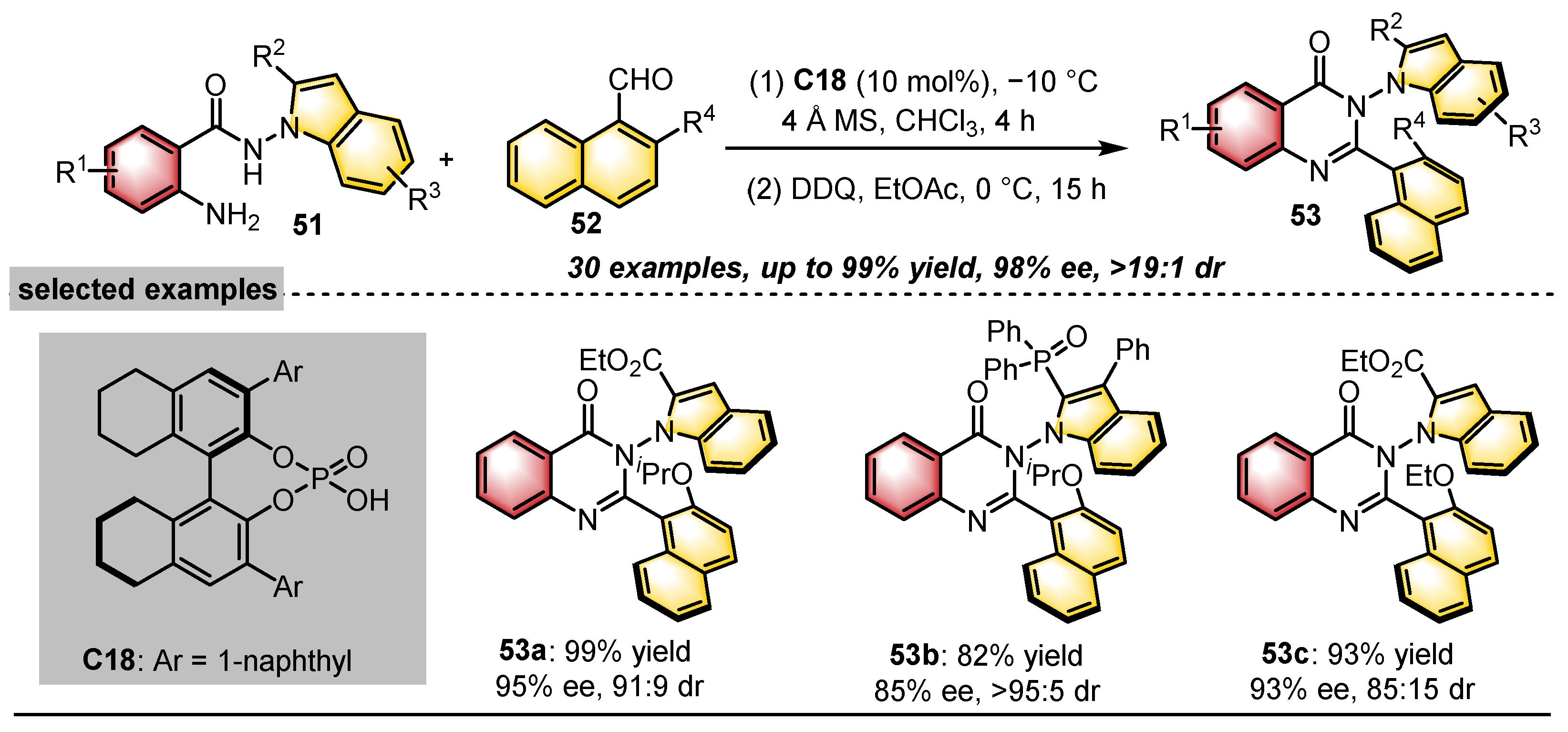
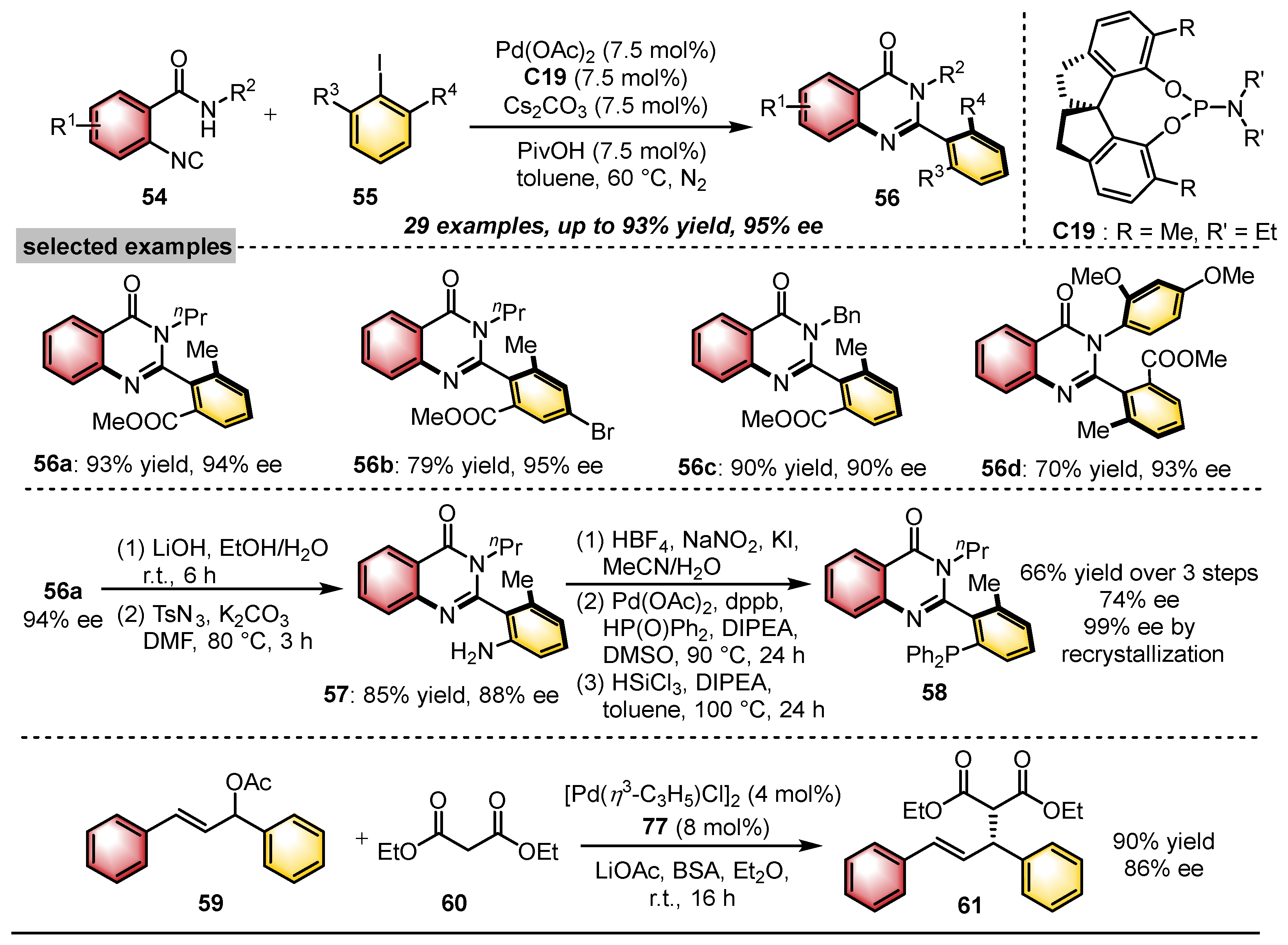
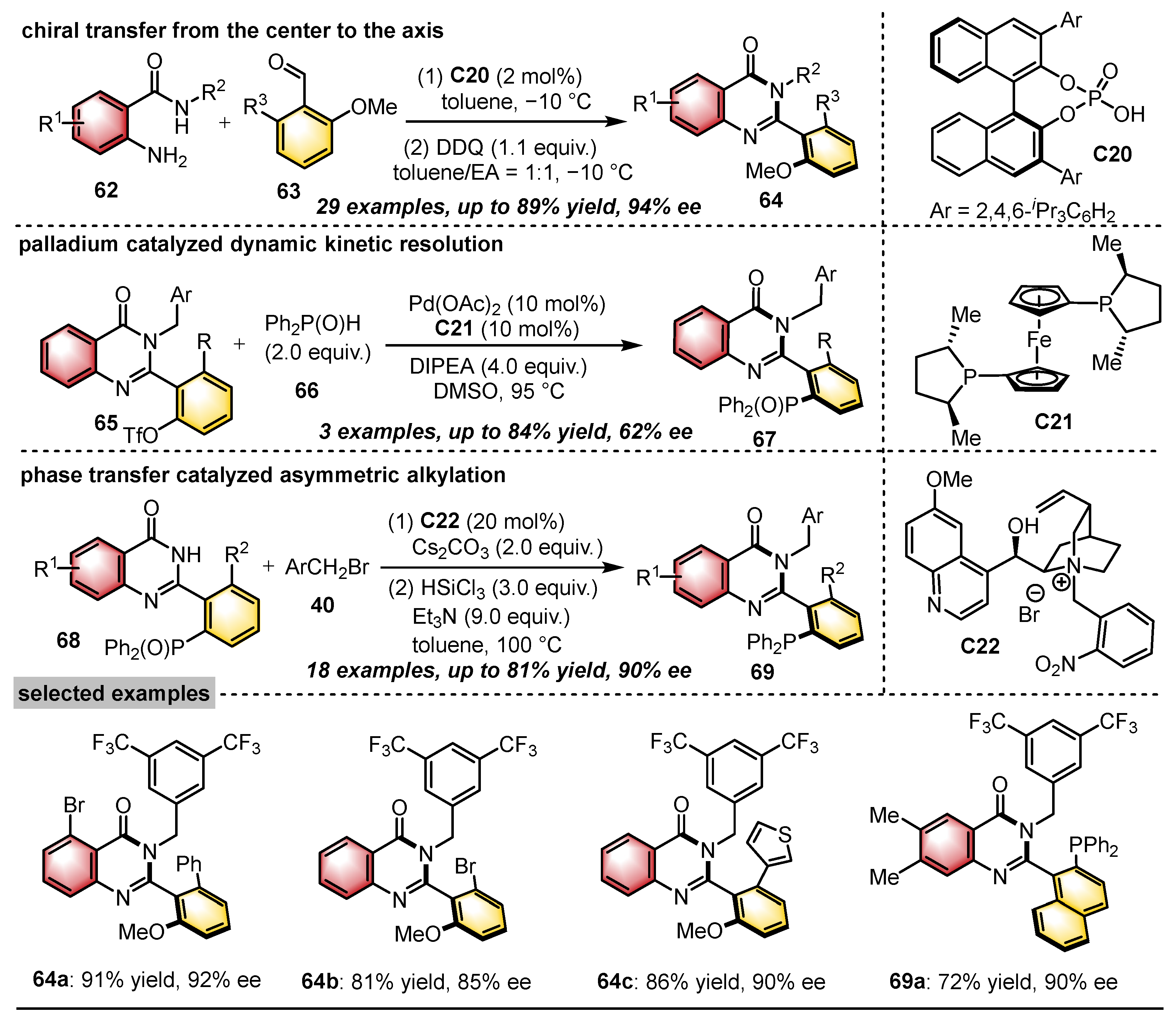
| Substrates | Products | Catalysts | Synthesis Strategies | References |
|---|---|---|---|---|
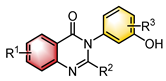 |  | Peptide | Atroposelective halogenation | [25] |
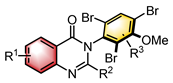
 |  | Enzyme | Atroposelective halogenation | [27] |
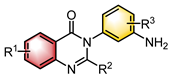
 |  | Pd(OAc)2 Chiral ligand | Desymmetrization | [28] |
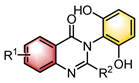 |  | CuI Chiral ligand | Desymmetrization | [31] |
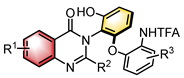
 |  | CPA | Condensation oxidation | [32] |
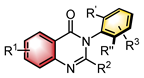
 | 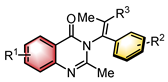 | [Ir(cod)Cl]2 Chiral ligand or Lewis base | N-H functionalization | [33] |
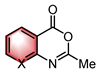 | 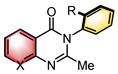 | CPA | Condensation | [34] |
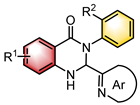 |  | [Ir]/CPA | Photoredox deracemization | [36] |
| Substrates | Products | Catalysts | Synthesis Strategies | References |
|---|---|---|---|---|
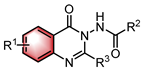 |  | Lewis base | N–H functionalization | [39] |
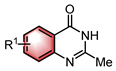
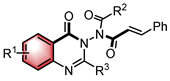 | Lewis base | N–H functionalization | [40] | |
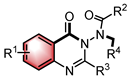 | PTC | N–H functionalization | [41] | |
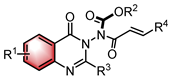 | NHC | N–H functionalization | [42] | |
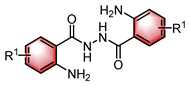 | 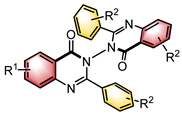 | CPA | Condensation oxidation | [43] |
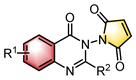 |  | Ag2CO3 Chiral ligand | Desymmetrization | [44] |
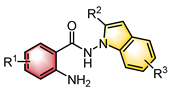 | 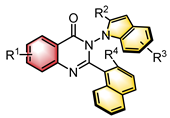 | CPA | Condensation oxidation | [45] |
| Substrates | Products | Catalysts | Synthesis Strategies | References |
|---|---|---|---|---|
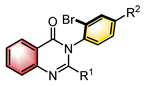
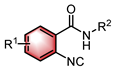 | 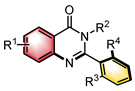 | Pd(OAc)2 Chiral ligand | Coupling cyclization | [45] |
 | 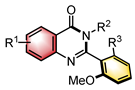 | CPA | Condensation oxidation | [46] |
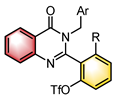 | 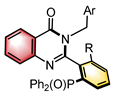 | Pd(OAc)2 Chiral ligand | Dynamic kinetic resolution | [46] |
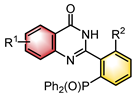 |  | PTC | N–H functionalization | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, J.; Yin, Y.; Jiang, Z. Recent Advances in Catalytic Atroposelective Synthesis of Axially Chiral Quinazolinones. Catalysts 2025, 15, 426. https://doi.org/10.3390/catal15050426
Liu Y, Wang J, Yin Y, Jiang Z. Recent Advances in Catalytic Atroposelective Synthesis of Axially Chiral Quinazolinones. Catalysts. 2025; 15(5):426. https://doi.org/10.3390/catal15050426
Chicago/Turabian StyleLiu, Yilin, Jiaoxue Wang, Yanli Yin, and Zhiyong Jiang. 2025. "Recent Advances in Catalytic Atroposelective Synthesis of Axially Chiral Quinazolinones" Catalysts 15, no. 5: 426. https://doi.org/10.3390/catal15050426
APA StyleLiu, Y., Wang, J., Yin, Y., & Jiang, Z. (2025). Recent Advances in Catalytic Atroposelective Synthesis of Axially Chiral Quinazolinones. Catalysts, 15(5), 426. https://doi.org/10.3390/catal15050426






